Preparation and Evaluation of Thermosensitive Liposomes Encapsulating I-125-Labeled Doxorubicin Derivatives for Auger Electron Therapy
Abstract
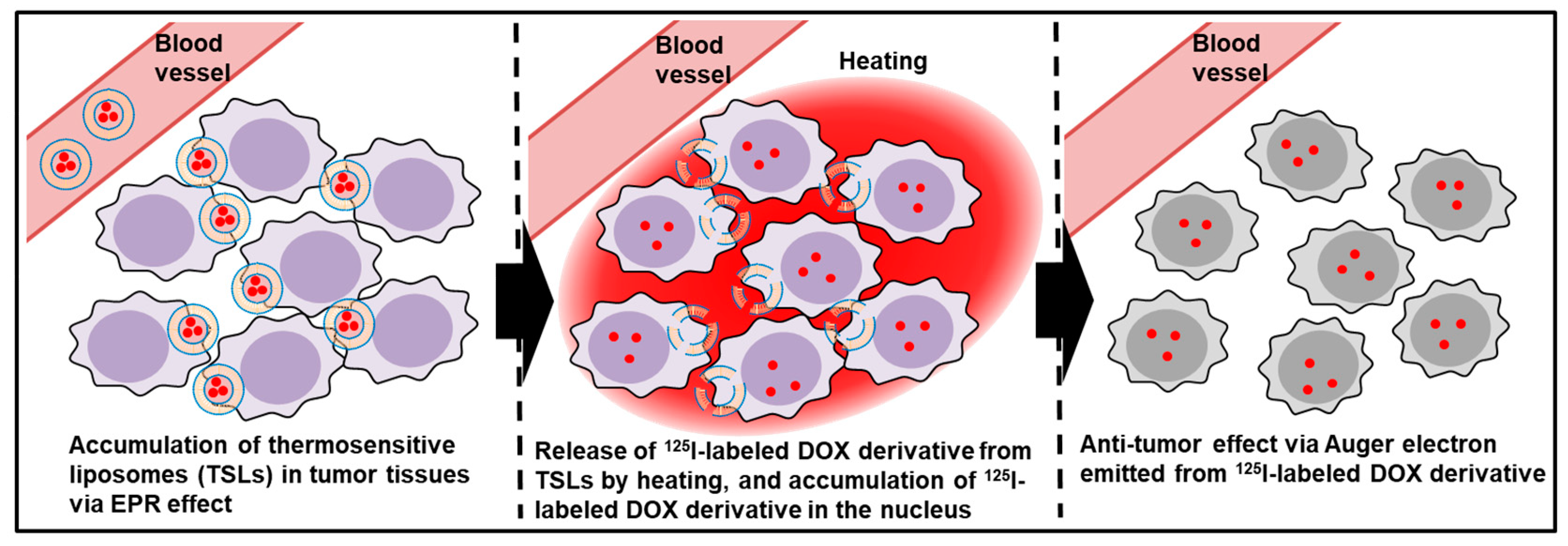
1. Introduction
2. Results and Discussion
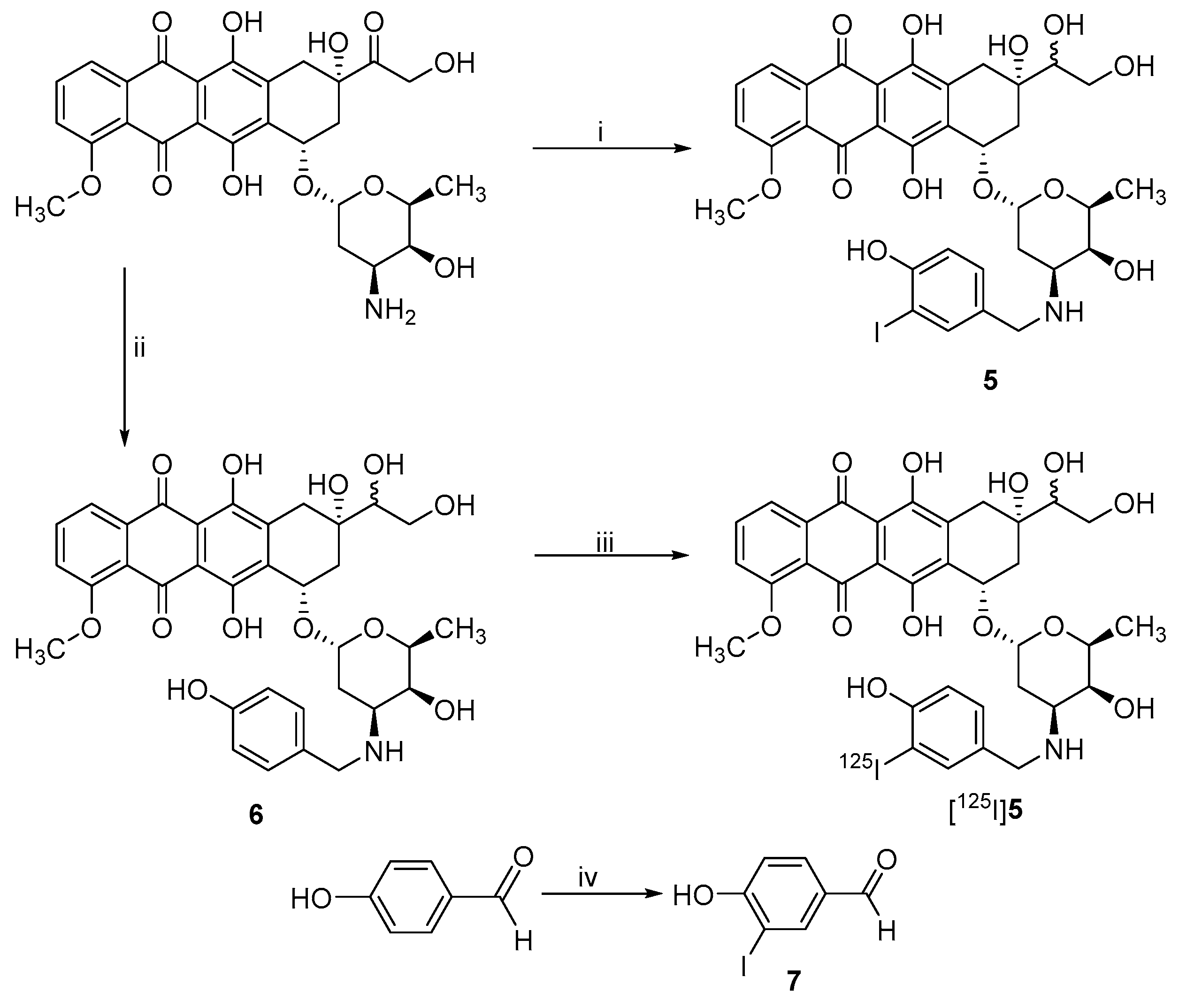
2.1. Synthesis of Non-Radioactive DOX Derivatives
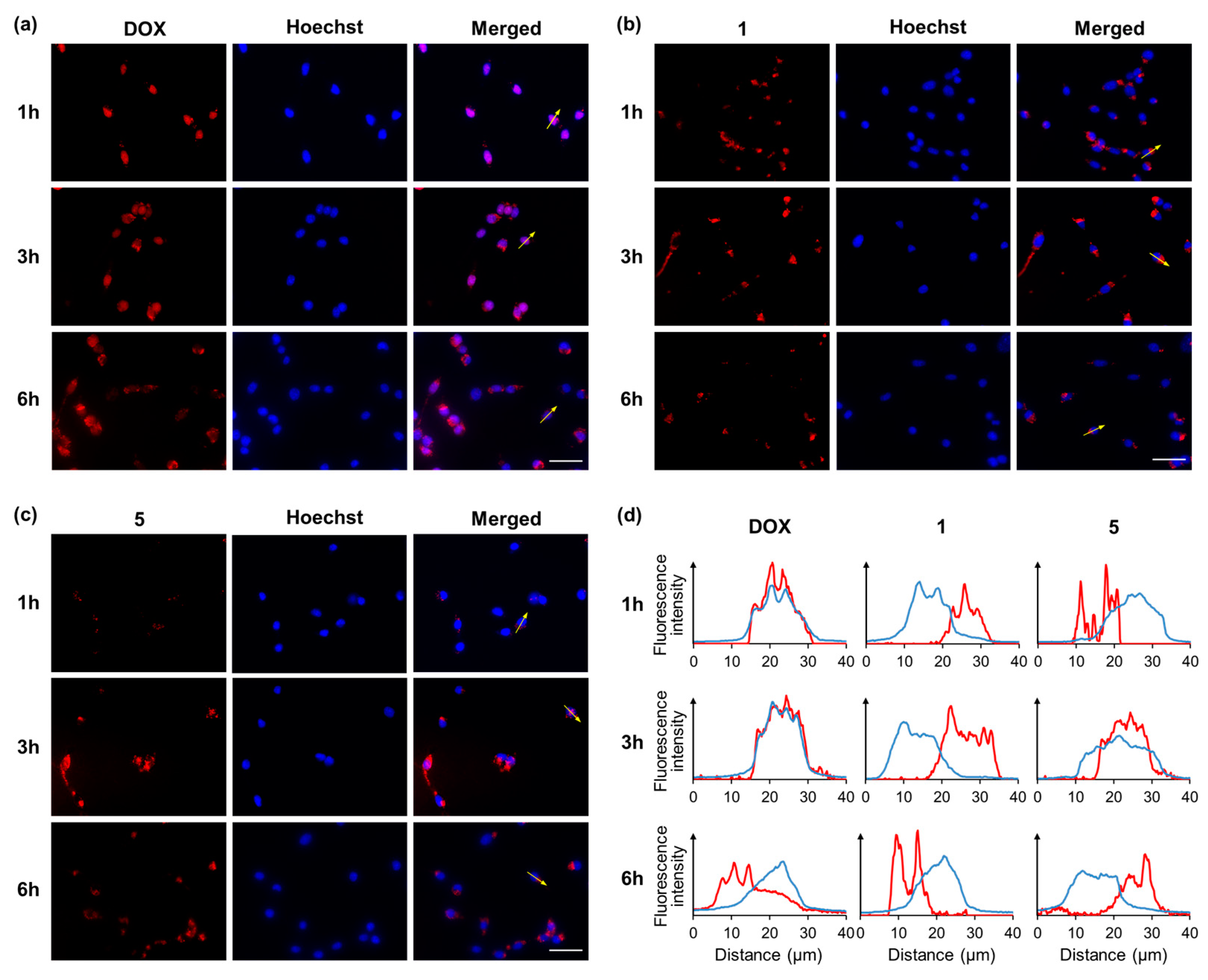
2.2. Intracellular Localization of DOX and DOX Derivatives
2.3. Radiolabeling
2.4. Determination of the Partition Coefficient
2.5. In Vitro Stability Assays
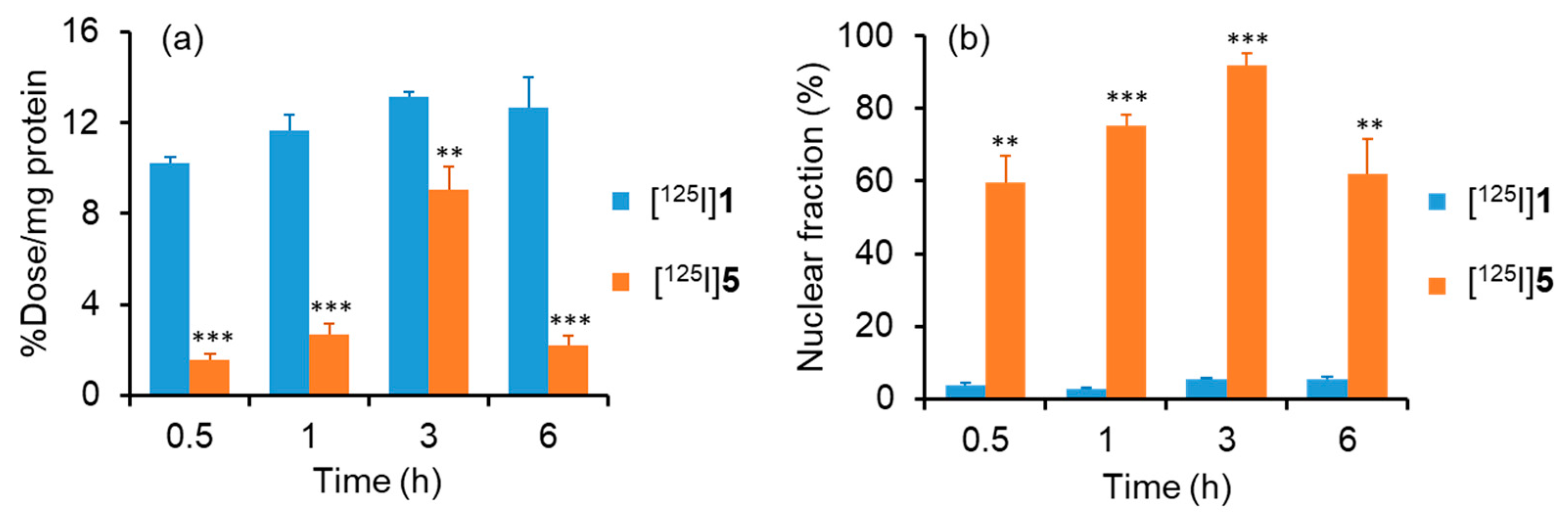
2.6. Cellular and Nuclear Uptake of [125I]1 and [125I]5
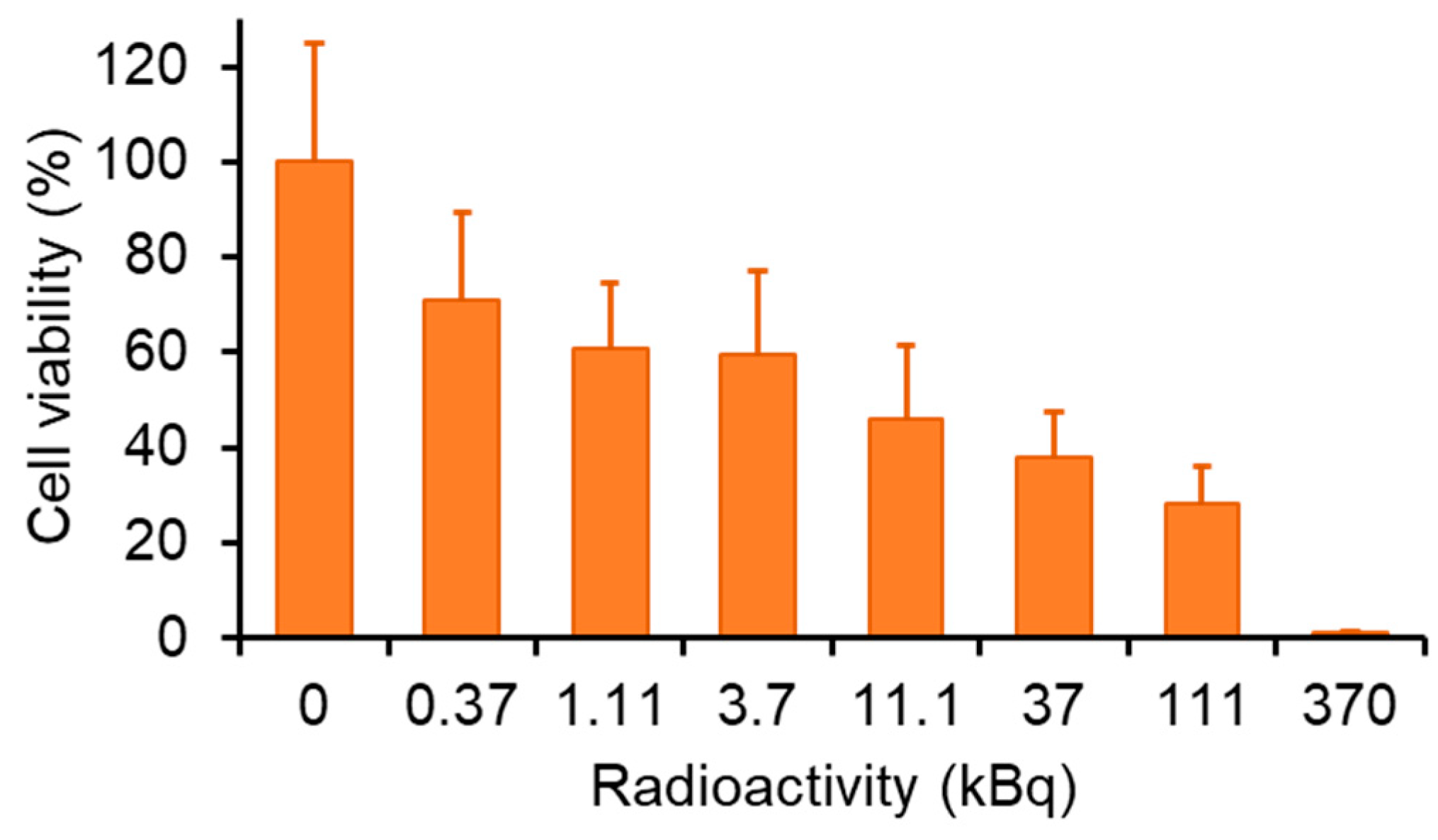
2.7. Auger Electron Therapy
2.8. Preperation of TSLs Encapsulating [125I]5
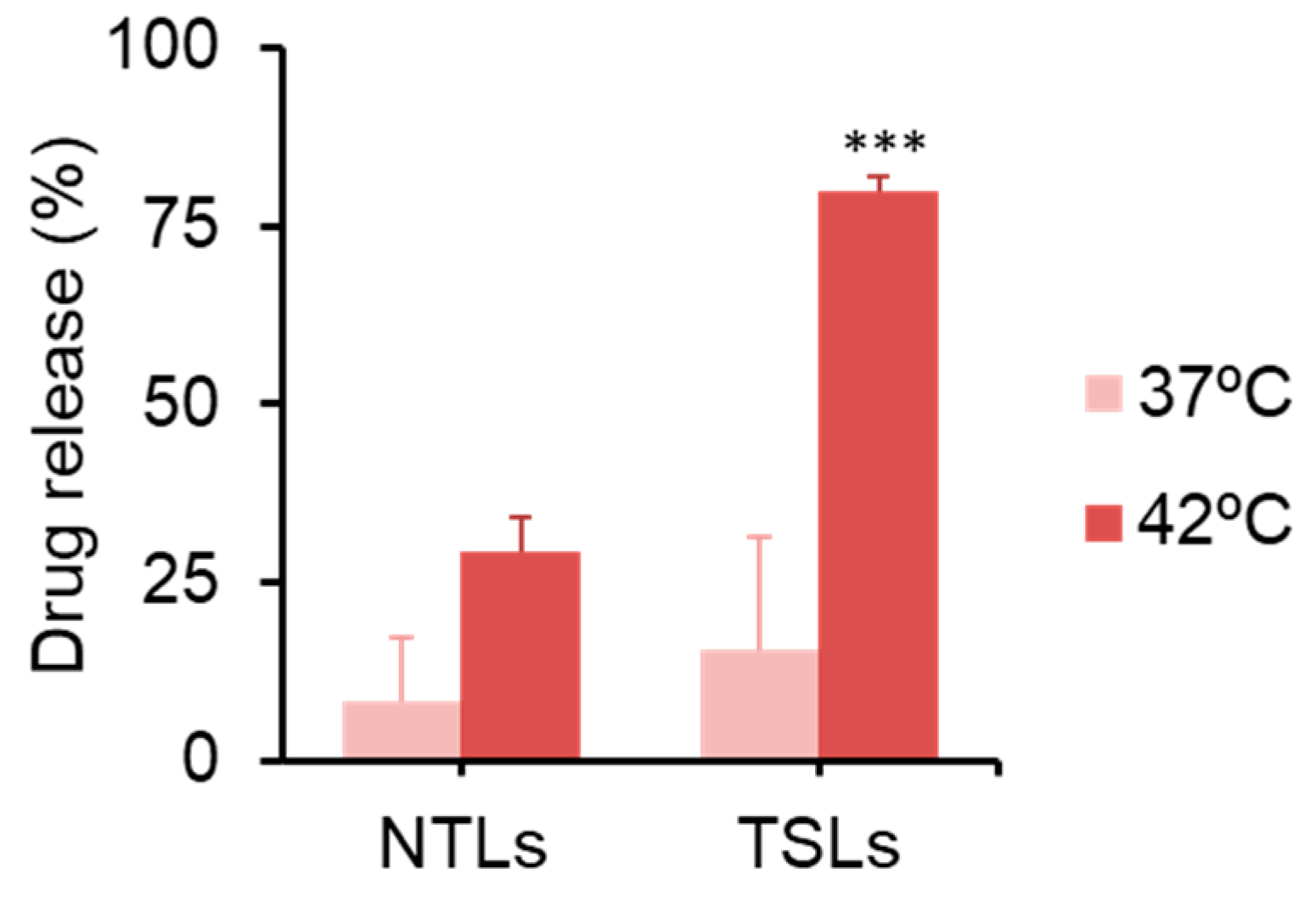
2.9. Drug Release Test
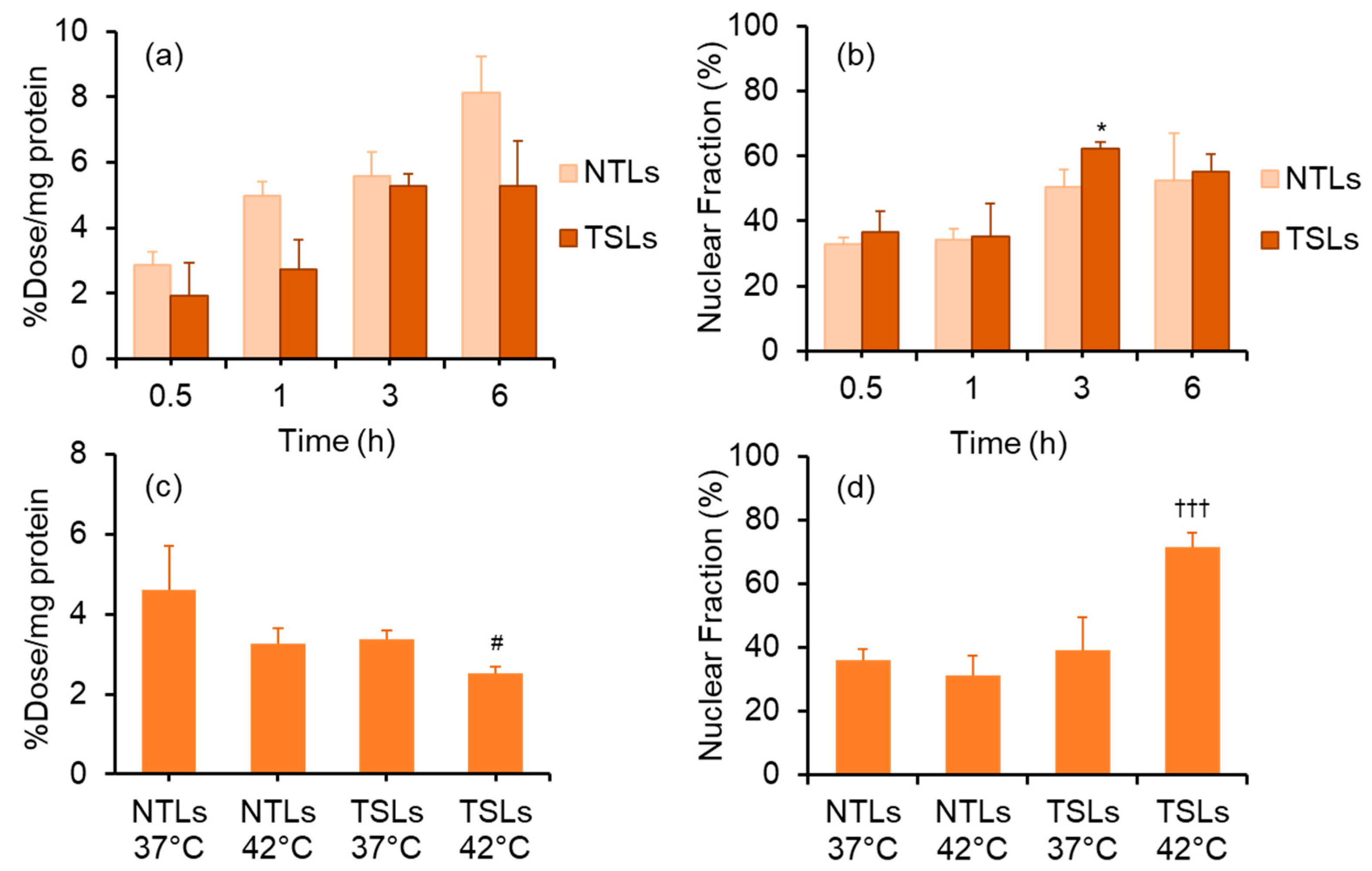
2.10. Cellular and Nuclear Uptake of TSLs Encapsulating [125I]5
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Non-Radioactive Compounds
3.2.1. Synthesis of 3-(tri-n-butylstannyl)benzoic Acid (4a)
3.2.2. Synthesis of N-Succinimidyl 3-(tri-n-butylstannyl)benzoate (ATE) (4b)
3.2.3. Synthesis of N-succinimidyl 3-iodobenzoate (SIB) (3)
3.2.4. Synthesis of 3’-N-3-Iodo-benzoyldoxorubicin (1)
3.2.5. Synthesis of 3’-N-3-Tributylstannylbenzoyldoxorubicin (2)
3.2.6. 4-Hydroxy-3-iodobenzaldehyde (7)
3.2.7. 3’-N-(4-Hydroxy-3-iodobenzyl)-13-(R/S)-dihydrodoxorubicin (5)
3.2.8. 3’-N-(4-Hydroxybenzyl) -13-(R/S)-dihydrodoxorubicin (6)
3.3. Synthesis of Radioactive Compounds
3.3.1. Radiosynthesis of 3’-N-3-iodo-benzoyldoxorubicin
3.3.2. Radiosynthesis of 3’-N-(4-hydroxy-3-iodobenzyl)doxorubicin
3.4. Intracellular Localization
3.5. Determination of Partition Coefficients
3.6. In Vitro Stability Assay
3.7. Cytotoxicity Assay
3.8. Preparation of Liposomes
3.9. Differential Scanning Calorimetry
3.10. Drug Encapsulation
3.11. Drug Release Test
3.12. Cellular and Nuclear Uptake Study
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Brady, D.; O’Sullivan, J.M.; Prise, K.M. What is the Role of the Bystander Response in Radionuclide Therapies? Front. Oncol. 2013, 3, 215. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.P.; Lozza, C.; Deshayes, E.; Boudousq, V.; Navarro-Teulon, I. Introduction to radiobiology of targeted radionuclide therapy. Front. Med. 2015, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Mishiro, K.; Hanaoka, H.; Yamaguchi, A.; Ogawa, K. Radiotheranostics with radiolanthanides: Design, development strategies, and medical applications. Coord. Chem. Rev. 2019, 383, 104–131. [Google Scholar] [CrossRef]
- Ogawa, K. Development of Diagnostic and Therapeutic Probes with Controlled Pharmacokinetics for Use in Radiotheranostics. Chem. Pharm. Bull. 2019, 67, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Kassis, A.I.; Adelstein, S.J. Radiobiologic principles in radionuclide therapy. J. Nucl. Med. 2005, 46 (Suppl. S1), 4S–12S. [Google Scholar]
- Ku, A.; Facca, V.J.; Cai, Z.; Reilly, R.M. Auger electrons for cancer therapy—A review. EJNMMI Radiopharm. Chem. 2019, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Costantini, D.L.; McLarty, K.; Lee, H.; Done, S.J.; Vallis, K.A.; Reilly, R.M. Antitumor effects and normal-tissue toxicity of 111In-nuclear localization sequence-trastuzumab in athymic mice bearing HER-positive human breast cancer xenografts. J. Nucl. Med. 2010, 51, 1084–1091. [Google Scholar] [CrossRef]
- Slastnikova, T.A.; Koumarianou, E.; Rosenkranz, A.A.; Vaidyanathan, G.; Lupanova, T.N.; Sobolev, A.S.; Zalutsky, M.R. Modular nanotransporters: A versatile approach for enhancing nuclear delivery and cytotoxicity of Auger electron-emitting 125I. EJNMMI Res. 2012, 2, 59. [Google Scholar] [CrossRef]
- Violet, J.A.; Farrugia, G.; Skene, C.; White, J.; Lobachevsky, P.; Martin, R. Triple targeting of Auger emitters using octreotate conjugated to a DNA-binding ligand and a nuclear localizing signal. Int. J. Radiat. Biol. 2016, 92, 707–715. [Google Scholar] [CrossRef]
- Ginj, M.; Hinni, K.; Tschumi, S.; Schulz, S.; Maecke, H.R. Trifunctional somatostatin-based derivatives designed for targeted radiotherapy using auger electron emitters. J. Nucl. Med. 2005, 46, 2097–2103. [Google Scholar]
- Lee, Y.; Thompson, D.H. Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1450. [Google Scholar] [CrossRef]
- Bi, H.; Xue, J.; Jiang, H.; Gao, S.; Yang, D.; Fang, Y.; Shi, K. Current developments in drug delivery with thermosensitive liposomes. Asian J. Pharm. Sci. 2019, 14, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.; Porter, T.M. Thermosensitive liposomes for localized delivery and triggered release of chemotherapy. J. Control. Release 2013, 169, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.H.; Wu, N.Z.; Hong, K.; Huang, S.K.; Dewhirst, M.W.; Papahadjopoulos, D. Thermosensitive liposomes: Extravasation and release of contents in tumor microvascular networks. Int. J. Radiat. Oncol. Biol. Phys. 1996, 36, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Kong, G.; Dewhirst, M.W. Hyperthermia and liposomes. Int. J. Hyperth. 1999, 15, 345–370. [Google Scholar]
- Ickenstein, L.M.; Edwards, K.; Sjoberg, S.; Carlsson, J.; Gedda, L. A novel 125I-labeled daunorubicin derivative for radionuclide-based cancer therapy. Nucl. Med. Biol. 2006, 33, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Fondell, A.; Edwards, K.; Unga, J.; Kullberg, E.; Park, J.W.; Gedda, L. In vitro evaluation and biodistribution of HER2-targeted liposomes loaded with an 125I-labelled DNA-intercalator. J. Drug Target. 2011, 19, 846–855. [Google Scholar] [CrossRef]
- Fondell, A.; Edwards, K.; Ickenstein, L.M.; Sjoberg, S.; Carlsson, J.; Gedda, L. Nuclisome: A novel concept for radionuclide therapy using targeting liposomes. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 114–123. [Google Scholar] [CrossRef]
- Arano, Y.; Wakisaka, K.; Ohmomo, Y.; Uezono, T.; Mukai, T.; Motonari, H.; Shiono, H.; Sakahara, H.; Konishi, J.; Tanaka, C.; et al. Maleimidoethyl 3-(tri-n-butylstannyl)hippurate: A useful radioiodination reagent for protein radiopharmaceuticals to enhance target selective radioactivity localization. J. Med. Chem. 1994, 37, 2609–2618. [Google Scholar] [CrossRef]
- Ogawa, K.; Takeda, T.; Yokokawa, M.; Yu, J.; Makino, A.; Kiyono, Y.; Shiba, K.; Kinuya, S.; Odani, A. Comparison of Radioiodine- or Radiobromine-Labeled RGD Peptides between Direct and Indirect Labeling Methods. Chem. Pharm. Bull. 2018, 66, 651–659. [Google Scholar] [CrossRef]
- Nunn, C.M.; Van Meervelt, L.; Zhang, S.D.; Moore, M.H.; Kennard, O. DNA-drug interactions. The crystal structures of d(TGTACA) and d(TGATCA) complexed with daunomycin. J. Mol. Biol. 1991, 222, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Egan, W.J.; Merz, K.M., Jr.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Shiba, K.; Akhter, N.; Yoshimoto, M.; Washiyama, K.; Kinuya, S.; Kawai, K.; Mori, H. Evaluation of radioiodinated vesamicol analogs for sigma receptor imaging in tumor and radionuclide receptor therapy. Cancer Sci. 2009, 100, 2188–2192. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Kanbara, H.; Kiyono, Y.; Kitamura, Y.; Kiwada, T.; Kozaka, T.; Kitamura, M.; Mori, T.; Shiba, K.; Odani, A. Development and evaluation of a radiobromine-labeled sigma ligand for tumor imaging. Nucl. Med. Biol. 2013, 40, 445–450. [Google Scholar] [CrossRef]
- Kannaka, K.; Sano, K.; Hagimori, M.; Yamasaki, T.; Munekane, M.; Mukai, T. Synthesis of an amphiphilic tetrazine derivative and its application as a liposomal component to accelerate release of encapsulated drugs. Bioorg. Med. Chem. 2019, 27, 3613–3618. [Google Scholar] [CrossRef]
- Mayer, L.D.; Cullis, P.R.; Bally, M.B. The Use of Transmembrane pH Gradient-Driven Drug Encapsulation in the Pharmacodynamic Evaluation of Liposomal Doxorubicin. J. Liposome Res. 1994, 4, 529–553. [Google Scholar] [CrossRef]
- Belchior, A.; Di Maria, S.; Fernandes, C.; Vaz, P.; Paulo, A.; Raposinho, P. Radiobiological and dosimetric assessment of DNA-intercalated 99mTc-complexes bearing acridine orange derivatives. EJNMMI Res. 2020, 10, 79. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elghobary, M.E.N.; Munekane, M.; Mishiro, K.; Fuchigami, T.; Ogawa, K. Preparation and Evaluation of Thermosensitive Liposomes Encapsulating I-125-Labeled Doxorubicin Derivatives for Auger Electron Therapy. Molecules 2023, 28, 1864. https://doi.org/10.3390/molecules28041864
Elghobary MEN, Munekane M, Mishiro K, Fuchigami T, Ogawa K. Preparation and Evaluation of Thermosensitive Liposomes Encapsulating I-125-Labeled Doxorubicin Derivatives for Auger Electron Therapy. Molecules. 2023; 28(4):1864. https://doi.org/10.3390/molecules28041864
Chicago/Turabian StyleElghobary, Mohamed Elsaid Nasr, Masayuki Munekane, Kenji Mishiro, Takeshi Fuchigami, and Kazuma Ogawa. 2023. "Preparation and Evaluation of Thermosensitive Liposomes Encapsulating I-125-Labeled Doxorubicin Derivatives for Auger Electron Therapy" Molecules 28, no. 4: 1864. https://doi.org/10.3390/molecules28041864
APA StyleElghobary, M. E. N., Munekane, M., Mishiro, K., Fuchigami, T., & Ogawa, K. (2023). Preparation and Evaluation of Thermosensitive Liposomes Encapsulating I-125-Labeled Doxorubicin Derivatives for Auger Electron Therapy. Molecules, 28(4), 1864. https://doi.org/10.3390/molecules28041864






