A Novel Near-Infrared Ytterbium Complex [Yb(DPPDA)2](DIPEA) with Φ = 0.46% and τobs = 105 μs
Abstract
:1. Introduction
2. Results and Discussion
2.1. FT-IR Analysis
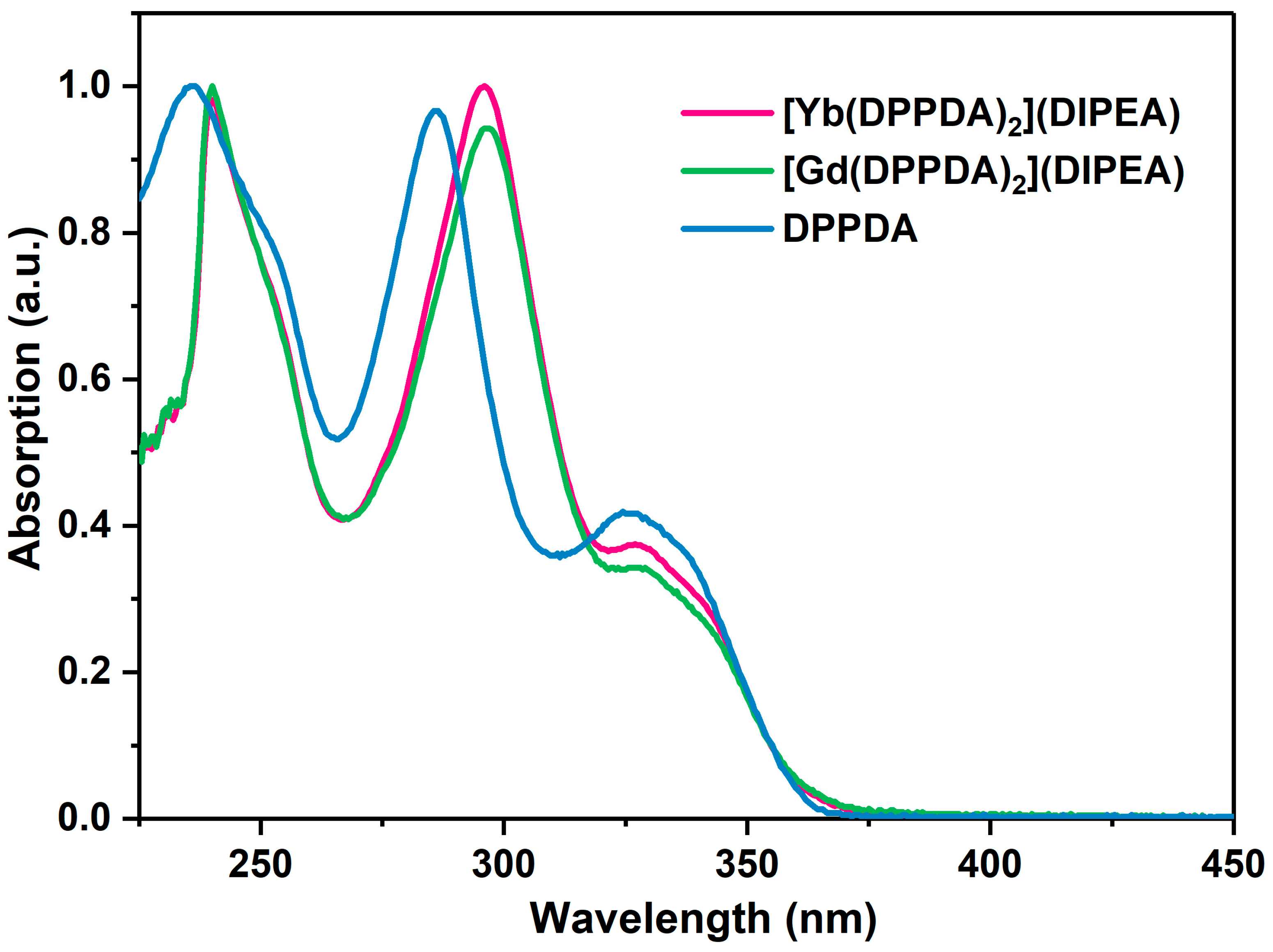
2.2. UV-Vis Analysis
2.3. Thermal Stability Characterization
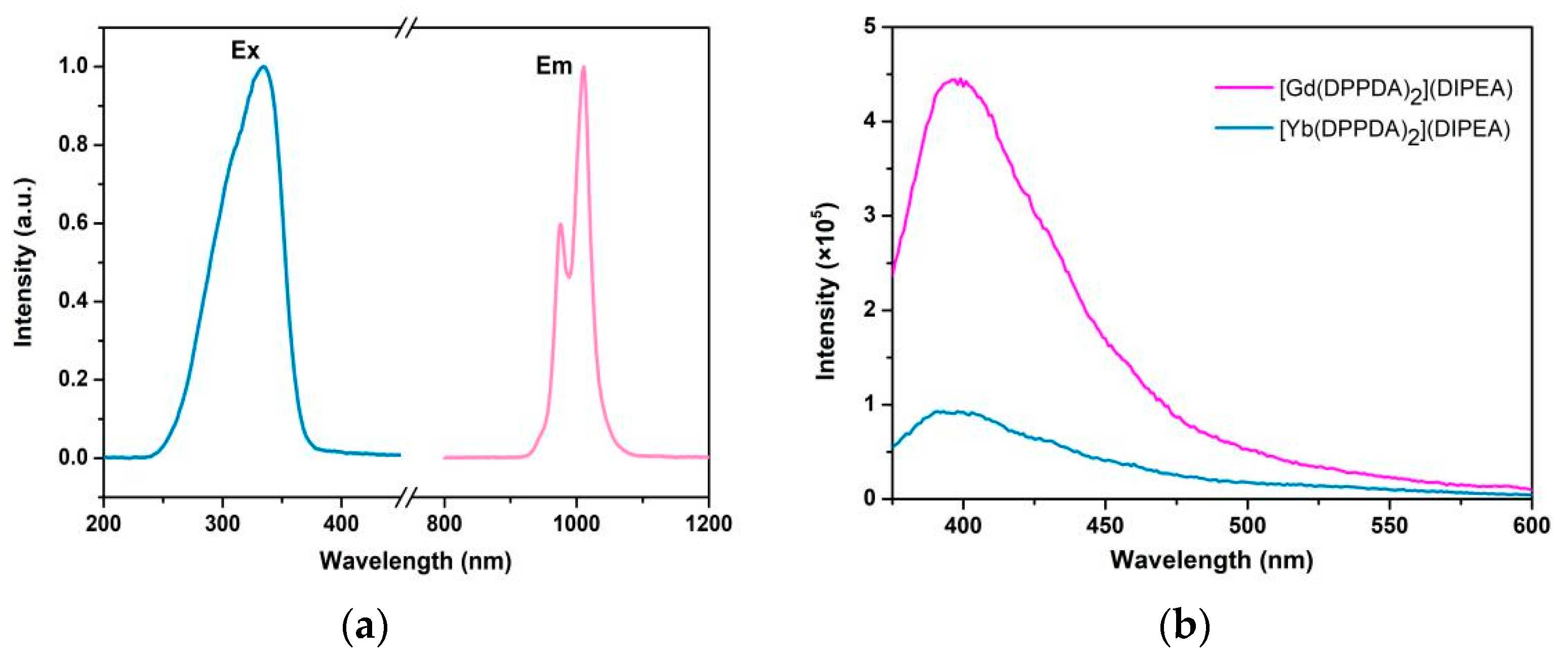
2.4. Luminescent Properties
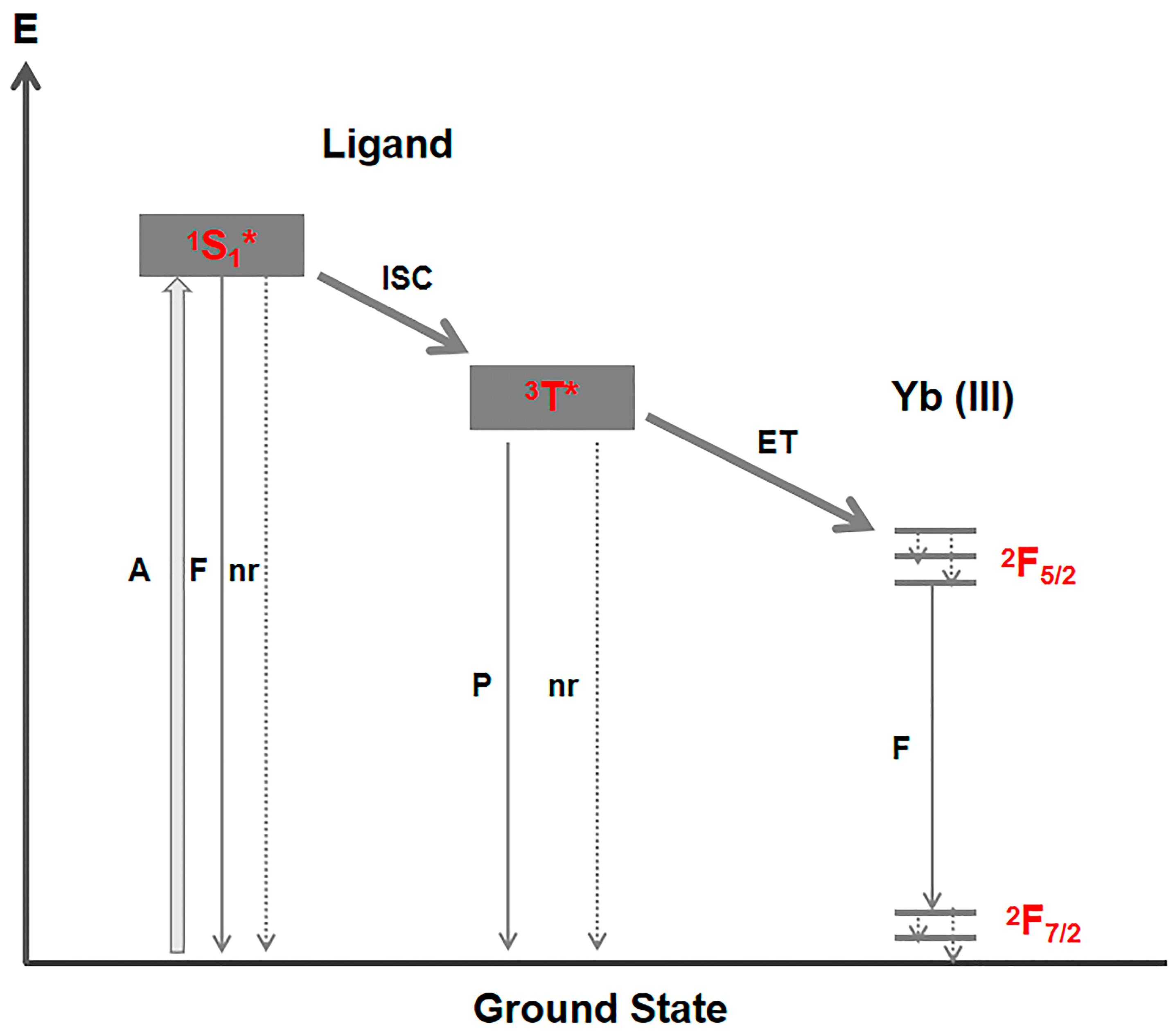
2.5. Luminescent Mechanisms
3. Materials and Methods
3.1. Drugs and Reagents
3.2. Instruments
3.3. Synthesis of 4,7-Diphenyl-1,10-Phenanthroline-2,9-Dicarboxylic Acid (DPPDA)
3.4. Synthesis of [Yb(DPPDA)2](DIPEA)
3.5. Synthesis of [Gd(DPPDA)2](DIPEA)
3.6. Method of Cyclic Voltammetry Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, S.; Zhou, L.; Zhang, H. Investigation progresses of rare earth complexes as emitters or sensitizers in organic light-emitting diodes. Light Sci. Appl. 2022, 11, 177. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2009, 39, 189–227. [Google Scholar] [CrossRef]
- Weissman, S.I. Intramolecular Energy Transfer the Fluorescence of Complexes of Europium. J. Chem. Phys. 1942, 10, 214–217. [Google Scholar] [CrossRef]
- Bulach, V.; Sguerra, F.; Hosseini, M.W. Porphyrin lanthanide complexes for NIR emission. Coord. Chem. Rev. 2012, 256, 1468–1478. [Google Scholar] [CrossRef]
- Varaksina, E.A.; Kiskin, M.A.; Lyssenko, K.A.; Puntus, L.N.; Korshunov, V.M.; Silva, G.S.; Freire, R.O.; Taydakov, I.V. Tuning the luminescence efficiency by perfluorination of side chains in Eu(3+) complexes with beta-diketones of the thiophene series. Phys. Chem. Chem. Phys. 2021, 23, 25748–25760. [Google Scholar]
- Zhao, Z.; Bian, M.; Lin, C.; Fu, X.; Yu, G.; Wei, H.; Liu, Z.; Bian, Z.; Huang, C. Efficient green OLEDs achieved by a terbium(III) complex with photoluminescent quantum yield close to 100%. Sci. China Chem. 2021, 64, 1504–1509. [Google Scholar] [CrossRef]
- Barry, D.E.; Caffrey, D.F.; Gunnlaugsson, T. Lanthanide-directed synthesis of luminescent self-assembly supramolecular structures and mechanically bonded systems from acyclic coordinating organic ligands. Chem. Soc. Rev. 2016, 45, 3244–3274. [Google Scholar] [CrossRef]
- Peng, X.-X.; Zhu, X.-F.; Zhang, J.-L. Near Infrared (NIR) imaging: Exploring biologically relevant chemical space for lanthanide complexes. J. Inorg. Biochem. 2020, 209, 111118. [Google Scholar] [CrossRef]
- Feng, L.; Li, C.; Liu, L.; Chen, X.; Jiang, G.; Wang, J.; Tang, B.Z. A Facile Structural Isomerization-Induced 3D Spatial D-A Interlocked Network for Achieving NIR-II Phototheranostic Agents. Angew. Chem. Int. Ed. 2022, 61, e202212673. [Google Scholar] [CrossRef]
- Mara, D.; Artizzu, F.; Smet, P.F.; Kaczmarek, A.M.; Van Hecke, K.; Van Deun, R. Vibrational Quenching in Near-Infrared Emitting Lanthanide Complexes: A Quantitative Experimental Study and Novel Insights. Chem. A Eur. J. 2019, 25, 15944–15956. [Google Scholar] [CrossRef]
- Jin, G.-Q.; Ning, Y.; Geng, J.-X.; Jiang, Z.-F.; Wang, Y.; Zhang, J.-L. Joining the journey to near infrared (NIR) imaging: The emerging role of lanthanides in the designing of molecular probes. Inorg. Chem. Front. 2019, 7, 289–299. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Ning, Y.; Meng, Y.-S.; Zhang, J.; Wu, Z.-Y.; Gao, S.; Zhang, J.-L. Highly near-IR emissive ytterbium(iii) complexes with unprecedented quantum yields. Chem. Sci. 2017, 8, 2702–2709. [Google Scholar] [CrossRef]
- Peng, Y.; Hu, J.X.; Lu, H.; Wilson, R.M.; Motevalli, M.; Hernández, I.; Gillin, W.P.; Wyatt, P.B.; Ye, H.Q. Functionalisation of ligands through click chemistry: Long-lived NIR emission from organic Er(iii) complexes with a perfluorinated core and a hydrogen-containing shell. RSC Adv. 2016, 7, 128–131. [Google Scholar] [CrossRef]
- Li, C.; Jiang, G.; Yu, J.; Ji, W.; Liu, L.; Zhang, P.; Du, J.; Zhan, C.; Wang, J.; Tang, B.Z. Fluorination Enhances NIR-II Emission and Photothermal Conversion Efficiency of Phototheranostic Agents for Imaging-Guided Cancer Therapy. Adv. Mater. 2022, 35, 2208229. [Google Scholar] [CrossRef]
- Kruck, C.; Nazari, P.; Dee, C.; Richards, B.S.; Turshatov, A.; Seitz, M. Efficient Ytterbium Near-Infrared Luminophore Based on a Nondeuterated Ligand. Inorg. Chem. 2019, 58, 6959–6965. [Google Scholar] [CrossRef]
- Zhang, J.; Petoud, S. Azulene-Moiety-Based Ligand for the Efficient Sensitization of Four Near-Infrared Luminescent Lanthanide Cations: Nd3+, Er3+, Tm3+, and Yb3+. Chem. A Eur. J. 2008, 14, 1264–1272. [Google Scholar] [CrossRef]
- Wartenberg, N.; Raccurt, O.; Bourgeat-Lami, E.; Imbert, D.; Mazzanti, M. Multicolour Optical Coding from a Series of Luminescent Lanthanide Complexes with a Unique Antenna. Chem. A Eur. J. 2013, 19, 3477–3482. [Google Scholar] [CrossRef]
- Zhang, J.; Badger, P.D.; Geib, S.J.; Petoud, S. Sensitization of Near-Infrared-Emitting Lanthanide Cations in Solution by Tropolonate Ligands. Angew. Chem. Int. Ed. 2005, 44, 2508–2512. [Google Scholar] [CrossRef]
- Hernández, I.; Zheng, Y.-X.; Motevalli, M.; Tan, R.H.C.; Gillin, W.P.; Wyatt, P.B. Efficient sensitized emission in Yb(iii) pentachlorotropolonate complexes. Chem. Commun. 2013, 49, 1933–1935. [Google Scholar] [CrossRef]
- Doffek, C.; Seitz, M. The Radiative Lifetime in Near-IR-Luminescent Ytterbium Cryptates: The Key to Extremely High Quantum Yields. Angew. Chem. Int. Ed. Engl. 2015, 54, 9719–9721. [Google Scholar]
- Salem, A. Fluorimetric determinations of nucleic acids using iron, osmium and samarium complexes of 4,7-diphenyl-1,10-phenanthroline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 65, 235–248. [Google Scholar] [CrossRef]
- Nie, D.; Chen, Z.; Bian, Z.; Zhou, J.; Liu, Z.; Chen, F.; Zhao, Y.; Huang, C. Energy transfer pathways in the carbazole functionalized β-diketonate europium complexes. New J. Chem. 2007, 31, 1639–1646. [Google Scholar] [CrossRef]
- Shavaleev, N.M.; Scopelliti, R.; Gumy, F.; Bünzli, J.-C.G. Surprisingly Bright Near-Infrared Luminescence and Short Radiative Lifetimes of Ytterbium in Hetero-Binuclear Yb−Na Chelates. Inorg. Chem. 2009, 48, 7937–7946. [Google Scholar] [CrossRef]
- Beeby, A.; Burton-Pye, B.P.; Faulkner, S.; Motson, G.R.; Jeffery, J.C.; McCleverty, J.A.; Ward, M.D. Synthesis and near-IR luminescence properties of neodymium(III) and ytterbium(III) complexes with poly(pyrazolyl)borate ligands. J. Chem. Soc. Dalton Trans. 2002, 31, 1923–1928. [Google Scholar] [CrossRef]
- Davies, G.M.; Aarons, R.J.; Motson, G.R.; Jeffery, J.C.; Adams, H.; Faulkner, S.; Ward, M.D. Structural and near-IR photophysical studies on ternary lanthanide complexes containing poly(pyrazolyl)borate and 1,3-diketonate ligands. Dalton Trans. 2004, 2, 1136–1144. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Chauvin, A.-S.; Kim, H.K.; Deiters, E.; Eliseeva, S.V. Lanthanide luminescence efficiency in eight- and nine-coordinate complexes: Role of the radiative lifetime. Coord. Chem. Rev. 2010, 254, 2623–2633. [Google Scholar] [CrossRef]
- Beeby, A.; Faulkner, S.; Williams, J.A.G. pH Dependence of the energy transfer mechanism in a phenanthridine-appended ytterbium complex. Part 2.1. J. Chem. Soc. Dalton Trans. 2002, 31, 1918–1922. [Google Scholar] [CrossRef]
- Horrocks, W.D.; Bolender, J.P.; Smith, W.D.; Supkowski, R.M. Photosensitized Near Infrared Luminescence of Ytterbium(III) in Proteins and Complexes Occurs via an Internal Redox Process. J. Am. Chem. Soc. 1997, 119, 5972–5973. [Google Scholar] [CrossRef]
- Borisova, N.E.; Kostin, A.A.; Reshetova, M.D.; Lyssenko, K.A.; Belova, E.V.; Myasoedov, B.F. The structurally rigid tetradentate N,N′,O,O′-ligands based on phenanthroline for binding of f-elements: The substituents vs. structures of the complexes. Inorg. Chim. Acta 2018, 478, 148–154. [Google Scholar]




| Solvent | CHCl3 | CDCl3 | CH3OH | CD3OD | Solid |
|---|---|---|---|---|---|
| τobs 1 (μs) | 49 | 78 | 10 | 105 | 31 |
| 2 (%) | 0.22 | 0.37 | 0.02 | 0.46 | 0.06 |
| Parameter | τobs (μs) | τrad (μs) | ηsens (%) | ||
|---|---|---|---|---|---|
| Value 1 | 105 | 840 | 12.5 | 0.46 | 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, G.; Zhang, D.; Wang, H.; Li, X.; Deng, R.; Zhou, S.; Tian, L.; Zhou, L. A Novel Near-Infrared Ytterbium Complex [Yb(DPPDA)2](DIPEA) with Φ = 0.46% and τobs = 105 μs. Molecules 2023, 28, 1632. https://doi.org/10.3390/molecules28041632
Ren G, Zhang D, Wang H, Li X, Deng R, Zhou S, Tian L, Zhou L. A Novel Near-Infrared Ytterbium Complex [Yb(DPPDA)2](DIPEA) with Φ = 0.46% and τobs = 105 μs. Molecules. 2023; 28(4):1632. https://doi.org/10.3390/molecules28041632
Chicago/Turabian StyleRen, Guozhu, Danyang Zhang, Hao Wang, Xiaofang Li, Ruiping Deng, Shihong Zhou, Long Tian, and Liang Zhou. 2023. "A Novel Near-Infrared Ytterbium Complex [Yb(DPPDA)2](DIPEA) with Φ = 0.46% and τobs = 105 μs" Molecules 28, no. 4: 1632. https://doi.org/10.3390/molecules28041632
APA StyleRen, G., Zhang, D., Wang, H., Li, X., Deng, R., Zhou, S., Tian, L., & Zhou, L. (2023). A Novel Near-Infrared Ytterbium Complex [Yb(DPPDA)2](DIPEA) with Φ = 0.46% and τobs = 105 μs. Molecules, 28(4), 1632. https://doi.org/10.3390/molecules28041632




