Abstract
A sequential Rh(III)-catalyzed C-H activation/annulation of N-hydroxybenzamides with propargylic acetates leading to the formation of NH-free isoquinolones is described. This reaction proceeds through a sequential C-H activation/alkyne insertion/intramolecular annulation/N-O bond cleavage procedure, affording a broad spectrum of products with diverse substituents in moderate-to-excellent yields. Notably, this protocol features the simultaneous formation of two new C-C/C-N bonds and one heterocycle in one pot with the release of water as the sole byproduct.
1. Introduction
Isoquinolone is a ubiquitous, structural motif that presents in various natural products, conjugated materials, and pharmaceuticals with a wide range of biological activities [1,2,3,4]. Meanwhile, isoquinolone derivatives, as versatile intermediates, provide wide access to a large variety of chemical molecules and drug structures in various organic transformations [5,6,7]. In this regard, the formation of isoquinolone derivatives has gained significant attention among synthetic and medicinal chemists [8,9,10,11,12,13,14]. Traditional methods to access these valuable derivatives involve the Bischler-Napieralski and Pictet-Spengler reactions [15,16], but they often suffer from the need for pre-activated substrates and harsh reaction conditions, which restrict their regioselectivity. Consequently, the development of efficient and atom/step-economical synthetic methods to construct these structures has attracted considerable attention from synthetic chemists.
In the last few decades, transition-metal-catalyzed C-H activation/annulations have been recognized as a powerful and straightforward approach for the synthesis of N-heterocycles and, therefore, have attracted increasing attention [17,18,19,20]. Recently, the transition-metal-catalyzed oxidative cyclization of N-substituted benzamides with symmetrical alkynes or diazos via C-H bond activation has been an efficient method for constructing N-protected isoquinolone derivatives [21,22,23,24,25,26,27,28]. Meanwhile, many other substrates bearing the N-directing group have also been used to construct an isoquinolone scaffold through the C-H activation/annulation strategy [29,30,31]. Despite these achievements, directing access to NH-free isoquinolone scaffolds via cascade C-H activation/annulation has attracted our great interest [32]. Ackermann reported the preparations of isoquinolone derivatives by Ru-catalyzed C-H functionalization/annulation of N-methoxybenzamides or N-hydroxybenzamides with alkynes in water in the presence of MesCO2K or 3-(CF3)C6H4CO2K as the cocatalytic additive (Scheme 1a) [33,34]. Later, a Rh(III)-catalyzed synthesis of isoquinolones via C-H activation/annulation of benzoylhydrazines and alkynes was further developed (Scheme 1b) [35]. In addition, Li described a highly efficient synthesis pathway by the reaction of iminopyridinium ylides with alkynes for the formation of an isoquinolone skeleton (Scheme 1c) [36]. Very recently, Wu disclosed a robust and convenient rhodium-catalyzed regioselective C-H activation/[4 + 2] annulation using propargyl alcohols as two-carbon synthons to construct 3-methylisoquinolones (Scheme 1d) [37]. Among the limited successful procedures with unsymmetric alkynes serving as the coupling partners, it is desirable to explore a new pathway for furnishing greater structural diversity of NH-free isoquinolone derivatives. We herein describe a novel rhodium(III)-catalyzed cascade C-H activation/annulation of N-hydroxybenzamides and propargylic acetates to generate NH-free 3-aryisoquinolones, releasing H2O as the sole byproduct (Scheme 1e).
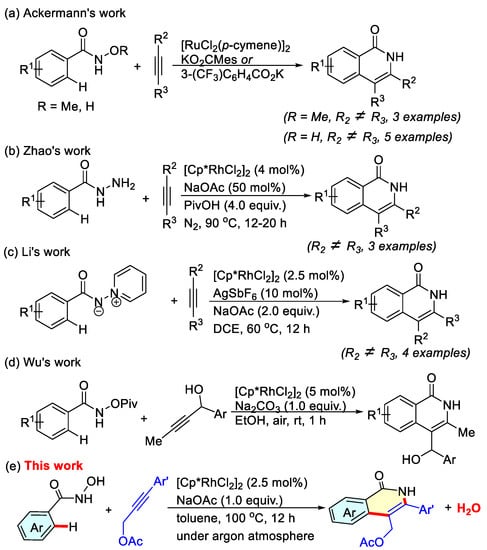
Scheme 1.
Synthesis of NH-free isoquinolones via C-H activation/annulation strategy.
2. Results and Discussion
Our study commenced with the reaction between N-hydroxybenzamide (1a) and 3-phenylprop-2-yn-1-yl acetate (2a) in the presence of [Cp * RhCl2]2 as catalyst (Table 1, entry 5). For experimental condition screening, the catalyst precursor [Cp * RhCl2]2 was fixed at 2.5 mol%, and, with addition of chloride abstracting reagent NaOAc (1.0 equiv.), the [4 + 2] annulated product 3aa was isolated in 84% yield after 12 h at 100 °C in toluene (Table 1, entry 5). The reaction was then performed in the presence of other transition metal catalysts, but none of them was as effective as [Cp * RhCl2]2 (Table 1, entries 1–4). Changing the catalyst loading failed to improve the reaction yield (Table 1, entries 6, 7). Investigation of the additive showed that NaOAc was the best choice (Table 1, entries 8–17). In addition, either decreasing or increasing the loading of additive did not improve this transformation (Table 1, entries 19, 20). Furthermore, several common organic solvents were investigated, and the results indicated that toluene favored this transformation (Table 1, entries 21–26). There was no extra benefit to the product yield at elevated reaction temperature and reaction time (Table 1, entries 27–30). Finally, we chose the reaction conditions of entry 5 as the optimal conditions.

Table 1.
Optimization of the reaction conditions a.
With the establishment of the optimum conditions, we first investigated the scope and generality of N-hydroxybenzamide substrates (Scheme 2a). It was found that a variety of substituted N-hydroxybenzamides reacted smoothly with 3-phenylprop-2-yn-1-yl acetate 2a to produce the corresponding isoquinolones in 41–89% yields. In general, N-hydroxybenzamides with either electron-donating (e.g., -Me, -Et, -t Bu, -OMe) or electron-withdrawing (e.g., -F, -Cl, -Br) groups at the para position of the benzene ring worked well with 2a to afford the corresponding products 3ba–3ha in 53–87% yields. Notably, various functional groups, including phenyl and even strong electron-withdrawing substituents -CN, -NO2, and -CF3, were tolerated well to supply the desired products 3ia–3la in 69–89% yields. Furthermore, meta- and ortho-substituted substrates were also tolerated regardless of the electronic property on the benzene ring, providing the desired isoquinolones 3ma–3ra in 41–78% yields. In addition, disubstituted N-hydroxybenzamides were also reactive to produce the corresponding products in 71–79% yields (products 3sa–3wa). Moreover, this transformation was further extended to N-hydroxy-2-naphthamide and N-hydroxythiophene-2-carboxamide substrates, giving the corresponding products 3xa and 3ya in 63% and 51% yields, respectively. Subsequently, we probed the scope of this transformation, employing propargylic acetates 2 bearing diverse substituents at the para position of the benzene ring and leading to the corresponding products 3ab–3ai in 49–89% yields (Scheme 2b). Encouragingly, the halo groups on either benzamide, as well as on the propargylic acetates moiety, were well tolerated to produce the target products, which may have potential applications in organic synthesis by further functionalization through Pd-catalyzed coupling reactions.

Scheme 2.
Substrate scope. Reaction conditions: N-hydroxybenzamides 1 (0.2 mmol), propargylic acetates 2 (0.2 mmol), [Cp * RhCl2]2 (2.5 mol%), and NaOAc (0.2 mmol) in toluene at 100 °C for 12 h under argon atmosphere. Isolated yields were reported.
To further prove the robustness and the general utility of this protocol, we carried out the reaction of N-hydroxybenzamide 1a and 3-phenylprop-2-yn-1-yl acetate 2a in gram-scale synthesis under the standard condition. This transformation was easily scaled up to 6 mmol (scaled up to 30 times), producing the desired product 3aa in 69% yield (Scheme 3a). Subsequently, insights into this cascade C-H activation/annulation were gained by performing control experiments to clarify the reaction mechanism. Treating N-hydroxybenzamide 1a with methanol-d4 under standard conditions for 2 h, a 74% deuterium was detected on the ortho C-H bond (Scheme 3b(i)), which indicated that the C-H activation process might be the reversible step. Next, a kinetic isotope effect (KIE) value of 1.08 was measured from two parallel reactions of N-hydroxybenzamide 1a or 1a-d5 with propargylic acetate 2a for 2 h under the standard conditions (Scheme 3b(ii)). The intermolecular competitive experiment of para-methoxyl- and para-trifluoromethyl-substituted N-hydroxybenzamide showed that the electron-donating group facilitated the reaction step, implying that C(sp2)-H bond cleavage might be the limiting step (Scheme 3b(iii)). As for the distinct propargylic acetates 2b and 2g, the electron-deficient propargylic acetate 2g delivered the product in a higher yield.
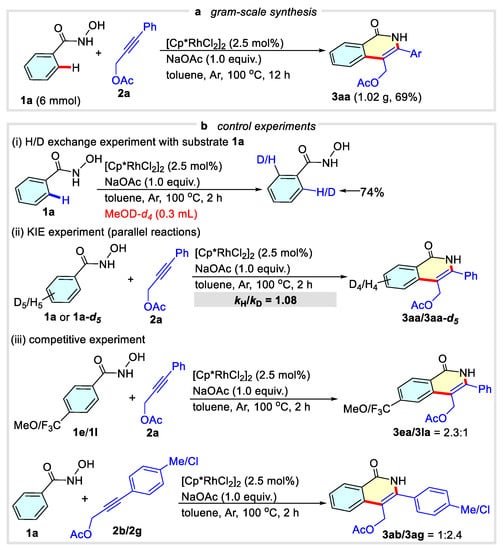
Scheme 3.
Gram-scale synthesis and control experiments.
On the basis of the above control experiments and relevant reports [33,36,38], a plausible mechanism for the Rh-catalyzed cascade C-H activation/annulation for the formation of NH-free isoquinolones is proposed in Scheme 4. Initially, the active catalyst was generated via ligand exchange, which mediated a facile C-H metalation process to give the five-membered rhodacycle A. Subsequent coordination of the alkyne to the rhodium center followed by the 1,2-insertion of the alkyne afforded the seven-membered rhodacycle C. Then, the metal migration and C-N bond formation of intermediate C afforded the intermediate D, which underwent the protonation to generate the desired product 3aa along with the release of a molecule of water.

Scheme 4.
Proposed mechanism for the formation of NH-free isoquinolones.
3. Materials and Methods
The detailed procedures for the synthesis and characterization of the products are given in Appendix A.
4. Conclusions
In conclusion, we developed an efficient and practical method to construct NH-free isoquinolones via Rh(III)-catalyzed C-H activation/annulation of N-hydroxybenzamides and propargylic acetates. A variety of N-hydroxybenzamides with a diverse array of substituents, irrespective of their electronic and steric nature, were tolerated well under the optimal conditions. Generation of H2O as the only byproduct makes this 100% carbon-efficient process attractive for the synthesis of isoquinolone derivatives. Moreover, the synthetic utility and practicability of the developed methodology in gram-scale synthesis were validated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27238553/s1, Characterization data for product 3, including 1H- and 13C-NMR spectroscopies, are available online. CCDC 2220211 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif or by emailing data_request@ccdc.cam.ac.uk or by contacting the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK; fax: +44-1223-336033.
Author Contributions
Conceptualization, X.H. and Y.S.; methodology, T.F. and S.Z.; formal analysis, S.K.; investigation, T.F. and S.Z.; data curation, T.Y. and K.T.; writing—original draft preparation, T.F. and Q.Y.; writing—review and editing, X.H. and Q.Y.; supervision, Q.Y., X.H. and Y.S.; project administration, X.H. and Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the Anhui Provincial Natural Science Foundation (no. 1808085MB41), the National Natural Science Foundation of China (no. 21772001), the University Synergy Innovation Program of Anhui Province (no. GXXT-2020-074), and the Excellent Young Talents Support Program of Education Administration of Anhui Province (no. gxyq2022233).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Experimental Section
Unless otherwise noted, all reagents were purchased from commercial suppliers and used without purification. All cascade reactions were performed in a resealable screw-capped Schlenk flask (approx. 15 mL volume) in the presence of a Teflon-coated magnetic stirrer bar (4 mm × 10 mm). Reactions were monitored by using thin-layer chromatography (TLC) on commercial silica gel plates (GF 254). Visualization of the developed plates was performed under UV lights (GF 254 nm). Flash column chromatography was performed on silica gel (200–300 mesh). 1H NMR spectra were recorded on a 500 MHz spectrometer, and 13C NMR spectra were recorded on a 125 MHz spectrometer (Supplementary Materials: 1H NMR and 13C NMR). Chemical shifts were expressed in parts per million (δ), and the signals were reported as s (singlet), d (doublet), dd (doublet of doublet), t (triplet), q (quartet), and m (multiplet), and coupling constants (J) were given in Hz. Chemical shifts as internal standards were referenced to CDCl3 (δ = 7.26 for 1H and δ = 77.16 for 13C NMR) as internal standard. HRMS analysis with a quadrupole time-of-flight mass spectrometer yielded ion mass/charge (m/z) ratios in atomic mass units. The melting points were measured using SGWX-4 melting point apparatus and were not corrected. The X-ray source used for the single-crystal X-ray diffraction analysis of compound 3aa was Mo Kα (λ = 0.71073 Å), and the thermal ellipsoid was drawn at the 30% probability level (Supplementary Materials: X-ray crystal data).
General procedure for the synthesis of isoquinolones 3 (product 3aa as an example). N-Hydroxybenzamides 1a (27.4 mg, 0.2 mmol), [Cp * RhCl2]2 (3.1 mg, 2.5 mol%), and NaOAc (16.4 mg, 0.2 mmol) were loaded into a Schlenk tube equipped with a Teflon-coated magnetic stir bar. The tube was evacuated and flushed with argon for three cycles. Propargylic acetates 2a (34.8 mg, 0.2 mmol) and toluene (2 mL) were then added, and the tube was placed into a preheated oil bath (100 °C) and stirred for 12 h. After the completion of the reaction, the reaction tube was allowed to cool to room temperature, extracted with CH2Cl2 (3 × 10 mL), and washed with brine. The organic layers were combined, dried over Na2SO4, filtered, and then evaporated under vacuum. The residue was purified using flash column chromatography with a silica gel (200–300 mesh) and using ethyl acetate and petroleum ether as the elution solvent to give desired product 3.
(1-Oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3aa). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 84% yield (49 mg); mp 163–164 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.58 (s, 1H), 8.29 (d, J = 8.0 Hz, 1H), 7.82–7.75 (m, 2H), 7.58–7.47 (m, 6H), 4.97 (s, 2H), 2.03 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.9, 162.2, 143.2, 137.5, 133.9, 133.4, 129.9, 129.8, 128.9, 127.5, 127.0, 125.8, 124.1, 106.8, 60.7, 21.2; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H16NO3 294.1125; Found 294.1127.
(6-Methyl-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ba). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 87% yield (53 mg); mp 166–167 °C; 1H NMR (500 MHz, CDCl3) δ 9.52 (s, 1H), 8.29 (d, J = 8.5 Hz, 1H), 7.52–7.47 (m, 6H), 7.36 (d, J = 8.0 Hz, 1H), 5.13 (s, 2H), 2.53 (s, 3H), 2.12 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 163.1, 144.4, 142.4, 142.3, 138.0, 134.3, 130.3, 129.3, 128.9, 128.3, 123.6, 108.0, 61.2, 22.7, 21.5; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H18NO3 308.1281; Found 308.1283.
(6-Ethyl-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ca). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 85% yield (54 mg); mp 172–173 °C; 1H NMR (500 MHz, CDCl3) δ 9.76 (s, 1H), 8.30 (d, J = 8.0 Hz, 1H), 7.50–7.48(m, 6H), 7.39 (d, J = 8.0 Hz, 1H), 5.15 (s, 2H), 2.82 (q, J = 7.5, 2H), 2.12 (s, 3H), 1.31 (t, J = 7.5 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 163.3, 150.5, 142.4, 138.1, 134.3, 130.3, 129.4, 129.3, 128.4, 127.8, 123.8, 122.4, 108.2, 61.2, 29.9, 21.5, 15.8; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C20H20NO3 322.1438; Found 322.1430.
(6-(tert-Butyl)-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3da). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 53% yield (37 mg); mp 174–175 °C; 1H NMR (500 MHz, CDCl3) δ 8.68 (s, 1H), 8.38 (d, J = 8.5 Hz, 1H), 7.74–7.68 (m, 1H), 7.64–7.57 (m, 1H), 7.54–7.47 (m, 5H), 5.21 (s, 2H), 2.10 (s, 3H), 1.41 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 171.3, 162.7, 157.3, 141.9, 137.6, 134.5, 130.3, 129.5, 129.2, 128.1, 125.4, 123.6, 120.1, 108.6, 61.0, 35.9, 31.5, 21.3; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C22H24NO3 350.1751; Found 350.1759.
(6-Methoxy-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ea). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 77% yield (49 mg); mp 172–173 °C; 1H NMR (500 MHz, CDCl3) δ 9.62 (s, 1H), 8.32 (d, J = 10.0 Hz, 1H), 7.51 (s, 5H), 7.14–7.05 (m, 2H), 5.14 (s, 2H), 3.92 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 164.1, 162.4, 142.8, 140.0, 134.4, 130.4, 130.4, 129.5, 129.2, 119.6, 116.1, 108.1, 105.9, 61.0, 55.9, 21.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H18NO4 324.1230; Found 324.1228.
(6-Fluoro-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3fa). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 73% yield (45 mg); mp 161–162 °C; 1H NMR (500 MHz, CDCl3) δ 9.00 (s, 1H), 8.42 (m, 1H), 7.53–7.47 (m, 6H), 7.35 (dd, J = 10.5 Hz, 2.0 Hz, 1H), 5.07 (s, 2H), 2.10 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.3, 166.4 (d, JC-F = 250.1 Hz), 162.4, 143.6, 140.6 (d, JC-F = 9.9 Hz), 133.7, 131.5 (d, JC-F = 10.1 Hz), 130.4, 129.2 (d, JC-F = 26.2 Hz), 115.9(d, JC-F = 23.1 Hz), 115.6, 109.4, 109.2, 60.8, 21.2; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H15FNO3 312.1030; Found 312.1022.
(6-Chloro-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ga). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 75% yield (49 mg); mp 174–175 °C; 1H NMR (500 MHz, CDCl3) δ 9.37 (s, 1H), 8.38 (d, J = 2.0 Hz, 1H), 7.70–7.64 (m, 2H), 7.54–7.48 (m, 5H), 5.13 (s, 2H), 2.10 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.3, 161.9, 142.4, 136.3, 134.1, 133.9, 133.5, 130.6, 129.5, 129.2, 127.8, 127.1, 125.7, 108.1, 60.8, 21.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H15ClNO3 328.0735; Found 328.0727.
(6-Bromo-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ha). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 71% yield (52 mg); mp 164–165 °C; 1H NMR (500 MHz, CDCl3) δ 9.20 (s, 1H), 8.26 (d, J = 9.0 Hz, 1H), 7.86 (d, J = 2.0 Hz, 1H), 7.64 (dd, J = 8.5, 2.0 Hz, 1H), 7.56–7.46 (m, 5H), 5.08 (s, 2H), 2.12 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.3, 162.5, 143.4, 139.5, 133.9, 130.7, 130.7, 130.0, 129.5, 129.2, 129.2, 126.8, 124.6, 107.5, 60.8, 21.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H15BrNO3 372.0230; Found 372.0235.
(1-Oxo-3,6-diphenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ia). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 89% yield (65 mg); mp 181–182 °C; 1H NMR (500 MHz, CDCl3) δ 8.68 (s, 1H), 8.52 (d, J = 8 Hz, 1H), 7.92 (s, 1H), 7.80 (dd, J = 8.5, 1Hz, 1H), 7.68 (d, J = 5.0 Hz, 2H), 7.49–7.55 (m, 7H), 7.44 (m, 1H), 5.21 (s, 2H), 2.12 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 163.1, 146.4, 142.8, 140.6, 138.3, 134.3, 130.4, 129.5, 129.4, 129.4, 129.2, 128.8, 127.9, 126.5, 124.7, 122.1, 108.5, 61.1, 21.5; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C24H20NO3 370.1438; Found 370.1439.
(6-Cyano-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ja). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 76% yield (48 mg); mp 205–206 °C; 1H NMR (500 MHz, CDCl3) δ 9.42 (s, 1H), 8.50 (d, J = 8.0 Hz, 1H), 8.05 (s, 1H), 7.74 (dd, J = 8.0, 1Hz, 1H), 7.59–7.52 (m, 6H), 5.12 (s, 2H), 2.13 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.1, 161.8, 144.1, 138.2, 133.5, 131.0, 129.5, 129.4, 129.2, 129.1, 128.9, 128.4, 118.6, 117.3, 107.6, 60.6, 21.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H15N2O3 319.1077; Found 319.1079.
(6-Nitro-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ka). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a yellow solid in 72% yield(48 mg); mp 191–192 °C; 1H NMR (500 MHz, CDCl3) δ 10.16 (s, 1H), 8.61 (s, 1H), 8.54 (d, J = 9.0 Hz, 1H), 8.39–8.21 (m, 1H), 7.60–7.50 (m, 5H), 5.19 (s, 2H), 2.12 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.1, 161.9, 151.3, 144.4, 138.9, 133.4, 131.0, 130.4, 129.6, 129.5, 129.2, 120.9, 119.8, 108.5, 60.7, 21.3; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H15N2O5 339.0975; Found 339.0971.
(1-Oxo-3-phenyl-6-(trifluoromethyl)-1,2-dihydroisoquinolin-4-yl)methyl acetate (3la). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 69% yield (50 mg); mp 193–194 °C; 1H NMR (500 MHz, CDCl3) δ 9.40 (s, 1H), 8.53 (d, J = 8.0 Hz, 1H), 7.99 (s, 1H), 7.74 (d, J = 8.5 Hz, 1H), 7.57–7.53 (m, 3H), 7.52–7.48 (m, 2H), 5.16 (s, 2H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.0, 162.5, 143.7, 137.9, 135.1 (q, JC-F = 32.5 Hz), 133.4, 130.4, 129.3, 129.1, 127.7, 125.18 (q, JC-F = 271.3 Hz), 123.0, 122.8, 121.1, 108.1, 60.6, 21.1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H15F3NO3 362.0999; Found 362.1007.
(7-Chloro-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ma). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 46% yield (30 mg); mp 177–178 °C; 1H NMR (500 MHz, CDCl3) δ 9.11 (s, 1H), 8.39 (d, J = 2.0 Hz, 1H), 7.70 (dd, J = 10.0, 5.0 Hz, 1H), 7.66 (d, J = 10.0 Hz, 1H), 7.57–7.51 (m, 3H), 7.49 (dd, J = 5.0, 1.5 Hz, 2H), 5.12 (s, 2H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.3, 161.9, 142.3, 136.3, 134.1, 133.9, 133.6, 130.6, 129.5, 129.2, 127.8, 127.1, 125.7, 108.1, 60.8, 21.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H15ClNO3 328.0735; Found 328.0741.
(7-Bromo-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3na). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 41% yield (30 mg); mp 172–173 °C; 1H NMR (500 MHz, CDCl3) δ 9.77 (s, 1H), 8.50 (s, 1H), 7.82 (dd, J = 5.0, 1.5 Hz, 1H), 7.58 (d, J = 10.0 Hz, 1H), 7.56–7.47 (m, 5H), 5.12 (s, 2H), 2.10 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.0, 161.6, 142.4, 136.6, 136.5, 133.8, 130.8, 130.5, 129.3, 129.0, 127.2, 125.6, 121.2, 107.9, 60.6, 21.2; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H15BrNO3 372.0230; Found 372.0231.
(8-Methyl-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3oa). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 78% yield (48 mg); mp 182–183 °C; 1H NMR (500 MHz, CDCl3) δ 10.77 (s, 1H), 7.56–7.48 (m, 7H), 7.24 (d, J = 7.0 Hz, 1H), 5.13 (s, 2H), 2.76 (s, 3H), 2.10 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.5, 164.8, 142.8, 139.7, 134.1, 132.7, 130.2, 130.0, 129.6, 129.1, 124.2, 121.7, 108.0, 61.7, 24.3, 21.5; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H18NO3 308.1281; Found 308.1286.
(8-Fluoro-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3pa). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a brownish yellow solid in 66% yield (41 mg); mp 164–165 °C; 1H NMR (500 MHz, CDCl3) δ 9.45 (s, 1H), 7.70–7.66 (m, 1H), 7.53–7.49 (m, 5H), 7.47 (d, J = 5.0 Hz, 1H), 7.17 (dd, J = 15.0 Hz, 5.0 Hz, 1H), 5.11 (s, 2H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.3, 163.3 (d, JC-F = 262.5 Hz), 160.6, 143.6, 140.8, 134.6 (d, JC-F = 10.0 Hz), 133.7, 130.6, 129.5, 129.3 (d, JC-F = 7.5 Hz), 119.7 (d, JC-F = 5.0 Hz), 115.1 (d, JC-F = 5.0 Hz), 114.2 (d, JC-F = 21.2 Hz), 107.5, 61.3, 21.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H15FNO3 312.1030; Found 312.1031.
(8-Chloro-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3qa). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a brownish yellow solid in 69% yield (45 mg); mp 171–172 °C; 1H NMR (500 MHz, CDCl3) δ 10.28 (s, 1H), 7.60–7.49 (m, 8H), 5.11 (s, 2H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 161.8, 143.8, 141.1, 136.4, 133.6, 133.2, 130.5, 130.4, 129.4, 129.4, 122.8, 122.4, 107.6, 61.4, 21.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H15ClNO3 328.0735; Found 328.0744.
(8-Bromo-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ra). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a brownish yellow solid in 62% yield (46 mg); mp 168–169 °C; 1H NMR (500 MHz, CDCl3) δ 10.21 (s, 1H), 7.76 (d, J = 7.5, 1H), 7.64 (dd, J = 8.5, 1.0 Hz, 1H), 7.55–7.50 (m, 5H), 7.47 (t, J = 8.0 Hz, 1H), 5.10 (s, 2H), 2.10 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 161.8, 143.6, 141.1, 134.3 133.7, 133.3, 130.4, 129.4, 123.8, 123.5, 123.3, 107.7, 61.4, 21.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H15BrNO3 372.0230; Found 372.0237.
(7,8-Dimethyl-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3sa). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 79% yield (51 mg); mp 198–199 °C; 1H NMR (500 MHz, CDCl3) δ 10.14 (s, 1H), 7.56–7.46 (m, 6H), 7.42 (d, J = 8.3 Hz, 1H), 5.12 (s, 2H), 2.78 (s, 3H), 2.42 (s, 3H), 2.10 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.5, 164.7, 141.6, 140.8, 137.8, 136.6, 135.3, 134.2, 130.0, 129.5, 129.2, 124.2, 120.9, 107.9, 61.7, 21.5, 21.4, 18.3; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C20H20NO3 322.1438; Found 322.1444.
(6,8-Dimethyl-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ta). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a light yellow solid in 71% yield (45 mg); mp 214–215 °C; 1H NMR (500 MHz, CDCl3) δ 10.75 (s, 1H), 7.56–7.45 (m, 5H), 7.28 (s, 1H), 7.07 (s, 1H), 5.10 (s, 2H), 2.71 (s, 3H), 2.44 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.6, 164.7, 143.1, 142.9, 142.6, 139.8, 134.3, 131.8, 129.9, 129.6, 129.1, 121.9, 121.6, 107.7, 61.8, 24.1, 22.4, 21.5; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C20H20NO3 322.1438; Found 322.1437.
(6-Fluoro-8-methyl-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ua). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 76% yield (49 mg); mp 182–183 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.43 (s, 1H), 7.53–7.48 (m, 3H), 7.48–7.39 (m, 2H), 7.30 (d, J = 10.0 Hz, 1H), 7.19 (d, J = 10.0 Hz, 1H), 4.88 (s, 2H), 2.84 (s, 3H), 2.02 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.4, 164.1 (d, J = 247.2 Hz), 162.5, 145.8 (d, J = 1.0 Hz), 144.2, 141.9 (d, J = 10.5 Hz), 133.1, 129.6, 129.2, 128.4, 120.6, 117.1 (d, J = 27.5 Hz), 107.0, 106.8, 106.0 (d, J = 5.0 Hz), 60.6, 23.7, 20.8; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H17FNO3 326.1187; Found 326.1179.
(6-Bromo-8-methyl-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3va). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 72% yield (55 mg); mp 180–181 °C; 1H NMR (500 MHz, DMSO-d6) δ 11.49 (s, 1H), 7.69 (s, 1H), 7.50 (s, 4H), 7.42 (d, J = 5.0 Hz, 2H), 4.88 (s, 2H), 2.80 (s, 3H), 2.01 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 170.8, 162.7, 144.7, 144.2, 140.9, 133.5, 132.1, 130.1, 129.7, 128.9, 126.8, 124.4, 123.0, 105.9, 60.8, 23.7, 21.2; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H17BrNO3 386.0386; Found 386.0377.
(6,8-Dichloro-1-oxo-3-phenyl-1,2-dihydroisoquinolin-4-yl)methyl acetate (3wa). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 73% yield (53 mg); mp 217–218 °C; 1H NMR (500 MHz, CDCl3) δ 9.74 (s, 1H), 7.57–7.25 (m, 7H), 5.05 (s, 2H), 2.12 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.2, 161.0, 144.8, 141.9, 139.3, 137.6, 133.3, 130.8, 130.3, 129.5, 129.2, 122.6, 120.9, 107.0, 61.2, 21.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H14Cl2NO3 362.0345; Found 362.0339.
(1-Oxo-3-phenyl-1,2-dihydrobenzo[g]isoquinolin-4-yl)methyl acetate (3xa). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a yellow solid in 63% yield (43 mg); mp 185–186 °C; 1H NMR (500 MHz, CDCl3) δ 9.24 (s, 1H), 9.03 (s, 1H), 8.15 (s, 1H), 8.07 (d, J = 8.0 Hz, 1H), 7.99 (d, J = 8.0 Hz, 1H), 7.66–7.60 (m, 1H), 7.59–7.50 (m, 6H), 5.26 (s, 2H), 2.14 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.6, 163.7, 141.1, 136.2, 134.6, 133.5, 131.9, 130.3, 129.8, 129.4129.3, 128.9, 128.6, 126.8, 124.1, 122.6, 108.2, 61.5, 21.5; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C22H18NO3 344.1281; Found 344.1277.
(7-Oxo-5-phenyl-6,7-dihydrothieno [2,3-c]pyridin-4-yl)methyl acetate (3ya). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 51% yield (30 mg); mp 158–159 °C; 1H NMR (500 MHz, CDCl3) δ 9.64 (s, 1H), 7.80 (d, J = 5.0 Hz, 1H), 7.53–7.51 (m, 5H), 7.38 (d, J = 5.0 Hz, 1H), 5.13 (s, 2H), 2.09 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.0, 158.9, 147.1, 142.8, 134.4, 133.1, 130.1, 129.1, 129.0, 124.4, 123.5, 108.0, 61.2, 21.1; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C16H14NO3S 300.0689; Found 300.0684.
(1-Oxo-3-(p-tolyl)-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ab). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 72% yield (44 mg); mp 163–164 °C; 1H NMR (500 MHz, CDCl3) δ 9.67 (s, 1H), 8.39 (d, J = 8.0 Hz, 1H), 7.76–7.68 (m, 2H), 7.54 –7.51 (m, 1H), 7.39 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 5.15 (s, 2H), 2.43 (s, 3H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 163.2, 142.5, 140.5, 138.0, 133.6, 131.3, 130.0, 129.2, 128.3, 127.1, 125.8, 123.8, 108.1, 61.2, 21.8, 21.5; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H18NO3 308.1281; Found 308.1279.
(3-(4-Ethylphenyl)-1-oxo-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ac). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 67% yield (43 mg); mp 165–166 °C; 1H NMR (500 MHz, CDCl3) δ 9.94 (s, 1H), 8.38 (d, J = 8.0 Hz, 1H), 7.76–7.68 (m, 2H), 7.51 (m, 1H), 7.43 (d, J = 8.0 Hz, 2H), 7.33 (d, J = 8.0 Hz, 2H), 5.17 (s, 2H), 2.73 (q, J = 5.0 Hz, 2H), 2.11 (s, 3H), 1.30 (t, J = 5.0 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 171.5, 163.5, 146.6, 142.7, 138.1, 133.6, 131.5, 129.4, 128.8, 128.3, 127.1, 125.8, 123.7, 108.0, 61.3, 29.1, 21.5, 15.7; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C20H20NO3 322.1438; Found 322.1436.
(3-(4-(tert-Butyl)phenyl)-1-oxo-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ad). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 49% yield (34 mg); mp 171–172 °C; 1H NMR (500 MHz, CDCl3) δ 8.42 (dd, J = 10.0, 5.0 Hz, 1H), 7.75–7.74 (m, 1H), 7.69 (d, J = 5.0 Hz, 1H), 7.53–7.51(m, 3H), 7.43 (dd, J = 5.0, 2.0 Hz, 2H), 5.18 (s, 2H), 2.13 (s, 3H), 1.38 (s, 11H); 13C NMR (125 MHz, CDCl3) δ 171.5, 162.9, 153.6, 142.4, 138.0, 133.7, 131.3, 129.0, 128.3, 127.2, 126.4, 125.9, 123.8, 108.0, 61.3, 35.3, 31.6, 21.5; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C22H24NO3 350.1751; Found 350.1754.
(3-(4-Methoxyphenyl)-1-oxo-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ae). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 76% yield (49 mg); mp 174–175 °C; 1H NMR (500 MHz, CDCl3) δ 8.77 (s, 1H), 8.45 (d, J = 7.5 Hz, 1H), 7.77–7.68 (m, 2H), 7.56–7.50 (m, 1H), 7.42 (d, J = 8.5 Hz, 2H), 7.02 (d, J = 8.5 Hz, 2H), 5.16 (s, 2H), 3.88 (s, 3H), 2.12 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.4, 163.0, 161.2, 142.0, 138.0, 133.7 130.6, 128.3, 127.2, 126.5, 125.8, 123.8, 114.9, 108.1, 61.3, 55.8, 21.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H18NO4 324.1230; Found 324.1225.
(3-(4-Fluorophenyl)-1-oxo-1,2-dihydroisoquinolin-4-yl)methyl acetate (3af). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 87% yield (54 mg); mp 193–194 °C; 1H NMR (500 MHz, CDCl3) δ 10.50 (s, 1H), 8.35 (d, J = 8.0 Hz, 1H), 7.78–7.70 (m, 2H), 7.59–7.47 (m, 3H), 7.22 (dd, J = 8.5, 8.5 Hz, 2H), 5.14 (s, 2H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.1, 164.1 (d, J = 243.8 Hz), 163.4, 141.2, 137.5, 133.5, 131.4 (d, J = 8.8 Hz), 123.0, 128.0, 127.2, 125.6, 123.6, 116.2 (d, J = 21.2 Hz), 108.4, 60.8, 21.2; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H15FNO3 312.1030; Found 312.1021.
(3-(4-Chlorophenyl)-1-oxo-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ag). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 84% yield (55 mg); mp 184–185 °C; 1H NMR (500 MHz, CDCl3) δ 10.57 (s, 1H), 8.34 (d, J = 8.0 Hz, 1H), 7.78–7.70 (m, 2H), 7.57–7.47 (m, 5H), 5.13 (s, 2H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.3, 163.6, 141.3, 137.8, 136.6, 133.8, 132.5, 131.0, 129.5, 128.2, 127.5, 125.8, 123.9, 108.7, 60.9, 21.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H15ClNO3 328.0735; Found 328.0736.
(3-(4-Bromophenyl)-1-oxo-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ah). This compound was purified by column chromatography (ethyl acetate/petroleum ether = 1:6) to provide a white solid in 79% yield (58 mg); mp 178–179 °C; 1H NMR (500 MHz, CDCl3) δ 10.55 (s, 1H), 8.35 (d, J = 8.0 Hz, 1H), 7.76 (dd, J = 7.5, 7.5 Hz, 1H), 7.71 (d, J = 8.0 Hz, 1H), 7.66 (d, J = 8.5 Hz, 2H), 7.56 (dd, 7.5, 7.5 Hz, 1H), 7.41(d, J = 8.5 Hz, 2H), 5.13 (s, 2H), 2.11 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.3, 163.6, 141.3, 137.8, 133.8, 132.9, 132.5, 131.2, 128.3, 127.5, 125.8, 124.9, 123.9, 108.7, 60.9, 21.4; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C18H15BrNO3 372.0230; Found 372.0225.
(1-Oxo-3-(4-(trifluoromethyl)phenyl)-1,2-dihydroisoquinolin-4-yl)methyl acetate (3ai). This compound was purified by column chromatography (EtOAc/petroleum ether = 1:6) to provide a white solid in 89% yield (64 mg); mp 195–196 °C; 1H NMR (500 MHz, CDCl3) δ 10.87 (s, 1H), 8.32–8.29 (m, 1H), 7.81–7.69 (m, 6H), 7.59–7.57 (m, 1H), 5.14 (s, 2H), 2.12 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 171.1, 163.5, 141.0, 137.6, 137.5, 133.9, 132.1 (q, J = 32.5 Hz), 130.2, 128.2, 127.7, 126.2, 125.9, 124.2 (q, J = 276.3 Hz), 108.9, 60.5, 21.2; HRMS (ESI-TOF) m/z: [M + H]+ Calcd for C19H15F3NO3 362.0999; Found 362.0992.
References
- Brookings, D.; Davenport, R.J.; Davis, J.; Galvin, F.C.A.; Lloyd, S.; Mack, S.R.; Owens, R.; Sabin, V.; Wynn, J. Novel nucleotide triphosphates as potent P2Y2 agonists. Bioorg. Med. Chem. Lett. 2007, 17, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, E.; Sooryakumar, D.; Agama, K.; Cushman, M.; Pommier, Y. Optimization of the Lactam Side Chain of 7-Azaindenoisoquinoline Topoisomerase I Inhibitors and Mechanism of Action Studies in Cancer Cells. J. Med. Chem. 2014, 57, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Bentley, K.W. β-Phenylethylamines and the isoquinoline alkaloids. Nat. Prod. Rep. 2006, 23, 444–463. [Google Scholar] [CrossRef]
- Bhadra, K.; Kumar, G.S. Isoquinoline Alkaloids and their Binding with DNA: Calorimetry and Thermal Analysis Applications. Mini-Rev. Med. Chem. 2010, 10, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Khadka, D.B.; Yang, S.H.; Cho, S.H.; Zhao, C.; Cho, W.-J. Synthesis of 12-oxobenzo[c]phenanthridinones and 4-substituted 3-arylisoquinolones via Vilsmeier–Haack reaction. Tetrahedron 2012, 68, 250–261. [Google Scholar] [CrossRef]
- Malkov, A.V.; Westwater, M.-M.; Gutnov, A.; Ramírez-López, P.; Friscourt, F.; Kadlčíková, A.; Hodačová, J.; Rankovic, Z.; Kotora, M.; Kočovský, P. New pyridine N-oxides as chiral organocatalysts in the asymmetric allylation of aromatic aldehydes. Tetrahedron 2008, 64, 11335–11348. [Google Scholar] [CrossRef]
- Thoi, V.S.; Kornienko, N.; Margarit, C.G.; Yang, P.; Chang, C.J. Visible-Light Photoredox Catalysis: Selective Reduction of Carbon Dioxide to Carbon Monoxide by a Nickel N-Heterocyclic Carbene–Isoquinoline Complex. J. Am. Chem. Soc. 2013, 135, 14413–14424. [Google Scholar] [CrossRef]
- Yu, D.-G.; Azambuja, F.; Glorius, F. α-MsO/TsO/Cl Ketones as Oxidized Alkyne Equivalents: Redox-Neutral Rhodium(III)-Catalyzed C-H Activation for the Synthesis of N-Heterocycles. Angew. Chem. Int. Ed. 2014, 53, 2754–2758. [Google Scholar] [CrossRef]
- Fang, Z.; Zheng, S.-C.; Guo, Z.; Guo, J.-Y.; Tan, B.; Liu, X.-Y. Asymmetric Synthesis of Axially Chiral Isoquinolones: Nickel-Catalyzed Denitrogenative Transannulation. Angew. Chem. Int. Ed. 2015, 54, 9528–9532. [Google Scholar] [CrossRef]
- Wu, J.-Q.; Zhang, S.-S.; Gao, H.; Qi, Z.; Zhou, C.-J.; Ji, W.-W.; Liu, Y.; Chen, Y.; Li, Q.; Li, X.; et al. Experimental and Theoretical Studies on Rhodium-Catalyzed Coupling of Benzamides with 2,2-Difluorovinyl Tosylate: Diverse Synthesis of Fluorinated Heterocycles. J. Am. Chem. Soc. 2017, 139, 3537–3545. [Google Scholar] [CrossRef]
- Huang, J.-R.; Bolm, C. Microwave-Assisted Synthesis of Heterocycles by Rhodium(III)-Catalyzed Annulation of N-Methoxyamides with α-Chloroaldehydes. Angew. Chem. Int. Ed. 2017, 56, 15921–15925. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zheng, G.; Yang, X.; Li, X. Rhodium(III)-catalyzed chemodivergent annulations between N-methoxybenzamides and sulfoxonium ylides via C–H activation. Chem. Commun. 2018, 54, 670–673. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Fang, X.; He, L.; Sun, J.; Zou, L.; Ma, W.; Ackermann, L. Cobaltaelectro-catalyzed oxidative allene annulation by electro-removable hydrazides. Chem. Commun. 2020, 56, 1393–1396. [Google Scholar] [CrossRef]
- Zhang, L.-B.; Geng, R.-S.; Wang, Z.-C.; Ren, G.-Y.; Wen, L.-R.; Li, M. Electrochemical intramolecular C–H/N–H functionalization for the synthesis of isoxazolidine-fused isoquinolin-1(2H)-ones. Green Chem. 2020, 22, 16–21. [Google Scholar] [CrossRef]
- Alvarez-Builla, J.; Vaquero, J.J.; Barluenga, J. Modern Heterocyclic Chemistry; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Glushkov, V.A.; Shklyaev, Y.V. Synthesis of 1(2H)-Isoquinolones. Chem. Heterocycl. Compd. 2001, 37, 663–687. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, B.; Zhang, J.; Yu, W.; Liu, Z.; Zhang, Y. Transition metal-catalyzed C–H bond functionalizations by the use of diverse directing groups. Org. Chem. Front. 2015, 2, 1107–1295. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, X.; Shen, C.; Xu, L.; Ding, L.; Zhong, G. Recent advances in chelation-assisted site- and stereoselective alkenyl C–H functionalization. Chem. Soc. Rev. 2021, 50, 3263–3314. [Google Scholar] [CrossRef]
- Kumar, S.; Nunewar, S.; Oluguttula, S.; Nanduri, S.; Kanchupalli, V. Recent advances in Rh(III)/Ir(III)-catalyzed C–H functionalization/annulation via carbene migratory insertion. Org. Biomol. Chem. 2021, 19, 1438–1458. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, X.; Lai, Y.; Zhang, Z. Recent advances in transition metal-catalyzed heteroannulative difunctionalization of alkenes via C–H activation for the synthesis of heterocycles. Org. Chem. Front. 2022, 9, 2256–2279. [Google Scholar] [CrossRef]
- Subhedar, D.D.; Mishra, A.A.; Bhanage, B.M. N-Methoxybenzamide: A Versatile Directing Group for Palladium-, Rhodium- and Ruthenium-Catalyzed C-H Bond Activations. Adv. Synth. Catal. 2019, 361, 4149–4195. [Google Scholar] [CrossRef]
- Hao, X.-Q.; Du, C.; Zhu, X.; Li, P.-X.; Zhang, J.-H.; Niu, J.-L.; Song, M.-P. Cobalt(II)-Catalyzed Decarboxylative C–H Activation/Annulation Cascades: Regioselective Access to Isoquinolones and Isoindolinones. Org. Lett. 2016, 18, 3610–3613. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yu, K.; Wang, B. Regioselective synthesis of multisubstituted isoquinolones and pyridones via Rh(III)-catalyzed annulation reactions. Chem. Commun. 2015, 51, 17277–17280. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, R.; Jeganmohan, M. Cobalt-catalyzed cyclization of benzamides with alkynes: A facile route to isoquinolones with hydrogen evolution. Org. Biomol. Chem. 2018, 16, 8384–8389. [Google Scholar] [CrossRef]
- Lin, C.; Shen, L. Co-catalyzed ortho-C–H functionalization/annulation of arenes and alkenes with alkynylsilanes: Access to isoquinolone and pyridine motifs. RSC Adv. 2019, 9, 30650–30654. [Google Scholar] [CrossRef] [PubMed]
- Thorat, V.H.; Aman, H.; Tsai, Y.-L.; Pallikonda, G.; Chuang, G.J.; Hsieh, J.-C. Cobalt-catalyzed coupling reactions of 2-halobenzamides with alkynes: Investigation of ligand-controlled dual pathways. Org. Chem. Front. 2021, 8, 6419–6426. [Google Scholar] [CrossRef]
- Allu, S.; Swamy, K.C.K. Ruthenium-Catalyzed Synthesis of Isoquinolones with 8-Aminoquinoline as a Bidentate Directing Group in C−H Functionalization. J. Org. Chem. 2014, 79, 3963–3972. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-W.; Zhu, Y.-Z.; Ou, J.-W.; Wang, Y.-P.; Zheng, J.-Y. Metal-Free Iodine(III)-Promoted Synthesis of Isoquinolones. J. Org. Chem. 2014, 79, 10988–10998. [Google Scholar] [CrossRef]
- Cera, G.; Haven, T.; Ackermann, L. Iron-catalyzed C–H/N–H activation by triazole guidance: Versatile alkyne annulation. Chem. Commun. 2017, 53, 6460–6463. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, W.-J.; Wang, J.; Ma, Q.; Shen, L.-J. Rhodium-catalyzed synthesis of substituted isoquinolones via a selective decarbonylation/alkyne insertion cascade of phthalimides. Org. Biomol. Chem. 2020, 18, 8219–8223. [Google Scholar] [CrossRef]
- He, Y.; Liao, X.-Z.; Dong, L.; Chen, F.-E. Rh(III)-Catalyzed three-component cascade annulation to produce the N-oxopropyl chain of isoquinolone derivatives. Org. Biomol. Chem. 2021, 19, 561–567. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, C.; Su, X.; Xia, W. Synthesis of isoquinolones by visible-light-induced deaminative [4+2] annulation reactions. Chem. Commun. 2020, 56, 5259–5262. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Fenner, S. Ruthenium-Catalyzed C-H/N-O Bond Functionalization: Green Isoquinolone Syntheses in Water. Org. Lett. 2011, 13, 6548–6551. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ackermann, L. Dehydrative C−H/N−OH Functionalizations in H2O by Ruthenium(II) Catalysis: Subtle Effect of Carboxylate Ligands and Mechanistic Insight. J. Org. Chem. 2014, 79, 12070–12082. [Google Scholar] [CrossRef]
- Yu, B.; Chen, Y.; Hong, M.; Duan, P.; Gan, S.; Chao, H.; Zhao, Z.; Zhao, J. Rhodium-catalyzed C–H activation of hydrazines leads to isoquinolones with tunable aggregation-induced emission properties. Chem. Commun. 2015, 51, 14365–14368. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, R.; Qi, Y.; Zhao, Q.; Yao, T. Rhodium(III)-catalyzed C–H activation/annulation of N-iminopyridinium ylides with alkynes and diazo compounds. Org. Chem. Front. 2021, 8, 1190–1196. [Google Scholar] [CrossRef]
- Meng, H.; Xu, H.; Zhou, Z.; Tang, Z.; Li, Y.; Zhou, Y.; Yi, W.; Wu, X. Recyclable rhodium-catalyzed C–H activation/[4 + 2] annulation with unconventional regioselectivity at ambient temperature: Experimental development and mechanistic insight. Green Chem. 2022, 24, 7012–7021. [Google Scholar] [CrossRef]
- Fukui, Y.; Liu, P.; Liu, Q.; He, Z.-T.; Wu, N.-Y.; Tian, P.; Lin, G.-Q. Tunable Arylative Cyclization of 1,6-Enynes Triggered by Rhodium(III)-Catalyzed C–H Activation. J. Am. Chem. Soc. 2014, 136, 15607–15614. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
