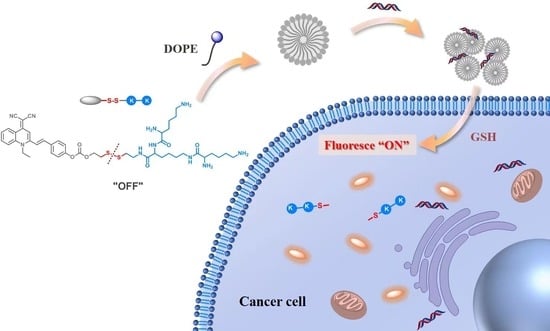
GSH-Activatable Aggregation-Induced Emission Cationic Lipid for Efficient Gene Delivery
Abstract
:1. Introduction
2. Result and Discussion
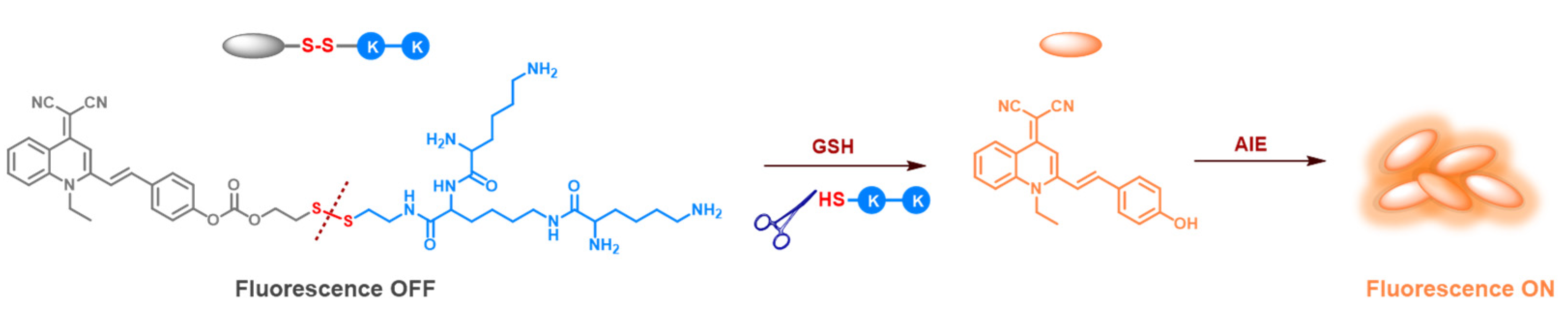
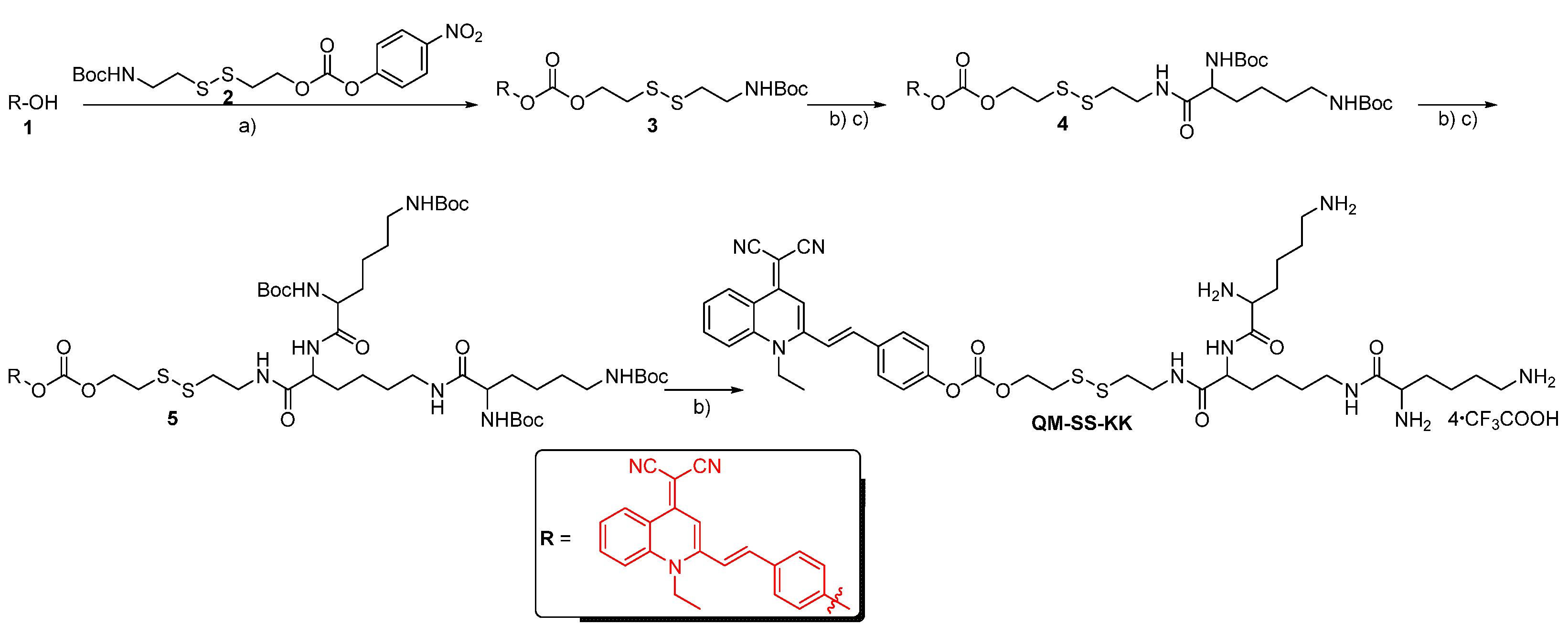
2.1. Syntheses of Lipid QM-SS-KK
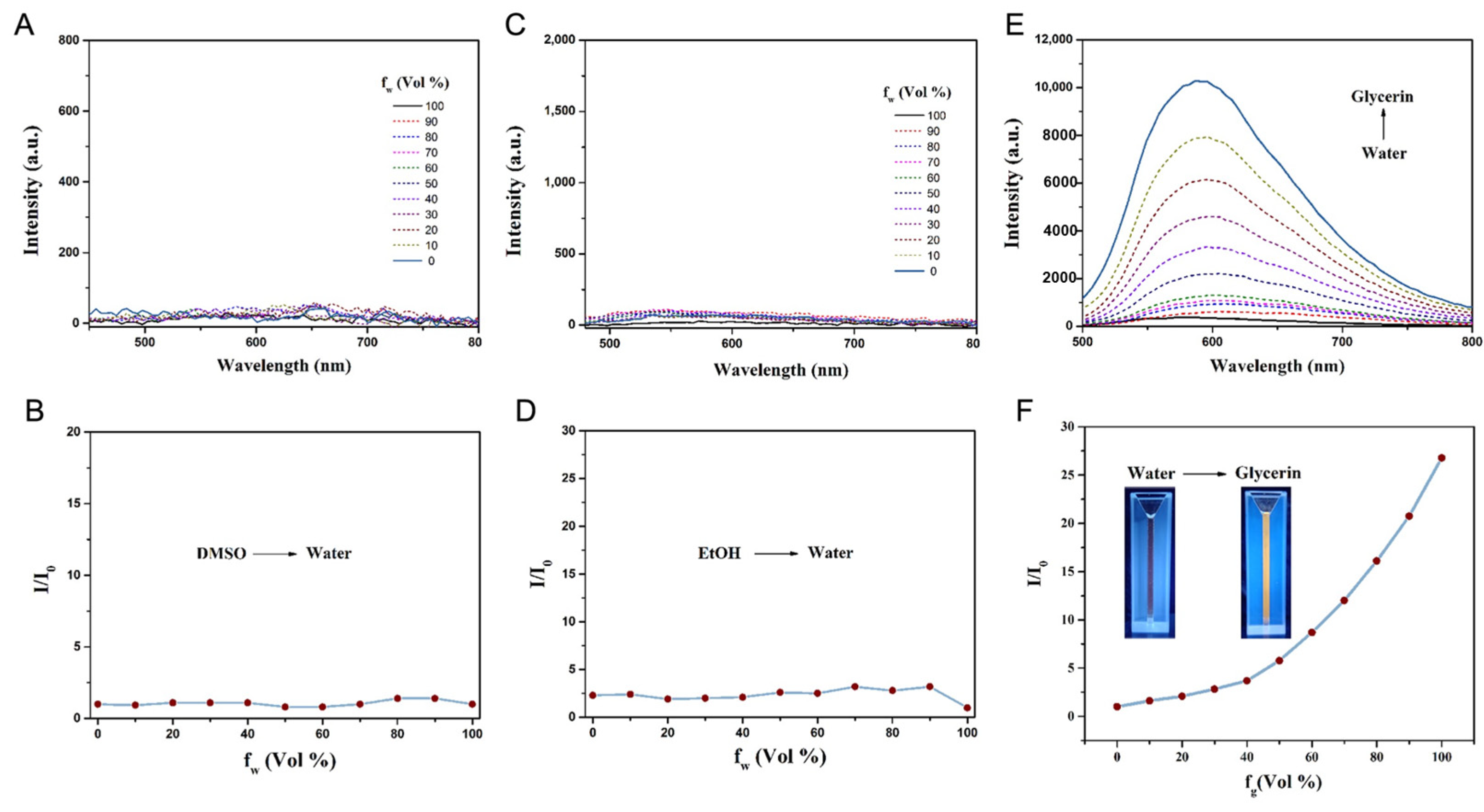
2.2. Photophysical Characterization
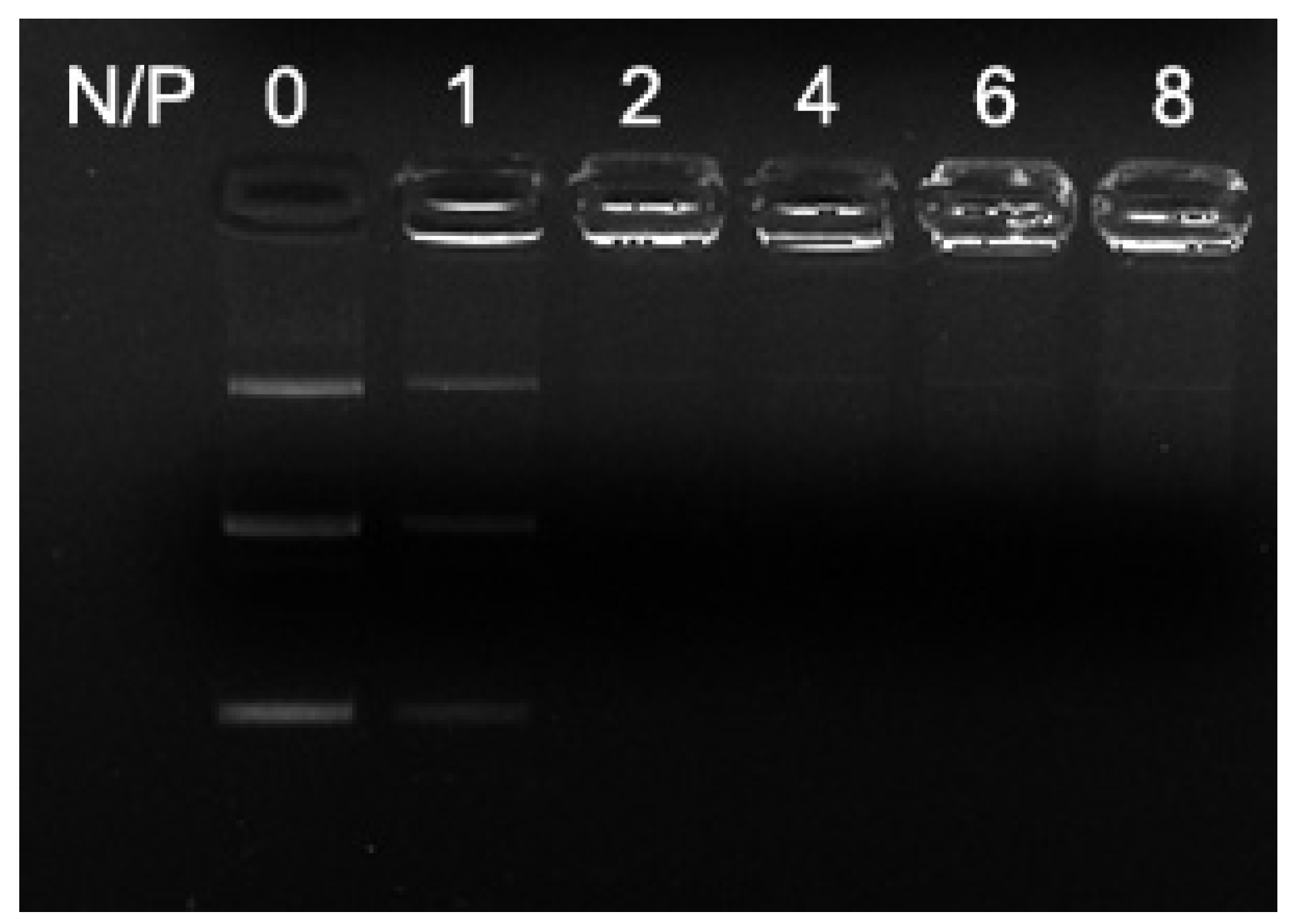
2.3. Agarose-Gel Retardant Assays of Liposomes and Lipoplexes
2.4. Hydrodynamic Radii and Zeta Potentials of Lipoplexes
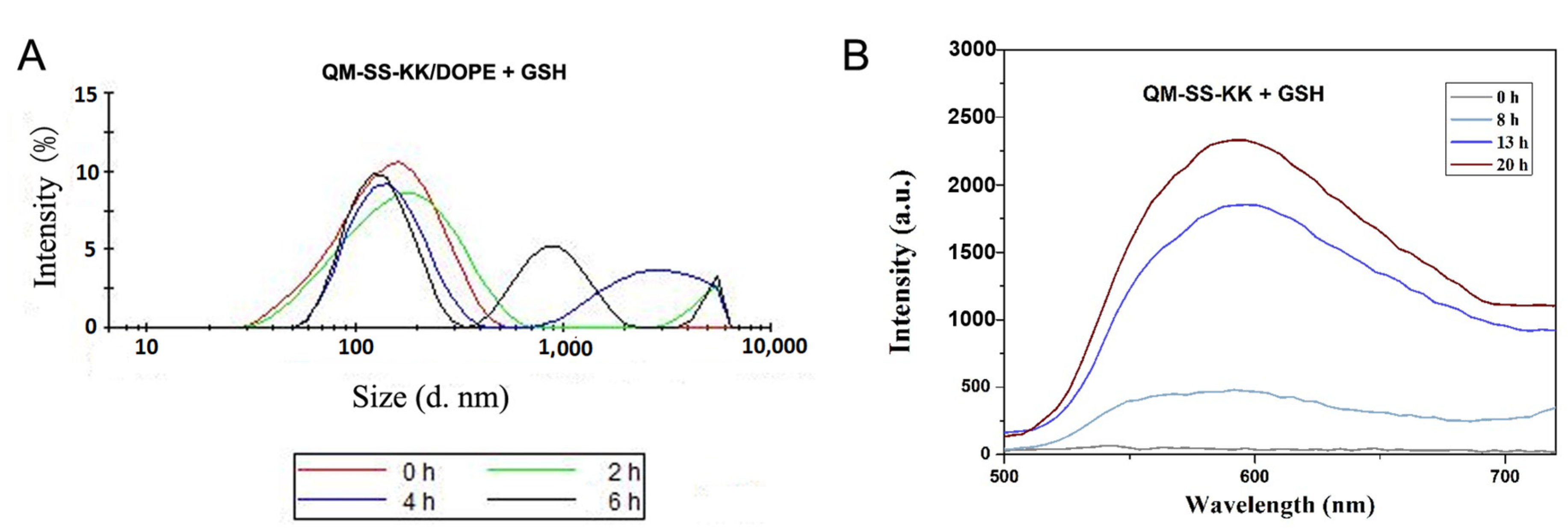
2.5. Reduction-Triggered Cleavage of Lipid and Liposome
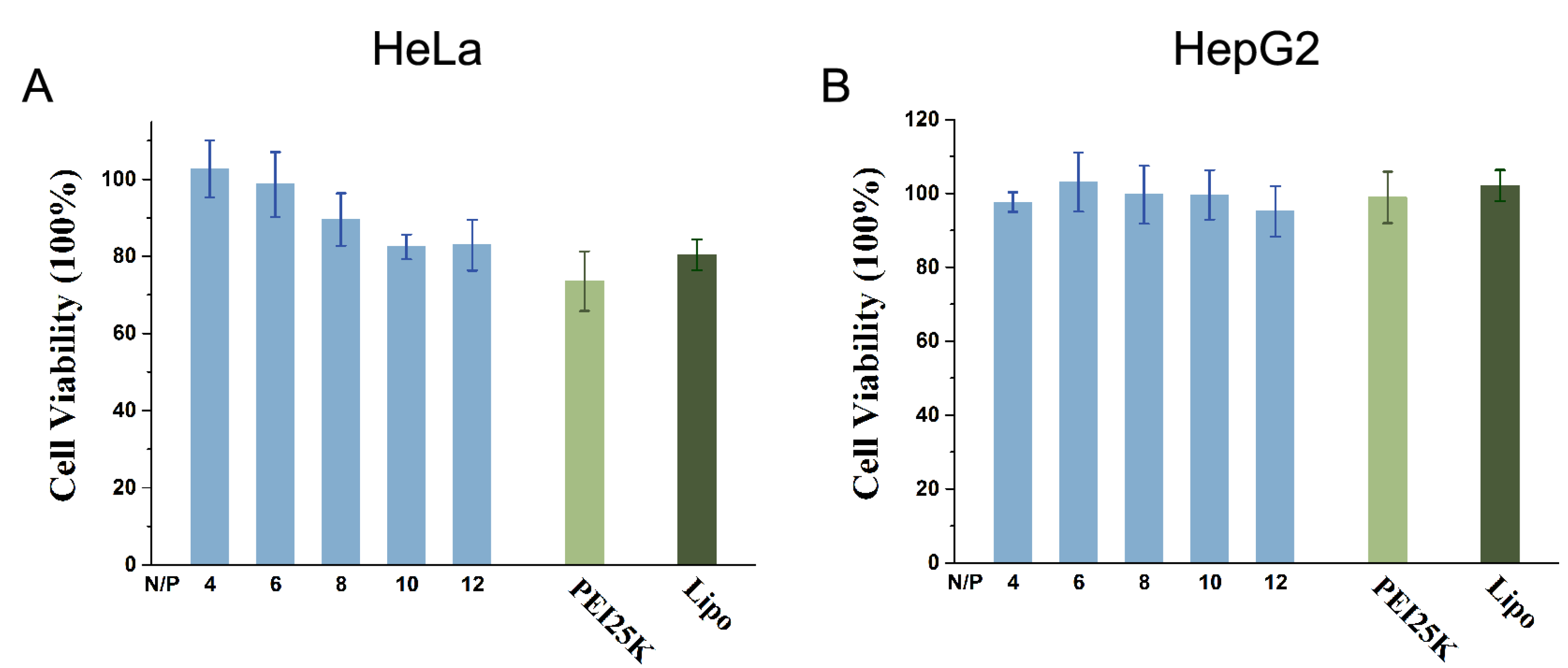
2.6. Cytotoxicity Assay
2.7. In Vitro Transfection
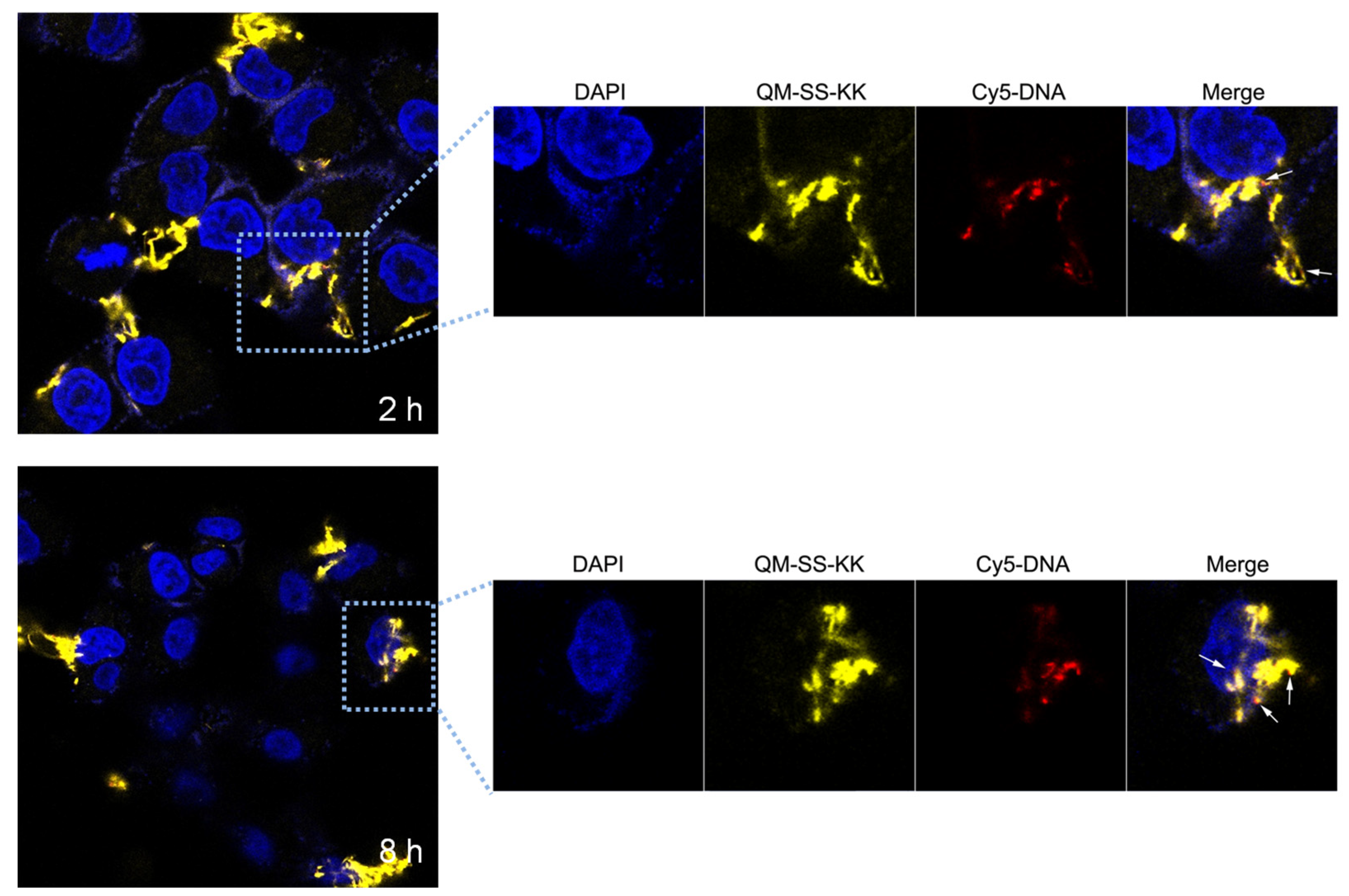
2.8. CLSM (Confocal Laser Scanning Microscopy) Measurements
3. Experimental Section
3.1. Materials
3.2. Synthesis of Lipid QM-SS-KK
3.2.1. Preparation of Compound 3
3.2.2. Preparation of Compound 4
3.2.3. Preparation of Compound 5
3.2.4. Preparation of Lipid QM-SS-KK
3.3. Preparation of the Cationic Liposomes
3.4. Agarose-Gel Retardation Assays
3.5. Fluorescence Assays
3.6. Lipoplex Particle Sizes and Zeta Potentials
3.7. MTT Cytotoxicity Assays
3.8. In Vitro Transfection Assays
3.9. Confocal Laser Scanning Microscopy (CLSM) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ediriweera, G.R.; Chen, L.; Yerbury, J.J.; Thurecht, K.J.; Vine, K.L. Non-viral vector-mediated gene therapy for ALS: Challenges and future perspectives. Mol. Pharm. 2021, 18, 2142–2160. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Z.; Zhang, J.; Guo, Y.; Pu, L.; Yu, X.Q. Fluorescent self-reporting vector with GSH reduction responsiveness for nucleic acid delivery. ACS Appl. Bio. Mater. 2021, 4, 5717–5726. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Ponti, F.; Campolungo, M.; Melchiori, C.; Bono, N.; Candiani, G. Cationic lipids for gene delivery: Many players, one goal. Chem. Phys. Lipids 2021, 235, 105032. [Google Scholar] [CrossRef]
- Qiu, M.; Li, Y.; Bloomer, H.; Xu, Q. Developing biodegradable lipid nanoparticles for intracellular mRNA delivery and genome editing. Acc. Chem. Res. 2021, 54, 4001–4011. [Google Scholar] [CrossRef]
- Ferhan, A.R.; Park, S.; Park, H.; Tae, H.; Jackman, J.A.; Cho, N.-J. Lipid nanoparticle technologies for nucleic acid delivery: A nanoarchitectonics perspective. Adv. Funct. Mater. 2022, 32, 2203669. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Wang, C.; Jankovic, K.E.; Dong, Y. Lipids and lipid derivatives for RNA delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef]
- Pezzoli, D.; Zanda, M.; Chiesa, R.; Candiani, G. The yin of exofacial protein sulfhydryls and the yang of intracellular glutathione in in vitro transfection with SS14 bioreducible lipoplexes. J. Control. Release 2013, 165, 44–53. [Google Scholar] [CrossRef]
- Zhi, D.; Zhang, S.; Cui, S.; Zhao, Y.; Wang, Y.; Zhao, D. The headgroup evolution of cationic lipids for gene delivery. Bioconjugate Chem. 2013, 24, 487–519. [Google Scholar] [CrossRef]
- Hadianamrei, R.; Zhao, X. Current state of the art in peptide-based gene delivery. J. Control. Release 2022, 343, 600–619. [Google Scholar] [CrossRef]
- Su, R.C.; Liu, Q.; Yi, W.J.; Zheng, L.T.; Zhao, Z.G. Lipoic acid functionalized amino acids cationic lipids as gene vectors. Bioorg. Med. Chem. Lett. 2016, 26, 4692–4697. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Su, R.C.; Yi, W.J.; Zheng, L.T.; Lu, S.S.; Zhao, Z.G. PH and reduction dual-responsive dipeptide cationic lipids with α-tocopherol hydrophobic tail for efficient gene delivery. Eur. J. Med. Chem. 2017, 129, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.T.; Yi, W.J.; Su, R.C.; Liu, Q.; Zhao, Z.G. Reducible amino acid based cationic lipids as highly efficient and serum-tolerant gene vectors. ChemPlusChem 2016, 81, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, L.; Hu, P.; Yang, Y.; Wei, W.; Deng, X.; Wang, L.; Tay, F.R.; Ma, J. Recent advances in stimulus-responsive nanocarriers for gene therapy. Adv. Sci. 2021, 8, 2100540. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Du, T.-T.; Yi, W.-J.; Liu, Q. Reducible amino acid based cationic lipids with a naphthalimide moiety as non-viral gene vehicles. J. Chem. Res. 2022, 46. [Google Scholar] [CrossRef]
- Wang, B.; Chen, P.; Zhang, J.; Chen, X.C.; Liu, Y.H.; Huang, Z.; Yu, Q.Y.; Zhang, J.H.; Zhang, W.; Wei, X.; et al. Self-assembled core-shell-corona multifunctional non-viral vector with AIE property for efficient hepatocyte-targeting gene delivery. Polym. Chem. 2017, 8, 7486–7498. [Google Scholar] [CrossRef]
- Liu, D.E.; Yan, X.; An, J.; Ma, J.; Gao, H. Construction of traceable cucurbituril-based virus-mimicking quaternary complexes with aggregation-induced emission for efficient gene transfection. J. Mater. Chem. B 2020, 8, 7475–7482. [Google Scholar] [CrossRef]
- Ding, A.-X.; Tan, Z.-L.; Shi, Y.-D.; Song, L.; Gong, B.; Lu, Z.-L. Gemini-type tetraphenylethylene amphiphiles containing [12]aneN3 and long hydrocarbon chains as nonviral gene vectors and gene delivery monitors. ACS Appl. Mater. Interfaces 2017, 9, 11546–11556. [Google Scholar] [CrossRef]
- Tang, F.; Wang, Q.; Gao, Y.N.; Zhang, Y.S.; Liang, Y.X.; Lu, Z.L.; Liu, R.; Ding, A.X. A NIR aggregation-induced emission fluoroamphiphile as visually trackable and serum-tolerant nonviral gene carrier. Bioconjugate Chem. 2022, 33, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Liu, M.X.; Liu, X.Y.; Sun, W.; Lu, Z.L.; Gao, Y.G.; He, L. Macrocyclic polyamine [12]aneN3 modified triphenylamine-pyrazine derivatives as efficient non-viral gene vectors with AIE and two-photon imaging properties. J. Mater. Chem. B 2020, 8, 3869–3879. [Google Scholar] [CrossRef]
- Suzuki, S.; Sasaki, S.; Sairi, A.S.; Iwai, R.; Tang, B.Z.; Konishi, G.I. Principles of aggregation-induced emission: Design of deactivation pathways for advanced AIEgens and applications. Angew. Chem. Int. Ed. 2020, 59, 9856–9867. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Jiang, W.; Yuan, L.; Duan, C.; Yuan, Q.; Long, Z.; Lou, X.D.; Xia, F. Recent advances in stimuli-responsive theranostic systems with aggregation-induced emission characteristics. Aggregate 2021, 2, 48–65. [Google Scholar] [CrossRef]
- Cai, X.; Liu, B. Aggregation-induced emission: Recent advances in materials and biomedical applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886. [Google Scholar] [CrossRef]
- Guo, Z.; Yan, C.; Zhu, W.-H. High-performance quinoline-malononitrile core as a building block for the diversity-oriented synthesis of AIEgens. Angew. Chem. Int. Ed. 2020, 59, 9812–9825. [Google Scholar] [CrossRef]
- Gu, K.; Qiu, W.; Guo, Z.; Yan, C.; Zhu, S.; Yao, D.; Shi, P.; Tian, H.; Zhu, W.H. An enzyme-activatable probe liberating AIEgens: On-site sensing and long-term tracking of β-galactosidase in ovarian cancer cells. Chem. Sci. 2018, 10, 398–405. [Google Scholar] [CrossRef]
- Fu, W.; Yan, C.; Guo, Z.; Zhang, J.; Zhang, H.; Tian, H.; Zhu, W.H. Rational Design of Near-Infrared Aggregation-Induced-Emission-Active Probes: In situ mapping of amyloid-β plaques with ultra sensitivity and high-fidelity. J. Am. Chem. Soc. 2019, 141, 3171–3177. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Q.; Chen, X.; Wang, Q.; Yan, C.; Zhao, X.; Zhao, W.; Zhu, W.-H. An enzyme-activatable aggregation-induced-emission probe: Intraoperative pathological fluorescent diagnosis of pancreatic cancer via specific cathepsin E. Adv. Mater. 2022, 34, 2107444. [Google Scholar] [CrossRef]
- Lyu, Y.; Chen, X.; Wang, Q.; Li, Q.; Wang, Q.; Li, X.; Zhu, Z.; Yan, C.; Zhao, X.; Zhu, W.-H. Monitoring autophagy with Atg4B protease-activated aggregation-induced emission probe. Adv. Funct. Mater. 2022, 32, 2108571. [Google Scholar] [CrossRef]
- Li, H.; Yao, Q.; Xu, F.; Li, Y.; Kim, D.; Chung, J.; Baek, G.; Wu, X.; Hillman, P.F.; Lee, E.Y.; et al. An activatable AIEgen probe for high-fidelity monitoring of overexpressed tumor enzyme activity and its application to surgical tumor excision. Angew Chem. Int. Ed. 2020, 59, 10186–10195. [Google Scholar] [CrossRef] [PubMed]
- Centillion Technology Holdings Corporation. Disulfide-Linked Reversible Terminators. U.S. Patent 2016/031416, 17 November 2016.
- Wang, D.; Tang, B.Z. Aggregation-induced emission luminogens for activity-based sensing. Acc. Chem. Res. 2019, 52, 2559–2570. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.-R.; Liu, Q.; Wang, D.; Deng, Y.-D.; Du, T.-T.; Yi, W.-J.; Yang, S.-T. GSH-Activatable Aggregation-Induced Emission Cationic Lipid for Efficient Gene Delivery. Molecules 2023, 28, 1645. https://doi.org/10.3390/molecules28041645
Yuan Y-R, Liu Q, Wang D, Deng Y-D, Du T-T, Yi W-J, Yang S-T. GSH-Activatable Aggregation-Induced Emission Cationic Lipid for Efficient Gene Delivery. Molecules. 2023; 28(4):1645. https://doi.org/10.3390/molecules28041645
Chicago/Turabian StyleYuan, Yue-Rui, Qiang Liu, Deyu Wang, Yu-Dan Deng, Ting-Ting Du, Wen-Jing Yi, and Sheng-Tao Yang. 2023. "GSH-Activatable Aggregation-Induced Emission Cationic Lipid for Efficient Gene Delivery" Molecules 28, no. 4: 1645. https://doi.org/10.3390/molecules28041645
APA StyleYuan, Y.-R., Liu, Q., Wang, D., Deng, Y.-D., Du, T.-T., Yi, W.-J., & Yang, S.-T. (2023). GSH-Activatable Aggregation-Induced Emission Cationic Lipid for Efficient Gene Delivery. Molecules, 28(4), 1645. https://doi.org/10.3390/molecules28041645