Fabrication of Hydroxy-Terminated Polybutadiene with Piezoelectric Property by Functionalized Branch Chain Modification
Abstract
:1. Introduction
2. Results
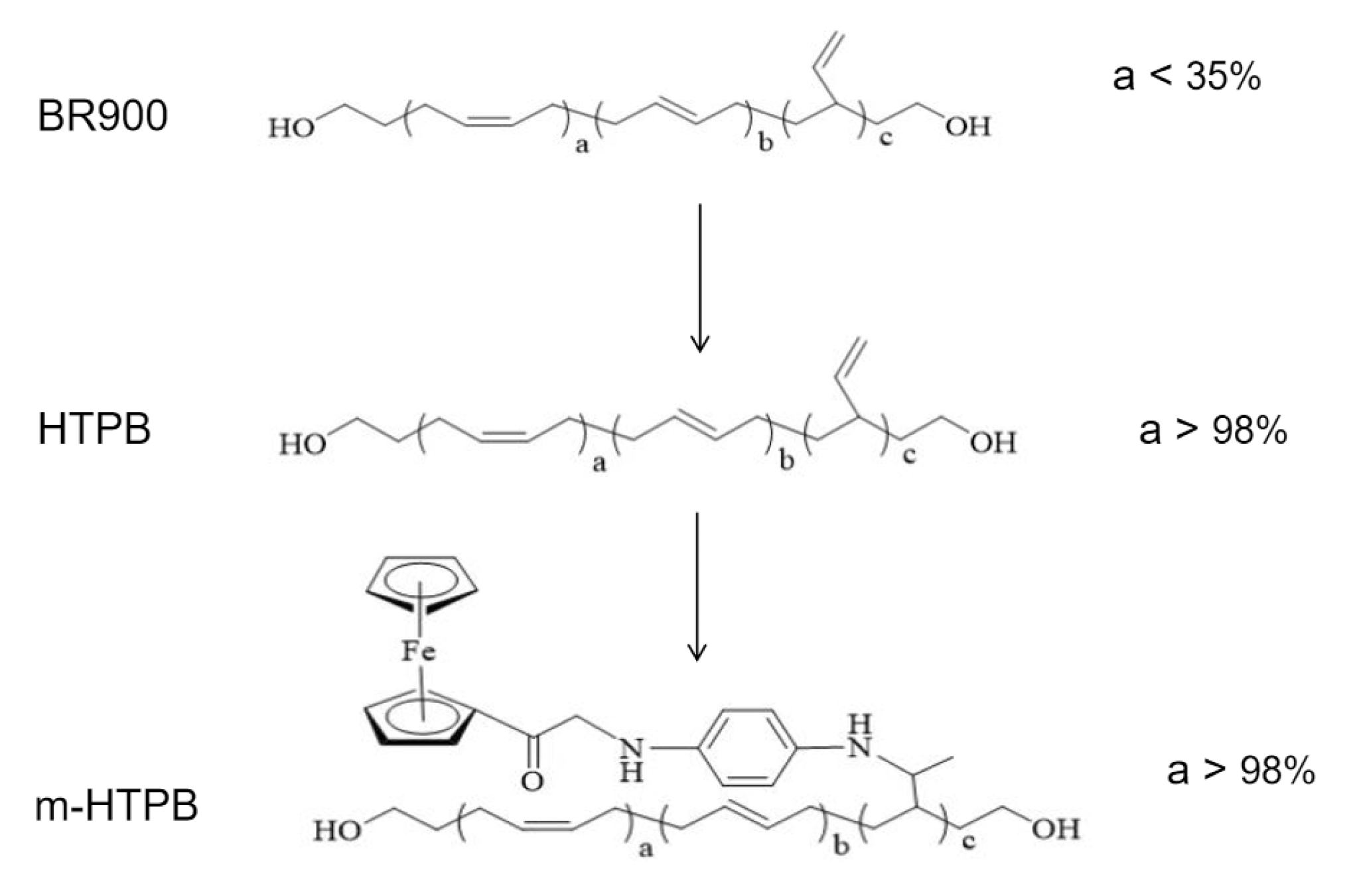
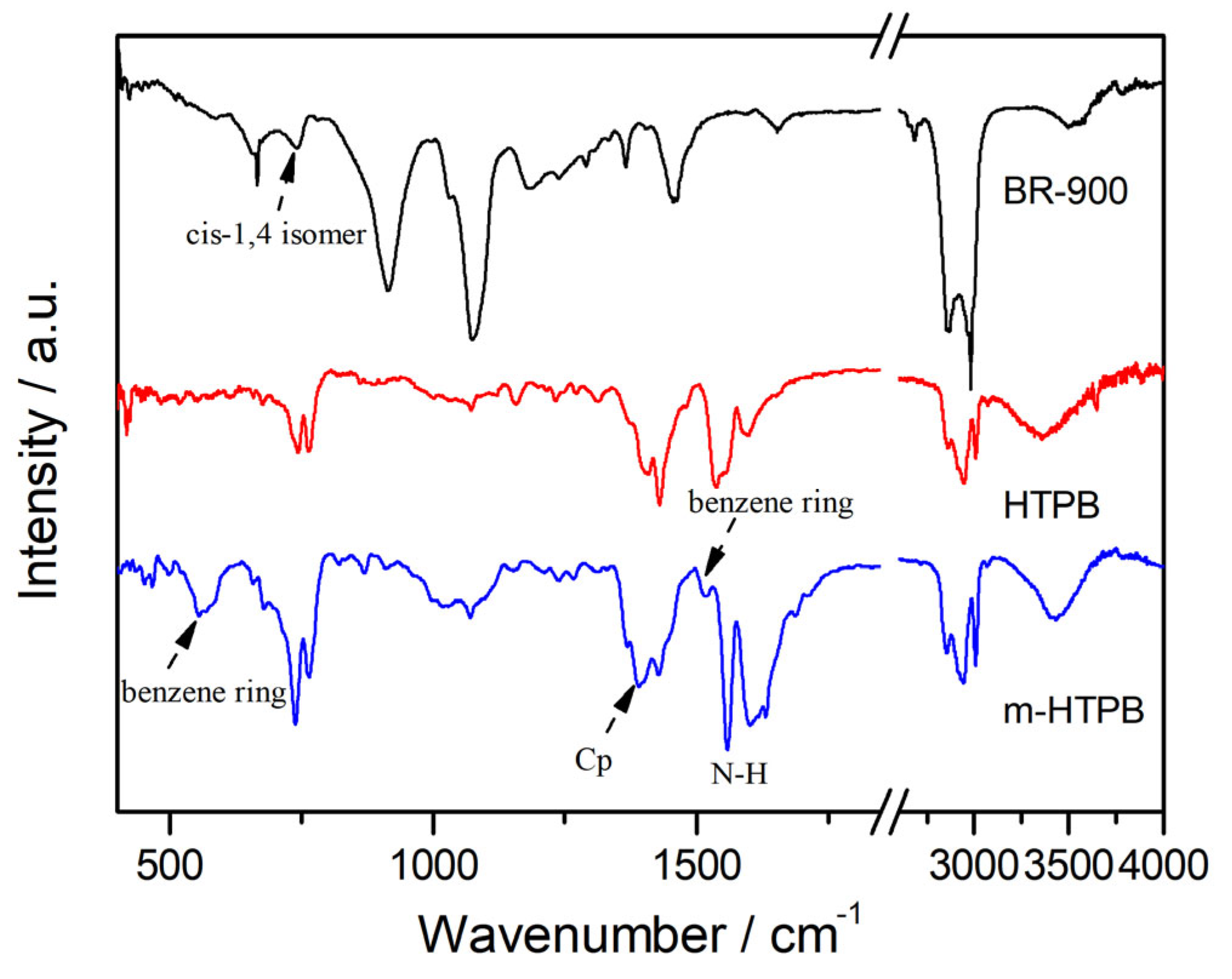
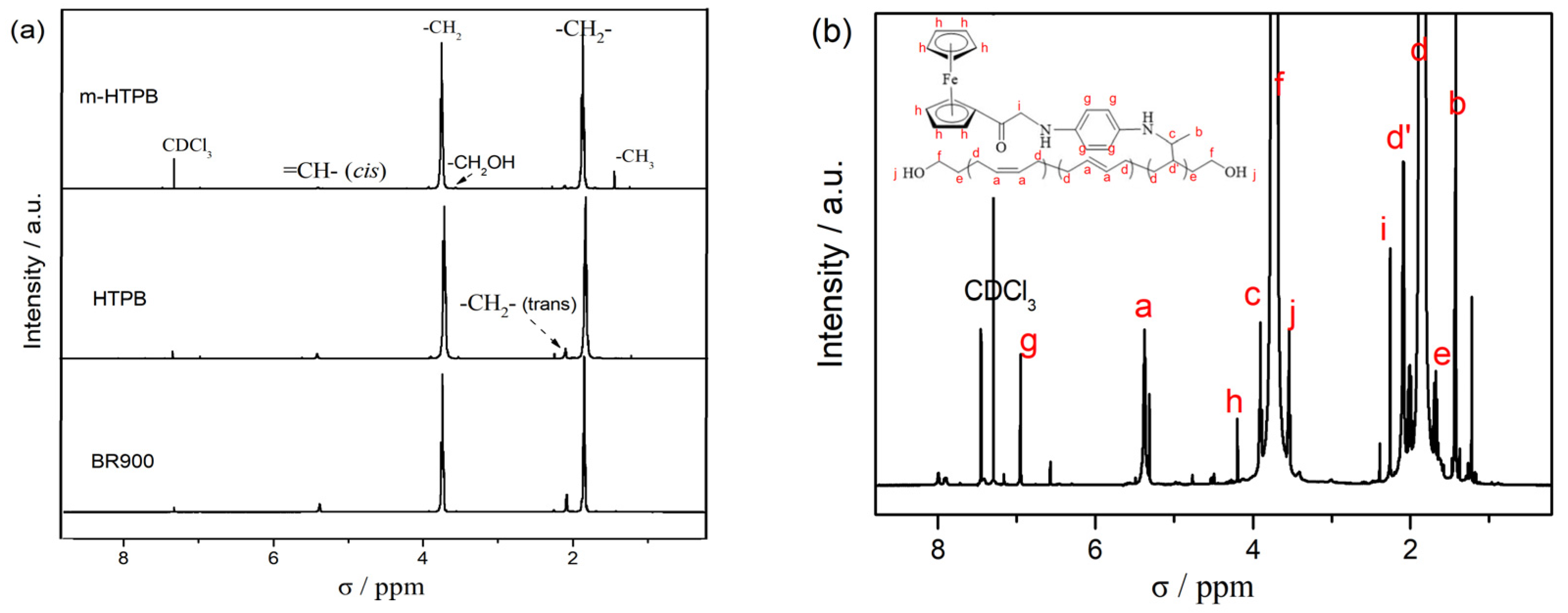
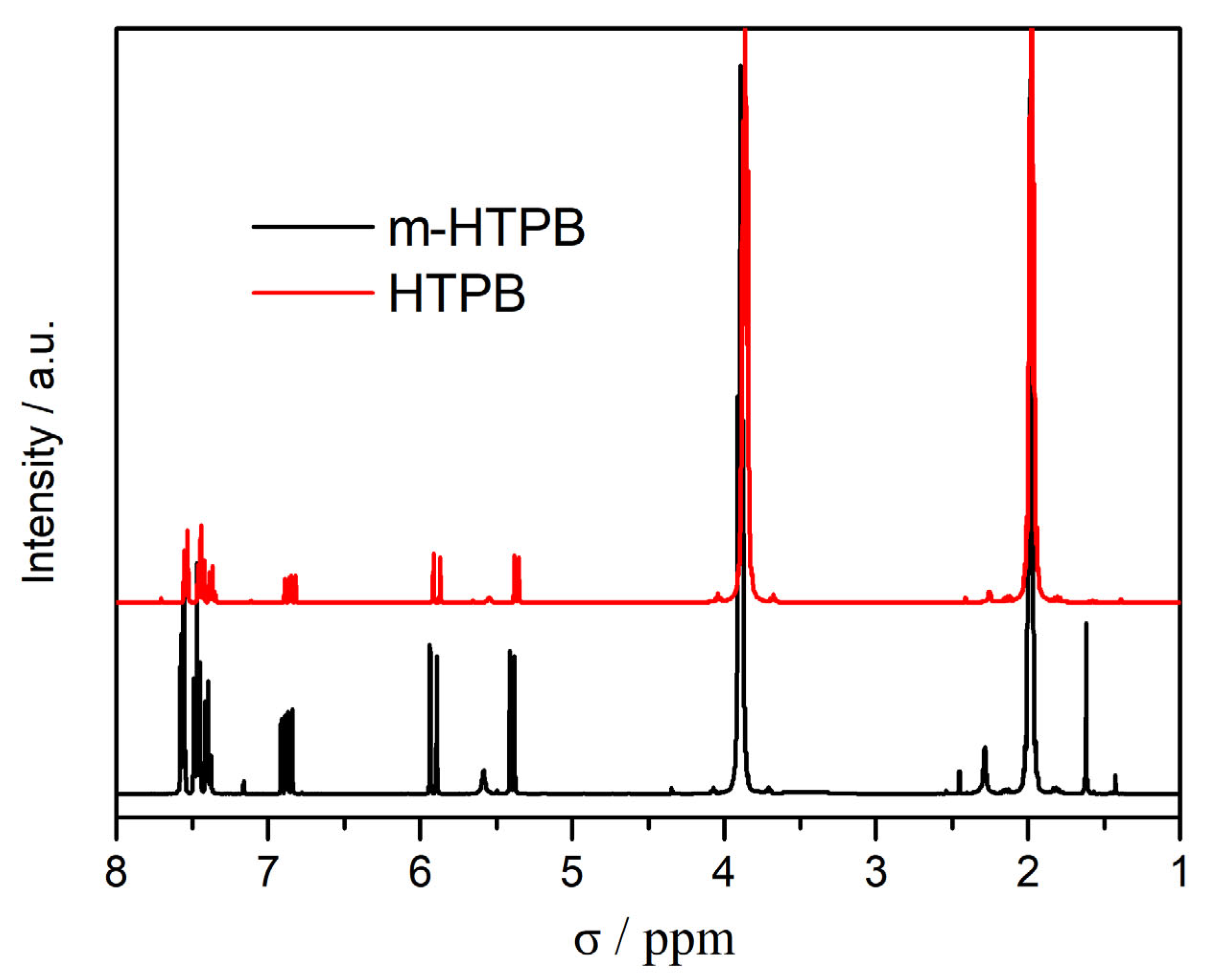
2.1. Characterization of m-HTPB
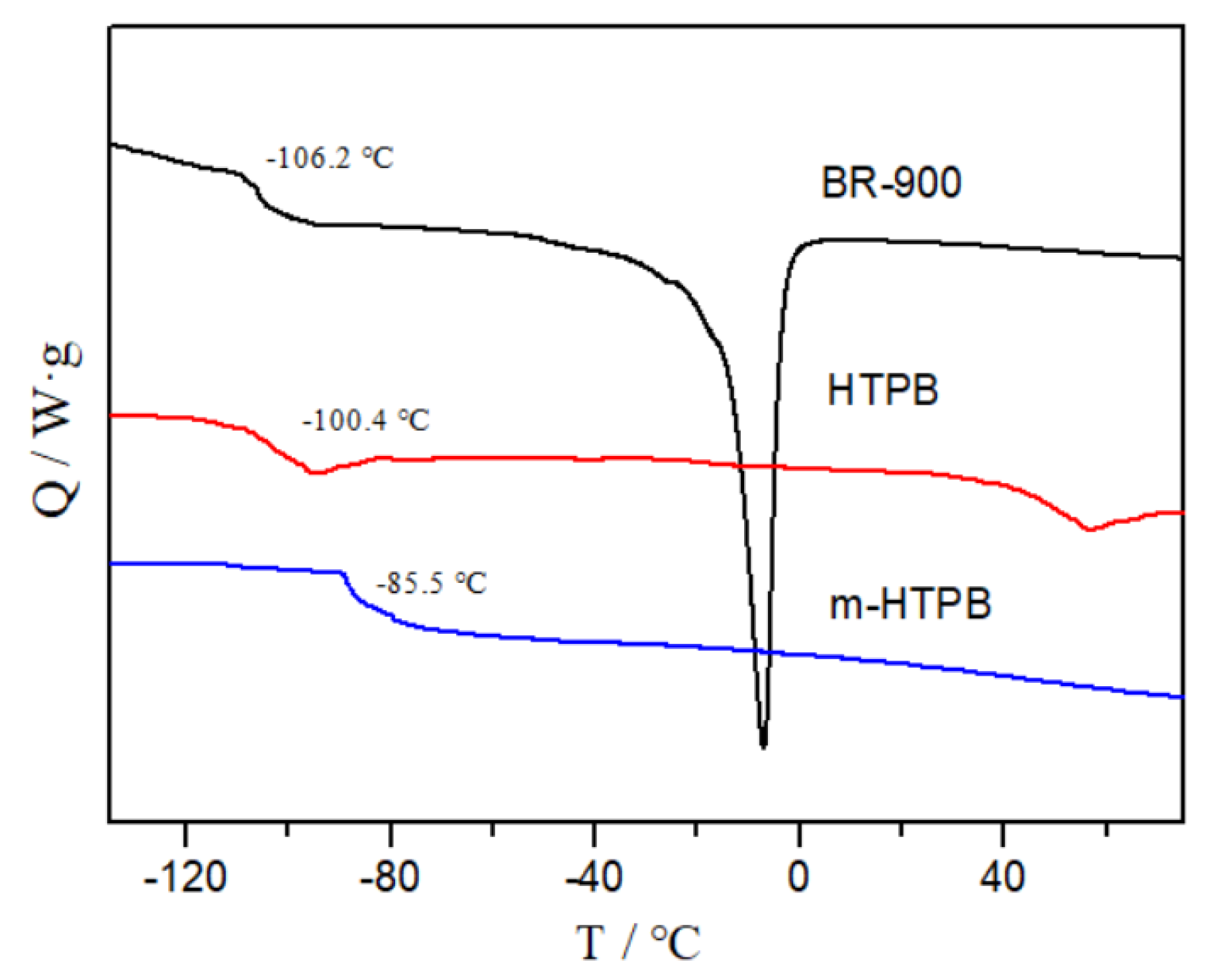
2.2. Thermal and Electrochemical Properties of m-HTPB
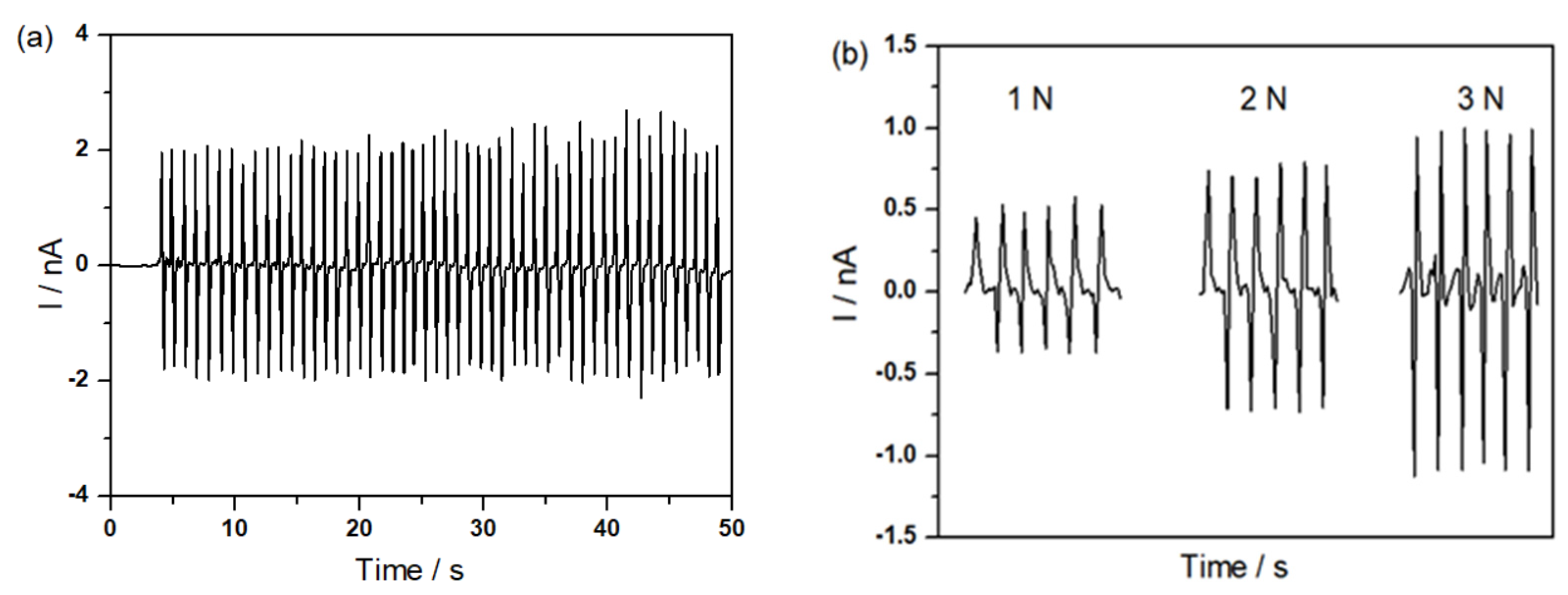
2.3. Piezoelectric Performance of m-HTPB
3. Materials and Methods
3.1. Materials
3.2. Preparation of m-HTPB
3.3. Preparation of m-HTPB-Based Piezoelectric Sensor
3.4. Characterizations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bai, Y.; Liu, Y.; Lv, H.; Shi, H.; Zhou, W.; Liu, Y.; Yu, D.-G. Processes of electrospun polyvinylidene fluoride-based nanofifibers, their piezoelectric properties, and several fantastic applications. Polymers 2022, 14, 4311. [Google Scholar] [CrossRef] [PubMed]
- Nivedhitha, D.; Jeyanthi, S. Polyvinylidene fluoride, an advanced futuristic smart polymer material: A comprehensive review. Polym. Adv. Technol. 2022, 1, 5914–5946. [Google Scholar] [CrossRef]
- Wang, X.; Meng, S.; Tebyetekerwa, M.; Li, Y.; Pionteck, J.; Sun, B.; Qin, Z.; Zhu, M. Highly sensitive and stretchable piezoresistive strain sensor based on conductive poly(styrene-butadiene-styrene)/few layer graphene composite fiber. Compos. A 2017, 105, 291–299. [Google Scholar] [CrossRef]
- Li, R.; Si, Y.; Zhu, Z.; Guo, Y.; Zhang, Y.; Pan, N.; Sun, G.; Pan, T. Supercapacitive iontronic nanofabric sensing. Adv. Mater. 2017, 29, 1700253–1700261. [Google Scholar] [CrossRef]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J. High performance piezoelectric devices based on aligned arrays of nanofifibers of poly (vinylideneflfluoride-co-triflfluoroethylene). Nat. Commun. 2013, 4, 1633–1643. [Google Scholar] [CrossRef]
- Karan, S.K.; Maiti, S.; Lee, J.H.; Mishra, Y.K.; Khatua, B.B.; Kim, J.K. Recent advances in self-powered tribo-/piezoelectric energy harvesters: All-in-one package for future smart technologies. Adv. Funct. Mater. 2020, 30, 2004446. [Google Scholar] [CrossRef]
- Gao, X.; Yang, J.; Wu, J.; Xin, X.; Li, Z.; Yuan, X.; Shen, X.; Dong, S. Piezoelectric actuators and motors: Materials, designs, and applications. Adv. Mater. Technol. 2020, 5, 1900716. [Google Scholar] [CrossRef]
- Jella, V.; Ippili, S.; Eom, J.H.; Pammi, S.V.N.; Jung, J.S.; Tran, V.D.; Nguyen, V.; Kirakosyan, A.; Yun, S.; Kim, D.; et al. A comprehensive review of flexible piezoelectric generators based on organic-inorganic metal halide perovskites. Nano Energy 2019, 57, 74–93. [Google Scholar] [CrossRef]
- Salim, M.; Salim, D.; Chandran, D.; Aljibori, H.S.; Kherbeet, A.S. Review of nano piezoelectric devices in biomedicine applications. Intell. Mater. Syst. Struct. 2018, 29, 2105. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, W.; Liu, J.; Zhao, M.; Li, M.; Xing, J.H. PVDF-based and its copolymer-based piezoelectric composites: Preparation methods and applications. J. Electr. Mater. 2022, 51, 5528–5549. [Google Scholar] [CrossRef]
- De Freitas, R.L.B.; Sakamoto, W.K.; Freitas, L.P.S.; Castro, F.; Antonio, P.; Filho, L.; Kitano, C.; de Carvalho, A.A. Characterization of PZT/PVDF composite fifilm as functional material. IEEE Sens. 2018, 18, 5067–5072. [Google Scholar] [CrossRef]
- Kang, H.B.; Han, C.S.; Pyun, J.C.; Ryu, W.H.; Kang, C.Y.; Cho, Y.S. (Na, K)NbO3 nanoparticle-embedded piezoelectric nanofiber composites for flexible nanogenerators. Compos. Sci. Technol. 2015, 111, 1–8. [Google Scholar] [CrossRef]
- Li, C.; Luo, W.; Liu, X.; Xu, D.; He, K. PMN-PT/PVDF nanocomposite for high output nanogenerator applications. Nanomaterials 2016, 6, 67. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Ribelles, J.L.G.; Lanceros-Mendez, S. Influence of processing conditions on polymorphism and nanofiber morphology of electroactive poly(vinylidene fluoride) electrospun membranes. Soft Mater. 2010, 8, 274–287. [Google Scholar] [CrossRef]
- Luo, Y.; Zhao, L.; Luo, G.; Li, M.; Han, X.; Xia, Y.; Li, Z.; Lin, Q.; Yang, P.; Dai, L.; et al. All electrospun fabrics based piezoelectric tactile sensor. Nanotechnol. 2022, 33, 415502–415513. [Google Scholar] [CrossRef]
- Gao, J.; Hu, M.; Dong, Y.; Li, R.K.Y. Graphite-nanoplatelet-decorated polymer nanofifiber with improved thermal, electrical, and mechanical properties. ACS Appl. Mater. Interf. 2013, 5, 7758–7764. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Huang, X.; Hu, M.; Xue, H.; Li, R.K.Y. Electrically conductive polymer nanofifiber composite with an ultralow percolation threshold for chemical vapour sensing. Compos. Sci. Technol 2018, 161, 135–142. [Google Scholar] [CrossRef]
- Raza, J.; Hami, A.; Khan, M.; Hussain, F.; Li, T.; Fazil, P.; Zada, A.; Wahab, Z.; Ali, A. Spectroscopic characterization of biosynthesized lead oxide (PbO) nanoparticles and their applications in PVC/graphite-PbO nanocomposites. Z. Phys. Chem. 2022, 236, 619–636. [Google Scholar] [CrossRef]
- Chen, K.; Ren, Q.; Li, J.; Chen, D.; Li, C. A highly stretchable and self-healing hydroxyterminated polybutadiene elastomer. J. Saudi Chem. Soci. 2020, 24, 1034–1041. [Google Scholar] [CrossRef]
- Chang, K.; Jia, H.; Gu, S.-Y. A transparent, highly stretchable, self-healing polyurethane based on disulfide bonds. Eur. Polym. 2019, 112, 822–831. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Yu, H.; Guan, X.; Deluca, L.T.; Zhang, W.; Shen, R.; Ye, Y. Combustion enhancement of hydroxylterminated polybutadiene by doping multiwall carbon nanotubes. Carbon 2019, 144, 472–480. [Google Scholar] [CrossRef]
- Malkappa, K.; Rao, B.N.; Suresh, G.; Ramana, C.V.; Jana, T. Organic/inorganic hybrid nanocolloids of water dispersible polyurethanes with antibacterial activity. Colloid Polym. Sci. 2018, 296, 95–106. [Google Scholar] [CrossRef]
- Dhabbe, K.I.; Nandagopal, S.; Maurya, M.; Bhattacharya, B. Quantitative estimation of purity of N-phenyl-β-naphthylamine (Nonox-D) by electrophilic bromination method. Analy. Chem. Indian J. 2008, 7, 435–438. [Google Scholar]
- Chen, S.-H.; Yu, K.-C.; Houng, S.-L.; Lai, J.-Y.; Membr, J. Gas transport properties of HTPB based polyurethane/cosalen membrane. J. Membr. Sci. 2000, 173, 99. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, J.; Hu, C.; Yang, B.; Ning, Z. Sustainable polyurethane networks with high self-healing and mechanical properties based on dual dynamic covalent bonds. Macromol. Chem. Phys. 2022, 224, 2200322–2200336. [Google Scholar] [CrossRef]
- Burelo, M.; Martínez, A.; Cruz-Morales, J.A.; Tlenkopatchev, M.A.; Gutierrez, S. Metathesis reaction from bio-based resources: Synthesis of diols and macrodiols using fatty alcohols, b-citronellol and natural rubber. Polym. Degrad. Stabil. 2019, 166, 202–212. [Google Scholar] [CrossRef]
- Zhou, Q.; Jie, S.; Li, B. Facile synthesis of novel HTPBs and EHTPBs with high cis-1,4 content and extremely low glass transition temperature. Polymer 2015, 67, 208–215. [Google Scholar] [CrossRef]
- Zhou, Q.; Jie, S.; Li, B. Preparation of hydroxyl-terminated polybutadiene with high cis-1,4 content. Ind. Eng. Chem. Res. 2014, 53, 17884–17893. [Google Scholar] [CrossRef]
- Moon, J.M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosen. Bioelectr. 2018, 102, 540–552. [Google Scholar] [CrossRef]
- Hamid, A.; Khan, M.; Hayat, A.; Raza, J.; Zada, A.; Ullah, A.; Raziq, F.; Li, T.; Hussain, F. Probing the physio-chemical appraisal of green synthesized PbO nanoparticles in PbO-PVC nanocomposite polymer membranes. Spectrochim. Acta Mol. Biomol. Spectrosc. 2020, 235, 118303–118310. [Google Scholar] [CrossRef]
- Hamid, A.; Khan, M.; Hussain, F.; Zada, A.; Li, T.; Alei, D.; Ali, A. Synthesis and physiochemical performances of PVC-sodium polyacrylate and PVC-sodium polyacrylate-graphite composite polymer membrane. Z. Phys. Chem. 2021, 235, 1791–1810. [Google Scholar] [CrossRef]
- Zhan, C.; Yu, G.; Lu, Y.; Wang, L.; Wujcik, E.; Wei, S. Conductive polymer nanocomposites: A critical review of modern advanced devices. J. Mater. Chem. C 2017, 5, 1569–1585. [Google Scholar] [CrossRef]
- Barcia, F.; Abraha, M.; Soares, B. Modification of epoxy resin by isocyanate-terminated polybutadiene. J. Appl. Polym. Sci. 2002, 83, 838–849. [Google Scholar] [CrossRef]
- Cherian, A.B.; Thachil, E.T. Block copolymers of unsaturated polyesters and functional elastomers. J. Appl. Polym. Sci. 2004, 94, 1956–1964. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, B.; Wang, G.; Tian, W.; Zhou, L.; Li, C. Fabrication of Hydroxy-Terminated Polybutadiene with Piezoelectric Property by Functionalized Branch Chain Modification. Molecules 2023, 28, 1810. https://doi.org/10.3390/molecules28041810
Yuan B, Wang G, Tian W, Zhou L, Li C. Fabrication of Hydroxy-Terminated Polybutadiene with Piezoelectric Property by Functionalized Branch Chain Modification. Molecules. 2023; 28(4):1810. https://doi.org/10.3390/molecules28041810
Chicago/Turabian StyleYuan, Bo, Guang Wang, Wenxue Tian, Li Zhou, and Chunxiang Li. 2023. "Fabrication of Hydroxy-Terminated Polybutadiene with Piezoelectric Property by Functionalized Branch Chain Modification" Molecules 28, no. 4: 1810. https://doi.org/10.3390/molecules28041810
APA StyleYuan, B., Wang, G., Tian, W., Zhou, L., & Li, C. (2023). Fabrication of Hydroxy-Terminated Polybutadiene with Piezoelectric Property by Functionalized Branch Chain Modification. Molecules, 28(4), 1810. https://doi.org/10.3390/molecules28041810




