Abstract
This study evaluates the applicability of enantioselective gas chromatography (eGC) and enantioselective comprehensive two-dimensional gas chromatography (eGC×GC) coupled with flame ionization detection for the stereospecific analysis of designated chiral monoterpenes within essential oils distilled from the leaves of Citrus hystrix (CH), C. limon (CL), C. pyriformis (CP), and C. microcarpa (CM). A cryogen-free solid-state modulator with a combination of enantioselective first-dimension and polar second-dimension column arrangements was used to resolve potential interferences in Citrus spp. leaf oils that can complicate the accurate determination of enantiomeric compositions. Interestingly, considerable variations were observed for the enantiomeric fractions (EFs) of the chiral terpenes. (+)-limonene was identified as the predominant enantiomer (60.3–98.9%) in all Citrus oils, (+)-linalool was the major enantiomer in CM (95.9%), (−)-terpenin-4-ol was the major isomer in CM (66.4%) and CP (61.1%), (−)-α-pinene was the dominant antipode in CL (55.5%) and CM (92.1%). CH contained (−)-citronellal (100%) as the pure enantiomer, while CL and CP have lower proportions (9.0–34.6%), and citronellal is absent in CM. The obtained enantiomeric compositions were compared and discussed with results from eGC using the same enantioselective column. To our knowledge, this work encapsulates the first report that details the EFs of these chiral monoterpenes in Citrus spp. leaf oil.
1. Introduction
The genus Citrus, belonging to the Rutaceae family, is represented by about 160 genera with 16 (Swingle System; [1]) to 162 (Tanaka System; [2]) species that are distributed throughout tropical and subtropical regions worldwide [3]. To date, Citrus has been much appreciated as one of the most important commercial fruit crops, with an estimated global production of lemons and limes above 9 million tons during the period of 2021–2022 [4]. In particular, the aromatic oil isolated from the fruit peels (commonly known as Citrus essential oil (EO)) of various citrus trees is highly valued in the pharmaceutical, food, and perfume industries [5,6]. Citrus spp. EOs have been reported to exhibit a wide spectrum of biological activity, including anti-carcinogenic, anti-bacterial, antioxidant, anti-fungal, anti-microbial, anti-spasmodic, anti-diabetic, anti-dermatophyte, and anti-inflammatory activities [7,8,9,10].
Chemically, Citrus EO constitutes a complex pool of bioactive secondary compounds that vary depending on their species, variety, cultivar, origin, climate, and others [11,12,13,14]. These aromatic Citrus oils normally consist predominantly of a complex mixture of monoterpenes and sesquiterpenes, whose relative concentrations vary according to species. Notably, the demand for genuine Citrus spp. EOs is continuously increasing worldwide, with selected Citrus spp. oils fetching higher market values (e.g., Citrus hystrix EO (>150 USD per kg) compared to Citrus sinensis EO (3.5–5.0 USD per kg) [15,16]. Consequently, many reports have been made on the occurrences of mislabeling or adulteration incidents, such as blending with other inexpensive EOs or the addition of lower-cost synthetic components [17,18,19,20]. In light of these phenomena, many analytical approaches (e.g., gas chromatography, isotope ratio mass spectrometry, and others) have been developed to authenticate and safeguard the quality of Citrus EOs [21,22,23,24].
It is known that plant terpenes are biosynthesized via a series of biogenetic pathways, and many of these compounds are present as different stereoisomers [25,26,27]. The assessment of the enantiomeric compositions of chiral terpene mixtures in plant-derived extracts is important in understanding the physiological and ecological roles of the biologically active enantiomers [28,29]. The chiral ratios of specific terpenic molecules may allow for the detection of EO adulteration, which is typically accomplished by the admixture of synthetic aromatic compounds. In this respect, single-dimensional (1D) enantioselective gas chromatography (eGC) has been used as one of the most powerful tools for the determination of the enantiomeric composition of chiral terpenes in plant EOs [30,31,32]. In particular, the development of various versatile chiral stationary phases in eGC has greatly facilitated the stereo-differentiation of chiral terpenes from a variety of EOs [33,34,35,36]. eGC has been successfully used in the past for the authentication of several Citrus spp. EOs, including C. aurantiifolia [37], C. reticulata [37], and C. limon [38]. Specifically, the enantiomeric distribution of chiral compounds can be described as enantiomeric excess (EE), enantiomeric ratio (ER), or enantiomeric fraction (EF), with EF as the preferred description of the relative amounts of the enantiomeric pairs [39].
Albeit eGC regularly provides sufficient resolution for chiral discrimination of chiral volatile organic compounds in EOs, a few studies have highlighted the applicability of enantioselective comprehensive two-dimensional gas chromatography (eGC×GC) for the interference-free ascertainment of ER or EE of volatile racemates in complex sample matrices [40,41]. In a typical eGC×GC arrangement, the first-dimension (1D) column generally consists of a chiral stationary phase, where chiral molecules are being differentiated into their respective enantiomers, and second-dimension (2D) achiral column phases will provide additional separation to address non-specific co-elution that may arise for the target enantiomers. The main advantages of GC×GC over conventional 1D GC correspond to greater peak capacity (i.e., improved resolution), in addition to the structured two-dimensional chromatogram that greatly facilitates peak identification. This high-resolution approach has been recently used for the quality control and/or authentication of C. aurantium [42], C. myrtifolia [43], and C. limon [44] EOs by accurately determining the EF, EE, or ER of chiral terpenic molecules.
Despite the numerous reports on the chiral analysis of Citrus spp. EOs, there are only a few studies that examined the enantiomeric composition of chiral terpenes of C. hystrix (Kaffir lime), C. pyriformis (Ponderosa lemon), and C. microcarpa (Calamansi lime) cultivated in Malaysia. Thus, this study aims to evaluate the applicability of cryogen-free thermal modulation-based enantioselective comprehensive two-dimensional gas chromatography–flame ionization detection (eGC×GC–FID) method for the stereoisomeric analysis of enantiomers of selected chiral terpenes in C. hystrix, C. pyriformis, C. limon, and C. microcarpa leaf EOs. To our knowledge, this is the first application that uses a solid-state modulator to effect the modulation of the 1D effluents for the enantiomeric analysis of chiral terpenes in Citrus spp. leaf oils. The chromatographic elution and/or separation behavior of different 1D chiral phases were investigated to achieve adequate enantio-resolution for the targeted enantiomers, while interfering compounds were further separated in the 2D achiral polar phase. EFs of α-pinene, limonene, citronellal, linalool, and terpinen-4-ol in Citrus spp. leaf oils were comparatively investigated using 1D eGC and eGC×GC methods. The prospect of using these chiral ratios to differentiate the analysed Citrus spp. leaf oils is discussed.
2. Results and Discussion
2.1. Enantioselective GC–FID Analysis of Citrus spp. Leaf EOs
The phytochemical compositions of steam-distilled C. hystrix, C. limon, C. pyriformis, and C. microcarpa leaf EOs have been recently reported [45]. α-Pinene, limonene, citronellal, linalool, and terpinen-4-ol were selected for the current study as these chiral monoterpenes (except citronellal) were found to be potentially present in all the studied Citrus spp. leaf oils. To justify the requirement of higher resolution eGC×GC separation approach for the enantiomeric analysis of Citrus spp. leaf oils, preliminary chiral analysis was first conducted using a one-dimensional eGC approach. It is known that the separation of enantiomorphic pairs of chiral terpenoids can be achieved using a chiral stationary phase, which typically consists of cyclodextrin derivatives solubilized in polysiloxane. Theoretically, enantiomorphic complexes formed by chiral monoterpenes and cyclodextrin derivatives result in different activity coefficients (i.e., different enantioseparation factors) that result in the separation of the enantiomers. As the chiral recognition is dependent on the chiral selectors within the stationary phase, different substituted β-cyclodextrin (β-CD) phases were evaluated. Amongst the five chiral monoterpenes studied, diacetyl tertbutylsilyl β-cyclodextrin (DAC-β-CD) phase resolves one pair of enantiomers, two pairs are resolved in diethyl tertbutylsilyl β-cyclodextrin (DET-β-CD), while dimethyl tertbutylsilyl β-cyclodextrin (DMT-β-CD) phase resolves only four enantiomeric pairs. All of the chiral terpenes were enantioseparated into their respective enantiomers using dimethyl pentyl-β-cyclodextrin (DMP-β-CD), as shown in Figure 1A.
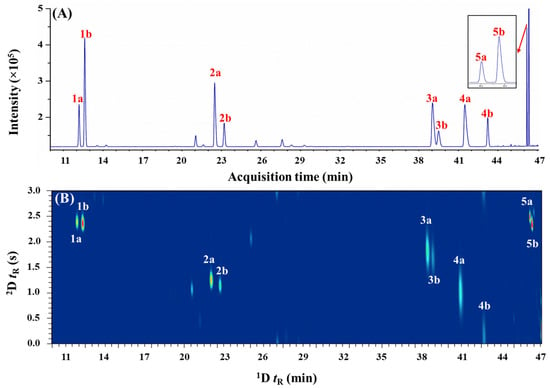
Figure 1.
The enantioseparation of standard mixtures using (A) eGC–FID and (B) eGC×GC–FID. 1a, (−)-α-pinene; 1b, (+)-α-pinene; 2a, (−)-limonene; 2b, (+)-limonene; 3a, (−)-citronellal; 3b, (+)-citronellal; 4a, (−)-linalool; 4b, (+)-linalool; 5a, (+)-terpinen-4-ol; and (5b), (−)-terpinen-4-ol.
The eGC–FID method was then applied for the enantiomeric analysis of different Citrus spp. leaf EOs. As expected, considerable co-elutions of the targeted enantiomeric pairs (except α-pinene) with other phytoconstituents were observed (Figure 2), making precise determination of the enantiomeric fractions difficult. In particular, it is challenging to accurately quantitate (±)-terpinen-4-ol fractions, as these compounds were significantly obscured by other compounds with resolution (Rs) < 0.7 (e.g., (−)-terpinen-4-ol for C. limon and C. pyriformis). Additionally, the enantiomeric assessment of limonene enantiomers for C. microcarpa is also compromised by the partial overlap of the (+)-limonene with an unassigned component of the oil (Figure 2B). Clearly, insufficient peak capacity and phase selectivity to separate the targeted optical isomers from other phytoconstituents within the leaf oils will result in ambiguities for the accurate determination of the enantiomeric fraction or enantiomeric excess specific to these optically active antipodes.
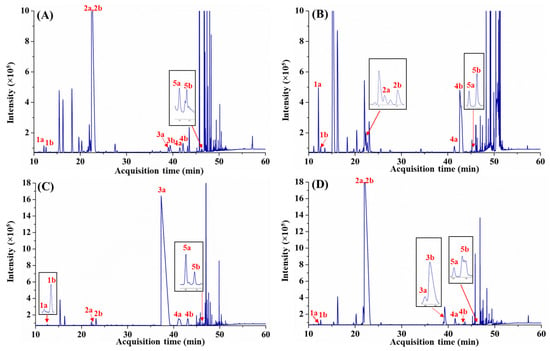
Figure 2.
Enantioanalysis of selected chiral monoterpenes in four Citrus spp. leaf EOs using eGC–FID. (A), C. limon; (B), C. microcarpa; (C), C. hystrix; and (D), C. pyriformis. 1a, (−)-α-pinene; 1b, (+)-α-pinene; 2a, (−)-limonene; 2b, (+)-limonene; 3a, (−)-citronellal; 3b, (+)-citronellal; 4a, (−)-linalool; 4b, (+)-linalool; 5a, (+)-terpinen-4-ol; and (5b), (−)-terpinen-4-ol.
2.2. eGC×GC–FID Analysis of Citrus spp. Leaf EOs
An enantioselective GC×GC–FID approach that provides better separation performance was evaluated for the enantioresolution of the chiral terpenes in Citrus spp. leaf oils. A combination of chiral × polar column sets were used to achieve the appropriate separation (Figure 1B) that approximate the difference in solutes vapor pressure and chiral recognition-based interactions, followed by polarity basis in 2D. Albeit theoretically, the 1D and 2D have different separation mechanisms, but it is important to note that partial correlation between the DMP-β-CD and SUPELCOWAX-10 phases will still exist, as the vapor pressure of volatile constituents still plays a notable role for all GC separations. The alternative arrangement of performing enantioseparation in 2D (i.e., GC×eGC; polar × chiral) was not investigated due to the difficulties in achieving successful stereoanalysis on the short 2D enantioselective column. For eGC×GC analysis, it is important to note that peak volume (i.e., the sum of responses across all modulated traces) is the only reliable measure to obtain the enantiomeric distributions. The 1D enantioselective column provided resolution of the enantiomers, and during the modulation events, different relative proportions of each enantiomer were sampled and rapidly delivered as pulsed peaks to the 2D column for additional achiral separation to resolve any potential interfering components. In this instance, it is important to note that for enantiomorphic pairs that displayed low enantioresolution in the 1D (e.g., citronellal; Rs < 1.5), the number of modulations across the 1D peak will be important for precise quantification of integrated responses of enantiomers. Thus, the effects of modulation ratio (MR) on EF were briefly investigated using citronellal enantiomers (1wh ~ 11.4 s; 1wb ~ 19.2 s) by varying the modulation periods (PM). The results showed that a PM of 3s provide an MR (calculated using MR = (wh × 1.6985)/PM) [46] value of ~6.4 that is adequate for accurate determination of EFs by reducing the relative proportion of “shared modulated peak” that constituted indeterminate composition of (+)- and (−)-citronellal. The “shared modulated peak” can be explained by the “intersect” region (i.e., incomplete separation in 1D) of both (+) and (−) enantiomers being sampled within the same modulation event and re-injected into the 2D SUPELCOWAX-10 column. At this point, the non-enantioselective 2D column provides no resolution of the (+) and (−) antipodes. Overall, a DMP-β-CD × SUPELCOWAX-10 column combination with a PM of 3s provides satisfactory separation of all the chiral monoterpenes from other potential interfering phytoconstituents within Citrus spp. leaf oils (Figure 3). In comparison to the eGC approach (Figure 2), the gain in phytoconstituent coverage in the 2D separation space can be readily observed. Albeit not using cryogens (liquid CO2 or N2) to modulate the 1D effluents, the peak focusing and compression effects of the solid-state modulator (SSM) were noteworthy, as evidenced by the narrow wb of the modulated peaks (0.11–0.57 s) as compared to the wb for eGC (3.12–24.42 s). Despite the SSM having an achiral modulation column to interface the 1D enantioselective and 2D SUPELCOWAX-10 columns, no significant loss of 1D enantioresolution or band broadening (average modulated wb of 0.29 s) issue was observed. Citronellal and linalool enantiomers displayed relatively broad modulated peaks (wb ~ 0.50 s) as compared to others (average wb ~ 0.15 s) due to their strong retention in the 2D polar stationary phase. From the obtained contour plots (Figure 3), it can be readily observed that interfering compounds that affect the accurate quantitation of peak volumes for respective chiral monoterpenes have been resolved via 2D separation. For instance, the (−)-terpinen-4-ol (Figure 3A) in C. limon leaf oil was successfully separated from an unknown component U1, with 2tR of 2.28 s and 2.65 s respectively. The (−)-limonene (2tR of 1.25 s) in C. microcarpa was resolved from an unknown compound U2 (Figure 3B; 2tR of 2.65 s), while three unidentified compounds U3, U4, and U5, that co-eluted with (−)-terpinen-4-ol (2tR of 2.33 s; approximate a quadruple component broad peak in eGC) in C. pyriformis were satisfactorily eluted at different retentions in 2D with 2tR of 2.72 s, 2.65 s, and 2.59 s, respectively. These results demonstrated that eGC×GC is a promising alternative to the one-dimensional eGC method that provides high-resolution enantioanalysis of chiral terpenes in Citrus spp. leaf oils.
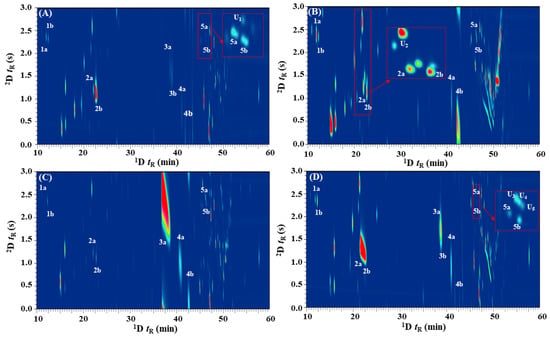
Figure 3.
The enantioanalysis of chiral monoterpenes in Citrus spp. leaf EOs using eGC×GC–FID. (A) C. limon; (B) C. microcarpa; (C) C. hystrix; and (D) C. pyriformis. 1a, (−)-α-pinene; 1b, (+)-α-pinene; 2a, (−)-limonene; 2b, (+)-limonene; 3a, (−)-citronellal; 3b, (+)-citronellal; 4a, (−)-linalool; 4b, (+)-linalool; 5a, (+)-terpinen-4-ol; and (5b), (−)-terpinen-4-ol.
2.3. Enantiomeric Distribution of Selected Chiral Monoterpenes in Citrus spp. Leaf EOs
The enantiomeric compositions obtained for α-pinene, limonene, citronellal, linalool, and terpinen-4-ol are crucial in safeguarding the quality and authenticity of Citrus spp. leaf oils, despite the fact that information concerning these aromatic oils remains scarce. Overall, the EFs determined using eGC–FID and eGC×GC–FID (Table 1) for chiral monoterpenes that do not suffer co-elutions (e.g., α-pinene) were generally comparable across all Citrus spp. oil samples with a variation of <0.7%. Thus, it is readily recognised that a simple 1D eGC–FID method should be adequate for enantiomeric analysis of less complex samples that comprise a lesser degree of co-elutions. However, compounds with significant overlapping with other matrix components (e.g., (−)-terpinen-4-ol in C. limon) exhibited significant differences in EF (variation of ~11.8%), suggesting possible overestimation or underestimation of enantiomeric compositions using the eGC–FID approach. Thus, the high complexity of Citrus spp. leaf EOs (Figure 2) with extensive chemical diversity of secondary compounds justified the need for a higher resolving power eGC×GC–FID method for correct estimation of enantiomeric excess. Results indicated that (+)-limonene consistently predominated in all analyzed Citrus spp. oils with EF > 60%, in which C. limon and C. pyriformis oils exhibited approximately similar EEs of 96.8% and 97.8%, respectively. In the case of citronellal, the (−) antipode was found to be enantiomerically pure (i.e., EE of 100%) in C. hystrix oil, while C. limon and C. pyriformis have the (+) antipode as the major enantiomer (EF > 65%). This compound was not found in C. microcarpa oil. Interestingly, α-pinene, linalool, and terpinen-4-ol displayed different EF across all analyzed Citrus species. (+)-α-Pinene predominated in C. hystrix and C. pyriformis (EF of 91.1% and 76.5%, respectively), while (−)-α-pinene was the major enantiomer in C. limon (55.5%) and C. microcarpa (92.1%). For linalool, the (−)-antipode predominates in C. limon (52.5%), C. hystrix (67.2%), and C. pyriformis (91.9%), while (+)-analogue dominate in C. microcarpa (95.9%). C. limon and C. hystrix displayed enantiomeric compositions of 53.3% and 70.4% for (+)-terpinen-4-ol, which are different from C. microcarpa (33.6%) and C. pyriformis (38.9%). In summary, notable differences were observed for the enantiomeric distributions of the investigated chiral compounds of C. limon, C. microcarpa, C. hystrix, and C. pyriformis leaf EOs. This suggested the potential for developing a stereoisomer distribution database to differentiate the analyzed Citrus leaf oils according to their species. Nevertheless, a more thorough study covering a larger representative sample size of Citrus spp. leaf oils that further evaluates the influences of geographical origin, harvesting period, and extraction method is warranted to validate the practicability and reliability of using chiral terpene distribution as a reference for differentiation of leaf oil from dissimilar Citrus species across different countries.

Table 1.
The enantiomeric composition (%) of selected chiral monoterpenes analyzed in Citrus spp. leaf EOs using the proposed eGC–FID and eGC×GC–FID methods.
3. Materials and Methods
3.1. Chemical and Reagents
(+)-α-Pinene (98%), α-pinene (98%), (S)-(−)-limonene (96%), dipentene, (S)-(−)-citronellal (96%), (±)-citronellal (≥95%), (−)-linalool (≥95%), linalool (97%), (−)-terpinen-4-ol (≥95%), and terpinen-4-ol (≥95%) were purchased from Sigma-Aldrich (Darmstadt, Germany). HPLC-grade n-hexane was supplied by QREC (Asia) Sdn. Bhd. (Selangor, Malaysia).
3.2. Citrus Leaf EO Samples
The Citrus leaves were sampled from selected plantation areas located at Batu Ferringhi, Penang (C. pyriformis), Gelugor, Penang (C. microcarpa), and Gemencheh, Negeri Sembilan (C. hystrix and C. limon). The Citrus leaf EOs were extracted by steam distilling the foliage of the Citrus plant for 3 h. The collected leaf oils were stored refrigerated in a glass vial at 4 °C until further analysis. Prior to eGC analysis, the leaf oils were diluted in n-hexane to the desired concentrations (0.5%, 1.0%, and 2.0% v/v).
3.3. eGC–FID System
eGC analyses were conducted on an Agilent Technologies 7890B GC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a flame ionization detector (FID), a 7693A autosampler, and a split/splitless inlet. The enantioseparation was evaluated using a series of enantioselective columns (DMT-β-CD, DET-β-CD, DMP-β-CD, and DAC-β-CD) supplied by MEGA S.r.l. (Legnano, Italy), and a MEGA-DEX DMP-β-CD capillary column of dimensions 25 m × 0.25 mm I.D. × 0.25 μm film thickness (df) was selected for further study. A range of oven ramp rates was investigated to determine the ramp rate that provides optimum enantioseparation and a shorter analysis time. The chromatographic conditions used were: oven temperature program of 40 °C (hold 2 min) to 60 °C at 25 °C min−1, followed by 1 °C min−1 to 80 °C, 0.5 °C min−1 to 90 °C, 20 °C min−1 to 130 °C, and 10 °C min−1 to 170 °C; injector temperature of 210 °C; FID temperature of 210 °C; helium (99.999%) as the carrier gas at a constant flow rate of 1.0 mL min−1 (approximately 26 cm s−1); injection volume of 1 µL; and a split ratio of 20:1.
3.4. eGC×GC–FID System
Separations were conducted on an Agilent 7890B GC system equipped with a FID, a 7693A autosampler, and a split/split-less inlet. The system was retrofitted with a solid-state thermal modulation system (SSM 1810, J&X Technologies, Shanghai, China). The chromatographic separation was performed using a MEGA-DEX DMP-β-CD (MEGA, Legnano, Italy; 25 m × 0.25 mm I.D. × 0.25 μm df) as the 1D column, and a SUPELCOWAX-10 (Supelco, Bellefonte, PA, USA) of dimensions 1.0 m × 0.1 mm I.D. × 0.1 μm df was used as the 2D column, connected by a SV series modulation column (J&X Technologies, Shanghai, China) coated with proprietary phase (no further elaboration by the manufacturer). A deactivated press-tight connector (Restek Corp., Bellefonte, PA, USA) was used to connect the capillary columns (1D and 2D) and the modulation column. The chromatographic conditions used were: oven temperature program of 40 °C (hold 2 min) to 60 °C at 25 °C min−1, followed by 1 °C min−1 to 80 °C, 0.5 °C min−1 to 90 °C, 20 °C min−1 to 130 °C, 10 °C min−1 to 170 °C, and 25 °C min−1 to 210 °C (hold 15 min); injection volume of 1 µL; injector temperature of 210 °C; detector temperature of 210 °C; sampling frequency of 100 Hz; helium (99.999%) at a flow rate of 1.0 mL min−1; injection volume of 1 μL; and a split ratio of 20:1. The entry and exit hot zones (i.e., micathermic heaters) of the modulator permit temperature programming from 50 °C to 320 °C. The cold trapping zone began at 9 °C, which was then ramped down to −51 °C at a rate of −50 °C/min to facilitate the trapping and focusing of the 1D effluents. The modulation was performed using a PM of 3 s, although other sampling durations were also evaluated.
3.5. Data Handling
Data processing was performed using Agilent Mass Hunter Qualitative Analysis 10.0 (Agilent Technologies, Santa Clara, CA, USA) for eGC–FID and eGC×GC–FID. The modulation platform was controlled using SSCenter software (v.2.6, J&X Technologies, Shanghai, China). Compound identification was performed based on the co-injection of respective standards to confirm their retentions in the eGC and eGC×GC methods. Acquired data from Agilent Mass Hunter Qualitative Analysis 10.0 software was exported and further processed using Origin 8 (OriginLab Corporation, Northampton, MA, USA). Canvas software (v.1.8, J&X Technologies, Shanghai, China) was used to generate the contour plots and facilitate further data processing.
4. Conclusions
This study evaluates the applicability of eGC–FID and eGC×GC–FID approaches for assessing the enantiomeric compositions of α-pinene, limonene, citronellal, linalool, and terpinen-4-ol in steam-distilled leaf oils derived from C. hystrix, C. limon, C. pyriformis, and C. microcarpa. Enantioseparation of all the targeted chiral terpenes from other interfering volatile secondary compounds was achievable using eGC×GC–FID with DMP-β-CD as the 1D and SUPELCOWAX-10 as the 2D. A modulation period of 3 s was found to provide sufficient modulations (as defined by MR) across the 1D effluents, allowing better accuracy for the determination of EFs. The summation of all the modulated peak volumes for each enantiomeric pair provided EF values close to those obtained from eGC–FID, provided that there are no significant co-elutions (e.g., α-pinene) with other phytoconstituents. On comparing the results obtained by eGC–FID with eGC×GC–FID, notable differences (≥11.8%) were observed for the EFs of terpinen-4-ol in C. limon and C. pyriformis leaf oils that showed considerable overlap with interfering components in eGC analysis. (+)-Limonene was identified as the predominant enantiomer (60.3–98.9%) in all Citrus spp. leaf oils, while (−)-linalool was the major enantiomer in C. limon (52.5%), C. hystrix (67.2%), and C. pyriformis (91.9%). For terpenin-4-ol, (+)-antipode was the predominant isomer in C. limon (53.3%) and C. hystrix (70.4%). (+)-α-Pinene was the major antipode in C. hystrix (91.1%) and C. pyriformis (76.5%). Notable differences were observed for (−)-citronellal in which C. hystrix was found to contain pure (−) isomer (EE of 100%), while both C. limon (65.4%) and C. pyriformis (91.0%) have the (+) antipode as the predominant enantiomer, and C. microcarpa indicates the absence of citronellal. The achieved results indicated differences in terms of the enantiomeric distributions of these chiral terpenes in different Citrus spp. leaf oils, which might be useful as references for Citrus EO producers, merchants, and consumers for quality control and further potential authentication purposes.
Author Contributions
Conceptualization, Y.F.W.; methodology, H.I.A.O. and A.Z.; formal analysis, H.I.A.O. and A.Z.; resources, Y.F.W., Z.Z., and X.G.; data curation, H.I.A.O. and A.Z.; writing—original draft preparation, A.Z. and H.I.A.O.; writing—review and editing, F.C., Z.Z., X.G., J.T.A., and Y.F.W.; supervision, Y.F.W.; funding acquisition, Y.F.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universiti Sains Malaysia for the APEX ERA Grant Scheme with Grant No.: 1001/PKIMIA/881002.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Acknowledgments
The support and assistance provided by Agilent Technologies (Malaysia) to Y.F.W. are acknowledged. MEGA S.r.l. is acknowledged for the provision of the enantioselective columns used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Citrus spp. EOs are available upon request.
References
- Swingle, W.T.; Reece, P.C. The Botany of Citrus and Its Wild Relatives. In The Citrus Industry; Reuther, W., Webber, H.J., Batchelor, L.D., Eds.; University of California Press: Berkeley, CA, USA, 1967; pp. 190–430. [Google Scholar]
- Tanaka, T. Fundamental Discussion of Citrus Classification. Stud. Citrol. 1977, 14, 1–6. [Google Scholar]
- Talon, M.; Wu, G.A.; Gmitter, F.G., Jr.; Rokhsar, D.S. The Origin of Citrus. In The Genus Citrus; Talon, M., Caruso, M., Gmitter, F.G., Jr., Eds.; Woodhead Publishing: Duxford, UK, 2020; pp. 9–32. ISBN 012812217X. [Google Scholar]
- United States Department of Agriculture. Citrus: World Markets and Trade; United States Department of Agriculture: Washinton, DC, USA, 2022. [Google Scholar]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Amparo Blázquez, M.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, E.; Laudicina, V.A.; Germanà, M.A. Current and Potential Use of Citrus Essential Oils. Curr. Org. Chem. 2013, 17, 3042–3049. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [PubMed]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus Essential Oils (CEOs) and Their Applications in Food: An Overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal Activity of Lemon (Citrus Lemon L.), Mandarin (Citrus Reticulata L.), Grapefruit (Citrus Paradisi L.) and Orange (Citrus Sinensis L.) Essential Oils. Food Control 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Li, Z.H.; Cai, M.; Liu, Y.S.; Sun, P.L.; Luo, S.L. Antibacterial Activity and Mechanisms of Essential Oil from Citrus Medica L. Var. Sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef]
- Hosni, K.; Zahed, N.; Chrif, R.; Abid, I.; Medfei, W.; Kallel, M.; Brahim, N.B.; Sebei, H. Composition of Peel Essential Oils from Four Selected Tunisian Citrus Species: Evidence for the Genotypic Influence. Food Chem. 2010, 123, 1098–1104. [Google Scholar] [CrossRef]
- Vekiari, S.A.; Protopapadakis, E.E.; Papadopoulou, P.; Papanicolaou, D.; Panou, C.; Vamvakias, M. Composition and Seasonal Variation of the Essential Oil from Leaves and Peel of a Cretan Lemon Variety. J. Agric. Food Chem. 2002, 50, 147–153. [Google Scholar] [CrossRef]
- Frizzo, C.D.; Lorenzo, D.; Dellacassa, E. Composition and Seasonal Variation of the Essential Oils from Two Mandarin Cultivars of Southern Brazil. J. Agric. Food Chem. 2004, 52, 3036–3041. [Google Scholar] [CrossRef]
- Paoli, M.; de Rocca Serra, D.; Tomi, F.; Luro, F.; Bighelli, A. Chemical Composition of the Leaf Essential Oil of Grapefruits (Citrus Paradisi Macf.) in Relation with the Genetic Origin. J. Essent. Oil Res. 2016, 28, 265–271. [Google Scholar] [CrossRef]
- da Camara, C.A.G.; Akhtar, Y.; Isman, M.B.; Seffrin, R.C.; Born, F.S. Repellent Activity of Essential Oils from Two Species of Citrus against Tetranychus Urticae in the Laboratory and Greenhouse. Crop Prot. 2015, 74, 110–115. [Google Scholar] [CrossRef]
- Efendi, D.; Budiarto, R.; Poerwanto, R.; Santosa, E.; Agusta, A. Relationship among Agroclimatic Variables, Soil and Leaves Nutrient Status with the Yield and Main Composition of Kaffir Lime (Citrus Hystrix Dc) Leaves Essential Oil. Metabolites 2021, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, J.; Ali, Z.; Avonto, C.; Khan, I.A. A Novel Approach for Lavender Essential Oil Authentication and Quality Assessment. J. Pharm. Biomed. Anal. 2021, 199, 114050. [Google Scholar] [CrossRef] [PubMed]
- Ojha, P.K.; Poudel, D.K.; Rokaya, A.; Satyal, R.; Setzer, W.N.; Satyal, P. Comparison of Volatile Constituents Present in Commercial and Lab-Distilled Frankincense (Boswellia Carteri) Essential Oils for Authentication. Plants 2022, 11, 2134. [Google Scholar] [CrossRef]
- Cuchet, A.; Jame, P.; Anchisi, A.; Schiets, F.; Oberlin, C.; Lefèvre, J.C.; Carénini, E.; Casabianca, H. Authentication of the Naturalness of Wintergreen (Gaultheria Genus) Essential Oils by Gas Chromatography, Isotope Ratio Mass Spectrometry and Radiocarbon Assessment. Ind. Crops Prod. 2019, 142, 111873. [Google Scholar] [CrossRef]
- Juliani, H.R.; Kapteyn, J.; Jones, D.; Koroch, A.R.; Wang, M.; Charles, D.; Simon, J.E. Application of Near-Infrared Spectroscopy in Quality Control and Determination of Adulteration of African Essential Oils. Phytochem. Anal. 2006, 17, 121–128. [Google Scholar] [CrossRef]
- Schipilliti, L.; Dugo, G.; Santi, L.; Dugo, P.; Mondello, L. Authentication of Bergamot Essential Oil by Gas Chromatography-Combustion-Isotope Ratio Mass Spectrometer (Gc-c-Irms). J. Essent. Oil Res. 2011, 23, 60–71. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Satyal, P.; Setzer, W.N. Authentication of Citrus Spp. Cold-Pressed Essential Oils by Their Oxygenated Heterocyclic Components. Molecules 2022, 27, 6277. [Google Scholar] [CrossRef]
- Masson, J.; Liberto, E.; Beolor, J.C.; Brevard, H.; Bicchi, C.; Rubiolo, P. Oxygenated Heterocyclic Compounds to Differentiate Citrus Spp. Essential Oils through Metabolomic Strategies. Food Chem. 2016, 206, 223–233. [Google Scholar] [CrossRef]
- Bounaas, K.; Bouzidi, N.; Daghbouche, Y.; Garrigues, S.; de la Guardia, M.; El Hattab, M. Essential Oil Counterfeit Identification through Middle Infrared Spectroscopy. Microchem. J. 2018, 139, 347–356. [Google Scholar] [CrossRef]
- Jahangeer, M.; Fatima, R.; Ashiq, M.; Basharat, A.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Therapeutic and Biomedical Potentialities of Terpenoids-A Review. J. Pure Appl. Microbiol. 2021, 15, 471–483. [Google Scholar] [CrossRef]
- Ninkuu, V.; Zhang, L.; Yan, J.; Fu, Z.; Yang, T.; Zeng, H. Biochemistry of Terpenes and Recent Advances in Plant Protection. Int. J. Mol. Sci. 2021, 22, 5710. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Terpenoids. In Pharmacognosy; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128020999. [Google Scholar]
- Luxová, A.; Urbanová, K.; Valterová, I.; Terzo, M.; Borg-Karlson, A.K. Absolute Configuration of Chiral Terpenes in Marking Pheromones of Bumblebees and Cuckoo Bumblebees. Chirality 2004, 16, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Kamaitytė-Bukelskienė, L.; Ložienė, K.; Labokas, J. Dynamics of Isomeric and Enantiomeric Fractions of Pinene in Essential Oil of Picea Abies Annual Needles during Growing Season. Molecules 2021, 26, 2138. [Google Scholar] [CrossRef]
- Chanotiya, C.S.; Pragadheesh, V.S.; Yadav, A.; Gupta, P.; Lal, R.K. Cyclodextrin-Based Gas Chromatography and GC/MS Methods for Determination of Chiral Pair Constituents in Mint Essential Oils. J. Essent. Oil Res. 2021, 33, 23–31. [Google Scholar] [CrossRef]
- Chanotiya, C.S.; Yadav, A. Enantioselective Capillary Gas Chromatography-Flame. Nat. Prod. Commun. 2009, 4, 7–10. [Google Scholar]
- Wong, Y.F.; Davies, N.W.; Chin, S.T.; Larkman, T.; Marriott, P.J. Enantiomeric Distribution of Selected Terpenes for Authenticity Assessment of Australian Melaleuca Alternifolia Oil. Ind. Crops Prod. 2015, 67, 475–483. [Google Scholar] [CrossRef]
- Huang, K.; Zhang, X.; Armstrong, D.W. Ionic Cyclodextrins in Ionic Liquid Matrices as Chiral Stationary Phases for Gas Chromatography. J. Chromatogr. A 2010, 1217, 5261–5273. [Google Scholar] [CrossRef]
- Schurig, V. Use of Derivatized Cyclodextrins as Chiral Selectors for the Separation of Enantiomers by Gas Chromatography. Ann. Pharm. Fr. 2010, 68, 82–98. [Google Scholar] [CrossRef]
- Bicchi, C.; Cagliero, C.; Liberto, E.; Sgorbini, B.; Martina, K.; Cravotto, G.; Rubiolo, P. New Asymmetrical Per-Substituted Cyclodextrins (2-O-Methyl-3-O-Ethyl- and 2-O-Ethyl-3-O-Methyl-6-O-t-Butyldimethylsilyl-β-Derivatives) as Chiral Selectors for Enantioselective Gas Chromatography in the Flavour and Fragrance Field. J. Chromatogr. A 2010, 1217, 1106–1113. [Google Scholar] [CrossRef]
- König, W.A.; Lutz, S.; Colberg, C.; Schmidt, N.; Wenz, G.; von der Bey, E.; Mosandl, A.; Günther, C.; Kustermann, A. Cyclodextrins as Chiral Stationary Phases in Capillary Gas Chromatography. Part III: Hexakis(3-O-acetyl-2,6-di-O-pentyl)-α-cyclodextrin. J. High Resolut. Chromatogr. 1988, 11, 621–625. [Google Scholar] [CrossRef]
- Fouad, H.A.; da Camara, C.A.G. Chemical Composition and Bioactivity of Peel Oils from Citrus Aurantiifolia and Citrus Reticulata and Enantiomers of Their Major Constituent against Sitophilus Zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 2017, 73, 30–36. [Google Scholar] [CrossRef]
- Schipilliti, L.; Dugo, P.; Bonaccorsi, I.; Mondello, L. Authenticity Control on Lemon Essential Oils Employing Gas Chromatography-Combustion-Isotope Ratio Mass Spectrometry (GC-C-IRMS). Food Chem. 2012, 131, 1523–1530. [Google Scholar] [CrossRef]
- de Geus, H.-J.; Wester, P.G.; de Boer, J.; Brinkman, U.A.T. Enantiomer Fractions Instead of Enantiomer Ratios. Chemosphere 2000, 41, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Krupcik, J.; Gorovenko, R.; Spanik, I.; Armstrong, D.W.; Sandra, P. Enantioselective Comprehensive Two-dimensional Gas Chromatography of Lavender Essential Oil. J. Sep. Sci. 2016, 39, 4667–4876. [Google Scholar] [CrossRef]
- Shellie, R.; Marriott, P.J. Comprehensive Two-Dimensional Gas Chromatography with Fast Enantioseparation. Anal. Chem. 2002, 74, 5426–5430. [Google Scholar] [CrossRef]
- Cuchet, A.; Anchisi, A.; Schiets, F.; Clément, Y.; Lantéri, P.; Bonnefoy, C.; Jame, P.; Carénini, E.; Casabianca, H. Determination of Enantiomeric and Stable Isotope Ratio Fingerprints of Active Secondary Metabolites in Neroli (Citrus Aurantium L.) Essential Oils for Authentication by Multidimensional Gas Chromatography and GC-C/P-IRMS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1185, 123003. [Google Scholar] [CrossRef]
- Salvo, A.; Costa, R.; Albergamo, A.; Arrigo, S.; Rotondo, A.; La Torre, G.L.; Mangano, V.; Dugo, G. An In-Depth Study of the Volatile Variability of Chinotto (Citrus Myrtifolia Raf.) Induced by the Extraction Procedure. Eur. Food Res. Technol. 2019, 245, 873–883. [Google Scholar] [CrossRef]
- Mondello, L.; Casilli, A.; Tranchida, P.Q.; Dugo, P.; Dugo, G. Comprehensive Two-dimensional GC for the Analysis of Citrus Essential Oils. Flavour Fragr. J. 2005, 20, 136–140. [Google Scholar] [CrossRef]
- Al Othman, H.I.; Alkatib, H.H.; Zaid, A.; Sasidharan, S.; Rahiman, S.S.F.; Lee, T.P.; Dimitrovski, G.; Althakafy, J.T.; Wong, Y.F. Phytochemical Composition, Antioxidant, and Antiproliferative Activities of Citrus hystrix, Citrus limon, Citrus pyriformis, and Citrus microcarpa Leaf Essential Oils against Human Cervical Cancer Cell Line. Plants 2022, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Khummueng, W.; Harynuk, J.; Marriott, P.J. Modulation Ratio in Comprehensive Two-Dimensional Gas Chromatography. Anal. Chem. 2006, 78, 4578–4587. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).