In Vitro Gastrointestinal Digestion Affects the Bioaccessibility of Bioactive Compounds in Hibiscus sabdariffa Beverages
Abstract
1. Introduction
2. Results
2.1. Initial Bioactive Compounds in Hibiscus Beverages
2.2. Bioactive Compounds Released during In Vitro Intestinal Digestion
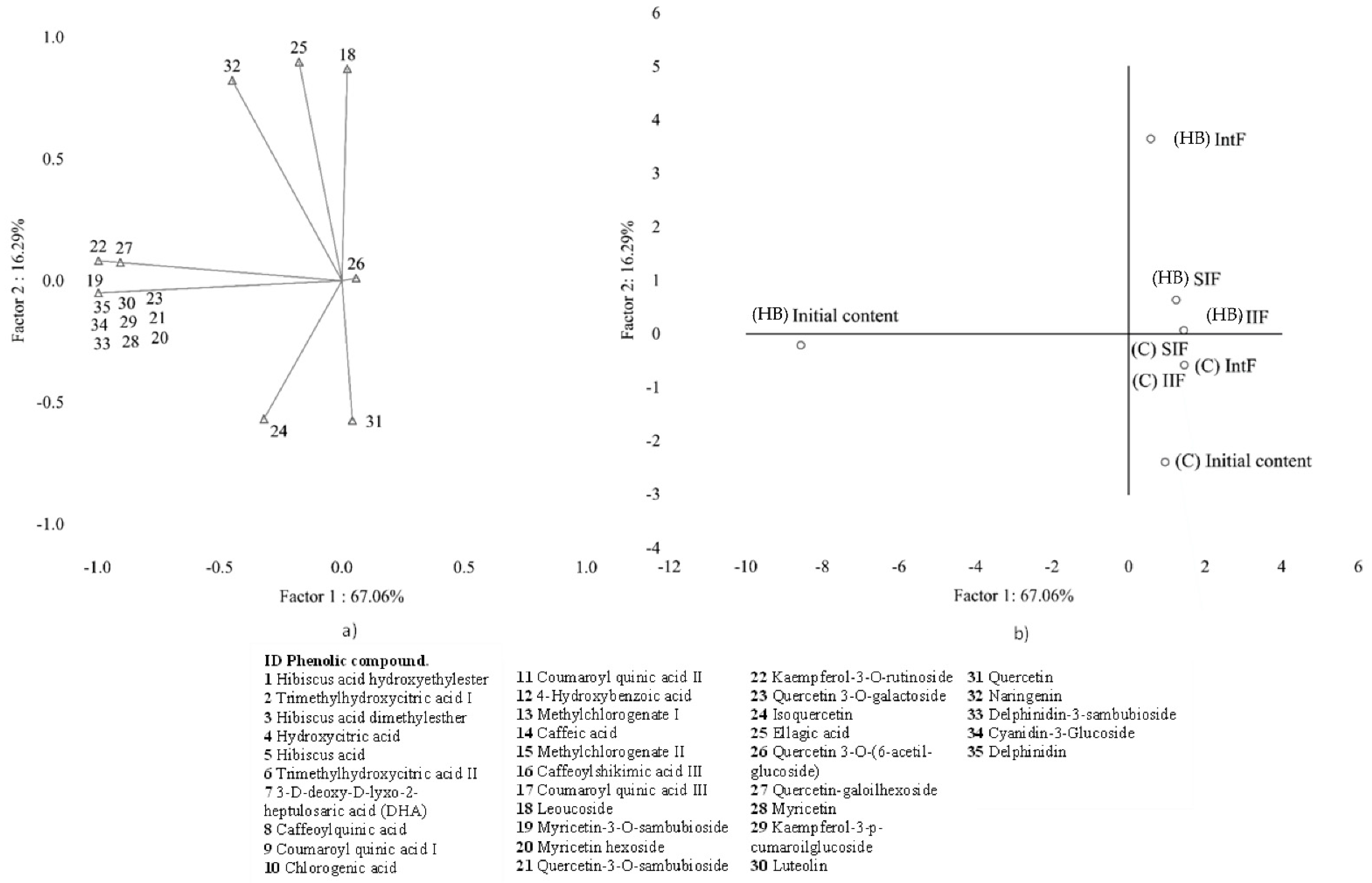
2.3. Initial Content and Indigestible Fractions after Gastrointestinal Digestion by Groups: Multivariate Data Analysis
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Sample Preparation
4.3. Bioaccessibility of Phenolic Compounds after In-Vitro Gastrointestinal Digestion
4.4. Determination of Phenolic Compounds and Organic Acids Profile by HPLC-DAD-ESI-MS
4.5. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Standard | Equation | R2 | LOQ 1 (μM/mL) |
|---|---|---|---|
| kaempferol | y = 24766x + 230022 | 0.9939 | 3.26 |
| p-coumaric acid | y = 90663x + 16308 | 0.9996 | 1.14 |
| Naringenin | y = 210592x + 130168 | 0.9965 | 3.34 |
| Garcinia acid | y = 7429.8x +24963 | 0.9979 | 1.00 |
| Quercetin | y = 1324209x + 78664 | 0.9979 | 2.58 |
References
- Monteiro, M.J.P.; Costa, A.I.A.; Tomlins, K.I.; Pintado, M.E. Quality improvement and new product development in the hibiscus beverage industry. In Processing and Sustainability of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 139–183. [Google Scholar]
- Cauich, I.; Rodríguez, J.; Fernández, V.; Ambrosio, V. Análisis de la rentabilidad de la producción de Flor de Jamaica (Hibiscus Sabdariffa). Panor. Económico 2020, 28, 94–101. [Google Scholar] [CrossRef]
- Sayago-Ayerdi, S.; Arranz, S.; Serrano, J.; Goñi, I. Dietary Fiber Content and Associated Antioxidant Compounds in Roselle Flower ( Hibiscus sabdariffa L.) Beverage. J. Agric. Food Chem. 2007, 55, 7886–7890. [Google Scholar] [CrossRef] [PubMed]
- Sáyago-Ayerdi, S.; Venema, K.; Tabernero, M.; Sarriá, B.; Bravo, L.; Mateos, R. Bioconversion of polyphenols and organic acids by gut microbiota of predigested Hibiscus sabdariffa L. calyces and Agave (A. tequilana Weber) fructans assessed in a dynamic in vitro model (TIM-2) of the human colon. Food Res. Int. 2021, 143, 110301. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Linares, I.; Fernández-Arroyo, S.; Arráez-Roman, D.; Palmeros-Suárez, P.A.; Del Val-Díaz, R.; Andrade-Gonzáles, I.; Fernández-Gutiérrez, A.; Gómez-Leyva, J.F.; Segura-Carretero, A. Characterization of phenolic compounds, anthocyanidin, antioxidant and antimicrobial activity of 25 varieties of Mexican Roselle (Hibiscus sabdariffa). Ind. Crop. Prod. 2015, 69, 385–394. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.-O. Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa L. on Obesity. Molecules 2019, 24, 210. [Google Scholar] [CrossRef]
- Sogo, T.; Terahara, N.; Hisanaga, A.; Kumamoto, T.; Yamashiro, T.; Wu, S.; Sakao, K.; Hou, D.X. Anti-inflammatory activity and molecular mechanism of delphinidin 3-sambubioside, a Hibiscus anthocyanin. BioFactors 2015, 41, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Awe, F.B.; Fagbemi, T.N.; Ifesan, B.O.T.; Badejo, A.A. Antioxidant properties of cold and hot water extracts of cocoa, Hibiscus flower extract, and ginger beverage blends. Food Res. Int. 2013, 52, 490–495. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; Castaño-Tostado, E.; Ramírez-de León, J.A.; Rocha-Guzmán, N.E.; Reynoso-Camacho, R. Effect of stevia and citric acid on the stability of phenolic compounds and in vitro antioxidant and antidiabetic capacity of a roselle (Hibiscus sabdariffa L.) beverage. Food Chem. 2015, 172, 885–892. [Google Scholar] [CrossRef]
- Carvalho de Castro, J.M.; Nascimento Alves, C.A.; de LimaSantos, K.; de Oliveira Silva, E.; Maria da Silva Araújo, Í.; Barros deVasconcelos, L. Elaboration of a mixed beverage from hibiscus and coconut water: An evaluation of bioactive and sensory properties. Int. J. Gastron. Food Sci. 2021, 23, 100284. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; Zúñiga-Hernández, A.; Torre, L.; Serrano-Altamirano, V.; Sánchez-Feria, C. Color en cálices de jamaica (Hibiscus sabdariffa L.) y su relación con características fisioquímicas de sus extractos acuosos. Rev. Chapingo Ser. Hortic. 2012, XVIII, 395–407. [Google Scholar] [CrossRef]
- Medina-Carrillo, R.E.; Sumaya-Martínez, M.T.; Machuca-Sánchez, M.L.; Sánchez-Herrera, L.M.; Balois-Morales, R.; Jiménez-Ruiz, E.I. Actividad antioxidante de extractos de cálices deshidratados de 64 variedades de jamaica (Hibiscus sabdariffa L.) en función de fenólicos y antocianinas totales. Rev. Cienc. Técnicas Agropecu. 2013, 22, 41–44. [Google Scholar]
- Ariza-Flores, R.; Serrano-Altamirano, V.; Navarro-Galindo, S.; Ovando-Cruz, M.E.; Vázquez-García, E.; Barrios-Ayala, A.; Michel-Aceves, A.C.; Guzmán-Maldonado, S.H.; Otero-Sánchez, M.A. Variedades mexicanas de jamaica (Hibiscus sabdariffa L.) ‘Alma Blanca’ y ‘Rosalíz’ de color claro, y ‘Cotzaltzin’ y ‘Tecoanapa’ de color rojo. Rev. Fitotec. Mex. 2014, 37, 181–185. [Google Scholar] [CrossRef]
- Duarte-Valenzuela, Z.; Gasga, V.M.; Montalvo-González, E.; Sayago-Ayerdi, S. Caracterización nutricional de 20 variedades mejoradas de jamaica (Hibiscus sabdariffa L.) cultivadas en méxico. Rev. Fitotec. Mex. 2016, 39, 199–206. [Google Scholar] [CrossRef]
- Kao, E.-S.; Hsu, J.-D.; Wang, C.-J.; Yang, S.-H.; Cheng, S.-Y.; Lee, H.-J. Polyphenols Extracted from Hibiscus sabdariffa L. Inhibited Lipopolysaccharide-Induced Inflammation by Improving Antioxidative Conditions and Regulating Cyclooxygenase-2 Expression. Biosci. Biotechnol. Biochem. 2009, 73, 385–390. [Google Scholar] [CrossRef]
- Alegbe, E.O.; Teralı, K.; Olofinsan, K.A.; Surgun, S.; Ogbaga, C.C.; Ajiboye, T.O. Antidiabetic activity-guided isolation of gallic and protocatechuic acids from Hibiscus sabdariffa calyxes. J. Food Biochem. 2019, 43, e12927. [Google Scholar] [CrossRef]
- Agunbiade, H.O.; Fagbemi, T.N.; Aderinola, T.A. Antioxidant properties of beverages from graded mixture of green/roasted coffee and hibiscus sabdariffa calyx flours. Appl. Food Res. 2022, 2, 100163. [Google Scholar] [CrossRef]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Inhibition of enzymes associated with obesity by the polyphenol-rich extracts of Hibiscus sabdariffa. Food Biosci. 2022, 50, 101992. [Google Scholar] [CrossRef]
- Eftekhari, A.; Khusro, A.; Ahmadian, E.; Dizaj, S.M.; Hasanzadeh, A.; Cucchiarini, M. Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: A comprehensive review. Arab. J. Chem. 2021, 14, 103106. [Google Scholar] [CrossRef]
- Gülçin, İ.; Gören, A.C.; Taslimi, P.; Alwasel, S.H.; Kılıc, O.; Bursal, E. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium)-analysis of its polyphenol contents by LC-MS/MS. Biocatal. Agric. Biotechnol. 2020, 23, 101441. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional beverages: The emerging side of functional foods: Commercial trends, research, and health implications. Compr. Rev. Food Sci. F. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Rodriguez-Roque, M.J.; Rojas-Graue, M.A.; Elez-Martinez, P.; Martin-Belloso, O. In vitro bioaccessibility of health-related compounds as affected by the formulation of fruit juice-and milk-based beverages. Food Res. Int. 2014, 62, 771–778. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; Blancas-Benitez, F.J.; Velderrain-Rodríguez, G.R.; Montalvo-González, E.; González-Aguilar, G.A.; Alvarez-Parrilla, E.; Sáyago-Ayerdi, S.G. Bioaccessibility of polyphenols released and associated to dietary fibre in calyces and decoction residues of Roselle (Hibiscus sabdariffa L.). J. Funct. Foods 2015, 18, 171–181. [Google Scholar] [CrossRef]
- Nignpense, B.E.; Latif, S.; Francis, N.; Blanchard, C.; Santhakumar, B.A. The impact of simulated gastrointestinal digestion on the bioaccessibility and antioxidant activity of purple rice phenolic compounds. Food Biosci. 2022, 47, 101706. [Google Scholar] [CrossRef]
- Durán-Castañeda, A.C.; Cardenas-Castro, A.P.; Pérez-Jiménez, J.; Pérez-Carvajal, A.M.; Sánchez-Burgos, J.A.; Mateos, R.; Sáyago-Ayerdi, S.G. Bioaccessibility of phenolic compounds in Psidium guajava L. varieties and P. friedrichsthalianum Nied. after gastrointestinal digestion. Food Chem. 2023, 400, 134046. [Google Scholar] [CrossRef]
- García-Niño, W.R.; Ibarra-Lara, L.; Cuevas-Magaña, M.Y.; Sánchez-Mendoza, A.; Armada, E. Protective activities of ellagic acid and urolithins against kidney toxicity of environmental pollutants: A review. Environ. Toxicol. Pharmacol. 2022, 95, 103960. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Rasheed, D.M.; Porzel, A.; Frolov, A.; El Seedi, H.R.; Wessjohann, L.A.; Farag, M.A. Comparative analysis of Hibiscus sabdariffa (roselle) hot and cold extracts in respect to their potential for α-glucosidase inhibition. Food Chem. 2018, 250, 236–244. [Google Scholar] [CrossRef]
- Umeoguaju, F.U.; Ephraim-Emmanuel, B.C.; Uba, J.O.; Bekibele, G.E.; Chigozie, N.; Orisakwe, O.E. Immunomodulatory and Mechanistic Considerations of Hibiscus sabdariffa (HS) in Dysfunctional Immune Responses: A Systematic Review. Front. Immunol. 2021, 12, 550670. [Google Scholar] [CrossRef] [PubMed]
- Verrelli, D.; Dallera, L.; Stendardo, M.; Monzani, S.; Pasqualato, S.; Giorgio, M.; Pallavi, R. Hydroxycitric Acid Inhibits Chronic Myelogenous Leukemia Growth through Activation of AMPK and mTOR Pathway. Nutrients 2022, 14, 2669. [Google Scholar] [CrossRef]
- Micucci, M.; Malaguti, M.; Gallina Toschi, T.; Di Lecce, G.; Aldini, R.; Angeletti, A.; Chiarini, A.; Budriesi, R.; Hrelia, S. Cardiac and Vascular Synergic Protective Effect of Olea europea L. Leaves and Hibiscus sabdariffa L. Flower Extracts. Oxid. Med. Cell Longev. 2015, 2015, 318125. [Google Scholar] [CrossRef] [PubMed]
- Zheoat, A.M.; Gray, A.I.; Igoli, J.O.; Ferro, V.A.; Drummond, R.M. Hibiscus acid from Hibiscus sabdariffa (Malvaceae) has a vasorelaxant effect on the rat aorta. Fitoterapia 2019, 134, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Torres, L.A.; Bernardino-Nicanor, A.; Gómez-Aldapa, C.A.; González-Montiel, S.; Rangel-Vargas, E.; Villagómez-Ibarra, J.R.; González-Cruz, L.; Cortés-López, H.; Castro-Rosas, J. Hibiscus Acid and Chromatographic Fractions from Hibiscus Sabdariffa Calyces: Antimicrobial Activity against Multidrug-Resistant Pathogenic Bacteria. Antibiotics 2019, 8, 218. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Liu, S.; Fei, D.; Zhang, M.; Zhang, Y. Effect of Quercetin-3-O-Sambubioside Isolated from Eucommia ulmoides Male Flowers on Spontaneous Activity and Convulsion Rate in Mice. Planta Med. 2014, 80, 974–977. [Google Scholar] [CrossRef]
- Popiolek-Kalisz, J.; Fornal, E. The Effects of Quercetin Supplementation on Blood Pressure—Meta-Analysis. Curr. Probl. Cardiol. 2022, 47, 101350. [Google Scholar] [CrossRef] [PubMed]
- Blancas-Benitez, F.J.; Pérez-Jiménez, J.; Montalvo-González, E.; González-Aguilar, G.A.; Sáyago-Ayerdi, S.G. In vitro evaluation of the kinetics of the release of phenolic compounds from guava (Psidium guajava L.) fruit. J. Funct. Foods 2018, 43, 139–145. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]



| ID | Tentative Compound | RT (min) | Molecular Formula | [M-H]− or [M]+ |
|---|---|---|---|---|
| Organic acids and related compounds | ||||
| 1 | Hibiscus acid hydroxyethyl ester | 3.07 | C8H12O8 | 235 |
| 2 | Trimethylhydroxycitric acid I | 3.12 | C9H14O8 | 249 |
| 3 | Hibiscus acid dimethylesther | 3.55 | C8H10O7 | 217 |
| 4 | Hydroxycitric acid | 3.83 | C6H8O8 | 207 |
| 5 | Hibiscus acid | 4.10 | C6H6O7 | 189 |
| 6 | Trimethylhydroxycitric acid II | 4.85 | C9H14O8 | 249 |
| 7 | 3-D-deoxy-D-lyxo-2-heptulosaric acid (DHA) | 4.86 | C7H10O8 | 221 |
| Phenolic acids and related compounds | ||||
| 8 | Caffeoylquinic acid | 11.93 | C16H18O9 | 353 |
| 9 | Coumaroyl quinic acid I | 13.64 | C16H18O8 | 337 |
| 10 | Chlorogenic acid | 13.75 | C16H18O9 | 353 |
| 11 | Coumaroyl quinic acid II | 15.84 | C16H18O8 | 337 |
| 12 | 4-Hydroxybenzoic acid | 14.57 | C7H6O3 | 138 |
| 13 | Methylchlorogenate I | 15.00 | C17H20O9 | 367 |
| 14 | Caffeic acid | 15.89 | C9H8O4 | 179 |
| 15 | Methylchlorogenate II | 16.50 | C17H20O9 | 367 |
| 16 | Caffeoylshikimic acid III | 17.49 | C16H16O8 | 335 |
| 17 | Coumaroyl quinic acid III | 20.85 | C16H18O8 | 337 |
| Flavonoids and related compounds | ||||
| 18 | Leoucoside | 13.49 | C26H28O15 | 579 |
| 19 | Myricetin-3-O-sambubioside | 15.51 | C26H28O17 | 612 |
| 20 | Myricetin hexoside | 16.53 | C21H20O13 | 480 |
| 21 | Quercetin-3-O-sambubioside | 16.97 | C26H28O16 | 596 |
| 22 | Kaempferol-3-O-rutinoside | 17.24 | C27H30O15 | 594 |
| 23 | Quercetin 3-O-galactoside | 17.49 | C21H20O12 | 464 |
| 24 | Isoquercetin | 17.74 | C21H20O12 | 463 |
| 25 | Ellagic acid | 17.83 | C14H6O8 | 301 |
| 26 | Quercetin 3-O-(6-acetil-glucoside) | 17.85 | C21H20O12 | 506 |
| 27 | Quercetin-galoilhexoside | 17.97 | C21H20O12 | 616 |
| 28 | Myricetin | 19.53 | C15H10O8 | 317 |
| 29 | Kaempferol-3-p-cumaroilglucoside | 19.93 | C30H26O13 | 594 |
| 30 | Luteolin | 20.34 | C15H10O6 | 286 |
| 31 | Quercetin | 20.80 | C15H10O7 | 301 |
| 32 | Naringenin | 21.38 | C15H12O5 | 272 |
| Anthocyanins and anthocyanidins | ||||
| 33 | Delphinidin-3-sambubioside | 11.90 | C26H29O16+ | 598 |
| 34 | Cyanidin-3-Glucoside | 12.32 | C15H11O7- | 302 |
| 35 | Delphinidin | 13.63 | C21H21O11+ | 550 |
| Beverages | ||||
|---|---|---|---|---|
| ID | Tentative Compound | RT (min) | Commercial | Hibiscus Beverage (HB) |
| Organic acids and related compounds | ||||
| 1 | Hibiscus acid hydroxyethyl ester | 3.07 | n.d. | 1.85 ± 1.07 |
| 2 | Trimethylhydroxycitric acid I | 3.12 | 1.31 ± 0.71 a | 0.51 ± 0.53 a |
| 3 | Hibiscus acid dimethylesther | 3.55 | 18.33 ± 0.4 a | 18.31 ± 5.36 a |
| 4 | Hydroxycitric acid | 3.83 | 7.21 ± 2.11 a | 31.25 ± 5.68 b |
| 5 | Hibiscus acid | 4.10 | 70.20 ± 11.50 a | 231.52 ± 50.96 b |
| 6 | Trimethylhydroxycitric acid II | 4.85 | 28.69 ± 0.84 a | 0.74 ± 0.07 b |
| 7 | 3-D-deoxy-D-lyxo-2-heptulosaric acid (DHA) | 4.86 | n.d. | 1.06 ± 0.40 |
| Total organic acids and related compounds mg/100 mL | 125.74 ± 22.0 a | 285.24 ± 54.15 b | ||
| Phenolic acids and hydroxycinnamic acids derivatives | ||||
| 8 | Caffeoylquinic acid | 11.93 | 7.09 ± 0.66 a | 6.59 ± 1.43 a |
| 9 | Coumaroyl quinic acid I | 13.64 | n.d. | 0.52 ± 0.15 |
| 10 | Chlorogenic acid | 13.75 | 0.21 ± 0.41 a | 3.36 ± 2.24 a |
| 11 | Coumaroyl quinic acid II | 15.84 | 0.54 ± 0.15 a | 0.15 ± 0.13 a |
| 12 | 4-Hydroxybenzoic acid | 14.57 | n.d. | 0.18 ± 0.31 |
| 13 | Methylchlorogenate I | 15.00 | n.d. | 0.97 ± 1.69 |
| 14 | Caffeic acid | 15.89 | n.d. | 0.42 ± 0.38 |
| 15 | Methylchlorogenate II | 16.50 | n.d. | 38.55 ± 23.66n |
| 16 | Caffeoylshikimic acid III | 17.49 | n.d. | 0.44 ± 0.10 |
| 17 | Coumaroyl quinic acid III | 20.85 | n.d. | 0.19 ± 0.19 |
| Total phenolic acids and related compounds mg/100 mL | 7.84 ± 1.72 a | 51.38 ± 26.10 b | ||
| Flavonoids and related compounds | ||||
| 18 | Leoucoside | 13.49 | n.d. | 0.04 ± 0.03 |
| 19 | Myricetin-3-O-sambubioside | 15.51 | n.d. | 5.40 ± 7.40 |
| 20 | Myricetin hexoside | 16.53 | n.d. | 1.01 ± 0.00 |
| 21 | Quercetin-3-O-sambubioside | 16.97 | n.d. | 4.78 ± 0.00 |
| 22 | Kaempferol-3-O-rutinoside | 17.24 | n.d. | 0.79 ± 0.00 |
| 23 | Quercetin 3-O-galactoside | 17.49 | n.d. | 0.04 ± 0.00 |
| 24 | Isoquercetin | 17.74 | 0.23 ± 0.07 a | 0.10 ± 0.11 a |
| 25 | Ellagic acid | 17.83 | n.d. | 0.98 ± 0.30 |
| 26 | Quercetin 3-O-(6-acetil-glucoside) | 17.85 | n.d. | 0.01 ± 0.00 |
| 27 | Quercetin-galloylhexoside | 17.97 | 2.76 ± 2.07 a | 7.03 ± 0.01 b |
| 28 | Myricetin | 19.53 | n.d. | 0.30 ± 0.43 |
| 29 | Kaempferol-3-p-cumaroilglucoside | 19.93 | n.d. | 0.13 ± 0.00 |
| 30 | Luteolin | 20.34 | n.d. | 0.01 ± 0.00 |
| 31 | Quercetin | 20.80 | 1.48 ± 0.59 a | 0.10 ± 0.04 b |
| 32 | Naringenin | 21.38 | n.d. | 0.03 ± 0.00 |
| Total flavonoids and related compounds mg/100 ml | 4.47 ± 0.90 a | 20.75 ± 0.54 b | ||
| Anthocyanins and anthocyanidins | ||||
| 33 | Delphinidin-3-sambubioside | 11.90 | n.d. | 39.88 ± 37.29 a |
| 34 | Cyanidin-3-glucoside | 12.32 | n.d. | 14.05 ± 12.40 a |
| 35 | Delphinidin | 13.63 | n.d. | 6.48 ± 3.87 a |
| Total anthocyanins and anthocyanidins mg/100 mL | n.d. | 60.41 ± 53.21 a | ||
| TOTAL (mg/100 mL) | 138.05 ± 50.24 a | 417.78 ± 45.85 b | ||
| Intestinal Fraction | Soluble Indigestible Fraction | Insoluble Indigestible Fraction | |||||
|---|---|---|---|---|---|---|---|
| ID | Tentative Compound | CB | HB | CB | HB | CB | HB |
| Organic acids and related compounds | |||||||
| 1 | Hibiscus acid hydroxyethyl ester | n.d. | <LOQ | 1.29 ± 0.31 a | 1.08 ± 0.12 a | n.d. | n.d. |
| 2 | Trimethylhydroxycitric acid I | n.d. | 1.14 ± 0.49 | 1.93 ± 0.69 a | 2.07 ± 0.29 a | n.d. | 0.33 ± 0.18 |
| 3 | Hibiscus acid dimethylesther | 10.56 ± 5.06 a | 2.58 ± 0.55 b | 19.46 ± 2.64 a | 3.77 ± 0.71 b | n.d. | n.d. |
| 4 | Hydroxycitric acid | n.d. | n.d. | n.d. | 1.48 ± 1.06 | 10.61 ± 1.87 a | 1.20 ± 0.61 b |
| 5 | Hibiscus acid | 16.00 ± 3.56 a | 3.37 ± 2.79 b | 2.20 ± 0.59 a | 9.16 ± 2.29 b | 13.33 ± 6.94 | n.d. |
| 6 | Trimethylhydroxycitric acid II | n.d. | <LOQ | n.d. | <LOQ | n.d. | n.d. |
| Total organic acids and related compounds | 26.56 ± 5.06 a | 7.50 ± 1.17 b | 24.88 ± 3.50 a | 17.67 ± 2.45 a | 23.94 ± 6.70 a | 1.53 ± 0.79 b | |
| Hydroxycinnamic acids and related compounds | |||||||
| 8 | Caffeoylquinic acid | n.d. | 7.90 ± 1.28 | n.d. | 0.13 ± 0.02 | n.d. | 6.99 ± 4.04 |
| 9 | Coumaroyl quinic acid III | n.d. | <LOD | n.d. | <LOQ | n.d. | 0.22 ± 0.12 |
| 10 | Chlorogenic acid | n.d. | 3.40 ± 1.07 | n.d. | n.d. | n.d. | n.d. |
| 13 | Coumaroyl quinic acid II | n.d. | 0.23 ± 0.03 | n.d. | n.d. | n.d. | 0.23 ± 0.17 |
| 14 | Caffeic acid | n.d. | 4.22 ± 0.85 | n.d. | n.d. | n.d. | 3.49 ± 2.02 |
| Total hydroxycinnamic acids and related compounds | - | 15.83 ± 3.34 b | - | 0.13 ± 0.02 | - | 10.93 ± 6.31 | |
| Flavonoids and related compounds | |||||||
| 18 | Leoucoside | n.d. | 0.74 ± 0.06 | n.d. | <LOQ | n.d. | n.d. |
| 28 | Quercetin-galloylhexoside | n.d. | 2.83 ± 0.06 | n.d. | <LOQ | n.d. | n.d. |
| 26 | Ellagic acid | n.d. | 2.45 ± 0.20 | n.d. | 1.29 ± 0.28 | n.d. | 1.07 ± 0.13 |
| 23 | Kaempferol-3-rutinoside | n.d. | 0.12 ± 0.03 | n.d. | <LOQ | n.d. | n.d. |
| 27 | Quercetin 3-O-(6-acetil-glucoside) | <LOQ | Nd | <LOQ | n.d. | <LOQ | <LOQ |
| 32 | Naringenin | n.d. | <LOQ | n.d. | n.d. | n.d. | n.d. |
| Total flavonoids and related compounds | - | 6.26 ± 0.16 | - | 1.29 ± 0.28 | - | ||
| TOTAL (mg/100 mL) | 26.56 ± 1.06 a | 29.59 ± 1.81 a | 24.88 ± 1.05 a | 19.40 ± 2.2 a | 23.94 ± 6.70 a | 13.65 ± 8.32 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Romero, J.d.J.; Arce-Reynoso, A.; Parra-Torres, C.G.; Zamora-Gasga, V.M.; Mendivil, E.J.; Sáyago-Ayerdi, S.G. In Vitro Gastrointestinal Digestion Affects the Bioaccessibility of Bioactive Compounds in Hibiscus sabdariffa Beverages. Molecules 2023, 28, 1824. https://doi.org/10.3390/molecules28041824
Rodríguez-Romero JdJ, Arce-Reynoso A, Parra-Torres CG, Zamora-Gasga VM, Mendivil EJ, Sáyago-Ayerdi SG. In Vitro Gastrointestinal Digestion Affects the Bioaccessibility of Bioactive Compounds in Hibiscus sabdariffa Beverages. Molecules. 2023; 28(4):1824. https://doi.org/10.3390/molecules28041824
Chicago/Turabian StyleRodríguez-Romero, José de Jesús, Alejandro Arce-Reynoso, Claudia G. Parra-Torres, Victor M. Zamora-Gasga, Edgar J. Mendivil, and Sonia G. Sáyago-Ayerdi. 2023. "In Vitro Gastrointestinal Digestion Affects the Bioaccessibility of Bioactive Compounds in Hibiscus sabdariffa Beverages" Molecules 28, no. 4: 1824. https://doi.org/10.3390/molecules28041824
APA StyleRodríguez-Romero, J. d. J., Arce-Reynoso, A., Parra-Torres, C. G., Zamora-Gasga, V. M., Mendivil, E. J., & Sáyago-Ayerdi, S. G. (2023). In Vitro Gastrointestinal Digestion Affects the Bioaccessibility of Bioactive Compounds in Hibiscus sabdariffa Beverages. Molecules, 28(4), 1824. https://doi.org/10.3390/molecules28041824





