A Status Review on Health-Promoting Properties and Global Regulation of Essential Oils
Abstract
1. Introduction
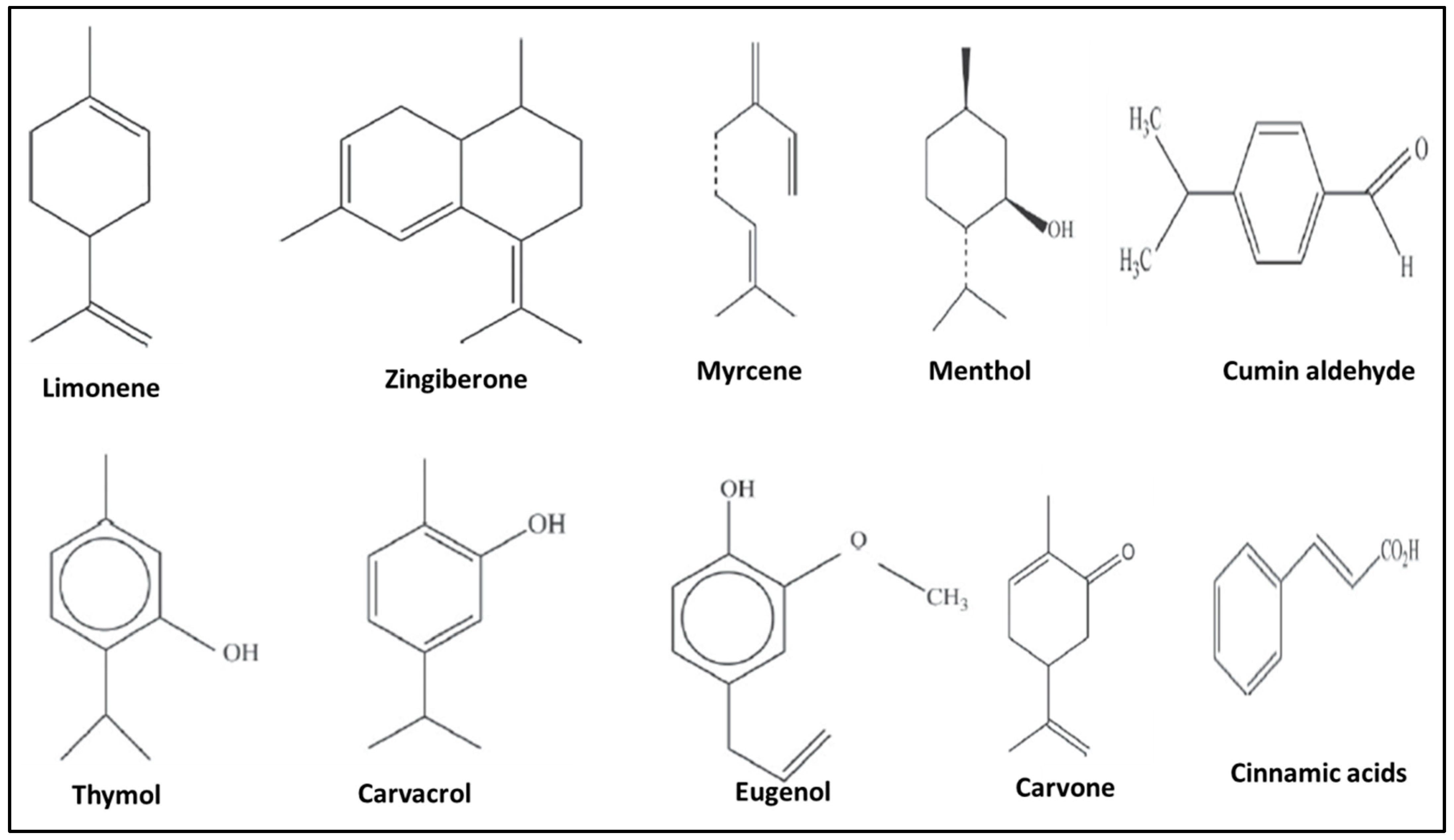
2. Extraction and Chemical Composition of EOs
3. Therapeutic Effects of EOs
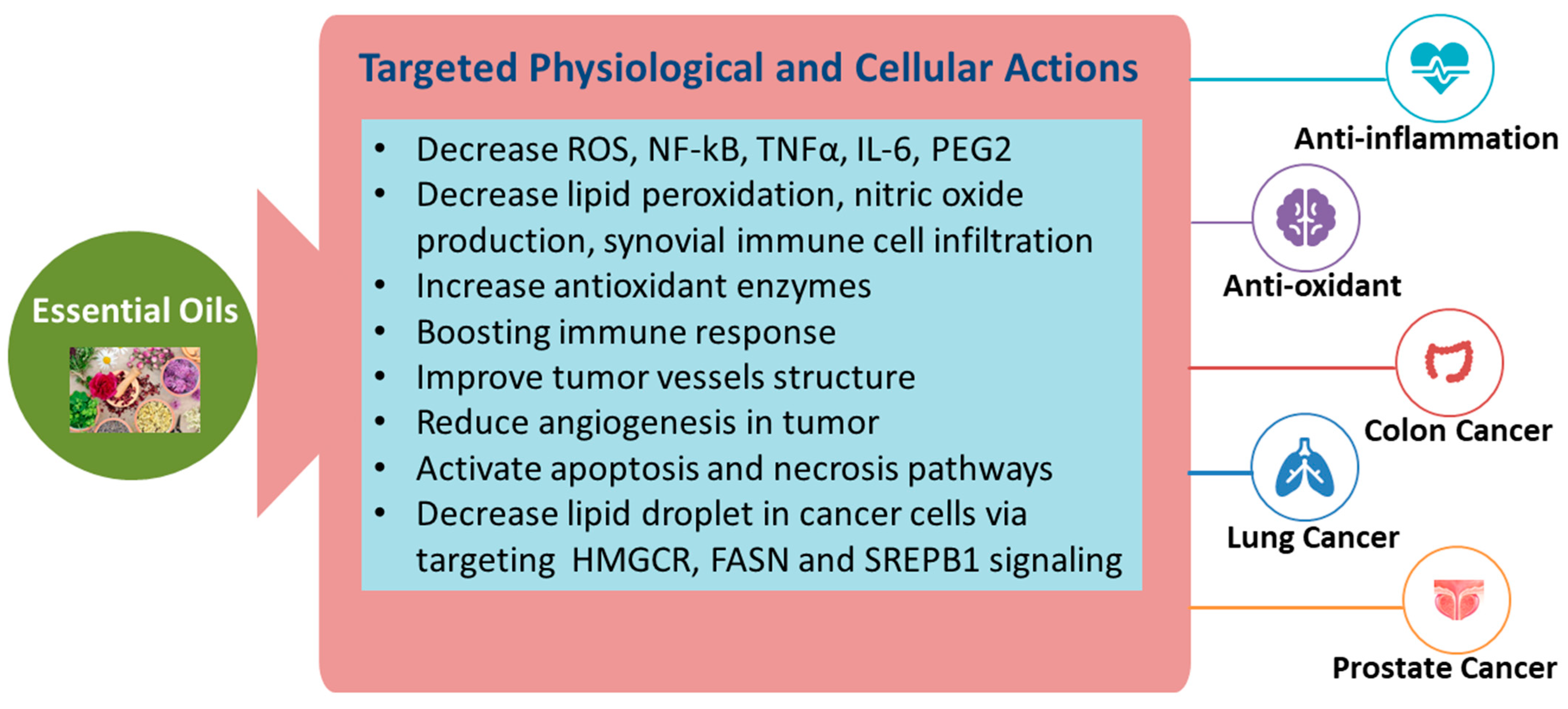
3.1. Antioxidant, Anti-Inflammatory, and Anti-Cancer Activities of Essential Oils
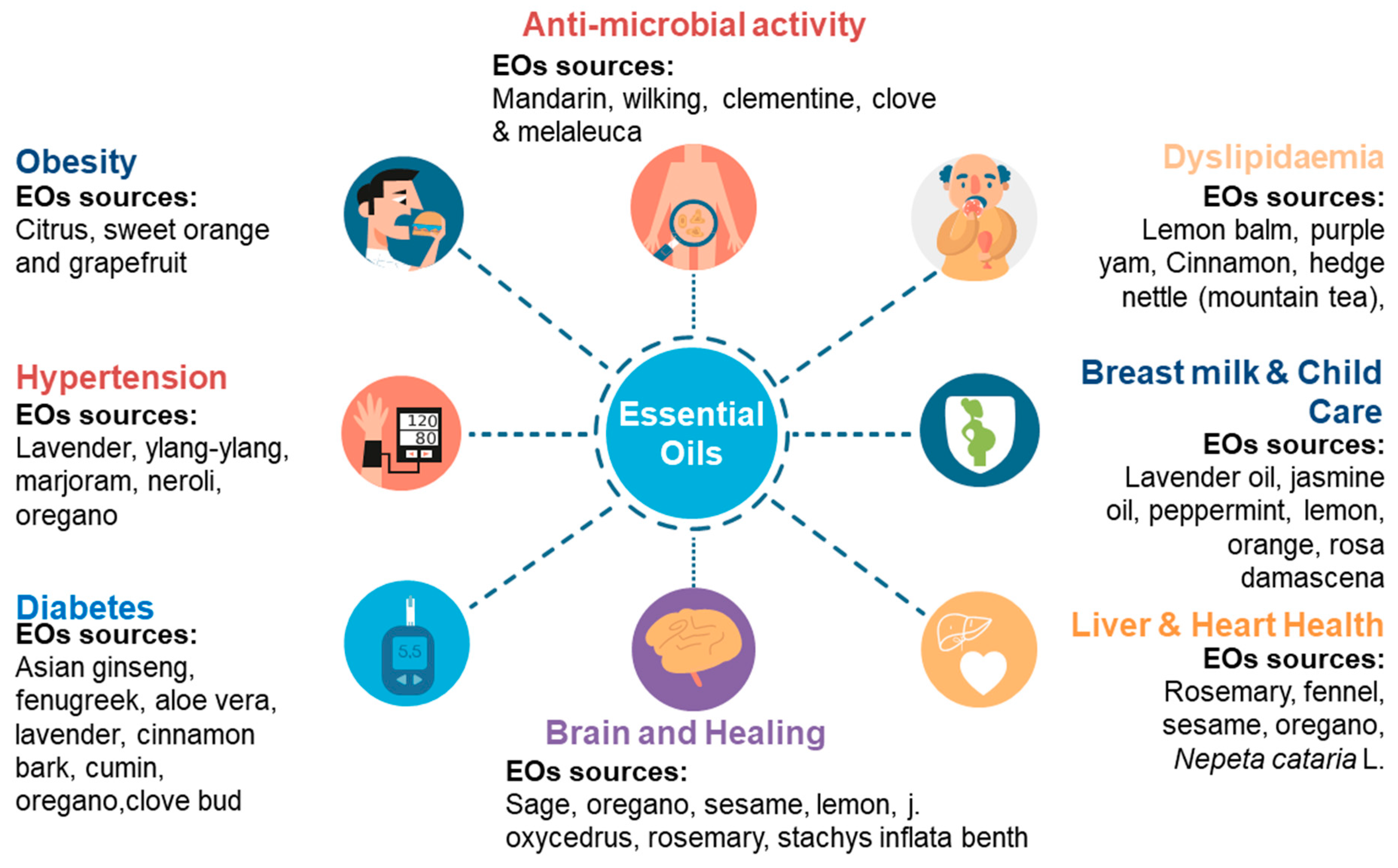
3.2. EOs: A Remedy for the Management of Metabolic Syndrome
3.2.1. EOs and Obesity
3.2.2. EOs and Diabetes
3.2.3. EOs and Hypertension
3.2.4. EO and Dyslipidemia
3.2.5. Dosage, Bioactive Metabolites, Therapeutic, and Adverse Effects of EOs
3.3. Enhance Breast Milk Production and Childcare
3.4. EOs: Natural Antibiotics
3.5. Other Beneficial Effects of EOs
4. Health-Associated Regulation and Consumption of EOs
- WHO hosts a digital platform (WHO Essential Medicines and Health Products Information Portal) that addresses the safety of plant materials, including EOs. The portal includes 5480 medicines and health products and is updated every month.
- The WHO regularly publishes guidelines regarding ‘good manufacturing product practices’ (GMP) for herbal medicines.
- The WHO issued four volumes titled ‘The WHO monographs on selected medicinal plants to provide scientific information on the safety, efficacy, and quality of EOs and other natural products’.
5. Limitation and Future Direction
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reuter, J.; Merfort, I.; Schempp, C.M. Botanicals in dermatology. Am. J. Clin. Dermatol. 2010, 11, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.; Jones, G. A contemporary introduction to essential oils: Chemistry, bioactivity and prospects for Australian agriculture. Agriculture 2015, 5, 48–102. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Corazza, M.; Borghi, A.; Lauriola, M.; Virgili, A. Use of topical herbal remedies and cosmetics: A questionnaire-based investigation in dermatology out-patients. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1298–1303. [Google Scholar] [PubMed]
- Yeshi, K.; Phurpa, W. Essential oils and their bioactive molecules in healthcare. In Herbal Biomolecules in Healthcare Applications; Academic Press: Cambridge, MA, USA, 2022; pp. 215–237. [Google Scholar]
- Lis-Balchin, M.; Deans, S.G.; Eaglesham, E. Relationship between bioactivity and chemical composition of commercial essential oils. Flavour Fragr. J. 1998, 13, 98–104. [Google Scholar] [CrossRef]
- Ly, T.T.G.; Yun, J.; Lee, D.-H.; Chung, J.-S.; Kwon, S.-M. Protective Effects and Benefits of Olive Oil and Its Extracts effects on Women’s Health. Nutrients 2021, 13, 4279. [Google Scholar] [PubMed]
- Fahmy, M.A.; Farghaly, A.A.; Hassan, E.E.; Hassan, E.M.; Hassan, Z.M.; Mahmoud, K.; Omara, E.A. Evaluation of the Anti-Cancer/Anti-Mutagenic Efficiency of Lavandula officinalis Essential Oil. Asian Pac. J. Cancer Prev. 2022, 23, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.; El Basuini, M.F.; Yilmaz, S.; Abdel-Latif, H.M.; Alagawany, M.; Kari, Z.A.; Abdul Razab, M.K.A.; Hamid, N.K.A.; Moonmanee, T.; Van Doan, H. Exploring the roles of dietary herbal essential oils in aquaculture: A review. Animals 2022, 12, 823. [Google Scholar]
- Radi, F.Z.; Bouhrim, M.; Mechchate, H.; Al-Zahrani, M.; Qurtam, A.A.; Aleissa, A.M.; Drioiche, A.; Handaq, N.; Zair, T. Phytochemical Analysis, Antimicrobial and Antioxidant Properties of Thymus zygis L. and Thymus willdenowii Boiss. Essential Oils. Plants 2021, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Kulig, M.; Galanty, A.; Grabowska, K.; Podolak, I. Assessment of safety and health-benefits of Citrus hystrix DC. peel essential oil, with regard to its bioactive constituents in an in vitro model of physiological and pathological skin conditions. Biomed. Pharmacother. 2022, 151, 113151. [Google Scholar] [PubMed]
- De Blasio, A.; D’Anneo, A.; Lauricella, M.; Emanuele, S.; Giuliano, M.; Pratelli, G.; Calvaruso, G.; Carlisi, D. The Beneficial Effects of Essential Oils in Anti-Obesity Treatment. Int. J. Mol. Sci. 2021, 22, 11832. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Preservation of aquatic food using edible films and coatings containing essential oils: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 66–105. [Google Scholar] [CrossRef]
- Zubair, M.; Shahzad, S.; Hussain, A.; Pradhan, R.A.; Arshad, M.; Ullah, A. Current Trends in the Utilization of Essential Oils for Polysaccharide-and Protein-Derived Food Packaging Materials. Polymers 2022, 14, 1146. [Google Scholar] [CrossRef] [PubMed]
- Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Evaluation of Placental Toxicity of Five Essential Oils and Their Potential Endocrine-Disrupting Effects. Curr. Issues Mol. Biol. 2022, 44, 2794–2810. [Google Scholar] [CrossRef] [PubMed]
- Sirousmehr, A.; Arbabi, J.; Asgharipour, M.R. Effect of drought stress levels and organic manures on yield, essential oil content and some morphological characteristics of sweet basil (Ocimum basilicum). Adv. Environ. Biol. 2014, 8, 880–885. [Google Scholar]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Okoh, O.O.; Sadimenko, A.P.; Afolayan, A.J. Comparative evaluation of the antibacterial activities of the essential oils of Rosmarinus officinalis L. obtained by hydrodistillation and solvent free microwave extraction methods. Food Chem. 2010, 120, 308–312. [Google Scholar] [CrossRef]
- Farhat, A.; Fabiano-Tixier, A.-S.; Visinoni, F.; Romdhane, M.; Chemat, F. A surprising method for green extraction of essential oil from dry spices: Microwave dry-diffusion and gravity. J. Chromatogr. A 2010, 1217, 7345–7350. [Google Scholar] [CrossRef]
- Masango, P. Cleaner production of essential oils by steam distillation. J. Clean. Prod. 2005, 13, 833–839. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A. Chemistry and bioactivity of essential oils. Lipids Essent Oils Antimicrob Agents 2011, 25, 203–238. [Google Scholar]
- Breitmaier, E. Terpenes: Flavors, Fragrances, Pharmaca, Pheromones; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Zuzarte, M.; Salgueiro, L. Essential oils chemistry. In Bioactive Essential Oils and Cancer; Springer: Berlin/Heidelberg, Germany, 2015; pp. 19–61. [Google Scholar]
- Moghaddam, M.; Mehdizadeh, L. Chemistry of essential oils and factors influencing their constituents. In Soft Chemistry and Food Fermentation; Elsevier: Amsterdam, The Netherlands, 2017; pp. 379–419. [Google Scholar]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Arumugam, G.; Swamy, M.K.; Sinniah, U.R. Plectranthus amboinicus (Lour.) Spreng: Botanical, phytochemical, pharmacological and nutritional significance. Molecules 2016, 21, 369. [Google Scholar] [CrossRef]
- Swamy, M.K.; Sinniah, U.R.; Akhtar, M. In vitro pharmacological activities and GC-MS analysis of different solvent extracts of Lantana camara leaves collected from tropical region of Malaysia. Evid. -Based Complement. Altern. Med. 2015, 2015, 506413. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Donati Sarti, M.; Campieri, M.; Valerii, M.C. Antioxidant, anti-inflammatory, and microbial-modulating activities of essential oils: Implications in colonic pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- De Lavor, É.M.; Fernandes, A.W.C.; de Andrade Teles, R.B.; Leal, A.E.B.P.; de Oliveira Júnior, R.G.; Gama e Silva, M.; De Oliveira, A.P.; Silva, J.C.; de Moura Fontes Araujo, M.T.; Coutinho, H.D.M. Essential oils and their major compounds in the treatment of chronic inflammation: A review of antioxidant potential in preclinical studies and molecular mechanisms. Oxidative Med. Cell. Longev. 2018, 2018, 6468593. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Sanzo, G.D.; Verardi, A.; Lopresto, C.G.; Pugliese, A.; Menichini, F.; Balducchi, R.; Calabrò, V. Chemical profile and antioxidant properties of extracts and essential oils from Citrus× limon (L.) burm. Cv. Femminello comune. Chem. Biodivers. 2016, 13, 571–581. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Lombardo, G.E.; Cirmi, S.; Musumeci, L.; Pergolizzi, S.; Maugeri, A.; Russo, C.; Mannucci, C.; Calapai, G.; Navarra, M. Mechanisms underlying the anti-inflammatory activity of bergamot essential oil and its antinociceptive effects. Plants 2020, 9, 704. [Google Scholar] [CrossRef]
- Afroz, M.; Zihad, S.N.K.; Uddin, S.J.; Rouf, R.; Rahman, M.S.; Islam, M.T.; Khan, I.N.; Ali, E.S.; Aziz, S.; Shilpi, J.A. A systematic review on antioxidant and antiinflammatory activity of Sesame (Sesamum indicum L.) oil and further confirmation of antiinflammatory activity by chemical profiling and molecular docking. Phytother. Res. 2019, 33, 2585–2608. [Google Scholar] [CrossRef]
- Rosalina, R.; Weerapreeyakul, N. An insight into sesamolin: Physicochemical properties, pharmacological activities, and future research prospects. Molecules 2021, 26, 5849. [Google Scholar] [CrossRef] [PubMed]
- Sankar, D.; Rao, M.R.; Sambandam, G.; Pugalendi, K. Effect of sesame oil on diuretics or ß-blockers in the modulation of blood pressure, anthropometry, lipid profile, and redox status. Yale J. Biol. Med. 2006, 79, 19. [Google Scholar] [PubMed]
- Jabbari, N.; Eftekhari, Z.; Roodbari, N.H.; Parivar, K. Evaluation of Encapsulated Eugenol by Chitosan Nanoparticles on the aggressive model of rheumatoid arthritis. Int. Immunopharmacol. 2020, 85, 106554. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity beyond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef]
- Jan, S.; Rashid, M.; Abd_Allah, E.F.; Ahmad, P. Biological efficacy of essential oils and plant extracts of cultivated and wild ecotypes of Origanum vulgare L. BioMed Res. Int. 2020, 2020, 8751718. [Google Scholar] [CrossRef] [PubMed]
- Boudries, H.; Loupassaki, S.; Ladjal Ettoumi, Y.; Souagui, S.; Bachir Bey, M.; Nabet, N.; Chikhoune, A.; Madani, K.; Chibane, M. Chemical profile, antimicrobial and antioxidant activities of Citrus reticulata and Citrus clementina (L.) essential oils. Int. Food Res. J. 2017, 24, 1782. [Google Scholar]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Yang, C.; Chen, H.; Chen, H.; Zhong, B.; Luo, X.; Chun, J. Antioxidant and anticancer activities of essential oil from Gannan navel orange peel. Molecules 2017, 22, 1391. [Google Scholar] [CrossRef]
- Thomas, V.; Giles, D.; PM Basavarajaswamy, G.; Kumar Das, A.; Patel, A. Coumarin derivatives as anti-inflammatory and anticancer agents. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2017, 17, 415–423. [Google Scholar] [CrossRef]
- Sylvestre, M.; Pichette, A.; Longtin, A.; Nagau, F.; Legault, J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J. Ethnopharmacol. 2006, 103, 99–102. [Google Scholar] [CrossRef]
- Feng, Y.; Deng, L.; Guo, H.; Zhao, Y.; Peng, F.; Wang, G.; Yu, C. The anti-colon cancer effects of essential oil of Curcuma phaeocaulis through tumour vessel normalisation. Front. Oncol. 2021, 4459, 728464. [Google Scholar] [CrossRef] [PubMed]
- Perumalsamy, H.; Shanmugam, R.; Kim, J.-R.; Anandapadmanaban, G.; Huq, M.; Dua, K.; Chellappan, D.K.; Yoon, T.H.; Balusamy, S.R. Nanoemulsion and Encapsulation Strategy of Hydrophobic Oregano Essential Oil Increased Human Prostate Cancer Cell Death via Apoptosis by Attenuating Lipid Metabolism. Bioinorg. Chem. Appl. 2022, 2022, 9569226. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Colnaghi, G.; Chemat, F.; Smadja, J.; Faoro, F.; Visinoni, F.A. Histo-cytochemistry and scanning electron microscopy of lavender glandular trichomes following conventional and microwave-assisted hydrodistillation of essential oils: A comparative study. Flavour Fragr. J. 2006, 21, 704–712. [Google Scholar] [CrossRef]
- Fornari, T.; Vicente, G.; Vázquez, E.; García-Risco, M.R.; Reglero, G. Isolation of essential oil from different plants and herbs by supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.d.A.; Andrade, L.N.; De Sousa, É.B.V.; De Sousa, D.P. Anti-ulcer activity of essential oil constituents. Molecules 2014, 19, 5717–5747. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Haran, G.H.; da Silva Calisto, V.K.; Jothi, G.; Quintans, J.d.S.S.; Cuevas, L.E.; Narain, N.; Júnior, L.J.Q.; Cipolotti, R. Essential oils and its bioactive compounds modulating cytokines: A systematic review on anti-asthmatic and immunomodulatory properties. Phytomedicine 2020, 73, 152854. [Google Scholar] [CrossRef]
- Sharma, P.R.; Mondhe, D.M.; Muthiah, S.; Pal, H.C.; Shahi, A.K.; Saxena, A.K.; Qazi, G.N. Anticancer activity of an essential oil from Cymbopogon flexuosus. Chem. -Biol. Interact. 2009, 179, 160–168. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The metabolic syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef]
- Sharma, P. Inflammation and the metabolic syndrome. Indian J. Clin. Biochem. 2011, 26, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Awadallah, S.; Hasan, H.; Attlee, A.; Raigangar, V.; Unnikannan, H.; Madkour, M.; Abraham, M.S.; Rashid, L.M. Waist circumference is a major determinant of oxidative stress in subjects with and without metabolic syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2541–2547. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Tripathi, P.; Pandey, R.; Srivatava, R.; Goswami, S. Alternative therapies useful in the management of diabetes: A systematic review. J. Pharm. Bioallied Sci. 2011, 3, 504. [Google Scholar] [PubMed]
- Rashed, A.A.; Mohd Nawi, M.N.; Sulaiman, K. Assessment of essential oil as a potential anti-obesity agent: A narrative review. J. EssEntial Oil Res. 2017, 29, 1–10. [Google Scholar] [CrossRef]
- Li, D.; Wu, H.; Dou, H. Weight loss effect of sweet orange essential oil microcapsules on obese SD rats induced by high-fat diet. Biosci. Biotechnol. Biochem. 2019, 83, 923–932. [Google Scholar] [CrossRef]
- Suryawanshi, J.A.S. An overview of Citrus aurantium used in treatment of various diseases. Afr. J. Plant Sci. 2011, 5, 390–395. [Google Scholar]
- Niijima, A.; Nagai, K. Effect of olfactory stimulation with flavor of grapefruit oil and lemon oil on the activity of sympathetic branch in the white adipose tissue of the epididymis. Exp. Biol. Med. 2003, 228, 1190–1192. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Heghes, S.C.; Filip, L.; Vostinaru, O.; Mogosan, C.; Miere, D.; Iuga, C.A.; Moldovan, M. Essential oil-bearing plants from Balkan Peninsula: Promising sources for new drug candidates for the prevention and treatment of diabetes mellitus and dyslipidemia. Front. Pharmacol. 2020, 11, 989. [Google Scholar] [CrossRef]
- Mowat, A.; Macsween, R.; Percy-Hobbs, L.; Foulis, A. Liver, biliary tract and pancreas. In Muir’s Textbook of Pathology, 13th ed.; MacSween, R., Whaley, K., Eds.; Arnold: London, UK, 1993; pp. 674–741. [Google Scholar]
- Rountree, R. Herb, drugs and blood sugar. Herbs Health 2001, 26. [Google Scholar]
- Sebai, H.; Selmi, S.; Rtibi, K.; Souli, A.; Gharbi, N.; Sakly, M. Lavender (Lavandula stoechas L.) essential oils attenuate hyperglycemia and protect against oxidative stress in alloxan-induced diabetic rats. Lipids Health Dis. 2013, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Talpur, N.; Echard, B.; Ingram, C.; Bagchi, D.; Preuss, H. Effects of a novel formulation of essential oils on glucose–insulin metabolism in diabetic and hypertensive rats: A pilot study. Diabetes Obes. Metab. 2005, 7, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Graves, D.J.; Anderson, R.A. Cinnamon extract regulates glucose transporter and insulin-signaling gene expression in mouse adipocytes. Phytomedicine 2010, 17, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Akinbola, I.A.; Ademosun, A.O.; Sanni, D.M.; Odubanjo, O.V.; Olasehinde, T.A.; Oyeleye, S.I. Essential oil from clove bud (Eugenia aromatica Kuntze) inhibit key enzymes relevant to the management of type-2 diabetes and some pro-oxidant induced lipid peroxidation in rats pancreas in vitro. J. Oleo Sci. 2015, 64, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.A.; Phinney, S.D. γ-Linolenate reduces weight regain in formerly obese humans. J. Nutr. 2007, 137, 1430–1435. [Google Scholar] [CrossRef]
- Gupta, R.; Guptha, S. Strategies for initial management of hypertension. Indian J. Med. Res. 2010, 132, 531. [Google Scholar]
- Kim, I.-H.; Kim, C.; Seong, K.; Hur, M.-H.; Lim, H.M.; Lee, M.S. Essential oil inhalation on blood pressure and salivary cortisol levels in prehypertensive and hypertensive subjects. Evid. -Based Complement. Altern. Med. 2012, 2012, 984203. [Google Scholar] [CrossRef]
- Hur, M.-H.; Oh, H.; Lee, M.S.; Kim, C.; Choi, A.-N.; Shin, G.-R. Effects of aromatherapy massage on blood pressure and lipid profile in Korean climacteric women. Int. J. Neurosci. 2007, 117, 1281–1287. [Google Scholar] [CrossRef]
- Aydin, Y.; Kutlay, Ö.; Ari, S.; Duman, S.; Uzuner, K.; Aydin, S. Hypotensive effects of carvacrol on the blood pressure of normotensive rats. Planta Med. 2007, 73, 1365–1371. [Google Scholar] [CrossRef]
- Earley, S.; Gonzales, A.L.; Garcia, Z.I. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol. Pharmacol. 2010, 77, 612–620. [Google Scholar] [CrossRef]
- Dantas, B.P.V.; Alves, Q.L.; de Assis, K.S.; Ribeiro, T.P.; de Almeida, M.M.; de Vasconcelos, A.P.; de Araújo, D.A.M.; de Andrade Braga, V.; de Medeiros, I.A.; Alencar, J.L. Participation of the TRP channel in the cardiovascular effects induced by carvacrol in normotensive rat. Vasc. Pharmacol. 2015, 67, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Almanaitytė, M.; Jurevičius, J.; Mačianskienė, R. Effect of carvacrol, TRP channels modulator, on cardiac electrical activity. BioMed Res. Int. 2020, 2020, 6456805. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.; Ayers, C.; Peterson, C.; Kansagara, D. Aromatherapy and Essential Oils: A Map of the Evidence; Department of Veterans Affairs (US): Washington, DC, USA, 2019. Available online: https://pubmed.ncbi.nlm.nih.gov/31851445/ (accessed on 14 November 2020).
- Li, T.; Teng, H.; An, F.; Huang, Q.; Chen, L.; Song, H. The beneficial effects of purple yam (Dioscorea alata L.) resistant starch on hyperlipidemia in high-fat-fed hamsters. Food Funct. 2019, 10, 2642–2650. [Google Scholar] [CrossRef]
- Ziegenfuss, T.N.; Hofheins, J.E.; Mendel, R.W.; Landis, J.; Anderson, R.A. Effects of a water-soluble cinnamon extract on body composition and features of the metabolic syndrome in pre-diabetic men and women. J. Int. Soc. Sport. Nutr. 2006, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Couturier, K.; Batandier, C.; Awada, M.; Hininger-Favier, I.; Canini, F.; Anderson, R.; Leverve, X.; Roussel, A.-M. Cinnamon improves insulin sensitivity and alters the body composition in an animal model of the metabolic syndrome. Arch. Biochem. Biophys. 2010, 501, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Jia, L.-N.; Honma, N.; Hosono, T.; Ariga, T.; Seki, T. Beneficial effects of cinnamon on the metabolic syndrome, inflammation, and pain, and mechanisms underlying these effects—A review. J. Tradit. Complement. Med. 2012, 2, 27–32. [Google Scholar] [CrossRef]
- Goren, A.C. Use of Stachys species (Mountain Tea) as herbal tea and food. Rec. Nat. Prod. 2014, 8, 71. [Google Scholar]
- Bahadori, M.B.; Maggi, F.; Zengin, G.; Asghari, B.; Eskandani, M. Essential oils of hedgenettles (Stachys inflata, S. lavandulifolia, and S. byzantina) have antioxidant, anti-Alzheimer, antidiabetic, and anti-obesity potential: A comparative study. Ind. Crops Prod. 2020, 145, 112089. [Google Scholar] [CrossRef]
- Weidner, C.; Wowro, S.J.; Freiwald, A.; Kodelja, V.; Abdel-Aziz, H.; Kelber, O.; Sauer, S. Lemon balm extract causes potent antihyperglycemic and antihyperlipidemic effects in insulin-resistant obese mice. Mol. Nutr. Food Res. 2014, 58, 903–907. [Google Scholar] [CrossRef]
- Cappello, G.; Spezzaferro, M.; Grossi, L.; Manzoli, L.; Marzio, L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: A prospective double blind placebo-controlled randomized trial. Dig. Liver Dis. 2007, 39, 530–536. [Google Scholar] [CrossRef]
- Singh, R.; Shushni, M.A.M.; Belkheir, A. Antibacterial and Antioxidant Activities of Mentha Piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef]
- Jabri, M.A.; Sakly, M.; Marzouki, L.; Sebai, H. Chamomile (Matricaria recutita L.) decoction extract inhibits in vitro intestinal glucose absorption and attenuates high fat diet-induced lipotoxicity and oxidative stress. Biomed. Pharmacother. 2017, 87, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, C.; Tsigrimani, D.; Tsiaka, T.; Lantzouraki, D.Z.; Strati, I.F.; Makris, C.; Tagkouli, D.; Proestos, C.; Sinanoglou, V.J.; Zoumpoulakis, P. Metabolic and antioxidant profiles of herbal infusions and decoctions. Food Chem. 2016, 211, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Rafraf, M.; Zemestani, M.; Asghari-Jafarabadi, M. Effectiveness of chamomile tea on glycemic control and serum lipid profile in patients with type 2 diabetes. J. Endocrinol. Investig. 2015, 38, 163–170. [Google Scholar] [CrossRef]
- Bayliak, M.M.; Dmytriv, T.R.; Melnychuk, A.V.; Strilets, N.V.; Storey, K.B.; Lushchak, V.I. Chamomile as a potential remedy for obesity and metabolic syndrome. EXCLI J. 2021, 20, 1261–1286. [Google Scholar] [PubMed]
- Hieu, T.H.; Dibas, M.; Surya, D.K.A.; Sherif, N.A.; Hashmi, M.U.; Mahmoud, M.; Trang, N.T.T.; Abdullah, L.; Nghia, T.L.B.; Hirayama, K.; et al. Therapeutic efficacy and safety of chamomile for state anxiety, generalized anxiety disorder, insomnia, and sleep quality: A systematic review and meta-analysis of randomized trials and quasi-randomized trials. Phytother. Res. 2019, 33, 1604–1615. [Google Scholar] [CrossRef]
- Zemestani, M.; Rafraf, M.; Asghari-Jafarabadi, M. Chamomile tea improves glycemic indices and antioxidants status in patients with type 2 diabetes mellitus. Nutrition 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Wu, L.; Guo, T.; Deng, R.; Liu, L.; Yu, Y. Apigenin ameliorates insulin resistance and lipid accumulation by endoplasmic reticulum stress and SREBP-1c/SREBP-2 pathway in palmitate-induced HepG2 Cells and highfat diet-fed mice. J. Pharmacol. Exp. Ther. 2021, 377, 146–156. [Google Scholar] [CrossRef]
- Al-Okbi, S.Y.; Hussein, A.M.S.; Elbakry, H.F.H.; Fouda, K.A.; Mahmoud, K.F.; Hassan, M.E. Health Benefits of Fennel, Rosemary Volatile Oils and their Nano-Forms in Dyslipidemic Rat Model. Pak. J. Biol. Sci. 2018, 21, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Hamden, K.; Keskes, H.; Belhaj, S.; Mnafgui, K.; Feki, A.; Allouche, N. Inhibitory potential of omega-3 fatty and fenugreek essential oil on key enzymes of carbohydrate-digestion and hypertension in diabetes rats. Lipids Health Dis. 2011, 10, 226. [Google Scholar] [CrossRef]
- Lai, Y.S.; Chen, W.C.; Ho, C.T.; Lu, K.H.; Lin, S.H.; Tseng, H.C.; Lin, S.Y.; Sheen, L.Y. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J. Agric. Food Chem. 2014, 62, 5897–5906. [Google Scholar] [CrossRef] [PubMed]
- Ebada, M.E. Essential oils of green cumin and chamomile partially protect against acute acetaminophen hepatotoxicity in rats. An. Da Acad. Bras. De Ciências 2018, 90, 2347–2358. [Google Scholar] [CrossRef] [PubMed]
- Rivaz, M.; Rahpeima, M.; Khademian, Z.; Dabbaghmanesh, M.H. The effects of aromatherapy massage with lavender essential oil on neuropathic pain and quality of life in diabetic patients: A randomized clinical trial. Complement. Ther. Clin. Pract. 2021, 44, 101430. [Google Scholar] [CrossRef]
- Nasiri, L.Z.; Hajimonfarednejad, M.; Riasatian, M.; Abolhassanzadeh, Z.; Iraji, A.; Vojoud, M.; Heydari, M.; Shams, M. Efficacy of inhaled Lavandula angustifolia Mill. Essential oil on sleep quality, quality of life and metabolic control in patients with diabetes mellitus type II and insomnia. J. Ethnopharmacol. 2020, 251, 112560. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Luque, L.; Badar, Z.; Kornic, S.; Danon, M. Prepubertal gynecomastia and chronic lavender exposure: Report of three cases. J. Pediatr. Endocrinol. Metab. 2016, 29, 103–107. [Google Scholar] [CrossRef]
- Henley, D.V.; Lipson, N.; Korach, K.S.; Bloch, C.A. Prepubertal Gynecomastia Linked to Lavender and Tea Tree Oils. N. Engl. J. Med. 2007, 356, 479–485. [Google Scholar] [CrossRef]
- Ramsey, J.T.; Li, Y.; Arao, Y.; Naidu, A.; Coons, L.A.; Diaz, A.; Korach, K.S. Lavender Products Associated with Premature Thelarche and Prepubertal Gynecomastia: Case Reports and Endocrine-Disrupting Chemical Activities. J. Clin. Endocrinol. Metab. 2019, 104, 5393–5405. [Google Scholar] [CrossRef]
- Kramer, M.S.; Chalmers, B.; Hodnett, E.D.; Sevkovskaya, Z.; Dzikovich, I.; Shapiro, S.; Collet, J.-P.; Vanilovich, I.; Mezen, I.; Ducruet, T. Promotion of Breastfeeding Intervention Trial (PROBIT): A randomized trial in the Republic of Belarus. JAMA 2001, 285, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I. Breastfeeding and the use of human milk. Pediatrics 2005, 115, 496–506. [Google Scholar] [PubMed]
- Zanardo, V.; Savona, V.; Cavallin, F.; D’Antona, D.; Giustardi, A.; Trevisanuto, D. Impaired lactation performance following elective delivery at term: Role of maternal levels of cortisol and prolactin. J. Matern. -Fetal Neonatal Med. 2012, 25, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Hector, D.; King, L.; Webb, K.; Heywood, P. Factors affecting breastfeeding practices. Applying a conceptual framework. New South Wales Public Health Bull. 2005, 16, 52–55. [Google Scholar]
- Zuppa, A.A.; Sindico, P.; Orchi, C.; Carducci, C.; Cardiello, V.; Catenazzi, P.; Romagnoli, C. Safety and efficacy of galactogogues: Substances that induce, maintain and increase breast milk production. J. Pharm. Pharm. Sci. 2010, 13, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Nice, F.J. Common herbs and foods used as galactogogues. ICAN: Infant Child Adolesc. Nutr. 2011, 3, 129–132. [Google Scholar] [CrossRef]
- Goksugur, S.B.; Karatas, Z. Breastfeeding and galactogogues agents. Acta Med. Anatolia 2014, 2, 113–118. [Google Scholar] [CrossRef]
- Matsumoto, T.; Asakura, H.; Hayashi, T. Does lavender aromatherapy alleviate premenstrual emotional symptoms? A randomized crossover trial. BioPsychoSocial Med. 2013, 7, 1–8. [Google Scholar] [CrossRef]
- Hossein Koulivand, P.; Khaleghi Ghadiri, M.; Gorji, A. Lavender and the Nervous System. Evid Based Complement. Alternat. Med. 2013, 2013, 681304. [Google Scholar]
- Agustie, P.R.; Hadisaputro, S.; Runjati, R.; Soejoenoes, A.; Mashudi, I.D.; Widyawati, M.N. Effect of oxytocin massage using lavender essential oil on prolactin level and breast milk production in primiparous mothers after caesarean delivery. Belitung Nurs. J. 2017, 3, 337–344. [Google Scholar] [CrossRef]
- Asazawa, K.; Kato, Y.; Yamaguchi, A.; Inoue, A. The effect of aromatherapy treatment on fatigue and relaxation for mothers during the early puerperal period in Japan: A pilot study. Int. J. Community Based Nurs. Midwifery 2017, 5, 365. [Google Scholar] [PubMed]
- Susanti, K.D.M.B.E.; Politeknik, J.K.; Politeknik, J.K. The Effect of Oxytocin Massage Method Using Lavender Essential Oils on The Smooth Production of Breast Milk at Mother Postpartum in Rejang Lebong Regency. In 1st International Conference on Inter-Professional Health Collaboration (ICIHC 2018); Atlantis Press: Amsterdam, The Netherlands, 2019; pp. 91–94. Available online: https://www.atlantis-press.com/proceedings/icihc-18/55916774 (accessed on 14 November 2020).
- Widyawati, M.N.; Hadisaputro, S.; Anies, A.; Soejoenoes, A. Effect of massage and aromatherapy on stress and prolactin level among primiparous puerperal mothers in Semarang, Central Java, Indonesia. Belitung Nurs. J. 2016, 2, 48–57. [Google Scholar] [CrossRef]
- Sabharwal, S.; Sudan, S.; Ranjan, V. Jasminum sambac linn (motia): A review. Int. J. Pharm. Res. Bio-Sci. 2013, 2, 108–130. [Google Scholar]
- Akbari, S.A.A.; Alamolhoda, S.H.; Baghban, A.A.; Mirabi, P. Effects of menthol essence and breast milk on the improvement of nipple fissures in breastfeeding women. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 629. [Google Scholar]
- Shuo-Shin, T.; Hsiu-Hung, W.; Fan-Hao, C. The effects of aromatherapy on postpartum women: A systematic review. J. Nurs. Res. 2020, 28, e96. [Google Scholar]
- Safajou, F.; Shahnazi, M.; Nazemiyeh, H. The effect of lemon inhalation aromatherapy on nausea and vomiting of pregnancy: A double-blinded, randomized, controlled clinical trial. Iran. Red Crescent Med. J. 2014, 16, e14360. [Google Scholar]
- Zahra, A.; Leila, M.S. Lavender aromatherapy massages in reducing labor pain and duration of labor: A randomized controlled trial. Afr. J. Pharm. Pharmacol. 2013, 7, 426–430. [Google Scholar] [CrossRef]
- Olapour, A.; Behaeen, K.; Akhondzadeh, R.; Soltani, F.; al Sadat Razavi, F.; Bekhradi, R. The effect of inhalation of aromatherapy blend containing lavender essential oil on cesarean postoperative pain. Anesthesiol. Pain Med. 2013, 3, 203. [Google Scholar] [CrossRef] [PubMed]
- Çetinkaya, B.; Başbakkal, Z. The effectiveness of aromatherapy massage using lavender oil as a treatment for infantile colic. Int. J. Nurs. Pract. 2012, 18, 164–169. [Google Scholar] [CrossRef]
- Field, T.; Field, T.; Cullen, C.; Largie, S.; Diego, M.; Schanberg, S.; Kuhn, C. Lavender bath oil reduces stress and crying and enhances sleep in very young infants. Early Hum. Dev. 2008, 84, 399–401. [Google Scholar] [CrossRef]
- Ghaderi, F.; Solhjou, N. The effects of lavender aromatherapy on stress and pain perception in children during dental treatment: A randomized clinical trial. Complement. Ther. Clin. Pract. 2020, 40, 101182. [Google Scholar] [CrossRef]
- Sezavar, M.; Ahmadi, R.; Shojaei, H.; Jafari, M.; Hashemi, I.; Attaei Nakhaie, A.R.; Nasibeh, R.; Zolala, S.; Ashrafinia, F.; Khojastehfard, Z. The Effect of Lavender Oil for Relief Painful Producer in Children and Infants: A Systematic Review. Int. J. Pediatr. 2020, 8, 11177–11185. [Google Scholar]
- Motaghi, M.; Borji, M.; Moradi, M. The effect of orange essence aromatherapy on anxiety in school-age children with diabetes. Biomed. Pharmacol. J. 2017, 10, 159–164. [Google Scholar]
- Keyhanmehr, A.S.; Movahhed, M.; Sahranavard, S.; Gachkar, L.; Hamdieh, M.; Afsharpaiman, S.; Nikfarjad, H. The effect of aromatherapy with Rosa damascena essential oil on sleep quality in children. Res. J. Pharmacogn. 2018, 5, 41–46. [Google Scholar]
- O’Flaherty, L.-A.; van Dijk, M.; Albertyn, R.; Millar, A.; Rode, H. Aromatherapy massage seems to enhance relaxation in children with burns: An observational pilot study. Burns 2012, 38, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Zorba, P.; Ozdemir, L. The preliminary effects of massage and inhalation aromatherapy on chemotherapy-induced acute nausea and vomiting: A quasi-randomized controlled pilot trial. Cancer Nurs. 2018, 41, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Kilina, A.; Kolesnikova, M. The efficacy of the application of essential oils for the prevention of acute respiratory diseases in organized groups of children. Vestn. Otorinolaringol. 2011, 5, 51–54. [Google Scholar]
- World Health Organization. The Future of Food Safety: Transforming Knowledge into Action for People, Economies and the Environment: Technical Summary by FAO and WHO. 2020. Available online: https://apps.who.int/iris/handle/10665/333621 (accessed on 14 November 2020).
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Silva, F.; Ferreira, S.; Duarte, A.; Mendonca, D.I.; Domingues, F.C. Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine 2011, 19, 42–47. [Google Scholar] [CrossRef]
- Fitzgerald, D.; Stratford, M.; Gasson, M.; Ueckert, J.; Bos, A.; Narbad, A. Mode of antimicrobial action of vanillin against Escherichia coli, Lactobacillus plantarum and Listeria innocua. J. Appl. Microbiol. 2004, 97, 104–113. [Google Scholar] [CrossRef]
- Osaili, T.M.; Hasan, F.; Dhanasekaran, D.K.; Obaid, R.S.; Al-Nabulsi, A.A.; Ayyash, M.; Karam, L.; Savvaidis, I.N.; Holley, R. Effect of active essential oils added to chicken tawook on the behaviour of Listeria monocytogenes, Salmonella spp. and Escherichia coli O157: H7 during storage. Int. J. Food Microbiol. 2021, 337, 108947. [Google Scholar] [CrossRef]
- Osaili, T.M.; Hasan, F.; Dhanasekaran, D.K.; Obaid, R.S.; Al-Nabulsi, A.A.; Karam, L.; Savvaidis, I.N.; Olaimat, A.N.; Ayyash, M.; Al-Holy, M. Effect of yogurt-based marinade combined with essential oils on the behavior of Listeria monocytogenes, Escherichia coli O157: H7 and Salmonella spp. in camel meat chunks during storage. Int. J. Food Microbiol. 2021, 343, 109106. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.N.; Rall, V.L.M.; Fernandes, A.A.H.; Ushimaru, P.I.; da Silva Probst, I.; Fernandes Jr, A. Essential oils against foodborne pathogens and spoilage bacteria in minced meat. Foodborne Pathog. Dis. 2009, 6, 725–728. [Google Scholar] [CrossRef]
- Chorianopoulos, N.; Kalpoutzakis, E.; Aligiannis, N.; Mitaku, S.; Nychas, G.-J.; Haroutounian, S.A. Essential oils of Satureja, Origanum, and Thymus species: Chemical composition and antibacterial activities against foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8261–8267. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E. Antibacterial activities of plant essential oils and their components against Escherichia coli O157: H7 and Salmonella enterica in apple juice. J. Agric. Food Chem. 2004, 52, 6042–6048. [Google Scholar] [CrossRef]
- Pereira dos Santos, E.; Nicácio, P.H.M.; Coêlho Barbosa, F.; Nunes da Silva, H.; Andrade, A.L.S.; Lia Fook, M.V.; de Lima Silva, S.M.; Farias Leite, I. Chitosan/essential oils formulations for potential use as wound dressing: Physical and antimicrobial properties. Materials 2019, 12, 2223. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Süntar, I.; Akkol, E.K.; Keleş, H.; Oktem, A.; Başer, K.H.C.; Yeşilada, E. A novel wound healing ointment: A formulation of Hypericum perforatum oil and sage and oregano essential oils based on traditional Turkish knowledge. J. Ethnopharmacol. 2011, 134, 89–96. [Google Scholar] [CrossRef]
- Tumen, I.; Süntar, I.; Keleş, H.; Küpeli Akkol, E. A therapeutic approach for wound healing by using essential oils of Cupressus and Juniperus species growing in Turkey. Evid.-Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Valizadeh, A.; Shirzad, M.; Pourmand, M.R.; Farahmandfar, M.; Sereshti, H.; Amani, A. Preparation and comparison of effects of different herbal oil ointments as wound-healing agents. Cells Tissues Organs 2019, 207, 177–186. [Google Scholar] [CrossRef]
- Ziaee, M.; Khorrami, A.; Ebrahimi, M.; Nourafcan, H.; Amiraslanzadeh, M.; Rameshrad, M.; Garjani, M.; Garjani, A. Cardioprotective effects of essential oil of Lavandula angustifolia on isoproterenol-induced acute myocardial infarction in rat. Iran. J. Pharm. Res. IJPR 2015, 14, 279. [Google Scholar]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Özbek, H.; Uğraş, S.; Dülger, H.; Bayram, I.; Tuncer, I.; Öztürk, G.; Öztürk, A. Hepatoprotective effect of Foeniculum vulgare essential oil. Fitoterapia 2003, 74, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, J.; Ma, J.; Qiao, F. Hepatoprotective effect of essential oils of Nepeta cataria L. on acetaminophen-induced liver dysfunction. Biosci. Rep. 2019, 39, BSR20190697. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef]
- Rocamora, C.R.; Ramasamy, K.; Lim, S.M.; Majeed, A.B.A.; Agatonovic-Kustrin, S. HPTLC based approach for bioassay-guided evaluation of antidiabetic and neuroprotective effects of eight essential oils of the Lamiaceae family plants. J. Pharm. Biomed. Anal. 2020, 178, 112909. [Google Scholar] [CrossRef]
- Juergens, U.R.; Dethlefsen, U.; Steinkamp, G.; Gillissen, A.; Repges, R.; Vetter, H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: A double-blind placebo-controlled trial. Respir. Med. 2003, 97, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Iravani, M. Clinical effects of Zataria multiflora essential oil on primary dysmenorrhea. J. Med. Plants 2009, 8, 54–60. [Google Scholar] [CrossRef]
- Tabatabaeichehr, M.; Mortazavi, H. The effectiveness of aromatherapy in the management of labor pain and anxiety: A systematic review. Ethiop. J. Health Sci. 2020, 30, 728281. [Google Scholar] [CrossRef]
- Bouyahya, A.; Lagrouh, F.; El Omari, N.; Bourais, I.; El Jemli, M.; Marmouzi, I.; Salhi, N.; Faouzi, M.E.A.; Belmehdi, O.; Dakka, N. Essential oils of Mentha viridis rich phenolic compounds show important antioxidant, antidiabetic, dermatoprotective, antidermatophyte and antibacterial properties. Biocatal. Agric. Biotechnol. 2020, 23, 101471. [Google Scholar] [CrossRef]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiologyopen 2017, 6, e00459. [Google Scholar] [CrossRef]
- Es, I.; Khaneghah, A.M.; Akbariirad, H. Global regulation of essential oils. In Essential Oils in Food Processing: Chemistry, Safety and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 327–338. [Google Scholar]
- Mortimer, S.; Reeder, M. Botanicals in dermatology: Essential oils, botanical allergens, and current regulatory practices. Dermatitis 2016, 27, 317–324. [Google Scholar] [CrossRef]
- Ajazuddin, S.S. Legal regulations of complementary and alternative medicines in different countries. Pharmacogn. Rev. 2012, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Food, U.; Administration, D. Is It a Cosmetic, a Drug, or Both? (Or Is It Soap?). Retrieved October 2016. Available online: https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap? (accessed on 5 October 2020).
- Japan External Trade Organization. Japanese Trade and Investment Statistics. 2011. Available online: https://www.jetro.go.jp/en/reports/statistics.html (accessed on 29 April 2014).
- Sydney, N. Therapeutic Goods Administration. 2014; Australian Government Department of Health. Available online: https://www.tga.gov.au (accessed on 29 April 2014).
- Government of Canada. Natural Health Products Regulations. Available online: http://laws-lois.justice.gc.ca/eng/regulations/SOR-2003-196/FullText.html (accessed on 19 December 2020).
- Wang, X.; Tao, N.; Ni, Y. Current development of functional foods in China. In Proceedings of the 2003 IFT Annual Meeting-Chicago, Chicago, IL, USA, 12–16 July 2003. [Google Scholar]
- Zhong-zhi, Q.; Dan, Y.; Liu, Y.; Peng, Y. Pharmacopoeia of the People’s Republic of China. A mildstone in development of China healthcare. Chin. Herb. Med. 2010, 2, 157–159. [Google Scholar]
- Parliament, E.; Union, t.C.o.t.E. Directive 2004/24/EC of the European Parliament and of the Council of 31 March 2004 amending, as regards traditional herbal medicinal products, Directive 2001/83/EC on the Community code relating to medicinal products for human use. Off. J. Eur. Union 2004, 136, 85–90. [Google Scholar]
- Rajagopalan, T. Traditional Herbal Medicines around the Globe: Modern Perspectives. The Indian Perspective Proceedings of the 10th General Assembly of WFPMM, Seoul, Korea. Swiss Pharma 1991, 13, 63–67. [Google Scholar]
- Aboushanab, T.; Khalil, M.; Al Ahmari, Y. The present state of complementary medicine regulation in Saudi Arabia. J. Integr. Med. 2019, 17, 147–149. [Google Scholar] [CrossRef]
- Robles, Y.R.; Peña, I.G.; Loquias, M.M.; Salenga, R.L.; Tan, K.C.; Ruamero, E.C., Jr. Regulatory issues on traditionally used herbal products, herbal medicines and food supplements in the Philippines. J. Asian Assoc. Sch. Pharm. 2012, 1, 170. [Google Scholar]
- Ngcobo, M.; Nkala, B.; Moodley, I.; Gqaleni, N. Recommendations for the development of regulatory guidelines for registration of traditional medicines in South Africa. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Gericke, N. The Regulation and Control of Traditional Herbal Medicines. An international overview with recommendations for the development of a South African Approach. Traditional Medicines Programme at the University of Cape Town. Working draft document, December 1995. Available online: http://apps.who.int/medicinedocs/pdf/whozip57e/whozip57e.pdf (accessed on 5 December 2011).
- FDA, US. US Food and Drug Administration Guidance for Industry: Botanical Drug Products. 2004. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/botanical-drug-development-guidance-industry (accessed on 15 August 2020).
- Food, U.; Administration, D. Dietary Supplements Guidance Documents & Regulatory Information. 2013. Available online: https://www.fda.gov/food/guidance-documents-regulatory-information-topic-food-and-dietary-supplements/dietary-supplements-guidance-documents-regulatory-information (accessed on 23 October 2020).
- Sharma, S. Current status of herbal product: Regulatory overview. J. Pharm. Bioallied Sci. 2015, 7, 293. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Benzie, I. An Introduction to Its History, Usage, Regulation, Current Trends, and Research Needs. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
| Essential Oil | Experimental Condition | Usage/Dosage | Duration | Bioactive Compounds | Therapeutic Effects | Possible Side Effects/Toxicity | Reference |
|---|---|---|---|---|---|---|---|
| Peppermint | 57 patients with IBS | Capsule/225 mg per day | 4 weeks | Polyphenols, flavonoids, tocopherols, menthone, and tanins | Significant reduction in the total IBS symptoms score | Not detected | [88,89] |
| Chamomile | HFD-fed Wistar rats | Water extract/100 mg/kg b.w. | 6 weeks | Phenolic compounds and terpenoids | Prevention of body weight gain; decrease in levels of serum TAG, TC, LPL, urea, and creatinine, ALT, and AST; decrease in MDA levels, increase in antioxidant enzyme activity (SOD, catalase, GPx) and GSH levels in the liver and kidney | Not detected | [90,91] |
| 64 patients with T2DM | Chamomile tea (3 g/150 mL hot water) three times per day | 8 weeks | Luteolin and apigenin | Decrease in HOMA-IR index, serum HbA1C, insulin, TAG, TC, and LDL levels; no changes in HDL levels Decrease in serum MDA, increase in serum total antioxidant capacity and antioxidant enzyme activities (SOD, GPx, and catalase) | Mild adverse event | [92,93,94,95] | |
| HFD-fed C57BL/6J mice | 10 mg/kg b.w. | 12 weeks | Apigenin | Decrease in body weight, visceral fat weight, plasma lipid levels (TAG, TC, LDL), postprandial glucose levels, and reduction of hepatic SREBP-1c and SREBP-2 expressions | No side effect | [96] | |
| Fennel and rosemary | 48 male albino rats | Volatile oils-15 and 7.5 mg/kg b.w. respectively | 4 weeks | Trans-anethole, terpenoids, estragole, fenchone, and limonene | Showed cardio and hepato- protective effect and safety towards kidney and blood sugar. Oxidative stress and inflammatory biomarkers were significantly improved | Not detected | [97] |
| Fenugreek | 60 diabetic male Wistar rats | 5% (w/w) | 8 weeks | Terpenenes | Glucose, triglyceride (TG), and total-cholesterol (TC) and LDL-cholesterol (LDL-C) levels decreased significantly in the plasma and liver of diabetic rats and increased the HDL-Cholesterol (HDL-Ch) level, modulated key enzyme related to hypertension | Not detected | [98] |
| Garlic | HFD-fed C57BL/6J mice | 50 mg/kg b.w. | 12 weeks | Diallyl disulfide, DADS | Exerted anti-obesity and anti-hyperlipidemic effects by reducing HFD-induced body weight gain and adipose tissue weight | Not detected | [99] |
| Cumin | Male rats with hepatotoxicity | 400 mg/kg | 2 weeks | Cuminaldehyde, α-pinene and γ-terpinene | Normalized acetaminophen-induced liver enzyme elevation and preserved liver structure | Not detected | [100] |
| Lavender | 75 diabetic neuropathic patients | Massage-2.5 cc of 3% lavender oil | 4 weeks | Linalool and linalyl acetate | Significantly increase the quality of life domain, reduce neuropathic pain | Not detected | [101] |
| 52 diabetic patients with insomnia | Inhalation | 4 weeks | Linalyl acetate and linalool | improve sleep quality and quantity, quality of life, and mood | Not detected | [102] |
| Country | Regulatory Agency |
|---|---|
| Australia | Therapeutic Goods Administration [166]. |
| Canada | Food and Drugs Act (FDA) and the Natural Health Product Regulations (the Regulations) [167] by the Natural and Non-prescription Health Product Directorate (NNHPD). |
| China | However, herbal medicinal products are governed by the current Drug Administration Law to meet certain requirements before they are marketed [168,169]. |
| European Union | The European Medicine Agency under directives 2001/83/EC and 2004/24/EC [170]. |
| India | Drugs and Cosmetics Act (D and C) of 1940 and Rules of 1945, department of AYUSH [171]. |
| Kingdom of Saudi Arabia (KSA) | The National Center for Complementary and Alternative Medicine (NCCAM) in Saudi Arabia, which is a part of the Ministry of Health (MOH) [172]. |
| Philippines | The Food and Drug Administration (FDA) is the government agency that has regulatory power over the production, distribution, and use of herbal products [173]. |
| South Africa | There are currently no guidelines or frameworks for the registration and regulation of traditional medicine (TM) and plant-based remedies in South Africa [174]. However, once a health-related claim is made for a finished product, it has to go through the full drug evaluation procedure at the Medicines Control Council (MCC) before marketing. Pharmaceutical standards need to be consistent with those of the United States Pharmacopoeia (USP) or the British Pharmacopoeia (BP) [175]. |
| United States of America (USA) | The botanical products are classified as a drug, food, or dietary supplement by the United States Food and Drug Administration (FDA) based on the claims or end-use. As per the FDA, the drug must be marketed under an approved New Drug Application (NDA) [176]. The FDA regulates dietary supplements under the Dietary Supplement Health and Education Act of 1994. These do not require premarket approval, and it’s the responsibility of the marketer to ensure the safety and labeling compliance of their products with the regulations. The claims need to comply with the regulatory guidelines issued by the FDA. The manufacturing of dietary supplements should be performed as per the current Good Manufacturing Practices (GMP) for dietary supplements [177]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osaili, T.M.; Dhanasekaran, D.K.; Zeb, F.; Faris, M.E.; Naja, F.; Radwan, H.; Cheikh Ismail, L.; Hasan, H.; Hashim, M.; Obaid, R.S. A Status Review on Health-Promoting Properties and Global Regulation of Essential Oils. Molecules 2023, 28, 1809. https://doi.org/10.3390/molecules28041809
Osaili TM, Dhanasekaran DK, Zeb F, Faris ME, Naja F, Radwan H, Cheikh Ismail L, Hasan H, Hashim M, Obaid RS. A Status Review on Health-Promoting Properties and Global Regulation of Essential Oils. Molecules. 2023; 28(4):1809. https://doi.org/10.3390/molecules28041809
Chicago/Turabian StyleOsaili, Tareq M., Dinesh Kumar Dhanasekaran, Falak Zeb, MoezAlIslam E. Faris, Farah Naja, Hadia Radwan, Leila Cheikh Ismail, Hayder Hasan, Mona Hashim, and Reyad Shaker Obaid. 2023. "A Status Review on Health-Promoting Properties and Global Regulation of Essential Oils" Molecules 28, no. 4: 1809. https://doi.org/10.3390/molecules28041809
APA StyleOsaili, T. M., Dhanasekaran, D. K., Zeb, F., Faris, M. E., Naja, F., Radwan, H., Cheikh Ismail, L., Hasan, H., Hashim, M., & Obaid, R. S. (2023). A Status Review on Health-Promoting Properties and Global Regulation of Essential Oils. Molecules, 28(4), 1809. https://doi.org/10.3390/molecules28041809










