Light Harvesting Nanoprobe for Trace Detection of Hg2+ in Water
Abstract
:1. Introduction
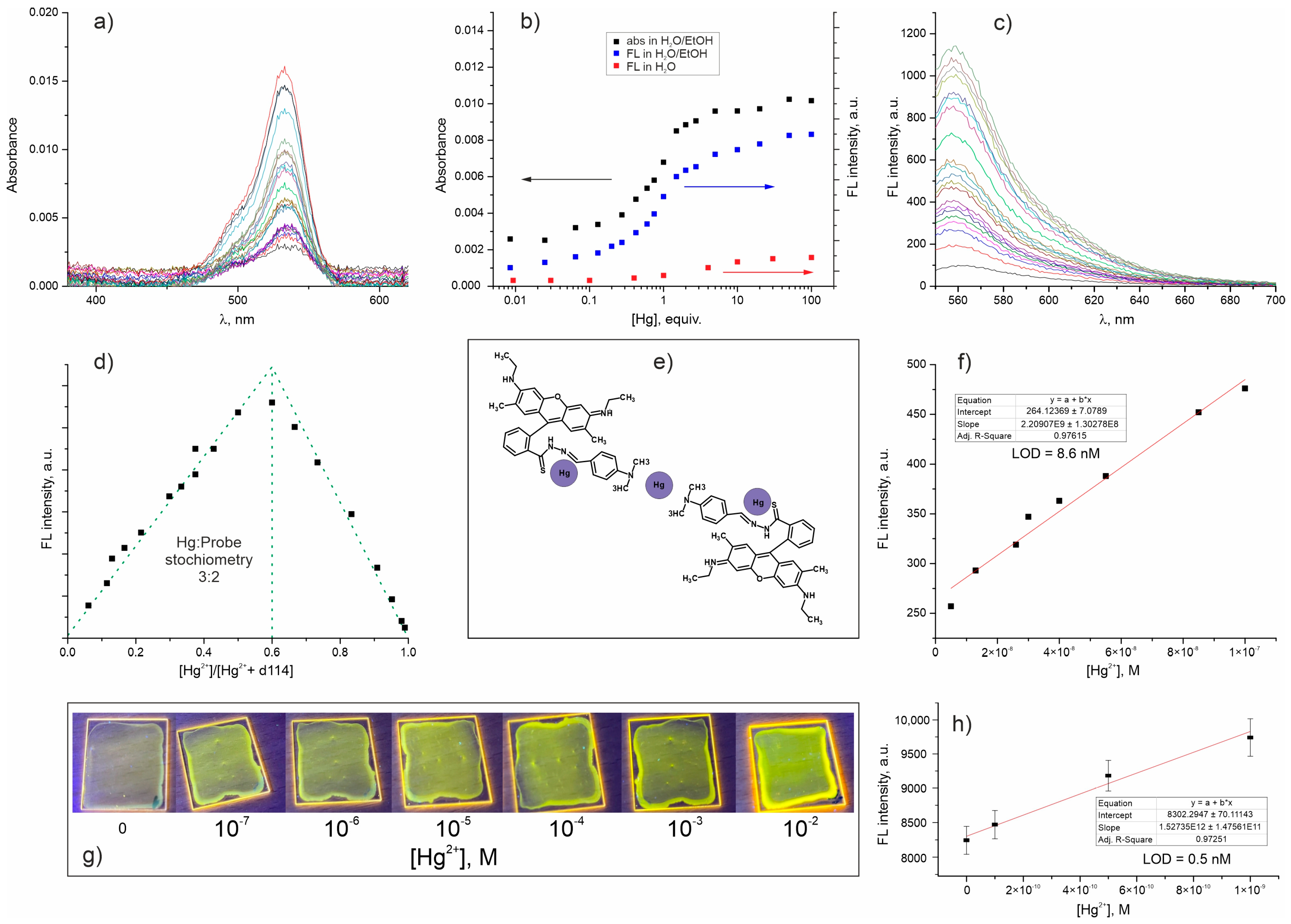
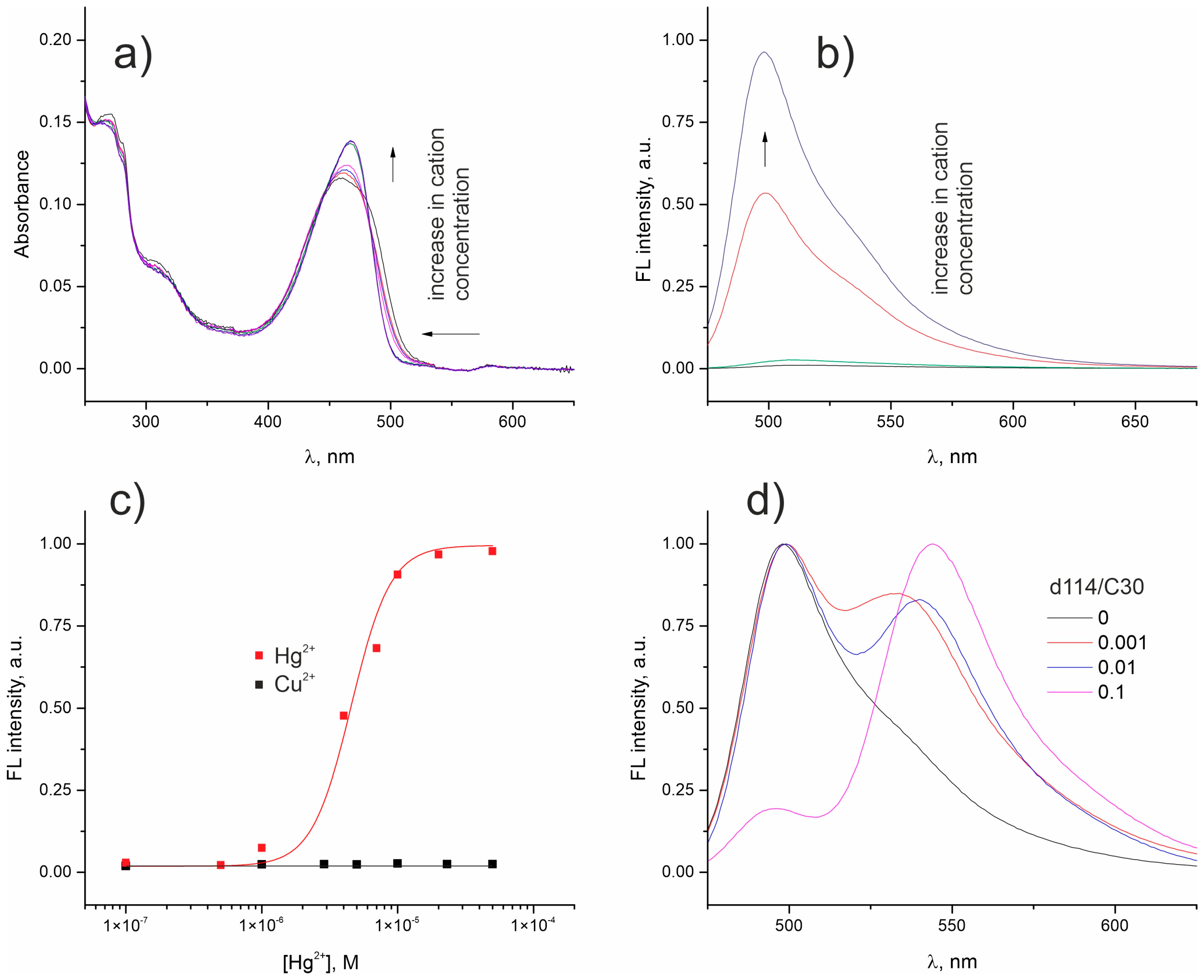
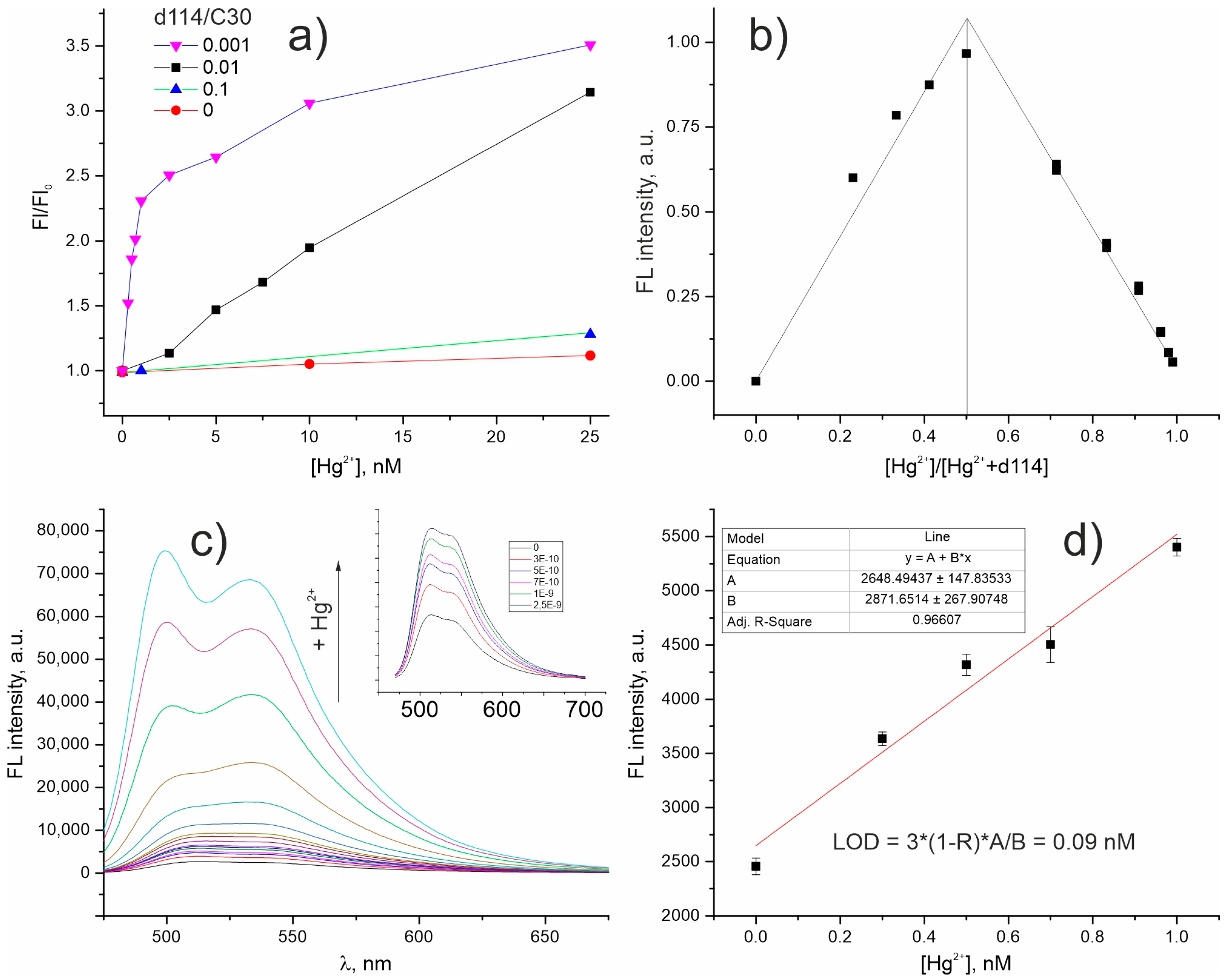
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Synthesis and Characterization of d114
3.3. Fabrication of the Sensing Coating Containing d114 on PMMA
3.4. Preparation of Fluorescent NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2017; p. 541.
- Jaywant, S.A.; Mahmood Arif, K. A Comprehensive Review of Microfluidic Water Quality Monitoring Sensors. Sensors 2019, 19, 4781. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, X.-J.; Lu, H.-Y.; Chen, C.-F. Tetrahydro[5]helicene thioimide-based fluorescent and chromogenic chemodosimeter for highly selective and sensitive detection of Hg2+. Sens. Actuators B Chem. 2014, 202, 583–587. [Google Scholar] [CrossRef]
- Fan, C.; He, S.; Liu, G.; Wang, L.; Song, S. A Portable and Power-Free Microfluidic Device for Rapid and Sensitive Lead (Pb2+) Detection. Sensors 2012, 12, 9467–9475. [Google Scholar] [CrossRef] [PubMed]
- Mitsai, E.; Kuchmizhak, A.; Pustovalov, E.; Sergeev, A.; Mironenko, A.; Bratskaya, S.; Linklater, D.P.; Balčytis, A.; Ivanova, E.; Juodkazis, S. Chemically non-perturbing SERS detection of a catalytic reaction with black silicon. Nanoscale 2018, 10, 9780–9787. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.S.; Bhosale, C.H.; Rajpure, K.Y. Oxidative degradation of acid orange 7 using Ag-doped zinc oxide thin films. J. Photochem. Photobiol. B Biol. 2012, 117, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Pavliuk, G.; Pavlov, D.; Mitsai, E.; Vitrik, O.; Mironenko, A.; Zakharenko, A.; Kulinich, S.A.; Juodkazis, S.; Bratskaya, S.; Zhizhchenko, A.; et al. Ultrasensitive SERS-based plasmonic sensor with analyte enrichment system produced by direct laser writing. Nanomaterials 2020, 10, 49. [Google Scholar] [CrossRef]
- Mitsai, E.; Naffouti, M.; David, T.; Abbarchi, M.; Hassayoun, L.; Storozhenko, D.; Mironenko, A.; Bratskaya, S.; Juodkazis, S.; Makarov, S.; et al. Si1-xGex nanoantennas with a tailored Raman response and light-to-heat conversion for advanced sensing applications. Nanoscale 2019, 11, 11634–11641. [Google Scholar] [CrossRef]
- Dostovalov, A.; Bronnikov, K.; Korolkov, V.; Babin, S.; Mitsai, E.; Mironenko, A.; Tutov, M.; Zhang, D.; Sugioka, K.; Maksimovic, J.; et al. Hierarchical anti-reflective laser-induced periodic surface structures (LIPSSs) on amorphous Si films for sensing applications. Nanoscale 2020, 12, 13431–13441. [Google Scholar] [CrossRef]
- Borodaenko, Y.; Gurbatov, S.; Tutov, M.; Zhizhchenko, A.; Kulinich, S.A.; Kuchmizhak, A.; Mironenko, A. Direct femtosecond laser fabrication of chemically functionalized ultra-black textures on silicon for sensing applications. Nanomaterials 2021, 11, 401. [Google Scholar] [CrossRef]
- Mironenko, A.Y.; Tutov, M.V.; Sergeev, A.A.; Mitsai, E.V.; Ustinov, A.Y.; Zhizhchenko, A.Y.; Linklater, D.P.; Bratskaya, S.Y.; Juodkazis, S.; Kuchmizhak, A.A. Ultratrace Nitroaromatic Vapor Detection via Surface-Enhanced Fluorescence on Carbazole-Terminated Black Silicon. ACS Sens. 2019, 4, 2879–2884. [Google Scholar] [CrossRef]
- Sergeeva, K.A.; Tutov, M.V.; Voznesenskiy, S.S.; Shamich, N.I.; Mironenko, A.Y.; Sergeev, A.A. Highly-sensitive fluorescent detection of chemical compounds via photonic nanojet excitation. Sens. Actuators B Chem. 2020, 305, 127354. [Google Scholar] [CrossRef]
- Tutov, M.V.; Sergeev, A.A.; Zadorozhny, P.A.; Bratskaya, S.Y.; Mironenko, A.Y. Dendrimeric rhodamine based fluorescent probe for selective detection of Au. Sens. Actuators B Chem. 2018, 273, 916–920. [Google Scholar] [CrossRef]
- Mironenko, A.Y.; Tutov, M.V.; Sergeev, A.A.; Bratskaya, S.Y. On/off rhodamine based fluorescent probe for detection of Au and Pd in aqueous solutions. Sens. Actuators B Chem. 2017, 246, 389–394. [Google Scholar] [CrossRef]
- Wang, L.; Ding, H.; Ran, X.; Tang, H.; Cao, D. Recent progress on reaction-based BODIPY probes for anion detection. Dye. Pigment. 2020, 172, 107857. [Google Scholar] [CrossRef]
- La, M.; Hao, Y.; Wang, Z.; Han, G.C.; Qu, L. Selective and Sensitive Detection of Cyanide Based on the Displacement Strategy Using a Water-Soluble Fluorescent Probe. J. Anal. Methods Chem. 2016, 2016, 1462013. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sedgwick, A.C.; Sun, X.; Bull, S.D.; He, X.-P.; James, T.D. Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species. Acc. Chem. Res. 2019, 52, 2582–2597. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Ha, J.; Cho, M.; Li, H.; Swamy, K.M.K.; Yoon, J. Recent developments of BODIPY-based colorimetric and fluorescent probes for the detection of reactive oxygen/nitrogen species and cancer diagnosis. Coord. Chem. Rev. 2021, 439, 213936. [Google Scholar] [CrossRef]
- Melnychuk, N.; Egloff, S.; Runser, A.; Reisch, A.; Klymchenko, A.S. Light-Harvesting Nanoparticle Probes for FRET-Based Detection of Oligonucleotides with Single-Molecule Sensitivity. Angew. Chemie Int. Ed. 2020, 59, 6811–6818. [Google Scholar] [CrossRef]
- Jurek, K.; Kabatc, J.; Kostrzewska, K.; Grabowska, M. New Fluorescence Probes for Biomolecules. Molecules 2015, 20, 13071–13079. [Google Scholar] [CrossRef]
- Kim, H.N.; Lee, M.H.; Kim, H.J.; Kim, J.S.; Yoon, J. A new trend in rhodamine-based chemosensors: Application of spirolactam ring-opening to sensing ions. Chem. Soc. Rev. 2008, 37, 1465. [Google Scholar] [CrossRef]
- Chen, X.; Pradhan, T.; Wang, F.; Kim, J.S.; Yoon, J. Fluorescent Chemosensors Based on Spiroring-Opening of Xanthenes and Related Derivatives. Chem. Rev. 2011, 112, 1910–1956. [Google Scholar] [CrossRef]
- Li, M.; Lu, H.-Y.; Liu, R.-L.; Chen, J.-D.; Chen, C.-F. Turn-On Fluorescent Sensor for Selective Detection of Zn 2+, Cd 2+, and Hg 2+ in Water. J. Org. Chem. 2012, 77, 3670–3673. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Marin, L.; Cheng, X. Water-soluble β-cyclodextrin based turn-on amplifying fluorescent probes for sensitive and selective detection of Hg2+/Hg+ ions. Sens. Actuators B Chem. 2023, 377, 133060. [Google Scholar] [CrossRef]
- Trofymchuk, K.; Reisch, A.; Didier, P.; Fras, F.; Gilliot, P.; Mely, Y.; Klymchenko, A.S. Giant light-harvesting nanoantenna for single-molecule detection in ambient light. Nat. Photonics 2017, 11, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Andreiuk, B.; Aparin, I.O.; Reisch, A.; Klymchenko, A.S. Bulky Barbiturates as Non-Toxic Ionic Dye Insulators for Enhanced Emission in Polymeric Nanoparticles. Chem. A Eur. J. 2021, 27, 12877–12883. [Google Scholar] [CrossRef]
- Shulov, I.; Oncul, S.; Reisch, A.; Arntz, Y.; Collot, M.; Mely, Y.; Klymchenko, A.S. Fluorinated counterion-enhanced emission of rhodamine aggregates: Ultrabright nanoparticles for bioimaging and light-harvesting. Nanoscale 2015, 7, 18198–18210. [Google Scholar] [CrossRef]
- Aparin, I.O.; Melnychuk, N.; Klymchenko, A.S. Ionic Aggregation-Induced Emission: Bulky Hydrophobic Counterions Light Up Dyes in Polymeric Nanoparticles. Adv. Opt. Mater. 2020, 8, 2000027. [Google Scholar] [CrossRef]
- Mironenko, A.Y.; Tutov, M.V.; Chepak, A.K.; Bratskaya, S.Y. FRET pumping of rhodamine-based probe in light-harvesting nanoparticles for highly sensitive detection of Cu2+. Anal. Chim. Acta 2022, 1229, 340388. [Google Scholar] [CrossRef]
- Mironenko, A.Y.; Tutov, M.V.; Chepak, A.K.; Zadorozhny, P.A.; Bratskaya, S.Y. A novel rhodamine-based turn-on probe for fluorescent detection of Au3+ and colorimetric detection of Cu2+. Tetrahedron 2019, 75, 1492–1496. [Google Scholar] [CrossRef]
- Water Quality Association. Mercury in Drinking Water. Available online: https://wqa.org/learn-about-water/common-contaminants/mercury (accessed on 30 October 2022).
- Adamoczky, A.; Nagy, L.; Nagy, M.; Zsuga, M.; Kéki, S. Conversion of isocyanide to amine in the presence of water and hg(Ii) ions: Kinetics and mechanism as detected by fluorescence spectroscopy and mass spectrometry. Int. J. Mol. Sci. 2020, 21, 5588. [Google Scholar] [CrossRef]
- Avramenko, V.; Bratskaya, S.; Zheleznov, V.; Sheveleva, I.; Voitenko, O.; Sergienko, V. Colloid stable sorbents for cesium removal: Preparation and application of latex particles functionalized with transition metals ferrocyanides. J. Hazard. Mater. 2011, 186, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Bratskaya, S.; Mironenko, A.; Koivula, R.; Synytska, A.; Musyanovych, A.; Simon, F.; Marinin, D.; Göbel, M.; Harjula, R.; Avramenko, V. Polymer-inorganic coatings containing nanosized sorbents selective to radionuclides. 2. Latex/tin oxide composites for cobalt fixation. ACS Appl. Mater. Interfaces 2014, 6, 22387–22392. [Google Scholar] [CrossRef] [PubMed]
- Bratskaya, S.; Musyanovych, A.; Zheleznov, V.; Synytska, A.; Marinin, D.; Simon, F.; Avramenko, V. Polymer-inorganic coatings containing nanosized sorbents selective to radionuclides. 1. Latex/Cobalt Hexacyanoferrate(II) composites for cesium fixation. ACS Appl. Mater. Interfaces 2014, 6, 16769–16776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Zhou, Y.; Yoon, J.; Kim, Y.; Kim, S.J.; Kim, J.S. Naphthalimide Modified Rhodamine Derivative: Ratiometric and Selective Fluorescent Sensor for Cu2+ Based on Two Different Approaches. Org. Lett. 2010, 12, 3852–3855. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chepak, A.; Balatskiy, D.; Tutov, M.; Mironenko, A.; Bratskaya, S. Light Harvesting Nanoprobe for Trace Detection of Hg2+ in Water. Molecules 2023, 28, 1633. https://doi.org/10.3390/molecules28041633
Chepak A, Balatskiy D, Tutov M, Mironenko A, Bratskaya S. Light Harvesting Nanoprobe for Trace Detection of Hg2+ in Water. Molecules. 2023; 28(4):1633. https://doi.org/10.3390/molecules28041633
Chicago/Turabian StyleChepak, Aleksandr, Denis Balatskiy, Mikhail Tutov, Aleksandr Mironenko, and Svetlana Bratskaya. 2023. "Light Harvesting Nanoprobe for Trace Detection of Hg2+ in Water" Molecules 28, no. 4: 1633. https://doi.org/10.3390/molecules28041633
APA StyleChepak, A., Balatskiy, D., Tutov, M., Mironenko, A., & Bratskaya, S. (2023). Light Harvesting Nanoprobe for Trace Detection of Hg2+ in Water. Molecules, 28(4), 1633. https://doi.org/10.3390/molecules28041633





