A Review for In Vitro and In Vivo Detection and Imaging of Gaseous Signal Molecule Carbon Monoxide by Fluorescent Probes
Abstract
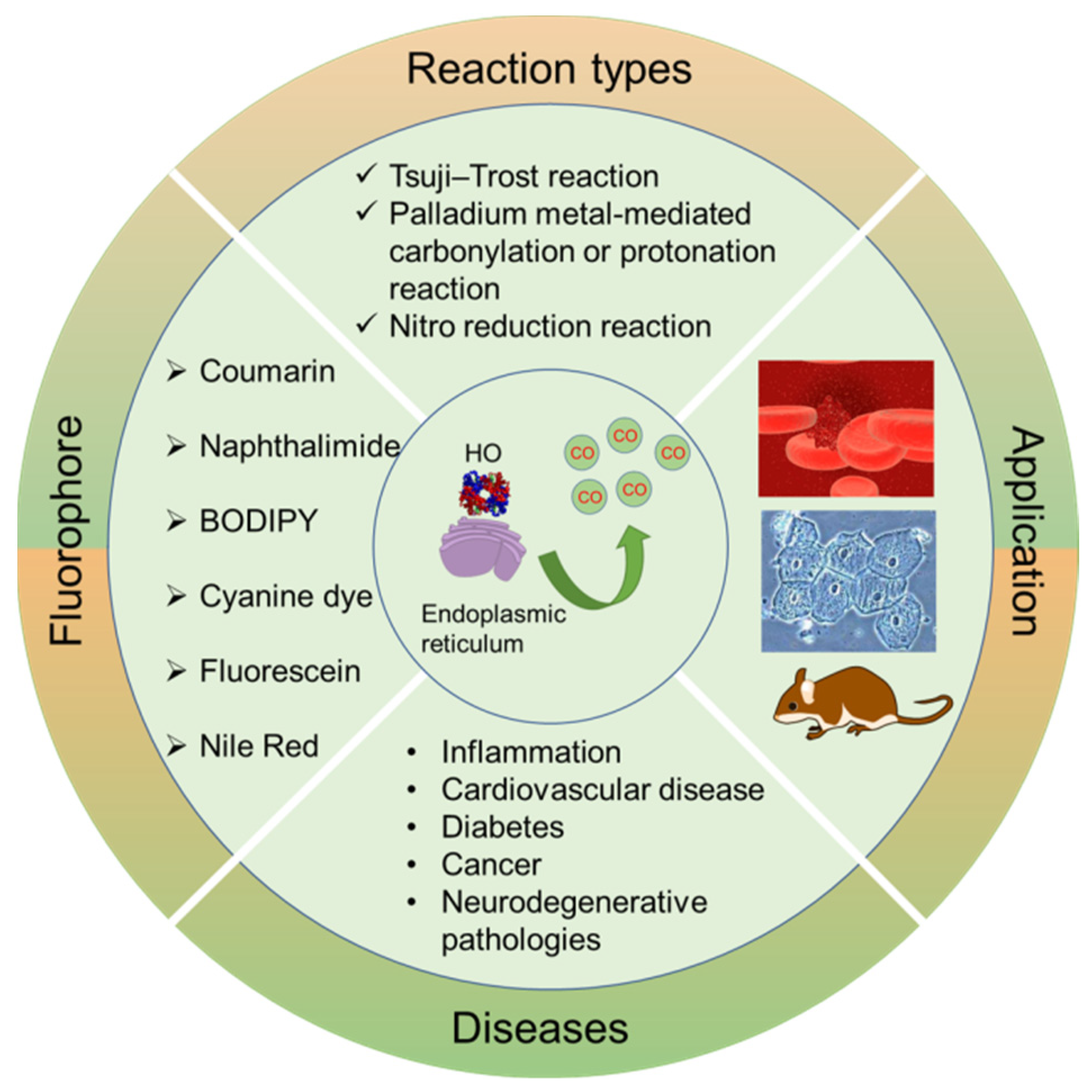
1. Introduction
2. Designing the Main Types of Chemical Reactions for CO Fluorescent Probes
2.1. Tsuji-Trost Reaction
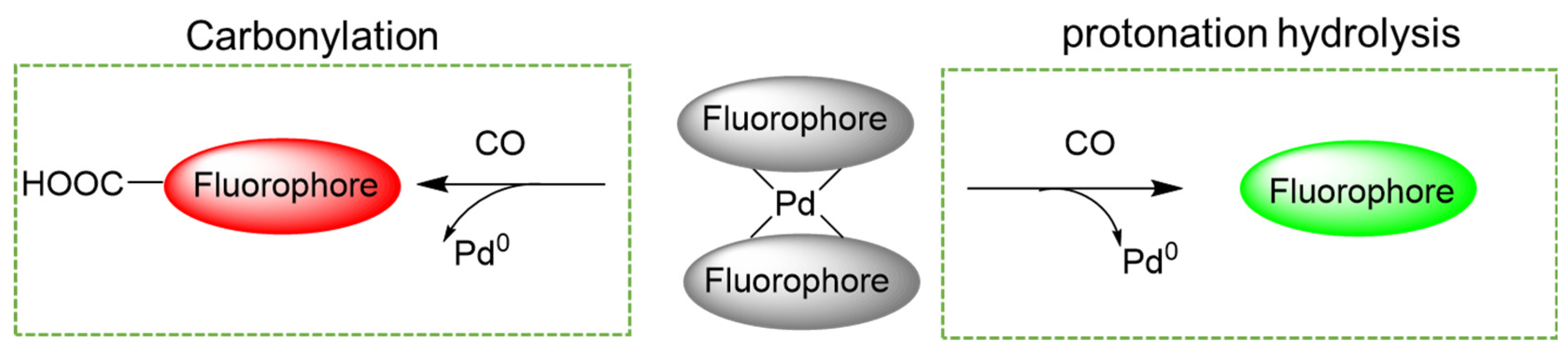
2.2. Palladium Metal-Mediated Carbonylation or Protonation Reaction
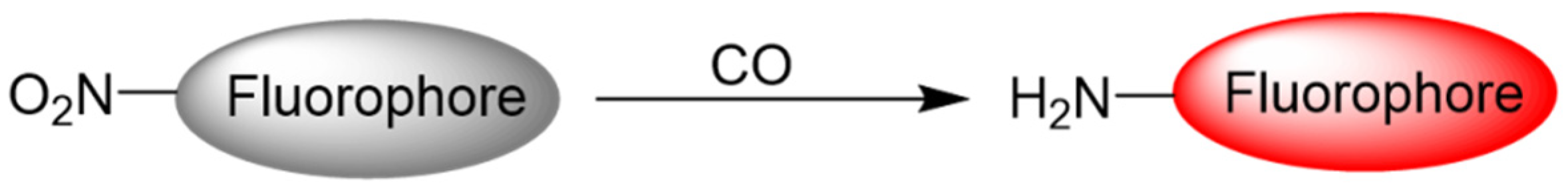
2.3. Reduction of Aromatic Nitro to Amine Reaction
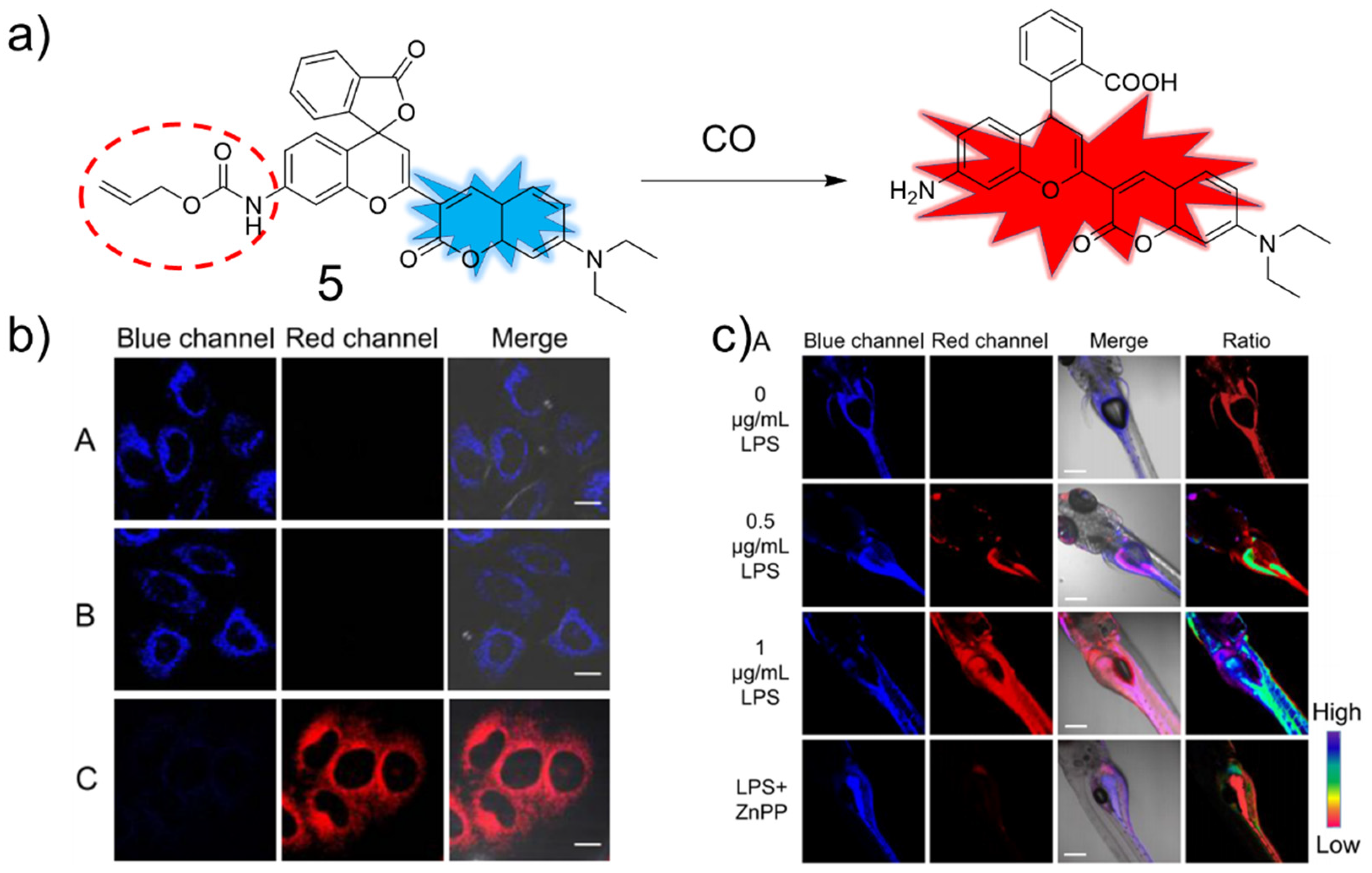
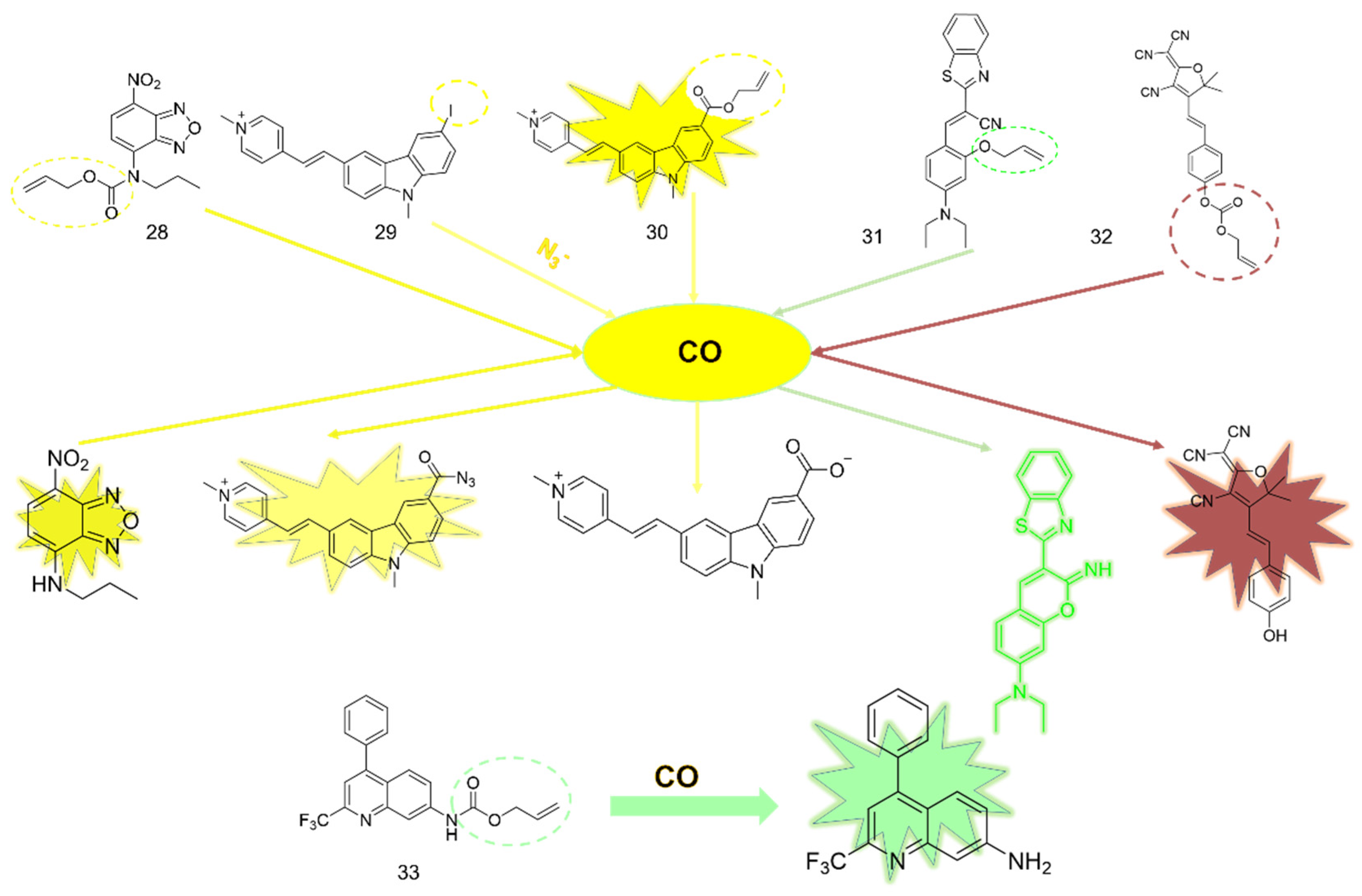
3. CO Fluorescent Probes Based on Tsuji-Trost Reaction
3.1. Coumarin as Fluorophore
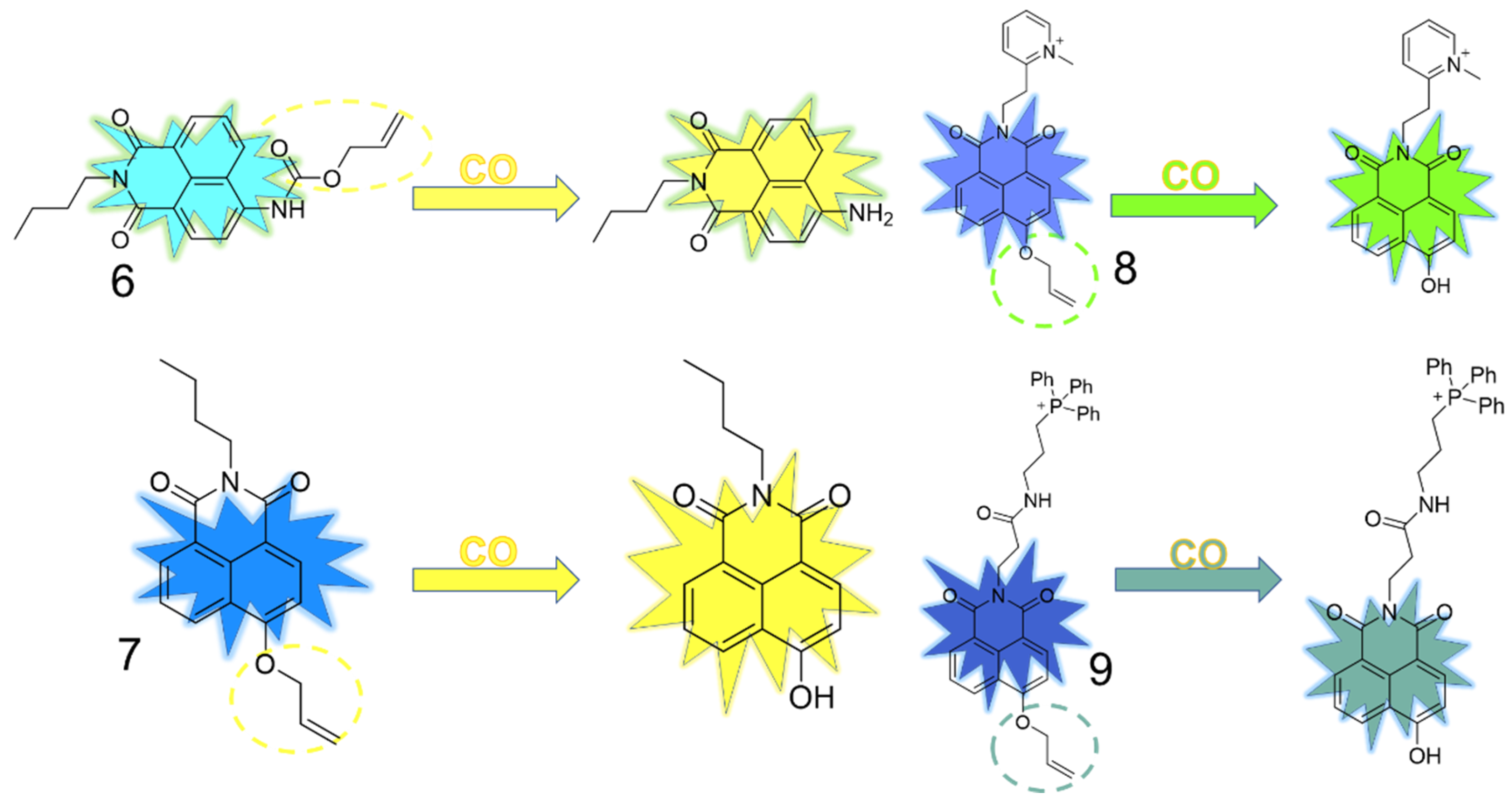
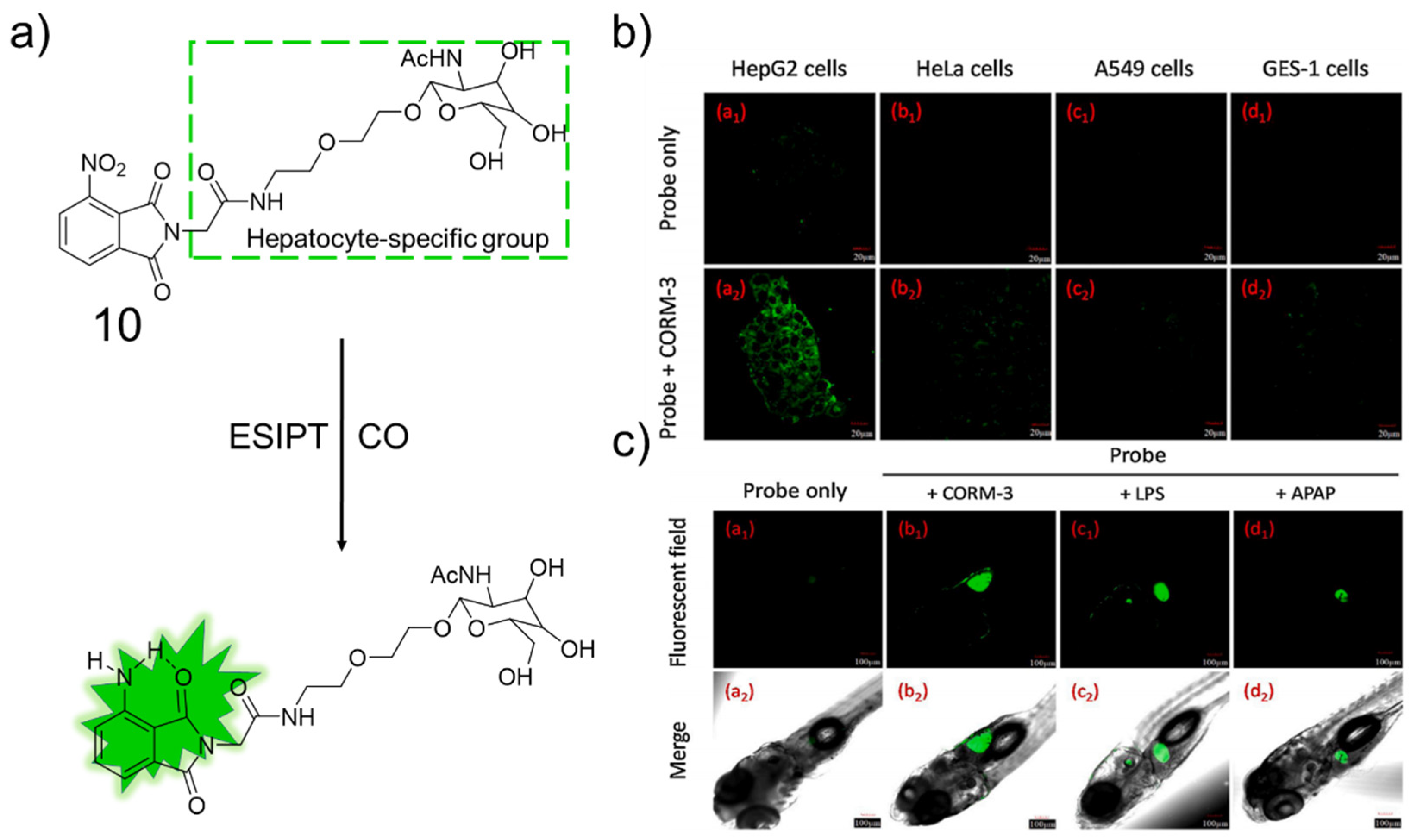
3.2. Naphthalimide as Fluorophore
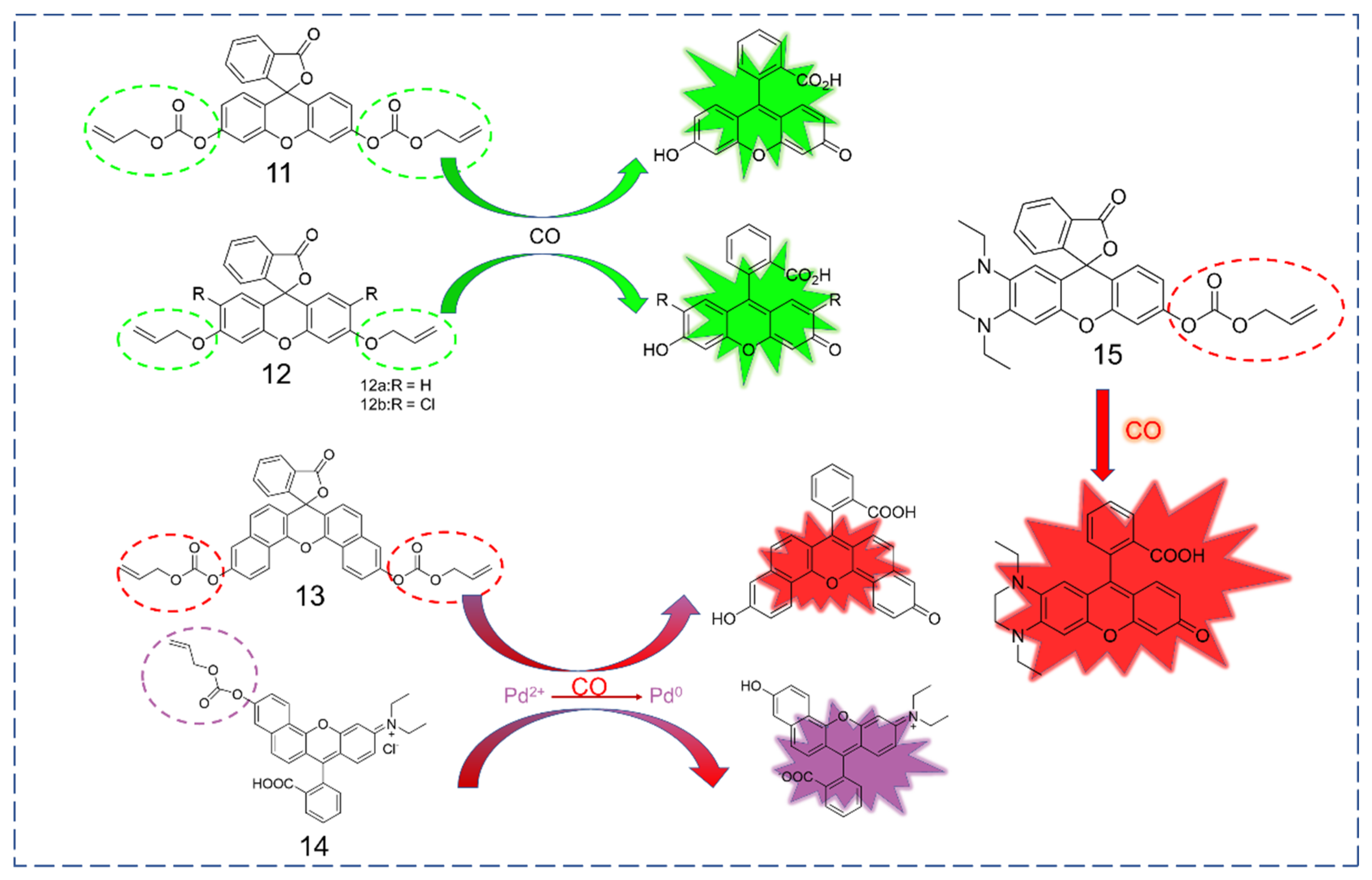
3.3. Xanthene as Fluorophore
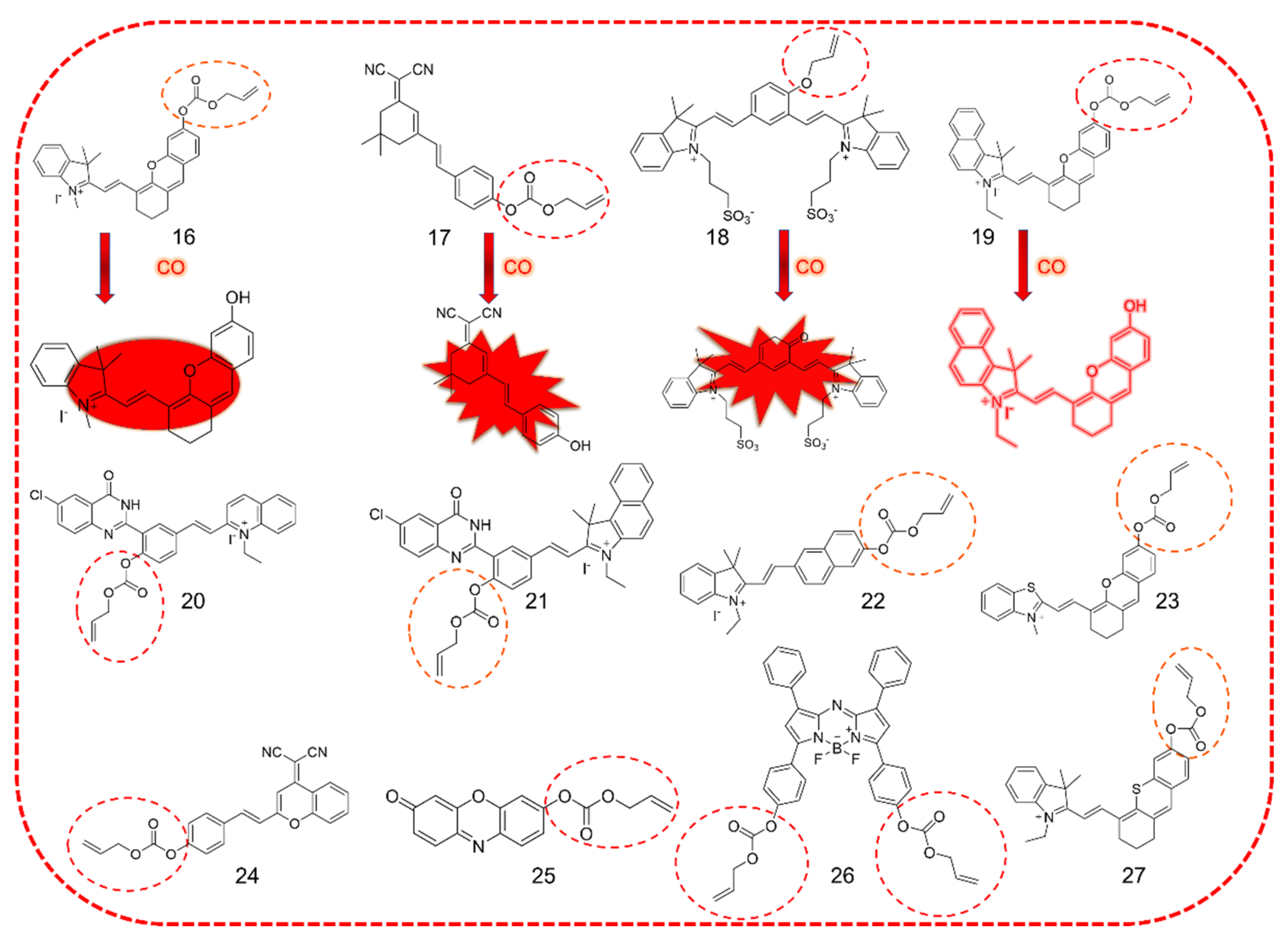
3.4. Near-Infrared Dyes as Fluorophore
3.5. Others
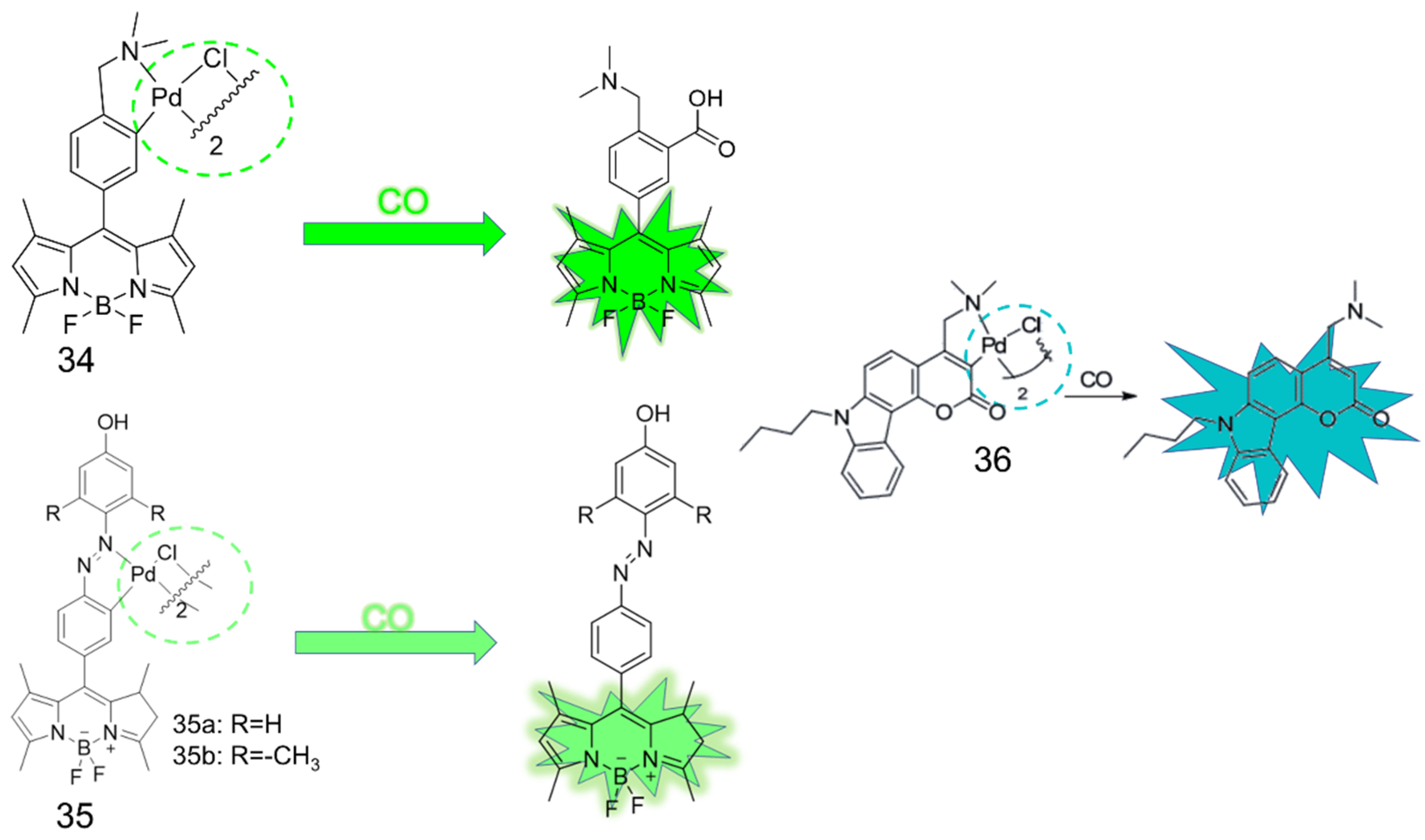
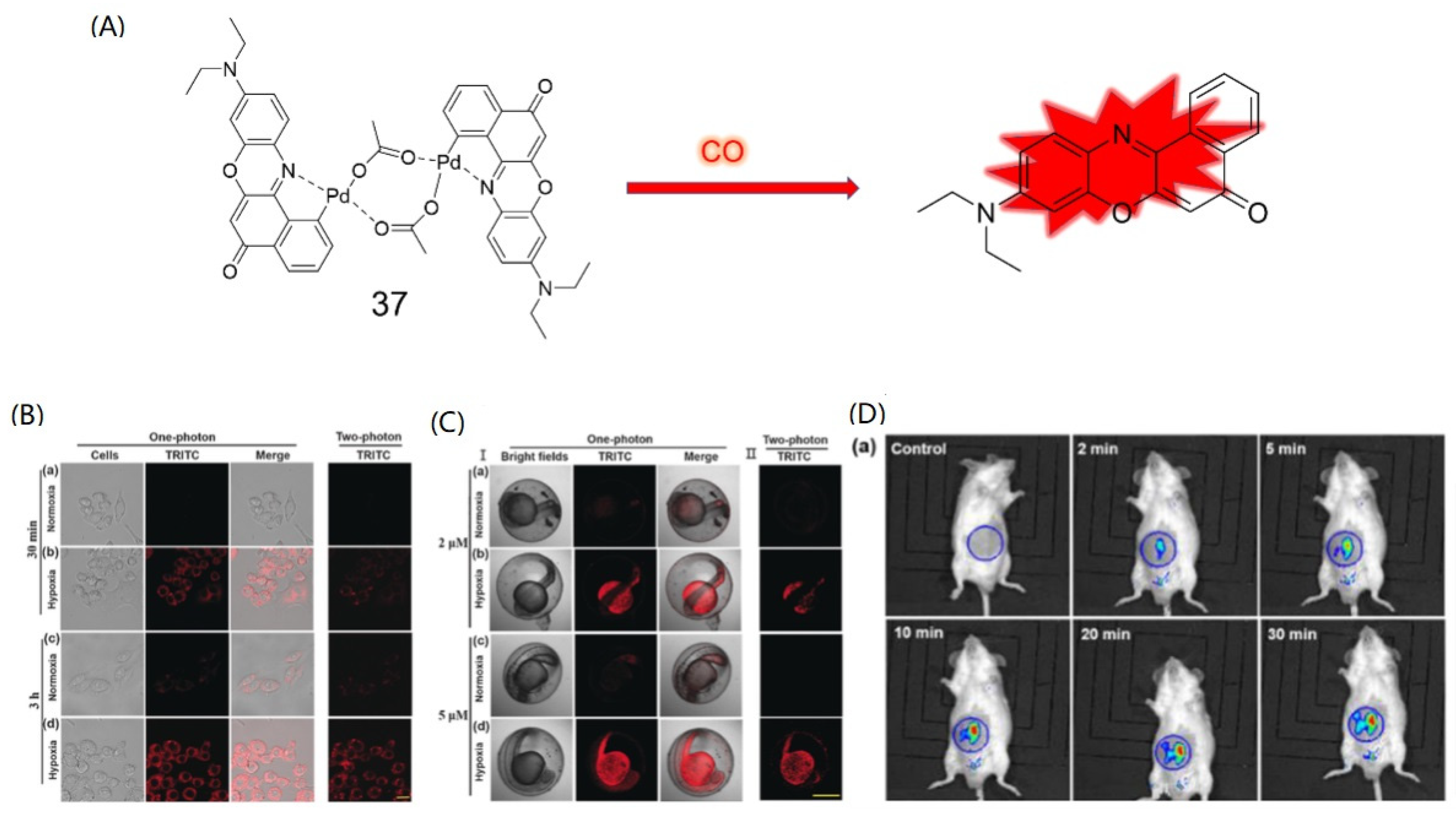
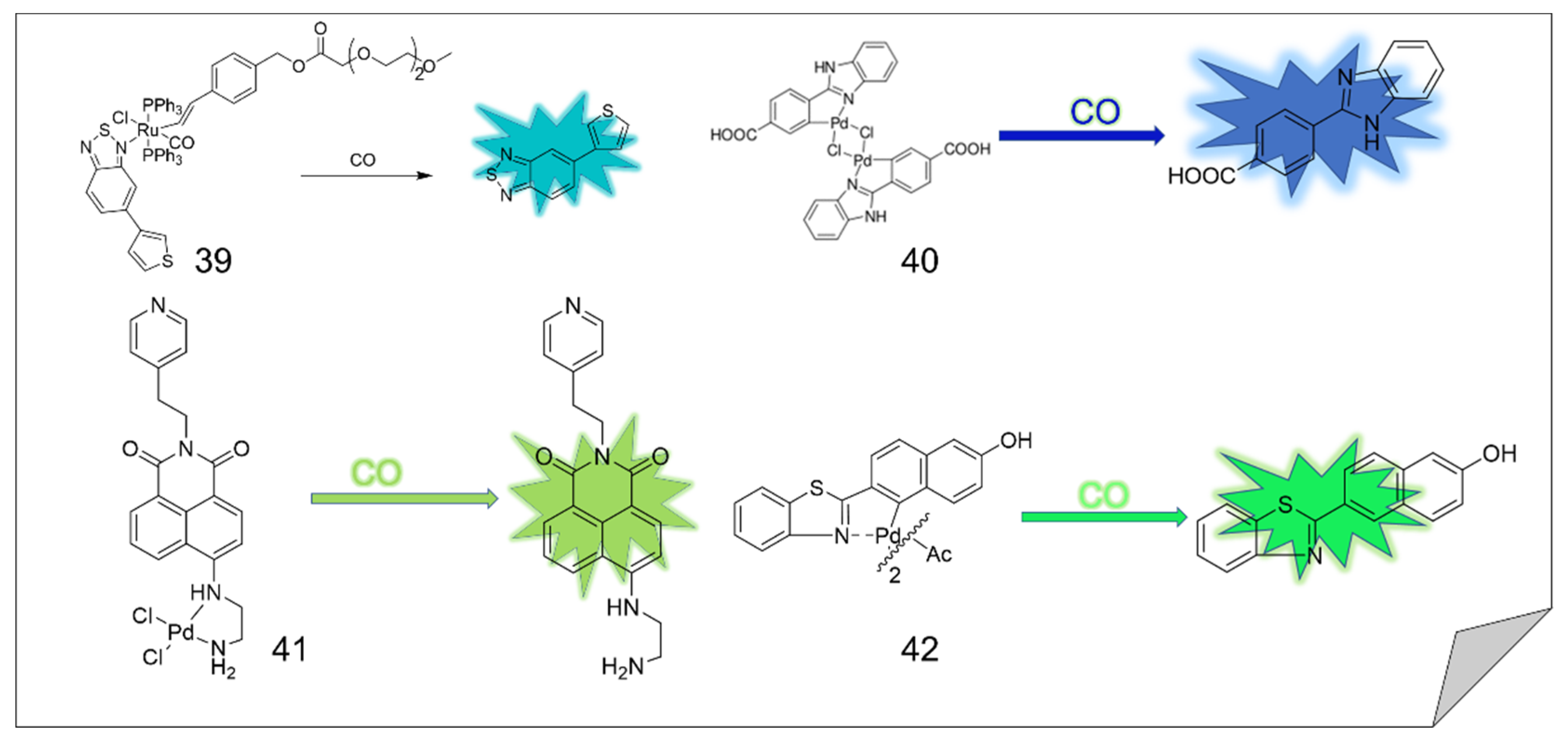
4. CO Fluorescence Probes Based on Pd-Mediated Carbonylation or Protonation Hydrolysis Reactions
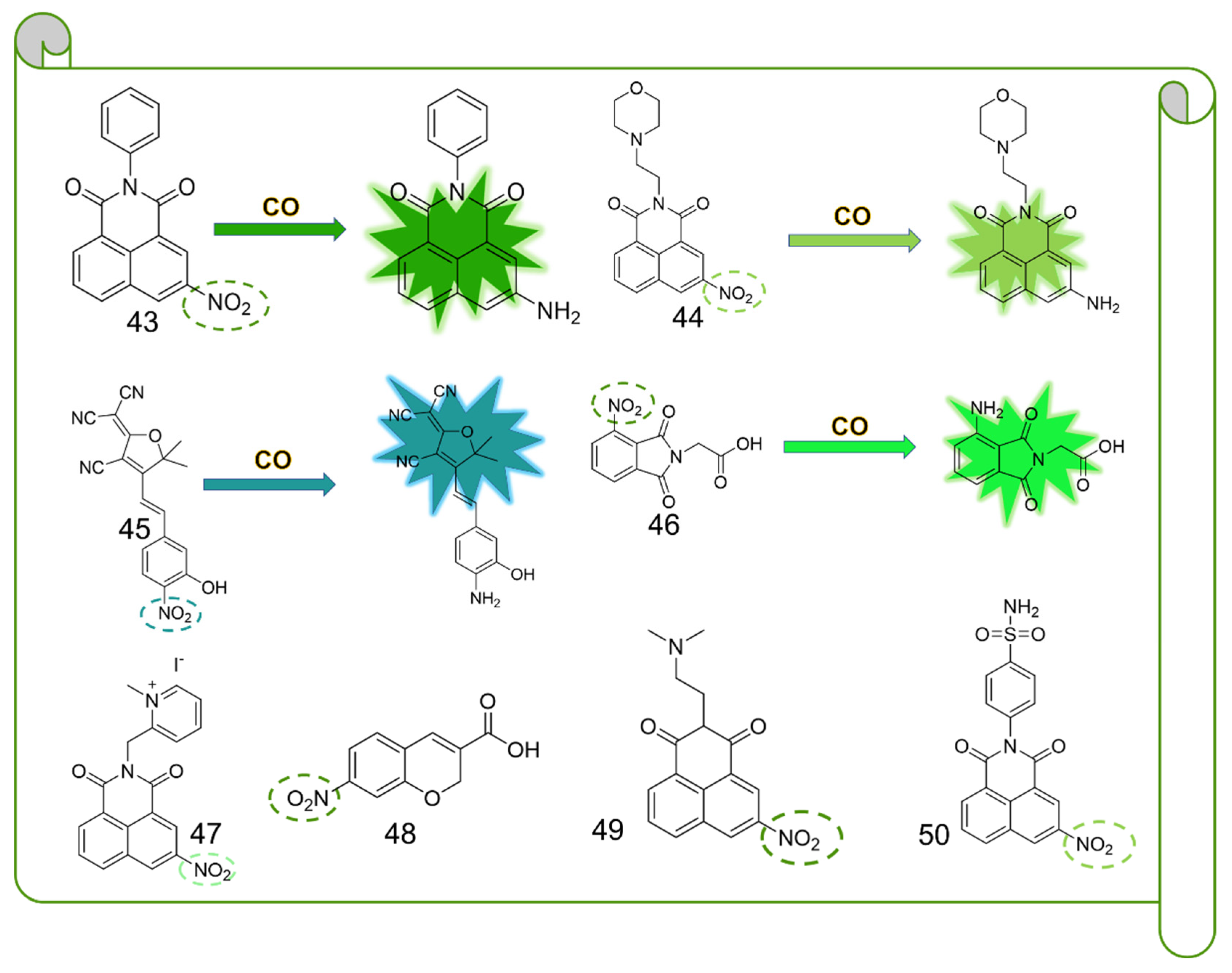
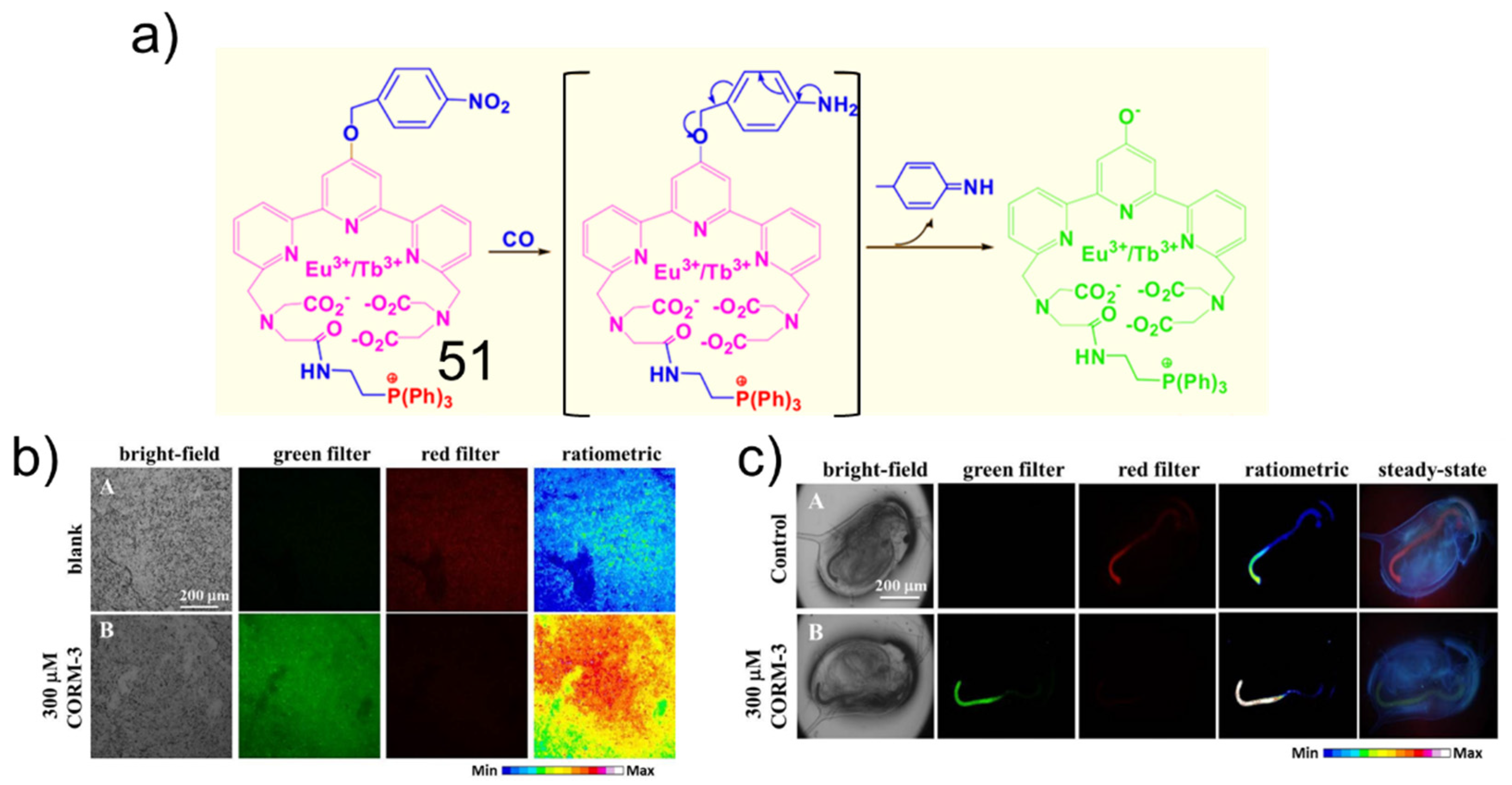
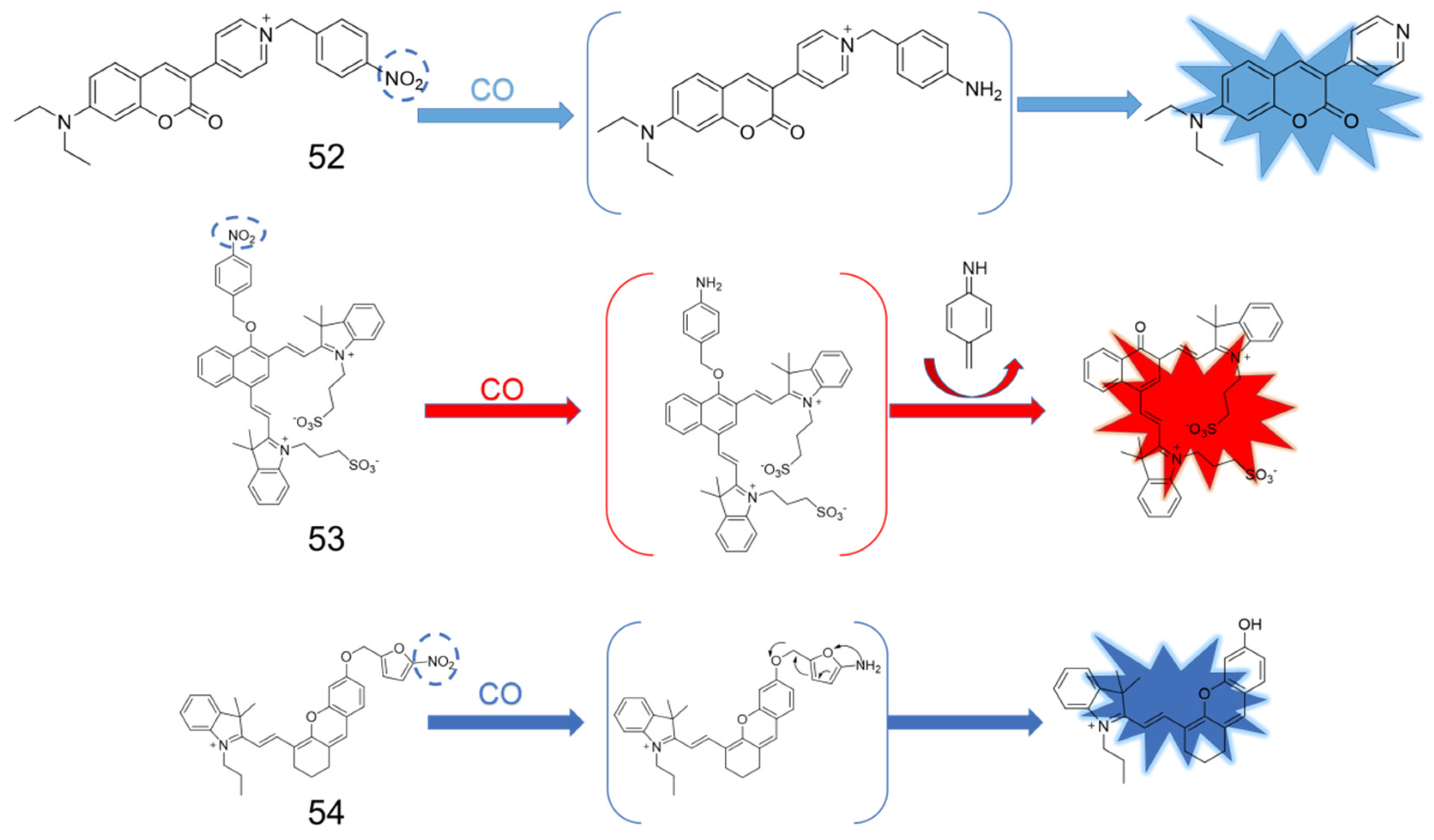
5. CO Fluorescent Probes Based on the Reduction in Nitro to Amine Reactions
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weaver, L.K. Clinical practice. Carbon monoxide poisoning. N. Engl. J. Med. 2009, 360, 1217–1225. [Google Scholar] [CrossRef]
- Murakawa, Y.; Nagai, M.; Mizutani, Y. Differences between Protein Dynamics of Hemoglobin upon Dissociation of Oxygen and Carbon Monoxide. J. Am. Chem. Soc. 2012, 134, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Strianese, M.; Pellecchia, C. Metal complexes as fluorescent probes for sensing biologically relevant gas molecules. Coord. Chem. Rev. 2016, 318, 16–28. [Google Scholar] [CrossRef]
- Verma, A.; Hirsch, D.J.; Glatt, C.E.; Ronnett, G.V.; Snyder, S.H. Carbon monoxide: A putative neural messenger. Science 1993, 259, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, R.; Chen, R.; Liang, X.; Song, W.; Lin, W. Activatable Photoacoustic Probe for In Situ Imaging of Endogenous Carbon Monoxide in the Murine Inflammation Model. Anal. Chem. 2021, 93, 8978–8985. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.-Q.; Xia, Y.-S.; Jiang, W.-L.; Wang, W.-X.; Tan, Z.-K.; Guo, K.-Y.; Mao, G.-J.; Li, C.-Y. A novel precipitating-fluorochrome-based fluorescent probe for monitoring carbon monoxide during drug-induced liver injury. Talanta 2022, 243, 123398. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, S.-Y.; Hong, S.; Chen, Q.-W.; Zeng, X.; Rong, L.; Zhong, Z.-L.; Zhang, X.-Z. Biomimetic carbon monoxide nanogenerator ameliorates streptozotocin induced type 1 diabetes in mice. Biomaterials 2020, 245, 119986. [Google Scholar] [CrossRef]
- Yue, L.; Tang, Y.; Huang, H.; Song, W.; Lin, W. A fluorogenic probe for detecting CO with the potential integration of diagnosis and therapy (IDT) for cancer. Sens. Actuators B Chem. 2021, 344, 130245. [Google Scholar] [CrossRef]
- Heylen, S.; Martens, J.A. Progress in the chromogenic detection of carbon monoxide. Angew. Chem. Int. Ed. 2010, 49, 7629–7630. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J. Simultaneous electrochemical detection of nitric oxide and carbon monoxide generated from mouse kidney organ tissues. Anal. Chem. 2007, 79, 7669–7675. [Google Scholar] [CrossRef]
- Marks, G.S.; Vreman, H.J.; McLaughlin, B.E.; Brien, J.F.; Nakatsu, K. Measurement of endogenous carbon monoxide formation in biological systems. Antioxid. Redox Signal. 2002, 4, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ma, Y.; Yin, J.; Lin, W. Strategies for designing organic fluorescent probes for biological imaging of reactive carbonyl species. Chem. Soc. Rev. 2019, 48, 4036–4048. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.-T.; Han, H.-H.; Sedgwick, A.C.; Zhu, G.-B.; Zang, Y.; Yang, X.-R.; Yoon, J.; James, T.D.; Li, J.; He, X.-P. Fluorescent probes for the detection of disease-associated biomarkers. Sci. Bull. 2022, 67, 853–878. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, X.; Sun, L.; Zhao, X.; He, Y.; Gao, G.; Han, W.; Zhou, J. Lysosome-targeting red fluorescent probe for broad carboxylesterases detection in breast cancer cells. Sens. Actuators B Chem. 2022, 33, 4229–4232. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, H.; Zhan, T.; Zhang, J.; Chen, B.; Qian, Z.; Zhang, G.; Zhang, W.; Zhou, J. A new mitochondrion targetable fluorescent probe for carbon monoxide-specific detection and live cell imaging. Chem. Commun. 2019, 55, 9444–9447. [Google Scholar] [CrossRef]
- Zhang, P.; Tian, Y.; Liu, H.; Ren, J.; Wang, H.; Zeng, R.; Long, Y.; Chen, J. In vivo imaging of hepatocellular nitric oxide using a hepatocyte-targeting fluorescent sensor. Chem. Commun. 2018, 54, 7231–7234. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, P.; Wang, H.; Yu, M.; Gao, Y.; Chen, J. Photoswitchable AIE nanoprobe for lysosomal hydrogen sulfide detection and reversible dual-color imaging. Sens. Actuators B Chem. 2018, 272, 340–347. [Google Scholar] [CrossRef]
- Pal, S.; Mukherjee, M.; Sen, B.; Mandal, S.K.; Lohar, S.; Chattopadhyay, P.; Dhara, K. A new fluorogenic probe for the selective detection of carbon monoxide in aqueous medium based on Pd(0) mediated reaction. Chem. Commun. 2015, 51, 4410–4413. [Google Scholar] [CrossRef]
- Feng, W.; Liu, D.; Zhai, Q.; Feng, G. Lighting up carbon monoxide in living cells by a readily available and highly sensitive colorimetric and fluorescent probe. Sens. Actuators B Chem. 2017, 240, 625–630. [Google Scholar] [CrossRef]
- Deng, Y.; Hong, J.; Zhou, E.; Feng, G. Near-infrared fluorescent probe with a super large stokes shift for tracking CO in living systems based on a novel coumarin-dicyanoisophorone hybrid. Dyes Pigments 2019, 170, 107634. [Google Scholar] [CrossRef]
- Fang, W.-L.; Tang, Y.-J.; Guo, X.-F.; Wang, H. A fluorescent probe for carbon monoxide based on allyl ether rather than allyl ester: A practical strategy to avoid the interference of esterase in cell imaging. Talanta 2019, 205, 120070. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.-L.; Wang, W.-X.; Mao, G.-J.; Yan, L.; Du, Y.; Li, Y.; Li, C.-Y. Construction of NIR and Ratiometric Fluorescent Probe for Monitoring Carbon Monoxide under Oxidative Stress in Zebrafish. Anal. Chem. 2021, 93, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Hong, J.; Feng, G. Colorimetric and ratiometric fluorescent detection of carbon monoxide in air, aqueous solution, and living cells by a naphthalimide-based probe. Sens. Actuators B Chem. 2017, 251, 389–395. [Google Scholar] [CrossRef]
- Wang, Z.; Geng, Z.; Zhao, Z.; Sheng, W.; Liu, C.; Lv, X.; He, Q.; Zhu, B. A highly specific and sensitive ratiometric fluorescent probe for carbon monoxide and its bioimaging applications. New J. Chem. 2018, 42, 14417–14423. [Google Scholar] [CrossRef]
- Zang, S.; Shu, W.; Shen, T.; Gao, C.; Tian, Y.; Jing, J.; Zhang, X. Palladium-triggered ratiometric probe reveals CO's cytoprotective effects in mitochondria. Dyes Pigments 2020, 173, 107861. [Google Scholar] [CrossRef]
- Du, F.; Qu, Y.; Li, M.; Tan, X. Mitochondria-targetable ratiometric fluorescence probe for carbon monoxide based on naphthalimide derivatives. Anal. Bioanal. Chem. 2021, 413, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jia, X.; Zhang, P.; Guo, Z.; Zhao, H.; Li, X.; Wei, C. A hepatocyte-specific fluorescent probe for imaging endogenous carbon monoxide release in vitro and in vivo. Sens. Actuators B Chem. 2021, 344, 130177. [Google Scholar] [CrossRef]
- Yan, J.-W.; Zhu, J.-Y.; Tan, Q.-F.; Zhou, L.-F.; Yao, P.-F.; Lu, Y.-T.; Tan, J.-H.; Zhang, L. Development of a colorimetric and NIR fluorescent dual probe for carbon monoxide. RSC Adv. 2016, 6, 65373–65376. [Google Scholar] [CrossRef]
- Feng, W.; Liu, D.; Feng, S.; Feng, G. A Readily Available Fluorescent Probe forCarbon Monoxide Imaging in Living Cells. Anal. Chem. 2016, 88, 10648–10653. [Google Scholar] [CrossRef]
- Feng, S.; Liu, D.; Feng, W.; Feng, G. Allyl Fluorescein Ethers as Promising Fluorescent Probes for Carbon Monoxide Imaging in Living Cells. Anal. Chem. 2017, 89, 3754–3760. [Google Scholar] [CrossRef]
- Hong, J.; Xia, Q.; Zhou, E.; Feng, G. NIR fluorescent probe based on a modified rhodol-dye with good water solubility and large Stokes shift for monitoring CO in living systems. Talanta 2020, 215, 120914. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Z.; Wang, R.; Yuan, R.; Liu, C.; Duan, Q.; Zhu, W.; Li, X.; Zhu, B. A mitochondria-targetable colorimetric and far-red fluorescent probe for the sensitive detection of carbon monoxide in living cells. Anal. Methods 2019, 11, 288–295. [Google Scholar] [CrossRef]
- Feng, W.; Feng, G. A readily available colorimetric and near-infrared fluorescent turn-on probe for detection of carbon monoxide in living cells and animals. Sens. Actuators B Chem. 2018, 255, 2314–2320. [Google Scholar] [CrossRef]
- Gong, S.; Hong, J.; Zhou, E.; Feng, G. A near-infrared fluorescent probe for imaging endogenous carbon monoxide in living systems with a large Stokes shift. Talanta 2019, 201, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Gong, S.; Feng, G. Rapid detection of CO in vitro and in vivo with a ratiometric probe showing near-infrared turn-on fluorescence, large Stokes shift, and high signal-tonoise ratio. Sens. Actuators B Chem. 2019, 301, 127075. [Google Scholar] [CrossRef]
- Li, S.-J.; Zhou, D.-Y.; Li, Y.-F.; Yang, B.; Ou-Yang, J.; Jie, J.; Liu, J.; Li, C.-Y. Mitochondria-targeted near-infrared fluorescent probe for the detection of carbon monoxide in vivo. Talanta 2018, 188, 691–700. [Google Scholar] [CrossRef]
- Xia, Y.-S.; Yan, L.; Mao, G.-J.; Jiang, W.-L.; Wang, W.-X.; Li, Y.; Jiang, Y.-Q.; Li, C.-Y. A novel HPQ-based fluorescent probe for the visualization of carbon monoxide in zebrafish. Sens. Actuators B Chem. 2021, 340, 129920. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X.; Tang, Y.; Li, M.; Yin, Y.; Lin, W. The development of a hemicyanine-based ratiometric CO fluorescent probe with long emission and its applications for imaging CO in vitro and in vivo. New J. Chem. 2020, 44, 12107–12112. [Google Scholar] [CrossRef]
- Yan, L.; Nan, D.; Lin, C.; Wan, Y.; Pan, Q.; Qi, Z. A near-infrared fluorescent probe for rapid detection of carbon monoxide in living cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 202, 284–289. [Google Scholar] [CrossRef]
- Biswas, B.; Venkateswarulu, M.; Sinha, S.; Girdhar, K.; Ghosh, S.; Chatterjee, S.; Mondal, P.; Ghosh, S. Long Range Emissive Water-Soluble Fluorogenic Molecular Platform for Imaging Carbon Monoxide in Live Cells. ACS Appl. Bio Mater. 2019, 2, 5427–5433. [Google Scholar] [CrossRef]
- Xu, Z.; Song, A.; Chen, F.W.H. Sensitive and effective imaging of carbon monoxide in living systems with a near-infrared fluorescent probe. RSC Adv. 2021, 11, 32203. [Google Scholar] [CrossRef] [PubMed]
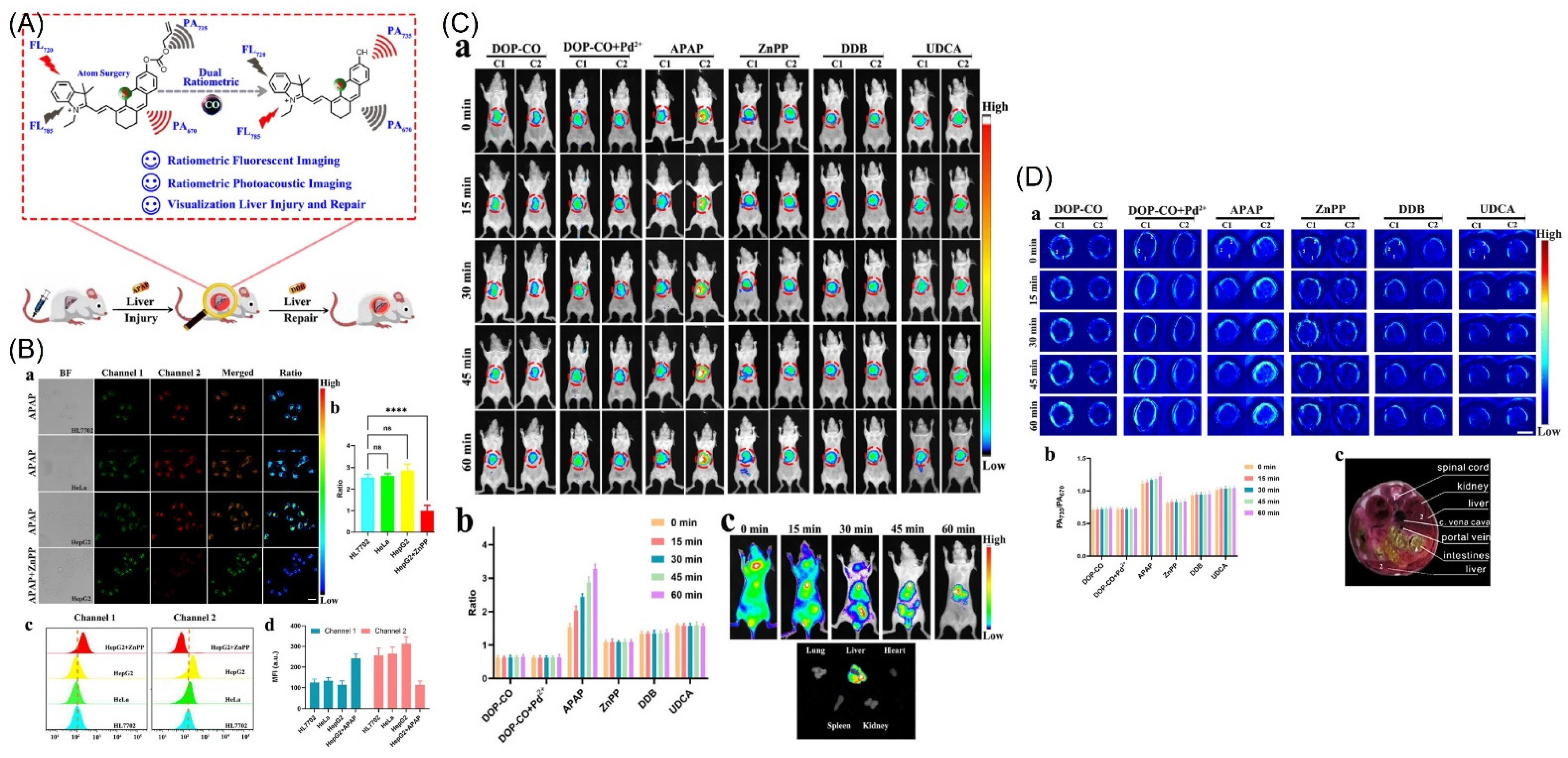
- Liu, L.; Xu, J.; Zhang, S.; Chen, H.; Wang, L.; Shen, X.-C.; Chen, H. Rational design of dual ratiometric photoacoustic and fluorescent probe for reliable imaging and quantitative detection of endogenous CO during drug-induced liver injury and repair. Sens. Actuators B Chem. 2022, 367, 132171. [Google Scholar] [CrossRef]
- Xu, Z.; Yan, J.; Li, J.; Yao, P.; Tan, J.; Zhang, L. A colorimetric and fluorescent turn-on probe for carbon monoxide and imaging in living cells. Tetrahedron Lett. 2016, 57, 2927–2930. [Google Scholar] [CrossRef]
- Shi, G.; Yoon, T.; Cha, S.; Kim, S.; Yousuf, M.; Ahmed, N.; Kim, D.; Kang, H.-W.; Kim, K.S. Turn-on and Turn-off Fluorescent Probes for Carbon Monoxide Detection and Blood Carboxyhemoglobin Determination. ACS Sens. 2018, 3, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, C.; Chen, Q.; Li, H.; Zhou, L.; Jiang, X.; Shi, M.; Zhang, P.; Jiang, G.; Tang, B.Z. An Easily Available Ratiometric Reaction-based AIE Probe for Carbon Monoxide Light-up Imaging. Anal. Chem. 2019, 91, 9388–9392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Z.; Liu, C.; Geng, Z.; Duan, Q.; Jia, P.; Li, Z.; Zhu, H.; Zhu, B.; Sheng, W. A long-wavelength ultrasensitive colorimetric fluorescent probe for carbon monoxide detection in living cells. Photochem. Photobiol. Sci. 2019, 18, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Feng, S.; Gong, S.; Feng, G. Golgi-targetable fluorescent probe for ratiometric imaging of CO in cells and zebrafish. Sens. Actuators B Chem. 2021, 347, 130631. [Google Scholar] [CrossRef]
- Michel, B.W.; Lippert, A.R.; Chang, C.J. Reaction-Based Fluorescent Probe for Selective Imaging of Carbon Monoxide in Living Cells Using a Palladium-Mediated Carbonylation. J. Am. Chem. Soc. 2012, 134, 15668–156671. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yang, J.; Xie, X.; Li, M.; Niu, J.; Tong, L.; Tang, B. A New Fluorescent Probe Based on Azobenzene-Cyclopalladium for the Selective Imaging of Endogenous Carbon Monoxide under Hypoxia Conditions. Anal. Chem. 2016, 88, 11154–11159. [Google Scholar] [CrossRef]
- Zheng, K.; Lin, W.; Tan, L.; Chen, H.; Cui, H. A unique carbazole–coumarin fused two-photon platform: Development of a robust two-photon fluorescent probe for imaging carbon monoxide in living tissues. Chem. Sci. 2014, 5, 3439–3448. [Google Scholar] [CrossRef]
- Liu, K.; Kong, X.; Ma, Y.; Lin, W. Rational design of a robust fluorescent probe for detecting endogenous carbon monoxide in living zebrafish embryos and mouse tissues. Angew. Chem. Int. Ed. Engl. 2017, 56, 13489–13492. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, H.-W.; Yin, X.; Yuan, L.; Zhang, S.-Y.H.X.-B. A cell membrane-anchored fluorescent probe for monitoring carbon monoxide release from living cells. Chem. Sci. 2018, 10, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Torre, C.; Toscani, A.; Marín-Hernández, C.; Robson, J.A.; Terencio, M.C.; White, A.J.P.; Alcaraz, M.J.; Wilton-Ely, J.D.E.T.; Martínez-Máñez, R.; Sancenón, F. Ex Vivo Tracking of Endogenous CO with a Ruthenium(II) Complex. J. Am. Chem. Soc. 2017, 139, 18484–18487. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yu, H.; Zhang, K.; Wang, S.; Hayat, T.; Alsaedi, A.; Huang, D. A Palladacycle based Fluorescence Turn-On Probe for Sensitive Detection of Carbon Monoxide. ACS Sens. 2018, 3, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Tikum, A.F.; Lim, W.; Fortibui, M.M.; Lee, S.; Park, S.; Kim, J. Palladium Probe Consisting of Naphthalimide and Ethylenediamine for Selective Turn-On Sensing of CO and Cell Imaging. Inorg Chem. 2021, 60, 7108–7114. [Google Scholar] [CrossRef] [PubMed]
- Zong, P.; Chen, Y.; Liu, K.; Bi, J.; Ren, M.; Wang, S.; Kong, F. Construction of a unique two-photon fluorescent probe and the application for endogenous CO detection in live organisms. Talanta 2022, 240, 123194. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Lohar, S.; Patra, A.; Ahmmed, E.; Mondal, S.; Bhakta, J.N.; Dhara, K.; Chattopadhyay, P. Naphthalimide-based fluorescence “turn-on” chemosensor for highly selective detection of carbon monoxide: Imaging applications in live cell. New J. Chem. 2018, 42, 13497–13502. [Google Scholar] [CrossRef]
- Dhara, K.; Lohar, S.; Patra, A.; Roy, P.; Saha, S.K.; Sadhukhan, G.C.; Chattopadhyay, P. A New Lysosome Targetable Turn-On Fluorogenic Probe for Carbon Monoxide Imaging in Living Cells. Anal. Chem. 2018, 90, 2933–2938. [Google Scholar] [CrossRef]
- Sarkar, A.; Fouzder, C.; Chakraborty, S.; Ahmmed, E.; Kundu, R.; Dam, S.; Chattopadhyay, P.; Dhara, K. A Nuclear-Localized Naphthalimide Based Fluorescent Light-Up Probe for Selective Detection of Carbon Monoxide in Living Cells. Chem. Res. Toxicol. 2020, 33, 651–656. [Google Scholar] [CrossRef]
- Tang, Z.; Song, B.; Ma, H.; Luo, T.; Guo, L.; Yuan, J. A Mitochondria-Targetable Ratiometric Time-Gated Luminescence Probe for Carbon Monoxide Based on Lanthanide Complexes. Anal. Chem. 2019, 91, 2939–2946. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.; Wang, X.; Duan, Q.; Jia, P.; Zhu, H.; Li, Z.; Zhang, X.; Ren, X.; Zhu, B.; et al. A metal-free near-infrared fluorescent probe for tracking the glucoseinduced fluctuations of carbon monoxide in living cells and zebrafish. Sens. Actuators B Chem. 2019, 291, 329–336. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C.; Chen, Y.; Zhuang, Z.; Tian, B.; Liu, C.; Jia, P.; Zhu, H.; Yu, Y.; Zhang, X.; et al. A novel Pd2+-free highly selective and ultrasensitive fluorescent probe for detecting CO-releasing molecule-2 in live cells and zebrafish. Dyes Pigments 2020, 174, 108040. [Google Scholar] [CrossRef]
- Feng, W.; Feng, S.; Feng, G. A fluorescent ESIPT probe for imaging CO releasing melecule-3 (CORM-3) in living systems. Anal. Chem. 2019, 91, 8602–8606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Gong, S.; Xia, Q.; Feng, G. In Vivo Imaging and Tracking Carbon Monoxide-Releasing Molecule-3 with an NIR Fluorescent Probe. ACS Sens. 2021, 6, 1312–1320. [Google Scholar] [CrossRef]
- Zhang, S.; Mu, X.; Zhu, J.; Yan, L. A metal-free coumarin-based fluorescent probe for the turn-on monitoring of carbon monoxide in an aqueous solution and living cells. Analyst 2021, 146, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, Z.; Li, Y.; Zhang, Y.; Wang, Q.; Zhang, C. A new metal-free near-infrared fluorescent probe based on nitrofuran for the detection and bioimaging of carbon monoxide releasing molecule-2 in vivo. Analyst 2022, 147, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, K.; Zeng, J.; Ding, Y.; Cheng, D.; He, L. Golgi-Targeting Fluorescent Probe for Monitoring CO-Releasing Molecule-3 In Vitro and In Vivo. ACS Omega 2022, 7, 9929–9935. [Google Scholar] [CrossRef] [PubMed]
- Alhalaby, H.; Principe, M.; Zaraket, H.; Vaiano, P.; Aliberti, A.; Quero, G.; Crescitelli, A.; Meo, V.D.; Esposito, E.; Consales, M.; et al. Design and Optimization of All-Dielectric Fluorescence Enhancing Metasurfaces: Towards Advanced Metasurface-Assisted Optrodes. Biosensors 2022, 12, 264. [Google Scholar] [CrossRef]
- Qu, J.-H.; Peeters, B.; Delport, F.; Vanhoorelbeke, K.; Lammertyn, J.; Spasic, D. Gold nanoparticle enhanced multiplexed biosensing on a fiber optic surface plasmon resonance probe. Biosens. Bioelectron. 2021, 192, 113549. [Google Scholar] [CrossRef]
- Liu, Y.; Guang, J.; Liu, C.; Bi, S.; Liu, Q.; Li, P.; Zhang, N.; Chen, S.; Yuan, H.; Zhou, D.; et al. Simple and Low-Cost Plasmonic Fiber-Optic Probe as SERS and Biosensing Platform. Adv. Opt. Mater. 2019, 7, 1900337. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Luo, K.; Tan, L.; Yang, Q.; Zhao, X.; Zhou, L. A Review for In Vitro and In Vivo Detection and Imaging of Gaseous Signal Molecule Carbon Monoxide by Fluorescent Probes. Molecules 2022, 27, 8842. https://doi.org/10.3390/molecules27248842
Xie C, Luo K, Tan L, Yang Q, Zhao X, Zhou L. A Review for In Vitro and In Vivo Detection and Imaging of Gaseous Signal Molecule Carbon Monoxide by Fluorescent Probes. Molecules. 2022; 27(24):8842. https://doi.org/10.3390/molecules27248842
Chicago/Turabian StyleXie, Can, Kun Luo, Libin Tan, Qiaomei Yang, Xiongjie Zhao, and Liyi Zhou. 2022. "A Review for In Vitro and In Vivo Detection and Imaging of Gaseous Signal Molecule Carbon Monoxide by Fluorescent Probes" Molecules 27, no. 24: 8842. https://doi.org/10.3390/molecules27248842
APA StyleXie, C., Luo, K., Tan, L., Yang, Q., Zhao, X., & Zhou, L. (2022). A Review for In Vitro and In Vivo Detection and Imaging of Gaseous Signal Molecule Carbon Monoxide by Fluorescent Probes. Molecules, 27(24), 8842. https://doi.org/10.3390/molecules27248842



