Quantification of Methylisothiazolinone and Methylchloroisothiazolinone Preservatives by High-Performance Liquid Chromatography
Abstract
1. Introduction
2. Results and Discussion
2.1. Results on Method Validation
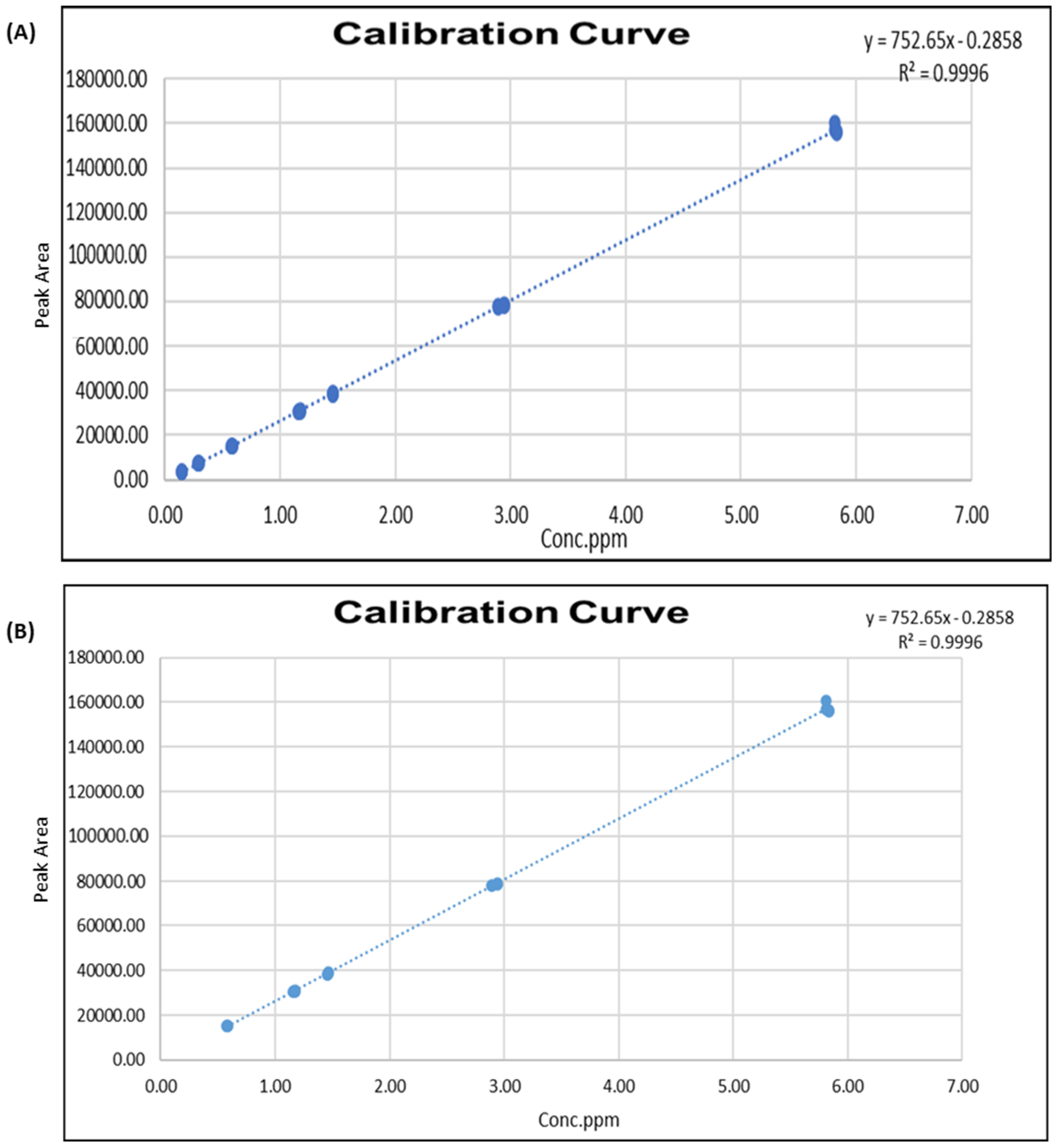
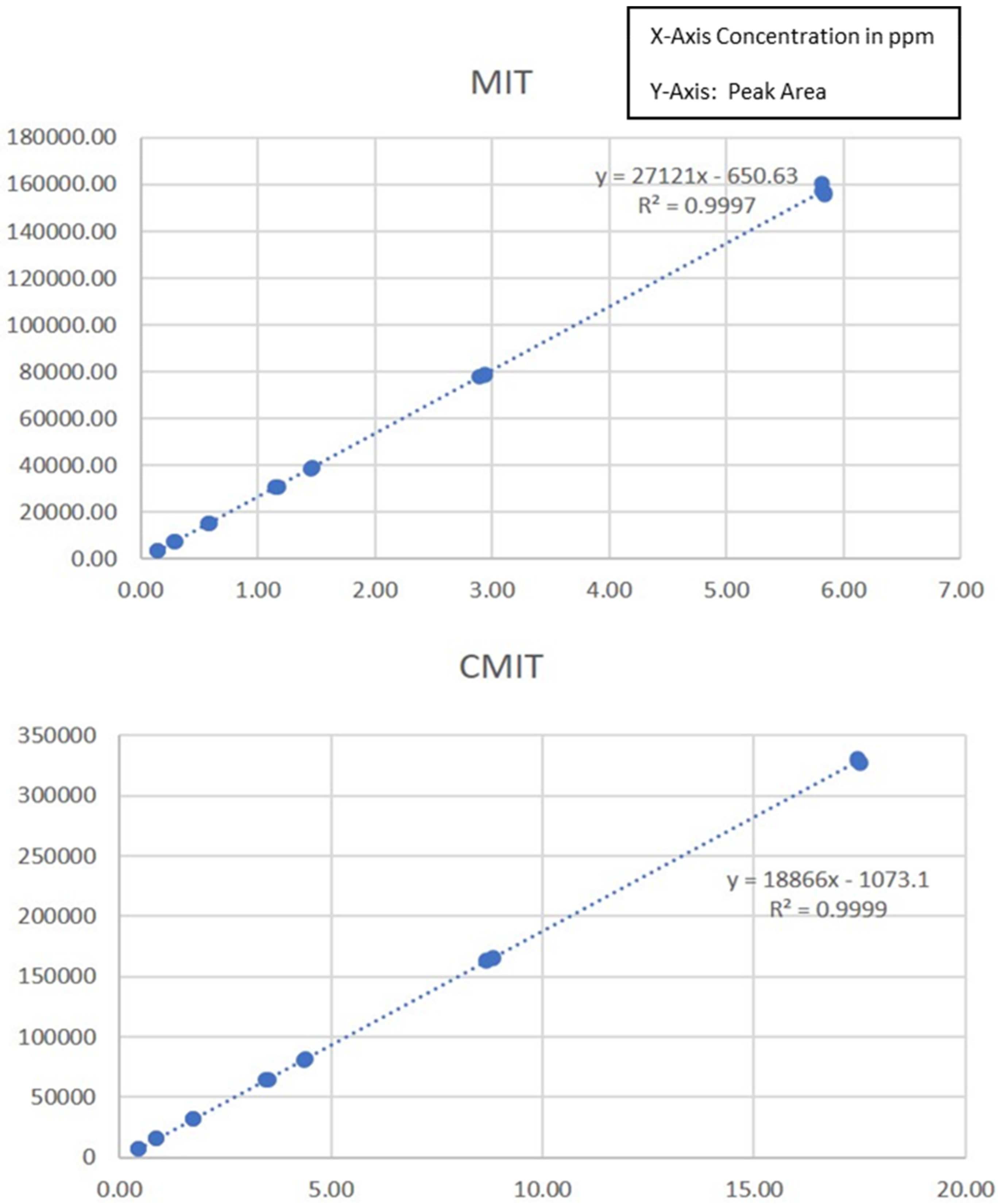
2.2. Linearity
2.3. Precision
3. Methodology
3.1. Materials
3.2. Methods
3.2.1. Sample Preparation
3.2.2. Optimization
Diluent Variations
Extraction Techniques
High-Performance Liquid Chromatography
- (i)
- methanol and water
- (ii)
- 0.01 M sodium phosphate buffer and acetonitrile
- (iii)
- water and acetonitrile
- (iv)
- methanol, acetonitrile, and 2% acetic acid
3.2.3. Detection by High-Performance Liquid Chromatography
3.2.4. Method Validation
Linearity
Precision
3.2.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cosmetica Italia. Consumption value of cosmetics and personal care in Europe from 2012 to 2019. In Rapporto Annuale; Cosmetics Europe, Cosmetica Italia: Milano, Italy, 2020; p. 26. [Google Scholar]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [PubMed]
- Strilets, O.P.; Petrovska, L.S.; Baranova, I.I.; Bespala, Y.O. A study of antimicrobial activity of foam-washing agent specimens at acidic pH values. Ann. Mechnikov Institude 2017, 2, 23–26. [Google Scholar]
- Yim, E.; Nole, K.L.B.; Tosti, A. Contact dermatitis caused by preservatives. Dermatitis 2014, 25, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.W. Parabens, oestrogenicity, underarm cosmetics and breast cancer: A perspective on a hypothesis. J. Appl. Toxicol. 2003, 23, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Sanders, F.T. Reregistration Eligibility Decision for 1,2-Benzisothiazolin-3-one (BIT); United States Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- Sanders, F.T. Reregistration Eligibility Decision for 2-octyl-3 (2H)-isothiazolone (OIT); United States Environmental Protection Agency: Washington, DC, USA, 2007. [Google Scholar]
- Aerts, O.; Meert, H.; Goossens, A.; Janssens, S.; Lambert, J.; Apers, S. Methylisothiazolinone in selected consumer products in Belgium: Adding fuel to the fire? Contact Dermat. 2015, 73, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Nieto, M.A.; Alcántara-Nicolás, F.; Melgar-Molero, V.; Pérez-Mesonero, R.; Vergara-Sánchez, A.; Martín-Fuentes, A.; González-Muñoz, P.; de Eusebio-Murillo, E. Conservantes en productos de higiene y cosméticos, medicamentos tópicos y productos de limpieza doméstica en España. Actas Dermosifiliogr. 2017, 108, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Horev, L.; Isaksson, M.; Engfeldt, M.; Persson, L.; Ingber, A.; Bruze, M. Preservatives in cosmetics in the Israeli market conform well to the EU legislation. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, M.; Persson, L. Occupational contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone through exposure to filler dust containing this preservative and with a positive patch test reaction to the dust. Contact Dermat. 2015, 1, 119–120. [Google Scholar] [CrossRef] [PubMed]
- El-Houri, R.B.; Christensen, L.P.; Persson, C.; Bruze, M.; Andersen, K.E. Methylisothiazolinone in a designer spectacle frame—A surprising finding. Contact Dermat. 2016, 75, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Aerts, O. Contact allergy caused by methylisothiazolinone and related isothiazolinones. Eur. J. Dermatol. 2017, 27, 115–122. [Google Scholar] [PubMed]
- Du, S.; McLaughlin, B.; Pal, S.; Aizenman, E. In vitro neurotoxicity of methylisothiazolinone, a commonly used industrial and household biocide, proceeds via a zinc and extracellular signal-regulated kinase mitogen-activated protein kinase-dependent pathway. J. Neurosci. 2022, 22, 7408–7416. [Google Scholar] [CrossRef] [PubMed]
- Spawn, A.; Aizenman, C.D. Abnormal visual processing and increased seizure susceptibility result from developmental exposure to the biocide methylisothiazolinone. Neuroscience 2012, 205, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Castanedo-Tardana, M.P.; Zug, K.A. Methylisothiazolinone. Dermatitis 2013, 24, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Wollseifen, H.R.; Kretschmer, T.; Riering, H. Analysis of Isothiazolinone Biocides in Cosmetic Products and Detergents by HPLC. 2012, p. 52355. Available online: https://atamankimya.com/sayfalaralfabe.asp?LanguageID=2&cid=3&id=2868&id2=727 (accessed on 10 September 2022).
- Baranowska, I.; Wojciechowska, I.; Solarz, N.; Krutysza, E. Determination of Preservatives in Cosmetics, Cleaning Agents and Pharmaceuticals Using Fast Liquid Chromatography. J. Chromatogr. Sci. 2014, 52, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Rosero-Moreano, M.; Canellas, E.; Nerín, C. Three-phase hollow-fiber liquid-phase microextraction combined with HPLC-UV for the determination of isothiazolinone biocides in adhesives used for food packaging materials. J. Sep. Sci. 2014, 37, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Dagnac, T.; Lores, M.; Garcia-Jares, C.; Sanchez-Prado, L.; Lamas, J.P.; Llompart, M. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography—Tandem mass spectrometry. J. Chromatogr. A 2012, 1270, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, J.B.; Canas, B.J.; Zhou, W.; Wang, P.G.; Krynitsky, A.J. Determination of methylisothiazolinone and methylchloroisothiazolinone in cosmetic products by ultra-high performance liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2015, 38, 2983–2988. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, I.; Wojciechowska, I. The Determination of Preservatives in Cosmetics and Environmental Waters by HPLC. Pol. J. Environ. Stud. 2013, 22, 1609–1625. [Google Scholar]
- Thi Huong Hoa, L.; Tran Ngoc Hung, V.; Thu Trang, D.; Nguyen Hung Thu, T.; Le, D.C. Development and Validation of an HPLC Method for Simultaneous Assay of MCI and MI in Shampoos Containing Plant Extracts. Int. J. Anal. Chem. 2019, 2019, 1851796. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, K.; Solomon, R.J. Organochlorine pesticides BHC and DDE in human blood in and around Madurai, India. Indian J. Clin. Biochem. 2006, 21, 169–172. [Google Scholar] [CrossRef] [PubMed]
- European Commission. COMMISSION REGULATION (EU) No 1003/2014. pp. 1–4. Available online: https://www.legislation.gov.uk/eur/2014/1003/2014-09-18 (accessed on 10 September 2022).
- European Commission. COMMISSION REGULATION (EU) 2016/1198. pp. 22–24. Available online: https://www.legislation.gov.uk/eur/2016/1198/contents (accessed on 10 September 2022).
- European Commission. COMMISSION REGULATION (EU) 2017/1224 of 6 July 2017 Amending Annex V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products. In Off. J. Eur. Union; 2017; L 174, pp. 16–18. Available online: https://eur-lex.europa.eu/eli/reg/2017/1224/oj (accessed on 10 September 2022).
- AOAC International. AOAC Official Method of Analysis. In Appendix F: Guidelines for Standard Method Performance Requirements; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
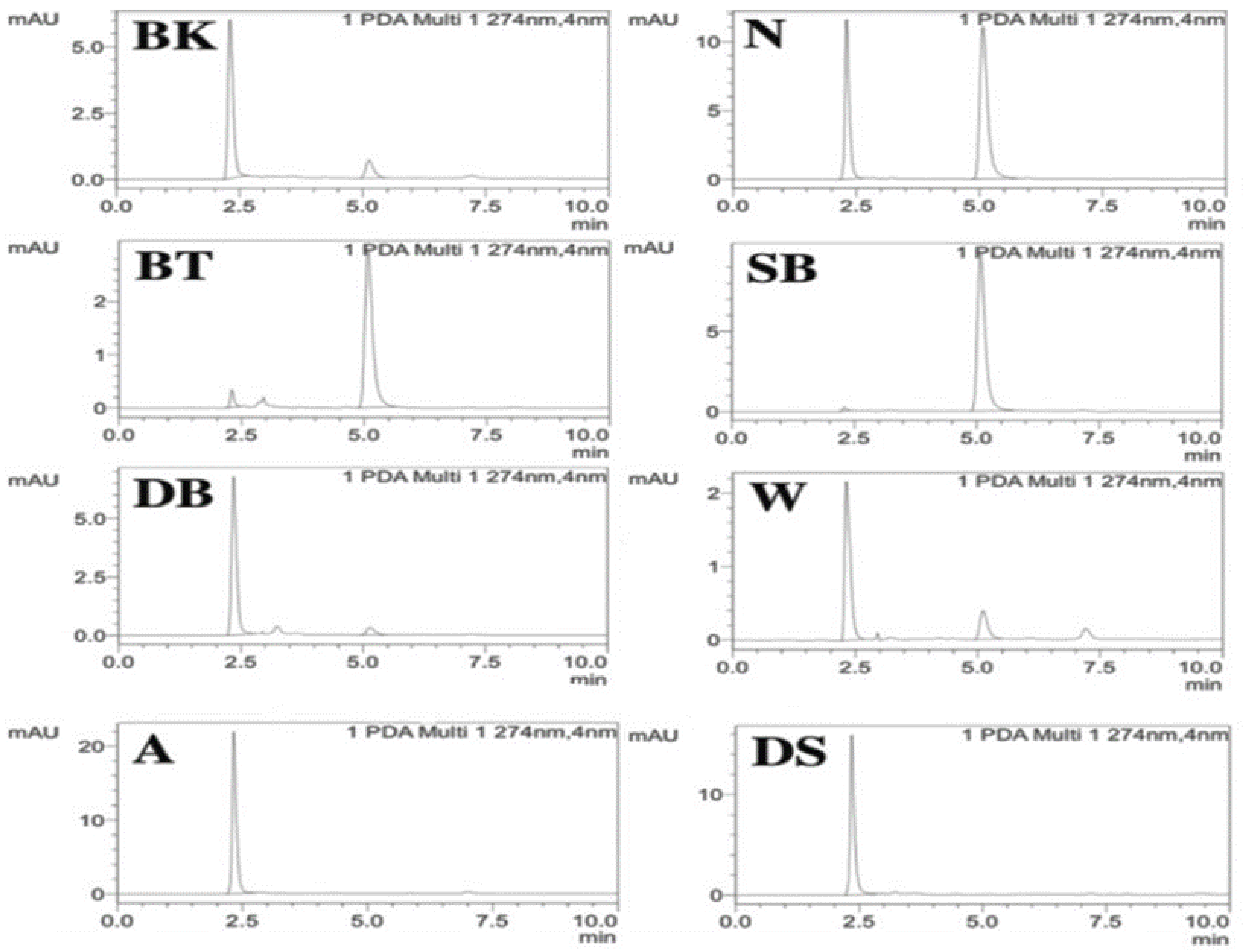
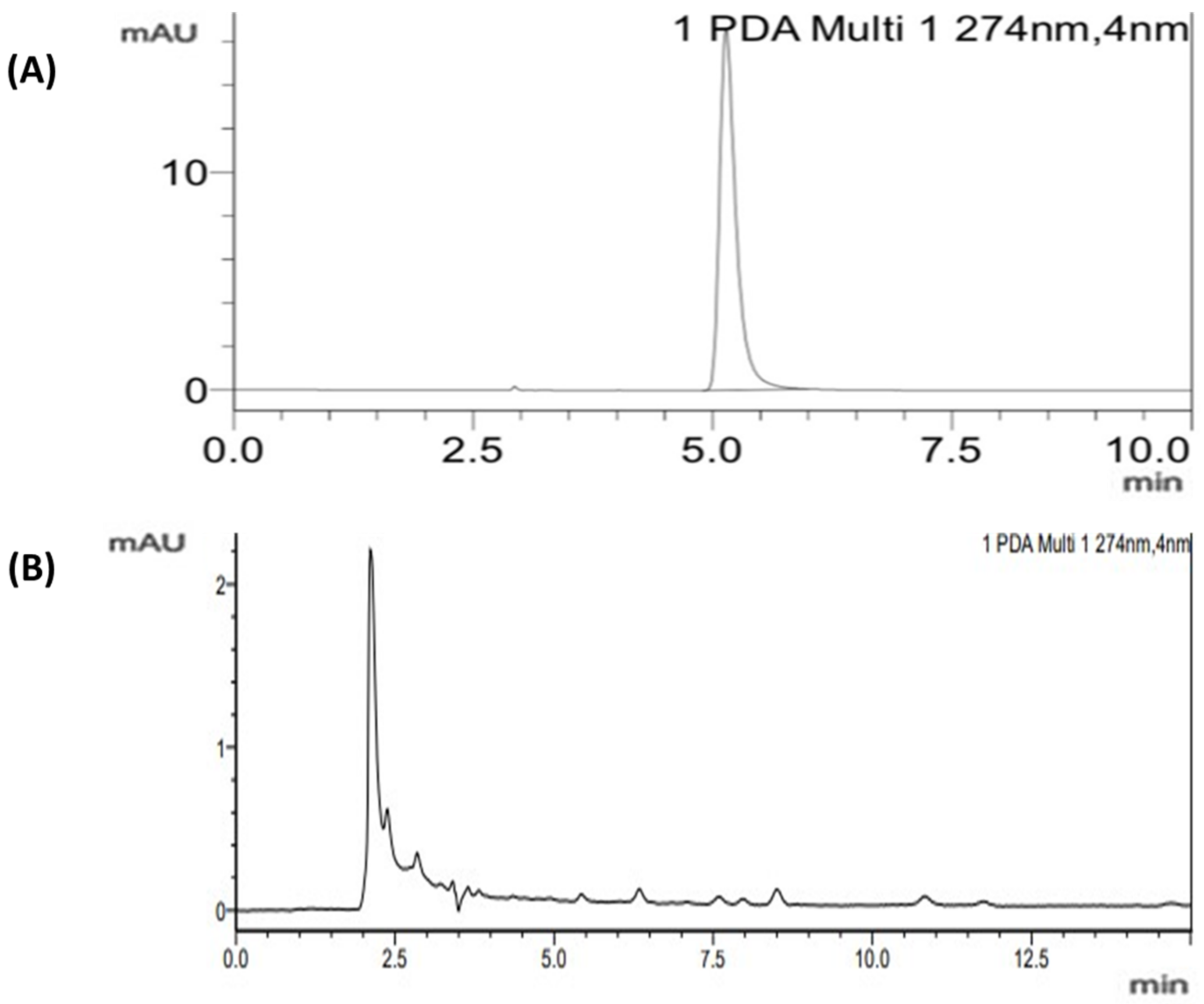
| S.No | Samples | Concentration (ppm) | Mean | Population SD | Standard Error | Sample SD |
|---|---|---|---|---|---|---|
| 1 | A | 0.2147 | 0.2146 | 0.0004249 | 0.0002345 | ±0.0005 |
| 0.2140 | ||||||
| 0.2150 | ||||||
| 2 | BK | 0.2240 | 0.2231 | 0.0006482 | 0.0003742 | ±0.0007 |
| 0.2226 | ||||||
| 0.2227 | ||||||
| 3 | BT | - | - | - | - | - |
| 4 | DB | 0.1241 | 0.1247 | 0.0004063 | 0.0002345 | ±0.0005 |
| 0.1250 | ||||||
| 0.1249 | ||||||
| 5 | DS | 0.1295 | 0.1294 | 0.0003699 | 0.00125753 | ±0.0004 |
| 0.1299 | ||||||
| 0.1289 | ||||||
| 6 | N | 2.9343 | 2.9090 | 0.0411234 | 0.0234726 | ±0.05 |
| 2.9418 | ||||||
| 2.8510 | ||||||
| 7 | SB | - | - | - | - | - |
| 8 | W | 0.1440 | 0.1495 | 0.0039189 | 0.0022626 | ±0.004 |
| 0.1521 | ||||||
| 0.1525 |
| S.No | Samples | Concentration (ppm) | Mean | Population SD | Standard Error | Sample SD |
|---|---|---|---|---|---|---|
| 1 | A | - | - | - | - | - |
| 2 | BK | |||||
| 3 | BT | 0.8275 | 0.8295 | 0.0021648 | 0.0012498 | ±0.002 |
| 0.8285 | ||||||
| 0.8325 | ||||||
| 4 | DB | - | - | - | - | - |
| 5 | DS | - | - | - | - | - |
| 6 | N | 3.1622 | 3.1652 | 0.0023686 | 0.0016298 | ±0.0021 |
| 3.1680 | ||||||
| 3.1652 | ||||||
| 7 | SB | 2.6561 | 2.8114 | 0.1710827 | 0.0987746 | ±0.2 |
| 3.0497 | ||||||
| 2.7283 | ||||||
| 8 | W | - | - | - | - | - |
| Validation Parameters | Results |
|---|---|
| Linearity | Fobs (a value of 0.00) was ˂F 95% (2.68) so the assumption of the validity of the linear dynamic range is accepted. |
| Precision | Repeatability limit r and intermediate precision limit R were estimated: r = 0.2% R = 2% |
| Bias | A recovery between 90 and 106% was found. |
| Working Range | The working range is defined from 0.15 ppm to 5.8 ppm for MIT, 0.44 ppm to 17.43 ppm for CMIT with an acceptable uncertainty |
| Measurement Uncertainty | C +/− 0.4% for MIT C +/− 0.03% for CMIT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, S.; Tabassum, H.; Abudawood, M.; Alrashoudi, R.; Alrashed, M.; Alsheikh, Y.A.; Alkaff, S.; Alghamdi, M.; Alenzi, N. Quantification of Methylisothiazolinone and Methylchloroisothiazolinone Preservatives by High-Performance Liquid Chromatography. Molecules 2023, 28, 1760. https://doi.org/10.3390/molecules28041760
Alanazi S, Tabassum H, Abudawood M, Alrashoudi R, Alrashed M, Alsheikh YA, Alkaff S, Alghamdi M, Alenzi N. Quantification of Methylisothiazolinone and Methylchloroisothiazolinone Preservatives by High-Performance Liquid Chromatography. Molecules. 2023; 28(4):1760. https://doi.org/10.3390/molecules28041760
Chicago/Turabian StyleAlanazi, Samyah, Hajera Tabassum, Manal Abudawood, Reem Alrashoudi, May Alrashed, Yazeed A. Alsheikh, Salma Alkaff, Manal Alghamdi, and Naif Alenzi. 2023. "Quantification of Methylisothiazolinone and Methylchloroisothiazolinone Preservatives by High-Performance Liquid Chromatography" Molecules 28, no. 4: 1760. https://doi.org/10.3390/molecules28041760
APA StyleAlanazi, S., Tabassum, H., Abudawood, M., Alrashoudi, R., Alrashed, M., Alsheikh, Y. A., Alkaff, S., Alghamdi, M., & Alenzi, N. (2023). Quantification of Methylisothiazolinone and Methylchloroisothiazolinone Preservatives by High-Performance Liquid Chromatography. Molecules, 28(4), 1760. https://doi.org/10.3390/molecules28041760







