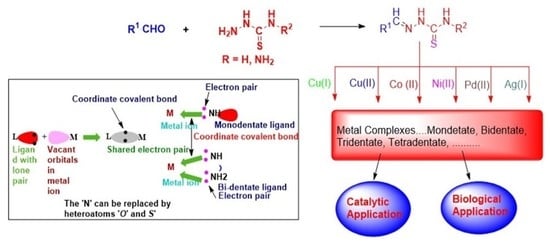
Transition Metal Complexes of Thiosemicarbazides, Thiocarbohydrazides, and Their Corresponding Carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)—A Review
Abstract
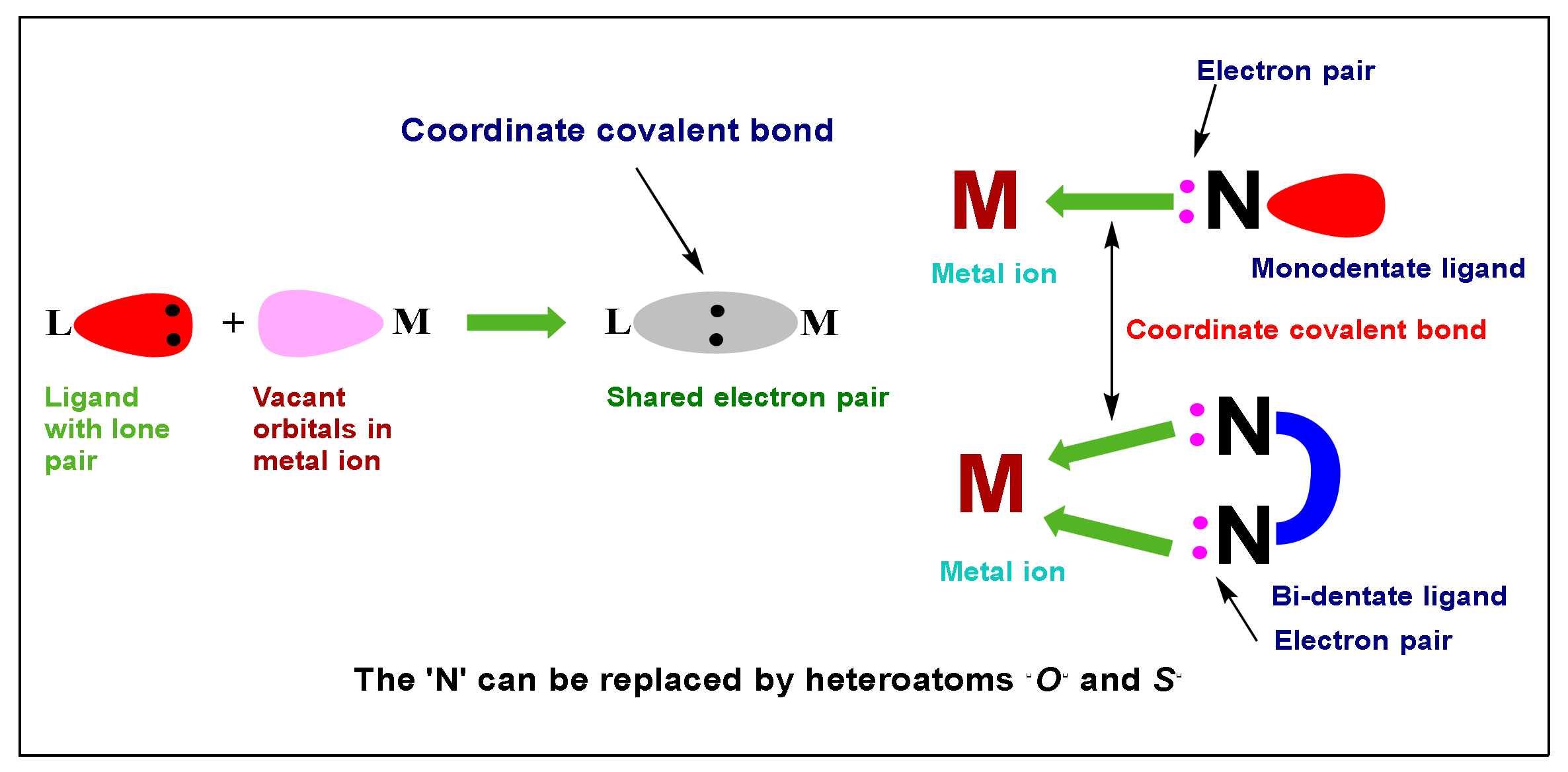
1. Introduction
- i.
- They have better coordination tendencies.
- ii.
- They form more stable complexes.
- iii.
- They have better selectivity.
- iv.
- They may form macrocyclic ligands.
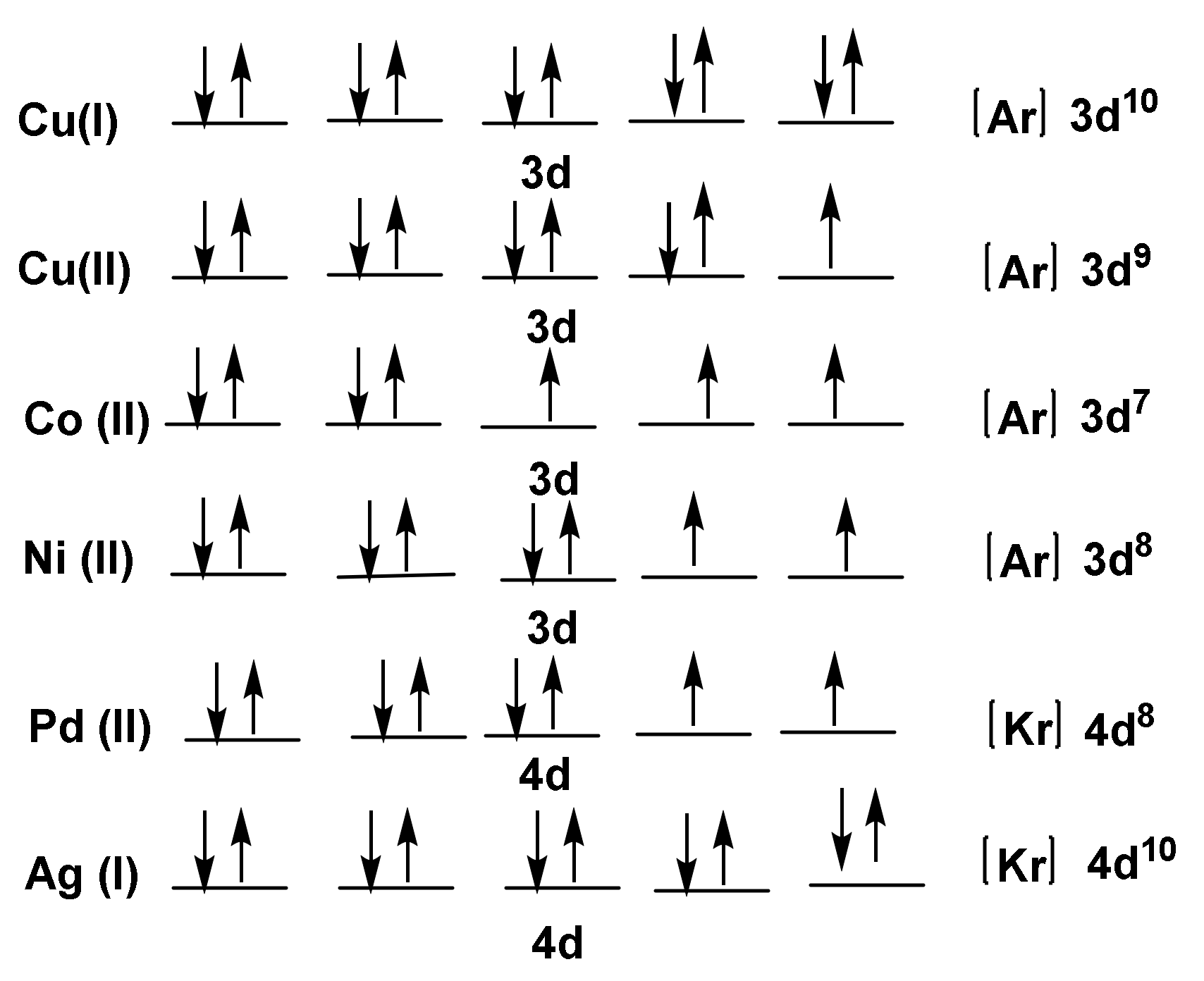
1.1. Electronic Configuration of the Assigned Metals
1.2. Stability of Transition Metal Complexes
1.3. Metal Complexes of Copper (Cu)
1.4. Metal Complexes of Cobalt (II)
1.5. Metal Complexes of Nickel(II)
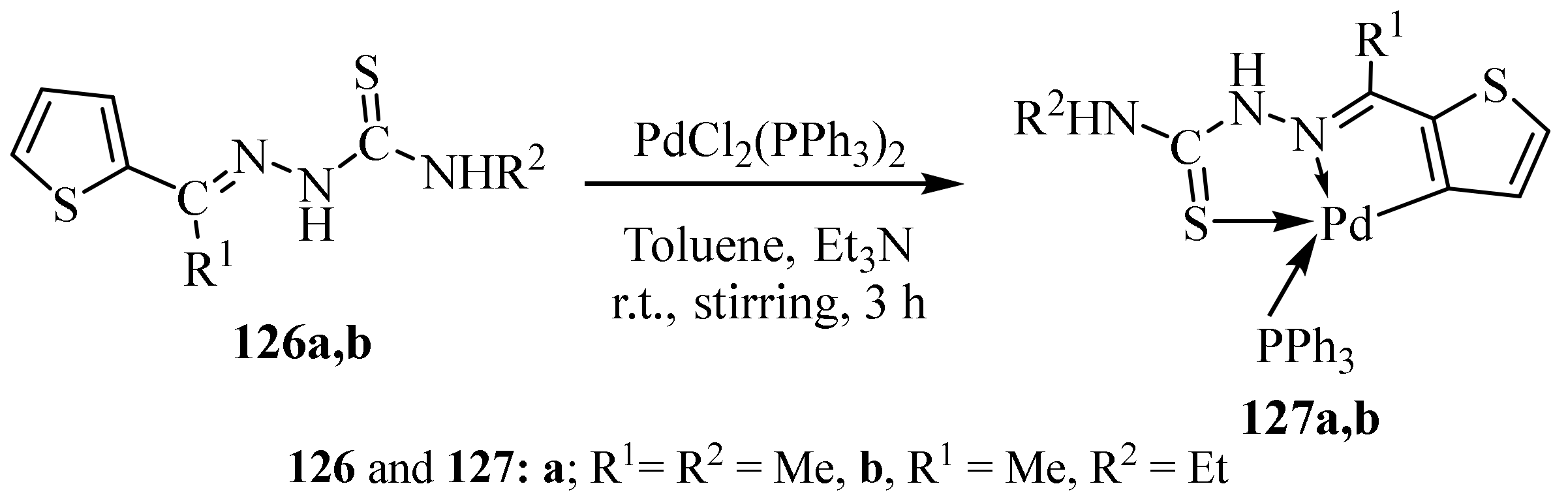
1.6. Metal Complexes of Palladium(II)
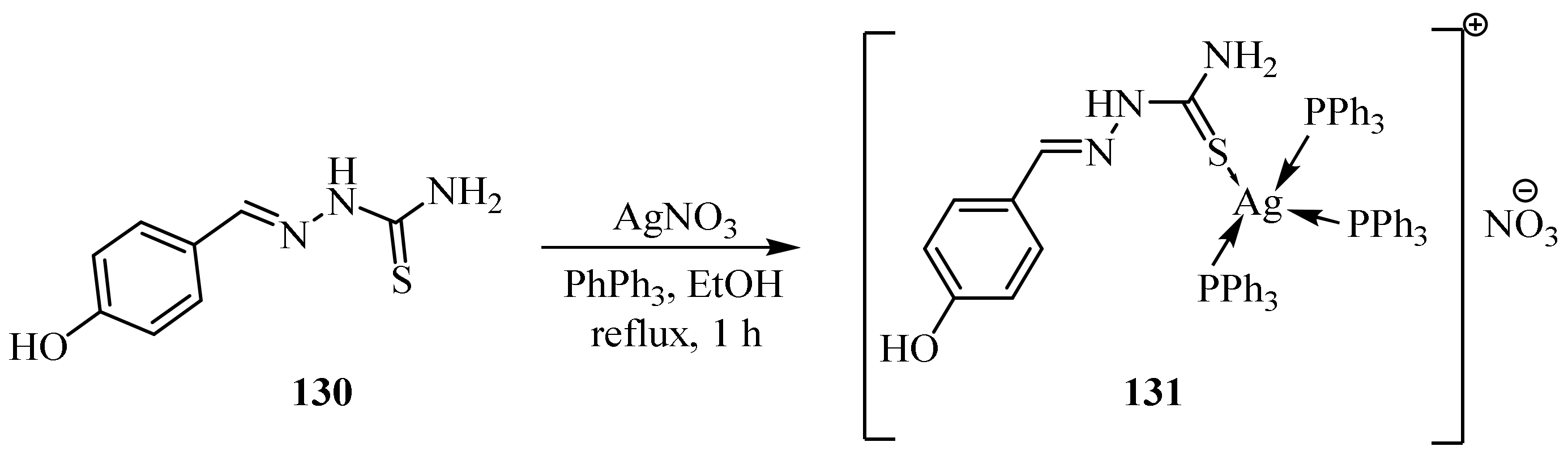
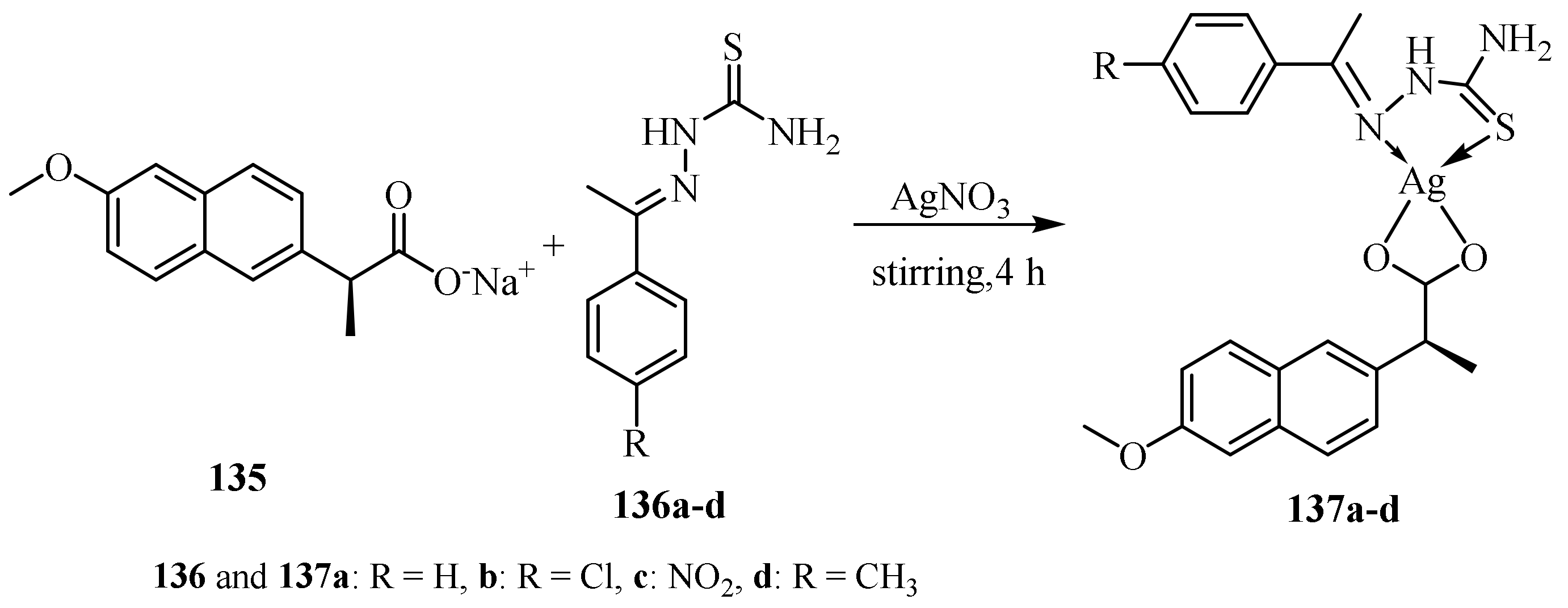
1.7. Metal Complexes of Silver (I)
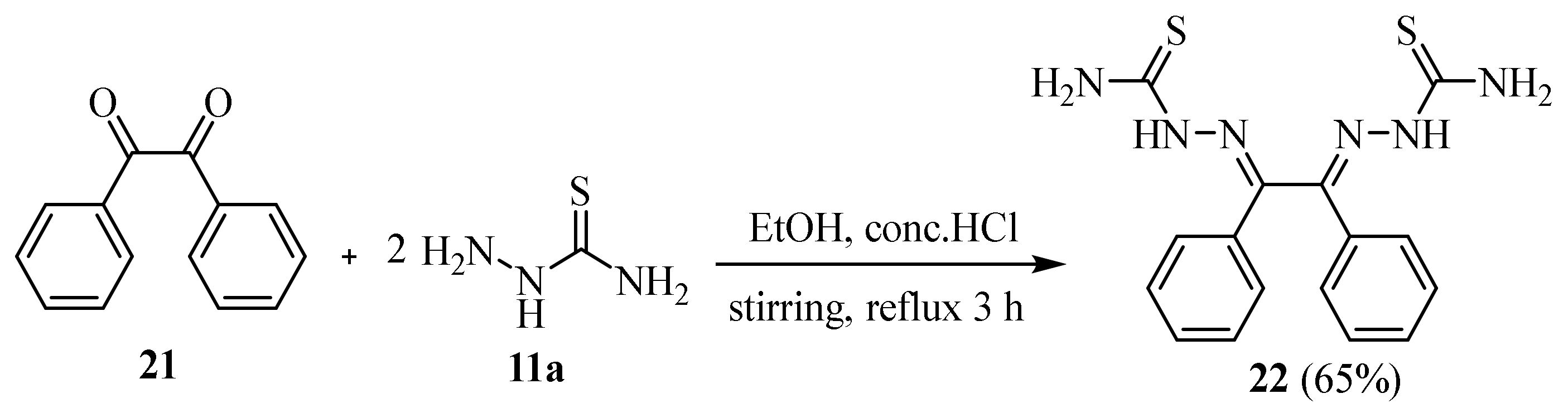
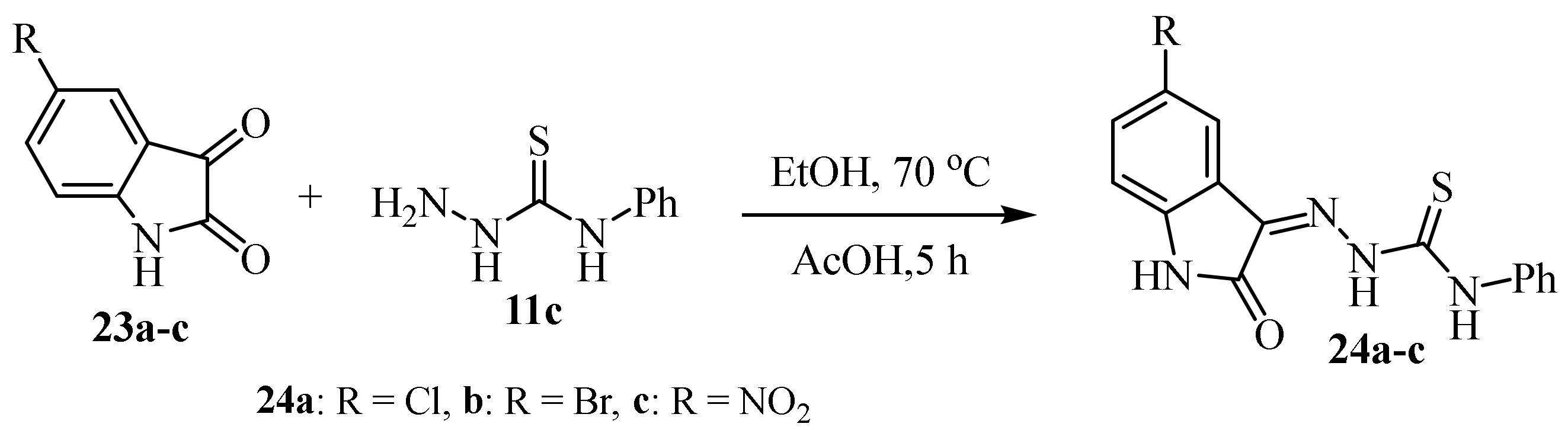
2. Synthesis of Thiosemicarbazide Derivatives
2.1. Using Carbon Disulphide
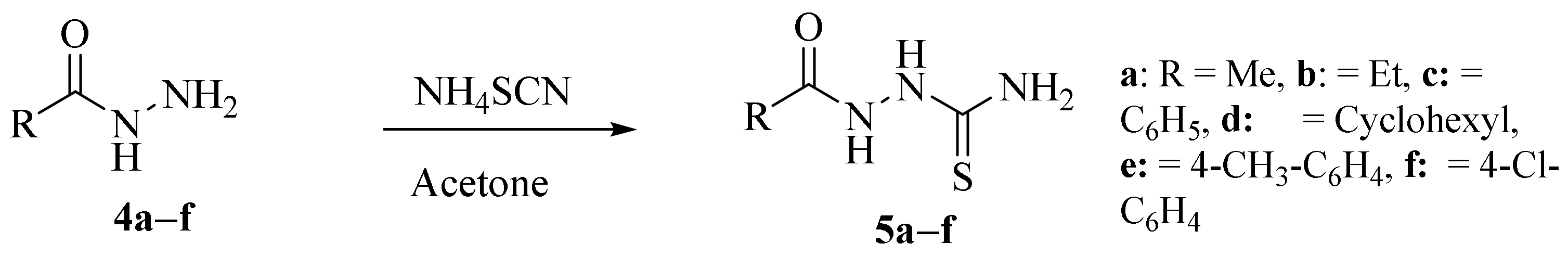
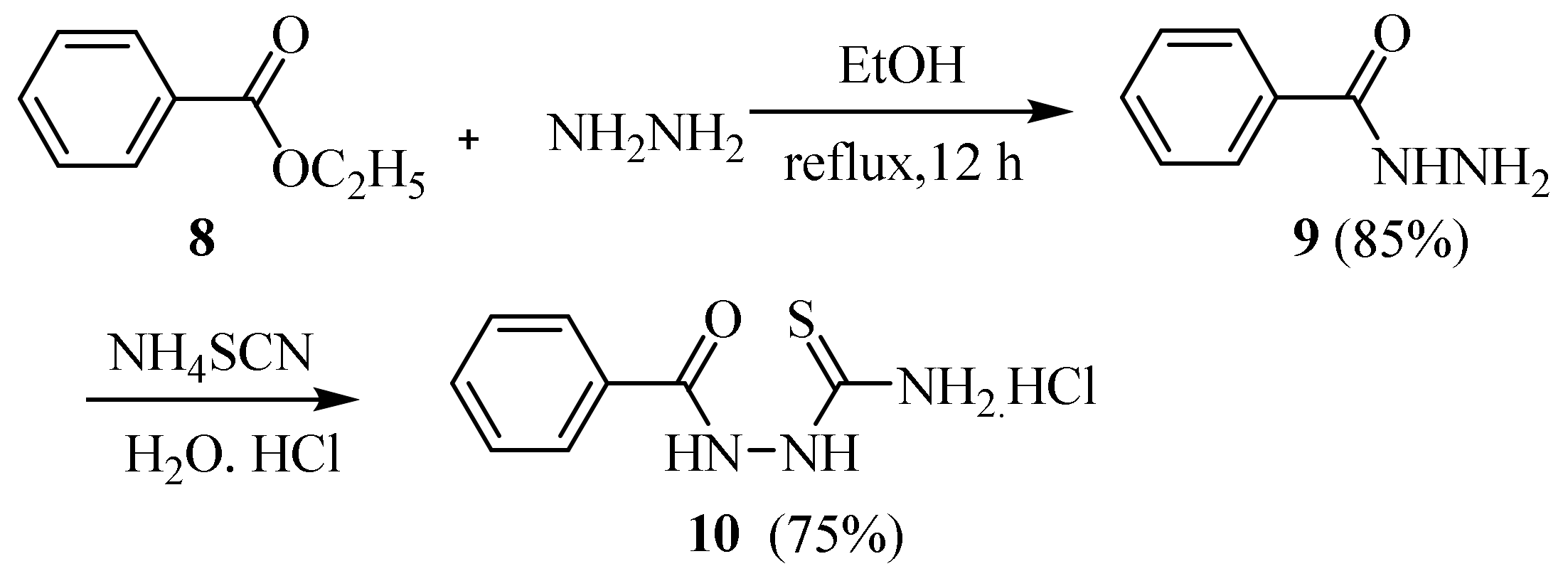
2.2. Using Ammonium Thiocyanate
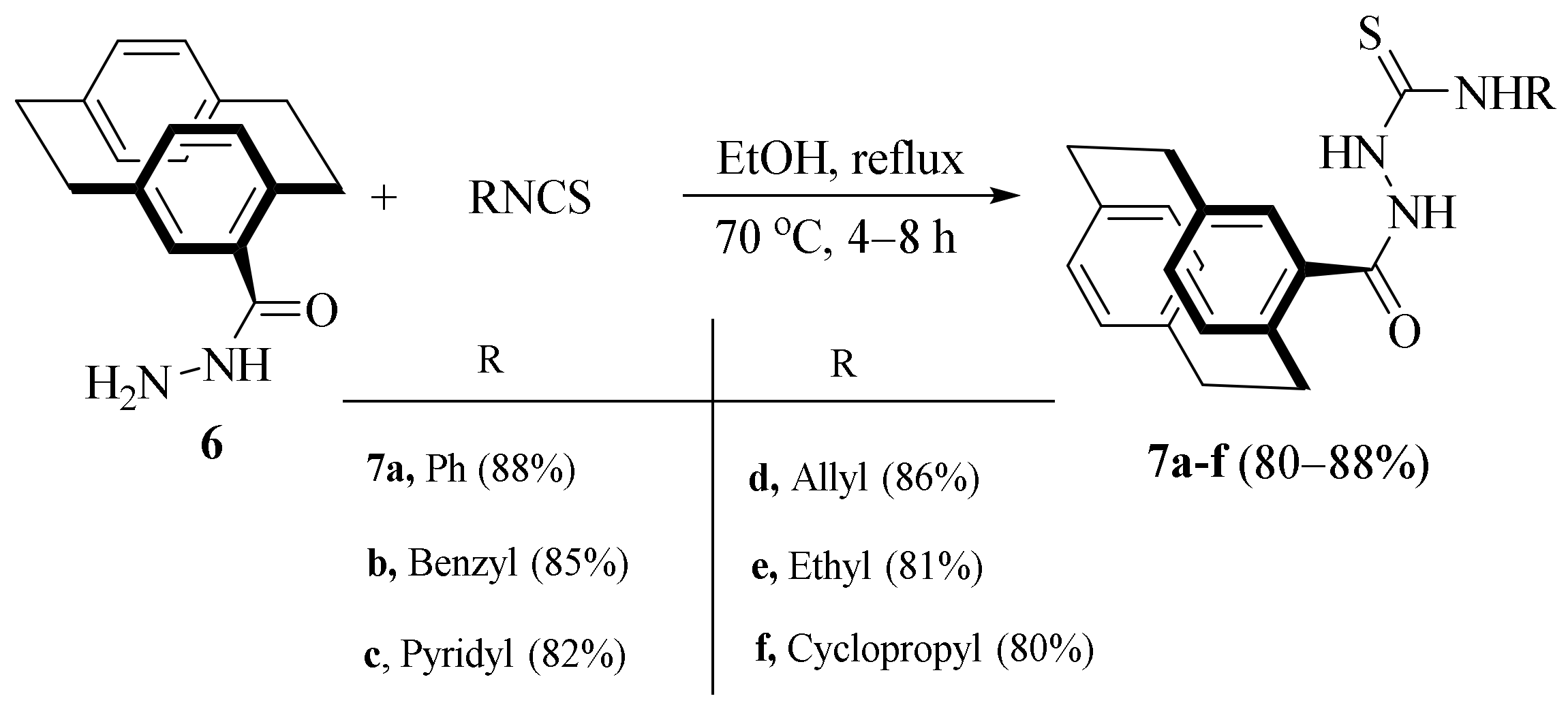
2.3. Reactions of Hydrazines with Substituted Isothiocyanate
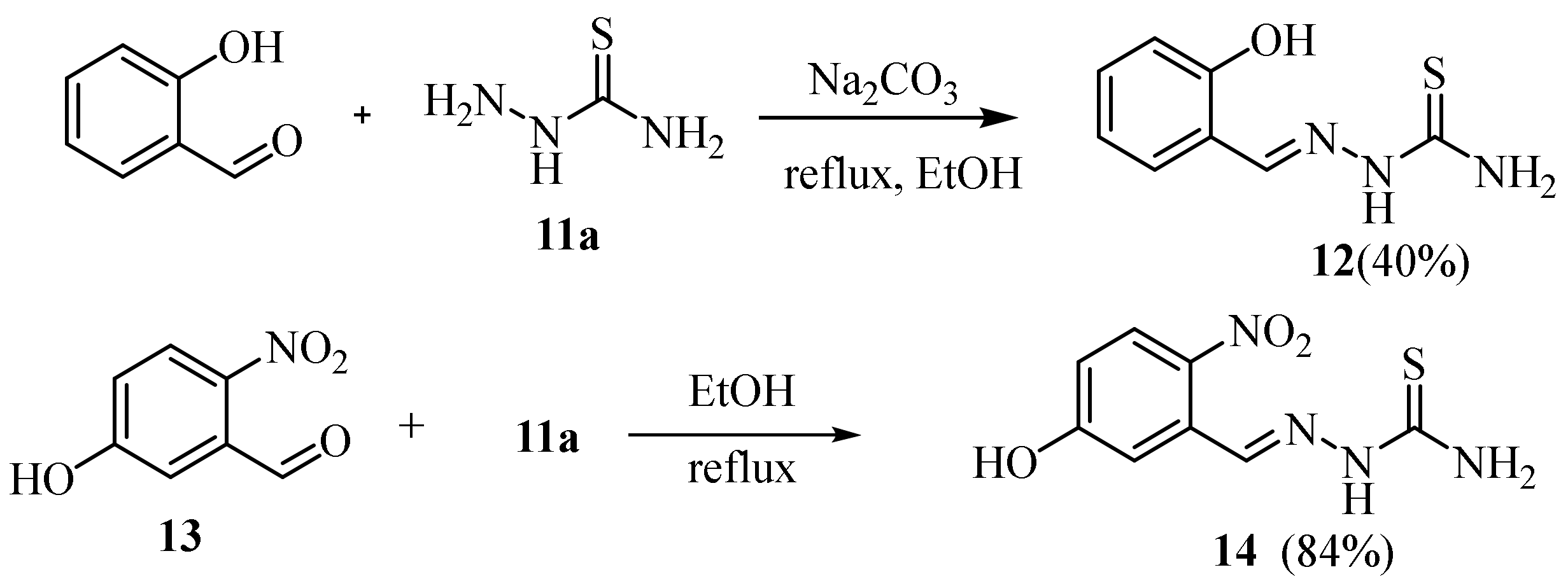
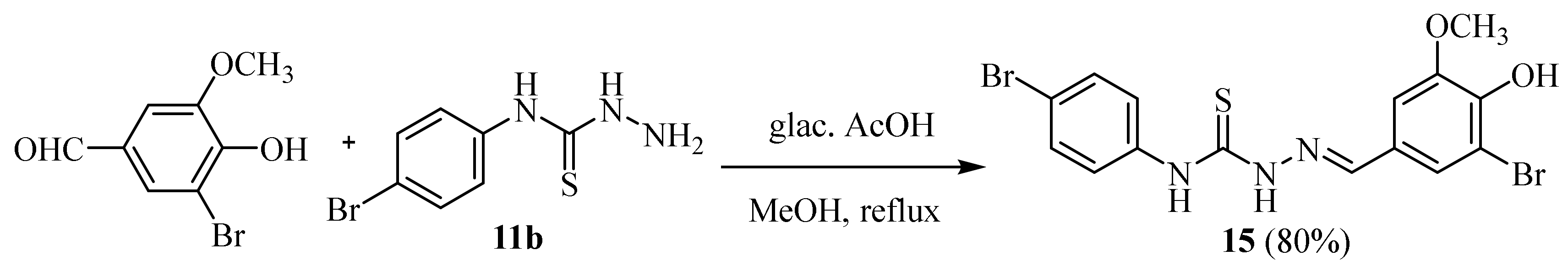
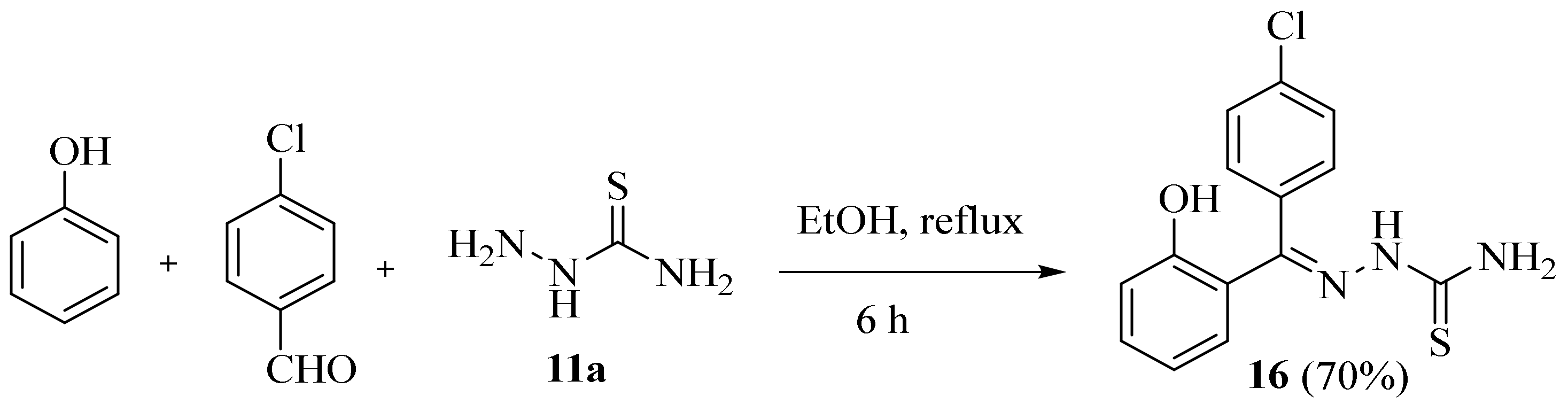
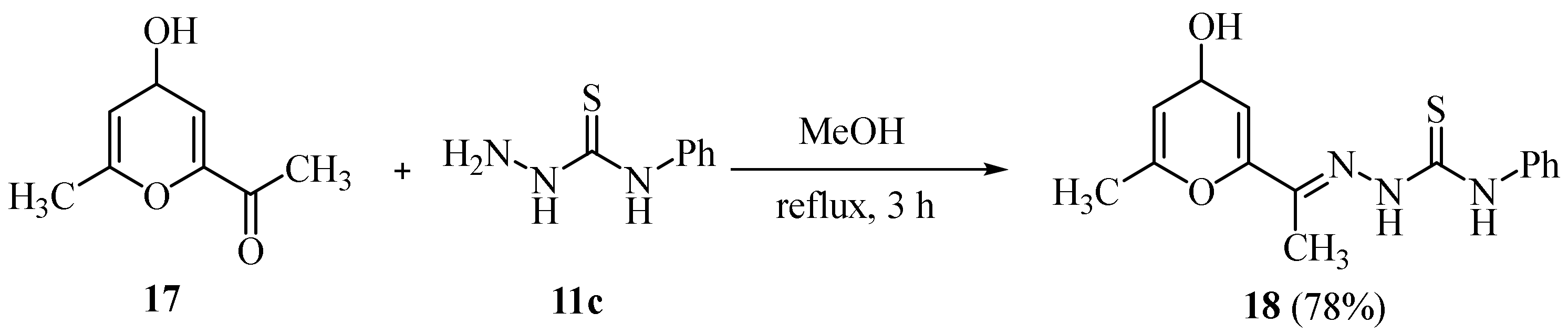
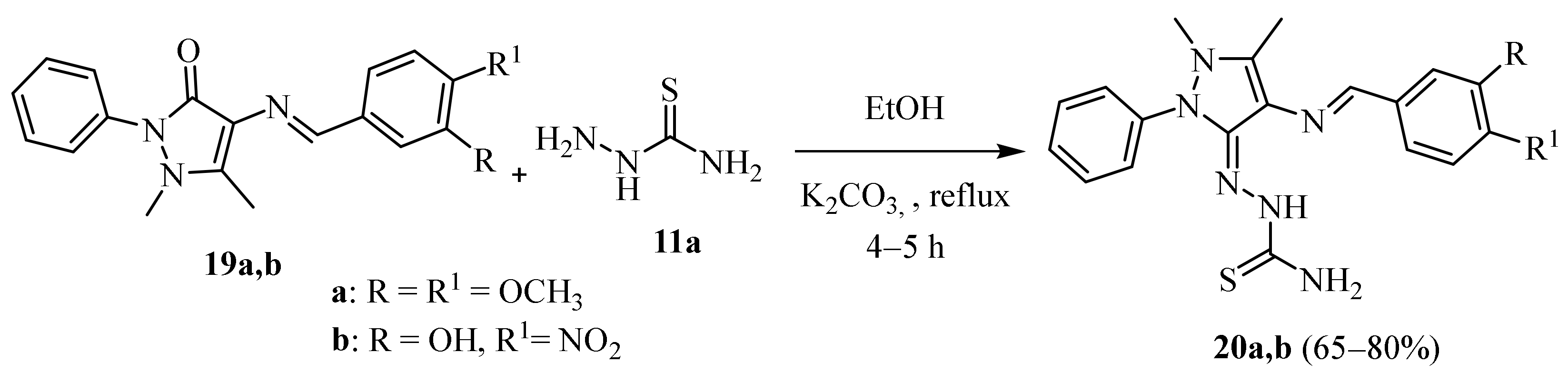
2.4. Representative Examples of Synthesized Thiosemicarbazones
3. Metal Complexes of Thiosemicarbazones and Thiocarbazones
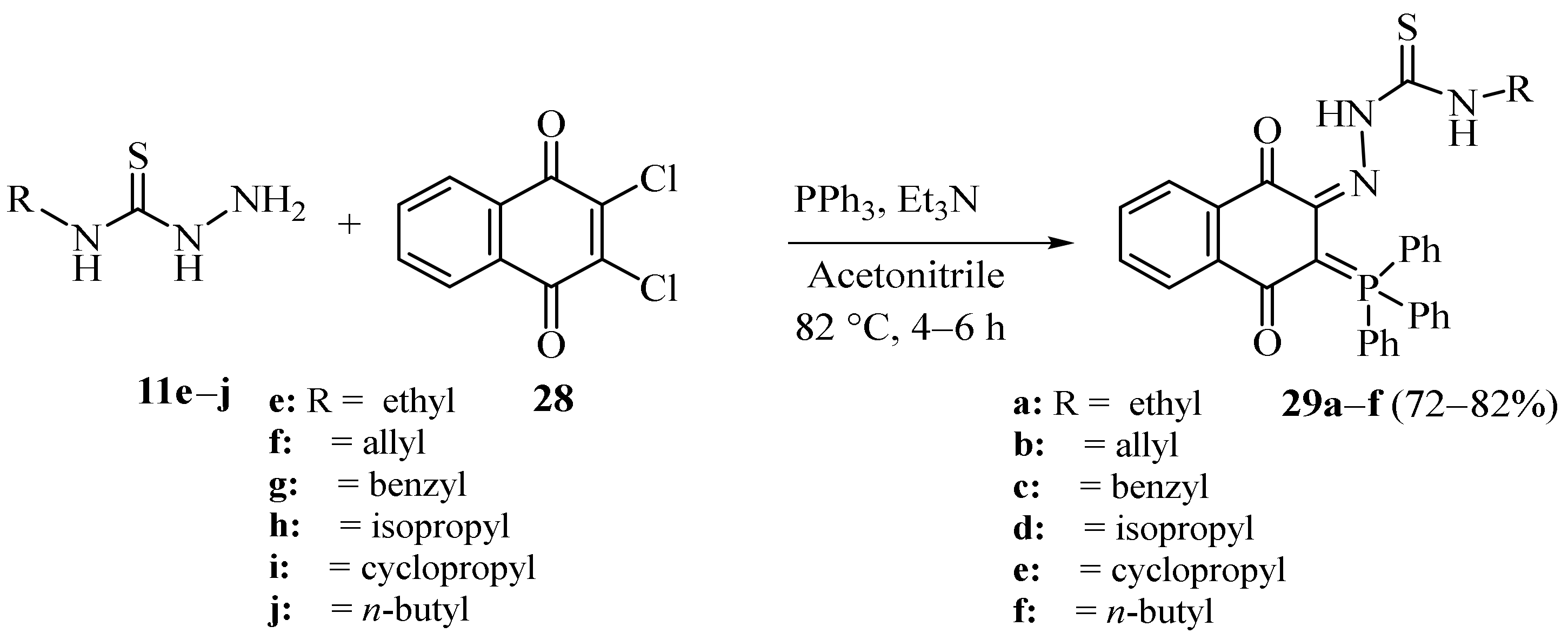
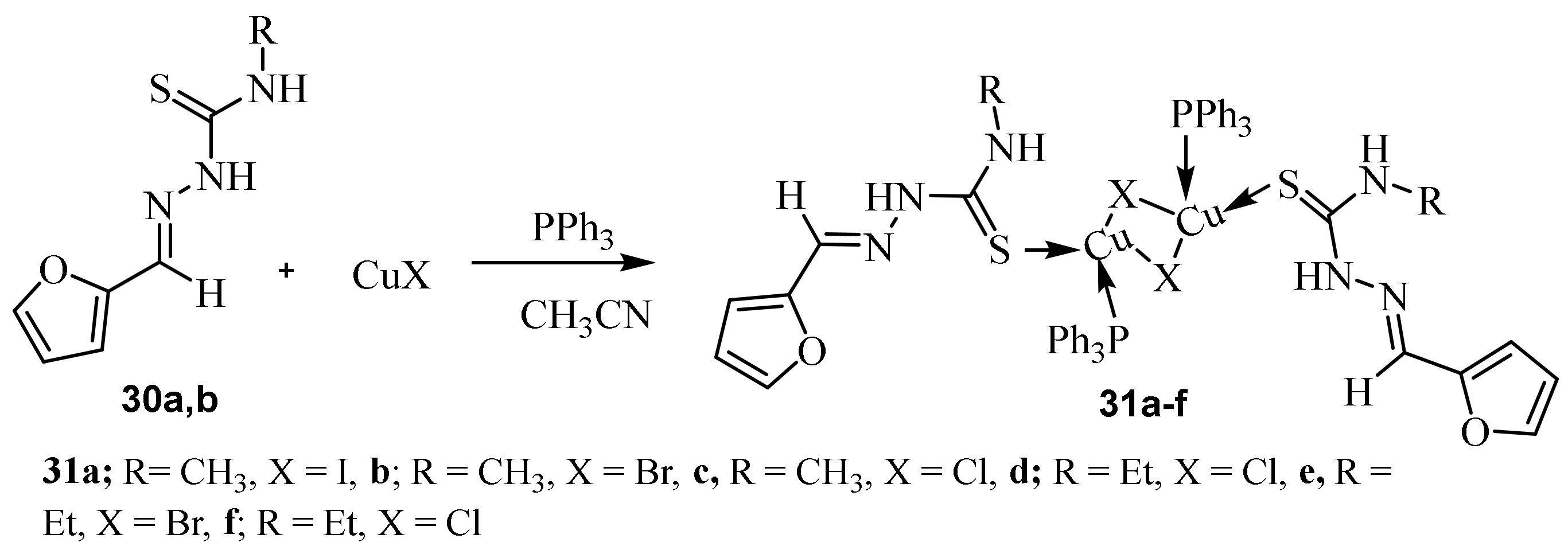
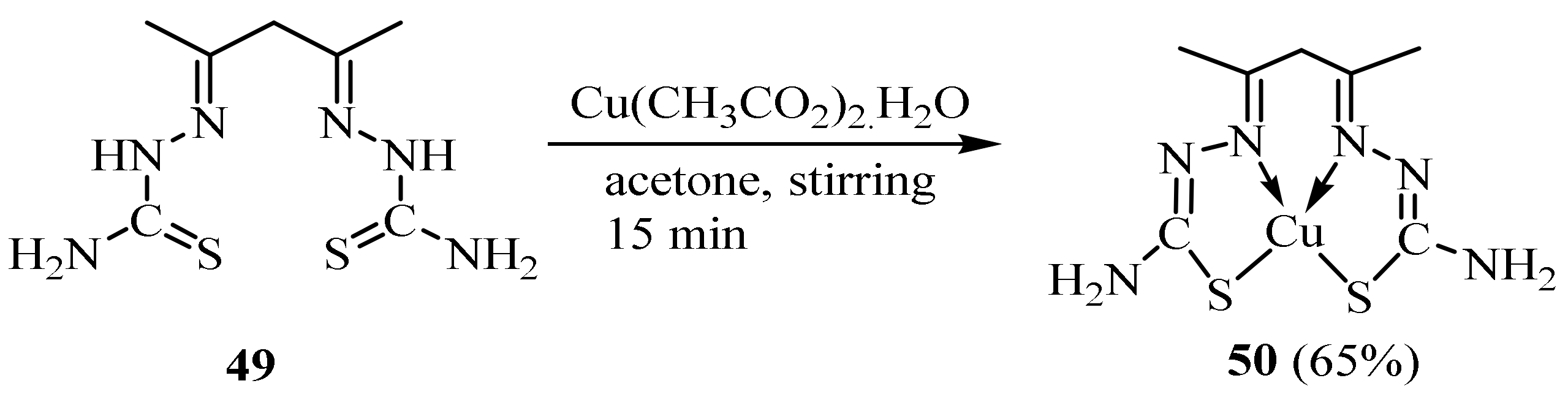
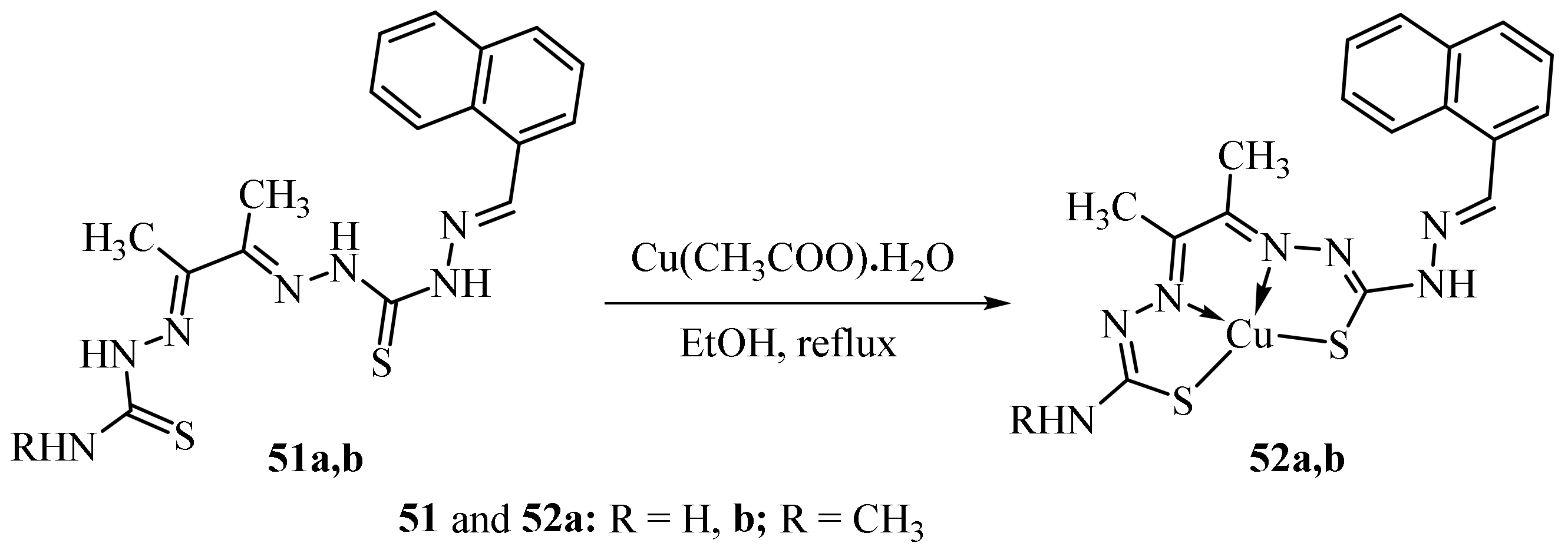
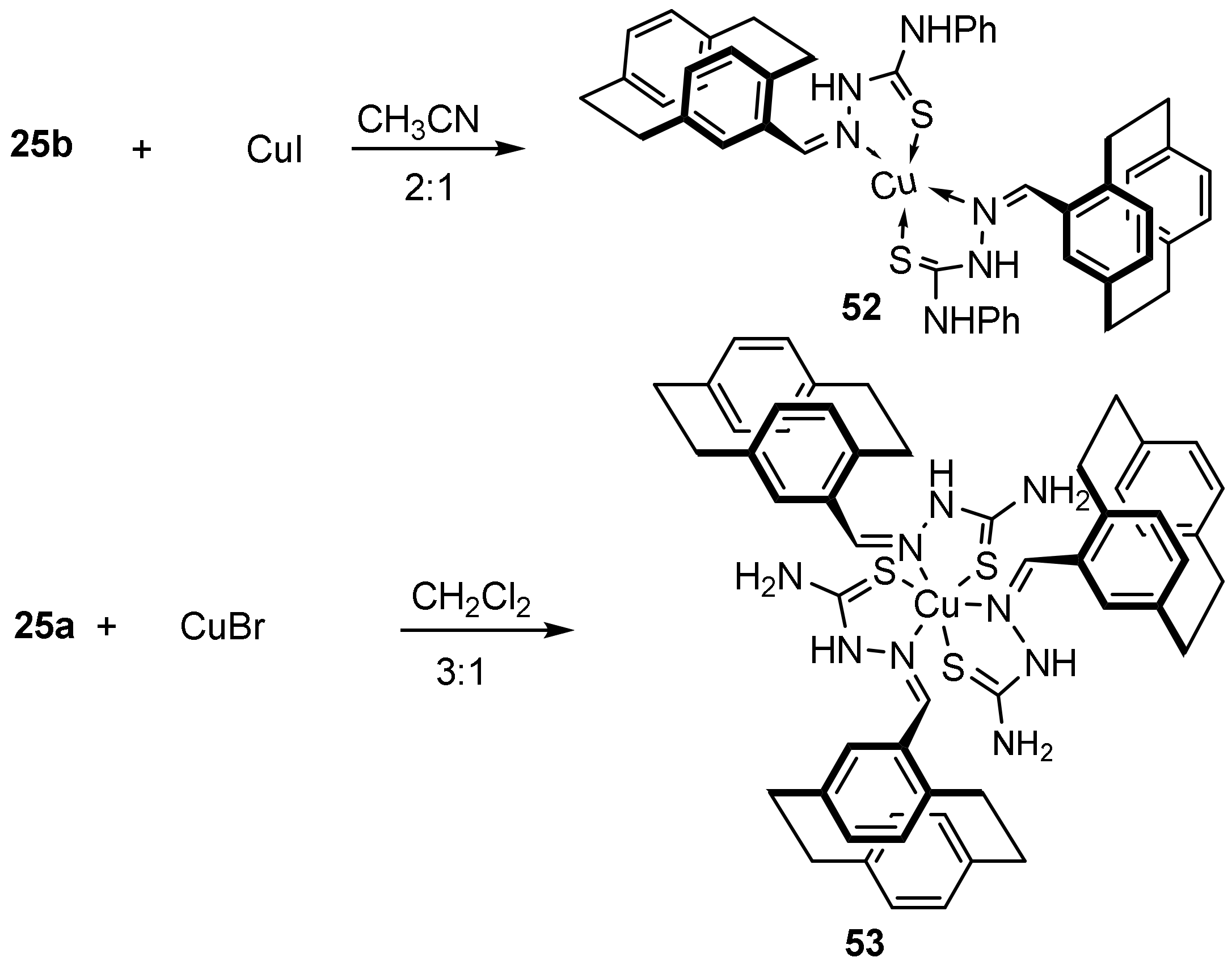
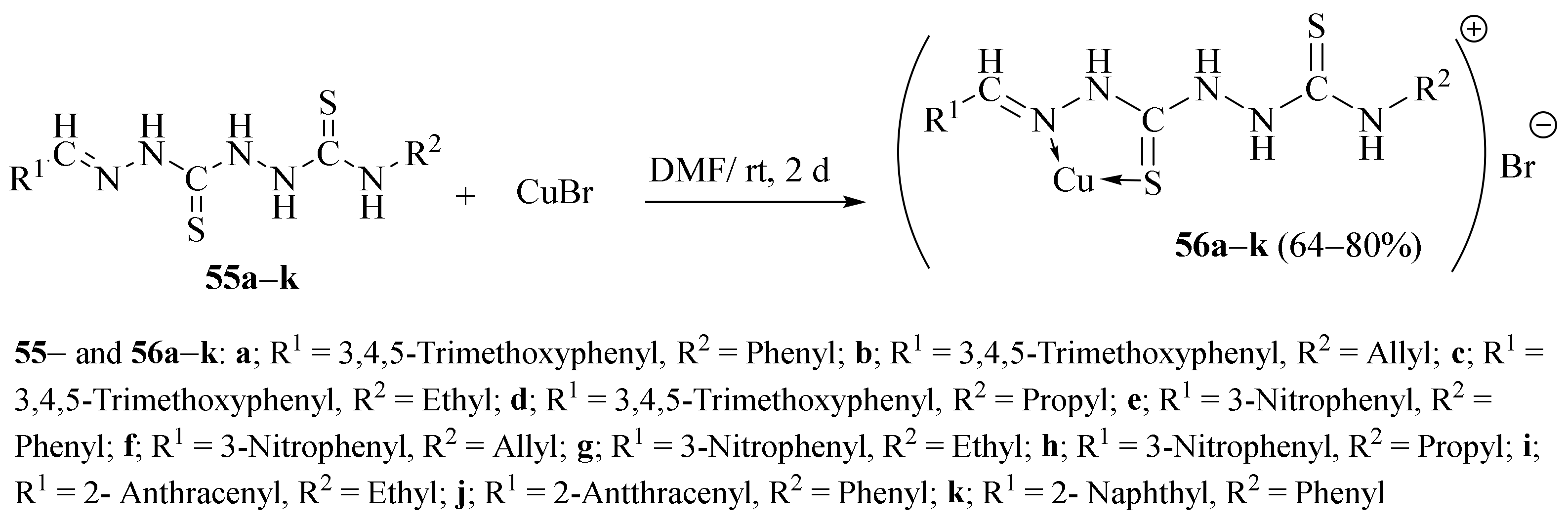
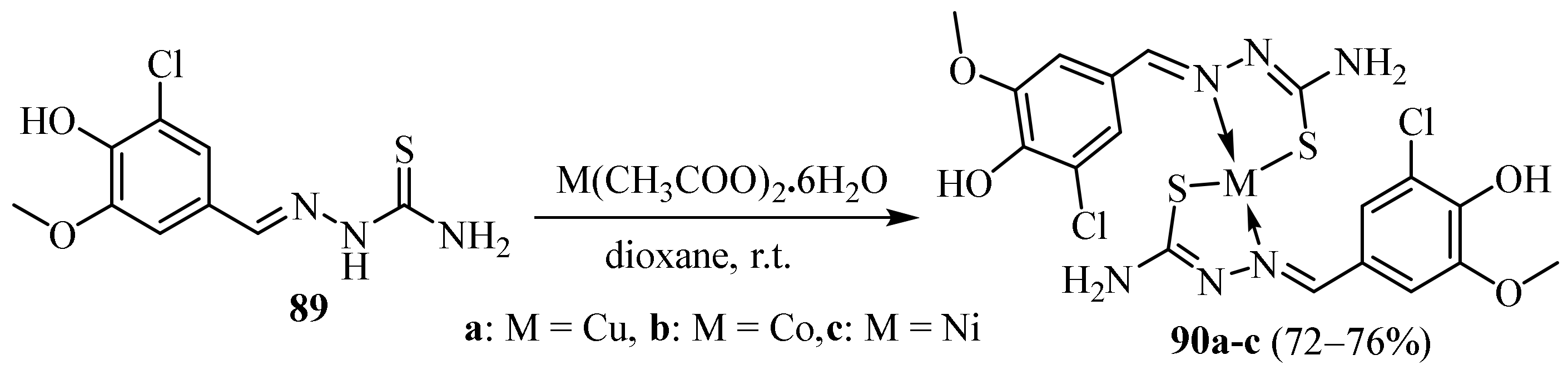
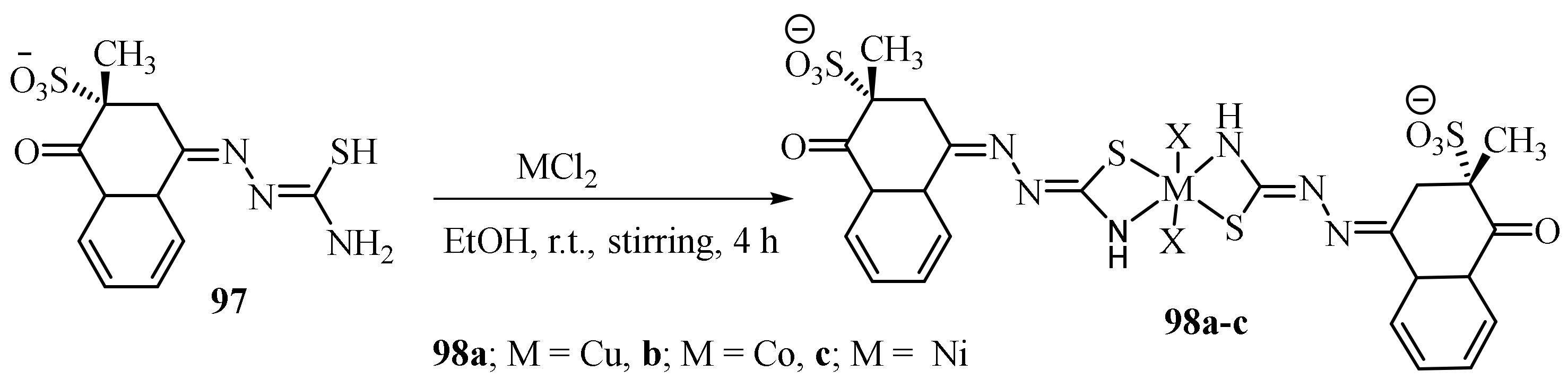
3.1. Cu Complexes of Thiosemicarbazides and Thiosemicarbazones
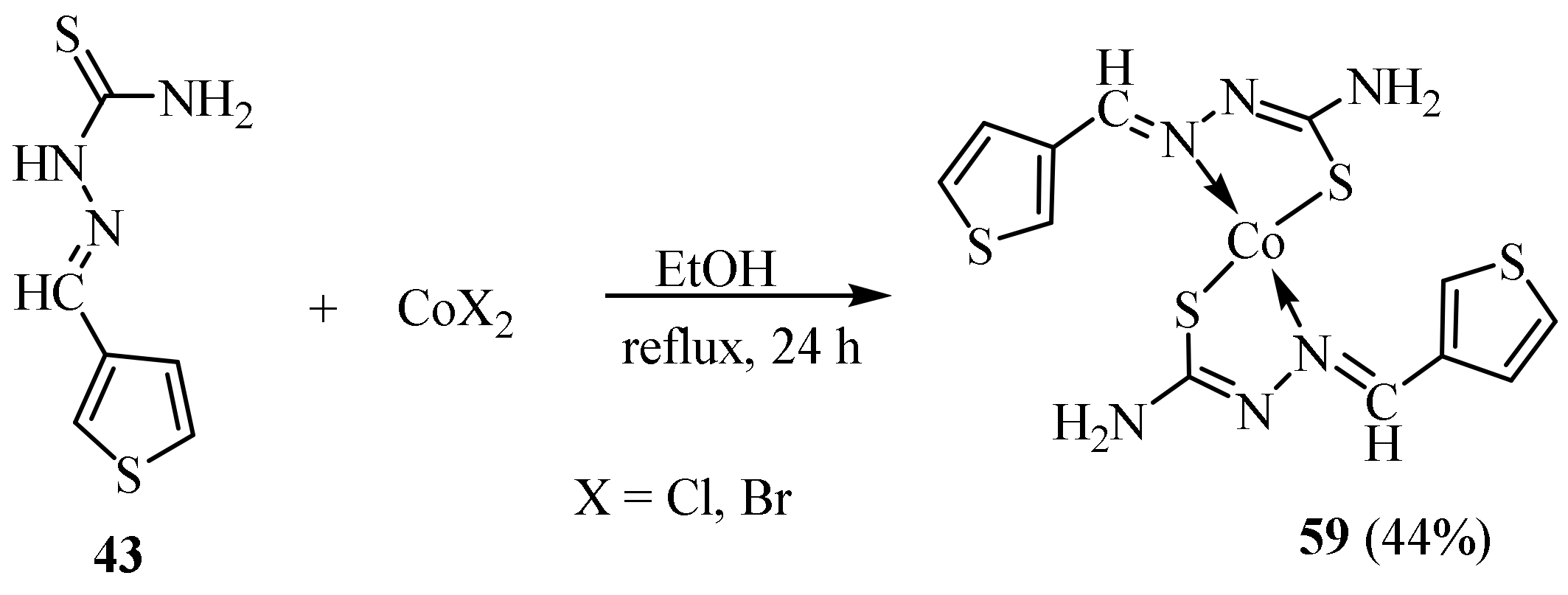
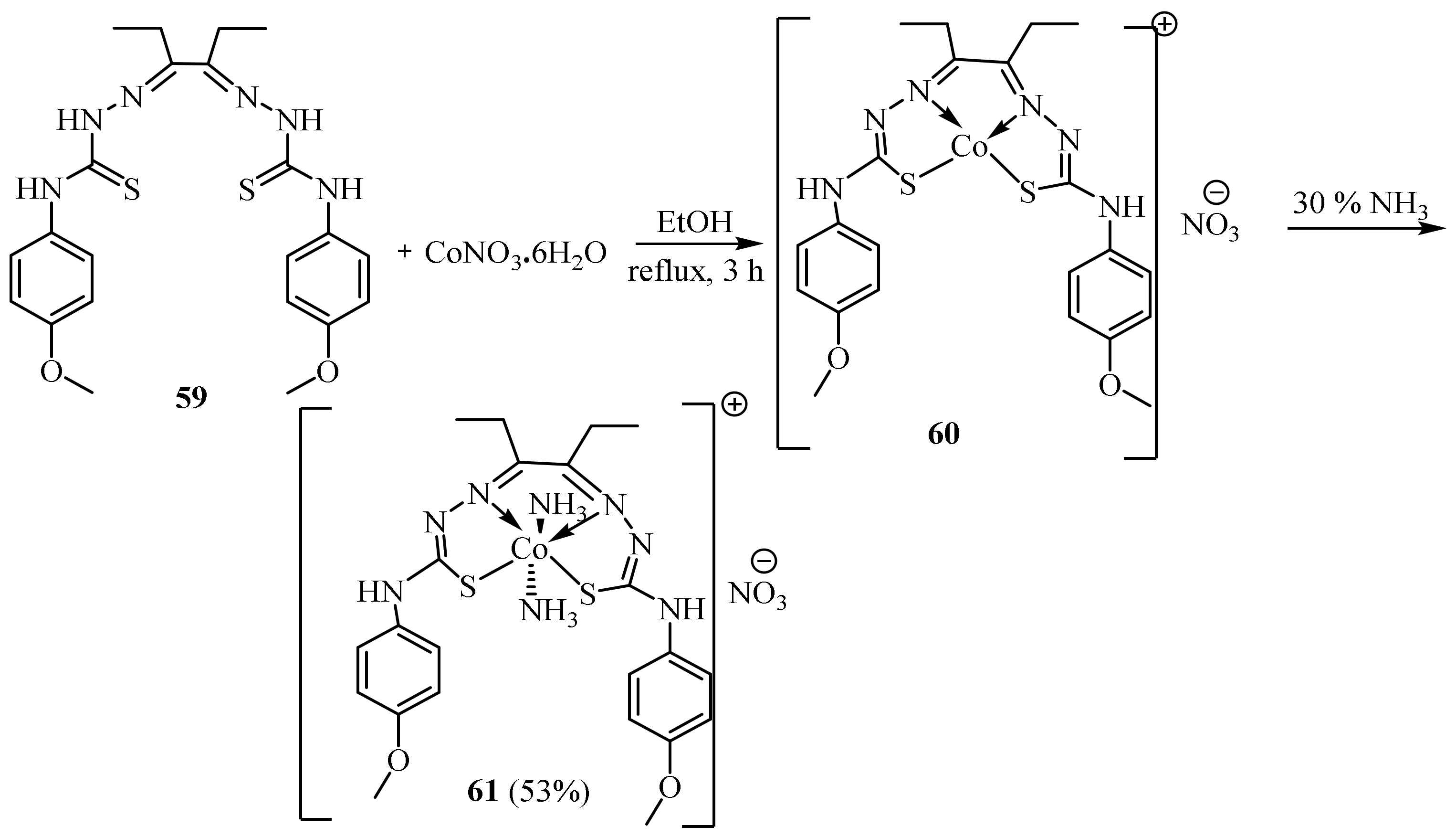
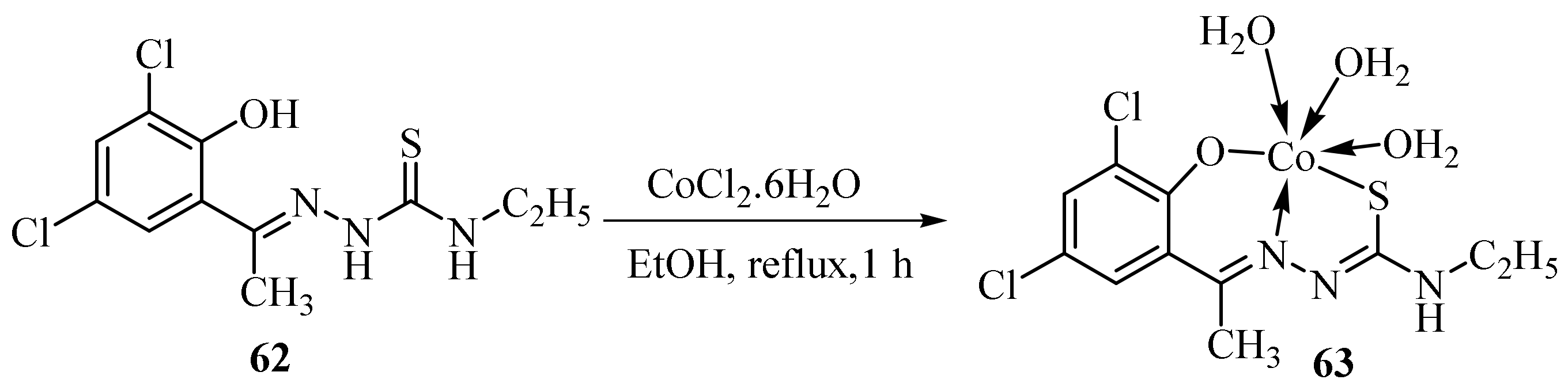
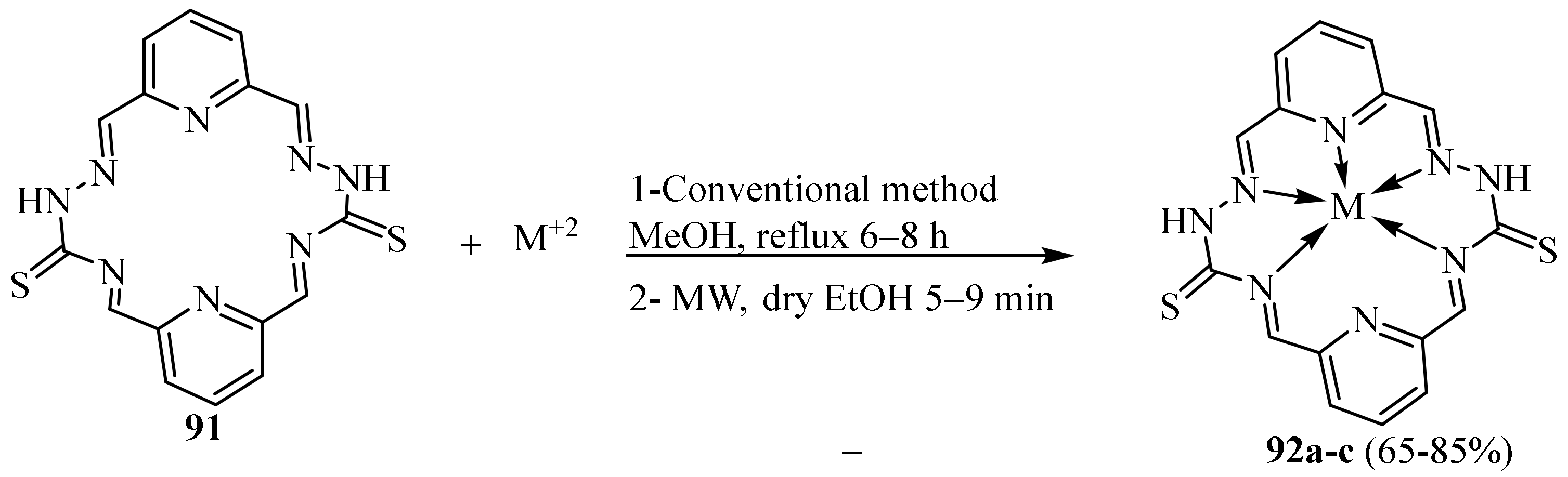
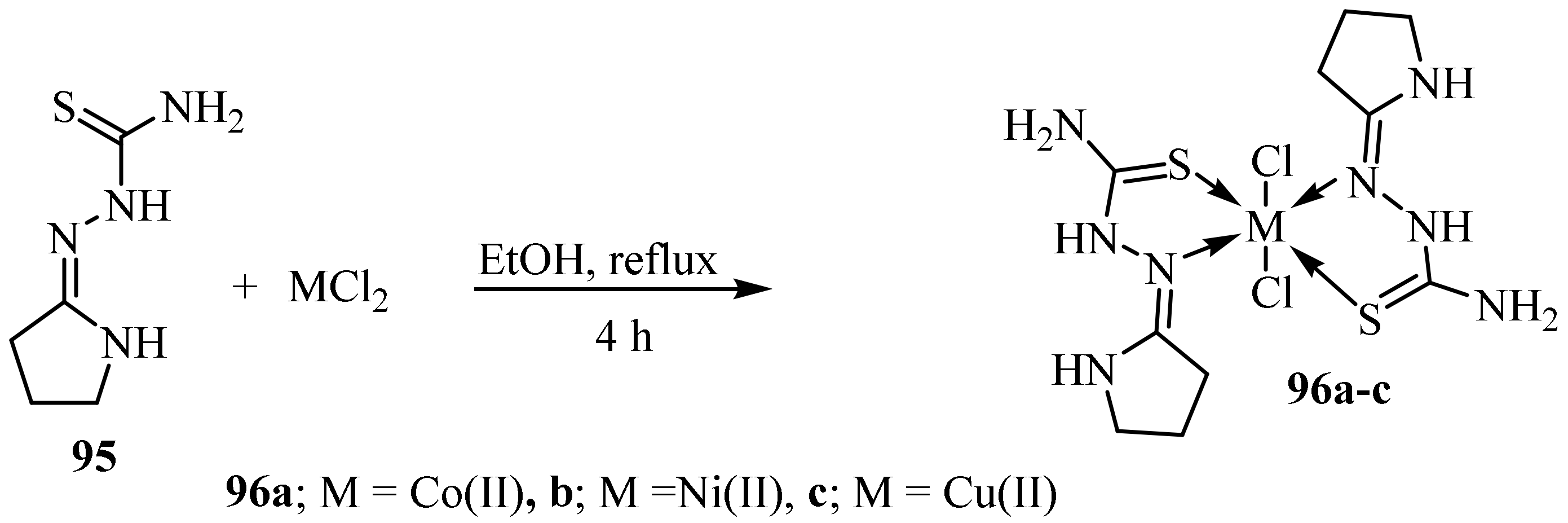
3.2. Co(II) Complexes of Thiosemicarbazides and Thiosemicarbazones
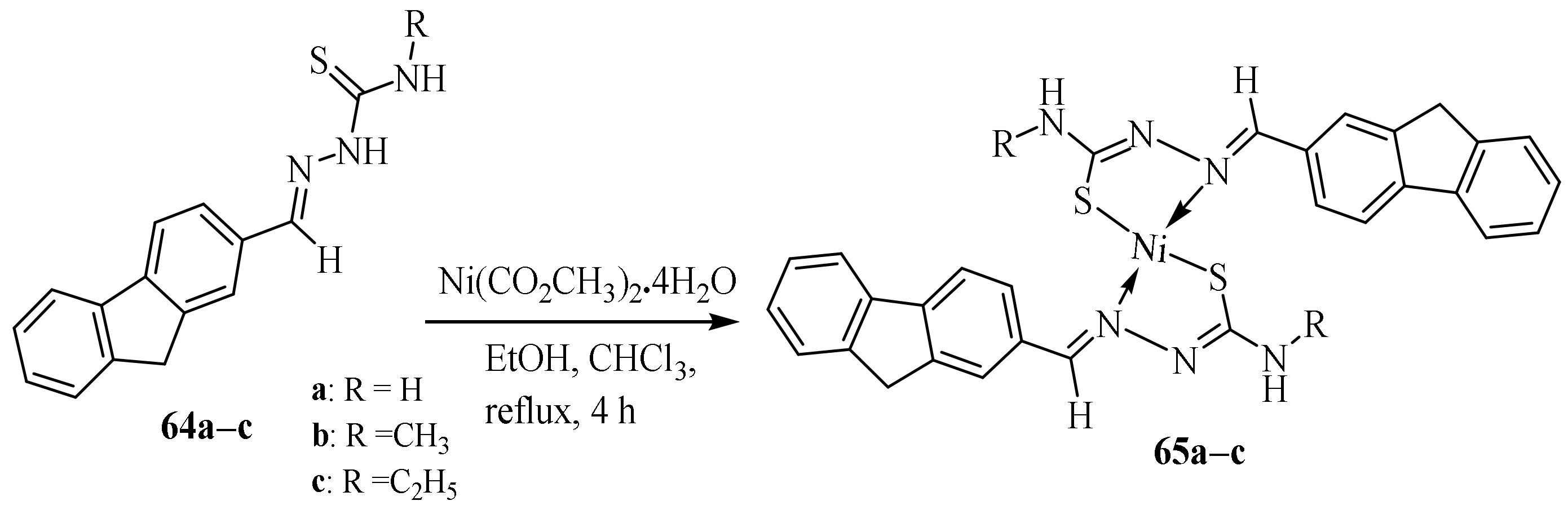
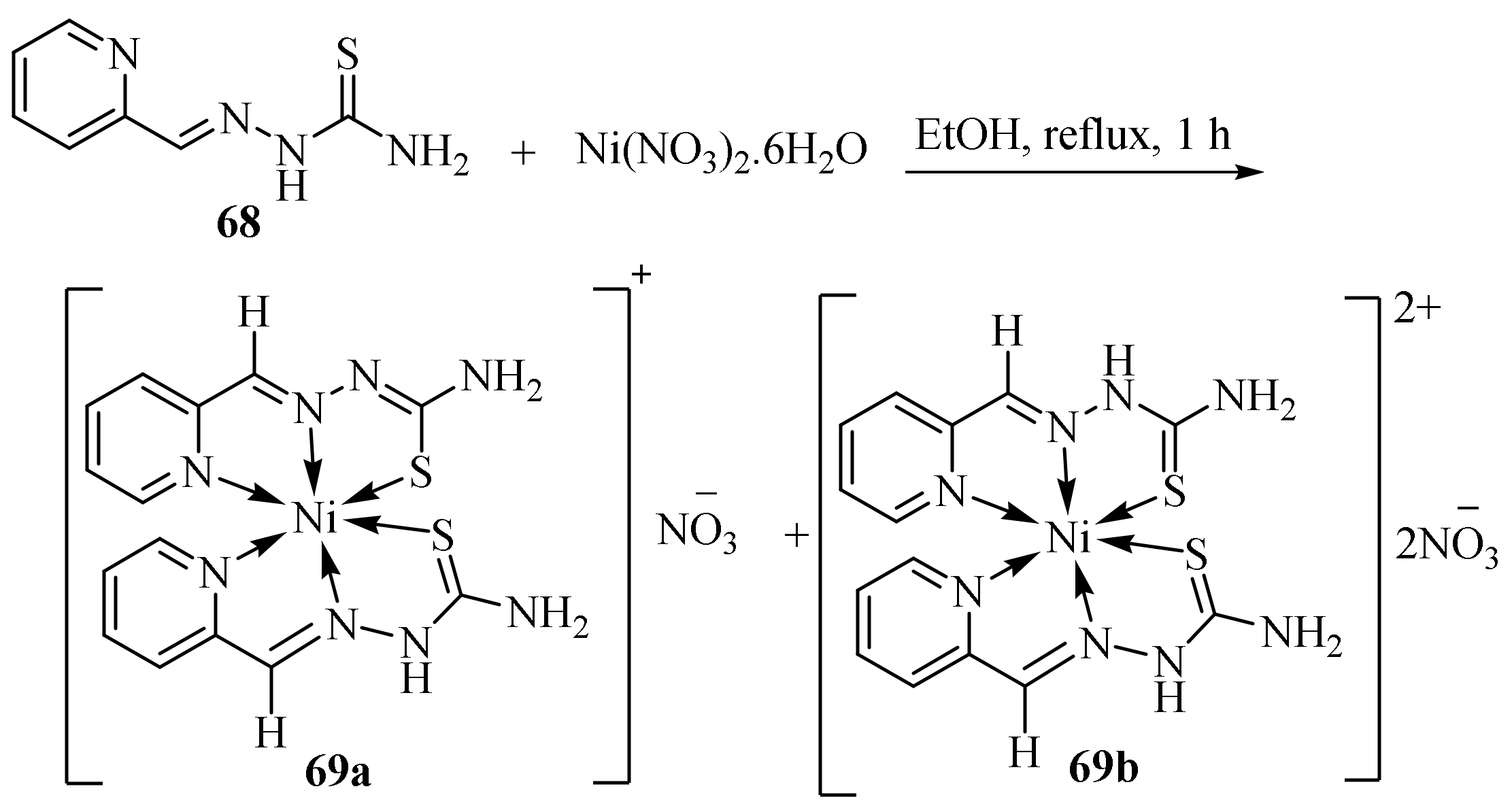
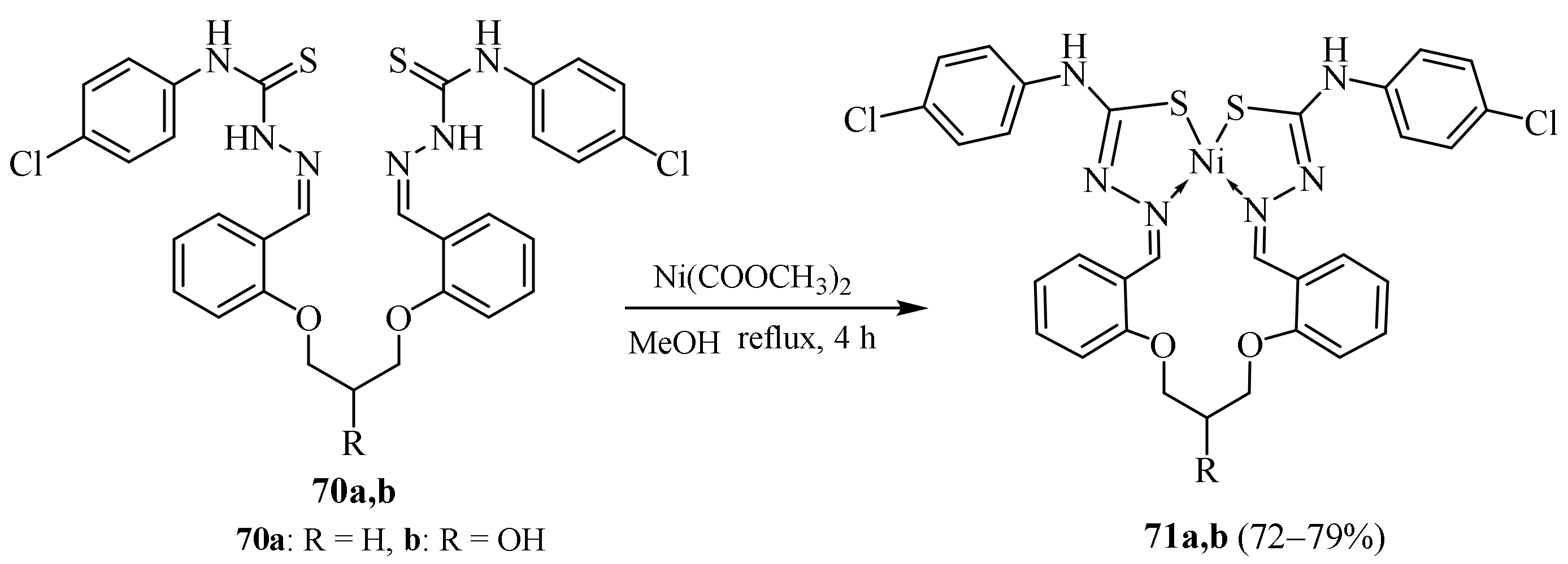
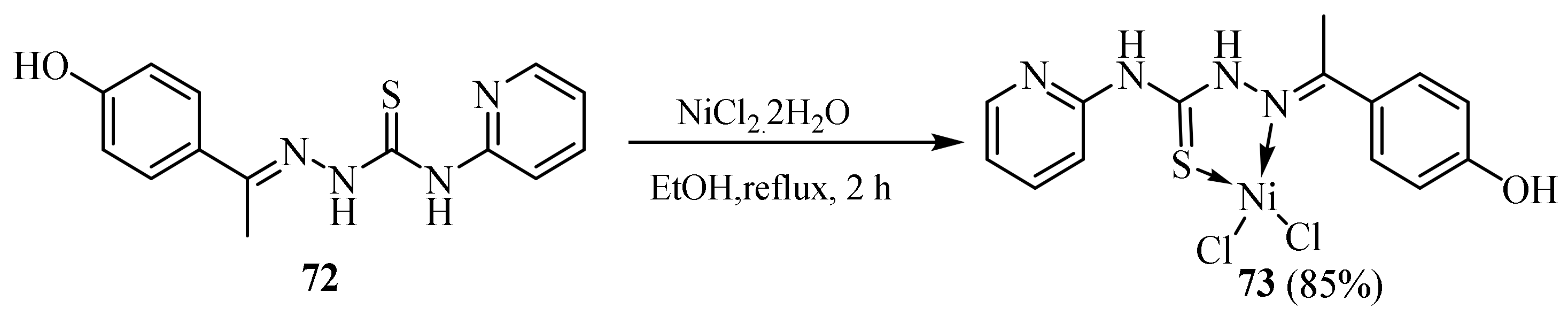
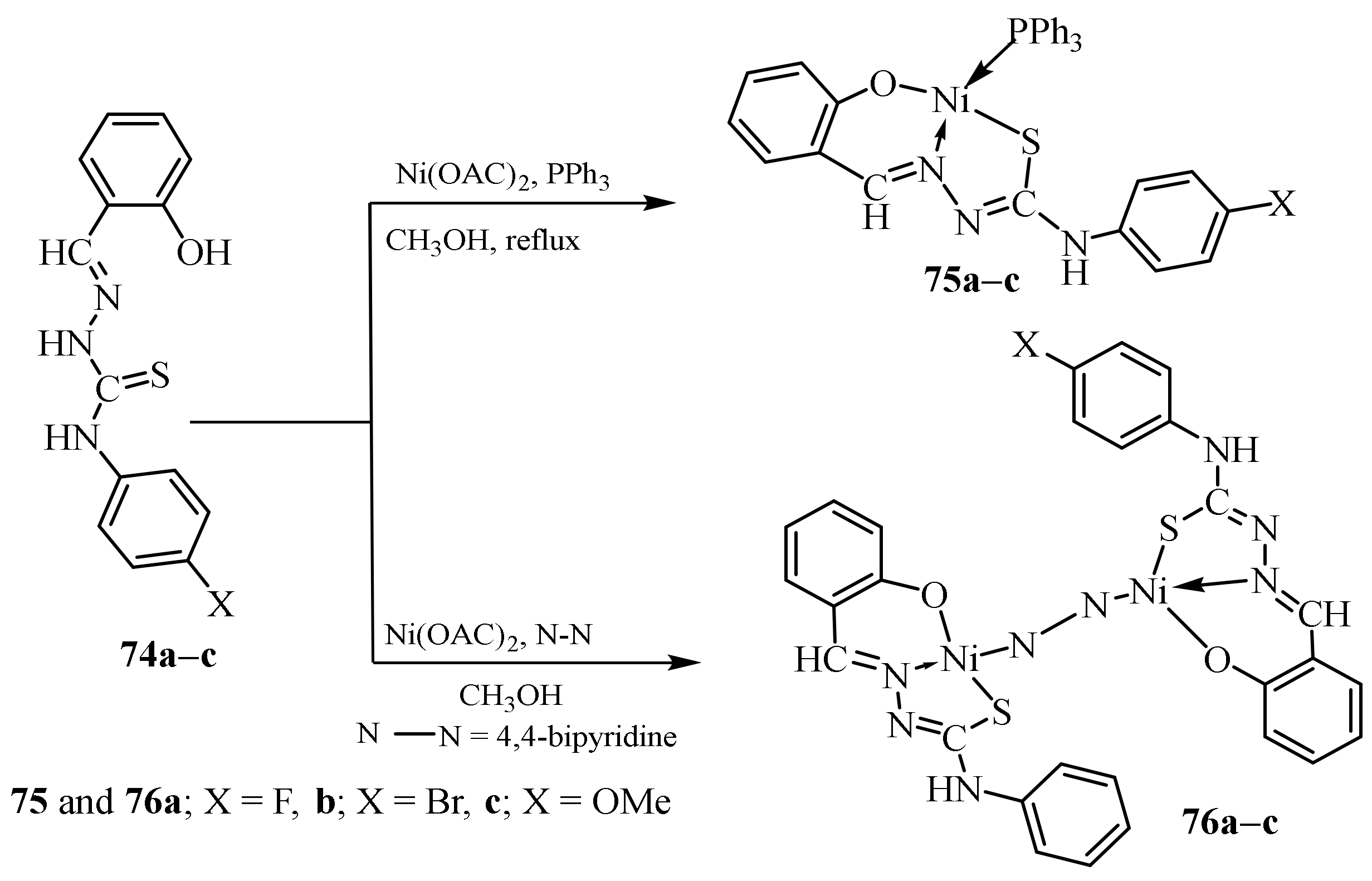
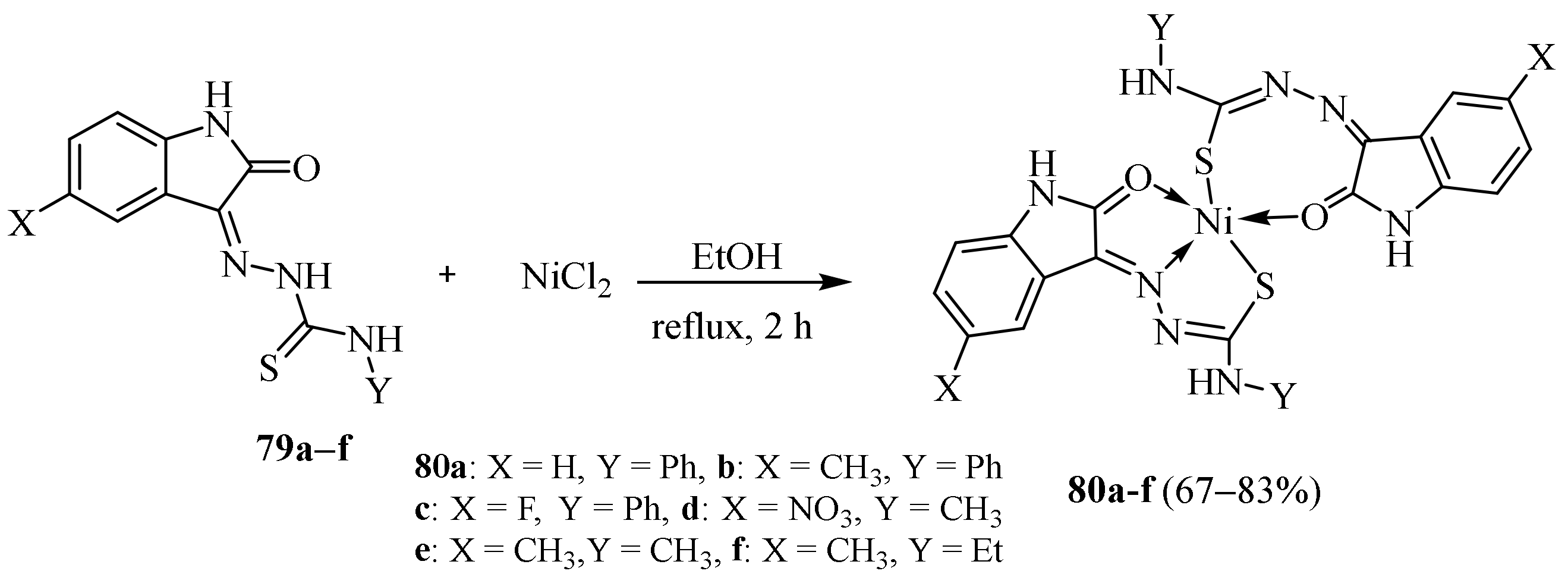
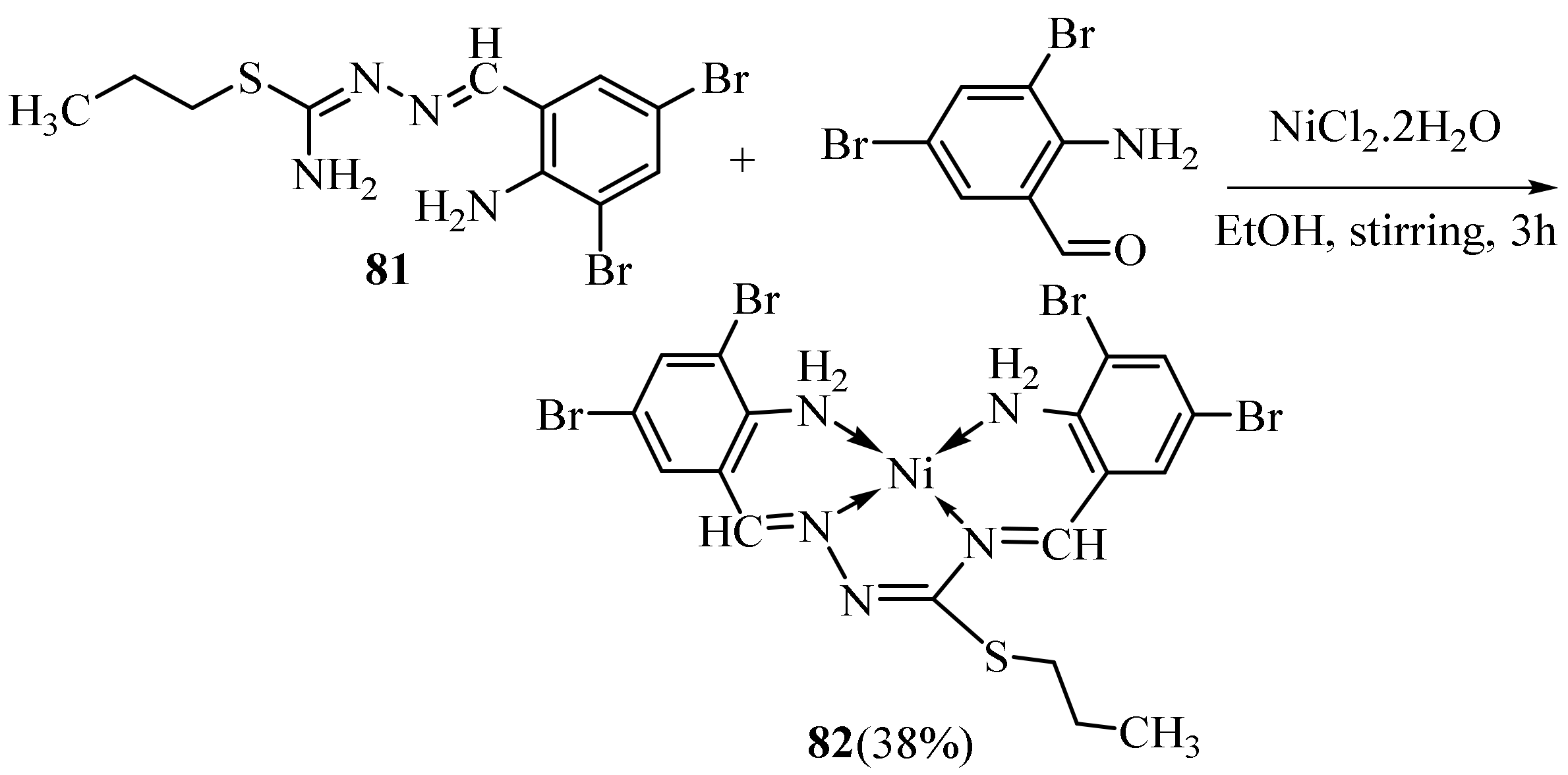
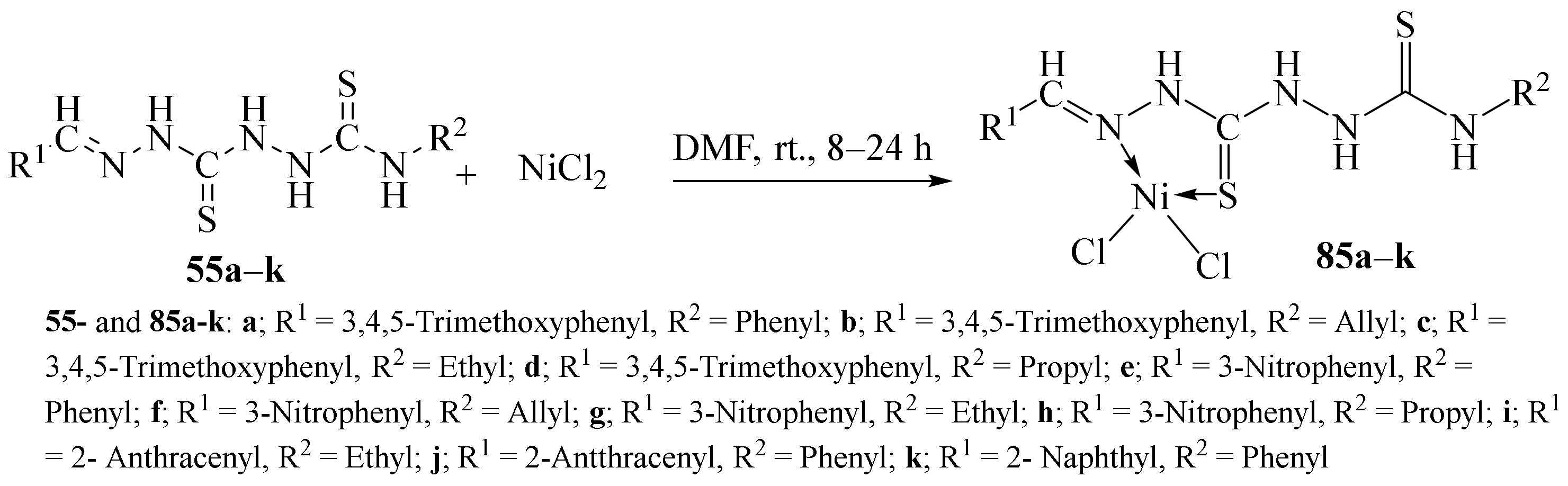
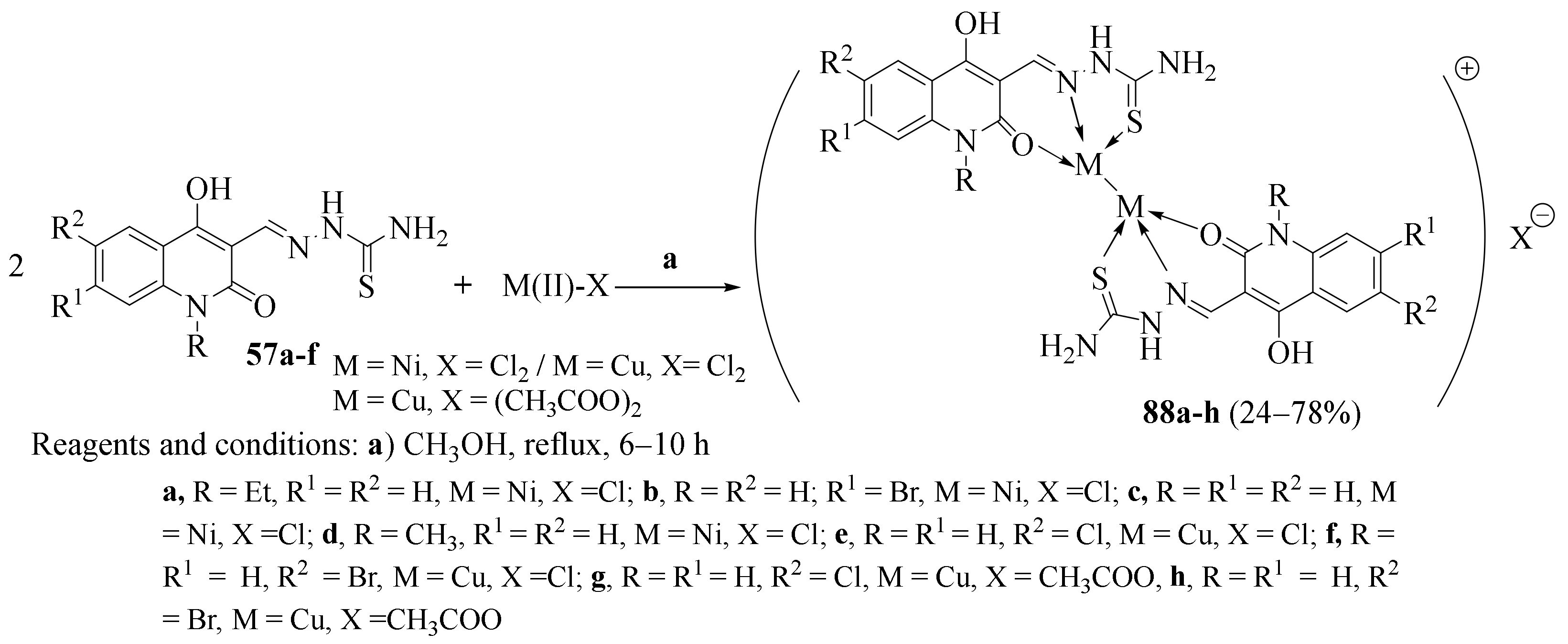
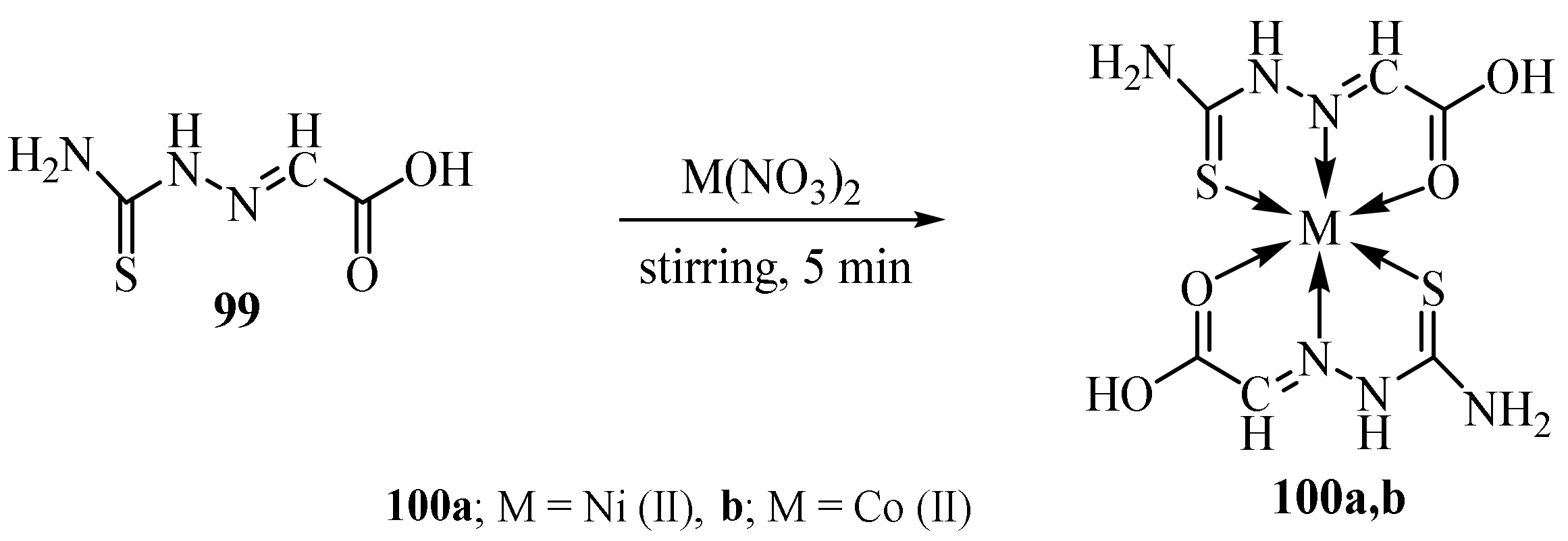
3.3. Ni (II) Complexes of Thiosemicarbazides and Thiosemicarbazones
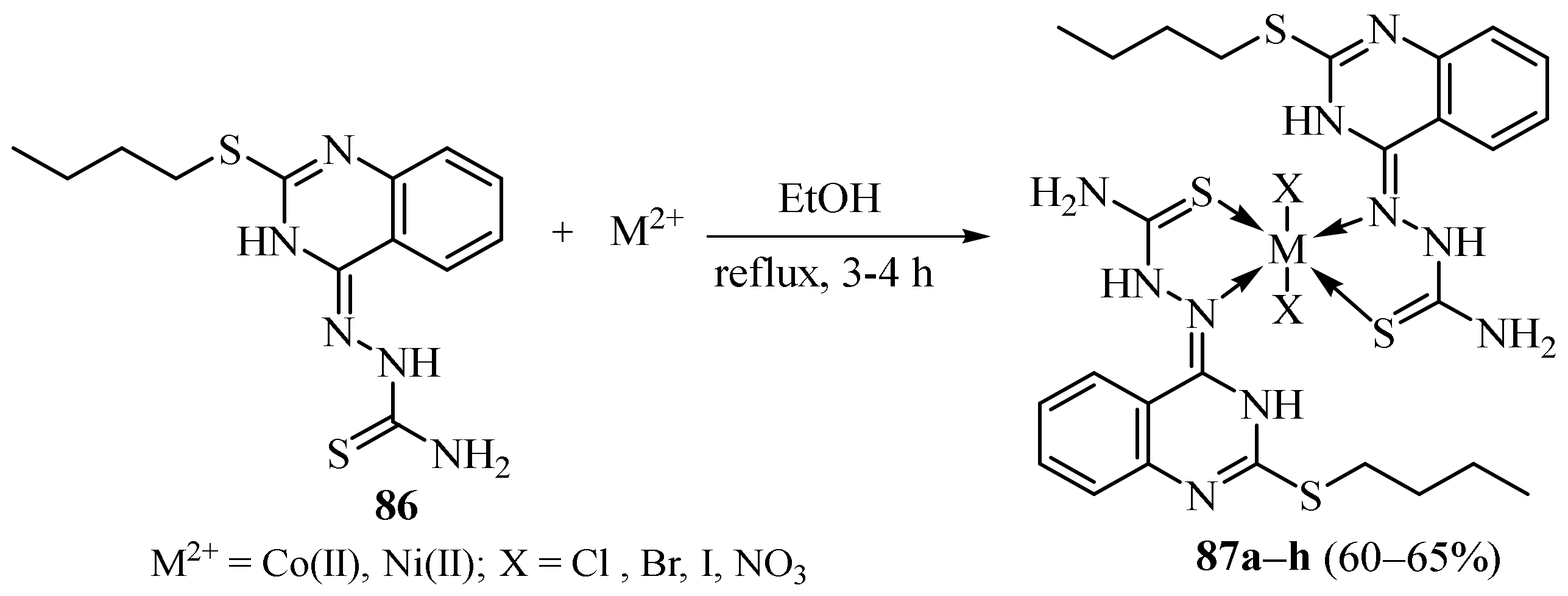
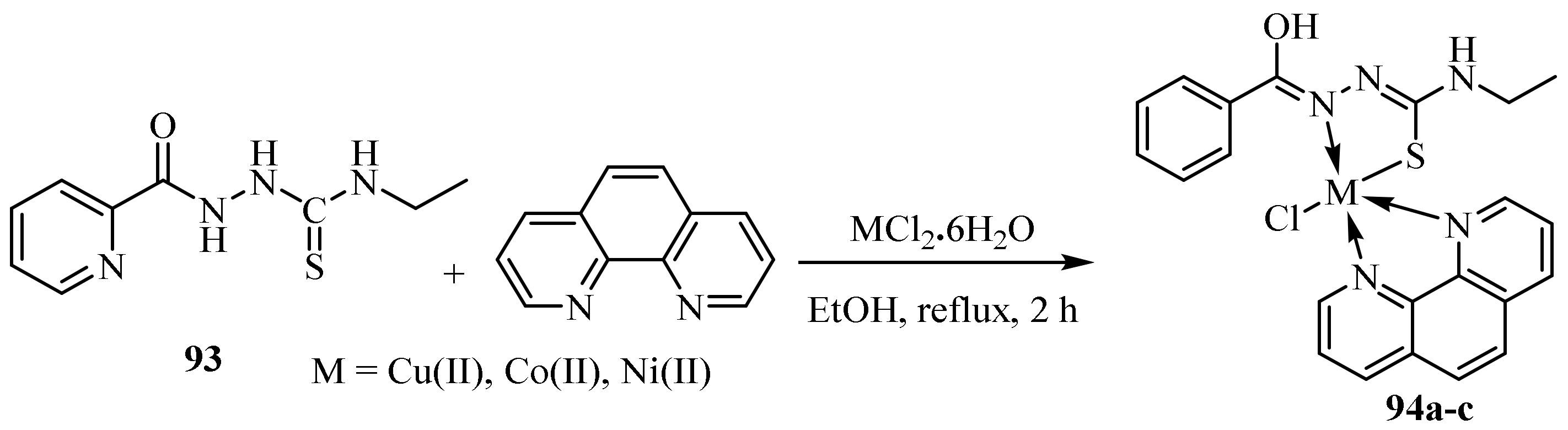
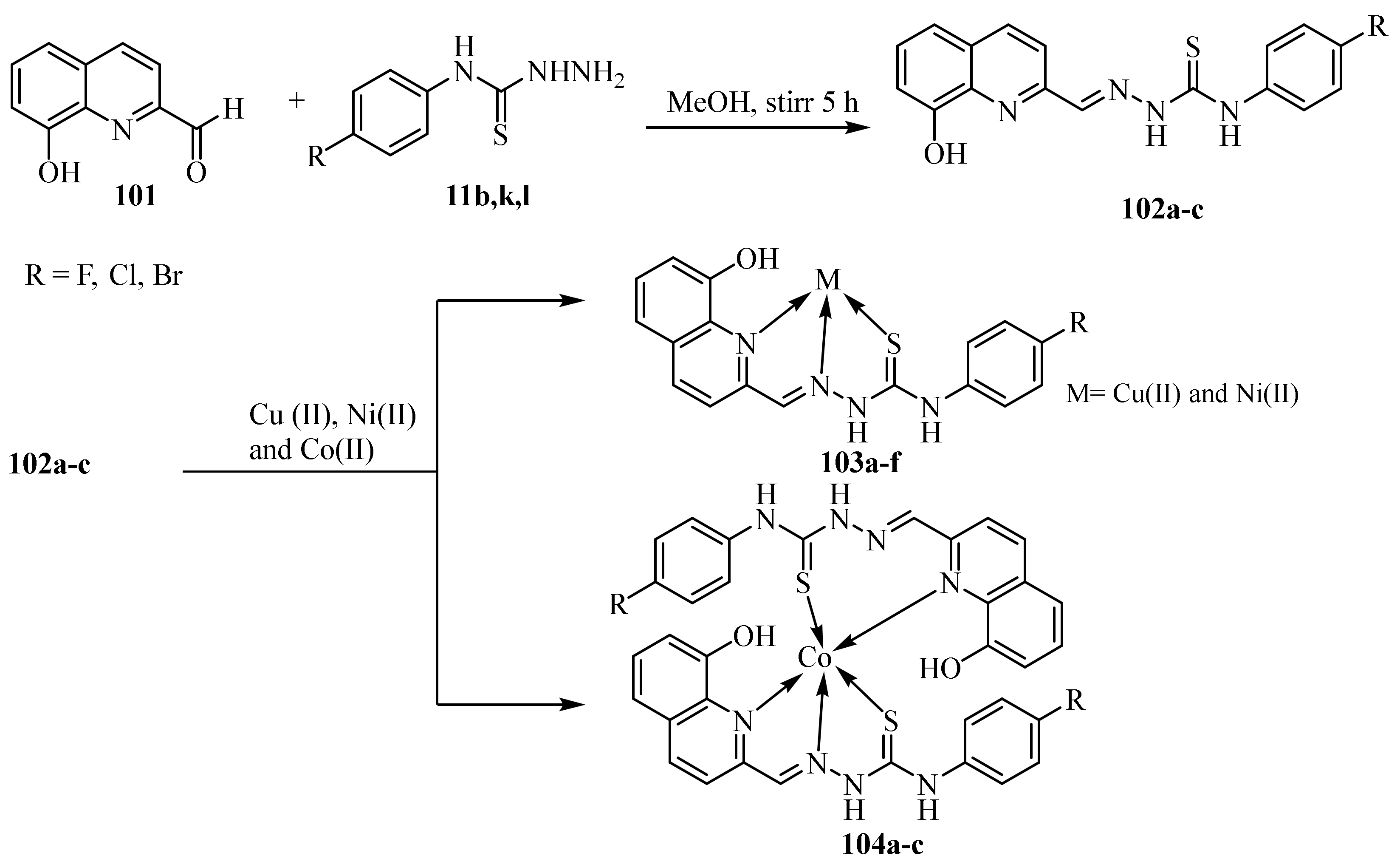
3.4. Cu(II), Co(II) and Ni (II) Complexes of Thiosemicarbazides and Thiosemicarbazones
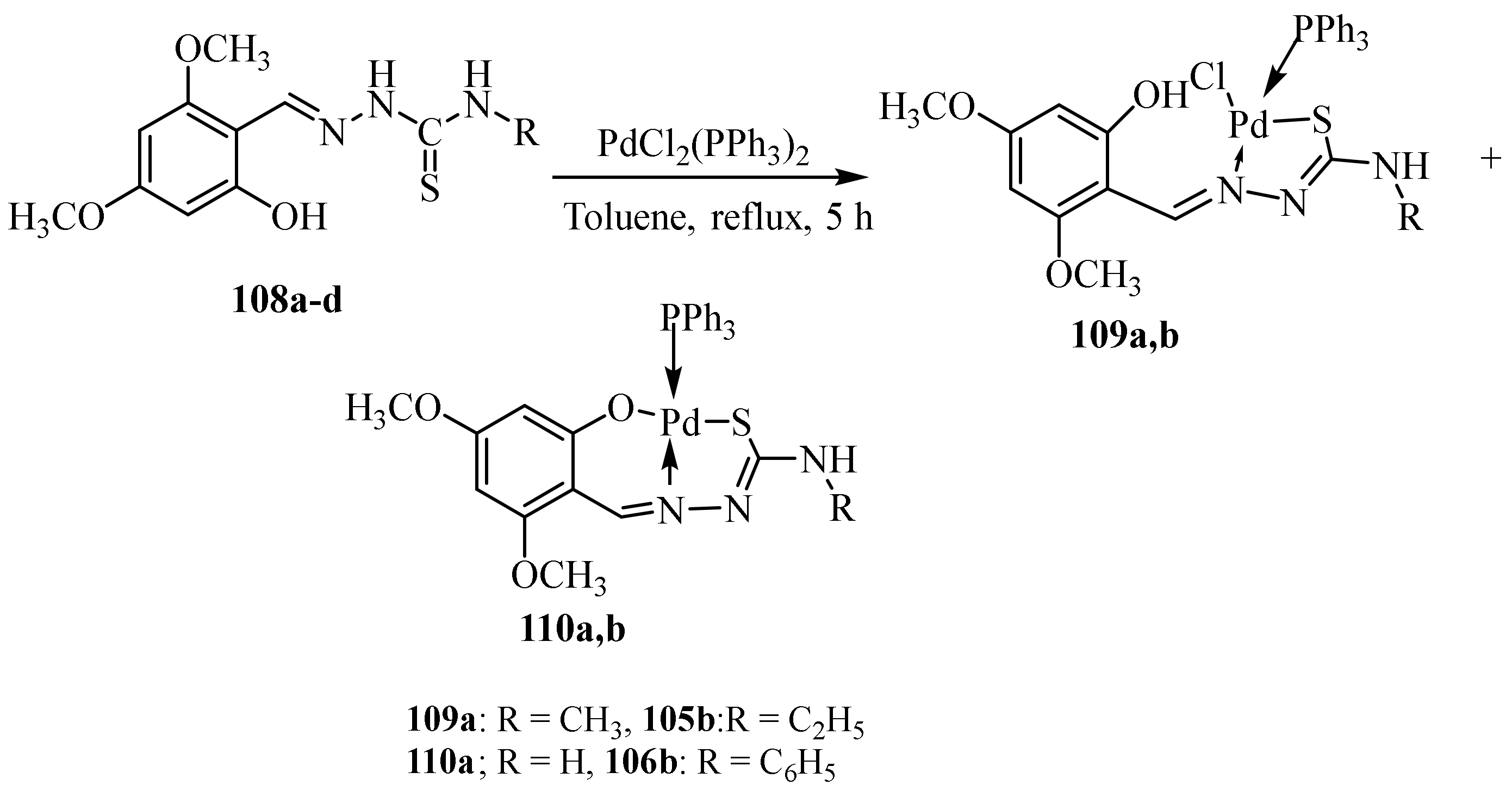
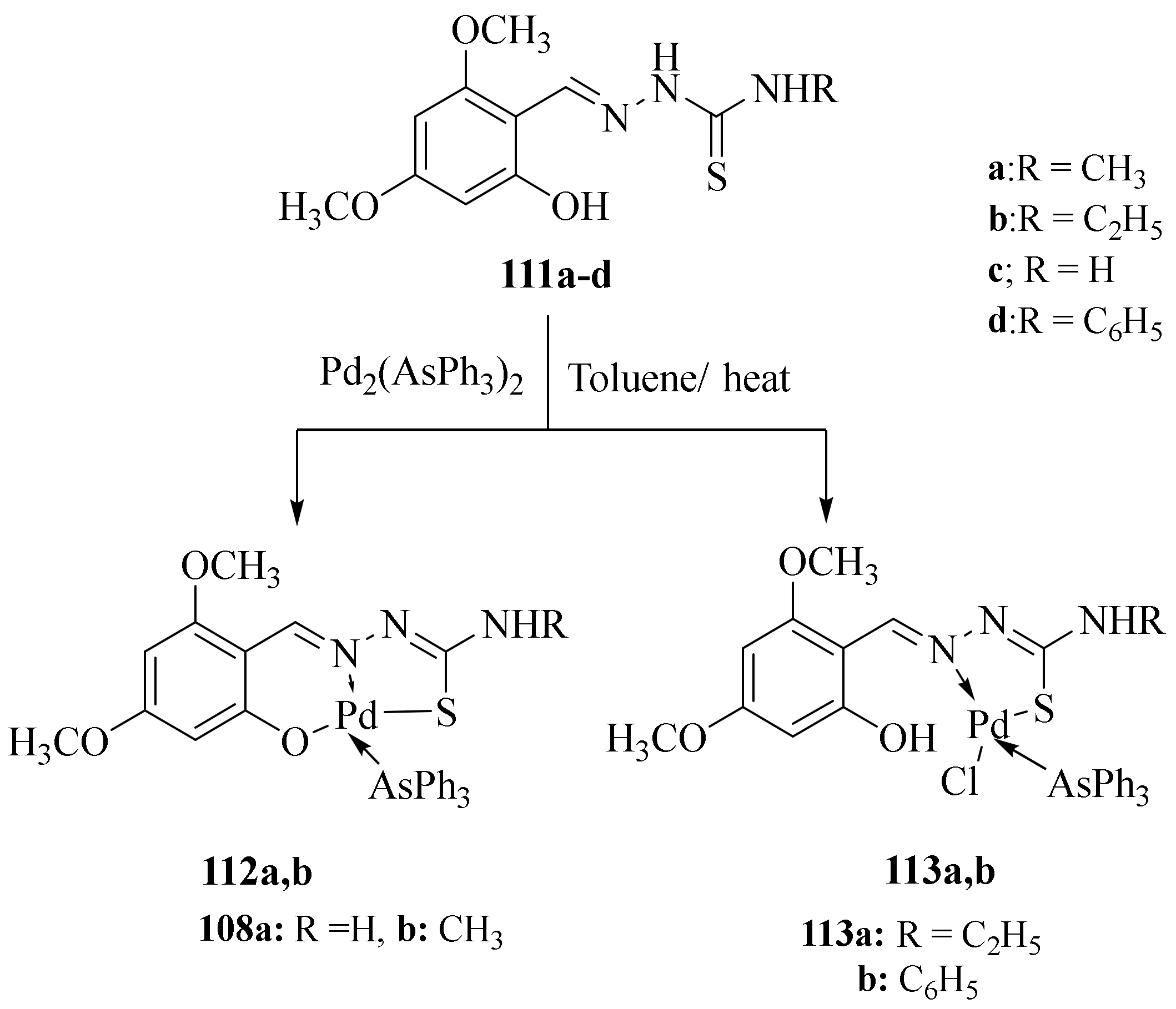
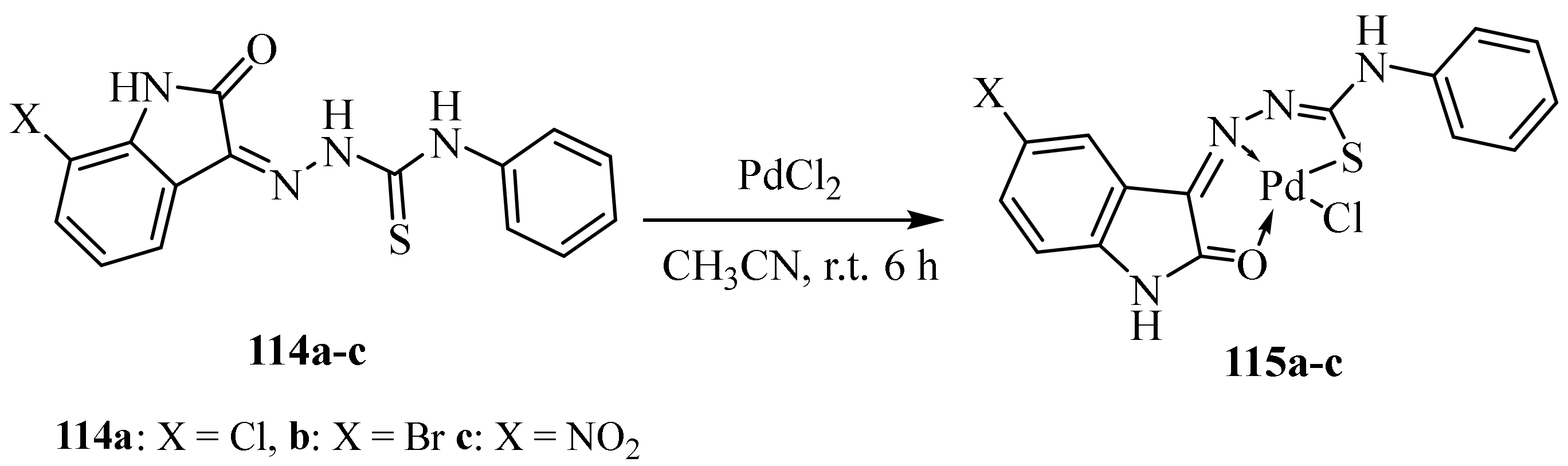
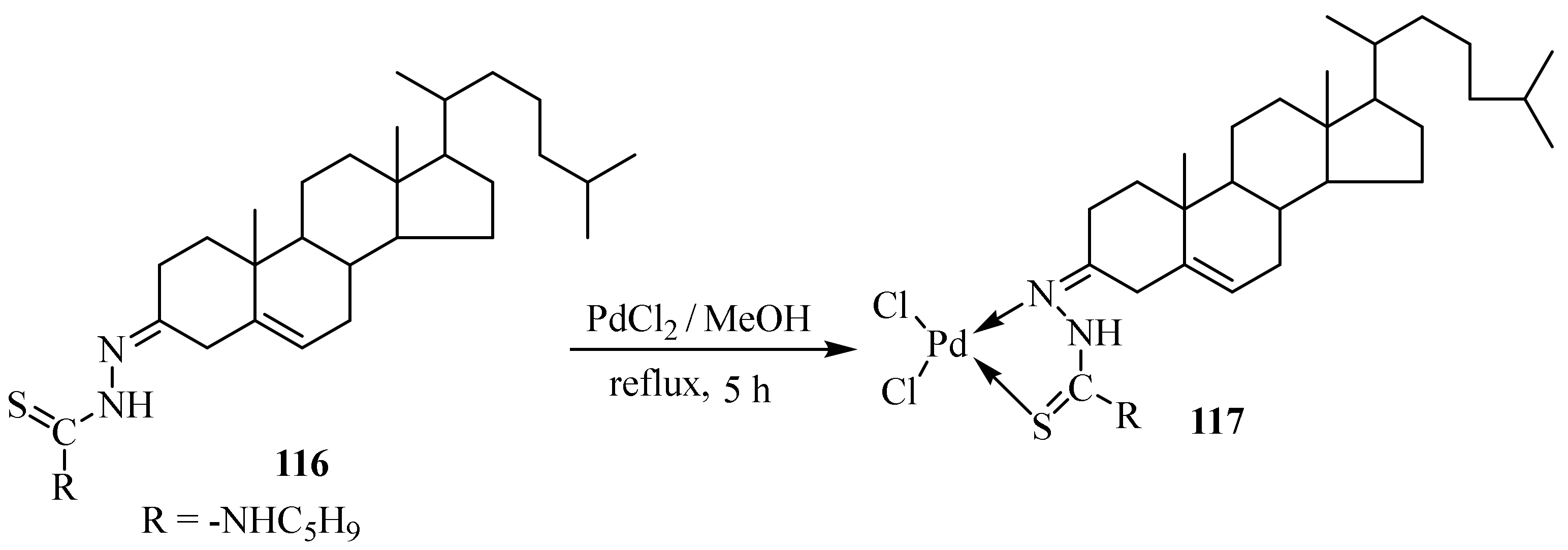
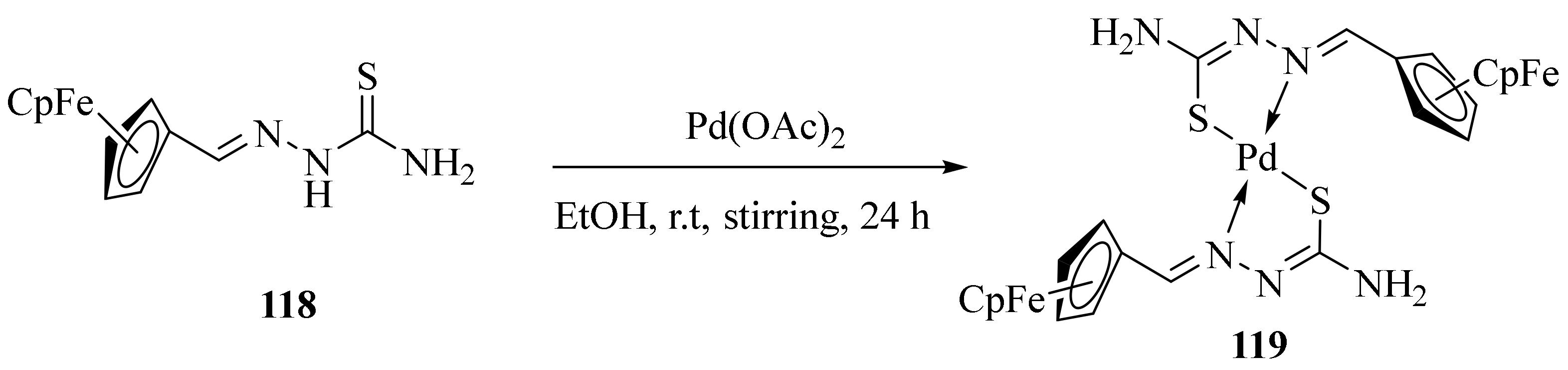
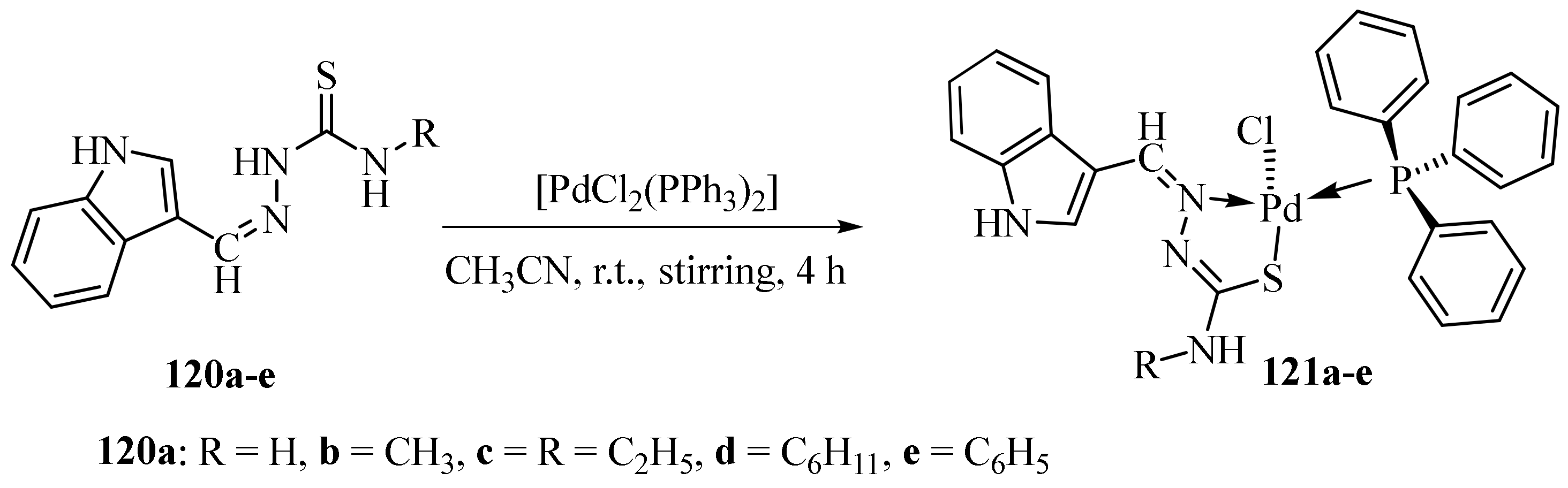
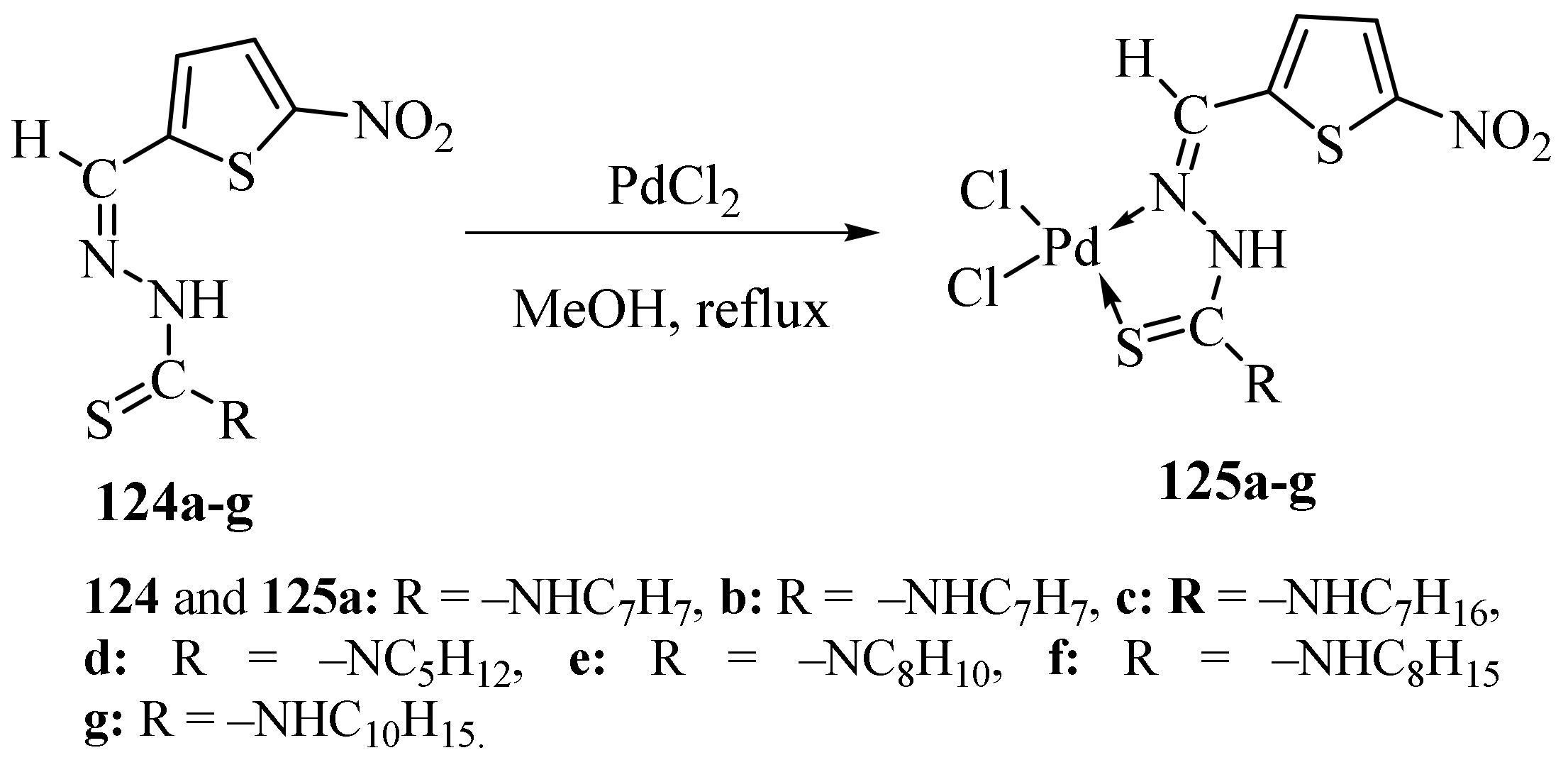
3.5. Pd(II) Complexes of Thiosemicarbazides and Thiosemicarbazones
Utility of 107 in Cross-Coupling Reaction
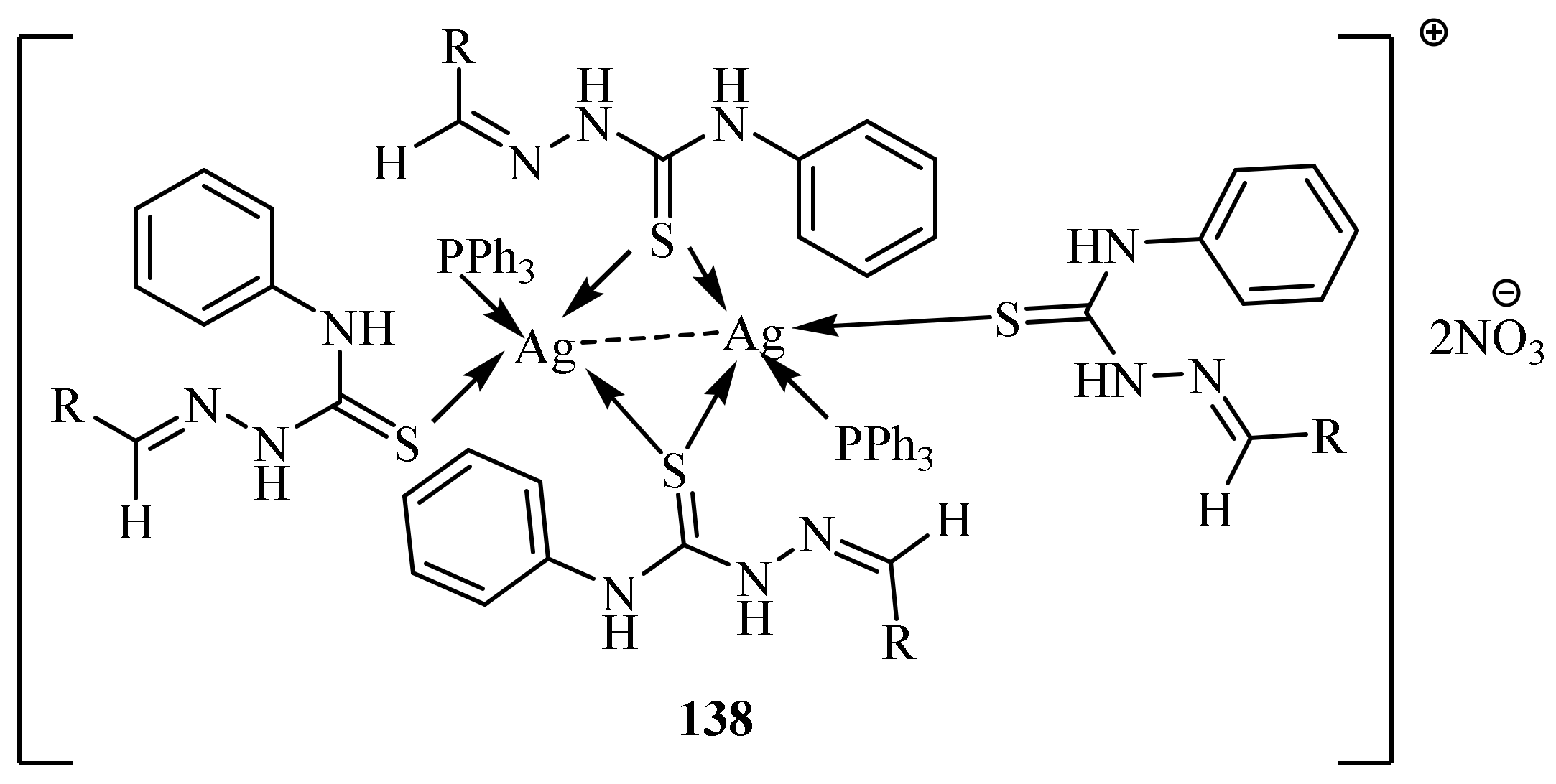
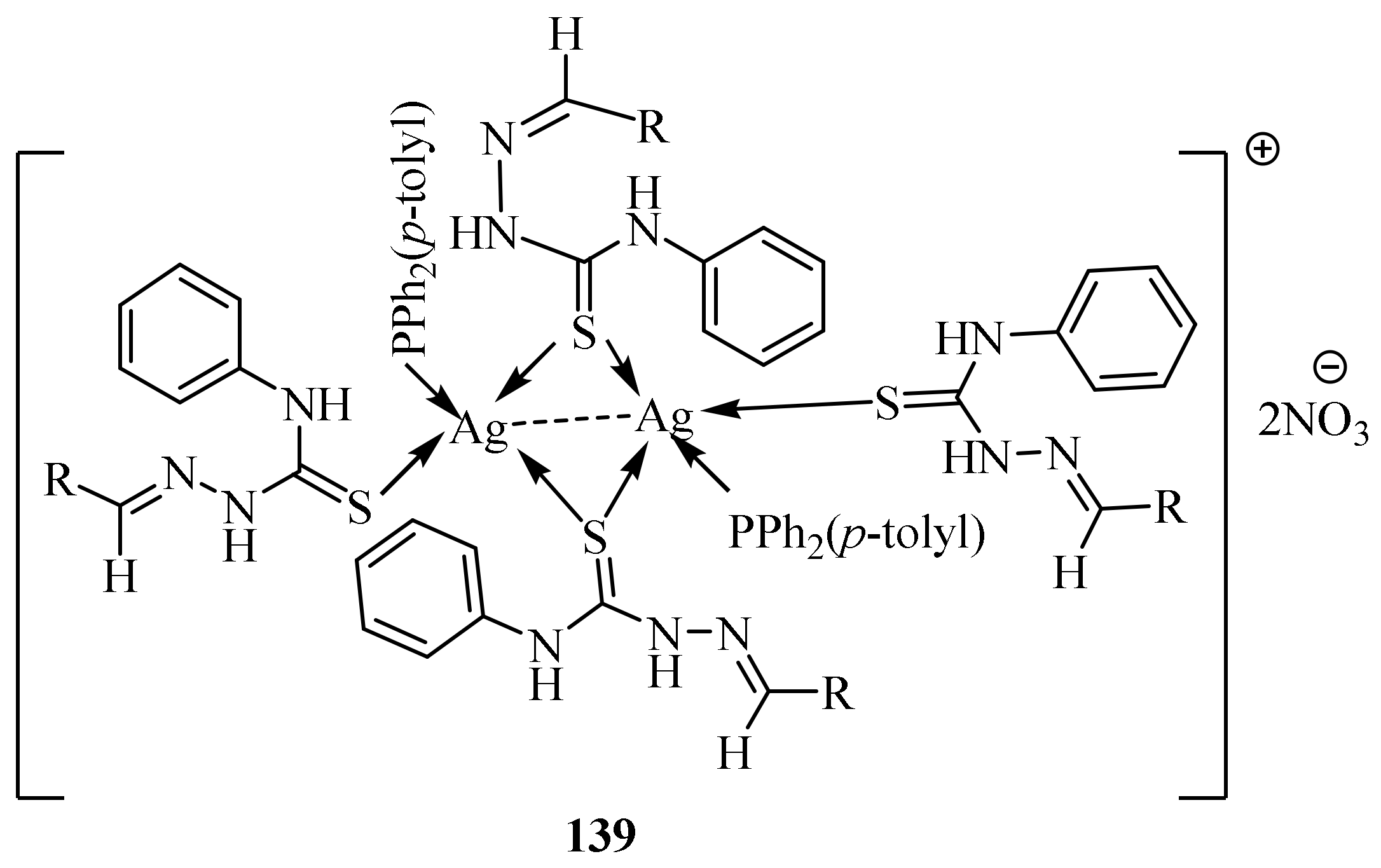
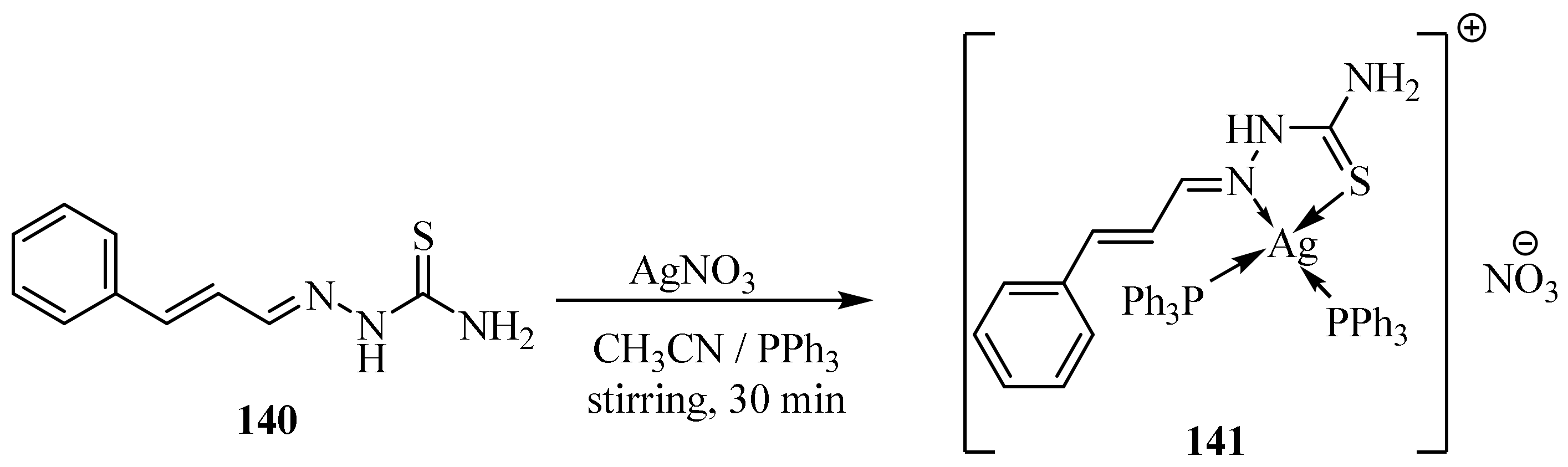
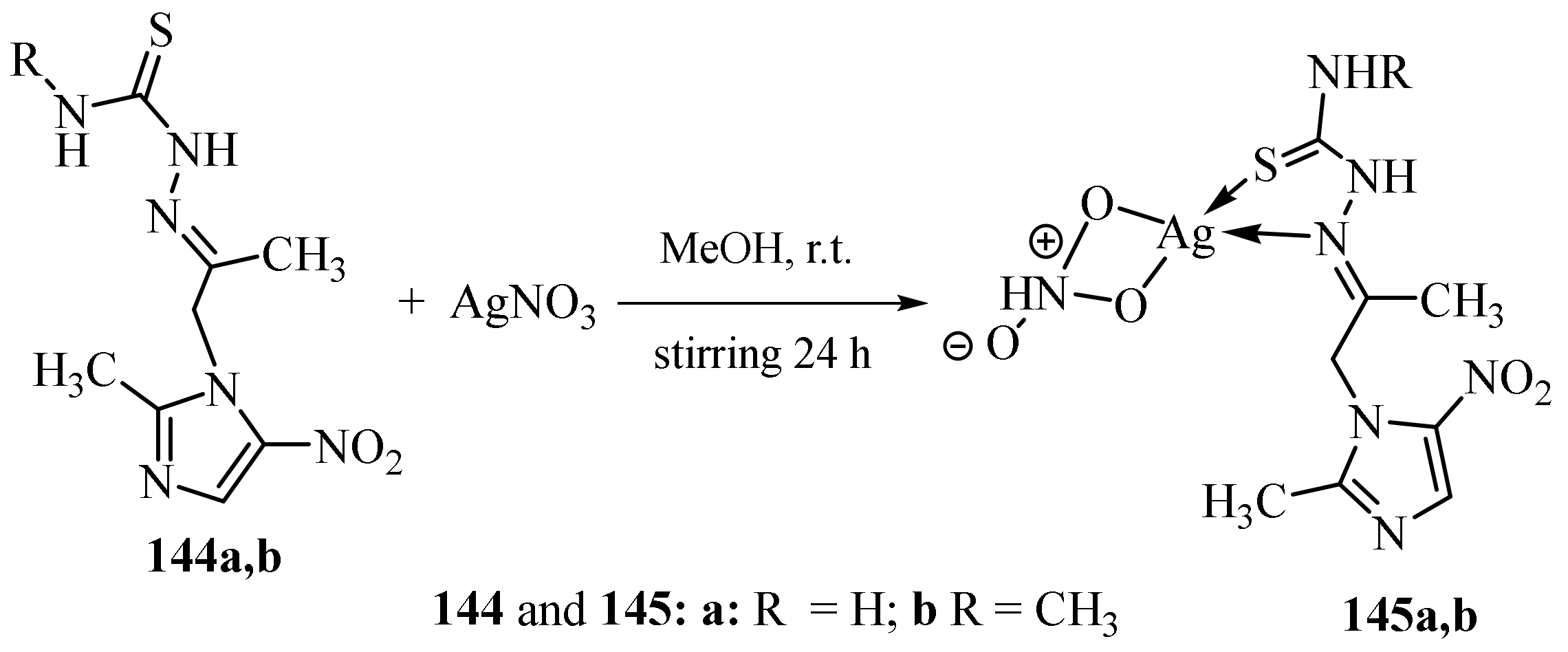
3.6. Ag(I) Complexes of Thiosemicarbazides and Thiosemicarbazones
4. Application of Thiosemicarbazone and Thiocarbazones Complexes of Transition Metals
4.1. Catalytic Applications
4.2. Biological Applications
- Adequately high thermodynamic stability to transport the metal to the active site;
- A good hydrolytically stability;
- A proper molecular weight. Low molecular weight compounds with no charge and very low water solubility have the advantage of being able to cross biological membranes by passive diffusion [130].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franz, K.J.; Metzler-Nolte, N. Introduction: Metals in medicine. Chem. Rev. 2019, 119, 727–729. [Google Scholar] [CrossRef]
- Frei, A.; Elliott, A.G.; Kan, A.; Dinh, H.; Bräse, S.; Bruce, A.E.; Bruce, M.R.; Chen, F.; Humaidy, D.; Jung, N. Metal Complexes as Antifungals?–From a Crowd-Sourced Compound Library to First In Vivo Experiments. JACS 2022, 2, 2277–2294. [Google Scholar] [CrossRef]
- Bagchi, A.; Mukherjee, P.; Raha, A. A review on transition metal complex-modern weapon in medicine. Int. J. Recent Adv. Pharm. Res 2015, 5, 171–180. [Google Scholar]
- Takaya, J. Catalysis using transition metal complexes featuring main group metal and metalloid compounds as supporting ligands. Chem. Sci. 2021, 12, 1964–1981. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Nolte, N.; Schatzschneider, U. Bioinorganic Chemistry. In Bioinorganic Chemistry; De Gruyter: Berlin, Germany, 2009. [Google Scholar] [CrossRef]
- Sahu, R.; Thakur, D. Schiff base: An overview of its medicinal chemistry potential for new drug molecules. Int. J. Pharm. Sci. Nanotechnol. 2012, 5, 1757–1764. [Google Scholar] [CrossRef]
- Golcu, A.; Tumer, M.; Demirelli, H.; Wheatley, R.A. Cd (II) and Cu (II) complexes of polydentate Schiff base ligands: Synthesis, characterization, properties and biological activity. Inorg. Chim. Acta 2005, 358, 1785–1797. [Google Scholar] [CrossRef]
- Padhye, S.; Afrasiabi, Z.; Sinn, E.; Fok, J.; Mehta, K.; Rath, N. Antitumor metallothiosemicarbazonates: Structure and antitumor activity of palladium complex of phenanthrenequinone thiosemicarbazone. Inorg. Chem. 2005, 44, 1154–1156. [Google Scholar] [CrossRef] [PubMed]
- Lainé, A.-L.; Passirani, C. Novel metal-based anticancer drugs: A new challenge in drug delivery. Curr. Opin. Pharm. 2012, 12, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sadler, P.J. Redox-active metal complexes for anticancer therapy. Eur. J. Inorg. Chem. 2017, 2017, 1541–1548. [Google Scholar] [CrossRef]
- Warra, A. Transition metal complexes and their application in drugs and cosmetics-a Review. J. Chem. Pharm. Res. 2011, 3, 951–958. [Google Scholar]
- Monro, S.; Colón, K.L.; Yin, H.; Roque III, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition metal complexes and photodynamic therapy from a tumor-centered approach: Challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 2018, 119, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Britz, A.; Gawelda, W.; Assefa, T.A.; Jamula, L.L.; Yarranton, J.T.; Galler, A.; Khakhulin, D.; Diez, M.; Harder, M.; Doumy, G. Using ultrafast X-ray spectroscopy to address questions in ligand-field theory: The excited state spin and structure of [Fe (dcpp) 2]2+. Inorg. Chem. 2019, 58, 9341–9350. [Google Scholar] [CrossRef] [PubMed]
- Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C. Copper in diseases and treatments, and copper-based anticancer strategies. Med. Res. Rev. 2010, 30, 708–749. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Muleta, F.; Alansi, T.; Eswaramoorthy, R. A review on synthesis characterization methods and biological activities of semicarbazone thiosemi-carbazone and their transition metal complexes. J. Nat. Sci. Res. 2019, 9, 33–46. [Google Scholar]
- Caselli, A.; Cesana, F.; Gallo, E.; Casati, N.; Macchi, P.; Sisti, M.; Celentano, G.; Cenini, S. Designing new ligands: Asymmetric cyclopropanation by Cu (I) complexes based on functionalised pyridine-containing macrocyclic ligands. Dalton Trans. 2008, 4202–4205. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Suzuki, H. Cobalt: Occurrence, Uses and Properties; Nova Publishers: Hauppauge, NY, USA, 2013. [Google Scholar]
- Pahonţu, E.; Julea, F.; Chumakov, Y.; Petrenco, P.; Roşu, T.; Gulea, A. Synthesis, characterization, crystal structure and antiproliferative activity studies of Cu (II), Ni (II) and Co (II) complexes with 4-benzoyl-5-pyrazolones derived compounds. J. Organomet. Chem. 2017, 836, 44–55. [Google Scholar] [CrossRef]
- Heffern, M.C.; Yamamoto, N.; Holbrook, R.J.; Eckermann, A.L.; Meade, T.J. Cobalt derivatives as promising therapeutic agents. Curr.Opin. Chem. Biol. 2013, 17, 189–196. [Google Scholar] [CrossRef]
- Yamada, S. Advancement in stereochemical aspects of Schiff base metal complexes. Coord. Chem. Rev. 1999, 190, 537–555. [Google Scholar] [CrossRef]
- Amirnasr, M.; Schenk, K.J.; Gorji, A.; Vafazadeh, R. Synthesis and spectroscopic characterization of [CoIII (salophen)(amine) 2] ClO4 (amine= morpholine, pyrrolidine, and piperidine) complexes. The crystal structures of [CoIII (salophen)(morpholine)2]ClO4 and [CoIII (salophen)(pyrrolidine) 2] ClO4. Polyhedron 2001, 20, 695–702. [Google Scholar] [CrossRef]
- Aly, M.M. Recent developments in the metallosupramolecular and molecular structures of the cobalt, iron and vanadium complexes of the dianionic tetradentate Schiff base ligands of salicylideneimine and acetylacetoneimine. J. Coord. Chem. 1998, 43, 89–113. [Google Scholar] [CrossRef]
- Hirota, S.; Kosugi, E.; Marzilli, L.G.; Yamauchi, O. The Co-CH3 bond in Shiff base B12 models: Influence of the trans and equatorial ligands as assessed by Fourier transform Raman spectroscopy. Inorg. Chim. Acta 1998, 275, 90–97. [Google Scholar] [CrossRef]
- Sawyer, D.T. The Chemistry and Activation of Dioxygen Species (O2, O2−. and HOOH) in Biology. In Oxygen Complexes and Oxygen Activation by Transition Metals; Springer: Boston, MA, USA, 1988; pp. 131–148. [Google Scholar]
- Henson, N.J.; Hay, P.J.; Redondo, A. Density functional theory studies of the binding of molecular oxygen with Schiff’s base complexes of cobalt. Inorg. Chem. 1999, 38, 1618–1626. [Google Scholar] [CrossRef]
- Bianchini, C.; Zoellner, R.W. Activation of dioxygen by cobalt group metal complexes. Adv. Inorg. Chem. 1996, 44, 263–339. [Google Scholar]
- Nagata, T.; Yorozu, K.; Yamada, T.; Mukaiyama, T. Enantioselective reduction of ketones with sodium borohydride, catalyzed by optically active (β-oxoaldiminato) cobalt (II) complexes. Angew. Chem. Int. Ed. Engl. 1995, 34, 2145–2147. [Google Scholar] [CrossRef]
- Böttcher, A.; Takeuchi, T.; Hardcastle, K.I.; Meade, T.J.; Gray, H.B.; Cwikel, D.; Kapon, M.; Dori, Z. Spectroscopy and electrochemistry of cobalt (III) Schiff base complexes. Inorg. Chem. 1997, 36, 2498–2504. [Google Scholar] [CrossRef]
- Sakiyama, H.; Kato, M.; Sasaki, S.; Tasaki, M.; Asato, E.; Koikawa, M. Synthesis and magnetic properties of a dinuclear manganese (II) complex with two manganese (II) ions of C2-twisted octahedral geometry. Polyhedron 2016, 111, 32–37. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Zangrando, E.; Kaminsky, W.; Garczarek, P.; Frontera, A. Solvent dependent nuclearity of manganese complexes with a polydentate hydrazone-based ligand and thiocyanate anions. Inorg. Chim. Acta 2017, 455, 204–212. [Google Scholar] [CrossRef]
- Nandi, S.; Das, K.; Datta, A.; Banerjee, D.; Roy, S.; Mondal, T.K.; Mandal, D.; Nanda, P.K.; Akitsu, T.; Tanaka, S. Synthesis, spectral elucidation, electrochemistry and DFT interpretation of manganese (II)-thioalkyl-arylazoimidazole complex. J. Mol. Struct. 2017, 1133, 574–579. [Google Scholar] [CrossRef]
- Ibrahim, O.B.; Mohamed, M.A.; Refat, M.S. Nano sized schiff base complexes with Mn (II), Co (II), Cu (II), Ni (II) and Zn (II) metals: Synthesis, spectroscopic and medicinal studies. Can. Chem. Trans 2014, 2, 108–121. [Google Scholar]
- Shafaatian, B.; Soleymanpour, A.; Oskouei, N.K.; Notash, B.; Rezvani, S.A. Synthesis, crystal structure, fluorescence and electrochemical studies of a new tridentate Schiff base ligand and its nickel (II) and palladium (II) complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, C.; Deacon, G.B.; Drago, P.R.; Erven, A.P.; Junk, P.C.; Luu, J.; Meyer, G.; Schmitz, S.; Ott, I.; Schur, J. Synthesis and antiproliferative activity of a series of new platinum and palladium diphosphane complexes. Dalton Trans. 2018, 47, 1918–1932. [Google Scholar] [CrossRef] [PubMed]
- Haribabu, J.; Tamizh, M.M.; Balachandran, C.; Arun, Y.; Bhuvanesh, N.S.; Endo, A.; Karvembu, R. Synthesis, structures and mechanistic pathways of anticancer activity of palladium (II) complexes with indole-3-carbaldehyde thiosemicarbazones. New J. Chem. 2018, 42, 10818–10832. [Google Scholar] [CrossRef]
- Lobana, T.S. Activation of C–H bonds of thiosemicarbazones by transition metals: Synthesis, structures and importance of cyclometallated compounds. RSC Adv. 2015, 5, 37231–37274. [Google Scholar] [CrossRef]
- Asiri, A.M.; Khan, S.A. Palladium (II) complexes of NS donor ligands derived from steroidal thiosemicarbazones as antibacterial agents. Molecules 2010, 15, 4784–4791. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M.D.; Ari, F.; Oral, A.Y.; Sarimahmut, M.; Kutlu, H.M.; Yilmaz, V.T.; Ulukaya, E. Promising anti-growth effects of palladium (II) saccharinate complex of terpyridine by inducing apoptosis on transformed fibroblasts in vitro. Bioorg. Med. Chem. 2013, 21, 4698–4705. [Google Scholar] [CrossRef]
- Özerkan, D.; Ertik, O.; Kaya, B.; Kuruca, S.E.; Yanardag, R.; Ülküseven, B. Novel palladium (II) complexes with tetradentate thiosemicarbazones. Synthesis, characterization, in vitro cytotoxicity and xanthine oxidase inhibition. Investig. New Drugs 2019, 37, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- West, D.X.; Ives, J.S.; Krejci, J.; Salberg, M.M.; Zumbahlen, T.L.; Bain, G.A.; Liberta, A.E.; Valdes-Martinez, J.; Hernandez-Ortiz, S.; Toscano, R.A. Copper (II) complexes of 2-benzoylpyridine 4N-substituted thiosemicarbazones. Polyhedron 1995, 14, 2189–2200. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 327, 349–359. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical uses of silver: History, myths, and scientific evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef]
- Raju, S.K.; Karunakaran, A.; Kumar, S.; Sekar, P.; Murugesan, M.; Karthikeyan, M. Silver Complexes as Anticancer Agents: A Perspective Review. Ger. J. Pharm. Biomater. 2022, 1, 6–28. [Google Scholar] [CrossRef]
- Paterson, B.M.; White, J.M.; Donnelly, P.S.; Koutsantonis, G. An icosanuclear silver (I) cluster supported by bis (thiosemicarbazonato) ligands. Aust. J. Chem. 2022, 75, 631–635. [Google Scholar] [CrossRef]
- Wilkinson, G.; Gillard, R.D. Comprehensive Coordination Chemistry The Synthesis, Reactions, Properties and Applications of Coordination Compounds V3 Main Group and Early Transition Elements; Pergamon Press: Oxford, UK, 1987. [Google Scholar]
- Lobana, T.S.; Khanna, S.; Sharma, R.; Hundal, G.; Sultana, R.; Chaudhary, M.; Butcher, R.; Castineiras, A. Versatility of thiosemicarbazones in the construction of monomers, dimers and hydrogen-bonded networks of silver (I) complexes. Cryst. Growth Des. 2008, 8, 1203–1212. [Google Scholar] [CrossRef]
- López-Torres, E.; Mendiola, M.A. Mercury complexes with the ligand benzaldehyde-N (4), N (4)-dimethylthiosemicarbazone. Inorg. Chim. Acta 2010, 363, 1275–1283. [Google Scholar] [CrossRef]
- Castineiras, A.; Pedrido, R. Novel fluorescent cationic silver thiosemicarbazone clusters containing different eight-membered Ag4S4 metallacycles. Inorg. Chem. 2009, 48, 4847–4855. [Google Scholar] [CrossRef]
- Metwally, M.A.; Bondock, S.; El-Azap, H.; Kandeel, E.-E.M. Thiosemicarbazides: Synthesis and reactions. J. Sulfur Chem. 2011, 32, 489–519. [Google Scholar] [CrossRef]
- Deng, J.; Yu, P.; Zhang, Z.; Wang, J.; Cai, J.; Wu, N.; Sun, H.; Liang, H.; Yang, F. Designing anticancer copper (II) complexes by optimizing 2-pyridine-thiosemicarbazone ligands. Eur. J. Med. Chem. 2018, 158, 442–452. [Google Scholar] [CrossRef]
- Alshammari, M.B.; Aly, A.A.; Bräse, S.; Nieger, M.; Abd El-Haleem, L.E. Efficient Synthesis of Various Substituted (Thio) Ureas, Semicarbazides, Thiosemicarbazides, Thiazolidones, and Oxadiazole Derived from [2.2] Paracyclophane. ACS Omega 2022, 7, 12879–12890. [Google Scholar] [CrossRef]
- Emara, A.A.; Seleem, H.; Madyan, A.M. Synthesis, spectroscopic investigations, and biological activity of metal complexes of N-benzoylthiosemicarbazide. J. Coord. Chem. 2009, 62, 2569–2582. [Google Scholar] [CrossRef]
- Joseph, J.; Mary, N.; Sidambaram, R. Synthesis, characterization, and antibacterial activity of the Schiff bases derived from thiosemicarbazide, Salicylaldehyde, 5-bromosalicylaldehyde and their copper (II) and nickel (II) complexes. Synth. React. Inorg. Met. -Org. Nano-Met. Chem. 2010, 40, 930–933. [Google Scholar] [CrossRef]
- Ferrari, M.B.; Capacchi, S.; Reffo, G.; Pelosi, G.; Tarasconi, P.; Albertini, R.; Pinelli, S.; Lunghi, P. Synthesis, structural characterization and biological activity of p-fluorobenzaldehyde thiosemicarbazones and of a nickel complex. J. Inorg. BioChem. 2000, 81, 89–97. [Google Scholar] [CrossRef]
- Tada, R. Synthesis and characterization of some new thiosemicarbazide. J. Chem. 2011, 3, 290–297. [Google Scholar]
- Umadevi, P.; Deepti, K.; Srinath, I.; Vijayalakshmi, G.; Tarakaramji, M. Synthesis and in-vitro antibacterial activity of some new urea, thiourea and thiosemicarbazide derivatives. Int. J. Pharm. Pharm. Sci 2012, 4, 379–383. [Google Scholar]
- Raja, M.U.; Gowri, N.; Ramesh, R. Synthesis, crystal structure and catalytic activity of ruthenium (II) carbonyl complexes containing ONO and ONS donor ligands. Polyhedron 2010, 29, 1175–1181. [Google Scholar] [CrossRef]
- Raman, N.; Selvan, A.; Manisankar, P. Spectral, magnetic, biocidal screening, DNA binding and photocleavage studies of mononuclear Cu (II) and Zn (II) metal complexes of tricoordinate heterocyclic Schiff base ligands of pyrazolone and semicarbazide/thiosemicarbazide based derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 76, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Raizada, S.; Tyagi, M.; Gautam, A. Synthesis, spectroscopic, and antimicrobial studies on bivalent nickel and copper complexes of bis (thiosemicrbazone). Bioinorg. Chem. Appl. 2007, 2007, 051483. [Google Scholar] [CrossRef]
- Munikumari, G.; Konakanchi, R.; Nishtala, V.B.; Ramesh, G.; Kotha, L.R.; Chandrasekhar, K.; Ramachandraiah, C. Palladium (II) complexes of 5-substituted isatin thiosemicarbazones: Synthesis, spectroscopic characterization, biological evaluation and in silico docking studies. Synth. Commun. 2019, 49, 146–158. [Google Scholar] [CrossRef]
- Aly, A.A.; Bräse, S.; Weis, P. Tridentate and bidentate copper complexes of [2.2] paracyclophanyl-substituted thiosemicarbazones, thiocarbazones, hydrazones and thioureas. J. Mol. Struct. 2019, 1178, 311–326. [Google Scholar] [CrossRef]
- Alshammari, M.B.; Aly, A.A.; Bräse, S.; Nieger, M.; Ibrahim, M.A.; Abd El-Haleem, L.E. Copper Complexes of 1, 4-Naphthoquinone Containing Thiosemicarbazide and Triphenylphosphine Oxide Moieties; Synthesis and Identification by NMR, IR, Mass, UV Spectra, and DFT Calculations. ACS Omega 2022, 7, 34463–34475. [Google Scholar] [CrossRef]
- Tian, Y.-P.; Duan, C.-Y.; Lu, Z.-L.; You, X.-Z.; Fun, H.-K.; Kandasamy, S. Crystal structure and spectroscopic studies on metal complexes containing ns donor ligands derived from S-benzyldithiocarbazate andp-dimethylaminobenzaldehyde. Polyhedron 1996, 15, 2263–2271. [Google Scholar] [CrossRef]
- Basuli, F.; Peng, S.-M.; Bhattacharya, S. Steric control of the coordination mode of the salicylaldehyde thiosemicarbazone ligand. Syntheses, structures, and redox properties of ruthenium and osmium complexes. Inorg. Chem. 1997, 36, 5645–5647. [Google Scholar] [CrossRef]
- Khan, S.A.; Asiri, A.M.; Al-Amry, K.; Malik, M.A. Synthesis, characterization, electrochemical studies, and in vitro antibacterial activity of novel thiosemicarbazone and its Cu (II), Ni (II), and Co (II) complexes. Sci. World J. 2014, 2014, 592375. [Google Scholar]
- Venkatesh, K.; Banothu Venkanna, K.; Sekhar, C.; Mukkanti, K. Synthesis, characterization & biological activity of some new thiosemicarbazide derivatives and their transition metal complexes. J. Chem. Pharm. Res. 2015, 7, 437–445. [Google Scholar]
- Bursal, E.; Turkan, F.; Buldurun, K.; Turan, N.; Aras, A.; Çolak, N.; Murahari, M.; Yergeri, M.C. Transition metal complexes of a multidentate Schiff base ligand containing pyridine: Synthesis, characterization, enzyme inhibitions, antioxidant properties, and molecular docking studies. Biometals 2021, 34, 393–406. [Google Scholar] [CrossRef]
- Suvarapu, L.N.; Somala, A.R.; Koduru, J.R.; Baek, S.O.; Ammireddy, V.R. A Critical Review on Analytical and Biological Applications of Thio-and Phenylthiosemicarbazones. Asian J. Chem. 2012, 24, 1889–1898. [Google Scholar]
- Bajaj, K.; Buchanan, R.M.; Grapperhaus, C.A. Antifungal activity of thiosemicarbazones, bis (thiosemicarbazones), and their metal complexes. J. Inorg. Biochem. 2021, 225, 111620. [Google Scholar] [CrossRef]
- Lobana, T.S.; Sharma, R.; Castineiras, A.; Kim, S.; Akitsu, T. Synthesis, crystal structure and DFT study of a dinuclear CuI complex with (E)-2-benzylidene-N-methylhydrazinecarbothioamide: Role of halogen-hydrogen bonds in copper-copper bridge bonding. J. Coord. Chem. 2021, 74, 942–954. [Google Scholar] [CrossRef]
- Khan, A.; Paul, K.; Singh, I.; Jasinski, J.P.; Smolenski, V.A.; Hotchkiss, E.P.; Kelley, P.T.; Shalit, Z.A.; Kaur, M.; Banerjee, S. Copper (I) and silver (I) complexes of anthraldehyde thiosemicarbazone: Synthesis, structure elucidation, in vitro anti-tuberculosis/cytotoxic activity and interactions with DNA/HSA. Dalton Trans. 2020, 49, 17350–17367. [Google Scholar] [CrossRef]
- Borges, A.P.; Carneiro, Z.A.; Prado, F.S.; Souza, J.R.; e Silva, L.H.F.; Oliveira, C.G.; Deflon, V.M.; de Albuquerque, S.; Leite, N.B.; Machado, A.E. Cu (I) complexes with thiosemicarbazides derived from p-toluenesulfohydrazide: Structural, luminescence and biological studies. Polyhedron 2018, 155, 170–179. [Google Scholar] [CrossRef]
- Pradhan, R.; Groner, V.M.; Johnson, N.A.; Zhang, Q.; Roll, M.F.; Moberly, J.G.; Waynant, K.V. Synthesis of an N, N-diethyl-tert-butylazothioformamide ligand and coordination studies with Copper (I) salts. Inorg. Chem. Commun. 2021, 124, 108393. [Google Scholar] [CrossRef]
- Jaafar, A.; Mansour, N.; Fix-Tailler, A.; Allain, M.; Faour, W.H.; Shebaby, W.N.; Tokajian, S.; El-Ghayoury, A.; Naoufal, D.; Larcher, G. Synthesis, Characterization, Antibacterial and Antifungal Activities Evaluation of Metal Complexes With Benzaldehyde-4-methylthiosemicarbazone Derivatives. Chem. Sel. 2022, 7, e202104497. [Google Scholar] [CrossRef]
- Nibila, T.; Soufeena, P.; Periyat, P.; Aravindakshan, K. Synthesis, structural characterization and biological activities of transition metal complexes derived from 2, 4-dihydroxybenzaldehyde n(4)-methyl (phenyl) thiosemicarbazone. J. Mol. Struct. 2021, 1231, 129938. [Google Scholar] [CrossRef]
- Yepseu, A.P.; Girardet, T.; Nyamen, L.D.; Fleutot, S.; Ketchemen, K.I.; Cleymand, F.; Ndifon, P.T. Copper (II) Heterocyclic Thiosemicarbazone Complexes as Single-Source Precursors for the Preparation of Cu9S5 Nanoparticles: Application in Photocatalytic Degradation of Methylene Blue. Catalysts 2022, 12, 61. [Google Scholar] [CrossRef]
- Tiwari, D.; Basnet, K.; Lamichhane, J.; Niraula, P.; Bhandari, S.; Yadav, P.N. Copper Complexes of Imidazole-2-carbaldehyde N (4)-Substituted Thiosemicarbazones: Synthesis, Characterization and Antimicrobial Activity. Asian J. Chem. 2016, 28, 2793–2797. [Google Scholar] [CrossRef]
- Salem, M.; Abbas, S.; Darwish, S.; Helal, M.; Aish, E. Synthesis of Novel Tridentate 5-Arylazosalicylaldehyde Thiosemicarbazone Derivatives and Their Complexes with Copper (II). Russ. J. Gen. Chem. 2021, 91, 1578–1583. [Google Scholar] [CrossRef]
- Rhoufal, F.; Guesmi, S.; Jouffret, L.; Ketatni, E.M.; Sergent, N.; Obbade, S.; Bentiss, F. Novel copper (II) and nickel (II) coordination complexes of 2, 4-pentanedione bis-thiosemicarbazone: Synthesis, structural characterization, computational studies, and magnetic properties. Inorg. Chem. Commun. 2022, 141, 109574. [Google Scholar] [CrossRef]
- Lim, S.; Price, K.A.; Chong, S.-F.; Paterson, B.M.; Caragounis, A.; Barnham, K.J.; Crouch, P.J.; Peach, J.M.; Dilworth, J.R.; White, A.R. Copper and zinc bis (thiosemicarbazonato) complexes with a fluorescent tag: Synthesis, radiolabelling with copper-64, cell uptake and fluorescence studies. JBIC J. Biol. Inorg. Chem. 2010, 15, 225–235. [Google Scholar] [CrossRef]
- Aly, A.A.; Abdallah, E.M.; Ahmed, S.A.; Awad, M.K.; Rabee, M.M.; Mostafa, S.M.; Bräse, S. Metal complexes of new thiocarbohydrazones of Cu (I), Co (II), and Ni (II); identification by NMR, IR, mass, UV spectra, and DFT calculations. J. Sulfur Chem. 2023, in press. [Google Scholar] [CrossRef]
- Aly, A.A.; Abdallah, E.M.; Ahmed, S.A.; Rabee, M.M.; Abdelhafez, E.-S.M. Metal complexes of thiosemicarbazones derived by 2-quinolones with Cu (I), Cu (II) and Ni (II); Identification by NMR, IR, ESI mass spectra and in silico approach as potential tools against SARS-CoV-2. J. Mol. Struct. 2022, 1265, 133480. [Google Scholar] [CrossRef]
- Alomar, K.; Khan, M.A.; Allain, M.; Bouet, G. Synthesis, crystal structure and characterization of 3-thiophene aldehyde thiosemicarbazone and its complexes with cobalt (II), nickel (II) and copper (II). Polyhedron 2009, 28, 1273–1280. [Google Scholar] [CrossRef]
- Marichelvam, T.; Murugan, G.; Holla, H.; Naranammalpuram Sundaram, V.N.; Palanimuthu, D. Electrocatalytic Oxidation of Hydrazine Using a Cobalt Bis (thiosemicarbazone) Complex. Top. Catal. 2023, 1–11. [Google Scholar] [CrossRef]
- Gujarathi, J. Synthesis, spectral and biological evaluation of Co (II) complexes derived from N (4) thiosemicarbazone. IJRAR-Int.J. Res. Anal. Rev. 2018, 5, 710–716. [Google Scholar]
- Savir, S.; Wei, Z.J.; Liew, J.W.K.; Vythilingam, I.; Lim, Y.A.L.; Saad, H.M.; Sim, K.S.; Tan, K.W. Synthesis, cytotoxicity and antimalarial activities of thiosemicarbazones and their nickel (II) complexes. J. Mol. Struct. 2020, 1211, 128090. [Google Scholar] [CrossRef]
- Savir, S.; Liew, J.W.K.; Vythilingam, I.; Lim, Y.A.L.; Tan, C.H.; Sim, K.S.; Lee, V.S.; Maah, M.J.; Tan, K.W. Nickel (II) complexes with polyhydroxybenzaldehyde and O, N, S tridentate thiosemicarbazone ligands: Synthesis, cytotoxicity, antimalarial activity, and molecular docking studies. J. Mol. Struct. 2021, 1242, 130815. [Google Scholar] [CrossRef]
- Graiff, C.; Canossa, S.; Predieri, G. Coordinative properties of the pyridine-2-carbaldehyde thiosemicarbazone ligand towards Ni (II). J. Struct. Chem. 2014, 55, 493–497. [Google Scholar] [CrossRef]
- Hosseini-Yazdi, S.A.; Hosseinpour, S.; Khandar, A.A.; Kassel, W.S.; Piro, N.A. Copper (II) and nickel (II) complexes with two new bis (thiosemicarbazone) ligands: Synthesis, characterization, X-ray crystal structures and their electrochemistry behavior. Inorganica. Chim. Acta 2015, 427, 124–130. [Google Scholar] [CrossRef]
- Yousef, T.; El-Reash, G.A.; El-Rakhawy, E. Structural, spectral, thermal and biological studies on (E)-2-(1-(4-hydroxyphenyl) ethylidene)-N-(pyridin-2-yl) hydrazinecarbothioamide and its metal complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Dinda, R.; Schmiesing, C.S.; Sinn, E.; Patil, Y.P.; Nethaji, M.; Stoeckli-Evans, H.; Acharyya, R. Mixed-ligand nickel (II) thiosemicarbazone complexes: Synthesis, characterization and biological evaluation. Polyhedron 2013, 50, 354–363. [Google Scholar]
- Selvamurugan, S.; Ramachandran, R.; Vijayan, P.; Manikandan, R.; Prakash, G.; Viswanathamurthi, P.; Velmurugan, K.; Nandhakumar, R.; Endo, A. Synthesis, crystal structure and biological evaluation of Ni (II) complexes containing 4-chromone-N(4)-substituted thiosemicarbazone ligands. Polyhedron 2016, 107, 57–67. [Google Scholar] [CrossRef]
- Ali, A.Q.; Teoh, S.G.; Eltayeb, N.E.; Ahamed, M.B.K.; Majid, A.A. Synthesis of nickel (II) complexes of isatin thiosemicarbazone derivatives: In vitro anti-cancer, DNA binding, and cleavage activities. J. Coord. Chem. 2014, 67, 3380–3400. [Google Scholar] [CrossRef]
- Eğlence-Bakır, S.; Celik, S.; Şahin, M.; Ozel, A.E.; Akyuz, S.; Ülküseven, B. Synthesis, molecular modelling, FT-IR, Raman and NMR characterization, molecular docking and ADMET study of new nickel (II) complex with an N4-tetradentate thiosemicarbazone. J. Biomol. Struct. Dyn. 2021, 39, 4212–4224. [Google Scholar] [CrossRef]
- Rai, B.; Sinha, P.; Singh, V.; Vidhyarthi, S.; Pandey, A. Structural and Spectroscopic Aspects of Schiff Base Metal Complexes of Cobalt (II), Nickel (II) and Copper (II). Orient. J. Chem. 2014, 30, 1429. [Google Scholar] [CrossRef]
- Damit, N.S.H.H.; Hamid, M.H.S.A.; Rahman, N.S.R.H.A.; Ilias, S.N.H.H.; Keasberry, N.A. Synthesis, structural characterisation and antibacterial activities of lead (II) and some transition metal complexes derived from quinoline-2-carboxaldehyde 4-methyl-3-thiosemicarbazone. Inorg. Chim. Acta 2021, 527, 120557. [Google Scholar] [CrossRef]
- Mahetar, J.G.; Mamtora, M.J.; Gondaliya, M.B.; Manawar, R.B.; Shah, M.K. Synthesis, characterization and antimicrobial activity of transition metal complexes of thiosemicarbazone bearing vanillin moiety. World J. Pharm. Res. 2014, 3, 4383–4392. [Google Scholar]
- Ahmed, M.F.A.; Mahammadyunus, V. Microwave synthesis and antimicrobial activity of some Copper (II), Cobalt (II), Nickel (II) and Chromium (III) complexes with Schiff base 2, 6-pyridinedicarboxaldehyde-Thiosemicarbazone. Orient. J. Chem. 2014, 30, 111–117. [Google Scholar] [CrossRef]
- Mohamed, E.E.; AbedelKarim, A.T.; Elmalik, Y.H.; Mohamed, A.E.; Aljahdali, M.S. Characterization and biological activity studies on some transition metal complexes of thiosemicarbazide derived from 2-picolinic acid hydrazide. Eur. J. Chem. 2014, 5, 252–259. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Mohamad, A.B. Antifungal and antioxidant activities of pyrrolidone thiosemicarbazone complexes. Bioinorg. Chem. Appl. 2012, 2012, 795812. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.A.; Wang, L.F.; Yang, R.D. Synthesis, characterization and antibacterial activities of manganese (II), cobalt (II), nickel (II), copper (II) and zinc (II) complexes with soluble vitamin K3 thiosemicarbazone. Transit. Met. Chem. 2003, 28, 395–398. [Google Scholar] [CrossRef]
- Huseynova, M.; Taslimi, P.; Medjidov, A.; Farzaliyev, V.; Aliyeva, M.; Gondolova, G.; Şahin, O.; Yalçın, B.; Sujayev, A.; Orman, E.B. Synthesis, characterization, crystal structure, electrochemical studies and biological evaluation of metal complexes with thiosemicarbazone of glyoxylic acid. Polyhedron 2018, 155, 25–33. [Google Scholar] [CrossRef]
- Kotian, A.; Kamat, V.; Naik, K.; Kokare, D.G.; Kumara, K.; Neratur, K.L.; Kumbar, V.; Bhat, K.; Revankar, V.K. 8-Hydroxyquinoline derived p-halo N4-phenyl substituted thiosemicarbazones: Crystal structures, spectral characterization and in vitro cytotoxic studies of their Co (III), Ni (II) and Cu (II) complexes. Bioorg. Chem. 2021, 112, 104962. [Google Scholar] [CrossRef]
- Baruah, J.; Gogoi, R.; Gogoi, N.; Borah, G. A thiosemicarbazone–palladium (II)–imidazole complex as an efficient pre-catalyst for Suzuki–Miyaura cross-coupling reactions at room temperature in aqueous media. Transit. Met. Chem. 2017, 42, 683–692. [Google Scholar] [CrossRef]
- Anu, D.; Naveen, P.; Devendhiran, T.; Shyamsivappan, S.; Kumarasamy, K.; Lin, M.C.; Frampton, C.S.; Kaveri, M. Synthesis, spectral characterization, X-ray crystallography and biological evaluations of Pd (II) complexes containing 4 (N)-substituted thiosemicarbazone. J. Coord. Chem. 2021, 74, 3153–3169. [Google Scholar] [CrossRef]
- Anu, D.; Naveen, P.; Rath, N.P.; Kaveri, M. Palladium (II) complexes containing substituted thiosemicarbazones. Synthesis, spectral characterization, X-ray crystallography, biomolecular interactions and in vitro cytotoxicity. J. Mol. Struct. 2020, 1206, 127703. [Google Scholar] [CrossRef]
- Khan, S.A.; Yusuf, M. Synthesis, spectral studies and in vitro antibacterial activity of steroidal thiosemicarbazone and their palladium (Pd (II)) complexes. Eur. J. Med. Chem. 2009, 44, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.; Huentupil, Y.; Rabanal, W.; Cisterna, J.; Brito, I.; Arancibia, R. Synthesis, characterization, X-ray structure, electrochemistry, photocatalytic activity and DFT studies of heterotrinuclear Ni (II), Pd (II) and Zn (II) complexes containing a formylferrocene thiosemicarbazone ligand. App. Organomet. Chem. 2020, 34, e5974. [Google Scholar] [CrossRef]
- Chellan, P.; Stringer, T.; Shokar, A.; Au, A.; Tam, C.; Cheng, L.W.; Smith, G.S.; Land, K.M. Antiprotozoal activity of palladium (II) salicylaldiminato thiosemicarbazone complexes on metronidazole resistant Trichomonas vaginalis. Inorg. Chem. Commun. 2019, 102, 1–4. [Google Scholar] [CrossRef]
- Bharti, N.; Sharma, S.; Naqvi, F.; Azam, A. New palladium (II) complexes of 5-nitrothiophene-2-carboxaldehyde thiosemicarbazones: Synthesis, spectral studies and in vitro anti-amoebic activity. Bioorg. Med. Chem. 2003, 11, 2923–2929. [Google Scholar] [CrossRef]
- Tweedy, B.G. Plant Extracts with Metal Ions as Potential Antimicrobial Agents. Phytopathology 1964, 55, 910. [Google Scholar]
- Lobana, T.S.; Kumari, P.; Butcher, R.J.; Akitsu, T.; Aritake, Y.; Perles, J.; Fernandez, F.J.; Vega, M.C. Thiosemicarbazonates of palladium (II): The presence of methyl/phenyl substituents (R2) at C2 carbon atom induces C–H activation of R1 rings of thiosemicarbazones {R1R2C2N3–N2H–C1(S)–N1HR3}. J. Organomet. Chem. 2012, 701, 17–26. [Google Scholar] [CrossRef]
- Ramachandran, E.; Raja, D.S.; Bhuvanesh, N.S.; Natarajan, K. Mixed ligand palladium (II) complexes of 6-methoxy-2-oxo-1,2-dihydroquinoline-3-carbaldehyde-4-N-substituted thiosemicarbazones with triphenylphosphine co-ligand: Synthesis, crystal structure and biological properties. Dalton Trans. 2012, 41, 13308–13323. [Google Scholar] [CrossRef] [PubMed]
- Dehno Khalaji, A.A.; Shahsavani, E.; Dusek, M.; Kucerakova, M.; Eigner, V. Synthesis, Characterization, and Crystal Structures of a Thiosemicarbazone Ligand and Its Silver (I) Complex. Iran. J. Chem. Chem. Eng. 2020, 39, 23–28. [Google Scholar]
- Sharma, P.; Nath, H.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Hussain, S.; Bhattacharyya, M.K. Biologically relevant unusual cooperative assemblies and fascinating infinite crown-like supramolecular nitrate–water hosts involving guest complex cations in bipyridine and phenanthroline-based Cu (ii) coordination compounds: Antiproliferative evaluation and theoretical studies. New J. Chem. 2021, 45, 8269–8282. [Google Scholar]
- Bharathi, S.; Mahendiran, D.; Kumar, R.S.; Choi, H.J.; Gajendiran, M.; Kim, K.; Rahiman, A.K. Silver (I) metallodrugs of thiosemicarbazones and naproxen: Biocompatibility, in vitro anti-proliferative activity and in silico interaction studies with EGFR, VEGFR2 and LOX receptors. Toxicol. Res. 2020, 9, 28–44. [Google Scholar] [CrossRef]
- Khir, N.A.F.M.; Razak, M.R.M.A.; Nordin, F.J.; Sofyan, N.R.F.M.; Rajab, N.F.; Sarip, R. Synthesis, Antiproliferative and Antimalarial Activities of Dinuclear Silver (I) Complexes with Triphenylphosphine and Thiosemicarbazones Ligands. Indones. J. Chem. 2021, 21, 575–587. [Google Scholar] [CrossRef]
- Abdul Halim, S.N.A.; Nordin, F.J.; Mohd Abd Razak, M.R.; Mohd Sofyan, N.R.F.; Abdul Halim, S.N.; Rajab, N.F.; Sarip, R. Synthesis, characterization, and evaluation of silver (I) complexes with mixed-ligands of thiosemicarbazones and diphenyl (p-tolyl) phosphine as biological agents. J. Coord. Chem. 2019, 72, 879–893. [Google Scholar] [CrossRef]
- Khalaji, A.D.; Shahsavani, E.; Feizi, N.; Kucerakova, M.; Dusek, M.; Mazandarani, R. Silver (I) thiosemicarbazone complex [Ag (catsc)(PPh3)2]NO3: Synthesis, characterization, crystal structure, and antibacterial study. C. R. Chim. 2017, 20, 534–539. [Google Scholar] [CrossRef]
- Wattanakanjana, Y.; Janwatthana, K.; Romyen, T.; Nimthong-Roldan, A. Crystal structures of copper (I) and silver (I) chloride complexes containing 4-phenylthiosemicarbazide and triphenylphosphine ligands. Asian J. Exp. Sci. 2021, 47, 28. [Google Scholar] [CrossRef]
- Oliveira, A.; Ferreira, J.F.; Farias, L.M.; Magalhães, P.P.; Teixeira, L.R.; Beraldo, H. Antimicrobial effects of silver (I) and bismuth (III) complexes with secnidazole-derived Schiff base ligands: The role of the nitro group reduction. J. Braz. Chem. Soc. 2019, 30, 2299–2307. [Google Scholar] [CrossRef]
- Schulte, G.; Luo, X.L.; Crabtree, R.H.; Zimmer, M. Functional Modeling of Ni, Fe Hydrogenases: A Nickel Complex in an N, O, S Environment. Angew. Chem. Int. Ed. 1991, 30, 193–194. [Google Scholar] [CrossRef]
- Kovala-Demertzi, D.; Yadav, P.N.; Demertzis, M.A.; Jasiski, J.P.; Andreadaki, F.J.; Kostas, I.D. First use of a palladium complex with a thiosemicarbazone ligand as catalyst precursor for the Heck reaction. Tetrahedron Lett. 2004, 45, 2923–2926. [Google Scholar] [CrossRef]
- Islam, M.; Hossain, D.; Mondal, P.; Tuhina, K.; Roy, A.S.; Mondal, S.; Mobarak, M. Synthesis, characterization, and catalytic activity of a polymer-supported copper (II) complex with a thiosemicarbazone ligand. Transit. Met. Chem. 2011, 36, 223–230. [Google Scholar] [CrossRef]
- Manikandan, R.; Anitha, P.; Prakash, G.; Vijayan, P.; Viswanathamurthi, P. Synthesis, spectral characterization and crystal structure of Ni (II) pyridoxal thiosemicarbazone complexes and their recyclable catalytic application in the nitroaldol (Henry) reaction in ionic liquid media. Polyhedron 2014, 81, 619–627. [Google Scholar] [CrossRef]
- Pelagatti, P.; Venturini, A.; Leporati, A.; Carcelli, M.; Costa, M.; Bacchi, A.; Pelizzi, G.; Pelizzi, C. Chemoselective homogeneous hydrogenation of phenylacetylene using thiosemicarbazone and thiobenzoylhydrazone palladium (II) complexes as catalysts. J. Chem. Soc. Dalton Trans. 1998, 2715–2722. [Google Scholar] [CrossRef]
- Kostas, I.D.; Steele, B.R. Thiosemicarbazone complexes of transition metals as catalysts for cross-coupling reactions. J. Catal. 2020, 10, 1107. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy–an update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.S.; Carnes, M.E.; Nell, B.P.; Zakharov, L.N.; Johnson, D.W. A facile route to old and new cyclophanes via self-assembly and capture. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Reis, D.C.; Despaigne, A.A.R.; Silva, J.G.D.; Silva, N.F.; Vilela, C.F.; Mendes, I.C.; Takahashi, J.A.; Beraldo, H. Structural studies and investigation on the activity of imidazole-derived thiosemicarbazones and hydrazones against crop-related fungi. Molecules 2013, 18, 12645–12662. [Google Scholar] [CrossRef]
- Beraldo, H.; Gambino, D. The wide pharmacological versatility of semicarbazones, thiosemicarba-zones and their metal complexes. Mini Rev. Med. Chem. 2004, 4, 31–39. [Google Scholar]
- Chohan, Z.H.; Praveen, M.; Ghaffar, A. Structural and biological behaviour of Co (II), Cu (II) and Ni (II) metal complexes of some amino acid derived Schiff-bases. Met. Based Drugs 1997, 4, 267–272. [Google Scholar] [CrossRef]
- Khan, M.; Ali, M.; Juyal, D. Ciprofloxacin metal complexes and their biological activities: A review. Pharma Innov. 2017, 6, 73. [Google Scholar]
- Khan, T.; Raza, S.; Lawrence, A.J. Medicinal Utility of Thiosemicarbazones with Special Reference to Mixed Ligand and Mixed Metal Complexes: A Review. Russ. J. Coord. Chem. 2022, 48, 877–895. [Google Scholar] [CrossRef]
- Metzler-Nolte, N.; Kraatz, H. Concepts and Models in Bioinorganic Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Liberta, A.E.; West, D.X. Antifungal and antitumor activity of heterocyclic thiosemicarbazones and their metal complexes: Current status. Biometals 1992, 5, 121–126. [Google Scholar] [CrossRef]
- Mathews, N.A.; Kurup, M.P. In vitro biomolecular interaction studies and cytotoxic activities of copper (II) and zinc (II) complexes bearing ONS donor thiosemicarbazones. Appl. Organomet. Chem. 2021, 35, e6056. [Google Scholar] [CrossRef]
- Singh, N.; Shrestha, S.; Shahi, N.; Choudhary, R.; Kumbhar, A.; Pokharel, Y.; Yadav, P. Anticancer potential of N (4) substituted 5-nitroisatin thiosemicarbazones and their copper (II) complexes. Rasayan J. Chem. 2021, 14, 1600–1610. [Google Scholar] [CrossRef]
- Akladios, F.N.; Andrew, S.D.; Parkinson, C.J. Cytotoxic activity of expanded coordination bis-thiosemicarbazones and copper complexes thereof. JBIC J. Biol. Inorg. Chem. 2016, 21, 931–944. [Google Scholar] [CrossRef]
- Khan, S.A.; Asiri, A.M. Multi-step synthesis, spectroscopic studies of biological active steroidal thiosemicarbazones and their palladium (II) complex as macromolecules. Int. J. Biol. Macromol. 2018, 107, 105–111. [Google Scholar] [CrossRef] [PubMed]
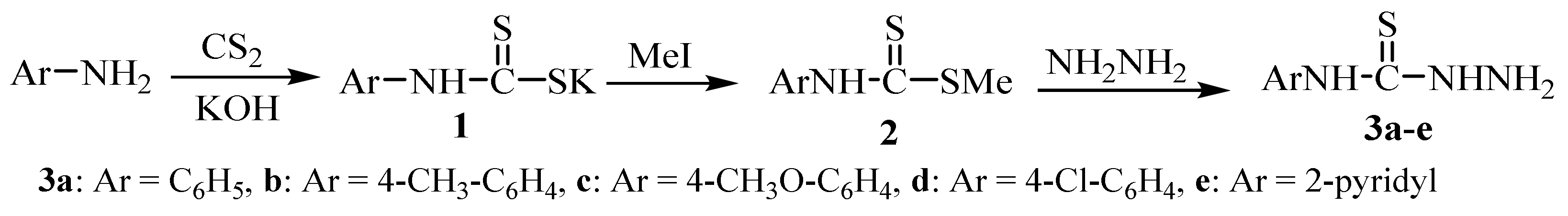

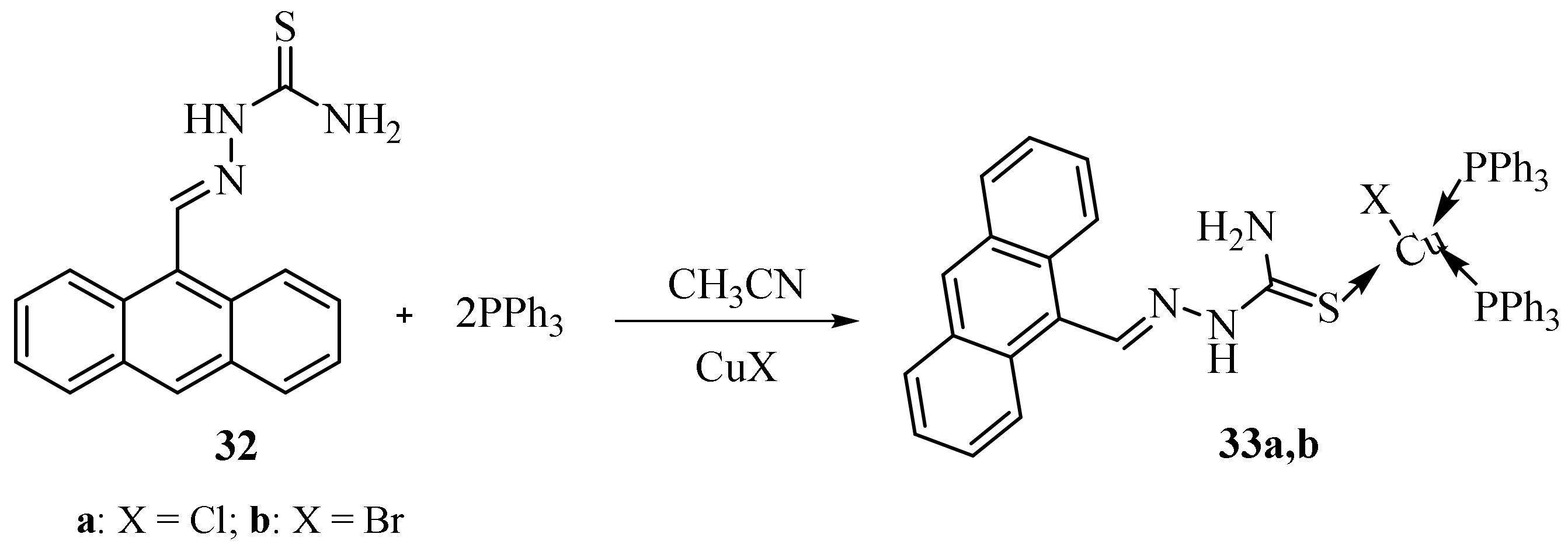
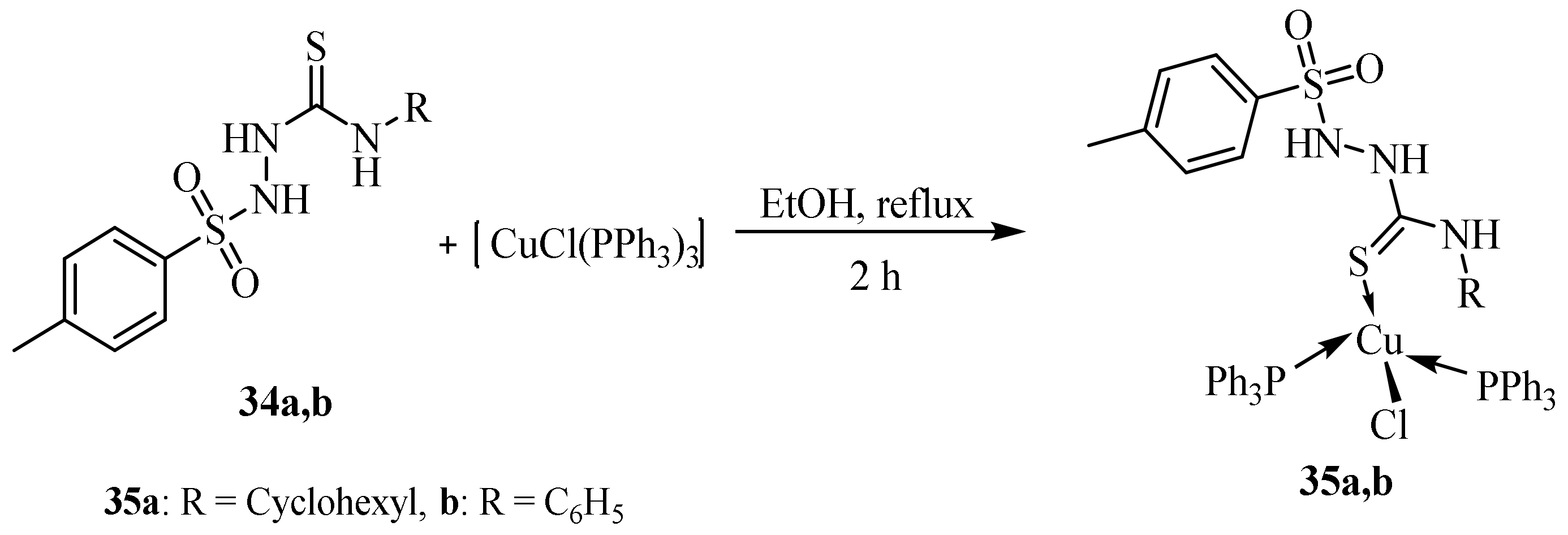
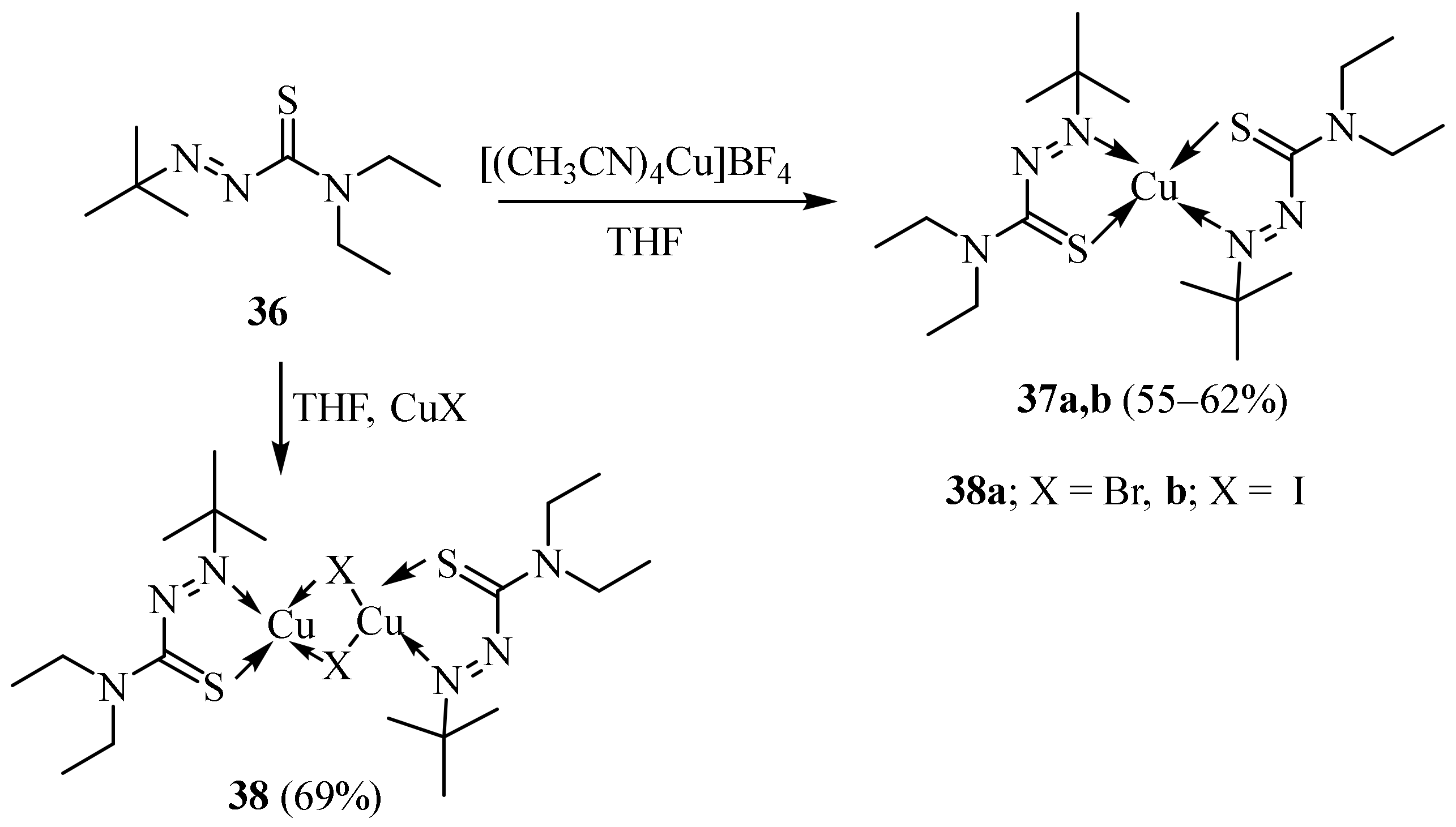
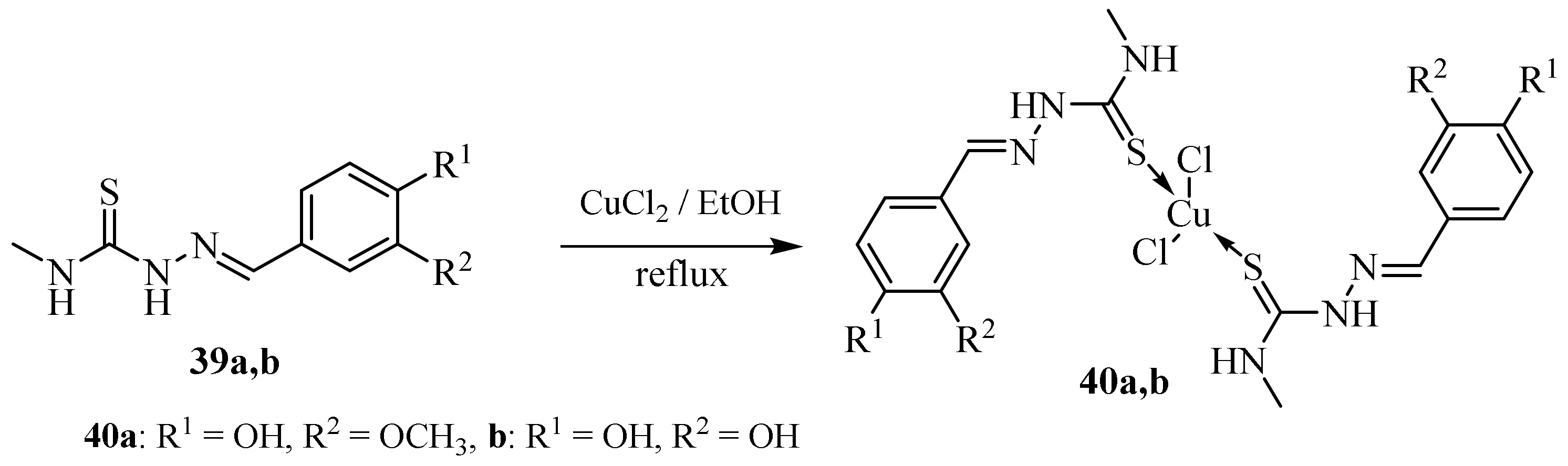
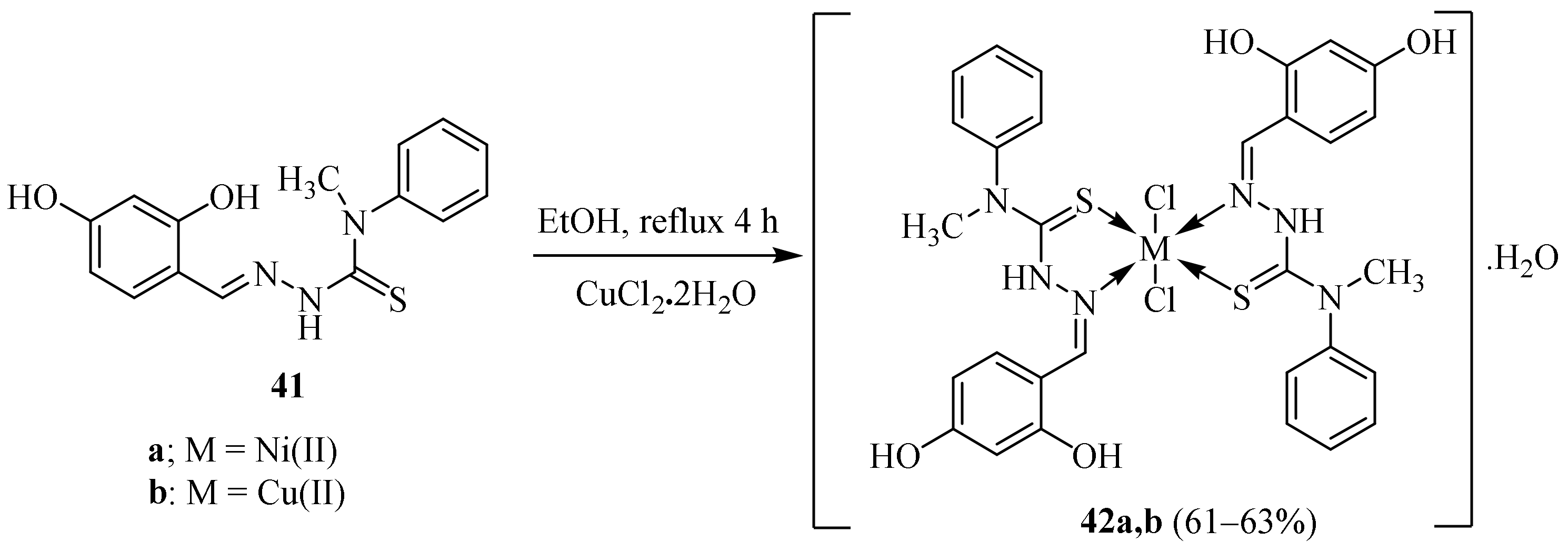
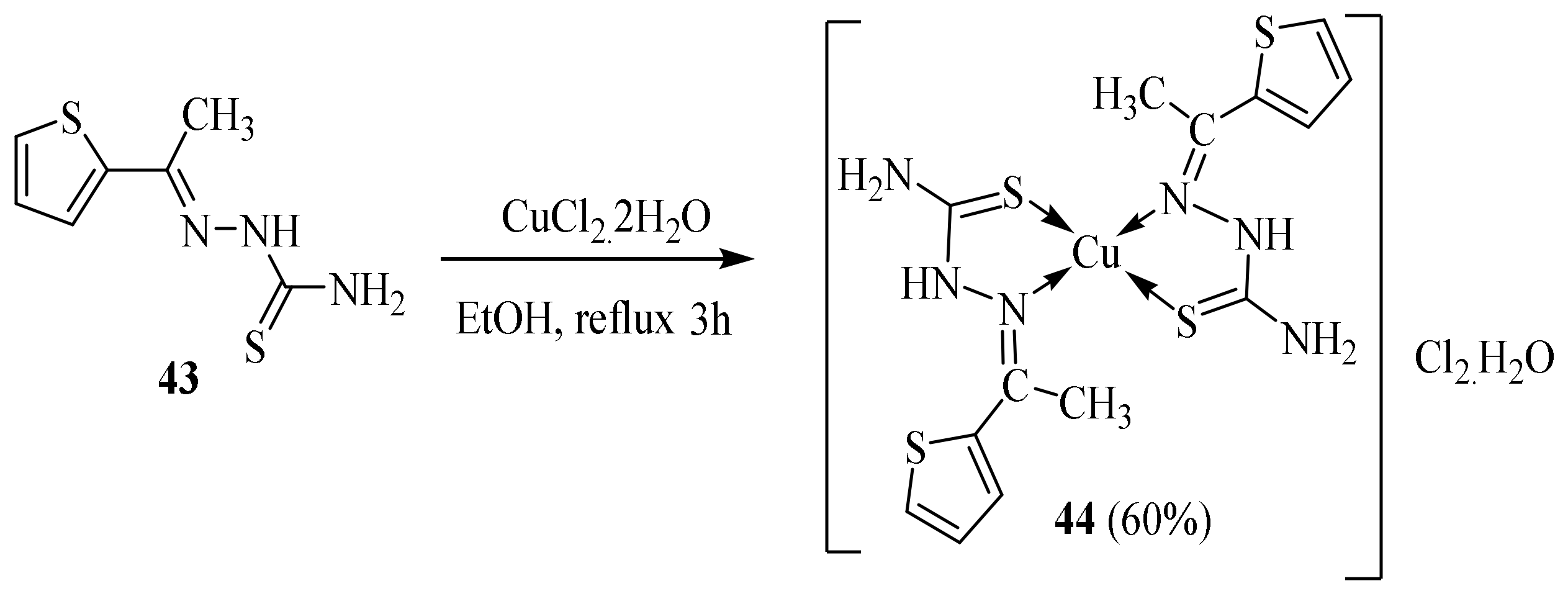
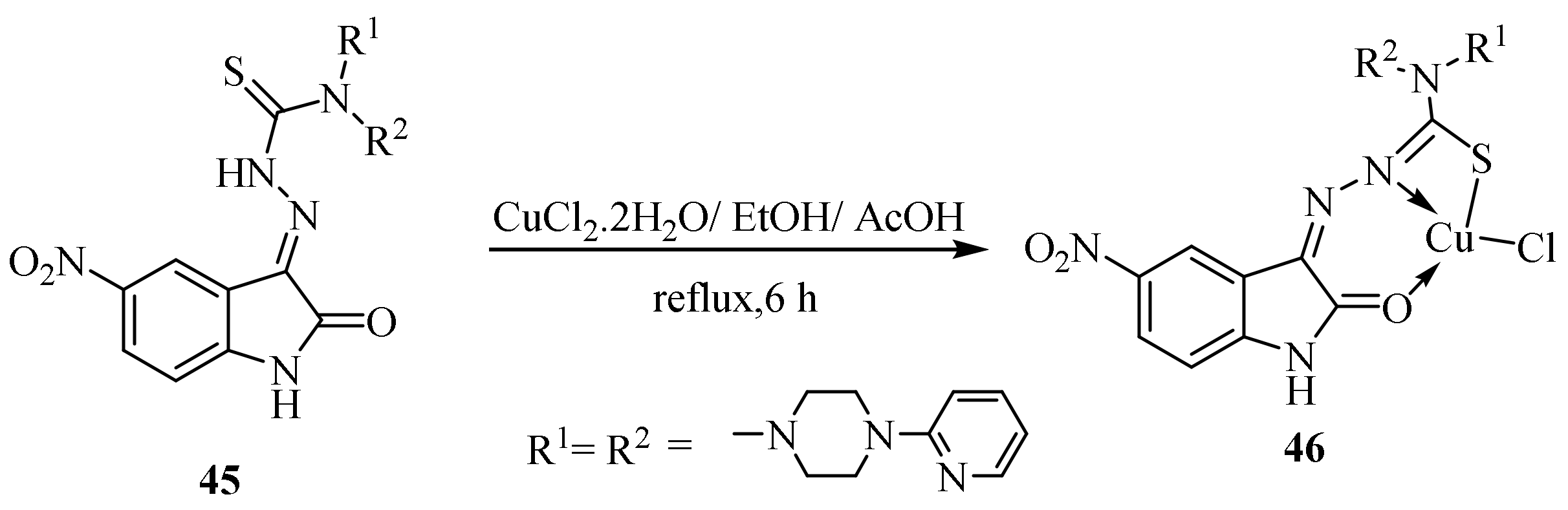
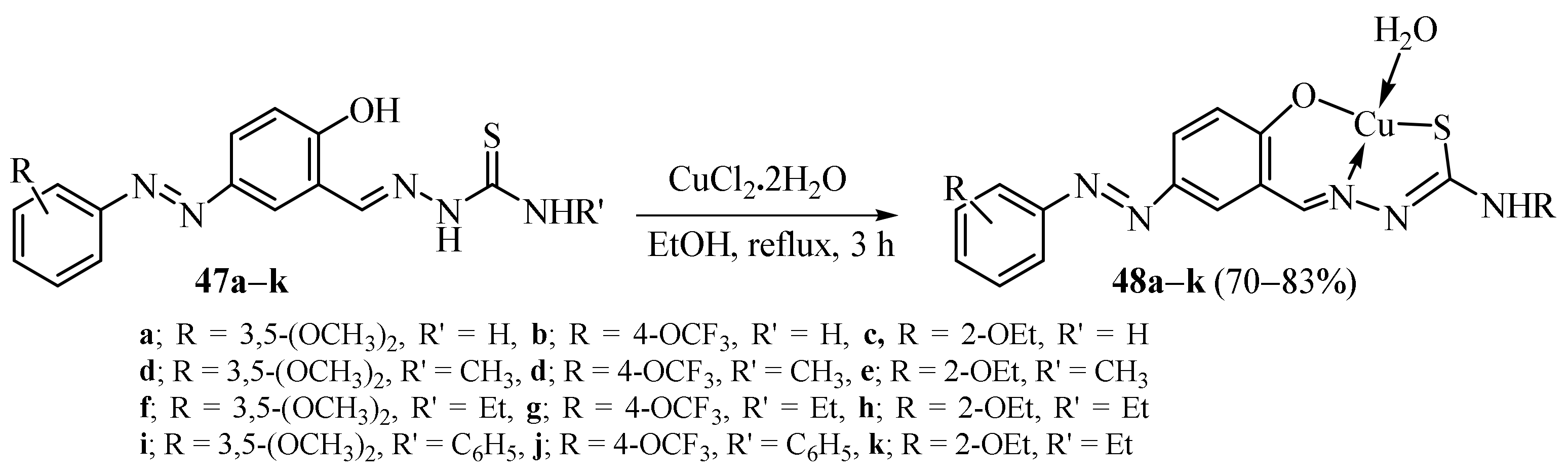
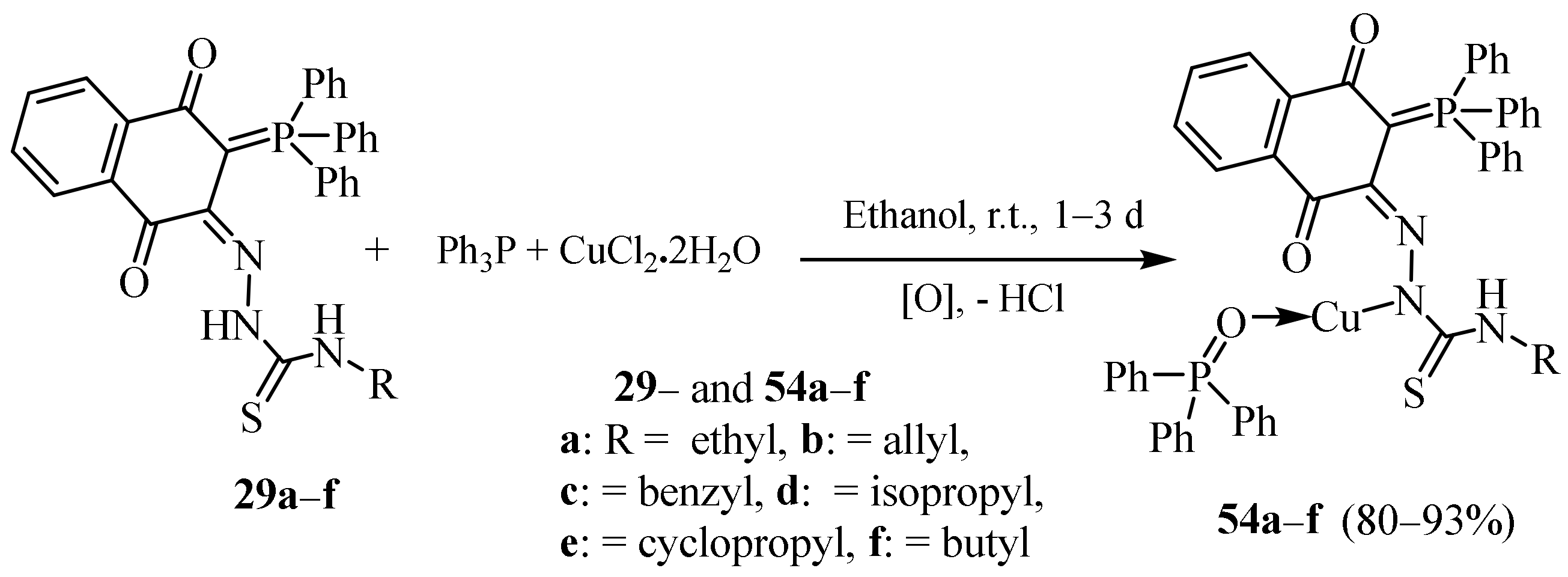






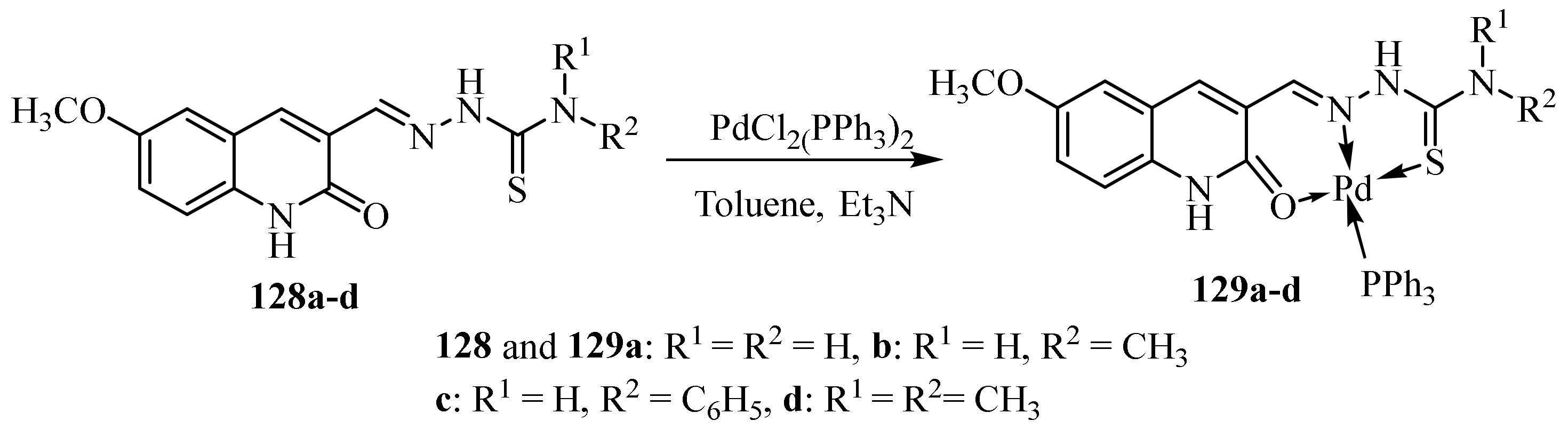


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aly, A.A.; Abdallah, E.M.; Ahmed, S.A.; Rabee, M.M.; Bräse, S. Transition Metal Complexes of Thiosemicarbazides, Thiocarbohydrazides, and Their Corresponding Carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)—A Review. Molecules 2023, 28, 1808. https://doi.org/10.3390/molecules28041808
Aly AA, Abdallah EM, Ahmed SA, Rabee MM, Bräse S. Transition Metal Complexes of Thiosemicarbazides, Thiocarbohydrazides, and Their Corresponding Carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)—A Review. Molecules. 2023; 28(4):1808. https://doi.org/10.3390/molecules28041808
Chicago/Turabian StyleAly, Ashraf A., Elham M. Abdallah, Salwa A. Ahmed, Mai M. Rabee, and Stefan Bräse. 2023. "Transition Metal Complexes of Thiosemicarbazides, Thiocarbohydrazides, and Their Corresponding Carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)—A Review" Molecules 28, no. 4: 1808. https://doi.org/10.3390/molecules28041808
APA StyleAly, A. A., Abdallah, E. M., Ahmed, S. A., Rabee, M. M., & Bräse, S. (2023). Transition Metal Complexes of Thiosemicarbazides, Thiocarbohydrazides, and Their Corresponding Carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)—A Review. Molecules, 28(4), 1808. https://doi.org/10.3390/molecules28041808