Comparison of In Vitro Estrogenic Activity of Polygoni multiflori Radix and Cynanchi wilfordii Radix via the Enhancement of ERα/β Expression in MCF7 Cells
Abstract
1. Introduction
2. Result and Discussion
2.1. Phytochemical Analysis Using HPLC
2.2. Total Phenolic and Total Flavonoid Contents
2.3. Antioxidant Activity: DPPH and Reducing Power Assays
2.4. The Proliferation of Human MCF-7 Cells
2.5. Effects of Plant Extracts on Cell Viability
2.6. Effect on Lipopolysaccharide-Induced Nitric Oxide (NO) Production
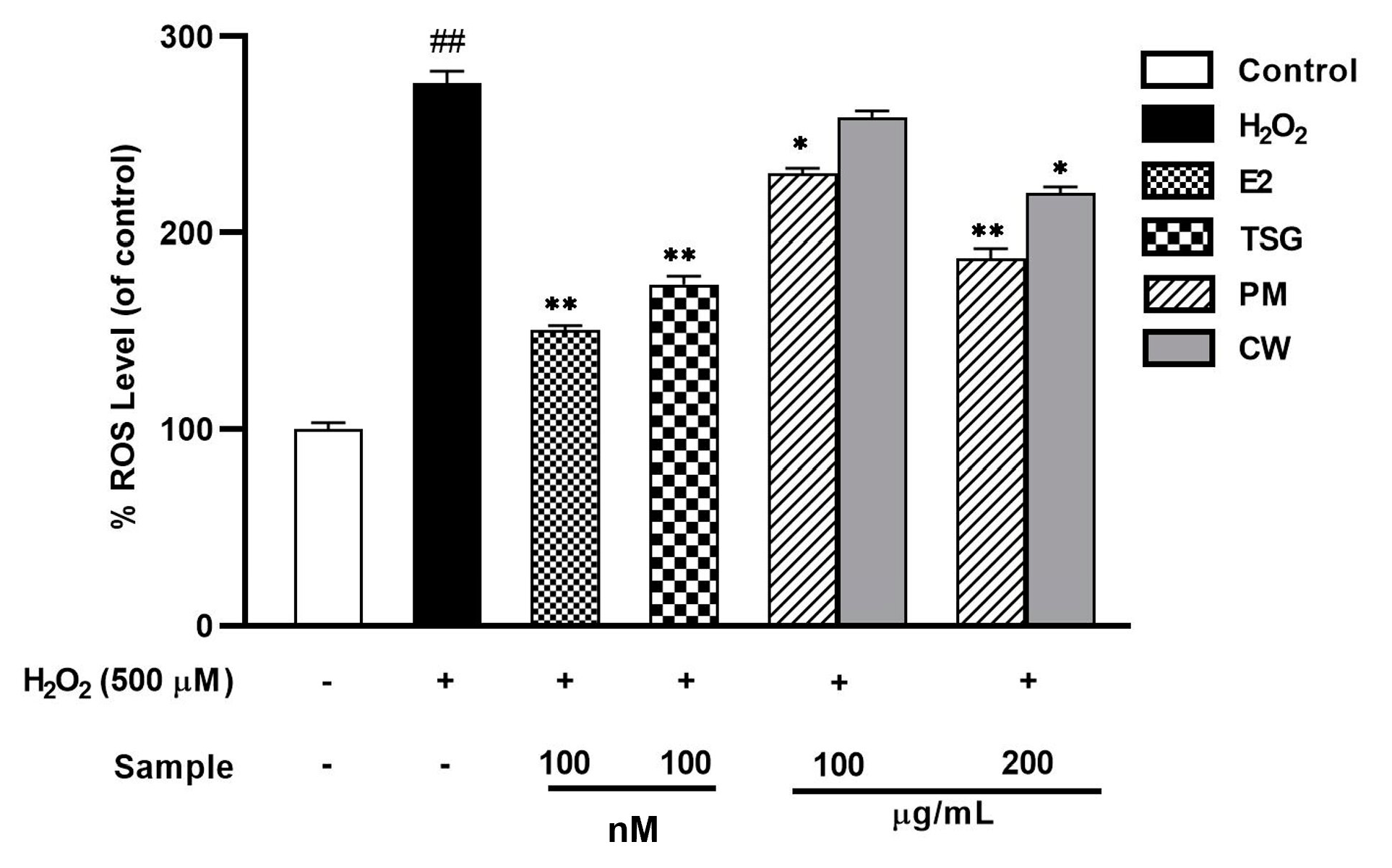
2.7. Suppression of Elevated Levels of Reactive Oxygen Species (ROS)
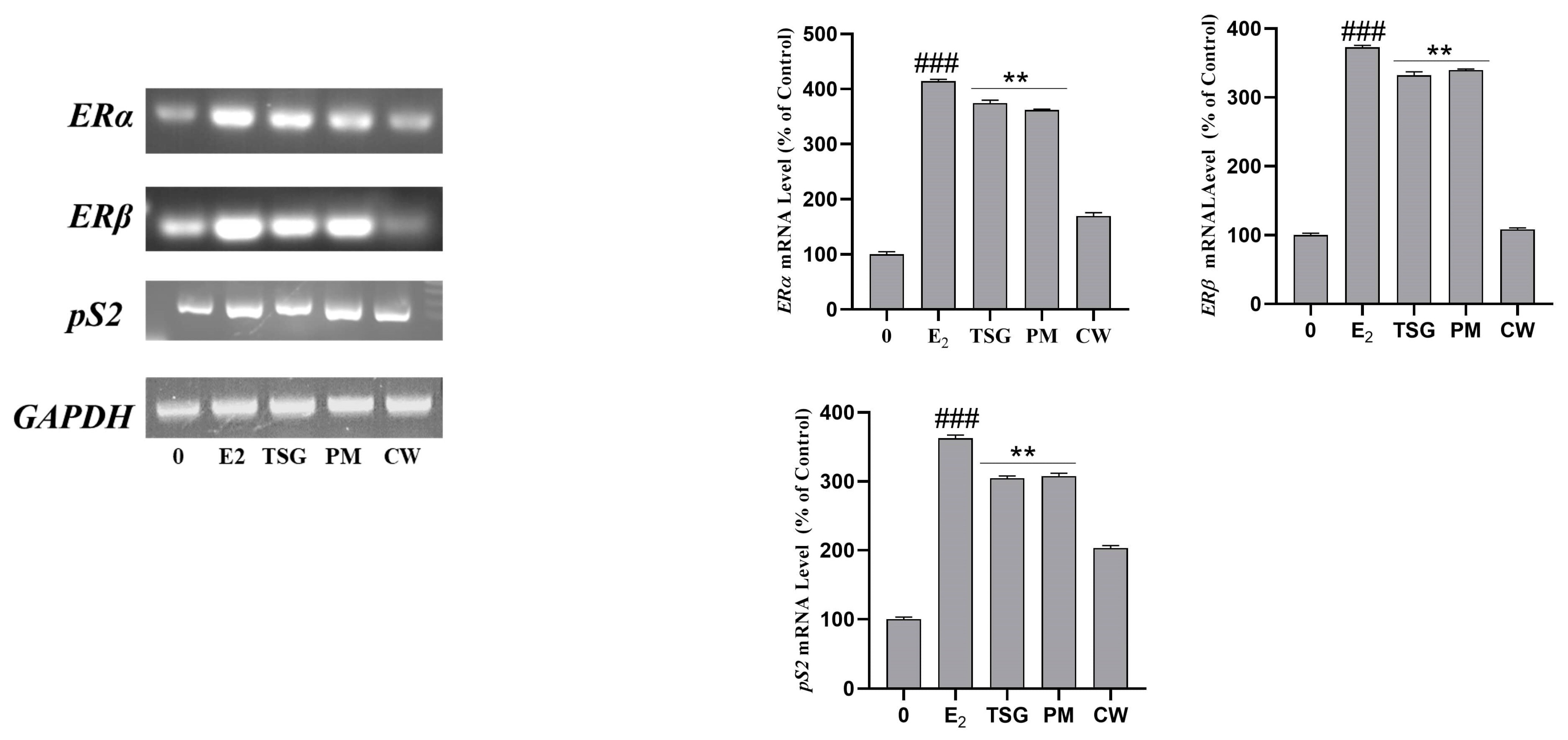
2.8. Estrogen Receptor mRNA Expression and Estrogenic Activity in Human MCF-7 Cells
3. Materials and Methods
3.1. Collection and Preparation of Plant Material Samples
3.2. Preparation of Standard Solutions
3.3. High-Performance Liquid Chromatography (HPLC)
3.4. Determination of Total Phenolic and Total Flavonoid Contents
3.5. DPPH Scavenging Assay
3.6. Chemical and Reagents for Cell Culture
3.7. Cell Culture
3.8. E-screen Assay
3.9. Cell Proliferation Assay
3.10. Cell Viability Assay
3.11. Measurement of Cellular ROS in HaCaT Cells
3.12. Measurement of Cellular NO Production in RAW 264.7 Cells
3.13. Gene Expression Analysis
| Genes | Forward Primers | Reverse Primers | Reference |
|---|---|---|---|
| ERα | CCGCTCATGATCAAACGCTCTAAG | GCCCTCTACACATTTTCCCTGGTT | [82] |
| ERβ | TTCCCAGCAATGTCACTAACTT | TTGAGGTTCCGCATACAGA | |
| pS2 | AATGGGCAGCCGTTAGGAAA | GCGCCCAATACGACCAAA |
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Han, E.-H.; Cho, K.; Goo, Y.; Kim, M.; Shin, Y.-W.; Kim, Y.-H.; Lee, S.-W. Development of molecular markers, based on chloroplast and ribosomal DNA regions, to discriminate three popular medicinal plant species, Cynanchum wilfordii, Cynanchum auriculatum, and Polygonum multiflorum. Mol. Biol. Rep. 2016, 43, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, L.; Han, T.; Chen, S.; Wang, J. Protective effects of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-d-glucoside, an active component of Polygonum multiflorum Thunb, on experimental colitis in mice. Eur. J. Pharmacol. 2008, 578, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Choi, H.G.; Li, Y.; Park, Y.M.; Lee, J.H.; Kim, D.H.; Lee, J.-H.; Son, J.K.; Na, M.; Lee, S.H. Chemical constituents of Cynanchum wilfordii and the chemotaxonomy of two species of the family Asclepiadacease, C. wilfordii and C. auriculatum. Arch. Pharmacal Res. 2011, 34, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, M.H.; Choi, Y.Y.; Hong, J.; Yang, W.M. Effects of Cynanchum wilfordii on osteoporosis with inhibition of bone resorption and induction of bone formation. Mol. Med. Rep. 2017, 17, 3758–3762. [Google Scholar] [CrossRef]
- Min-Kyeoung, K.; Hongtao, W.; Yeon-Ju, K.; Subramaniyam, S.; Deok-Chun, Y. Molecular authentication by multiplex-PCR of three similar medicinal plant species: Cynanchum wilfordii, Cynanchum auriculatum and Polygonum multiflorum (Fallopia multiflorum). J. Med. Plants Res. 2013, 4, 2584–2589. [Google Scholar]
- Kim, Y.; Choi, H.; Shin, J.; Jo, A.; Lee, K.-E.; Cho, S.-S.; Hwang, Y.-P.; Choi, C. Molecular Discrimination of Cynanchum wilfordii and Cynanchum auriculatum by InDel Markers of Chloroplast DNA. Molecules 2018, 23, 1337. [Google Scholar] [CrossRef]
- Alamgir, A.N.M. Molecular Pharmacognosy—A New Borderline Discipline Between Molecular Biology and Pharmacognosy. In Therapeutic Use of Medicinal Plants and Their Extracts; Springer: Berlin/Heidelberg, Germany, 2018; Volume 2, pp. 665–720. [Google Scholar]
- Lee, M.-S.; Hxiao, H.-J. Rapid and sensitive authentication of Polygonum multiflorum (He-Shou-Wu) of Chinese medicinal crop using specific isothermal nucleic acid amplification. Ind. Crop. Prod. 2018, 129, 281–289. [Google Scholar] [CrossRef]
- Santoro, N.; Roeca, C.; Peters, B.A.; Neal-Perry, G. The menopause transition: Signs, symptoms, and management options. J. Clin. Endocrinol. Metab. 2021, 106, 1–15. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jin, B.; Lee, S.; Oh, J.-Y.; Song, J.; Lee, D.; Kim, Y.-S.; Kim, H. A Herbal Formula HT051, a Combination of Pueraria lobata and Rehmannia glutinosa, Prevents Postmenopausal Obesity in Ovariectomized Rats. Evidence-Based Complement. Altern. Med. 2017, 2017, 8641535. [Google Scholar] [CrossRef]
- Kharb, R.; Haider, K.; Neha, K.; Yar, M.S. Aromatase inhibitors: Role in postmenopausal breast cancer. Arch. der Pharm. 2020, 353, 2000081. [Google Scholar] [CrossRef]
- Guthrie, A.K.; Larson, J.C.; Ensrud, E.K.; Anderson, G.L.; Carpenter, J.S.; Freeman, E.W.; Joffe, H.; LaCroix, A.Z.; E Manson, J.; Morin, C.M.; et al. Effects of Pharmacologic and Nonpharmacologic Interventions on Insomnia Symptoms and Self-reported Sleep Quality in Women With Hot Flashes: A Pooled Analysis of Individual Participant Data From Four MsFLASH Trials. Sleep 2018, 41, zsx190. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.J.B.P.; Endocrinology, R.C. Postmenopausal osteoporosis: Assessment and management. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 739–757. [Google Scholar] [CrossRef]
- Ko, S.-H.; Kim, H.-S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-C.; Arthur, R.; Iyengar, N.M.; Kamensky, V.; Xue, X.; Wassertheil-Smoller, S.; A Allison, M.; Shadyab, A.H.; A Wild, R.; Sun, Y.; et al. Association between regional body fat and cardiovascular disease risk among postmenopausal women with normal body mass index. Eur. Hear. J. 2019, 40, 2849–2855. [Google Scholar] [CrossRef]
- McCarthy, M.; Raval, A.P. The peri-menopause in a woman’s life: A systemic inflammatory phase that enables later neurodegenerative disease. J. Neuroinflammation 2020, 17, 317. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, C.M.; Romão, F.; Castelo-Branco, C. Menopause and aging: Changes in the immune system—A review. Maturitas 2010, 67, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Freedman, M.A. Quality of life and menopause: The role of estrogen. J. Women’s Health 2002, 11, 703–718. [Google Scholar] [CrossRef]
- MacGregor, E.A. Migraine, menopause and hormone replacement therapy. Post Reprod. Health 2018, 24, 11–18. [Google Scholar] [CrossRef]
- Locklear, T.D.; Huang, Y.; Frasor, J.; Doyle, B.J.; Perez, A.; Gomez-Laurito, J.; Mahady, G.B. Estrogenic and progestagenic effects of extracts of Justicia pectoralis Jacq., an herbal medicine from Costa Rica used for the treatment of menopause and PMS. Maturitas 2010, 66, 315–322. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Zhang, Y.; Chen, J.; Liang, X. In vitro estrogenic activities of Chinese medicinal plants traditionally used for the management of menopausal symptoms. J. Ethnopharmacol. 2005, 98, 295–300. [Google Scholar] [CrossRef]
- Genwali, G.R.; Acharya, P.P.; Rajbhandari, M. Isolation of Gallic Acid and Estimation of Total Phenolic Content in Some Medicinal Plants and Their Antioxidant Activity. Nepal J. Sci. Technol. 2013, 14, 95–102. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.; Ferreira, I.C.J.F.c. Bioactivity of phenolic acids: Metabolites versus parent com-pounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Mera, I.F.G.; Falconí, D.E.G.; Córdova, V.M. Secondary metabolites in plants: Main classes, phytochemical analysis and pharmacological activities. Bionatura 2019, 4, 1000–1009. [Google Scholar] [CrossRef]
- Wang, L.; Cai, F.; Zhao, W.; Tian, J.; Kong, D.; Sun, X.; Liu, Q.; Chen, Y.; An, Y.; Wang, F.J.M. Cynanchum auriculatum Royle ex Wight., Cynanchum bungei Decne. and Cynanchum wilfordii (Maxim.) Hemsl.: Current Research and Prospects. Molecules 2021, 26, 7065. [Google Scholar] [CrossRef]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2014, 159, 158–183. [Google Scholar] [CrossRef]
- Qian, J.; Hou, M.; Wu, X.; Dai, C.; Sun, J.; Dong, L.J.B. Pharmacotherapy. A review on the extraction, purification, detection, and pharmacological effects of 2, 3, 5, 4’-tetrahydroxystilbene-2-O-β-d-glucoside from Polygonum multiflorum. Biomed. Pharmacother. 2020, 124, 109923. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Ni, J. Huyiligeqi Emodin: A Review of its Pharmacology, Toxicity and Pharmacokinetics. Phytotherapy Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef]
- Phuyal, N.; Jha, P.K.; Raturi, P.P.; Rajbhandary, S. Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. Sci. World J. 2020, 2020, 8780704. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Abd-El-Aziz, N.M.; Hifnawy, M.S.; El-Ashmawy, A.A.; Lotfy, R.A.; Younis, I.Y. Application of Box-Behnken design for optimization of phenolics extraction from Leontodon hispidulus in relation to its antioxidant, anti-inflammatory and cytotoxic activities. Sci. Rep. 2022, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.-I.; Matsui, T. Nitric oxide, a janus-faced therapeutic target for diabetic microangiopathy—Friend or foe? Pharm.-Log. Res. 2011, 64, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Peña-Bautista, C.; Baquero, M.; Vento, M.; Cháfer-Pericás, C. Free radicals in Alzheimer’s disease: Lipid peroxidation biomarkers. Clin. Chim. Acta 2019, 491, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Farag, R.S.; Abdel-Latif, M.S.; El Baky, H.H.A.; Tawfeek, L.S. Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotechnol. Rep. 2020, 28, e00536. [Google Scholar] [CrossRef] [PubMed]
- Affat, S.S.J.U.o.T.-Q.J.o.S. Classifications, advantages, disadvantages, toxicity effects of natural and synthetic dyes: A review. Univ. Thi-Qar J. Sci. 2021, 8, 130–135. [Google Scholar]
- Lin, T.-K.; Chen, S.-D.; Lin, K.-J.; Chuang, Y.-C. Seizure-Induced Oxidative Stress in Status Epilepticus: Is Antioxidant Beneficial? Antioxidants 2020, 9, 1029. [Google Scholar] [CrossRef]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef]
- Wang, J.; Chung, M.H.; Xue, B.; Ma, H.; Ma, C.; Hattori, M. Estrogenic and Antiestrogenic Activities of Phloridzin. Biol. Pharm. Bull. 2010, 33, 592–597. [Google Scholar] [CrossRef]
- Zeng, M.; Li, M.; Li, M.; Zhang, B.; Li, B.; Zhang, L.; Feng, W.; Zheng, X. 2-Phenylacetamide Isolated from the Seeds of Lepidium apetalum and Its Estrogen-Like Effects In Vitro and In Vivo. Molecules 2018, 23, 2293. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Lou, Y.-J. Proliferation-stimulating effects of icaritin and desmethylicaritin in MCF-7 cells. Eur. J. Pharmacol. 2004, 504, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Min, J.I.A.; Yuan, L.I.; Hai-Liang, X.I.N.; Ting-Ting, H.O.U.; Zhang, N.D.; Hong-Tao, X.U.; Zhang, Q.Y.; Lu-Ping, Q.I.N. Estrogenic activity of osthole and imperatorin in MCF-7 cells and their osteoblastic effects in Saos-2 cells. Chin. J. Nat. Med. 2016, 14, 413–420. [Google Scholar]
- Ahn, H.-N.; Jeong, S.-Y.; Bae, G.-U.; Chang, M.; Zhang, D.; Liu, X.; Pei, Y.; Chin, Y.-W.; Lee, J.; Oh, S.-R.; et al. Selective Estrogen Receptor Modulation by Larrea nitida on MCF-7 Cell Proliferation and Immature Rat Uterus. Biomol. Ther. 2014, 22, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Hwang, Y.-H.; Yee, S.-T.J.M.i.D.D. Estrogenic activities of 2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-D-glucoside and physcion in MCF-7 cells. Med. Drug Discov. 2021, 9, 100072. [Google Scholar] [CrossRef]
- Matsuda, H.; Shimoda, H.; Morikawa, T.; Yoshikawa, M. Phytoestrogens from the roots of Polygonum cuspidatum (polygonaceae): Structure-Requirement of hydroxyanthraquinones for estrogenic activity. Bioorganic Med. Chem. Lett. 2001, 11, 1839–1842. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Lee, S.R.; Kim, B.; Hong, J.-H.; Jang, Y.S.; Lee, D.E.; Pang, C.; Kang, K.S.; Kim, K.H. Estrogenic Activity of 4-Hydroxy-Benzoic Acid from Acer tegmentosum via Estrogen Receptor α-Dependent Sig-naling Pathways. Plants 2022, 11, 3387. [Google Scholar] [CrossRef]
- Jung, S.; Lee, M.-S.; Choi, A.-J.; Kim, C.-T.; Kim, Y. Anti-Inflammatory Effects of High Hydrostatic Pressure Extract of Mulberry (Morus alba) Fruit on LPS-Stimulated RAW264.7 Cells. Molecules 2019, 24, 1425. [Google Scholar] [CrossRef]
- Spiller, F.; Formiga, R.O.; Coimbra, J.F.D.S.; Alves-Filho, J.C.; Cunha, T.M.; Cunha, F.Q. Targeting nitric oxide as a key modulator of sepsis, arthritis and pain. Nitric Oxide 2019, 89, 32–40. [Google Scholar] [CrossRef]
- Pratap, U.P.; Patil, A.; Sharma, H.R.; Hima, L.; Chockalingam, R.; Hariharan, M.M.; Shitoot, S.; Priyanka, H.P.; ThyagaRajan, S. Estrogen-induced neuroprotective and anti-inflammatory effects are dependent on the brain areas of middle-aged female rats. Brain Res. Bull. 2016, 124, 238–253. [Google Scholar] [CrossRef]
- Kolb-Bachofen, V.; Kuhn, A.; Suschek, C. The role of nitric oxide. Rheumatology 2006, 45 (suppl. s3), iii17–iii19. [Google Scholar] [CrossRef]
- Brezani, V.; Smejkal, K.; Hosek, J.; Tomasova, V.J.C.M.C. Anti-inflammatory natural prenylated phenolic com-pounds-potential lead substances. Curr. Med. Chem. 2018, 25, 1094–1159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gupta, S.; Yadav, T.C.; Pruthi, V.; Varadwaj, P.K.; Goel, N. Extrapolation of phenolic compounds as multi-target agents against cancer and inflammation. J. Biomol. Struct. Dyn. 2018, 37, 2355–2369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, Q.; Yang, Y.; Ai, X.; Chen, S.; Song, Y.J.A.d.P. Synthesis and anti-inflammatory effects of novel emodin deriv-atives bearing azole moieties. Arch. Der Pharm. 2020, 353, 1900264. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-W.; Lee, Y.-H.; Chen, L.-G.; Lee, C.-J.; Wang, C.-C.J.M. In vitro and in vivo anti-osteoarthritis effects of 2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-d-Glucoside from Polygonum multiflorum. Molecules 2018, 23, 571. [Google Scholar] [CrossRef] [PubMed]
- Sarangarajan, R.; Meera, S.; Rukkumani, R.; Sankar, P.; Anuradha, G. Antioxidants: Friend or foe? Asian Pac. J. Trop. Med. 2017, 10, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Al-Rawas, A.M.; Al-Maqbali, M.; Al-Saleh, M.; Enriquez, M.B.; Al-Siyabi, S.; Al-Hashmi, K.; Al-Lawati, I.; Bulthuis, M.L.C.; et al. Systemic Oxidative Stress Is Increased in Postmenopausal Women and Independently Associates with Homocysteine Levels. Int. J. Mol. Sci. 2020, 21, 314. [Google Scholar] [CrossRef] [PubMed]
- Morena, M.; Delbosc, S.; Dupuy, A.-M.; Canaud, B.; Cristol, J.-P. Overproduction of reactive oxygen species in end-stage renal disease patients: A potential component of hemodialysis-associated inflammation. Hemodial. Int. 2005, 9, 37–46. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2019, 467, 1–12. [Google Scholar] [CrossRef]
- Bito, T.; Nishigori, C. Impact of reactive oxygen species on keratinocyte signaling pathways. J. Dermatol. Sci. 2012, 68, 3–8. [Google Scholar] [CrossRef]
- Monteiro, R.; Teixeira, D.; Calhau, C. Estrogen Signaling in Metabolic Inflammation. Mediat. Inflamm. 2014, 2014, 615917. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F.; Li, C.; Otkur, W.; Hayashi, T.; Mizuno, K.; Ikejima, T. Silibinin treatment protects human skin cells from UVB injury through upregulation of estrogen receptors. J. Photochem. Photobiol. B Biol. 2021, 216, 112147. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-M.; Lei, S.-N.; Tang, W.; Xun, M.-H.; Zhao, Z.-W.; Cheng, M.-Y.; Zhang, X.-D.; Wang, W.J.M. Optimization of Ultra-sound-Assisted Cellulase Extraction from Nymphaea hybrid Flower and Biological Activities: Antioxidant Activity, Protec-tive Effect against ROS Oxidative Damage in HaCaT Cells and Inhibition of Melanin Production in B16 Cells. Molecules 2022, 27, 1914. [Google Scholar] [CrossRef] [PubMed]
- Moo-Huchin, V.M.; Moo-Huchin, M.I.; Estrada-León, R.J.; Cuevas-Glory, L.; Estrada-Mota, I.A.; Ortiz-Vázquez, E.; Betancur-Ancona, D.; Sauri-Duch, E. Antioxidant compounds, antioxidant activity and phenolic content in peel from three tropical fruits from Yucatan, Mexico. Food Chem. 2015, 166, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar]
- Tanwar, A.K.; Dhiman, N.; Kumar, A.; Jaitak, V. Engagement of phytoestrogens in breast cancer suppression: Structural classification and mechanistic approach. Eur. J. Med. Chem. 2021, 213, 113037. [Google Scholar] [CrossRef] [PubMed]
- Sellitto, A.; D’Agostino, Y.; Alexandrova, E.; Lamberti, J.; Pecoraro, G.; Memoli, D.; Rocco, D.; Coviello, E.; Giurato, G.; Nassa, G.; et al. Insights into the Role of Estrogen Receptor β in Triple-Negative Breast Cancer. Cancers 2020, 12, 1477. [Google Scholar] [CrossRef]
- Desmawati, D.; Sulastri, D.J.O.a.M.j.o.m.s. Phytoestrogens and their health effect. Open Access Maced. J. Med. Sci. 2019, 7, 495. [Google Scholar] [CrossRef]
- Lee, D.; Ko, Y.; Pang, C.; Ko, Y.-J.; Choi, Y.-K.; Kim, K.H.; Kang, K.S.J.M. Estrogenic Activity of Mycoestrogen (3 β, 5 α, 22 E)-Ergost-22-en-3-ol via Estrogen Receptor α-Dependent Signaling Pathways in MCF-7 Cells. Molecules 2021, 27, 36. [Google Scholar] [CrossRef]
- Nanashima, N.; Horie, K.; Tomisawa, T.; Chiba, M.; Nakano, M.; Fujita, T.; Maeda, H.; Kitajima, M.; Takamagi, S.; Uchiyama, D.; et al. Phytoestrogenic activity of blackcurrant (Ribes nigrum) anthocyanins is mediated through estrogen receptor alpha. Mol. Nutr. Food Res. 2015, 59, 2419–2431. [Google Scholar] [CrossRef]
- Njamen, D.; Djiogue, S.; Zingue, S.; Mvondo, M.A.; Nkeh-Chungag, B.N. In vivo and in vitro estrogenic activity of extracts from Erythrina poeppigiana (Fabaceae). J. Complement. Integr. Med. 2013, 10, 63–73. [Google Scholar] [CrossRef]
- Nguyen, K.-N.H.; Nguyen, N.-V.T.; Kim, K.H. Determination of phenolic acids and flavonoids in leaves, calyces, and fruits of Physalis angulata L. in Viet Nam. Pharmacia 2021, 68, 501–509. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Bayertai; Tang, S.; Zhou, X. Analysis of gallic acid and ellagic acid in leaves of Elaeagnus angustifolia L. from different habitats and times in Xinjiang by HPLC with cluster analysis. Acta Chromatogr. 2021, 33, 195–201. [Google Scholar] [CrossRef]
- Akter, R.; Kwak, G.-Y.; Ahn, J.C.; Mathiyalagan, R.; Ramadhania, Z.M.; Yang, D.C.; Kang, S.C. Protective Effect and Potential Antioxidant Role of Kakadu Plum Extracts on Alcohol-Induced Oxidative Damage in HepG2 Cells. Appl. Sci. 2021, 12, 236. [Google Scholar] [CrossRef]
- Akter, R.; Ling, L.; Rupa, E.J.; KyuPark, J.; Mathiyalagan, R.; Nahar, J.; Won, L.J.; Hyun, K.D.; Murugesan, M.; Yang, D.C.; et al. Binary Effects of Gynostemma Gold Nanoparticles on Obesity and Inflammation via Downregulation of PPARγ/CEPBα and TNF-α Gene Expression. Molecules 2022, 27, 2795. [Google Scholar] [CrossRef]
- Villalobos, M.; Olea, N.; Brotons, J.A.; Olea-Serrano, M.F.; Ruiz de Almodovar, J.; Pedraza, V.J.E.h.p. The E-screen assay: A comparison of different MCF7 cell stocks. Envrion. Health Perspect 1995, 103, 844–850. [Google Scholar] [CrossRef]
- Chang, B.Y.; Kim, D.S.; Kim, H.S.; Kim, S.Y. Evaluation of estrogenic potential by herbal formula, HPC 03 for in vitro and in vivo. Reproduction 2018, 155, 103–113. [Google Scholar] [CrossRef]
- You, W.; Ahn, J.; Boopathi, V.; Arunkumar, L.; Rupa, E.; Akter, R.; Kong, B.; Lee, G.; Yang, D.; Kang, S.; et al. Enhanced Antiobesity Efficacy of Tryptophan Using the Nanoformulation of Dendropanax morbifera Extract Mediated with ZnO Nanoparticle. Materials 2021, 14, 824. [Google Scholar] [CrossRef]
- Ramadhania, Z.M.; Nahar, J.; Ahn, J.C.; Yang, D.U.; Kim, J.H.; Lee, D.W.; Kong, B.M.; Mathiyalagan, R.; Rupa, E.J.; Akter, R.; et al. Terminalia ferdinandiana (Kakadu Plum)-Mediated Bio-Synthesized ZnO Nanoparticles for Enhancement of Anti-Lung Cancer and Anti-Inflammatory Activities. Appl. Sci. 2022, 12, 3081. [Google Scholar] [CrossRef]
- Ahn, S.; Singh, P.; Jang, M.; Kim, Y.-J.; Castro-Aceituno, V.; Simu, S.Y.; Kim, Y.J.; Yang, D.-C. Gold nanoflowers synthesized using Acanthopanacis cortex extract inhibit inflammatory mediators in LPS-induced RAW264.7 macrophages via NF-κB and AP-1 pathways. Colloids Surf. B Biointerfaces 2018, 162, 398–404. [Google Scholar] [CrossRef]
- Hazman, M.J.B.-S.U.J.o.B.; Sciences, A. Gel express: A novel frugal method quantifies gene relative expression in conventional RT-PCR. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Akter, R.; Ahn, J.C.; Nahar, J.; Awais, M.; Ramadhania, Z.M.; Oh, S.-W.; Oh, J.-H.; Kong, B.M.; Rupa, E.J.; Lee, D.W.J.F.i.P. Pomegranate juice fermented by tannin acyl hydrolase and Lactobacillus vespulae DCY75 enhance estrogen receptor ex-pression and anti-inflammatory effect. Front. Pharmacol. 2022, 13, 1010103. [Google Scholar] [CrossRef] [PubMed]
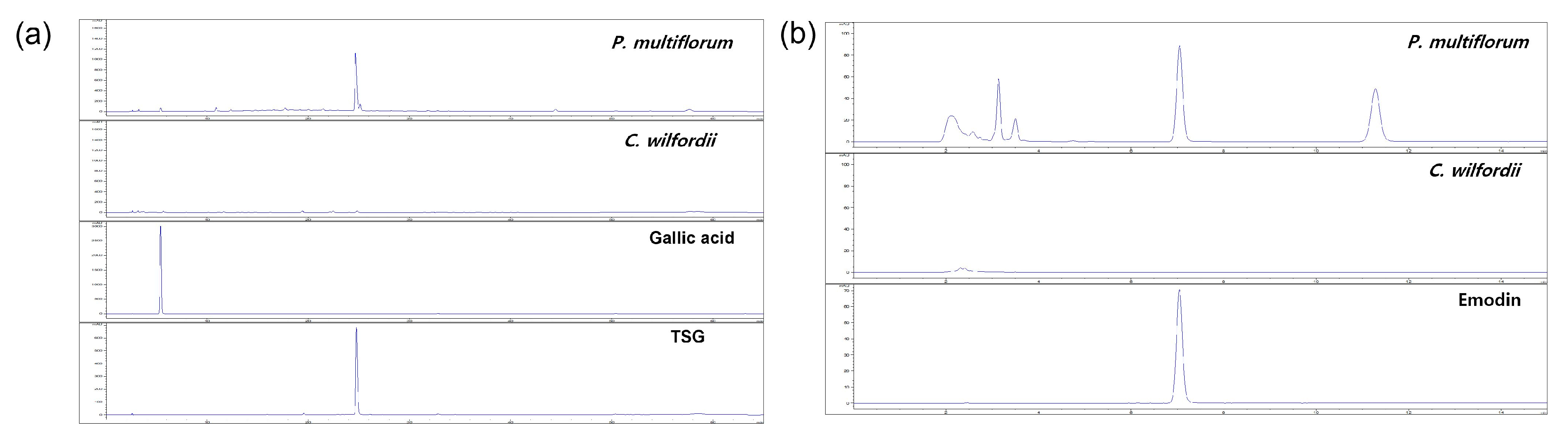
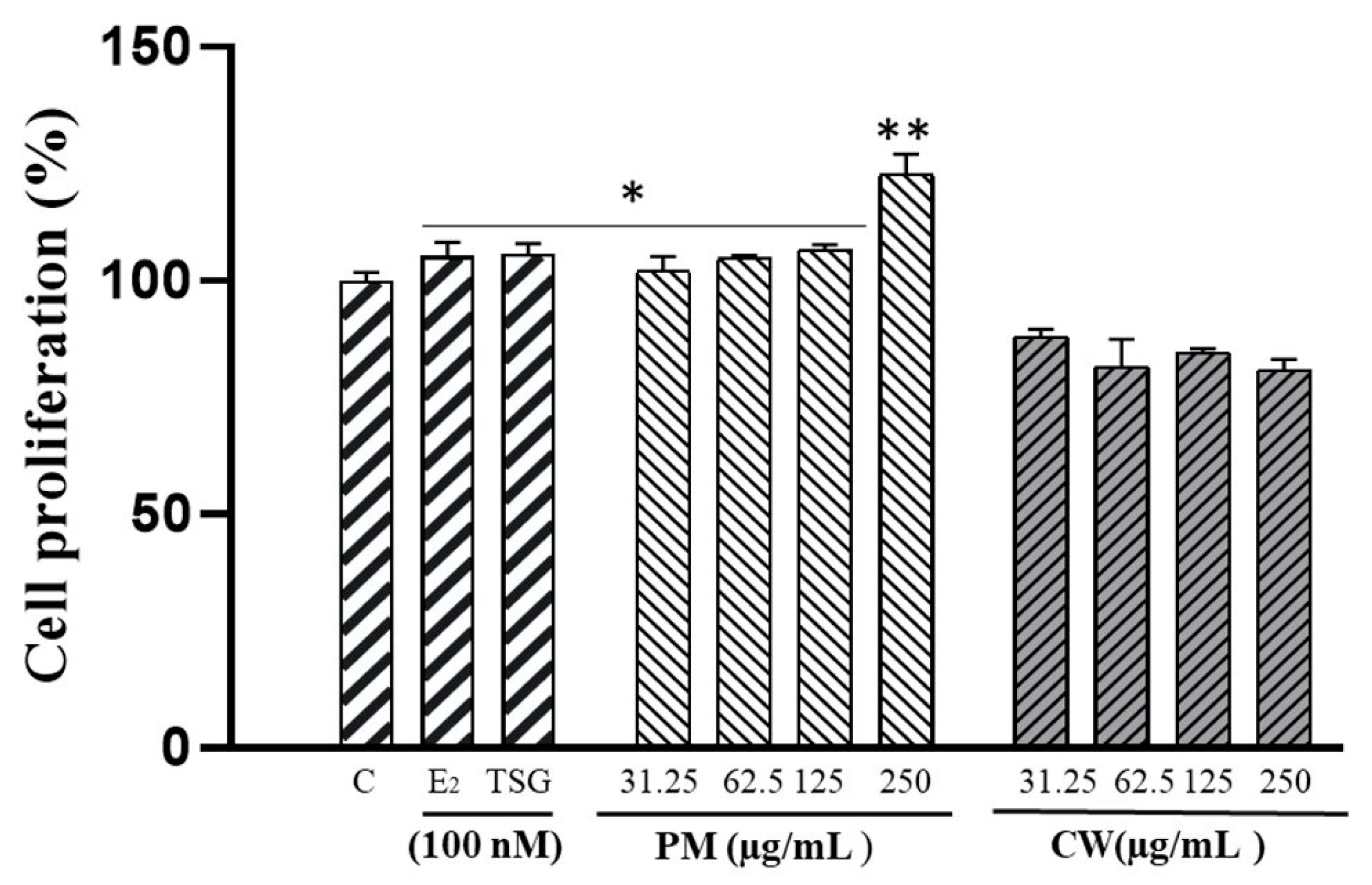
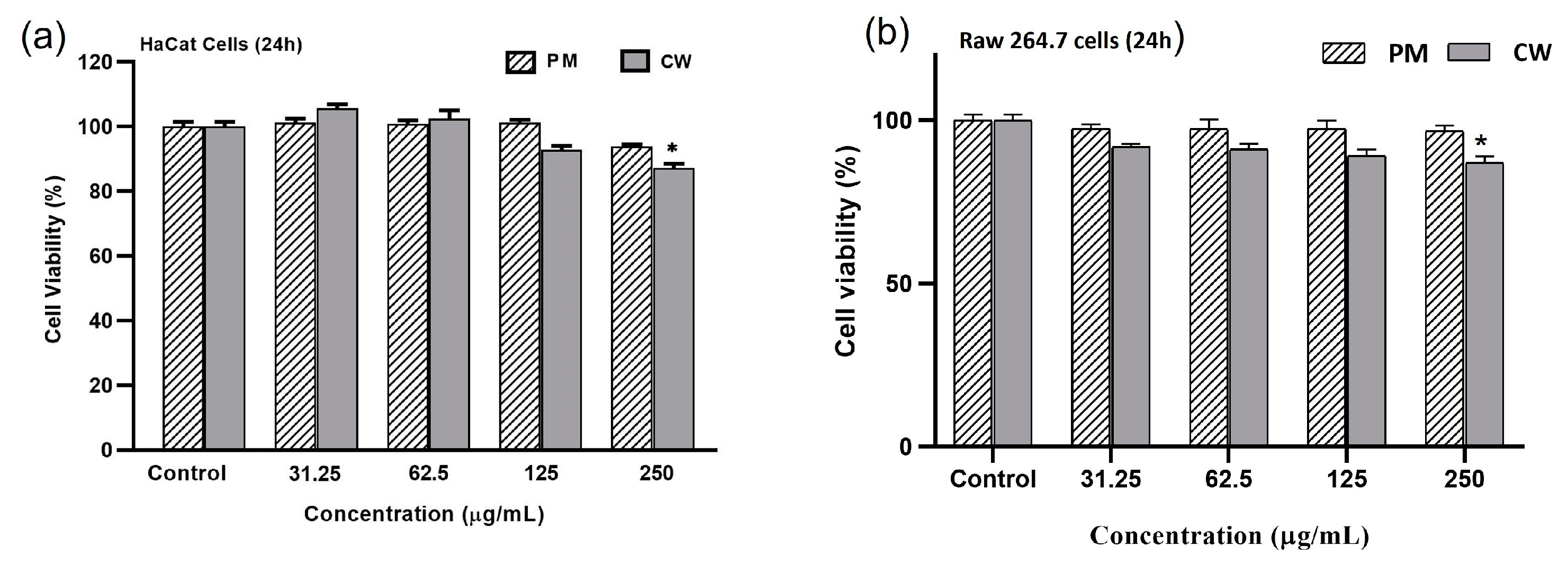
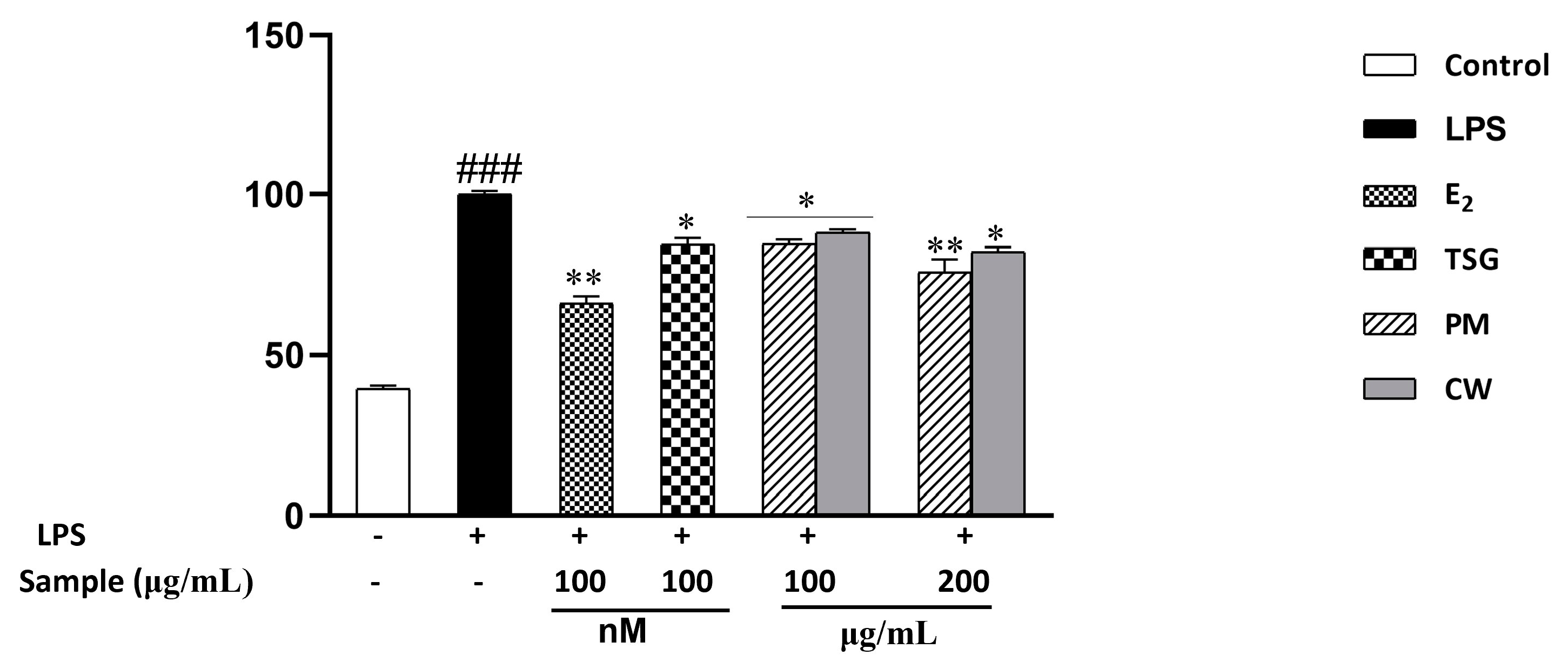
| Samples | Contents (mg/g DW) | ||
|---|---|---|---|
| Gallic Acid | TSG | Emodin | |
| P. multiflorum | 0.17 ± 0.016 | 39.01 ± 0.280 | 0.84 ± 0.003 |
| C. wilfordii | ND | 1.18 ± 0.155 | ND |
| Samples | TPC (µg GAE/mg Extract) | TFC (µg RE/mg Extract) |
|---|---|---|
| PM | 14.03 ± 0.03 a | 4.81 ± 0.01 b |
| CW | 2.08 ± 0.01 c | 5.84 ± 0.03 a |
| Samples | In Vitro Antioxidant | |
|---|---|---|
| DPPH (µg GAE/mg Extract) | Reducing Power (µg GAE/mg Extract) | |
| PM | 0.95 ± 0.01 a | 3.37 ± 0.01 a |
| CW | 0.81 ± 0.01 b | 1.80 ± 0.10 c |
| Standard | Solvent | Regression Equations | R2 | Linearity Range |
|---|---|---|---|---|
| Gallic acid | y = 14941x + 118.73 | 0.9997 | 0.03125–1 mg mL−1 | |
| TSG | Methanol | y = 1537.6x + 8.8448 | 0.9998 | 0.015625–1 mg mL−1 |
| Emodin | y = 5667x − 55.038 | 0.9985 | 0.015625–1 mg mL−1 |
| System/Condition | Gallic Acid and TSG | Emodin |
|---|---|---|
| Flow Rate | 1.0 mL/min | 1.0 mL/min |
| Wavelength | 260 nm | 436 nm |
| Injection Volume | 5 µL | 5 µL |
| Solvents | Gradient eluent | Isocratic eluent: |
| A: Methanol | A: 0.1% Phosphoric acid in water | |
| B: 0.1% Acetic acid in water | B: Methanol | |
| Column Temperature | 35 °C | 30 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, R.; Yang, D.U.; Ahn, J.C.; Awais, M.; Nahar, J.; Ramadhania, Z.M.; Kim, J.Y.; Lee, G.J.; Kwak, G.-Y.; Lee, D.W.; et al. Comparison of In Vitro Estrogenic Activity of Polygoni multiflori Radix and Cynanchi wilfordii Radix via the Enhancement of ERα/β Expression in MCF7 Cells. Molecules 2023, 28, 2199. https://doi.org/10.3390/molecules28052199
Akter R, Yang DU, Ahn JC, Awais M, Nahar J, Ramadhania ZM, Kim JY, Lee GJ, Kwak G-Y, Lee DW, et al. Comparison of In Vitro Estrogenic Activity of Polygoni multiflori Radix and Cynanchi wilfordii Radix via the Enhancement of ERα/β Expression in MCF7 Cells. Molecules. 2023; 28(5):2199. https://doi.org/10.3390/molecules28052199
Chicago/Turabian StyleAkter, Reshmi, Dong Uk Yang, Jong Chan Ahn, Muhammad Awais, Jinnatun Nahar, Zelika Mega Ramadhania, Jong Yun Kim, Gyong Jai Lee, Gi-Young Kwak, Dong Wook Lee, and et al. 2023. "Comparison of In Vitro Estrogenic Activity of Polygoni multiflori Radix and Cynanchi wilfordii Radix via the Enhancement of ERα/β Expression in MCF7 Cells" Molecules 28, no. 5: 2199. https://doi.org/10.3390/molecules28052199
APA StyleAkter, R., Yang, D. U., Ahn, J. C., Awais, M., Nahar, J., Ramadhania, Z. M., Kim, J. Y., Lee, G. J., Kwak, G.-Y., Lee, D. W., Kong, B. M., Yang, D. C., & Jung, S.-K. (2023). Comparison of In Vitro Estrogenic Activity of Polygoni multiflori Radix and Cynanchi wilfordii Radix via the Enhancement of ERα/β Expression in MCF7 Cells. Molecules, 28(5), 2199. https://doi.org/10.3390/molecules28052199










