Recent Advances in the Synthesis of 3,n-Fused Tricyclic Indole Skeletons via Palladium-Catalyzed Domino Reactions
Abstract
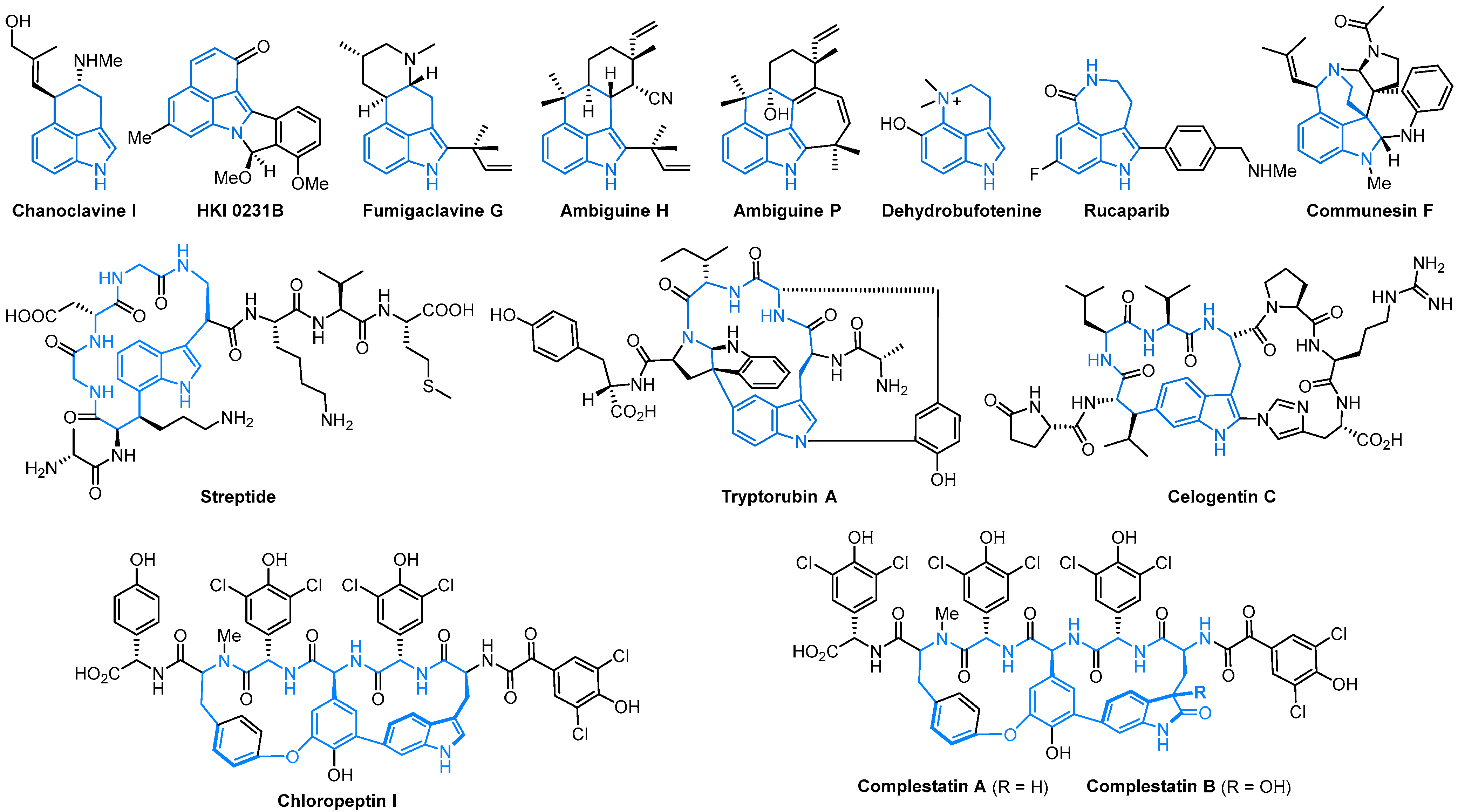
1. Introduction
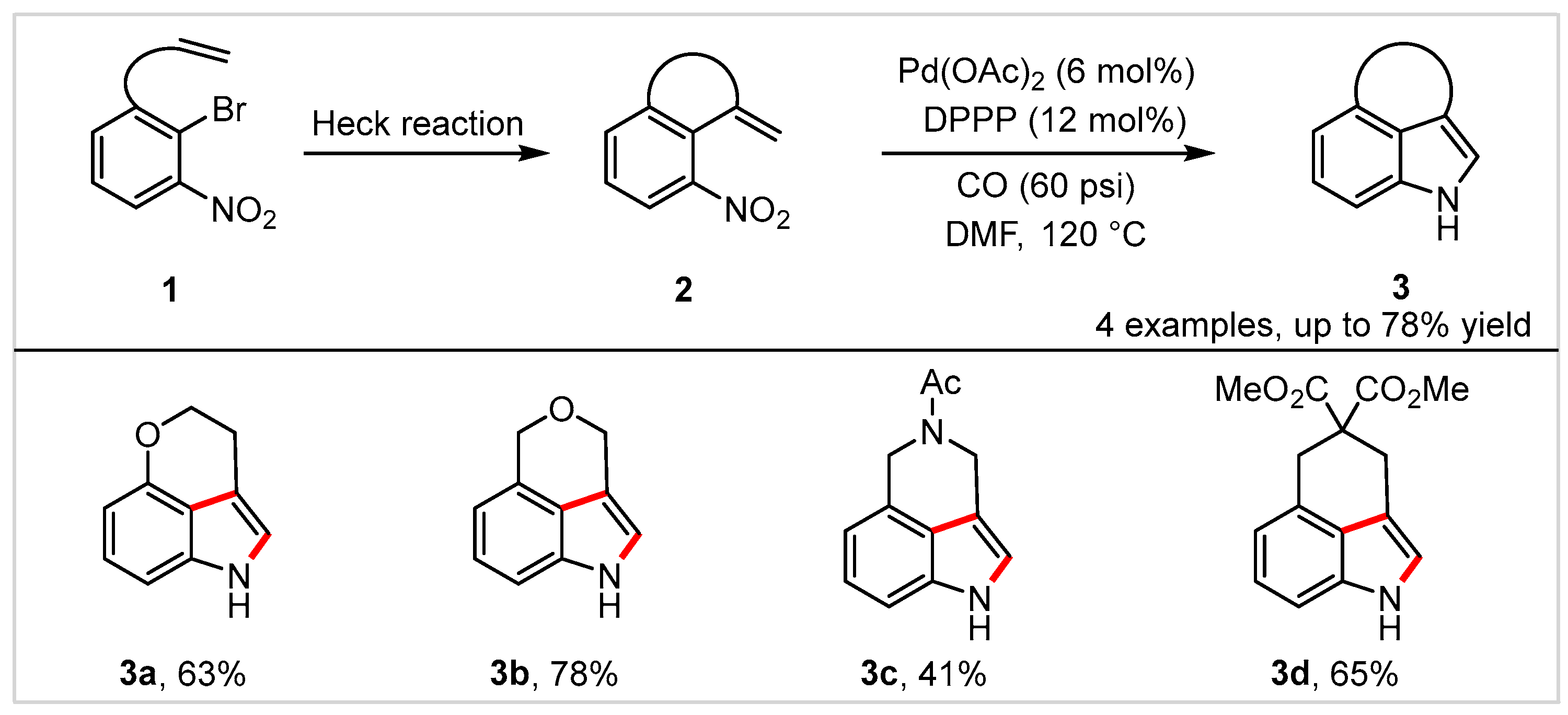
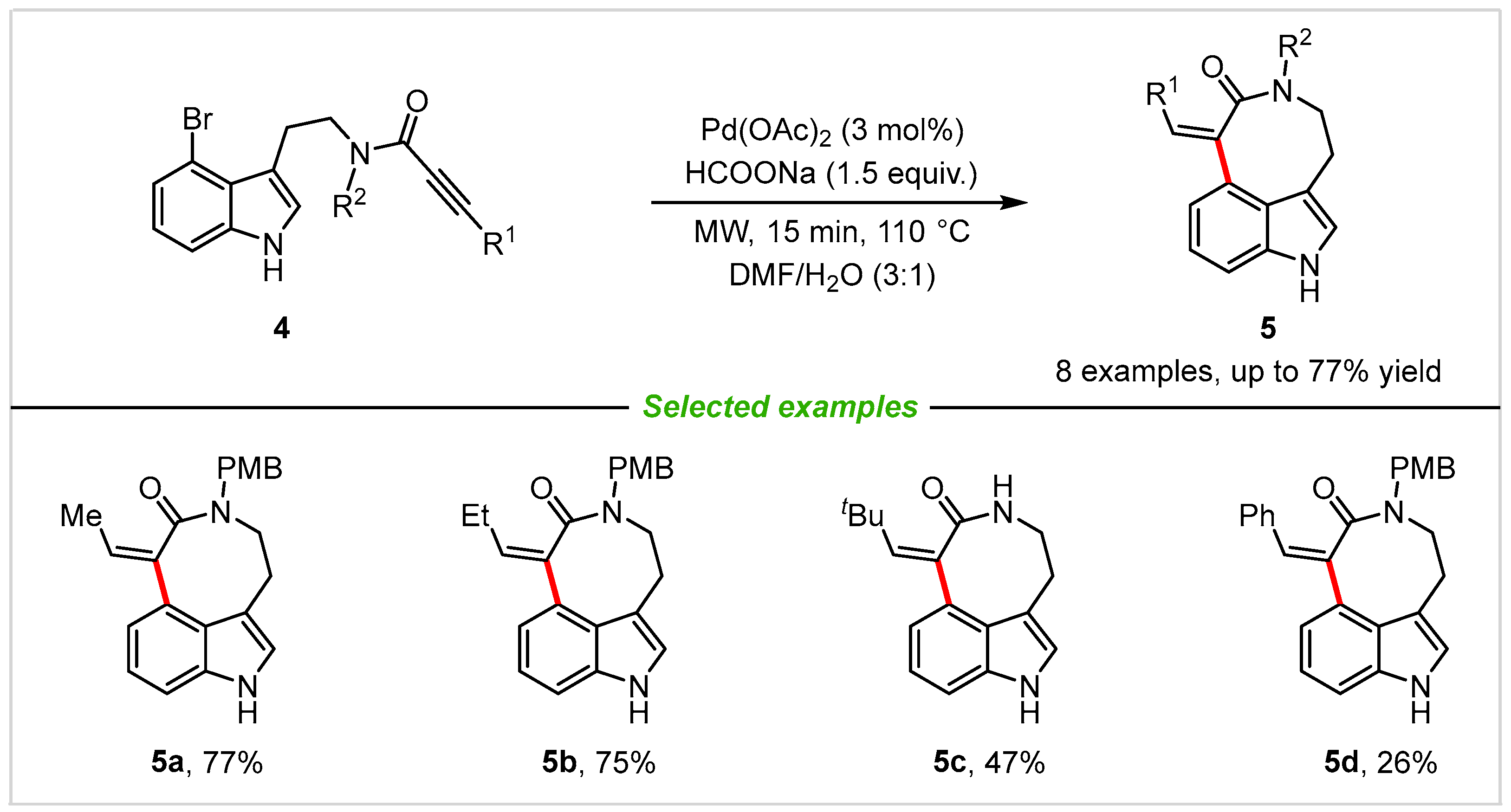
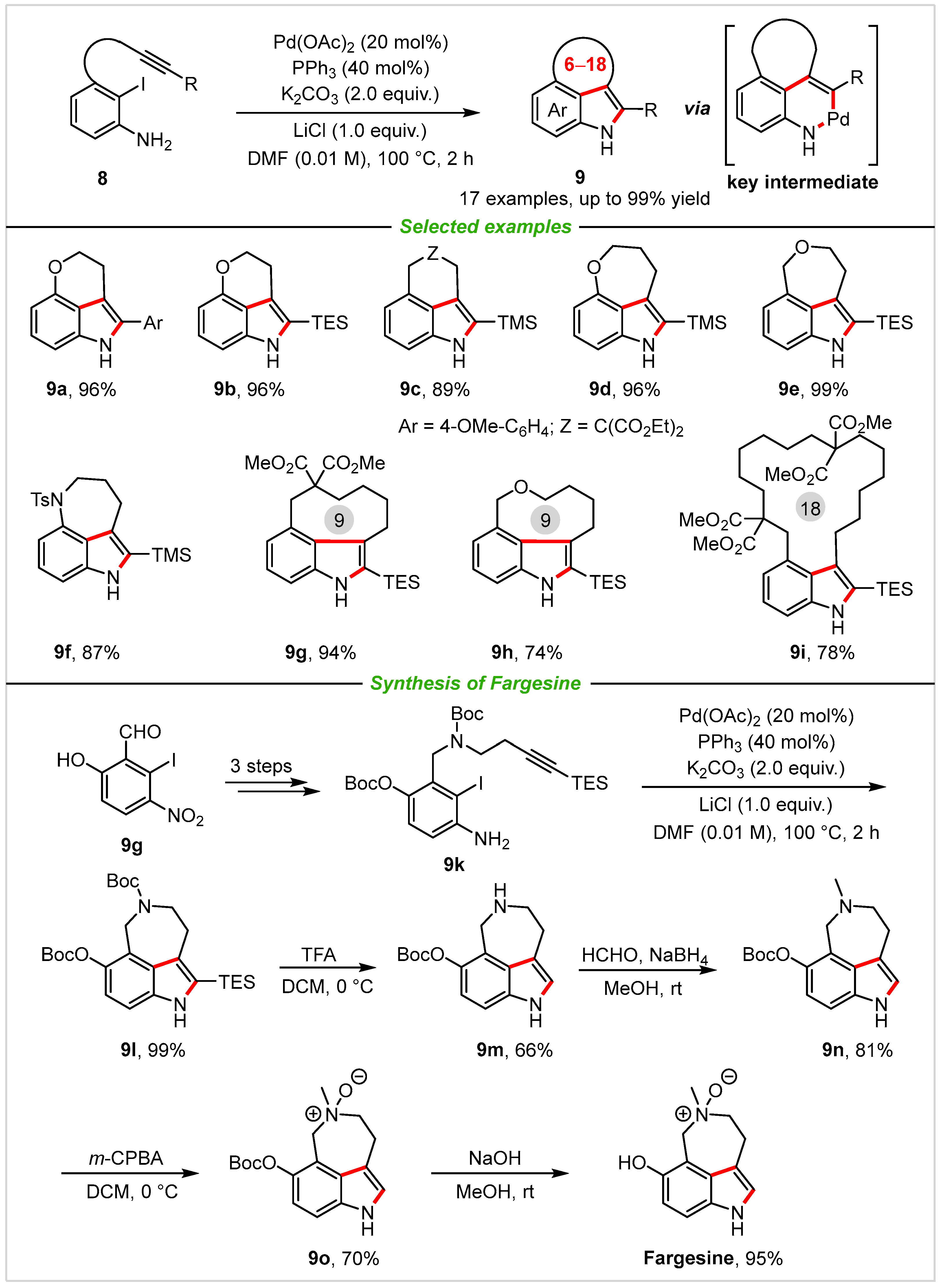
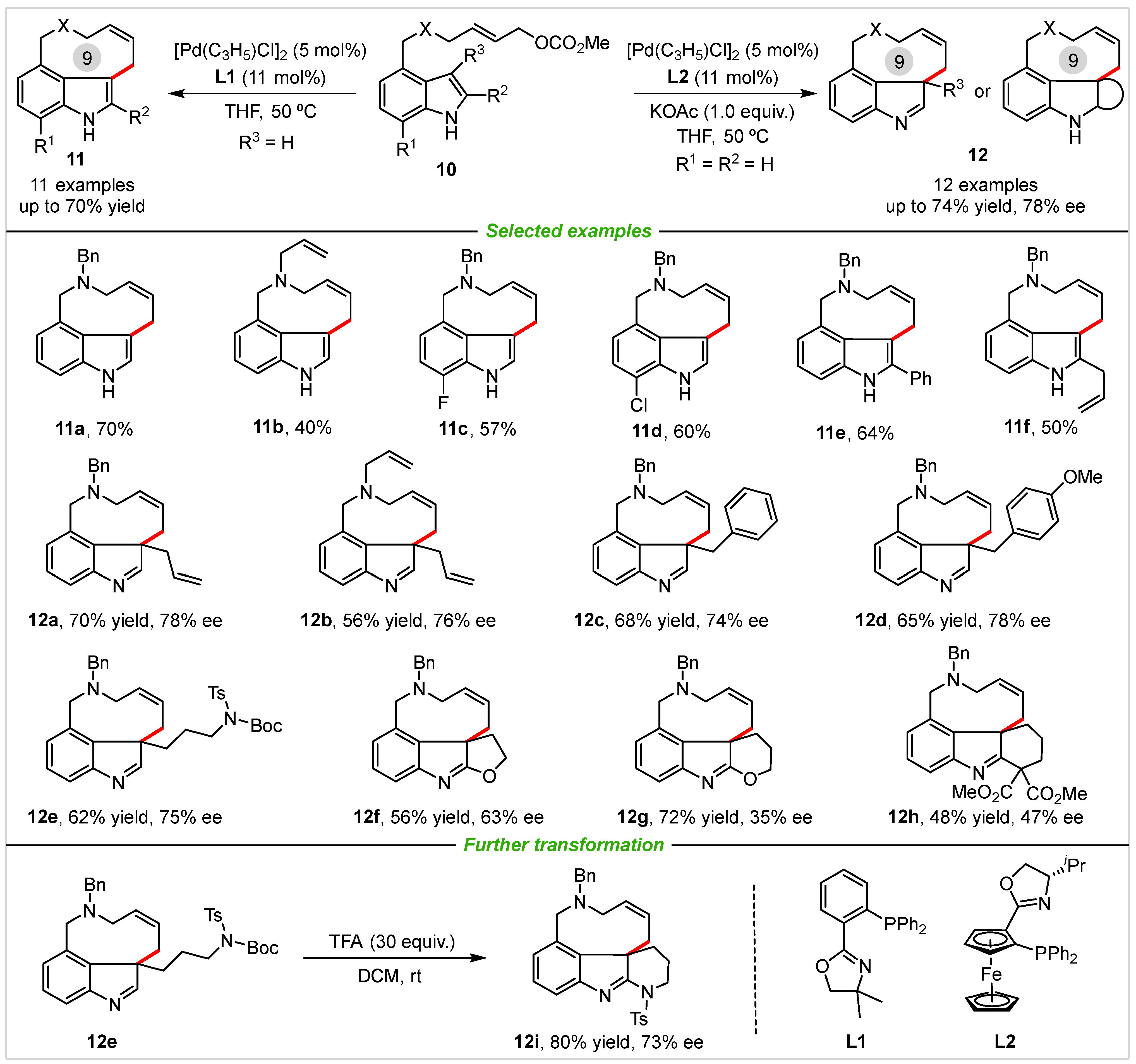
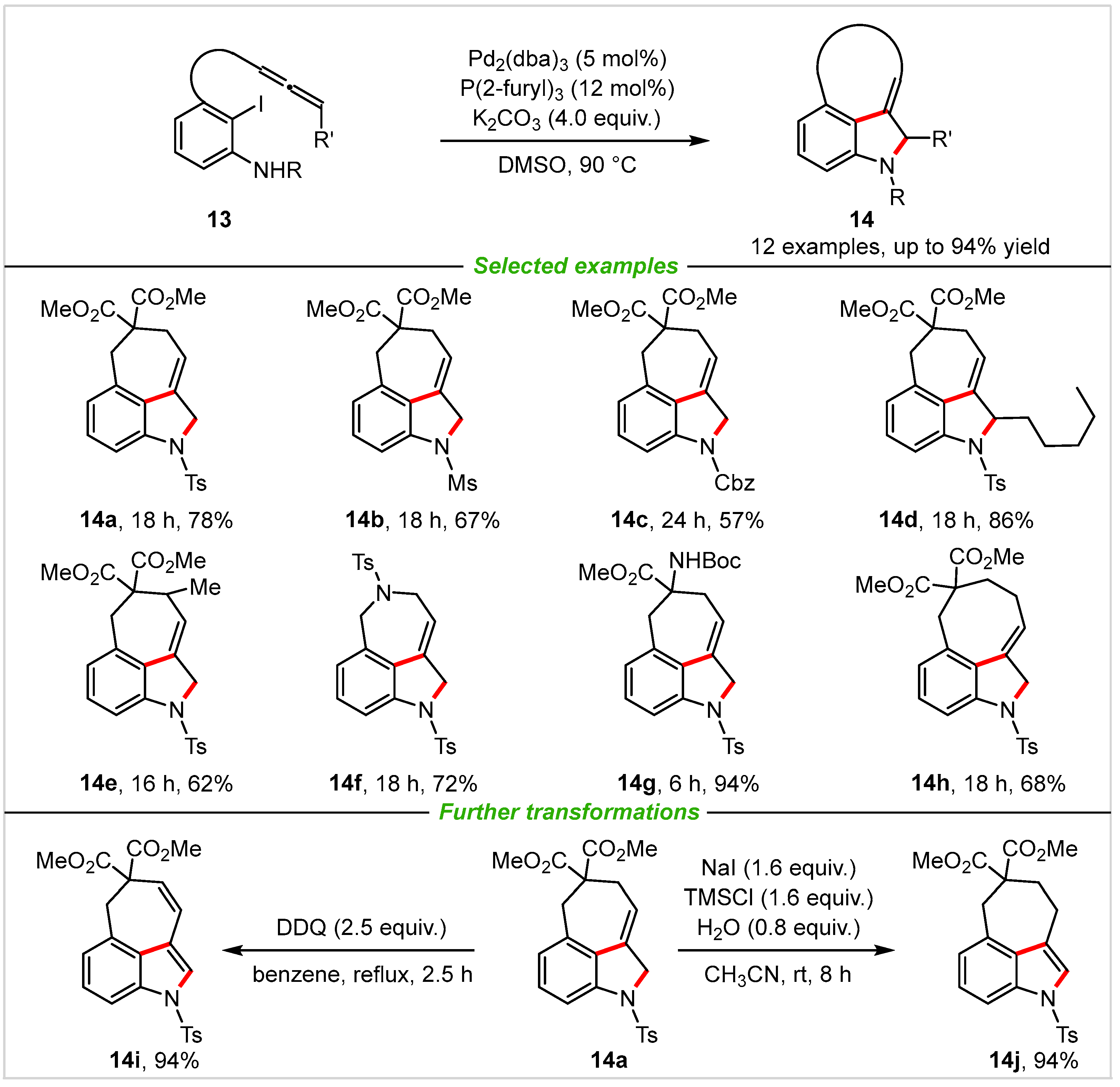
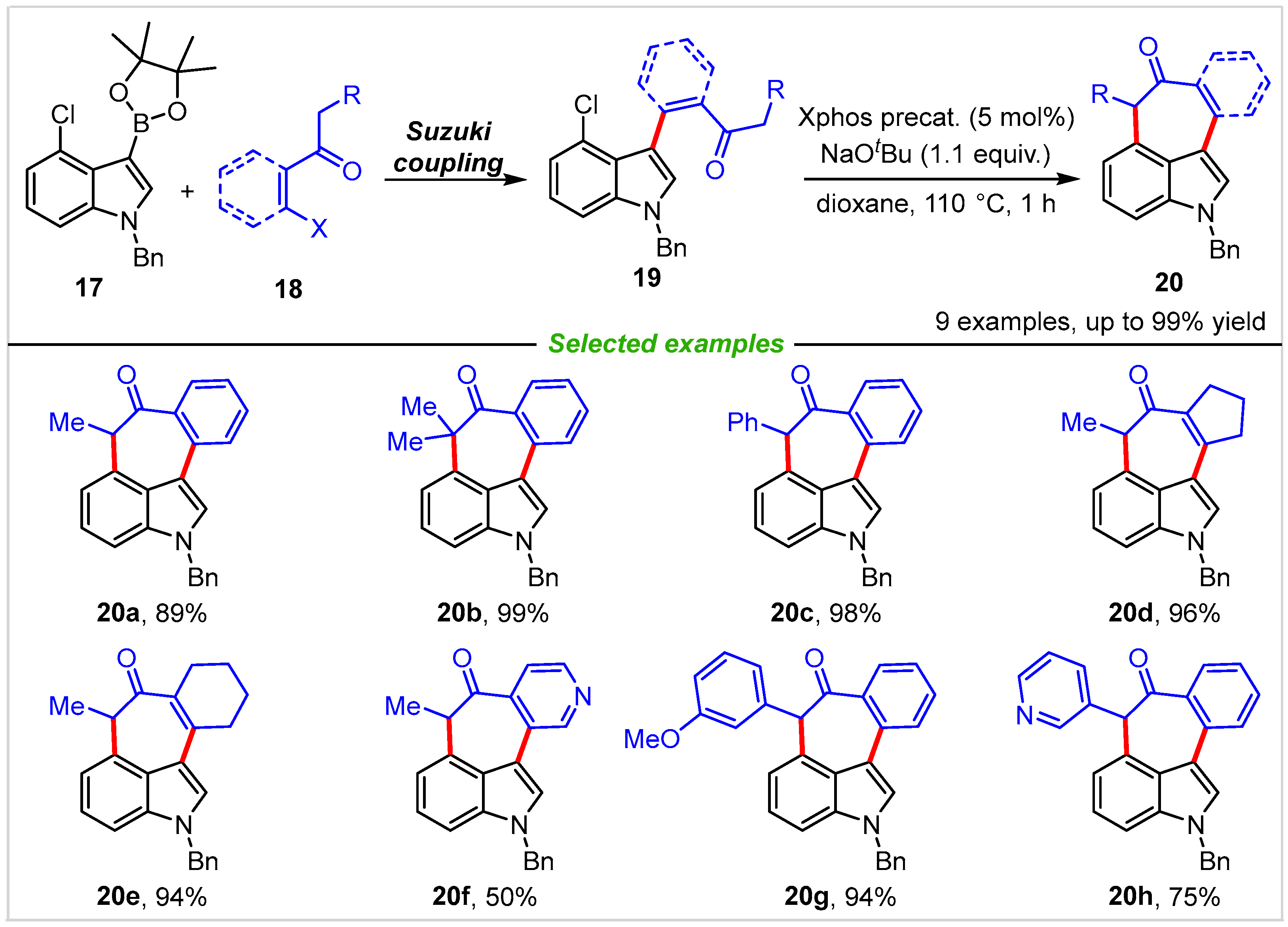
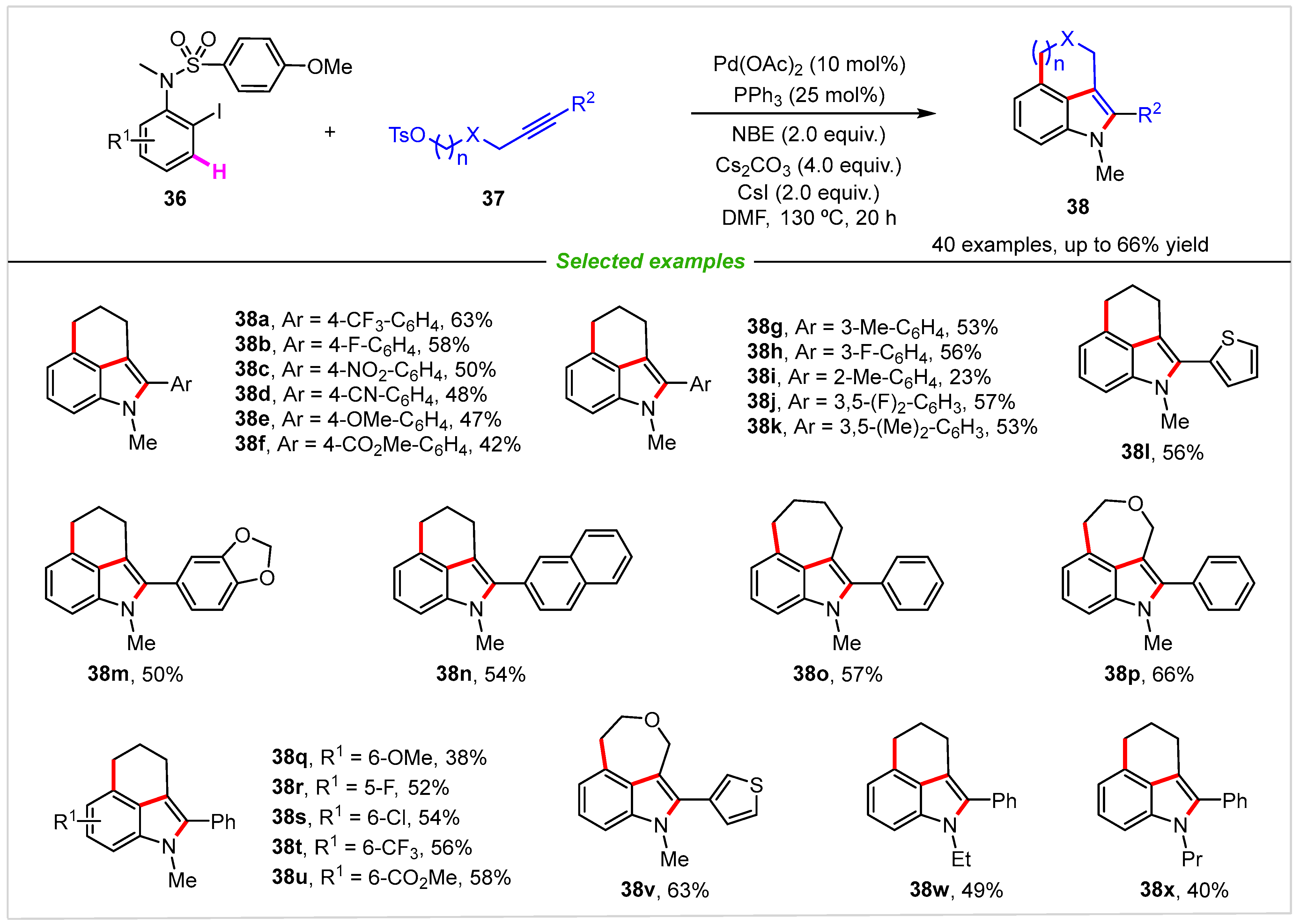
2. Intramolecular Cyclization
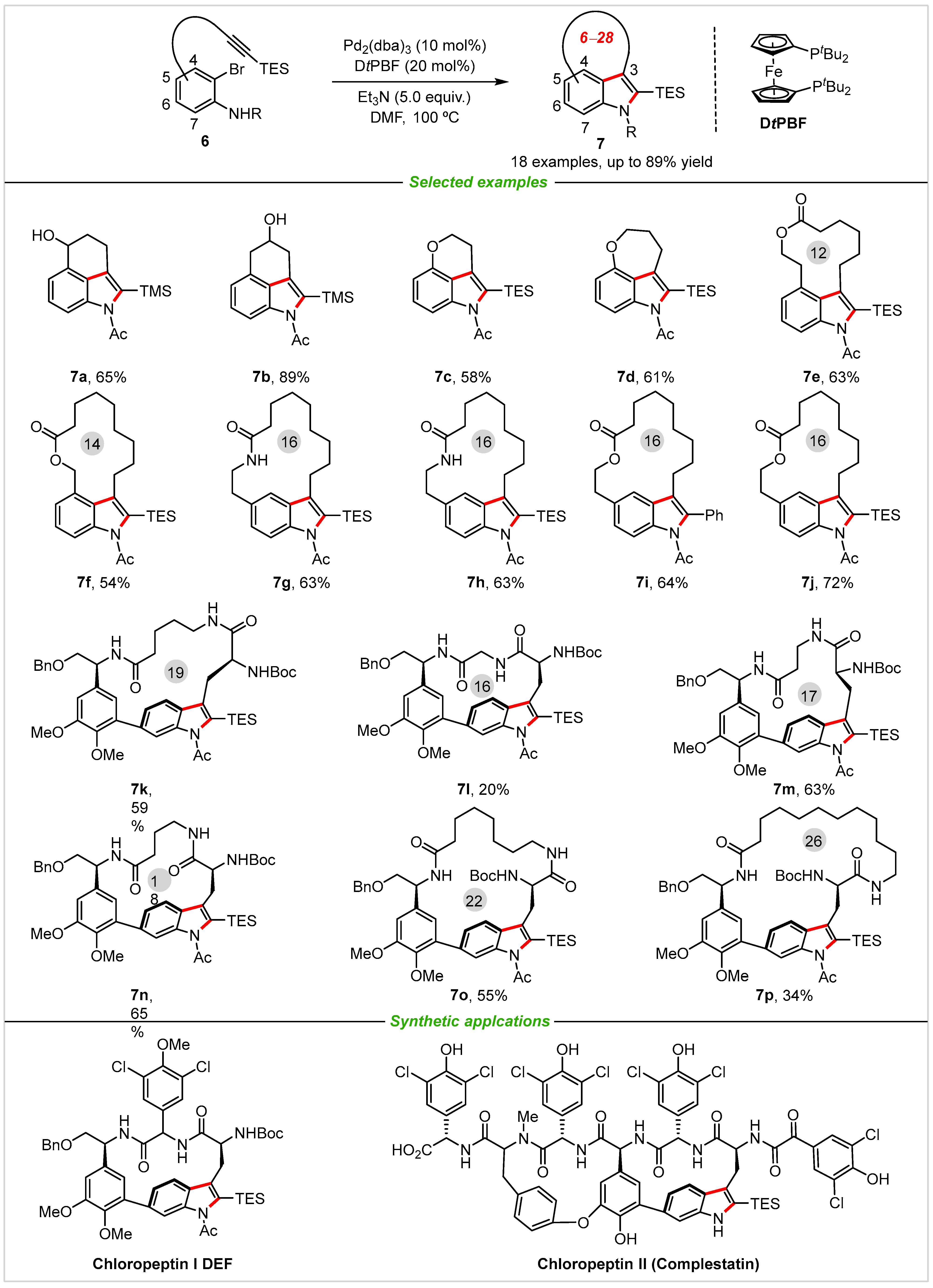
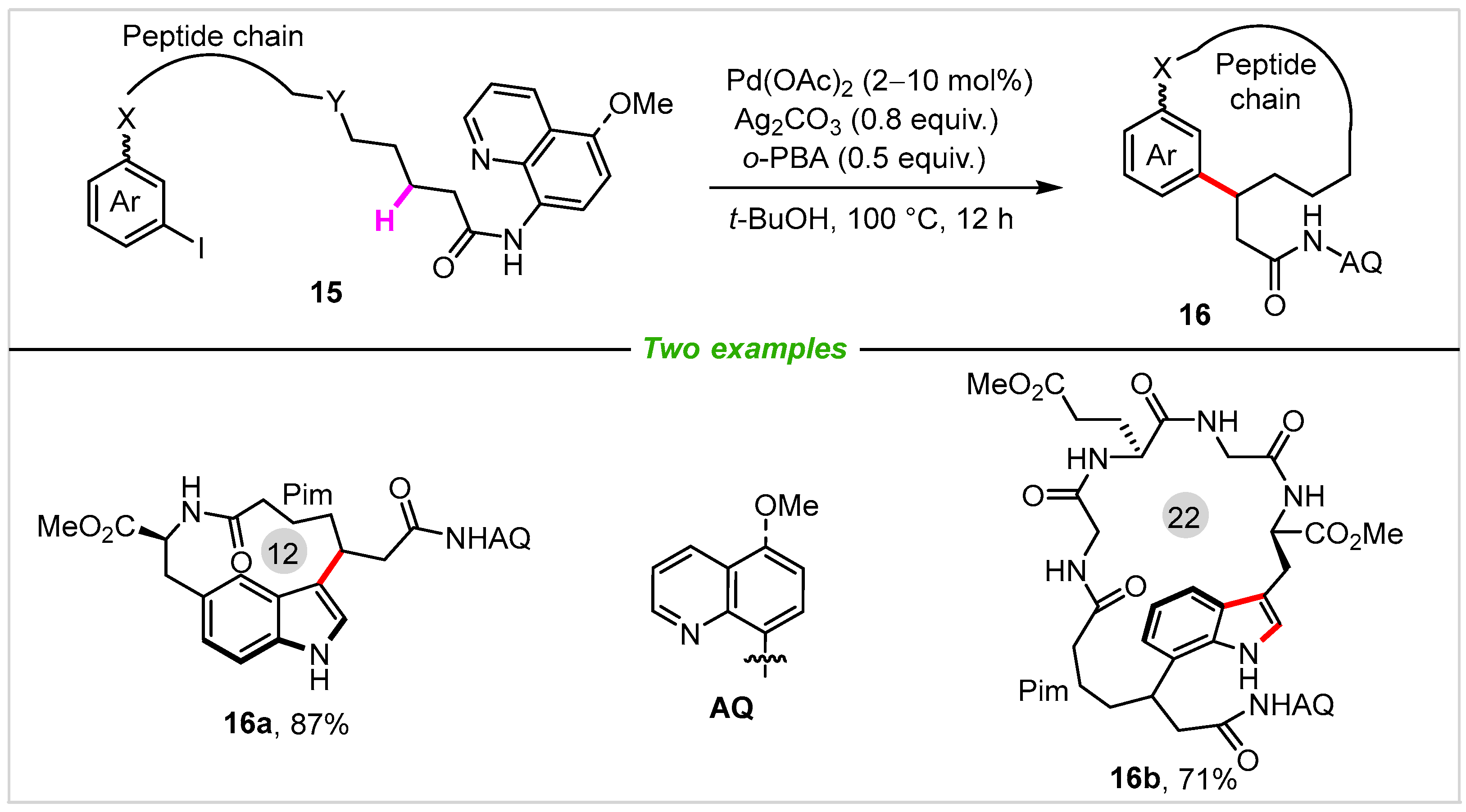
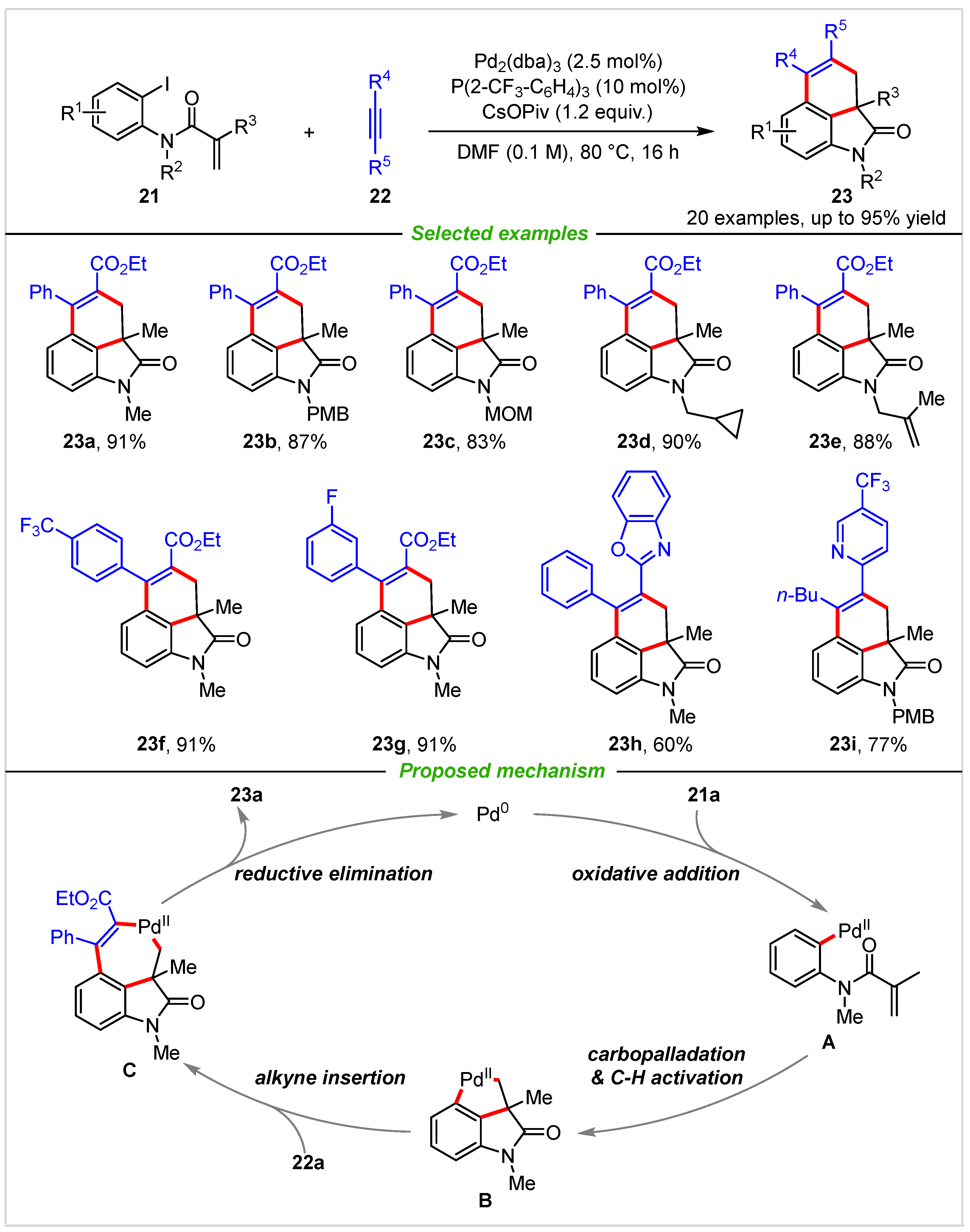
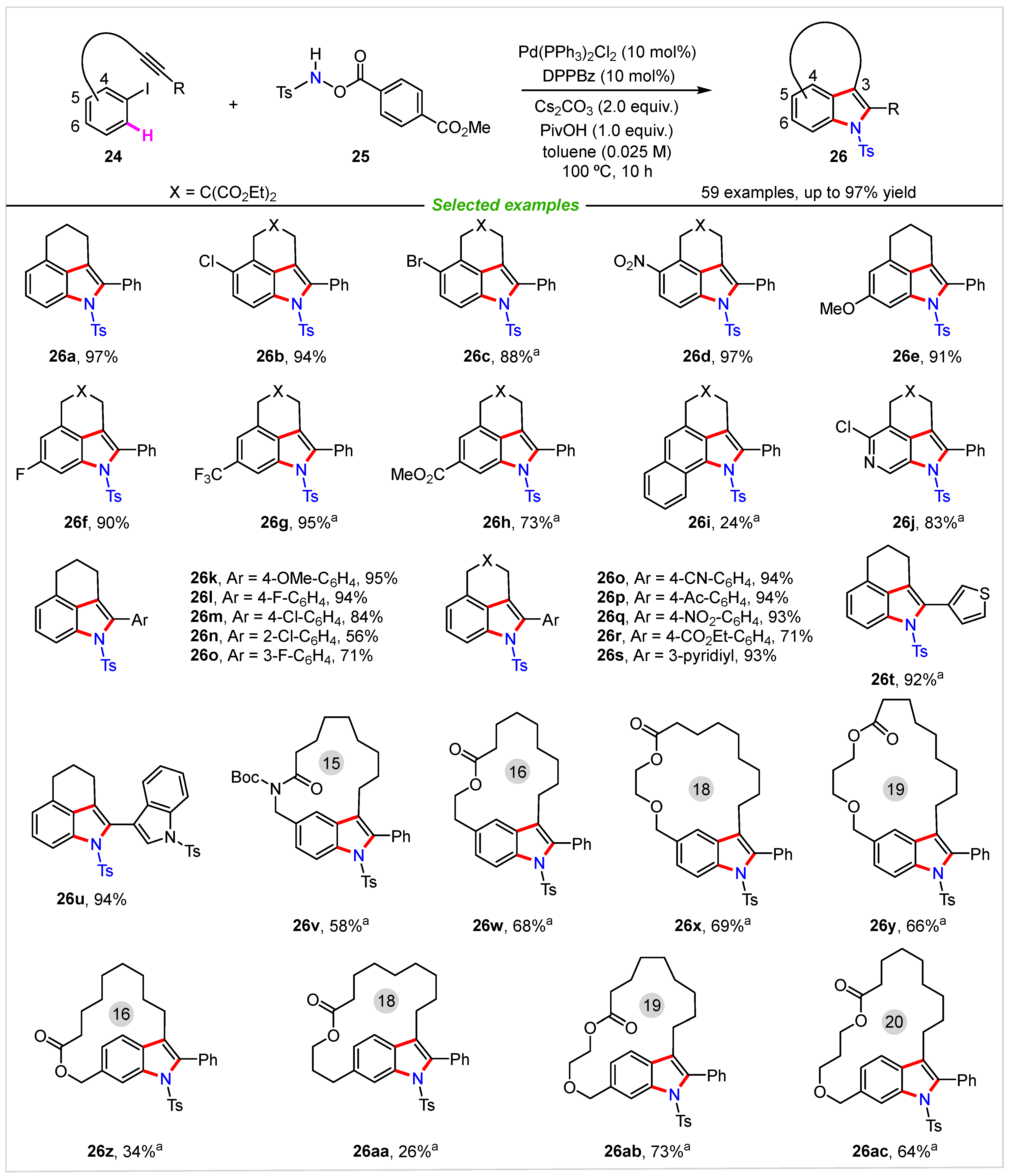
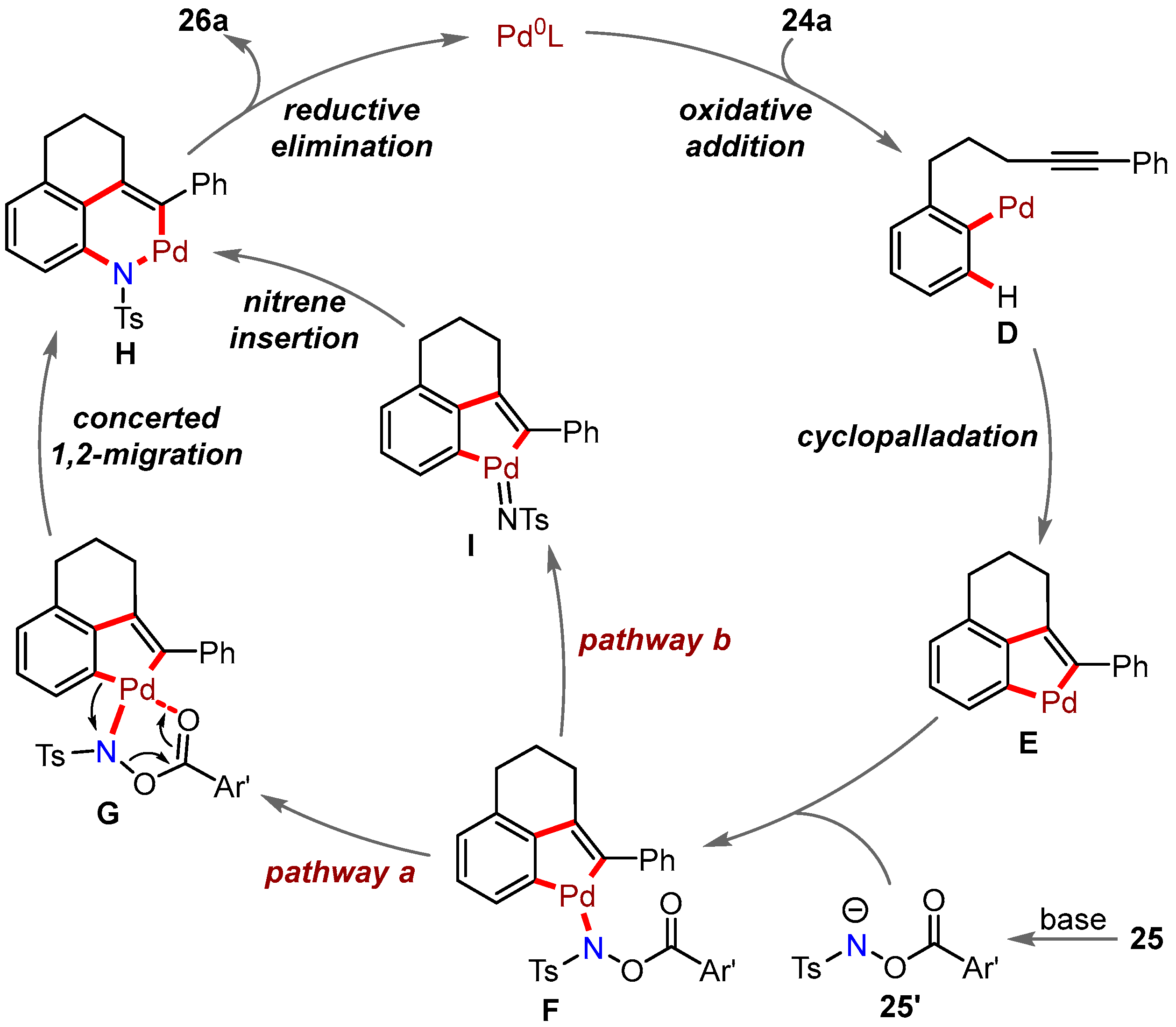
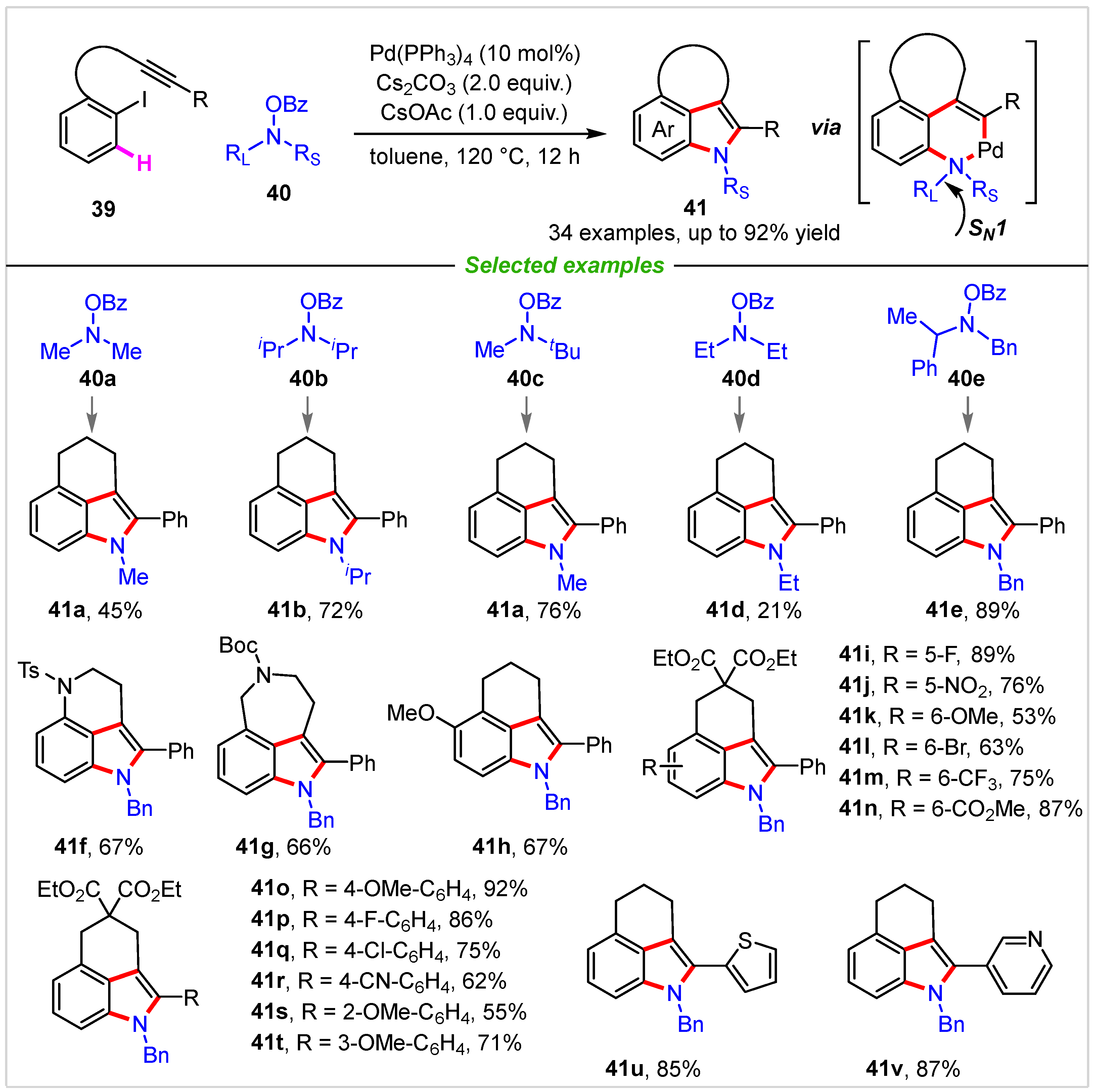
3. Intermolecular Cyclization
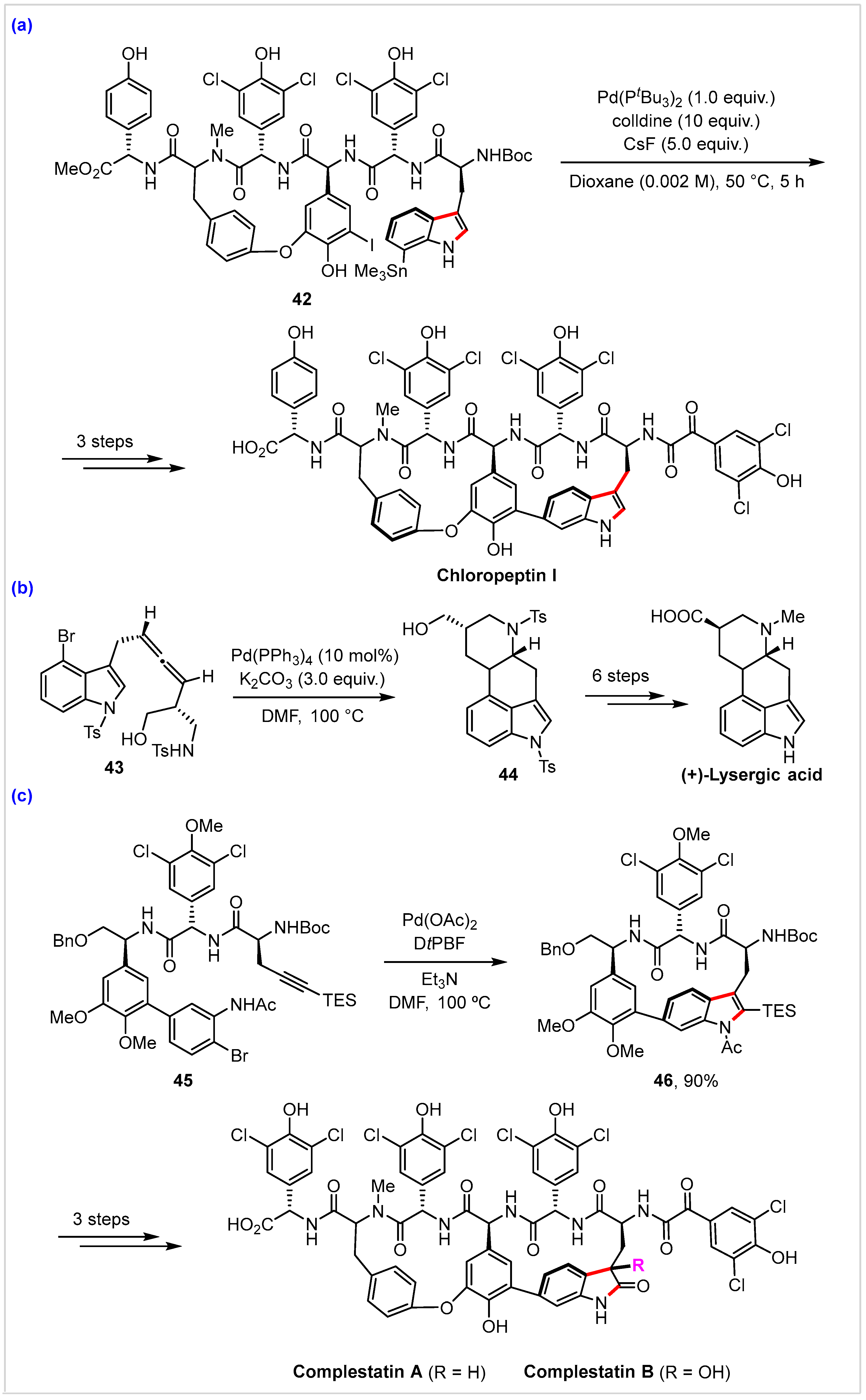
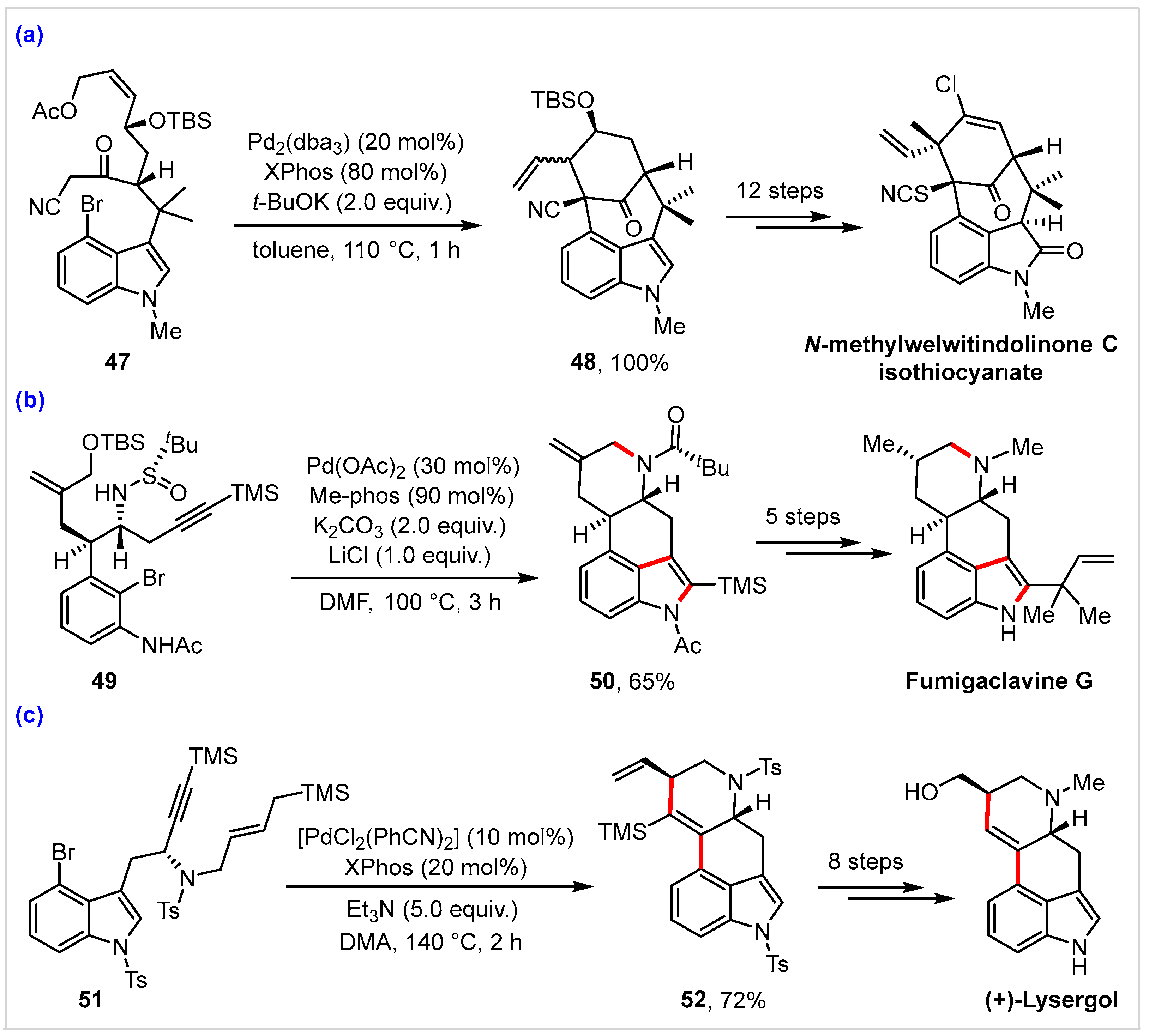
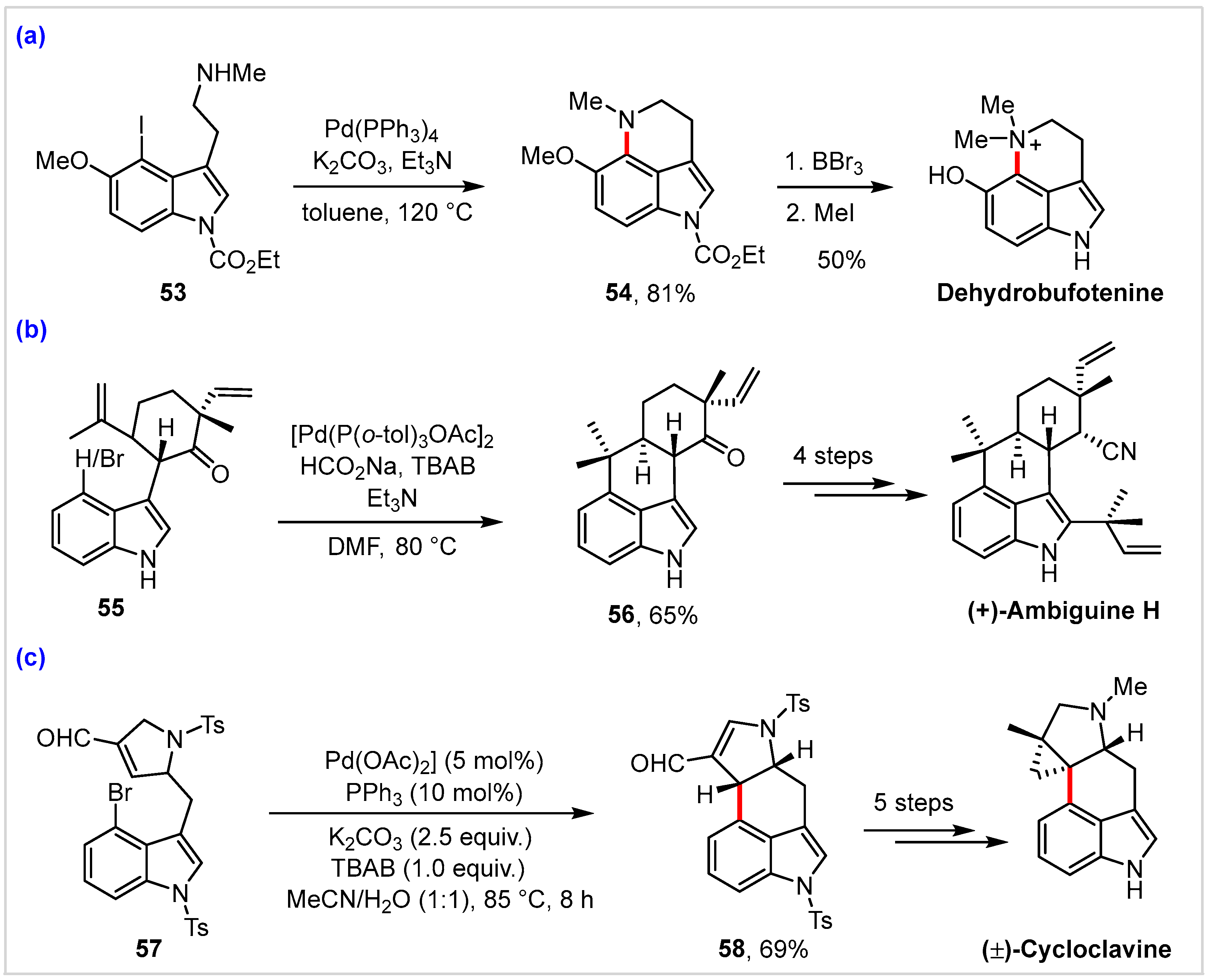
4. Application in Total Synthesis of Representative Natural Products
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DtBPF | 1,1′-Bis(di-tert-butylphosphino)ferrocene |
| TES | triethylsilyl |
| TMS | trimethylsilyl |
| DMF | N,N-dimethylformamide |
| DMA | N,N-dimethylacetamide |
| DCM | dichloromethane |
| THF | tetrahydrofuran |
| DMSO | dimethyl sulfoxide |
| TFA | trifluoroacetic acid |
| dba | dibenzylideneacetone |
| m-CPBA | m-chloroperbenzoic acid |
| Me-phos | dicyclohexyl(2′-methyl-[1,1′-biphenyl]-2-yl)phosphane |
| DPPP | 1,3-bis(diphenylphosphaneyl)propane |
| TBAB | tetrabutylammonium bromide |
| SN1 | unimolecular nucleophilic substitution |
| Boc | tert-butoxycarbonyl |
| SCN | isothiocyanato |
| DDQ | 4,5-dichloro-3,6-dioxocyclohexa-1,4-diene-1,2-dicarbonitrile |
| OTBS | tert-butyldimethylsilyloxy |
| PCC | pyridinium chlorochromate |
| PMB | para-methoxlphenyl |
| DFT | density functional theory |
| o-PBA | ortho-phenyl benzoic acid |
| DPPBz | 1,2-Bis(diphenylphosphino)benzene |
| NBE | norbornene |
| NMR | nuclear magnetic resonance |
| HRMS | high resolution mass spectrometry |
| Xphos | dicyclohexyl(2′,4′,6′-triisopropyl-[1,1′-biphenyl]-2-yl)phosphane |
References
- Cacchi, S.; Fabrizi, G. Synthesis and functionalization of indoles through palladium-catalyzed reactions. Chem. Rev. 2005, 105, 2873–2920. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, G.R.; Kuethe, J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef]
- Gribble, G.W. Recentdevelopments in indole ring synthesis—Methodology and applications. J. Chem. Soc. Perkin Trans. 2000, 1, 1045–1075. [Google Scholar] [CrossRef]
- Prabagar, B.; Yang, Y.; Shi, Z. Site-selective C-H functionalization to access the arene backbone of indoles and quinolines. Chem. Soc. Rev. 2021, 50, 11249–11269. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, K.; Ma, D. Oxidative coupling strategies for the synthesis of indole alkaloids. Chem. Soc. Rev. 2018, 47, 8018–8029. [Google Scholar] [CrossRef] [PubMed]
- Bariwal, J.; Voskressensky, L.G.; Van der Eycken, E.V. Recent advances in spirocyclization of indole derivatives. Chem. Soc. Rev. 2018, 47, 3831–3848. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, T.; Harada, S.; Nakajima, M. Synthetic methods for 3,4-fused tricyclic indoles via indole ring formation. Asian J. Org. Chem. 2018, 7, 1730–1742. [Google Scholar] [CrossRef]
- Connon, R.; Guiry, P.J. Recent advances in the development of one-pot/multistep syntheses of 3,4-annulated indoles. Tetrahedron Lett. 2020, 61, 151696. [Google Scholar] [CrossRef]
- Scopton, A.; Kelly, T.R. Synthesis of HKI 0231B. J. Org. Chem. 2005, 70, 10004–10012. [Google Scholar] [CrossRef]
- Ginnon, W.F.; Benigni, J.D.; Suzuki, J.; Daly, J.W. The synthesis of dehydrobufotenine. Tetrahedron Lett. 1967, 8, 1531–1533. [Google Scholar] [CrossRef]
- Sahu, S.; Das, B.; Maji, M.S. Stereodivergent total synthesis of hapalindoles, fischerindoles, hapalonamide H, and ambiguine H alkaloids by developing a biomimetic, redox-neutral, cascade Prins-type cyclization. Org. Lett. 2018, 20, 6485–6489. [Google Scholar] [CrossRef] [PubMed]
- Wyche, T.P.; Ruzzini, A.C.; Schwab, L.; Currie, C.R.; Clardy, J. Tryptorubin A: A polycyclic peptide from a fungus-derived streptomycete. J. Am. Chem. Soc. 2017, 139, 12899–12902. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Litvinov, D.N.; He, L.; Banerjee, B.; Castle, S.L. Total synthesis of celogentin C. Angew. Chem. Int. Ed. 2009, 48, 6104–6107. [Google Scholar] [CrossRef]
- Garfunkle, J.; Kimball, F.S.; Trzupek, J.D.; Takizawa, S.; Shimamura, H.; Tomishima, M.; Boger, D.L. Total synthesis of chloropeptin II (complestatin) and chloropeptin I. J. Am. Chem. Soc. 2009, 131, 16036–16038. [Google Scholar] [CrossRef]
- Isley, N.A.; Endo, Y.; Wu, Z.-C.; Covington, B.C.; Bushin, L.B.; Seyedsayamdost, M.R.; Boger, D.L. Total synthesis and stereochemical assignment of streptide. J. Am. Chem. Soc. 2019, 141, 17361–17369. [Google Scholar] [CrossRef] [PubMed]
- Horwell, D.C.; Nichols, P.D.; Ratcliffe, G.S.; Roberts, E. Synthesis of conformationally constrained tryptophan derivatives. J. Org. Chem. 1994, 59, 4418–4423. [Google Scholar] [CrossRef]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd metal catalysts for cross-couplings and related reactions in the 21st century: A critical review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef]
- Shetgaonkar, S.E.; Mamgain, R.; Kikushima, K.; Dohi, T.; Singh, F.V. Palladium-catalyzed organic reactions involving hypervalent iodine reagents. Molecules 2022, 27, 3900. [Google Scholar] [CrossRef]
- Heck, R.F. Acylation, methylation, and carboxyalkylation of olefins by Group VIII metal derivative. J. Am. Chem. Soc. 1968, 90, 5518–5526. [Google Scholar] [CrossRef]
- Söderberg, B.C.; Rector, S.R.; O’Neil, S.N. Palladium-catalyzed synthesis of fused indoles. Tetrahenron Lett. 1999, 40, 3657–3660. [Google Scholar] [CrossRef]
- Söderberg, B.C.; Hubbard, J.W.; Rector, S.R.; O’Neil, S.N. Synthesis offused indoles by sequential palladium-catalyzed Heck reaction and N-heteroannulation. Tetrahedron 2005, 61, 3637–3649. [Google Scholar] [CrossRef]
- Peshkov, V.A.; Van Hove, S.; Donets, P.A.; Pereshivko, O.P.; Van Hecke, K.; Van Meervelt, L.; Van der Eycken, E.V. Synthesis of the azocino[cd]indole framework through Pd-catalyzed intramolecular acetylene hydroarylation. Eur. J. Org. Chem. 2011, 2011, 1837–1840. [Google Scholar] [CrossRef]
- Larock, R.C.; Yum, E.K. Synthesis of indoles via palladium-catalyzed heteroannulation of internal alkynes. J. Am. Chem. Soc. 1991, 113, 6689–6690. [Google Scholar] [CrossRef]
- Larock, R.C.; Yum, E.K.; Refvik, M.D. Synthesis of 2,3-disubstituted indoles via palladium-catalyzed annulation of internal alkynes. J. Org. Chem. 1998, 63, 7652–7662. [Google Scholar] [CrossRef]
- Breazzano, S.P.; Poudel, Y.B.; Boger, D.L. A Pd(0)-mediated indole (macro)cyclization reaction. J. Am. Chem. Soc. 2013, 135, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Mohr, P.J.; Halcomb, R.L. Total synthesis of (+)-phomactin A using a B-alkyl Suzuki macrocyclization. J. Am. Chem. Soc. 2003, 125, 1712–1713. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Palladium-catalyzed cross-coupling reactions in total synthesis. Angew. Chem. Int. Ed. 2005, 44, 4442–4489. [Google Scholar] [CrossRef]
- Shan, D.; Gao, Y.; Jia, Y. Intramolecular Larock indole synthesis: Preparation of 3,4-fused tricyclic indoles and total synthesis of fargesine. Angew. Chem. Int. Ed. 2013, 52, 4902–4905. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shan, D.; Jia, Y. Intramolecular Larock indole synthesis for the preparation of tricyclic indoles and its application in the synthesis of tetrahydropyrroloquinoline and fargesine. Tetrahedron 2014, 70, 5136–5141. [Google Scholar] [CrossRef]
- Zeng, J.; Sun, G.; Yao, W.; Zhu, Y.; Wang, R.; Cai, L.; Liu, K.; Zhang, Q.; Liu, X.-W.; Wan, Q. 3-Aminodeoxypyranoses in glycosylation: Diversity-oriented synthesis and assembly in oligosaccharides. Angew. Chem. Int. Ed. 2017, 56, 5227–5231. [Google Scholar] [CrossRef]
- Xu, Q.-L.; Dai, L.-X.; You, S.-L. Diversity oriented synthesis of indole-based peri-annulated compounds via allylic alkylation reactions. Chem. Sci. 2013, 4, 97–102. [Google Scholar] [CrossRef]
- Byun, S.; Farah, A.O.; Wise, H.R.; Katchmar, A.; Cheong, P.H.Y.; Scheidt, K.A. Enantioselective copper-catalyzed borylative amidation of allenes. J. Am. Chem. Soc. 2022, 144, 22850–22857. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dixon, D.J. Stereoselective spirolactam synthesis via palladium catalyzed arylative allene carbocyclization cascades. Org. Lett. 2010, 12, 3784–3787. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, S. Pd(0)-catalyzed insertion−cyclization reaction of 2,3-allenols with aryl or alkenyl halides. Diastereoselective synthesis of highly optically active trans-2,3-disubstituted vinylic oxiranes. J. Am. Chem. Soc. 1999, 121, 7943–7944. [Google Scholar] [CrossRef]
- Chen, S.; Gao, Z.; Zhao, H.; Li, B. Palladium-catalyzed regio- and stereoselective arylesterification of ferrocenylallene: A synthetic route to ferrocene-containing disubstituted E-allylic esters. J. Org. Chem. 2014, 79, 1481–1486. [Google Scholar] [CrossRef]
- Lee, M.; Nguyen, M.; Brandt, C.; Kaminsky, W.; Lalic, G. Catalytic Hydroalkylation of allenes. Angew. Chem. Int. Ed. 2017, 56, 15703–15707. [Google Scholar] [CrossRef]
- Nakano, S.; Inoue, N.; Hamada, Y.; Nemoto, T. Pd-catalyzed cascade cyclization by intramolecular Heck insertion of an allene-allylic amination sequence: Application to the synthesis of 3,4-fused tricyclic indoles. Org. Lett. 2015, 17, 2622–2625. [Google Scholar] [CrossRef]
- Walsh, C.T.; O’Brien, R.V.; Khosla, C. Nonproteinogenic amino acid building blocks for nonribosomal peptide and hybrid polyketide scaffolds. Angew. Chem. Int. Ed. 2013, 52, 7098–7124. [Google Scholar] [CrossRef]
- Cardote, T.A.F.; Ciulli, A. Cyclic and macrocyclic peptides as chemical tools to recognise protein surfaces and probe protein–protein interactions. ChemMedChem 2016, 11, 787–794. [Google Scholar] [CrossRef]
- Marsault, E.; Peterson, M.L. Macrocycles are great cycles: Applications, opportunities, and challenges of synthetic macrocycles in drug discovery. J. Med. Chem. 2011, 54, 1961–2004. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, G.; Sun, M.; Mahankali, M.; Ma, Y.; Zhang, M.; Hua, W.; Hu, Y.; Wang, Q.; Chen, J.; et al. A general strategy for synthesis of cyclophane-braced peptide macrocycles via palladium-catalysed intramolecular sp3 C-H arylation. Nat. Chem. 2018, 10, 540–548. [Google Scholar] [CrossRef]
- Bellina, F.; Rossi, R. Transition metal-catalyzed direct arylation of substrates with activated sp3-hybridized C-H bonds and some of their synthetic equivalents with aryl halides and pseudohalides. Chem. Rev. 2010, 110, 1082–1146. [Google Scholar] [CrossRef]
- Hellal, M.; Singh, S.; Cuny, G.D. Synthesis of tetracyclic indoles via intramolecular α-arylation of ketones. J. Org. Chem. 2012, 77, 4123–4130. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.F.; Marchese, A.D.; Lautens, M. Palladium-catalyzed synthesis of dihydrobenzoindolones via C-H bond activation and alkyne insertion. Org. Lett. 2018, 20, 4367–4370. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Hao, J.; Yu, J.; Ma, X.; Liu, J.; Luan, X. Hydroxylamines as bifunctional single-nitrogen sources for the rapid assembly of diverse tricyclic indole scaffolds. J. Am. Chem. Soc. 2020, 142, 6698–6707. [Google Scholar] [CrossRef]
- Qin, H.; Xu, Z.; Cui, Y.; Jia, Y. Total Synthesis of (±)-Decursivine and (±)-Serotobenine: A Witkop photocyclization/elimination/O-Michael addition cascade approach. Angew. Chem. Int. Ed. 2011, 50, 4447–4449. [Google Scholar] [CrossRef]
- Smith, A.B.; Kanoh, N.; Ishiyama, H.; Minakawa, N.; Rainier, J.D.; Hartz, R.A.; Cho, Y.S.; Cui, H.; Moser, W.H. Tremorgenic Indole Alkaloids. The total synthesis of (−)-penitrem D. J. Am. Chem. Soc. 2003, 125, 8228–8237. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.-H.; Chan, A.S.C.; Yu, W.-Y. Pd-catalyzed intermolecular ortho-C-H amidation of anilides by N-nosyloxycarbamate. J. Am. Chem. Soc. 2010, 132, 12862–12864. [Google Scholar] [CrossRef]
- Zheng, H.; Zhu, Y.; Shi, Y. Palladium(0)-catalyzed Heck reaction/C-H activation/amination sequence with diaziridinone: A facile approach to indolines. Angew. Chem. Int. Ed. 2014, 53, 11280–11284. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Z.; Qi, W.; Ji, X.; Cheng, C.; Zhang, Y. Synthesis of indolines by palladium-catalyzed intermolecular amination of unactivated C(sp3)-H bonds. Org. Lett. 2019, 21, 6508–6512. [Google Scholar] [CrossRef]
- Cheng, C.; Zuo, X.; Tu, D.; Wan, B.; Zhang, Y. Synthesis of 3,4-fused tricyclic indoles through cascade carbopalladation and C–H amination: Development and total synthesis of rucaparib. Org. Lett. 2020, 22, 4985–4989. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, L.; Quatrale, A.E.; Mantuano, P.; Leo, M.G.; Silvestris, N.; Rolland, J.F.; Carioggia, E.; Lioce, M.; Paradiso, A.; Azzariti, A. Optimize radiochemotherapy in pancreatic cancer: PARP inhibitors a new therapeutic opportunity. Mol. Oncol. 2013, 7, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Plummer, R.; Lorigan, P.; Steven, N.; Scott, L.; Middleton, M.R.; Wilson, R.H.; Mulligan, E.; Curtin, N.; Wang, D.; Dewji, R.; et al. A phase II study of the potent PARP inhibitor, rucaparib (PF-01367338, AG014699), with temozolomide in patients with metastatic melanoma demonstrating evidence of chemopotentiation. Cancer Chemother. Pharmacol. 2013, 71, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, J.; Zhong, T.; Zheng, X.; Zhou, J.; Jiang, X.; Yu, C. Palladium-catalyzed [2 + 2 + 1] annulation of alkyne-tethered aryl iodides with diaziridinone: Synthesis of 3,4-fused tricyclic indoles. J. Org. Chem. 2020, 85, 10823–10834. [Google Scholar] [CrossRef]
- Wang, W.; Yang, M.; Han, D.; He, Q.; Fan, R. Tandem palladium catalysis for rapid construction of 3,4-fused tricyclic indoles. Adv. Synth. Catal. 2020, 362, 1281–1285. [Google Scholar] [CrossRef]
- Catellani, M.; Frignani, F.; Rangoni, A. A complex catalytic cycle leading to a regioselective synthesis of o,o′-disubstituted vinylarenes. Angew. Chem. Int. Ed. 1997, 36, 119–122. [Google Scholar] [CrossRef]
- Wang, J.; Dong, G. Palladium/norbornene cooperative catalysis. Chem. Rev. 2019, 119, 7478–7528. [Google Scholar] [CrossRef]
- Zhang, B.-S.; Wang, F.; Gou, X.-Y.; Yang, Y.-H.; Jia, W.-Y.; Liang, Y.-M.; Wang, X.-C.; Li, Y.; Quan, Z.-J. Palladium-catalyzed synthesis of tricyclic indoles via a N–S bond cleavage strategy. Org. Lett. 2021, 23, 7518–7523. [Google Scholar] [CrossRef]
- Wu, J.; Li, L.; Liu, M.; Bai, L.; Luan, X. Selective C(sp3)-N bond cleavage of N,N-dialkyl tertiary amines with the loss of a large alkyl group via an SN1 pathway. Angew. Chem. Int. Ed. 2022, 61, e202113820. [Google Scholar]
- Gouda, H.; Matsuzaki, K.; Tanaka, H.; Hirono, S.; Ōmura, S.; McCauley, J.A.; Sprengeler, P.A.; Furst, G.T.; Smith, A.B. Stereostructure of (−)-chloropeptin I, a novel inhibitor of gp120−CD4 binding, via high-temperature molecular dynamics, Monte Carlo conformational searching, and NMR spectroscopy. J. Am. Chem. Soc. 1996, 118, 13087–13088. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Ikeda, H.; Ogino, T.; Matsumoto, A.; Woodruff, H.B.; Tanaka, H.; Omura, S. Chloropeptin-I and chloropeptin-II, novel inhibitors against GP120-CD4 binding from Streptomyces sp. J. Antibiot. 1994, 47, 1173–1174. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Ogino, T.; Sunazuka, T.; Tanaka, H.; Omura, S. Chloropeptins, new anti-HIV antibiotics inhibiting gp120-CD4 binding from Streptomyces sp. II. Structure elucidation of chloropeptin I. J. Antibiot. 1997, 50, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Jung, J.-K.; Liu, T.; Kuntz, K.W.; Snapper, M.L.; Hoveyda, A.H. Total synthesis of anti-HIV agent chloropeptin I. J. Am. Chem. Soc. 2003, 125, 9032–9034. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.N.J.A.; van Dongen, P.W.J.; Vree, T.B.; Hekster, Y.A.; van Roosmalen, J. Ergot alkaloids. Drugs 1998, 56, 523–535. [Google Scholar] [CrossRef]
- Moldvai, I.; Temesvári-Major, E.; Incze, M.; Szentirmay, É.; Gács-Baitz, E.; Szántay, C. Enantioefficient synthesis of α-ergocryptine: First direct synthesis of (+)-lysergic acid. J. Org. Chem. 2004, 69, 5993–6000. [Google Scholar] [CrossRef]
- Kurokawa, T.; Isomura, M.; Tokuyama, H.; Fukuyama, T. Synthesis of lysergic acid methyl ester via the double cyclization strategy. Synlett 2009, 2009, 775–778. [Google Scholar]
- Inoue, T.; Yokoshima, S.; Fukuyama, T. Synthetic studies toward (+)-lysergic acid: Construction of the tetracyclic ergoline skeleton. Hetetocycles 2009, 79, 373–378. [Google Scholar]
- Iwata, A.; Inuki, S.; Oishi, S.; Fujii, N.; Ohno, H. Formal total synthesis of (+)-lysergic acid via zinc(II)-mediated regioselective ring-opening reduction of 2-alkynyl-3-indolyloxirane. J. Org. Chem. 2011, 76, 5506–5512. [Google Scholar] [CrossRef]
- Singh, S.B.; Jayasuriya, H.; Salituro, G.M.; Zink, D.L.; Shafiee, A.; Heimbuch, B.; Silverman, K.C.; Lingham, R.B.; Genilloud, O.; Teran, A.; et al. The complestatins as HIV-1 integrase inhibitors. Efficient isolation, structure elucidation, and inhibitory activities of isocomplestatin, chloropeptin I, new complestatins, A and B, and acid-hydrolysis products of chloropeptin I. J. Nat. Prod. 2001, 64, 874–882. [Google Scholar] [CrossRef]
- Breazzano, S.P.; Boger, D.L. Synthesis and stereochemical determination of complestatin A and B (neuroprotectin A and B). J. Am. Chem. Soc. 2011, 133, 18495–18502. [Google Scholar] [CrossRef]
- Stratmann, K.; Moore, R.E.; Bonjouklian, R.; Deeter, J.B.; Patterson, G.M.L.; Shaffer, S.; Smith, C.D.; Smitka, T.A. Welwitindolinones, unusual alkaloids from the blue-green algae Hapalosiphon welwitschii and Westiella intricata. Relationship to fischerindoles and hapalinodoles. J. Am. Chem. Soc. 1994, 116, 9935–9942. [Google Scholar] [CrossRef]
- Jimenez, J.I.; Huber, U.; Moore, R.E.; Patterson, G.M.L. Oxidized welwitindolinones from terrestrial Fischerella spp. J. Nat. Prod. 1999, 62, 569–572. [Google Scholar] [CrossRef]
- Komine, K.; Nomura, Y.; Ishihara, J.; Hatakeyama, S. Total synthesis of (−)-N-methylwelwitindolinone C isothiocyanate based on a Pd-catalyzed tandem enolate coupling strategy. Org. Lett. 2015, 17, 3918–3921. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Shan, D.; Pitchakuntla, M.; Ma, Y.; Jia, Y. Total syntheses of festuclavine, pyroclavine, costaclavine, epi-costaclavine, pibocin A, 9-deacetoxyfumigaclavine C, fumigaclavine G, and dihydrosetoclavine. Org. Lett. 2017, 19, 3323–3326. [Google Scholar] [CrossRef] [PubMed]
- Milde, B.; Pawliczek, M.; Jones, P.G.; Werz, D.B. Enantioselective total synthesis of (+)-lysergol: A formal anti-carbopalladation/Heck cascade as the key step. Org. Lett. 2017, 19, 1914–1917. [Google Scholar] [CrossRef] [PubMed]
- Marki, F.; Robertson, A.V.; Witkop, B. Dehydrobufotenine, a novel type of tricyclic serotonin metabolite from Bufo Marinus. J. Am. Chem. Soc. 1961, 83, 3341–3342. [Google Scholar] [CrossRef]
- Peat, A.J.; Buchwald, S.L. Novel Syntheses of Tetrahydropyrroloquinolines: Applications to alkaloid synthesis. J. Am. Chem. Soc. 1996, 118, 1028–1030. [Google Scholar] [CrossRef]
- Baran, P.S.; Maimone, T.J.; Richter, J.M. Total synthesis of marine natural products without using protecting groups. Nature 2007, 446, 404–408. [Google Scholar] [CrossRef]
- Stauffacher, D.; Niklaus, P.; Tscherter, H.; Weber, H.P.; Hofmann, A. Cycloclavin, ein neues alkaloid aus Ipomoea hildebrandtii vatke—71: Mutterkornalkaloide. Tetrahedron 1969, 25, 5879–5887. [Google Scholar] [CrossRef]
- Incze, M.; Dörnyei, G.; Moldvai, I.; Temesvaári-Major, E.; Egyed, O.; Szaántay, C. New routes to clavine-type ergot alkaloids. Part 2: Synthesis of the last, so far not yet synthesized member of the clavine alkaloid family, (±)-cycloclavine. Tetrahedron 2008, 64, 2924–2929. [Google Scholar] [CrossRef]
- Petronijevic, F.R.; Wipf, P. Total synthesis of (±)-cycloclavine and (±)-5-epi-cycloclavine. J. Am. Chem. Soc. 2011, 133, 7704–7707. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, J.-T.; Zhang, H.-L.; Shi, Z.-F.; Wen, J.; Cao, X.-P. Formal synthesis of (±)-cycloclavine. J. Org. Chem. 2014, 79, 122–127. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Zhu, X.; Liu, X.; He, F.; Yang, G.; Xu, C.; Yang, X. Recent Advances in the Synthesis of 3,n-Fused Tricyclic Indole Skeletons via Palladium-Catalyzed Domino Reactions. Molecules 2023, 28, 1647. https://doi.org/10.3390/molecules28041647
Fan L, Zhu X, Liu X, He F, Yang G, Xu C, Yang X. Recent Advances in the Synthesis of 3,n-Fused Tricyclic Indole Skeletons via Palladium-Catalyzed Domino Reactions. Molecules. 2023; 28(4):1647. https://doi.org/10.3390/molecules28041647
Chicago/Turabian StyleFan, Liangxin, Xinxin Zhu, Xingyuan Liu, Fangyu He, Guoyu Yang, Cuilian Xu, and Xifa Yang. 2023. "Recent Advances in the Synthesis of 3,n-Fused Tricyclic Indole Skeletons via Palladium-Catalyzed Domino Reactions" Molecules 28, no. 4: 1647. https://doi.org/10.3390/molecules28041647
APA StyleFan, L., Zhu, X., Liu, X., He, F., Yang, G., Xu, C., & Yang, X. (2023). Recent Advances in the Synthesis of 3,n-Fused Tricyclic Indole Skeletons via Palladium-Catalyzed Domino Reactions. Molecules, 28(4), 1647. https://doi.org/10.3390/molecules28041647




