Emerging Biopharmaceuticals from Pimpinella Genus
Abstract
1. Introduction
2. Folk-Medicine Application
3. Phytochemistry
3.1. Phenylpropanoids
3.2. Terpenoids
3.3. Flavonoids and Their Glycosides
3.4. Coumarins
3.5. Sterols
3.6. Organic Acids
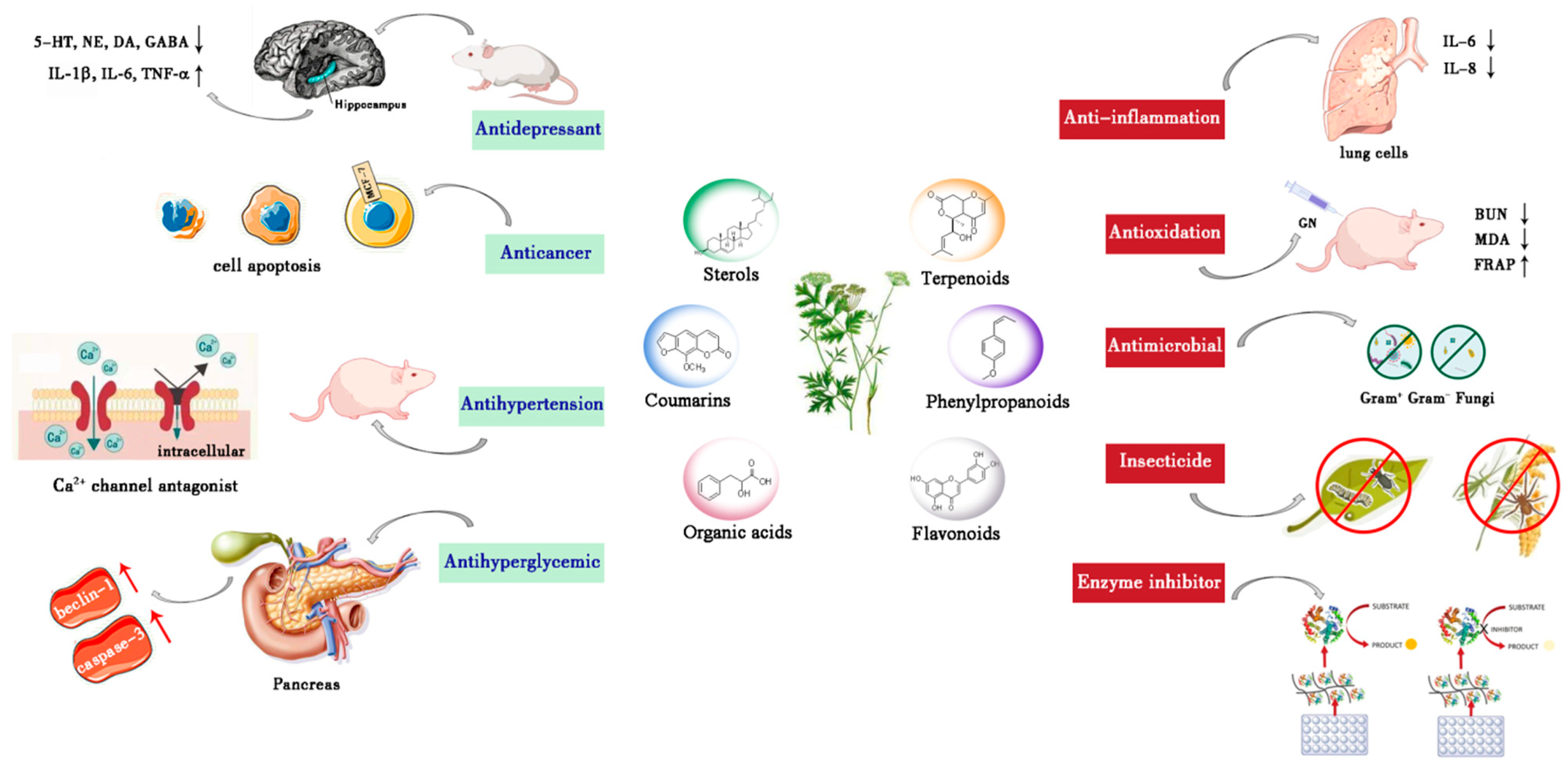
4. Pharmacology
4.1. Antioxidant Activity
4.2. Antibacterial Activity
4.3. Anti-Inflammatory Activity
4.4. Anti-Tumor Activity
4.5. Hypoglycemic Activity
4.6. Hypotensive Activity
4.7. Insecticidal Activity
4.8. Enzymes Inhibitory Activity
4.9. Antidepressant Activity
4.10. Other Activities
5. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APAP | acetaminophen |
| NAPQI | N-acetyl-p-benzoquinonimine |
| GSH | glutathione |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| MDA | malondialdehyde |
| ALP | alkaline phosphatase |
| IL-1 | interleukin-1 |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| IL-10 | interleukin-10 |
| TNF-α | tumor necrosis-α |
| PGE2 | prostaglandin E-2 |
| LPS | lipopolysaccharide |
| NALF | nasal lavage fluid |
| Th1 | T-helper type 1 cell |
| Th2 | T-helper type 2 cell |
| Treg | regulatory T cell |
| SOD | superoxide dismutase |
| STZ | streptozotocin |
| AChE | acetylcholinesterase |
| BChE | butyrylcholinesterase |
| CYP | cytochrome P |
| GSTs | glutathione transferases |
| RALDHs | retinaldehyde dehydrogenases |
| 5-HT | 5-hydroxytryptamine |
| DA | dopamine |
| NE | norepinephrine |
| GSH-Px | glutathione peroxidase |
| CAT | catalase |
| NO | nitric oxide |
References
- National Mittee. Pharmacopoeia of People’s Republic of China; The Medicine Science and Technology Press of China: Beijing, China, 2020. [Google Scholar]
- Nasır, A.; Yabalak, E. Investigation of antioxidant, antibacterial, antiviral, chemical composition, and traditionalmedicinal properties of the extracts and essentialoils of the Pimpinella species from a broad perspective: A review. J. Essent. Oil Res. 2021, 33, 411–426. [Google Scholar] [CrossRef]
- Cinbilgel, I.; Eren, O.; Duman, H.; Gokceoglu, M. Pimpinella ibradiensis (Apiaceae), an unusual new species from Turkey. Phytotaxa 2015, 217, 164–172. [Google Scholar] [CrossRef]
- Ertekin, A.; Kaya, O. A new record species for the flora of Turkey, Pimpinella nephrophylla Rech. f. & H. Riedl. (Apiaceae). Ot Sist. Bot. Derg. 2005, 12, 13–18. [Google Scholar]
- Massalin, H.; Pu, C. A lock-free multiprocessor OS Kernel (Abstract). ACM SIGOPS Oper. Syst. Rev. 1992, 26, 8. [Google Scholar] [CrossRef]
- Elmassry, M.M.; Kormod, L.; Labib, R.M.; Mohamed, A. Metabolome based volatiles mapping of roasted umbelliferous fruits aroma via HS-SPME GC/MS and peroxide levels analyses. J. Chromatogr. B 2018, 1099, 117–126. [Google Scholar] [CrossRef]
- Koriem, K.M.M.; Arbid, M.S.; El-Gendy, N.F. The protective role of anise oil in oxidative stress and genotoxicity produced in favism. J. Diet Suppl. 2016, 13, 505–521. [Google Scholar] [CrossRef]
- SAl-wendawi, A.; Gharb, L.A.; Al-ghrery, R.S. Antioxidant, antibacterial and antibiofilm potentials of anise (Pimpinella anisum) seeds extracted essential oils. Iraqi J. Agric. Sci. 2021, 52, 348–358. [Google Scholar] [CrossRef]
- Ghlissi, Z.; Kallel, R.; Krichen, F.; Hakim, A.; Zeghal, K.; Boudawara, T.; Bougatef, A.; Sahnoun, Z. Polysaccharide from Pimpinella anisum seeds: Structural characterization, anti-inflammatory and laser burn wound healing in mice. Int. J. Biol. Macromol. 2020, 156, 1530–1538. [Google Scholar] [CrossRef]
- Mahmoud, A.; Mustafa, G.; Ebrahim, S.S.; Roshana, S.; Mohmmad K., S. Pimpinelol, a novel atypical Sesquiterpene lactone from Pimpinella haussknechtii fruits with evaluation of endoplasmic reticulum stress in breast cancer cells. Fitoterapia 2018, 129, 198–202. [Google Scholar]
- Bonesi, M.; Saab, A.M.; Tenuta, M.C.; Leporini, M.; Saab, M.J.; Loizzo, M.R.; Tundis, R. Screening of traditional Lebanese medicinal plants as antioxidantsand inhibitors of key enzymes linked to type 2 diabetes. Plant Biosyst. 2020, 154, 656–662. [Google Scholar] [CrossRef]
- Lu, J.; Qian, W.H.; Guan, S.; Deng, X.M.; Song, X.F.; Liu, X.Y.; Wang, D.C. Extraction and isolation of antioxidant components from Pimpinella brachycarpa. Occup. Health 2011, 27, 967–969. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shin, Y.J.; Kang, R.L. Two new sesquiterpenes from the aerial parts of Pimpinella brachycarpa NAKAI. B Korean Chem. Soc. 2013, 34, 2215–2217. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, H.N.; Kang, M.J.; Choe, E.; Auh, J.H.; Kim, J.I. Chamnamul [Pimpinella brachycarpa (Kom.) Nakai] ameliorates hyperglycemia and improves antioxidant status in mice fed a high-fat, high-sucrose diet. Nutr. Res. Pract. 2013, 7, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K. Chemical composition of the essential oil of the flowering aerial parts of Pimpinella monoica. Nat. Prod. Commun. 2013, 8, 1643–1644. [Google Scholar] [CrossRef]
- Ozbek, H.; Guvenalp, Z.; K-Uz, A.; Kazaz, C.; Demirezer, L.O. Trinorguaian and germacradiene type sesquiterpenes along with flavonoids from the herbs of Pimpinella cappadocica Boiss. & Bal. Phytochem. Lett. 2015, 11, 74–79. [Google Scholar]
- Ozbek, H.; Guvenalp, Z.; K-Uz, A.; Kazaz, C.; Demirezer, L.O. β-hydroxydihydrochalcone and flavonoid glycosides along with triterpene saponin and sesquiterpene from the herbs of Pimpinella rhodantha boiss. Nat. Prod. Res. 2016, 30, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Hritcu, L.; Dogan, G.; Hayta, S.; Bagci, E. The effects of inhaled Pimpinella peregrina essential oil on scopolamine-induced memory impairment, anxiety, and depression in laboratory rats. Mol. Neurobiol. 2016, 53, 6557–6567. [Google Scholar] [CrossRef]
- Askari, F.; Sefidkon, F.; Teimouri, M. Chemical composition and antimicrobial activity of Pimpinella khorasanica L. engstr and oil in Iran. J. Essent. Oil Bear. Plants 2013, 16, 265–269. [Google Scholar] [CrossRef]
- Saab, A.M.; Tacchini, M.; Sacchetti, G.; Contini, C.; Schulz, H.; Lampronti, I.; Gambari, R.; Makhlouf, H.; Tannoury, M.; Venditti, A.; et al. Phytochemical analysis and potential naturalcompounds against SARS-CoV-2/COVID-19 inessential oils derived from medicinal plantsoriginating from Lebanon. An information note. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2022, 156, 855–864. [Google Scholar] [CrossRef]
- Abouzid, S.F.; Mohamed, A.A. Survey on medicinal plants and spices used inBeni-Sueif, Upper Egypt. J. Ethnobiol. Ethnomed. 2011, 7, 18. [Google Scholar] [CrossRef]
- Kreydiyyeh, S.I.; Usta, J.; Knio, K.; Markossian, S.; Dagher, S. Aniseed oil increases glucose absorption and reduces urine output in the rat. Life Sci. 2003, 74, 663–673. [Google Scholar] [CrossRef]
- Yoney, A.; Prieto, J.M.; Lardos, A.; Heinrich, M. Ethnopharmacy of Turkish-speaking cypriots in Greater London. Phytother Res. 2010, 24, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Picon, P.D.; Picon, R.V.; Costa, A.F.; Sander, G.B.; Amaral, K.M.; Aboy, A.L.; Henriques, A.T. Randomized clinical trial of a phytotherapic compound containing Pimpinella anisum, Foeniculum vulgare, Sambucus nigra, and Cassia augustifolia for chronic constipation. BMC Complement. Altern. Med. 2010, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Anli, R.E.; Bayram, M. Traditional aniseed-flavored spirit drinks. Food Rev. Int. 2010, 26, 246–269. [Google Scholar] [CrossRef]
- Hammer, K.; Laghetti, G.; Cifarelli, S.; Spahillari, M.; Perrino, P. Pimpinella anisoides Briganti. Genet. Resour. Crop Evol. 2000, 47, 223–225. [Google Scholar] [CrossRef]
- Kubeczka, K.H.; Massow, F.V.; Formacek, V.; Smith, M.A.R. A new type of phenylpropane from the essential fruit oil of Pimpinella anisum L. Z. Für Nat. B 1976, 31, 283–284. [Google Scholar] [CrossRef]
- Reichling, J.; Kemmerer, B.; S-Gurth, H. Biosynthesis of pseudoisoeugenols in tissue cultures of Pimpinella anisum. Pharm. World Sci. 1995, 17, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.T.; Schnoes, H.K.; Lichtenstein, E.P. 4-Methoxy-2-(trans-1-propenyl) phenyl (±)-2-methylbutanoate from anise plants. Phytochemistry 1977, 16, 615–616. [Google Scholar] [CrossRef]
- Qiao, B.L.; Wang, C.D.; Mi, C.F.; Li, F.X.; Shi, H.L.; Gaodao, C.Z. Isolation and identification of llungianin A and llungianin B from Pimpinella thellungiana Wolff root. Acta Pharm. Sin. 1997, 32, 56–58. [Google Scholar]
- Qiao, B.L.; Wang, C.D.; Mi, C.F. Study on chemical constituents of Pimpinella thellungiana Wolff root. Chin Bull Bot. 1998, 40, 88–90. [Google Scholar]
- Shi, H.L.; Li, F.X.; Mi, C.F.; Qiao, B.L.; Wang, C.D. Study on chemical constituents of Pimpinella thellungiana Wolff root. China J. Chin. Mater. Med. 1998, 23, 421–422. [Google Scholar]
- Qiao, B.L.; Wang, C.D.; Li, F.X.; Mi, C.F.; Shi, H.L. Study on the chemical constituents of Pimpinella thellungiana Wolff rootIII: Isolation and identification of llungianin F. Chin. Trad. Herb. Drug 1998, 29, 3–4. [Google Scholar]
- Qiao, B.L.; Wang, C.D.; Li, F.X.; Mi, C.F.; Shi, H.L. Separation and identification of thellungianin G from the root of Pimpinella thellungiana Wolff. China J. Chin. Mater Med. 1999, 24, 551–552. [Google Scholar]
- Lu, L.; Zhai, X.; Li, X.; Wang, S.; Zhang, L.; Wang, L.; Jin, X.; Liang, L.; Deng, Z.; Li, Z.; et al. Met1-Specific Motifs Conserved in OTUB Subfamily of Green Plants Enable Rice OTUB1 to Hydrolyse Met1 Ubiquitin Chains. Nat. Commun. 2022, 13, 4672. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Kirimer, N.; Baser, K.H.C.; Bedir, E.; Khan, I.A.; Wedge, D.E. Gas chromatographic-mass spectrometric analysis of essential oils from Pimpinella aurea, Pimpinella corymbosa, Pimpinella peregrina and Pimpinella puberula gathered from Eastern and Southern Turkey. J. Chromatogr. A 2005, 1097, 192–198. [Google Scholar] [CrossRef]
- Sun, S.-J.; Deng, P.; Peng, C.-E.; Ji, H.-Y.; Mao, L.-F.; Peng, L.-Z. Extraction, Structure and Immunoregulatory Activity of Low Molecular Weight Polysaccharide from Dendrobium Officinale. Polymers 2022, 14, 2899. [Google Scholar] [CrossRef]
- Stahl, E.; Herting, D. Die verteilung von inhaltsstoffen in drei Pimpinella-arten. Phytochemistry 1976, 15, 999–1001. [Google Scholar] [CrossRef]
- Dev, V.; Mathela, C.S.; Melkani, A.B.; Pope, N.M.; Sturm, N.S.; Bottini, A.T. Diesters of 2-(E-3-methyloxiranyl)-hydroquinone from Pimpinella diversifolia. Phytochemistry 1989, 28, 1531–1532. [Google Scholar] [CrossRef]
- Macías, M.J.; Martín, V.; Grande, M.; Kubeczka, K.H. Phenylpropanoids from Pimpinella villosa. Phytochemistry 1994, 37, 539–542. [Google Scholar] [CrossRef]
- Wang, C.D.; Ding, K.; Wu, Y.H.; Guo, W.B.; Chen, J.; Yuan, Z.Z. Study on chemical constituents of Pimpinella thellungiana Wolff root. Acta Pharm. Sin. 1983, 18, 522–524. [Google Scholar]
- Qiao, B.L.; Wang, C.D.; Li, F.X.; Shi, H.L.; Mi, C.F. Isolation and identification of llungianin H from Pimpinella thellungiana Wolff root. Chin. Trad. Herb. Drug 2000, 31, 161–162. [Google Scholar]
- Shi, H.L.; Mi, C.F.; Qiao, B.L.; Li, F.X.; Wang, C.D.; Liu, Z. Study on chemical constituents of Pimpinella thellungiana Wolff root. Acta Pharm. Sin. 1998, 21, 236–237. [Google Scholar]
- V-Negueruela, A.; P-Alonso, M.J.; Perez, P.L.; Palá-Paúl, J.; Sanz, J. Analysis by gas chromatography-mass spectrometry of the essentialoil from the aerial parts of Pimpinella junoniae Ceb. & Ort.,gathered in La Gomera, Canary Islands, Spain. J. Chromatogr. A 2003, 1011, 241–244. [Google Scholar]
- Delazar, A.; Biglari, F.; Esnaashari, S.; Nazemiyeh, H.; Talebpour, A.H.; Nahar, L.; Sarker, S.D. GC-MS analysis of the essential oils, and the isolation of phenylpropanoid derivatives from the aerial parts of Pimpinella aurea. Phytochemistry 2006, 67, 2176–2181. [Google Scholar] [CrossRef]
- Ksouda, G.; Sellimi, S.; Merlier, F.; Falcimaigne-cordin, A.; Thomasset, B.; Nasri, M.; Hajji, M. Composition, antibacterial and antioxidant activities of Pimpinella saxifraga essential oil and application to cheese preservation as coating additive. Food Chem. 2019, 28, 47–56. [Google Scholar] [CrossRef]
- Vuckovic, I.; Stikovic, S.; Stesevi, D.; Jadranin, M.; Trifunovic, S. Phytochemical investigation of Pimpinella serbica. J. Serb. Chem. Soc. 2021, 86, 1241–1247. [Google Scholar] [CrossRef]
- Tepe, B.; Akpulat, H.A.; Sokmen, M.; Daferera, D.; Yumrutas, O.; Aydin, E.; Polissiou, M.; Sokmen, A. Screening of the antioxidative and antimicrobial properties of the essential oils of Pimpinella anisetum and Pimpinella flabellifolia from Turkey. Food Chem. 2006, 97, 719–724. [Google Scholar] [CrossRef]
- Chang, X.; Kang, W.Y. Antioxidant and α-glucosidase inhibitory compoundsfrom Pimpinella candolleana Wight et Arn. Med. Chem Res. 2012, 21, 4324–4329. [Google Scholar] [CrossRef]
- Rebey, I.B.; Bourgou, S.; Wannes, W.A.; Selami, I.H.; Tounsi, M.S.; Marzouk, B.; Fauconnier, M.L.; Ksouri, R. Comparative assessment of phytochemical profiles and antioxidant properties of Tunisian and Egyptian anise (Pimpinella anisum L.) seeds. Plant Biosyst. -Int. J. Deal. All Asp. Plant Biol. 2017, 152, 971–978. [Google Scholar]
- Karik, U.; Demirbolat, I. Chemical composition, antioxidant and antimicrobial activities of Pimpinellaenguezekensis: Anovel species from Anatolia, Turkey-fruit essential oil. J. Essent. Oil Bear. Plants 2020, 23, 356–362. [Google Scholar] [CrossRef]
- Adel, M.; Dadar, M.; Zorriehzahra, M.J.; Elahi, R.; Stadtlander, T. Antifungal activity and chemical composition of Iranian medicinal herbs against fish pathogenic fungus, Saprolegnia parasitica. Iran J. Fish Sci. 2020, 19, 3239–3254. [Google Scholar]
- Xu, X.W.; Lin, G.X.; Lin, C.L. Study on chemical components of essentialoil from Zhejiang Pimpinella diversifolia. China Phar. 2012, 21, 3–4. [Google Scholar]
- Suleimen, E.M.; Ibataev, Z.A.; Iskakova, Z.B.; Dudkin, R.V.; Gorovoi, P.G.; Aistova, E.V. Constituent composition and biological activity of essential oil from Pimpinella thellungiana. Chem. Nat. Compd. 2017, 53, 169–172. [Google Scholar] [CrossRef]
- Balbino, S.; Repajic, M.; Obranovic, M.; Medved, A.M.; Tonkovi, P.; Dragovi-Uzelac, V. Characterization of lipid fraction of Apiaceae family seed spices: Impact of species and extraction method. J. Appl. Res. Med. Aromat Plants 2021, 25, 100326. [Google Scholar] [CrossRef]
- Farzaneh, V.; Gominho, J.; Pereira, H.; Carvalho, I.S. Screening of the antioxidant and enzyme inhibition potentials of portuguese Pimpinella anisum L. seeds by GC-MS. Food Anal. Method 2018, 11, 2645–2656. [Google Scholar] [CrossRef]
- Tavallali, V.; Rahmati, S.; Bahmanzadegan, A. Antioxidant activity, polyphenolic contentsand essential oil composition of Pimpinella anisum L. as affected by zinc fertilizer. J. Sci. Food Agr. 2017, 97, 4883–4889. [Google Scholar] [CrossRef] [PubMed]
- Fitsiou, E.; Mitropoulou, G.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Bardouki, H.; Panayiotidis, M.; Galanis, A.; Kourkoutas, Y.; Chlichlia, K.; et al. Phytochemical profile and evaluation of the biological activities of essential oils derived fromthe Greek aromatic plant species Ocimum basilicum, Mentha spicata, Pimpinella anisum and Fortunella margarita. Molecules 2016, 21, 1069. [Google Scholar] [CrossRef]
- Matusinsky, P.; Zouhar, M.; Pavela, R.; Novy, P. Antifungal effect of five essential oils against important pathogenic fungi of cereals. Ind. Crop. Prod. 2015, 67, 208–215. [Google Scholar] [CrossRef]
- Xue, K.F.; Wang, J.Z. Isolation and identification of new flavonoidglycosides in Pimpinella thellungiana Wolff. Chin. Trad. Herb. Drug 1992, 23, 451–452. [Google Scholar]
- Wang, C.D.; Ding, K.; Guo, W.B.; Wu, Y.H. Study on chemical constituents of Pimpinella thellungiana Wolff. Chin. Trad. Herb. Drug 1980, 11, 344. [Google Scholar]
- Wang, C.D.; Guo, W.B.; Ding, K.; Wu, Y.H. Study on chemical constituents of Pimpinella thellungiana Wolff (II). J. Shanxi Med. 1981, 10, 61–62. [Google Scholar]
- Cui, X.M.; Ren, H.; Hu, J.; Chen, J.; Meng, X.; Mao, Z.Y.; Chen, Z.Y. Study on HPLC fingerprint and determination of 10 components of Pimpinella thellungiana Wolff. Lishizhen Med. Mater. Med. Res. 2020, 31, 2313–2316. [Google Scholar]
- Liu, R.; Tai, G.; Pei, X.L.; Wang, R.; Zhang, S.R.; Pei, M.R. Determination of nine components in Yanghongshan by HPLC. Chin. J. Pharm. Anal. 2020, 40, 1097–1103. [Google Scholar]
- Zengin, G.; Sinan, K.I.; Ak, G.; Mahomoodally, F.M.; Custódio, L. Chemical profile, antioxidant, antimicrobial, enzyme inhibitory, and cytotoxicity of seven Apiaceae species from Turkey: A comparative study. Ind. Crop. Prod. 2020, 153, 112572. [Google Scholar] [CrossRef]
- Topcagic, A.; Zeljkovic, S.A.; Kezic, M.; Sofi, E. Fatty acids and phenolic compounds composition of anise seed. J. Food Process Pres. 2021, 46, e15872. [Google Scholar] [CrossRef]
- Qiao, B.L.; Wang, C.D.; Shi, H.L.; Mi, C.F.; Li, F.X. Study on chemical constituents of Pimpinella thellungiana Wolff root (I). Chin. Trad. Herb. Drug 1996, 27, 136–138. [Google Scholar]
- Pradhan, P.; Luthria, D.L.; Banerji, A. Pimolin anew class of natural product from Pimpinella monoica: A novel dimericfurochromone. Bioorgan. Med. Chem. Lett. 1994, 20, 2425–2428. [Google Scholar] [CrossRef]
- Pradhan, P.; Banerji, A. Novel cyclobutane fused furochromoneoligomers from the seeds of Pimpinella monoica Dalz. Tetrahedron 1998, 54, 14541–14548. [Google Scholar] [CrossRef]
- Alehaideb, Z.; M-Nasri, S. Determination of benchmark doses for linear furanocoumarin consumption associated with inhibition of cytochrome P450 1A2 isoenzyme activity in healthy human adults. Toxicol Rep. 2021, 8, 1437–1444. [Google Scholar] [CrossRef]
- Taddeo, V.A.; Epifano, F.; Preziuso, F.; Fiorito, S.; Caron, N.; Rives, A.; Medina, P.; Poirot, M.; Silvente-Poirot, S.; Genovese, S. HPLC analysis and skin whitening effects of umbellipren in-containing extracts of Anethum graveolens, Pimpinella anisum, and Ferulago campestris. Molecules 2019, 24, 501. [Google Scholar] [CrossRef]
- Taddeo, V.A.; Genovese, S.; Medina, P.; Palmisano, R.; Epifano, F.; Fiorito, S. Quantification of biologically active O-prenylated and unprenylate phenylpropanoids in dill (Anethum graveolens), anise (Pimpinella anisum), and wild celery (Angelica archangelica). J. Pharm. Biomed. 2017, 134, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; W-Hajnos, M.; Podgorski, R. Comparison of matrix solid-phase dispersion and liquid-solid extraction methods followed by solid-phase extraction in the analysis of selected furanocoumarins from Pimpinella roots by HPLC-DAD. Acta Chromatogr. 2015, 27, 687–696. [Google Scholar] [CrossRef]
- Saini, R.K.; Song, M.H.; Yu, J.W.; Shang, X.; Keum, Y. Phytosterol profiling of Apiaceae family seeds spices using GC-MS. Foods 2021, 10, 2378. [Google Scholar] [CrossRef]
- Liang, G.Y.; Wang, D.P.; Xu, B.X. Study on chemical constituents of folk medicine P. candolleana. Guizhou Sci. 2003, 21, 58–60. [Google Scholar]
- Jing, L.; Qian, W.; Xu, L.; Hung, G.; Cong, W.; Wang, Z.; Deng, X.; Wang, D.; Guan, S. Phytochemical composition and toxicity of an antioxidant extract from Pimpinella brachycarpa (Kom.) Nakai. Environ. Toxicol. Pharmacol. 2012, 34, 409–415. [Google Scholar]
- Rebey, I.B.; Bourgou, S.; Detry, P.; Wannes, W.A.; Kenny, T.; Ksouri, R.; Sellami, H.I.; Fauconnier, M. Green extraction of fennel and anise edible oils using bio-based solvent and supercritical fluid: Assessment of chemical composition, antioxidant property, and oxidative stability. Food Bioprocess Tech. 2019, 12, 1798–1807. [Google Scholar] [CrossRef]
- Kozlowska, M.; Gruczynska, E.; Scibisz, I.; Rudzińska, M. Fatty acids and sterols composition, and antioxidant activity of oilsextracted from plant seeds. Food Chem. 2016, 213, 450–456. [Google Scholar] [CrossRef]
- Xue, K.F.; Ma, B.; Wang, J.Z. Separation and identification of thellungianol from Pimpinella thellungiana. Chin. Trad. Herb. Drug 1998, 29, 1–2. [Google Scholar]
- Cui, X.M.; Shi, H.L.; Ren, H. Content determination of nine constituents in different medicinal parts of Pimpinella thellungiana. Chin. J. Exp. Trad. Med. Form 2019, 25, 97–103. [Google Scholar]
- Liu, R.; Wang, R.; Pei, K.; Zhang, S.R.; Pei, M.R. Study on serum pharmacochemistry of Pimpinella thellungiana whole herb with roots by UHPLC-Q-Orbitrap HRMS. Chin. Trad. Herb. Drug 2020, 26, 145–151. [Google Scholar]
- Lee, S.Y.; Moon, E.; Kim, S.Y.; Lee, K.R. Quinic acid derivatives from Pimpinella brachycarpa exert anti-neuroinflammatory activity in lipopolysaccharide-induced microglia. Bioorg. Med. Chem. Lett. 2013, 23, 2140–2144. [Google Scholar] [CrossRef]
- Tepe, A.S.; Tepe, B. Traditional use, biological activity potential and toxicityof Pimpinella species. Ind. Crop. Prod. 2015, 69, 153–166. [Google Scholar] [CrossRef]
- Ashtiyani, S.C.; Seddigh, A.; Najafi, H.; Hossaini, N.; Avan, A.; Akabray, A.; Manian, M.; Nedaeinia, R. Pimpinella anisum L.ethanolic extract ameliorates the gentamicin-induced nephrotoxicity in rats. Nephrology 2017, 22, 133–138. [Google Scholar] [CrossRef]
- Ghosh, A.; Saleh-e-ln, M.M.; Abukawsar, M.M.; Ahsan, M.A.; Rahim, M.M.; Bhuiyan, N.H.; Roy, S.K.; Naher, S. Characterization of quality and pharmacological assessment of Pimpinella anisum L. (Anise) seeds cultivars. J. Food Meas. Charact. 2019, 13, 2672–2685. [Google Scholar] [CrossRef]
- E-Sayed, S.M.; Ahmed, N.; Selim, S.; Ai-Khalaf, A.A.; Nahhas, N.E.; Abdel-Hafez, S.H.; Sayed, S.; Emam, H.M.; Ibrahim, M.A.R. Acaricidal and antioxidant activities of anise oil (Pimpinella anisum) and the oil’s effect on protease and acetylcholinesterase in the two-spotted spider mite (Tetranychus urticae Koch). Agriculture 2022, 12, 224. [Google Scholar] [CrossRef]
- OAli, A.A.; El-Naggar, M.E.; Abdel-Aziz, M.S.; Saleh, D.I.; Abu-Saied, M.A.; EI-Sayed, W.A. Facile synthesis of natural anise-based nanoemulsions and their antimicrobial activity. Polymers 2021, 13, 2009. [Google Scholar]
- Wahyuningrum, R.; Utami, P.I.; Dhiani, B.A. Screening of potential free radicals scavenger and antibacterial activities of purwoceng (Pimpinella alpina Molk). Trop Life Sci. Res. 2016, 27, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, H.; Sarrafi, Y.; Salehi, P. Antioxidant and antidiabetic activities of 11 herbalplants from Hyrcania region, Iran. J. Food Drug Anal. 2016, 24, 179–188. [Google Scholar] [CrossRef]
- IAhmed, A.M.; Matthaus, B.; Ozcan, M.M.; Juhaimi, F.A.; Ghafoor, K.; Babiker, E.E.; Osman, M.A.; Alqah, H.A.S. Determination of bioactive lipid and antioxidant activity of Onobrychis, Pimpinella, Trifolium, and Phleum spp. seed and oils. J. Oleo Sci. 2020, 69, 1367–1371. [Google Scholar] [CrossRef]
- Yang, R.; Hou, E.; Cheng, W.; Yan, X.; Zhang, T.; Li, S.; Yao, H.; Liu, J.; Guo, Y. Membrane-Targeting Neolignan-Antimicrobial Peptide Mimic Conjugates to Combat Methicillin-Resistant Staphylococcus aureus (MRSA) Infections. J. Med. Chem. 2022, 65, 16879–16892. [Google Scholar] [CrossRef]
- Condò, C.; Anacarso, I.; Sabia, C.; Iseppi, R.; Anfelli, I.; Forti, L.; Niederhäusern, S.; Bondi, M.; Messi, P. Antimicrobial activity of spices essential oils andits effectiveness on mature biofilms of humanpathogens. Nat. Prod. Res. 2020, 34, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Tarighi, S.; Taheri, P. Effects of plant essential oils on growth and virulence factors of Erwinia amylovora. J. Plant Pathol. 2020, 102, 409–419. [Google Scholar] [CrossRef]
- Gafitanu, C.A.; Filip, D.; Cernatescu, C. Formulation and evaluation of anise-based bioadhesive vaginal gels. Biomed. Pharmacother. 2016, 83, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.R.; Shokrib, H.; Saffarian, Z. Anti-fungal activity of some native essential oils against emerging multidrug resistant human nondermatophytic moulds. J. Herb. Med. 2020, 23, 100370. [Google Scholar] [CrossRef]
- Trifan, A.; Luca, S.V.; Bostanaru, A.; Brebu, M.; Jităreanu, A.; Cristina, R.; Skalicka-Woźniak, K.; Granica, S.; Czerwińska, M.E.; Kruk, A.; et al. Apiaceae essential oils: Boosters of terbinafine activity against dermatophytes and potent anti-inflammatory effectors. Plants 2021, 10, 2378. [Google Scholar] [CrossRef] [PubMed]
- Obaid, A.J.; Al-Janabi, J.K.A.; Taj-Aldin, W.R. Bioactivities of anethole, astragalin and cryptochlorogenic acid extracted from anise oil and moringa oleifera on the keratinase gene expression of Trichophyton rubrum. J. Pure Appl. Microbiol. 2020, 14, 615–626. [Google Scholar] [CrossRef]
- Ferdes, M.; Juhaimi, F.A.; Ozcan, M.M.; Ghafoor, K. Inhibitory effect of some plant essential oils on growth of Aspergillusniger, Aspergillus oryzae, Mucor pusillus and Fusarium oxysporum. S. Afr. J. Bot. 2017, 113, 457–460. [Google Scholar] [CrossRef]
- Radaelli, M.; Silva, B.P.; Weidlich, L.; Hoehne, L.; Flach, A.; Mendonça, A.C.; Ethur, E.M. Antimicrobial activities of six essential oils commonly used as condiments in Brazil against Clostridium perfringens. Braz. J. Microbiol. 2016, 47, 424–430. [Google Scholar] [CrossRef]
- Hu, F.; Tu, X.F.; Thakur, K.; Hu, F.; Li, X.; Zhang, Y.; Zhang, J.; Wei, Z. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef]
- Khoury, R.E.; Atoui, A.; Verheecke, C.; Maroun, R.; Khoury, A.E.; Mathieu, F. Essential oils modulate gene expression and ochratoxin aproduction in Aspergillus carbonarius. Toxins 2016, 8, 242. [Google Scholar] [CrossRef]
- Noori, N.; Khanjari, A.; Rezaeigolestani, M.; Karabagias, I.K.; Mokhtari, S. Development of antibacterial biocomposites based on poly(lactic acid) with spice essential oil (Pimpinella anisum) for Food Applications. Polymers 2021, 13, 3791. [Google Scholar] [CrossRef]
- Ghazya, O.; Fouad, M.; Saleh, H.; Kholif, A.E.; Morsy, T.A. Ultrasound-assisted preparation of anise extract nanoemulsion and itsbioactivity against different pathogenic bacteria. Food Chem. 2021, 341, 128259. [Google Scholar] [CrossRef]
- Topuz, O.K.; Ozvural, E.B.; Zhao, Q.; Huang, Q.; Chikindas, M.; Gölükçü, M. Physical and antimicrobial properties of anise oil loaded nanoemulsionson the survival of foodborne pathogens. Food Chem. 2016, 203, 117–123. [Google Scholar] [CrossRef]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Deepika; Dubey, N.K. Nanostructured Pimpinella anisum essential oil as novel green food preservative against fungal infestation, aflatoxin B1 contamination and deterioration of nutritional qualities. Food Chem. 2021, 344, 128574. [Google Scholar] [CrossRef] [PubMed]
- Domiciano, T.P.; Dalalio, M.M.O.; Silva, E.L.; Ritter, A.M.V.; Estevão-Silva, C.F.; Ramos, F.S.; Caparroz-Assef, S.M.; Cuman, R.K.N.; Bersani-Amado, C.A. Inhibitory effect of anethole in nonimmune acute inflammation. N-S Arch Pharmacol. 2013, 386, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Iannarellia, R.; Marinellia, O.; Morelli, M.B.; Santoni, G.; Amantini, C.; Nabissi, M.; Maggi, F. Aniseed (Pimpinella anisum L.) essential oil reduces pro-inflammatory cytokines and stimulates mucus secretion in primary airway bronchial andtracheal epithelial cell lines. Ind. Crop. Prod. 2018, 144, 81–86. [Google Scholar] [CrossRef]
- Kfoury, M.; Borgie, M.; Verdin, A.; Ledoux, F.; Courcot, D.; Auezova, L.; Fourmentin, S. Essential oil components decrease pulmonary and hepatic cellsinflammation induced by air pollution particulate matter. Environ. Chem. Lett. 2016, 14, 345–351. [Google Scholar] [CrossRef]
- Liao, C.S.; Han, Y.Y.; Chen, Z.J.; Baigude, H. The extract of black cumin, licorice, anise, and black tea alleviates OVA-induced allergicrhinitis in mouse via balancing activity of helper T cells in lung. Allergy Asthma Cl. Im. 2021, 17, 1. [Google Scholar]
- A-Reheem, M.A.T.; Oraby, M.M. Anti-microbial, cytotoxicity, and necrotic ripostesof Pimpinella anisum essential oil. Ann. Arg. Sci. 2015, 60, 335–340. [Google Scholar]
- Zhang, C.; Li, J.; Xiao, M.; Wang, D.; Qu, Y.; Zou, L.; Zheng, C.; Zhang, J. Oral colon-targeted mucoadhesive micelles with enzyme-responsive controlled release of curcumin for ulcerative colitis therapy. Chin. Chem. Lett. 2022, 33, 4924–4929. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Lirussi, F.; Garrido, C.; Ye, X.-Y.; Xie, T. Dual inhibitors of histone deacetylases and other cancer-related targets: A pharmacological perspective. Biochem. Pharmacol. 2020, 182, 114224. [Google Scholar] [CrossRef]
- Faried, M.A.; El-Mehi, A.E.S. Aqueous anise extract alleviated the pancreatic changes in streptozotocin-induced diabetic rat model via modulation of hyperglycaemia, oxidative stress, apoptosis and autophagy: A biochemical, histological and immunohistochemical study. Folia Morphol. 2020, 78, 489–502. [Google Scholar] [CrossRef]
- Hashemnia, M.; Nikousefat, Z.; Mohammadalipour, A.; Zangeneh, M.; Zangeneh, A. Wound healing activity of Pimpinella anisum methanolic extract in streptozotocin-induced diabetic rats. J. Wound Care 2019, 28, 26–36. [Google Scholar] [CrossRef]
- Ren, L.P.; Zhang, X.D.; Lei, J.T. Anti-hypertensive effect of different extracts of Pimpinella brachycarpa. Acta Nutr. Sin. 2017, 39, 607–609. [Google Scholar]
- Pontes, V.C.B.; Rodrigues, D.P.; Caetano, A.; Gamberini, M.T. Preclinical investigation of the cardiovascular actions induced by aqueousextract of Pimpinella anisum L. seeds in rats. J. Ethnopharmacol. 2019, 237, 74–80. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Canale, A.; Senthil-Nathan, S.; Maggi, F. Not just popular spices! Essential oils from Cuminum cyminum and Pimpinella anisum are toxic to insect pests and vectors without affecting non-targetinvertebrates. Ind. Crop. Prod. 2018, 124, 236–243. [Google Scholar] [CrossRef]
- Kostic, I.; Lazarevic, J.; Jovanovic, D.Š.; Kostić, M.; Marković, T.; Milanović, S. Potential of essential oils from anise, dill and fennel seedsfor the gypsy moth control. Plants 2021, 10, 2194. [Google Scholar] [CrossRef] [PubMed]
- Kavallieratos, N.G.; Boukouvala, M.C.; Ntalli, N.; Skourti, A.; Karagianni, E.S.; Nika, E.P.; Kontodimas, D.C.; Cappellacci, L.; Petrelli, R.; Cianfaglione, K.; et al. Effectiveness of eight essential oils against two key stored-product beetles, Prostephanus truncatus (Horn) and Trogoderma granarium Everts. Food Chem. Toxicol. 2020, 139, 111255. [Google Scholar] [CrossRef] [PubMed]
- Skuhrovec, J.; Douda, O.; Zouhar, M.; Maňasová, M.; Božik, M.; Klouček, P. Insecticidal and behavioral effect of microparticles of Pimpinella anisum essential oil on larvae of Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2020, 113, 255–262. [Google Scholar]
- Willow, J.; Sulg, S.; Kaurilind, E.; Silva, A.I.; Kaasik, R.; Smagghe, G.; Veromann, E. Evaluating the effect of seven plant essential oils on pollen beetle (Brassicogethes aeneus) survival and mobility. Crop. Prot. 2020, 134, 105181. [Google Scholar] [CrossRef]
- Lee, H.E.; Hong, S.J.; Hasan, N.; Baek, E.J.; Kim, J.T.; Kim, Y.; Park, M. Repellent efficacy of essential oils and plant extracts against Tribolium castaneum and Plodia interpunctella. Entomol. Res. 2020, 50, 450–459. [Google Scholar] [CrossRef]
- Nikoletta, N.; Despoina, Z.; Maria, A.D.; Efimia, P.M.; Urania, M.P.; Nikilaos, M. Anise, parsley and rocket as nematicidal soil amendments and their impacton non-target soil organisms. Appl. Soil Ecol. 2019, 143, 17–25. [Google Scholar]
- Ikbal, C.; Pavela, R. Essential oils as active ingredients of botanical insecticides against aphids. J. Pest Sci. 2019, 92, 971–986. [Google Scholar] [CrossRef]
- Mudroncekova, S.; Ferencik, J.; Gruľová, D.; Barta, M. Insecticidal and repellent effects of plant essential oils against Ips typographus. J. Pest Sci. 2019, 92, 595–608. [Google Scholar] [CrossRef]
- Erdemir, T.; Erler, F. Repellent, oviposition-deterrent and egg-hatching inhibitoryeffects of some plant essential oils against citrus mealybug, Planococcus citri Risso (Hemiptera: Pseudococcidae). J. Plant Dis. Prot. 2017, 124, 473–479. [Google Scholar] [CrossRef]
- Hategekimana, A.; Erler, F. Fecundity and fertility inhibition effects of some plant essential oils and their major components against Acanthoscelides obtectus Say (Coleoptera: Bruchidae). J. Plant Dis. Protect 2020, 127, 615–623. [Google Scholar] [CrossRef]
- Draz, K.A.; Tabikha, R.M.; Eldosouky, M.I.; Darwish, A.A.; Abdelnasser, M. Biotoxicity of essential oils and their nano-emulsions against the coleopteran stored product insect pests Sitophilus oryzae L. and Tribolium castaneum herbst. Int. J. Pest Manag. 2022. [Google Scholar] [CrossRef]
- Giunti, G.; Laudani, F.; Presti, E. Contact toxicity and ovideterrent activity of three essential oil-based nano-emulsions against the olive fruit fly Bactrocera oleae. Horticulturae 2022, 8, 240. [Google Scholar] [CrossRef]
- C-Tejero, M.; Guirao, P.; P-Villalobos, M.J. Aphicidal activity of farnesol against the green peach aphid-Myzus persicae. Pest Manag. Sci. 2022, 78, 2714–2721. [Google Scholar] [CrossRef] [PubMed]
- Palermo, D.; Giunti, G.; Laudani, F.; Palmeri, V.; Campolo, O. Essential oil-based nano-biopesticides: Formulation and bioactivity against the confused flour beetle Tribolium Confusum. Sustain. 2021, 13, 9746. [Google Scholar] [CrossRef]
- Hashem, A.S.; Ramadan, M.M.; A-Hady, A.A.A.; Sut, S.; Maggi, F.; Acqua, S.D. Pimpinella anisum essential oil nanoemulsion toxicity against Tribolium castaneum? Shedding lighton its interactions with aspartate aminotransferaseand alanine aminotransferase by molecular docking. Molecules 2020, 25, 4841. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.S.; Awadalla, S.S.; Zayed, G.M.; Maggi, F.; Benelli, G. Pimpinella anisum essential oil nanoemulsions against Tribolium castaneum-insecticidal activity and mode of action. Environ. Sci. Pollut. R 2018, 25, 18802–18812. [Google Scholar] [CrossRef] [PubMed]
- Laojun, S.; Damapong, P.; Peerada, D.; Wallapa, W.; Nantana, S.; Thavatchai, K.; Tanawat, C. Efficacy of commercial botanical pure essential oils of garlic (Allium sativum) and anise (Pimpinella anisum) against larvae of the mosquito Aedes aegypti. J. App. Biol. Biotech. 2020, 8, 88–92. [Google Scholar]
- Chantawee, A.; Soonwera, M. Larvicidal, pupicidal and oviposition deterrent activities of essential oils from Umbelliferae plants against house fly Musca domestica. Asian S Pac. J. Trop. Med. 2018, 11, 621–629. [Google Scholar] [CrossRef]
- Elmhalli, F.; Palsson, K.; Örberg, J.; Grand, G. Acaricidal properties of ylang-ylang oil and star anise oil against nymphs of Ixodes ricinus (Acari: Ixodidae). Exp. Appl. Acarol. 2018, 76, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Iannarelli, R.; Petrelli, R.; Cappellacci, L.; Cianfaglione, K.; Afshar, F.H.; Nicoletti, M.; Canale, A.; Maggi, F. Synergized mixtures of Apiaceae essential oils and relatedplant-borne compounds: Larvicidal effectiveness on the filariasisvector Culex quinquefasciatus Say. Ind Crop. Prod. 2017, 96, 186–195. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Cianfaglione, K.; Bajalan, I.; Morshedloo, M.R.; Lupidi, G.; Romano, D.; et al. Microemulsions for delivery of Apiaceae essential oils-Towards highlyeffective and eco-friendly mosquito larvicides? Ind. Crop. Prod. 2019, 129, 631–640. [Google Scholar] [CrossRef]
- Showler, A.T.; Harlien, J.L. Effects of the botanical compound p-anisaldehyde on horn fly (Diptera: Muscidae) repellency, mortality, and reproduction. J. Med. Entomol. 2018, 55, 183–192. [Google Scholar] [CrossRef]
- Showler, A.T.; Harlien, J.L. Botanical compound p-anisaldehyde repels larval lone star tick (Acari: Ixodidae), and halts reproduction by gravid adults. J. Med. Entomol. 2018, 55, 200–209. [Google Scholar] [CrossRef]
- S-Gomez, S.; Pagan, R.; Pavela, R.; Mazzara, E.; Spinozzi, E.; Marinelli, O.; Zeppa, L.; Morshedloo, M.R.; Maggi, F.; Canale, A. Lethal and sublethal effects of essential oil-loaded zein nanocapsules on a zoonotic disease vector mosquito, and their non-target impact. Ind. Crop. Prod. 2022, 176, 114413. [Google Scholar] [CrossRef]
- Chan, O.H.; Hwang, J.Y.; Lee, Y.A.; Song, M.; Kwon, O.K.; Sim, J.H.; Kim, S.; Song, K.; Lee, S. The inhibitory effects of the ethanolic extract of Pimpinella brachycarpa on cytochrome P450 enzymes in humans. J. Korean Soc. Appl. Bi 2014, 57, 113–116. [Google Scholar]
- Chronopoulou, E.G.; Ataya, F.; Labrou, N.E. A microplate-based platform with immobilized human glutathione transferase A1-1 for high-throughput screening of plant-origin inhibitors. Curr. Pharm. Biotechno 2018, 19, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Bou-Salah, L.; Benarous, K.; Linani, A.; Bombarada, I.; Yousfi, M. In vitro and in silico inhibition studies of five essential oils on both enzymeshuman and bovine xanthine oxidase. Ind. Crop. Prop. 2020, 143, 111949. [Google Scholar]
- Bui, T.B.C.; Nosaki, S.; Kokawa, M.; Xu, Y.Q.; Kitamura, Y.; Tasnokura, M.; Hachimura, S.; Miyakawa, T. Evaluation of spice and herb as phyto-derivedselective modulators of human retinaldehydedehydrogenases using a simple in vitro method. Biosci. Rep. 2021, 41, BSR20210491. [Google Scholar] [CrossRef]
- Koriem, K.M.M.; Fadl, N.N.; El-Zayat, S.R.; Hosny, E.N.; El-Azma, M.H. Geranium oil and anise oil inhibitbrain cerebral cortex andhippocampus inflammation indepressed animal model. Nutr. Food Sci. 2021, 2, 439–456. [Google Scholar]
- El-Shamy, K.A.; Koriem, K.M.M.; Fadl, N.N.; El-Azma, M.H.A.; Arbid, M.S.S.; Morsy, F.A.; El-Zayat, S.R.; Hosny, E.N.; Youness, E.R. Oral supplementation with geranium oil or aniseoil ameliorates depressed rat-related symptomsthrough oils antioxidant effects. J. Complement Integr. Med. 2019, 17, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Mosaffa-Jahromi, M.; Tamaddon, A.; Afsharypuor, S.; Salehi, A.; Seradj, S.H.; Pasalar, M.; Jafari, P.; Lankarani, K.B. Effectiveness of anise oil for treatment of mild to moderate depression in patients with irritable bowel syndrome: Arandomized active and placebo-controlled clinical trial. J. Evid-Based Compl. Alt Med. 2017, 22, 41–46. [Google Scholar] [CrossRef]
- Ntalli, N.; Michaelakis, A.; Eloh, K.; Papachristos, D.P.; Wejnerowski, L.; Caboni, P.; Cerbin, S. Biocidal effect of (E)-anethole on the cyanobacterium Aphanizomenon gracile Lemmermann. J. Appl. Phycol. 2017, 29, 1297–1305. [Google Scholar] [CrossRef]
- A-Pancevska, N.; Kungulovski, D.; N-Bogdanov, M. Comparative study of essential oils from fennel fruits and anise fruits: Chemical composition and in vitro antimicrobial activity. Maced. J. Chem. Chen En 2021, 40, 241–252. [Google Scholar]
- Samojlik, I.; Mijatović, V.; Petković, S.; Škrbić, B.; Božin, B. The influence ofessential oil of aniseed (Pimpinella anisum, L.)on drug effects on the central nervous system. Fitoterapia 2012, 83, 1466–1473. [Google Scholar] [CrossRef]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Elbouzidi, A.; Bousta, D. Assessment of antidepressant-like, anxiolytic effects and impact on memory of Pimpinella anisum L. total extract on swiss blbino mice. Plants 2021, 10, 1573. [Google Scholar] [CrossRef]
- Alotaibi, M.F. Pimpinella anisum extract attenuates spontaneous and agonist-induceduterine contraction in term-pregnant rats. J. Ethnopharmacol. 2020, 254, 112730. [Google Scholar] [CrossRef] [PubMed]
- Mosavata, S.H.; Jaberib, A.R.; Sobhani, Z.; Mosaffa-Jahromi, M.; Iraji, A.; Moayedfard, A. Efficacy of Anise (Pimpinella anisum L.) oil for migraine headache: A pilotrandomized placebo-controlled clinical trial. J. Ethnopharmacol. 2019, 236, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, M.; Khalili, D.; Tehrani, F.R.; Amin, G.; Negarandeh, R. Could anise decrease the intensity ofpremenstrual syndrome symptoms incomparison to placebo? A double-blindrandomized clinical trial. J. Complement. Integr. Med. 2020, 17, 20190077. [Google Scholar] [CrossRef] [PubMed]

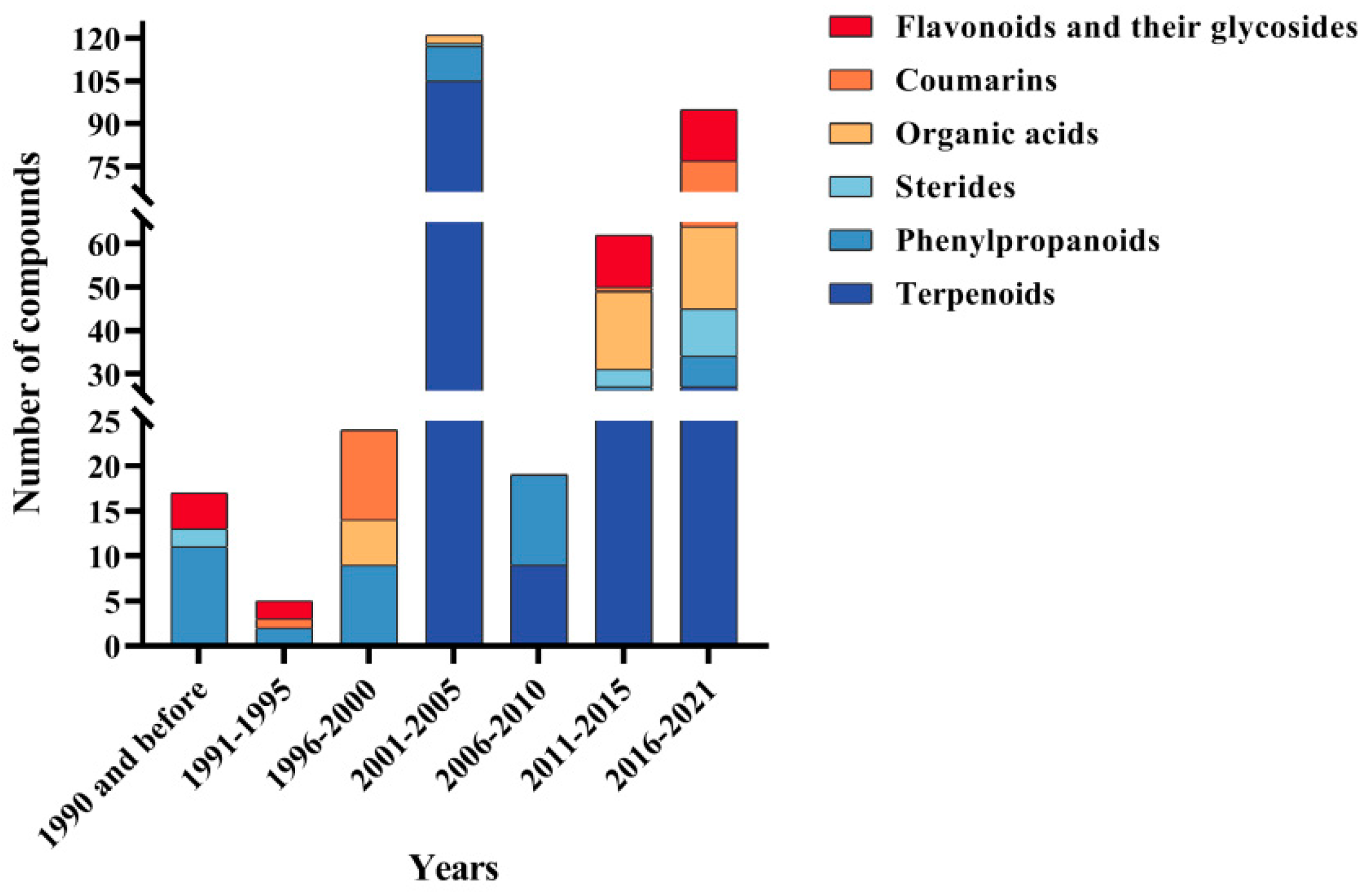
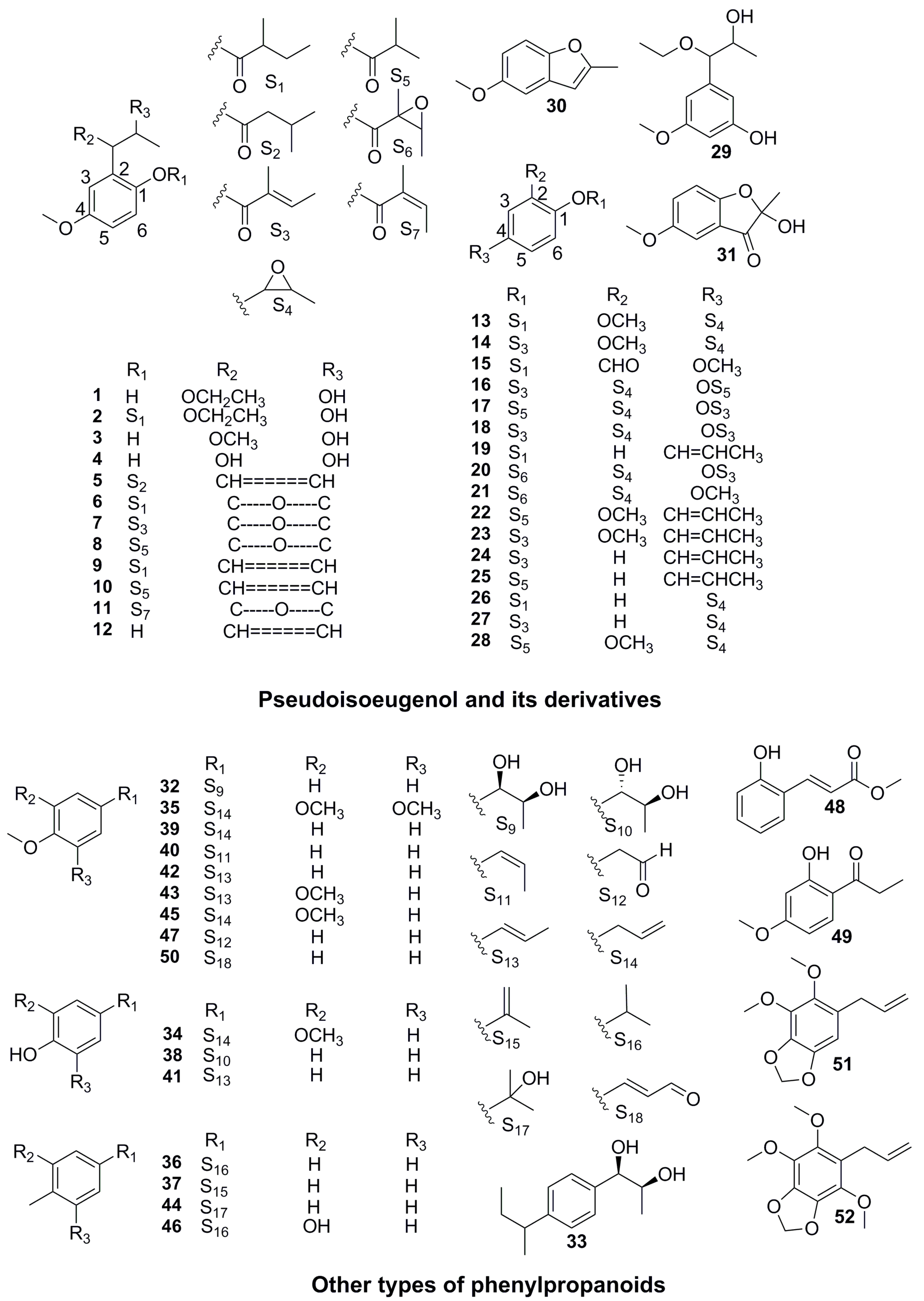
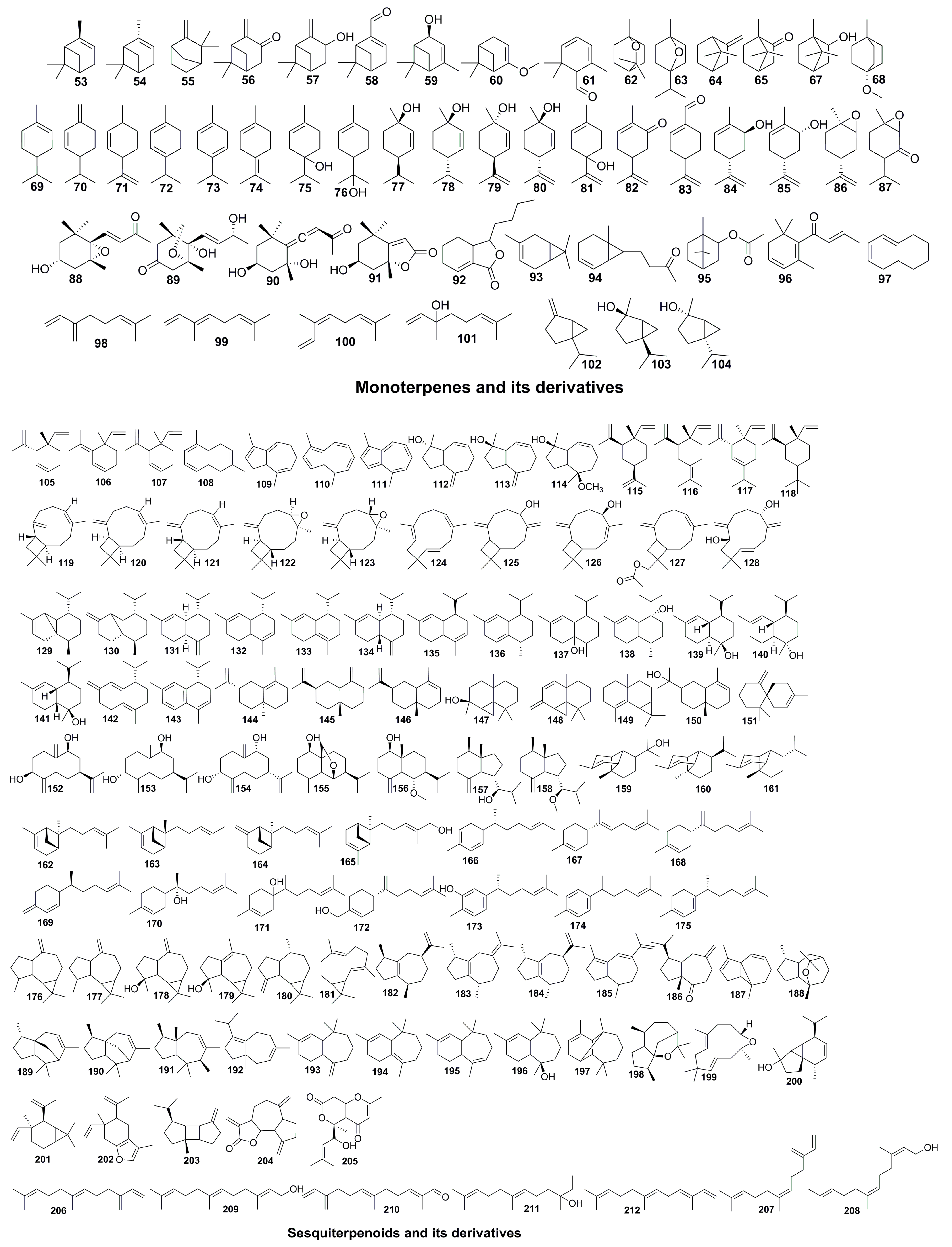

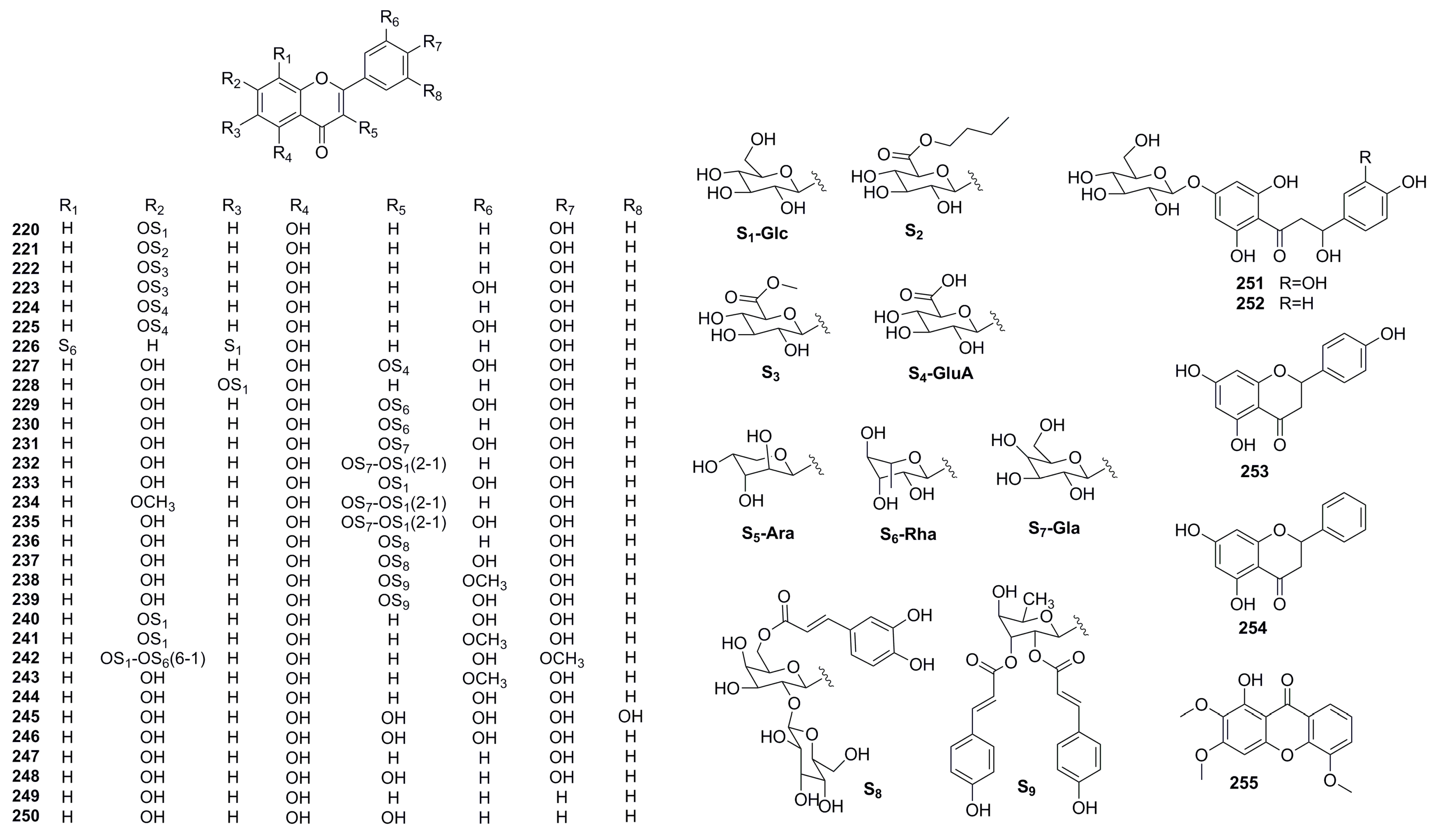
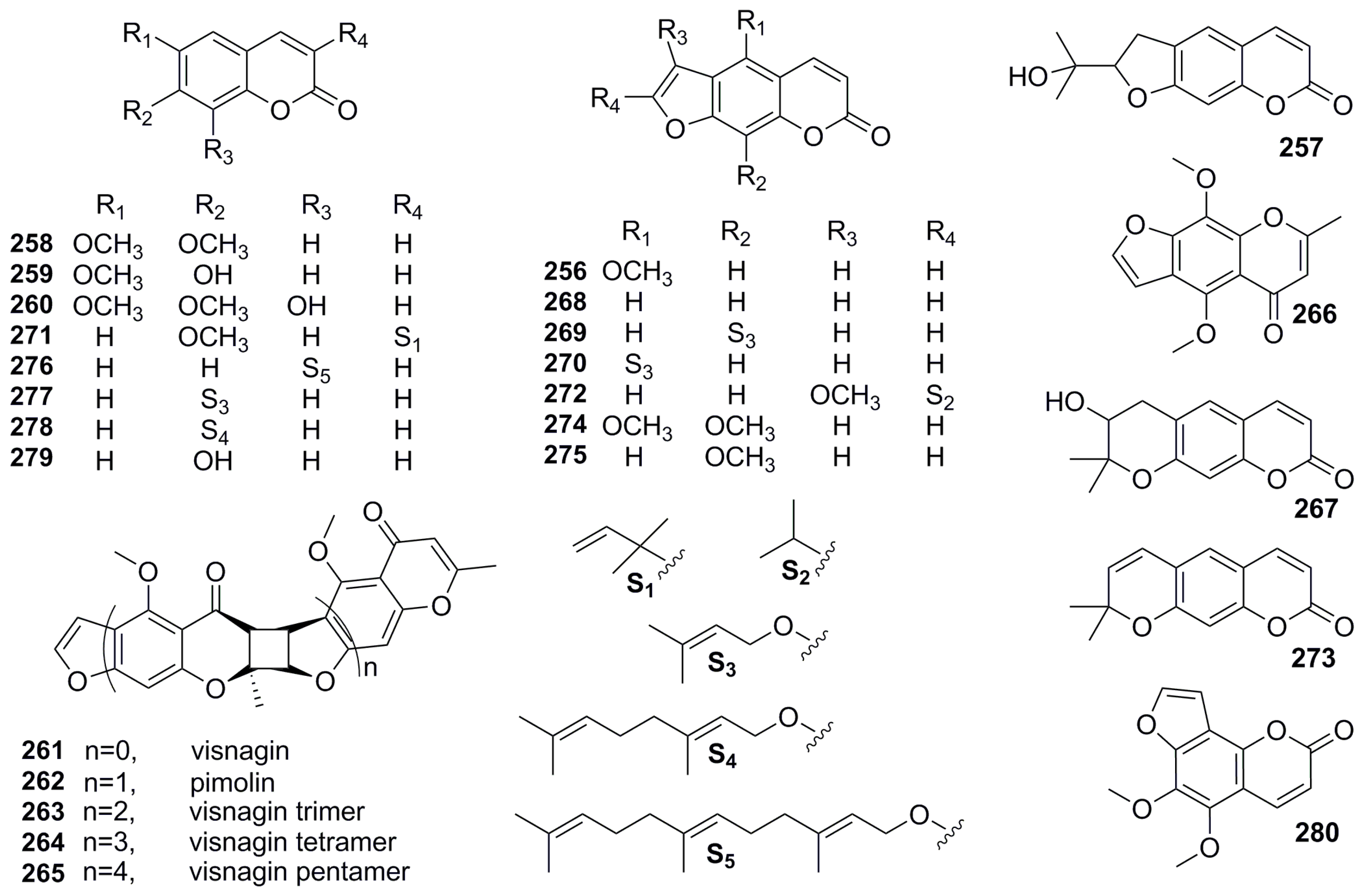
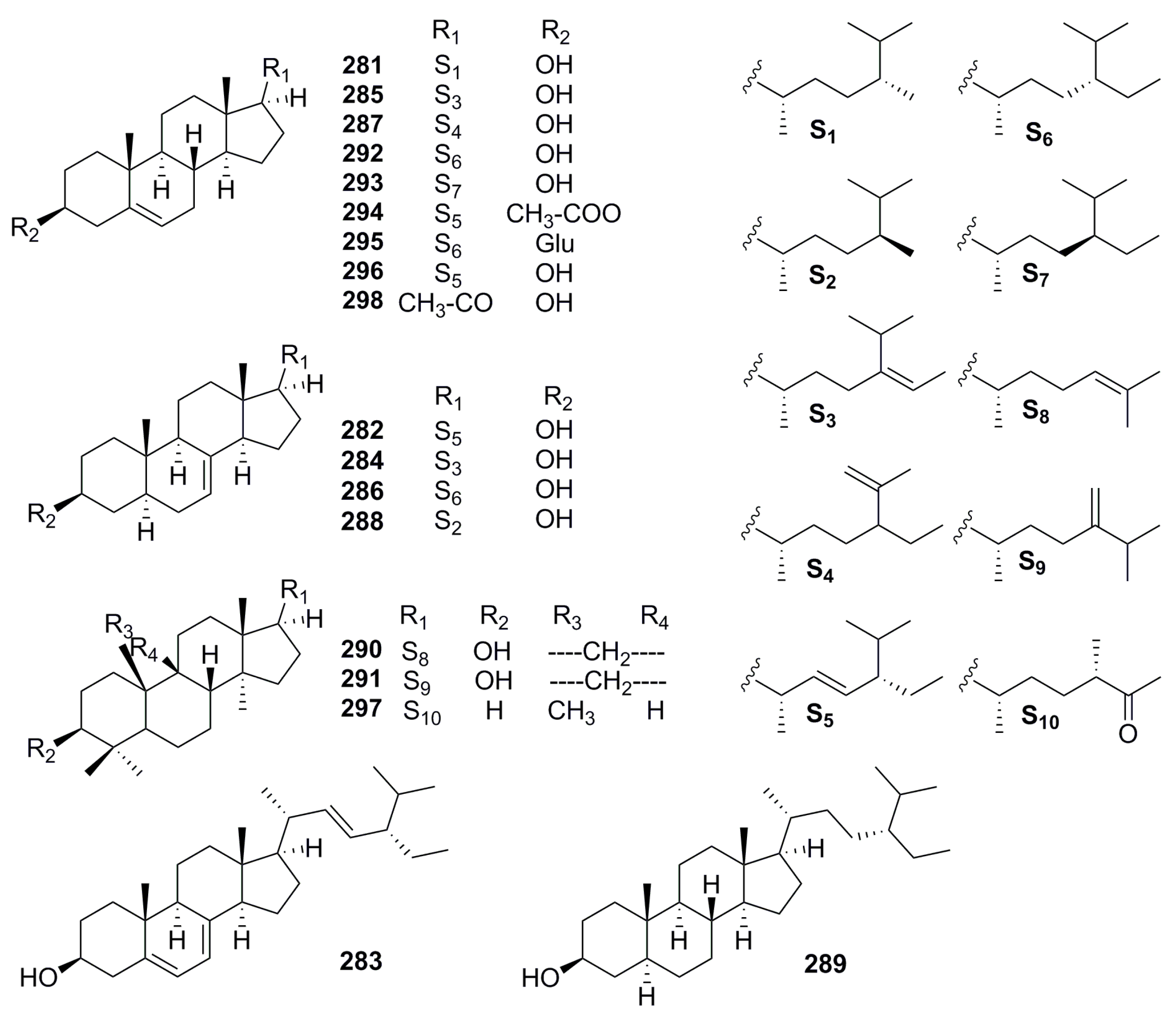
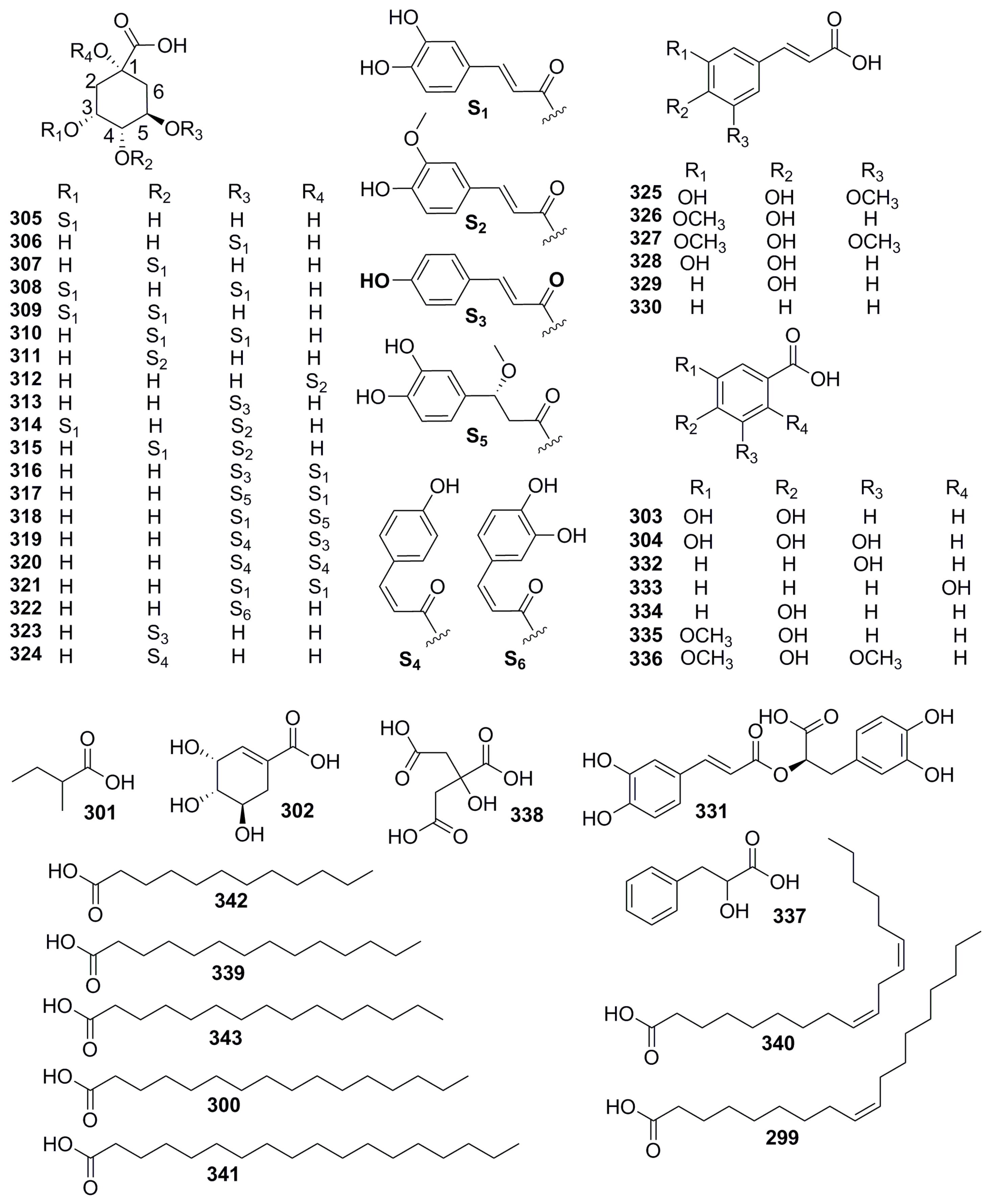
| Part | Pimpinella spp. | Folk-Medicine Applications | Country/Region | Reference |
|---|---|---|---|---|
| Aerial parts | P. diversifolia | Cold, dyspepsia, dysentery, and diarrhea | China | [12] |
| P. candollean | Chest pain, stomach pain, rheumatism, muscle and bone pain, and used as wild vegetables | China | [12] | |
| P. thellungiana | Anticoagulation | Chian | [12] | |
| P. brachycarpa | Gastrointestinal disturbances, bronchial asthma, insomnia, persistent cough, and used as vegetables | Korean | [13] [14] | |
| P. cappadocica | Carminative and digestive | Turkey | [16] | |
| P. anisum | Renal colic, gastrointestinal colic, and upper respiratory tract disease | Egypt | [21] | |
| P. anisum | Renal colic, gastrointestinal colic, and upper respiratory tract disease | Lebanon | [22] | |
| P. anisum | Used as a tea to treat constipation | Brazil | [24] | |
| Seeds | P. brachycarpa | Gastrointestinal disturbances, bronchial asthma, insomnia, persistent cough, and used as vegetables | Korean | [14] |
| P. monoica | Stomachache | India | [15] | |
| P. rhodantha | Sedative, expectorant, and increase lactation | Turkey | [17] | |
| P. peregrine | Carminative, digestive, and increase lactation | Turkey | [18] | |
| P. khorasanica | Carminative, digestive, and increase lactation | Turkey | [19] | |
| P. anisum | Epilepsy | Iran | [20] | |
| P. anisum | Insect repellents, stomach-cramping sedatives, diuretics, and urinary tract disinfectants | England | [23] | |
| P. anisum | Used as plant spice to produce spirits drinks and confectionery | Spain France | [25] [25] | |
| Essential oil | P. anisum | Carminative, aromatic, disinfectant, and diuretic | Iran | [20] |
| No. | Name | Formula | Mol. Wt. | Species | Reference |
|---|---|---|---|---|---|
| 1 | 2-(1′-ethoxy-2′-hydroxy)propyl-4-methoxyphenol (llungianin A) | C12H18O4 | 226 | P. thellungiana | [30] |
| 2 | 2-(1′-ethoxy-2′-hydroxy)propyl-4-methoxyphenyl-2-methyl-butyrate (llungianin B) | C17H26O5 | 310 | P. thellungiana | [30] |
| 3 | 2-(1′-methoxy-2′-hydroxy) propyl-4-methoxyphenol (llungianin E) | C11H16O4 | 212 | P. thellungiana | [31] |
| 4 | 2-(1′,2′-dihydroxy)propyl-4-methoxyphenol | C10H14O4 | 198 | P. thellungiana | [32] |
| 5 | 4-methoxy-2-propenyl-phenyl-(3′-methyl) butanoate | C15H20O3 | 248 | P. thellungiana | [33] |
| 6 | 2-(1′,2′-epoxy)propyl-4-methoxypheryl-(2″-methyl)-butyrate (llungianin G) | C15H20O4 | 264 | P. thellungiana P. saxifraga | [34] [46] |
| 7 | 4-methoxy-2-(3-methyloxiranyl) phenyl-2-methylbutenate | C15H18O4 | 262 | P. diversifolia P. aurea P. peregrina | [35] [45] |
| 8 | 4-methoxy-2-(3-methyloxiranyl)phenyl isobutyrate | C14H18O4 | 250 | P. diversifolia P. peregrina | [35] [36] |
| 9 | 4-methoxy-1-propenyl-phenyl-(2′-methyl) butanoate | C15H20O3 | 248 | P. anisum | [29] |
| 10 | 4-methoxy-2-(1-propenyl)-phenylisobutyrate | C14H18O3 | 234 | P. peregrina | [36] |
| 11 | 4-methoxy-2-(3-methyloxiranyl)-phenylangelate | C15H18O4 | 262 | P. peregrina | [36] |
| 12 | pseudoisoeugenol | C10H12O2 | 164 | P. saxifraga | [37] |
| 13 | 2-methoxy-4-(3-methyloxiranyl) phenyl 2-methyl butanoate | C15H20O4 | 264 | P. saxifraga | [37] |
| 14 | 2-methoxy-4-(3-methyloxiranyl) phenyl2-methyl butenate | C15H18O4 | 262 | P. saxifraga | [38] |
| 15 | 4-methoxy-2-fromylphenyl-(2′-methyl) butanoate | C13H16O4 | 236 | P. anisum | [29] |
| 16 | 1-angelyloxy-2-(3-methyloxiranyl)-4-isobutyryloxybenzene | C18H22O5 | 318 | P. diversifolia | [39] |
| 17 | l-isobuty-ryloxy-2-(3-methyloxiranyl)-4-angelyloxybenzene | C18H22O5 | 318 | P. diversifolia | [39] |
| 18 | 1,4-diangelyoxy-2-(3-methyloxiranyl)benzene | C19H22O5 | 330 | P. diversifolia | [39] |
| 19 | 4-propenyl-phenyl-2-methyl butanoate (llungianin F) | C15H20O2 | 232 | P. thellungiana | [33] |
| 20 | 4-(2-methyl-2-butenoyl)oxy)-2-(3-methyloxiran-2-yl)-phenyl2-methyl-2,3-epoxybutanoate | C19H22O6 | 346 | P. villosa | [40] |
| 21 | 4-(2-methyl-2-butenoyloxy)-2-(3-methyloxiran-2-yl)-phenyl 2-methyl-2-butenoate | C15H18O5 | 278 | P. villosa | [40] |
| 22 | 2-methoxy-4-prop-1-enylphenyl isobutyrate | C14H18O3 | 234 | P. junoniae P. aurea | [44] [45] |
| 23 | pseudoisoeugenyltiglate | C15H18O3 | 246 | P. junoniae | [44] |
| 24 | 4-(1-propenyl)-phenyl tiglate | C14H16O2 | 216 | P. aurea | [45] |
| 25 | 4-(1-propenyl)-phenylisobutyrate | C13H16O2 | 204 | P. corymbosa | [36] |
| 26 | 4-(3-methyloxiranyl)-phenyl-2-methylbutyrate | C14H18O3 | 234 | P. aurea | [45] |
| 27 | 4-(3-Methyloxiranyl)-phenyltiglate | C14H16O3 | 232 | P. aurea | [45] |
| 28 | epoxypseudoisoeugenyl-2-methyl butyrate | C14H18O4 | 250 | P. corymbosa P. peregrina P. puberula | [36] |
| 29 | 5-(1′-ethoxy-2′-hydroxy)propyl-3-methoxyphenol | C12H18O4 | 226 | P. thellungiana | [41] |
| 30 | 5-methoxy-2-methyl benzofuran (llungianin H) | C10H10O2 | 162 | P. thellungiana P. junoniae P. peregrina | [42] [44] [36] |
| 31 | 2-methyl-2-hydroxy-5-methoxy berzo (d) hydrofuran-3-one | C10H10O4 | 194 | P. thellungiana | [43] |
| 32 | erythro-1′-(4-methoxyphenyl)-propan-1′,2′-diol | C10H14O3 | 182 | P. aurea | [45] |
| 33 | erythro-1′-[4-(sec-butyl)-phenyl]-propan-1′,2′-diol | C13H20O2 | 208 | P. aurea | [45] |
| 34 | eugenol | C10H12O2 | 164 | P. puberula | [36] |
| 35 | elemicine | C12H16O3 | 208 | P. puberula | [36] |
| 36 | p-cymene | C10H14 | 134 | P. anisetum P. aurea P. corymbosa | [48] [45] |
| 37 | α,p-dimethylstyrene | C10H12 | 132 | P. aurea | [45] |
| 38 | 1-(4-hydroxyphenyl)-1,2-ethanediol | C8H10O3 | 154 | P. candolleana | [49] |
| 39 | methyl chavicol | C10H12O | 148 | P. anisetum P. anisum | [48] [50] |
| 40 | cis-anethole | C10H12O | 148 | P. anisetum P. flabellifolia P. saxifrage P. anisum | [48] [46] [50] |
| 41 | 4-propenylphenol | C9H10O | 134 | P. thellungiana | [41] |
| 42 | trans-anethole | C10H12O | 148 | P. anisetum P. flabellifolia P. aurea P. corymbosa P. peregrine P. anisum | [48] [36] [50] |
| 43 | methyl isoeugenol | C11H14O2 | 178 | P. flabellifolia | [48] |
| 44 | p-cymen-8-ol | C10H14O | 150 | P. junoniae P. aurea | [44] [45] |
| 45 | methyl eugenol | C11H14O2 | 178 | P. corymbosa P. puberula | [36] |
| 46 | carvacrol | C11H14O2 | 178 | P. aurea P. corymbosa P. puberula | [36] |
| 47 | p-anisaldehyde | C10H12O2 | 164 | P. saxifrage P. anisum | [46] [50] |
| 48 | methyl-O-coumarate | C10H10O3 | 178 | P. saxifraga | [46] |
| 49 | 1-(2-hydroxy-4-methoxyphenyl)propan1-one | C10H12O3 | 180 | P. saxifraga | [46] |
| 50 | 4-methoxycinnamaldehyde | C10H12O3 | 180 | P. saxifraga | [46] |
| 51 | dillapiole | C12H14O4 | 222 | P. saxifrage P. serbica | [46] [47] |
| 52 | nothoapiole | C13H16O5 | 252 | P. serbica | [47] |
| No. | Type | Name | Formula | Mol. Wt. | Species | Reference |
|---|---|---|---|---|---|---|
| 53 | Monoterpenoid | α-pinene | C10H16 | 136 | P. aurea P. corymbosa P. peregrina P. puberula P. junoniae P. anisetum P. flabellifolia P. anisum P. affinis P. monoica P. thellungiana | [36] [44] [48] [57] [52] [15] [54] |
| 54 | Monoterpenoid | β-pinene | C10H16 | 136 | P. aurea P. corymbosa P. puberula P. flabellifolia P. anisum P. monoica P. thellungiana | [36] [48] [6] [15] [54] |
| 55 | Monoterpenoid | camphene | C10H16 | 136 | P. aurea P. corymbosa P. flabellifolia | [36] [48] |
| 56 | Monoterpenoid | pinocarvone | C10H14O | 150 | P. aurea | [36] |
| 57 | Monoterpenoid | pinocarveol | C10H16O | 152 | P. aurea P. thellungiana | [36] [54] |
| 58 | Monoterpenoid | myrtenal | C10H14O | 150 | P. corymbosa P. thellungiana | [36] [54] |
| 59 | Monoterpenoid | trans-verbenol | C10H16O | 152 | P. corymbosa P. peregrine P. monoica | [36] [15] |
| 60 | Monoterpenoid | myrtenol | C10H16O | 152 | P. aurea | [36] |
| 61 | Monoterpenoid | safranal | C10H14O | 150 | P. anisum | [57] |
| 62 | Monoterpenoid | 1,8-cineole | C10H18O | 154 | P. anisum | [57] |
| 63 | Monoterpenoid | 1,4-cineole | C10H18O | 154 | P. thellungiana | [54] |
| 64 | Monoterpenoid | α-fenchene | C10H16 | 136 | P. monoica | [15] |
| 65 | Monoterpenoid | camphor | C10H16O | 152 | P. anisum | [58] |
| 67 | Monoterpenoid | borneol | C10H18O | 154 | P. anisum P. monoica | [58] [15] |
| 68 | Monoterpenoid | 1-methoxy-4-methylbicyclo[2.2.2]octane | C10H18O | 154 | P. thellungiana | [54] |
| 69 | Monoterpenoid | α-phellandrene | C10H16 | 136 | P. flabellifolia P. anisum | [48] [57] |
| 70 | Monoterpenoid | β-phellandrene | C10H16 | 136 | P. aurea P. puberula P. junoniae P. anisum | [36] [44] [58] |
| 71 | Monoterpenoid | Limonene | C10H16 | 136 | P. aurea P. corymbosa P. puberula P. anisetum P. flabellifolia P. anisum P. enguezekensis P. affinis P. monoica | [36] [48] [57] [51] [52] [15] |
| 72 | Monoterpenoid | α-terpinene | C10H16 | 136 | P. aurea P. puberula P. anisum P. monoica | [36] [50] [15] |
| 73 | Monoterpenoid | γ-terpinene | C10H16 | 136 | P. aurea P. flabellifolia P. anisum P. enguezekensis P. monoica | [36] [48] [6] [51] [15] |
| 74 | Monoterpenoid | Terpinolene | C10H16 | 136 | P. aurea P. junoniae P. anisum P. monoica | [36] [44] [58] [15] |
| 75 | Monoterpenoid | terpinen-4-ol | C10H18O | 154 | P. aurea P. junoniae P. flabellifolia P. anisum | [36] [44] [48] [57] |
| 76 | Monoterpenoid | α-terpineol | C10H18O | 154 | P. junoniae P. flabellifolia P. anisum P. monoica | [44] [48] [57] [15] |
| 77 | Monoterpenoid | trans-p-menth-2-en-1-ol | C10H18O | 154 | P. aurea | [36] |
| 78 | Monoterpenoid | cis-p-menth-2-en-1-ol | C10H18O | 154 | P. aurea | [36] |
| 79 | Monoterpenoid | trans-p-mentha-2,8-dien-1-ol | C10H16O | 152 | P. puberula P. flabellifolia | [36] [48] |
| 80 | Monoterpenoid | cis-p-mentha-2,8-dien-1-ol | C10H16O | 152 | P. puberula P. flabellifolia | [36] [48] |
| 81 | Monoterpenoid | p-mentha-1,8-dien-4-ol | C10H16O | 152 | P. aurea | [36] |
| 82 | Monoterpenoid | carvone | C10H14O | 150 | P. puberula P. anisum P. enguezekensis | [36] [6] [51] |
| 83 | Monoterpenoid | perilla aldehyde | C10H14O | 150 | P. puberula | [36] |
| 84 | Monoterpenoid | trans-carveol | C10H16O | 152 | P. puberula P. anisum | [36] [57] |
| 85 | Monoterpenoid | cis-carveol | C10H16O | 152 | P. anisum | [57] |
| 86 | Monoterpenoid | cis-1,2-limonene epoxide | C10H16O | 152 | P. puberula | [36] |
| 87 | Monoterpenoid | piperitone oxide | C10H16O2 | 168 | P. thellungiana | [54] |
| 88 | Monoterpenoid | 3-hydroxy-5,6-epoxy-7-megastigmen-9-one | C13H20O3 | 224 | P. brachycarpa | [13] |
| 89 | Monoterpenoid | (1R,6R,9R)-6,9,11-trihydroxy-4-megastigmen-3-one | C13H20O4 | 240 | P. brachycarpa | [13] |
| 90 | Monoterpenoid | grasshopper ketone | C13H20O3 | 224 | P. brachycarpa | [13] |
| 91 | Monoterpenoid | loliolide | C11H16O3 | 196 | P. brachycarpa | [13] |
| 92 | Monoterpenoid | sedanolide | C12H18O2 | 194 | P. puberula | [36] |
| 93 | Monoterpenoid | δ-3-carene | C10H16 | 136 | P. aurea P. corymbosa P. puberula P. anisum P. enguezekensis | [36] [59] [51] |
| 94 | Monoterpenoid | traginone | C12H18O | 178 | P. puberula | [36] |
| 95 | Monoterpenoid | bornyl acetate | C12H20O2 | 196 | P. aurea P. puberula | [36] |
| 96 | Monoterpenoid | trans-β-damascenone | C13H18O | 190 | P. puberula | [36] |
| 97 | Monoterpenoid | cyclodecadiene | C10H16 | 136 | P. diversifolia | [53] |
| 98 | Monoterpenoid | β-myrcene | C10H16 | 136 | P. aurea P. corymbosa P. puberula P. anisetum P. flabellifolia P. anisum P. affinis P. monoica | [36] [48] [59] [52] [15] |
| 99 | Monoterpenoid | trans-β-ocimene | C10H16 | 136 | P. aurea P. anisum P. monoica | [36] [58] [15] |
| 100 | Monoterpenoid | cis-β-ocimene | C10H16 | 136 | P. anisum P. affinis P. monoica | [58] [52] [15] |
| 101 | Monoterpenoid | Linalool | C10H18O | 154 | P. junoniae P. flabellifolia P. anisum P. enguezekensis P. affinis P. diversifolia | [44] [48] [50] [51] [52] [53] |
| 102 | Monoterpenoid | sabinene | C10H16 | 136 | P. aurea P. corymbosa P. puberula P. flabellifolia P. anisum P. monoica | [36] [48] [57] [15] |
| 103 | Monoterpenoid | trans-sabinene hydrate | C10H18O | 154 | P. aurea | [36] |
| 104 | Monoterpenoid | cis-sabinene hydrate | C10H18O | 154 | P. aurea | [36] |
| 105 | C12-sesquiterpenes | isogeijerene | C12H18 | 162 | P. corymbosa P. puberula | [36] |
| 106 | C12-sesquiterpenes | isogeijerene C | C12H18 | 162 | P. puberula | [36] |
| 107 | C12-sesquiterpenes | geijerene | C12H18 | 152 | P. aurea P. corymbosa P. peregrina P. puberula P. anisetum P. anisum P. affinis P. khorasanica P. thellungiana | [36] [48] [50] [52] [19] [54] |
| 108 | C12-sesquiterpenes | pregeijerene | C12H18 | 162 | P. corymbosa P. puberula P. affinis P. khorasanica | [36] [52] [19] |
| 109 | C12-sesquiterpenes | 3,10-dihydro-1,4-dimethylazulene | C12H14 | 158 | P. puberula | [36] |
| 110 | C12-sesquiterpenes | 4,10-dihydro-1,4-dimethylazulene | C12H14 | 158 | P. corymbosa | [36] |
| 111 | C12-sesquiterpenes | 1,4-dimethylazulene | C12H12 | 156 | P. corymbosa | [36] |
| 112 | C12-sesquiterpenes | 8-epi-dictamnol | C12H18O | 178 | P. puberula | [36] |
| 113 | C12-sesquiterpenes | dictamnol | C12H18O | 178 | P. puberula P. affinis | [36] [52] |
| 114 | C12-sesquiterpenes | 1α, 5α-dimethyl-4α, 10α-bicyclo [0,3,5] dec-8-en-5β-methoxy-1β-ol | C13H22O2 | 210 | P. cappadocica | [16] |
| 115 | Sesquiterpenes | β-elemene | C15H24 | 204 | P. aurea P. corymbosa P. anisum P. diversifolia | [36] [50] [53] |
| 116 | Sesquiterpenes | γ-elemene | C15H24 | 204 | P. flabellifolia P. monoica | [48] [15] |
| 117 | Sesquiterpenes | δ-elemene | C15H24 | 204 | P. corymbosa P. anisum P. enguezekensis P. affinis | [36] [50] [51] [52] |
| 118 | Sesquiterpenes | elemol | C15H26O | 222 | P. puberula | [36] |
| 119 | Sesquiterpenes | β-caryophyllene | C15H24 | 204 | P. aurea P. corymbosa P. peregrina P. puberula P. anisetum P. anisum P. monoica P. diversifolia | [36] [48] [57] [15] [53] |
| 120 | Sesquiterpenes | 9-epi-β-caryophyllene | C15H24 | 204 | P. peregrina | [36] |
| 121 | Sesquiterpenes | isocaryophyllene | C15H24 | 204 | P. peregrina | [36] |
| 122 | Sesquiterpenes | isocaryophyllene oxide | C15H24O | 220 | P. corymbosa P. peregrina | [36] |
| 123 | Sesquiterpenes | caryophyllene oxide | C15H24O | 220 | P. aurea P. corymbosa P. peregrina P. puberula P. monoica P. diversifolia P. thellungiana | [36] [15] [53] [54] |
| 124 | Sesquiterpenes | α-humulene | C15H24 | 204 | P. corymbosa P. peregrine P. monoica P. diversifolia | [36] [15] [53] |
| 125 | Sesquiterpenes | caryophylladienol II | C15H24O | 220 | P. peregrine | [36] |
| 126 | Sesquiterpenes | caryophyllenol II | C15H24O | 220 | P. puberula | [36] |
| 127 | Sesquiterpenes | 12-hydroxy-β-caryophylleneacetate | C17H26O2 | 262 | P. aurea P. corymbosa | [36] |
| 128 | Sesquiterpenes | (2R*,6S*)-2,6-dihydroxyhumlaobtusa | C15H24O2 | 236 | P. brachycarpa | [13] |
| 129 | Sesquiterpenes | α-cubebene | C15H24 | 204 | P. corymbosa P. junoniae P. monoica | [36] [44] [15] |
| 130 | Sesquiterpenes | β-cubebene | C15H24 | 204 | P. corymbosa P. junoniae P. affinis P. monoica P. diversifolia | [36] [44] [52] [15] [53] |
| 131 | Sesquiterpenes | γ-muurolene | C15H24 | 204 | P. aurea P. corymbosa P. peregrine P. junoniae P. enguezekensis | [36] [44] [51] |
| 132 | Sesquiterpenes | α-cadinene | C15H24 | 204 | P. corymbosa | [36] |
| 133 | Sesquiterpenes | δ-cadinene | C15H24 | 204 | P. corymbosa P. anisetum P. anisum P. monoica P. diversifolia | [36] [48] [58] [15] [53] |
| 134 | Sesquiterpenes | γ-cadinene | C15H24 | 204 | P. junoniae P. monoica | [44] [15] |
| 135 | Sesquiterpenes | α-amorphene | C15H24 | 204 | P. aurea | [45] |
| 136 | Sesquiterpenes | cadina-1,4-diene | C15H24 | 204 | P. corymbosa | [36] |
| 137 | Sesquiterpenes | 1-epi-cubenol | C15H26O | 222 | P. corymbosa | [36] |
| 138 | Sesquiterpenes | cis-cadin-4-en-7-ol | C15H26O | 222 | P. aurea | [45] |
| 139 | Sesquiterpenes | T-cadinol | C15H26O | 222 | P. corymbosa | [36] |
| 140 | Sesquiterpenes | α-cadinol | C15H26O | 222 | P. corymbosa P. anisum | [36] [59] |
| 141 | Sesquiterpenes | T-muurolol | C15H26O | 222 | P. corymbosa | [36] |
| 142 | Sesquiterpenes | germacrene D | C15H24 | 204 | P. aurea P. corymbosa P. peregrina P. puberula P. anisetum P. anisum P. enguezekensis P. affinis P. monoica P. thellungiana | [36] [48] [50] [51] [52] [15] [54] |
| 143 | Sesquiterpenes | α-calacorene | C15H20 | 200 | P. corymbosa P. anisum P. monoica | [36] [58] [15] |
| 144 | Sesquiterpenes | 4,11-selinadiene | C15H24 | 204 | P. saxifraga | [46] |
| 145 | Sesquiterpenes | β-selinene | C15H24 | 204 | P. saxifrage P. anisum | [46] [57] |
| 146 | Sesquiterpenes | α-selinene | C15H24 | 204 | P. monoica | [15] |
| 147 | Sesquiterpenes | thujopsan-2-α-ol | C15H26O | 222 | P. aurea | [45] |
| 148 | Sesquiterpenes | Thujpsadiene | C15H22 | 202 | P. saxifraga | [46] |
| 149 | Sesquiterpenes | cyclopropa[a]naphthalene | C15H24 | 204 | P. diversifolia | [53] |
| 150 | Sesquiterpenes | 7-epi-α-eudesmol | C15H26O | 222 | P. aurea | [45] |
| 151 | Sesquiterpenes | β-chamigrene | C15H24 | 204 | P. anisum P. diversifolia | [57] [53] |
| 152 | Sesquiterpenes | (3S,7S,9S)-3,9-dihydroxygermacra-4(15),10(14),11(12)-triene | C15H24O2 | 236 | P. brachycarpa | [13] |
| 153 | Sesquiterpenes | (3R,7S,9S)-3,9-dihydroxygermacra-4(15),10(14),11(12)-triene | C15H24O2 | 236 | P. brachycarpa | [13] |
| 154 | Sesquiterpenes | (3R,7R,9R)-3,9-dihydroxygermacra-4(15),10(14),11(12)-triene | C15H24O2 | 236 | P. brachycarpa | [13] |
| 155 | Sesquiterpenes | 6β,14-epoxyeudesm-4(15)-en-1β-ol | C15H24O2 | 236 | P. brachycarpa | [13] |
| 156 | Sesquiterpenes | 6β-methoxyeudesm-4(15)-en-1β-ol | C16H28O2 | 252 | P. brachycarpa | [13] |
| 157 | Sesquiterpenes | (7R*)-opposit-4(15)-ene-1β,7-diol | C16H28O | 236 | P. brachycarpa | [13] |
| 158 | Sesquiterpenes | 7β-methoxy-4(14)-oppositen-1β-ol | C17H30O | 250 | P. brachycarpa | [13] |
| 159 | Sesquiterpenes | α-copaene-11-ol | C15H24O | 220 | P. corymbosa | [36] |
| 160 | Sesquiterpenes | α-ylangene | C15H24 | 204 | P. anisetum P. anisum | [48] [58] |
| 161 | Sesquiterpenes | α-copaene | C15H24 | 204 | P. aurea P. corymbosa P. peregrine P. junoniae P. anisum P. monoica P. thellungiana | [36] [44] [58] [15] [54] |
| 162 | Sesquiterpenes | trans-α-bergamotene | C15H24 | 204 | P. peregrine P. junoniae P. anisum | [36] [44] [59] |
| 163 | Sesquiterpenes | cis-α-bergamotene | C15H24 | 204 | P. peregrina | [36] |
| 164 | Sesquiterpenes | trans-β-bergamotene | C15H24 | 204 | P. peregrina | [36] |
| 165 | Sesquiterpenes | trans-α-bergamotol | C15H24O | 220 | P. corymbosa | [36] |
| 166 | Sesquiterpenes | α-zingiberene | C15H24 | 204 | P. corymbosa P. junoniae P. anisetum P. anisum P. enguezekensis P. khorasanica P. diversifolia | [36] [44] [48] [50] [51] [19] [53] |
| 167 | Sesquiterpenes | trans-α-bisabolene | C15H24 | 204 | P. corymbosa | [36] |
| 168 | Sesquiterpenes | β-bisabolene | C15H24 | 204 | P. corymbosa P. peregrina P. puberula P. junoniae P. aurea P. anisetum P. anisum P. enguezekensis P. khorasanica P. diversifolia P. thellungiana | [36] [44] [45] [48] [50] [51] [19] [53] [54] |
| 169 | Sesquiterpenes | β-sesquiphellandrene | C15H24 | 204 | P. peregrine P. junoniae P. diversifolia P. thellungiana | [36] [44] [53] [54] |
| 170 | Sesquiterpenes | α-bisabolol | C15H26O | 222 | P. aurea P. corymbosa P. peregrine P. junoniae P. thellungiana | [36] [44] [54] |
| 171 | Sesquiterpenes | β-bisabolol | C15H26O | 222 | P. aurea | [45] |
| 172 | Sesquiterpenes | β-bisabolenol | C15H24O | 220 | P. aurea | [36] |
| 173 | Sesquiterpenes | xanthorrhizol | C15H22O | 218 | P. junoniae | [44] |
| 174 | Sesquiterpenes | α-curcumene | C15H22 | 202 | P. junoniae P. anisum P. khorasanica P. thellungiana | [44] [57] [19] [54] |
| 175 | Sesquiterpenes | γ-curcumene | C15H24 | 202 | P. thellungiana | [54] |
| 176 | Sesquiterpenes | dehydro aromadendrene | C15H22 | 204 | P. monoica | [15] |
| 177 | Sesquiterpenes | aromadendrene | C15H24 | 204 | P. anisetum P. diversifolia | [48] [53] |
| 178 | Sesquiterpenes | spathulenol | C15H24O | 220 | P. corymbosa P. junoniae P. aurea P. anisetum P. thellungiana | [36] [44] [45] [48] [54] |
| 179 | Sesquiterpenes | isospathulenol | C15H24O | 220 | P. thellungiana | [54] |
| 180 | Sesquiterpenes | β-gurjunene | C15H24 | 204 | P. junoniae | [44] |
| 181 | Sesquiterpenes | bicyclogermacrene | C15H24 | 204 | P. aurea P. corymbosa P. peregrina P. flabellifolia | [36] [48] |
| 182 | Sesquiterpenes | α-guaiene | C15H24 | 204 | P. diversifolia | [54] |
| 183 | Sesquiterpenes | cis-β-guaiene | C15H24 | 204 | P. junoniae | [44] |
| 184 | Sesquiterpenes | trans-β-guaiene | C15H24 | 204 | P. junoniae | [44] |
| 185 | Sesquiterpenes | 4,6-guaiadiene | C15H22 | 202 | P. corymbosa P. peregrina | [36] |
| 186 | Sesquiterpenes | salvial-4(14)-en-1-one | C15H24O | 220 | P. thellungiana | [54] |
| 187 | Sesquiterpenes | clavukerin B | C12H16 | 160 | P. corymbosa | [36] |
| 188 | Sesquiterpenes | kessane | C15H26O | 222 | P. aurea | [36] |
| 189 | Sesquiterpenes | α-cedrene | C15H24 | 204 | P. monoica P. diversifolia | [15] [53] |
| 190 | Sesquiterpenes | 2-epi-α-funebrene | C15H24 | 204 | P. monoica | [15] |
| 191 | Sesquiterpenes | diepi-α-cedrene | C16H28 | 220 | P. anisum | [50] |
| 192 | Sesquiterpenes | daucene | C15H24 | 204 | P. monoica | [15] |
| 193 | Sesquiterpenes | α-himachalene | C15H24 | 204 | P. corymbosa P. anisum P. enguezekensis | [36] [50] [51] |
| 194 | Sesquiterpenes | β-himachalene | C15H24 | 204 | P. anisum | [50] |
| 195 | Sesquiterpenes | γ-himachalene | C15H24 | 204 | P. corymbosa P. anisetum P. anisum | [36] [48] [50] |
| 196 | Sesquiterpenes | himachalol | C15H26O | 222 | P. corymbosa P. peregrina P. aurea | [36] [45] |
| 197 | Sesquiterpenes | α-longipinene | C15H24 | 204 | P. anisum P. thellungiana | [58] [54] |
| 198 | Sesquiterpenes | guaioxide | C15H26O | 222 | P. aurea | [36] |
| 199 | Sesquiterpenes | humulene epoxide II | C15H24O | 220 | P. peregrina | [36] |
| 200 | Sesquiterpenes | epi-cubebol | C15H24O | 220 | P. corymbosa | [36] |
| 201 | Sesquiterpenes | bicycloelemene | C15H24 | 204 | P. aurea P. corymbosa | [36] |
| 202 | Sesquiterpenes | isofuranogermacrene | C15H20O | 204 | P. diversifolia | [53] |
| 203 | Sesquiterpenes | β-bourbonene | C15H24 | 204 | P. corymbosa P. junoniae P. anisum | [36] [44] [58] |
| 204 | Sesquiterpenes | dehydrocostus lactone | C15H18O2 | 230 | P. puberula | [36] |
| 205 | Sesquiterpenes | Pimpinelol | C15H20O5 | 280 | P. haussknechtii | [10] |
| 206 | Sesquiterpenes | trans-β-farnesene | C15H24 | 204 | P. peregrine P. junoniae P. khorasanica P. diversifolia | [36] [44] [19] [53] |
| 207 | Sesquiterpenes | cis-β-farnesene | C15H24 | 204 | P. aurea P. corymbosa P. peregrine P. thellungiana | [36] [54] |
| 208 | Sesquiterpenes | cis, cis-farnesol | C15H26O | 222 | P. junoniae | [44] |
| 209 | Sesquiterpenes | trans, trans-farnesol | C15H26O | 222 | P. junoniae | [44] |
| 210 | Sesquiterpenes | sinensal | C15H22O | 218 | P. peregrina | [36] |
| 211 | Sesquiterpenes | nerolidol | C15H26O | 222 | P. anisum P. diversifolia | [59] [53] |
| 212 | Sesquiterpenes | cis,trans-α-farnesene | C15H24 | 204 | P. thellungiana | [54] |
| 213 | Triterpenoids | ursolic acid | C30H48O3 | 456 | P. anisum | [56] |
| 214 | Triterpenoids | oleanolic acid | C30H48O3 | 456 | P. anisum | [56] |
| 215 | Triterpenoids | betulinic acid | C30H48O3 | 456 | P. anisum | [56] |
| 216 | Triterpenoids | lupeol | C30H50O | 426 | P. anisum | [56] |
| 217 | Triterpenoids | α-amyrin | C30H50O | 426 | P. anisum | [55] |
| 218 | Triterpenoids | β-amyrin | C30H50O | 426 | P. anisum | [55] |
| 219 | Triterpenoids | saikogenin F-3-O-{β-D- glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→4)-β-D-glucopyranosyl-(1→3)]-β-D-fucopyranoside} | C53H86O22 | 1075 | P. rhodantha | [17] |
| No. | Name | Formula | Mol. Wt. | Species | Reference |
|---|---|---|---|---|---|
| 220 | apigenin-7-O-glucoside | C21H20O10 | 432 | P. thellungiana | [60] |
| 221 | apigenin-7-O-β-D-butylglucuronide | C25H26O10 | 502 | P. thellungiana | [60] |
| 222 | apigenin-7-O-methylglucuronide | C20H22O11 | 460 | P. thellungiana | [61] |
| 223 | luteolin-7-O-methylglucuronide | C22H20O12 | 476 | P. thellungiana | [61] |
| 224 | apigenin-7-O-glucuronide | C21H18O11 | 446 | P. thellungiana | [62] |
| 225 | luteolin-7-O-glucuronide | C21H18O12 | 462 | P. thellungiana | [62] |
| 226 | schaftoside | C26H28O14 | 564 | P. thellungiana | [63] |
| 227 | quercetin-3-O-glucuronide | C21H18O13 | 478 | P. thellungiana | [64] |
| 228 | isovitexin | C21H20O10 | 432 | P. candolleana | [49] |
| 229 | quercetin-3-O-rhamnoside | C21H20O11 | 448 | P. brachycarpa | [12] |
| 230 | kaempferol-3-O-rhamnoside | C22H22O10 | 446 | P. cappadocica | [16] |
| 231 | quercetin-3-O-galactoside | C21H20O12 | 464 | P. cappadocica | [16] |
| 232 | kaempferol-3-O-(2″-O-glucopyranosyl)-galactoside | C27H30O16 | 610 | P. cappadocica | [16] |
| 233 | quercetin-3-O-glucoside | C21H20O12 | 464 | P. cappadocica | [16] |
| 234 | rhamnositrin-3-O-(2″-O-glucopyranosyl)-galactoside | C28H30O18 | 624 | P. cappadocica | [16] |
| 235 | quercetin-3-O-(2″-O-glucopyranosyl)-galactoside | C27H30O17 | 626 | P. cappadocica | [16] |
| 236 | kaempferol-3-O-(2″-O-β-D-glucopyranosyl-6″-O-caffeoyl)-β-D-galactopyranoside (erzurumin) | C36H36O19 | 772 | P. cappadocica | [16] |
| 237 | quercetin-3-O-(2″-O-β-D-glucopyranosyl-6″-O-caffeoyl)-β-D-galactopyranoside (ilicanin) | C36H36O20 | 788 | P. cappadocica | [16] |
| 238 | quercetin-3′-methylether-3-O-α-L-(2″,3″-di-O-trans-coumaroyl)-rhamnopyranoside | C40H34O15 | 754 | P. rhodantha | [17] |
| 239 | quercetin-3-O-α-L-(2″,3″-di-O-trans-coumaroyl)-rhamnopyranoside | C39H34O13 | 740 | P. rhodantha | [17] |
| 240 | luteolin-7-O-glucoside | C21H20O11 | 302 | P. anthriscoides | [65] |
| 241 | chrysoeriol-7-O-glucoside | C22H22O11 | 462 | P. anthriscoides | [65] |
| 242 | diosmetin-7-O-rutinoside | C28H32O15 | 608 | P. anthriscoides | [65] |
| 243 | chrysoeriol | C16H12O6 | 300 | P. anthriscoides | [65] |
| 244 | luteolin | C15H10O6 | 286 | P. candolleana | [49] |
| 245 | myricetin | C15H10O8 | 318 | P. anisum | [66] |
| 246 | quercetin | C15H10O7 | 302 | P. anisum | [66] |
| 247 | apigenin | C15H10O5 | 270 | P. anisum | [66] |
| 248 | kaempferol | C15H10O6 | 286 | P. anisum | [66] |
| 249 | chrysin | C15H10O4 | 254 | P. anisum | [66] |
| 250 | galangin | C15H10O5 | 270 | P. anisum | [66] |
| 251 | (βR)-β, 3, 4, 2′, 6′ -pentahydroxy-4′-O-β-D-glucosyldihydrochalcone (ziganin) | C21H24O12 | 468 | P. rhodantha | [17] |
| 252 | 3-hydroxy-p-phlorizin | C21H24O11 | 452 | P. rhodantha | [17] |
| 253 | naringenin | C15H12O5 | 272 | P. anisum | [66] |
| 254 | pinocembrin | C15H12O4 | 256 | P. anisum | [66] |
| 255 | 1-hydroxy-2, 3, 5-trimethoxyxathone | C16H14O6 | 302 | P. candolleana | [49] |
| No. | Name | Formula | Mol. Wt. | Species | Reference |
|---|---|---|---|---|---|
| 256 | bergapten | C12H8O4 | 216 | P. thellungiana | [67] |
| 257 | marmesin | C14H4O4 | 246 | P. thellungiana | [67] |
| 258 | scoparone | C11H10O4 | 206 | P. thellungiana | [67] |
| 259 | scopoletin | C10H8O4 | 192 | P. thellungiana | [67] |
| 260 | isofraxidin | C11H10O5 | 222 | P. thellungiana | [67] |
| 261 | visnagin | C13H10O4 | 230 | P. monoica | [69] |
| 262 | pimolin | C26H20O8 | 460 | P. monoica | [68] |
| 263 | visnagintrimer | C39H30O12 | 690 | P. monoica | [69] |
| 264 | visnagin tetramer | C52H40O16 | 920 | P. monoica | [69] |
| 265 | visnagin pentamer | C65H50O20 | 1150 | P. monoica | [69] |
| 266 | khellin | C14H12O5 | 260 | P. monoica | [69] |
| 267 | aegelinol | C14H14O4 | 246 | P. anthriscoides | [65] |
| 268 | psoralen | C11H6O3 | 186 | P. anthriscoides | [65] |
| 269 | imperatorin | C16H14O4 | 270 | P. anthriscoides | [65] |
| 270 | isoimperatorin | C16H14O4 | 270 | P. anthriscoides | [65] |
| 271 | 3-(1, 1-dimethylallyl) herniarin | C15H16O3 | 228 | P. anthriscoides | [65] |
| 272 | peucedanin | C15H14O4 | 258 | P. anthriscoides | [65] |
| 273 | xanthyletin | C14H12O3 | 228 | P. anthriscoides P. anthriscoides | [65] [65] |
| 274 | isopimpinellin | C13H10O5 | 246 | P. anisum | [70] |
| 275 | methoxsalen | C12H8O4 | 216 | P. anisum | [70] |
| 276 | umbelliprenin | C24H30O3 | 366 | P. anisum | [71] |
| 277 | 7-. prenyloxycoumarin | C14H14O3 | 230 | P. anisum | [72] |
| 278 | auraptene | C19H22O3 | 298 | P. anisum | [72] |
| 279 | umbelliferone | C9H6O3 | 162 | P. anisum | [72] |
| 280 | pimpinellin | C13H10O5 | 246 | P. anisum | [73] |
| No. | Name | Formula | Mol. Wt. | Species | Reference |
|---|---|---|---|---|---|
| 281 | campesterol | C28H48O | 400 | P. anisum | [74] |
| 282 | α-spinasterol | C29H48O | 412 | P. anisum | [74] |
| 283 | stigmasta-5,7,22-trien-3-ol | C29H46O | 410 | P. anisum | [74] |
| 284 | Δ7-avenasterol | C29H48O | 412 | P. anisum | [74] |
| 285 | Δ5-avenasterol | C29H48O | 412 | P. anisum | [74] |
| 286 | Δ7-stigmastenol | C29H50O | 414 | P. anisum | [55] |
| 287 | Δ5,23-stigmastadienol | C29H48O | 412 | P. anisum | [55] |
| 288 | Δ7-campesterol | C28H48O | 400 | P. anisum | [77] |
| 289 | sitostanol | C29H52O | 416 | P. anisum | [77] |
| 290 | cycloartenol | C30H50O | 426 | P. anisum | [78] |
| 291 | 24-methylenecycloartenol | C31H52O | 440 | P. anisum | [78] |
| 292 | b-sitosterol | C30H52O | 428 | P. thellungiana P. candolleana P. brachycarpa | [41] [75] [76] |
| 293 | g-sitosterol | C29H50O | 414 | P. thellungiana | [41] |
| 294 | stigmasta-5, 22-dien-3-olacetate | C31H50O2 | 454 | P. candolleana | [49] |
| 295 | daucosterol | C35H60O6 | 576 | P. candolleana | [49] |
| 296 | stigmasterol | C29H48O | 412 | P. candolleana | [75] |
| 297 | 24ζ-methyl-5R-lanosta-25-one | C30H52O | 428 | P. brachycarpa | [76] |
| 298 | pregnenolone | C21H32O2 | 316 | P. brachycarpa | [76] |
| No. | Name | Formula | Mol. Wt. | Species | Reference |
|---|---|---|---|---|---|
| 299 | oleic acid | C18H34O2 | 282 | P. thellungiana | [42] |
| 300 | palmitic acid | C16H32O2 | 256 | P. thellungiana P. aurea | [42] [36] |
| 301 | 2-methylbutanoic acid | C5H10O2 | 102 | P. thellungiana | [34] |
| 302 | shikimic acid | C7H10O5 | 174 | P. thellungiana | [79] |
| 303 | 3,4-dihydroxybenzoic acid | C7H6O4 | 154 | P. thellungiana P. aurea | [34] [36] |
| 304 | gallic acid | C7H6O5 | 170 | P. thellungiana P. aurea | [80] [36] |
| 305 | 3-O-trans-caffeoylquinic acid | C16H18O9 | 354 | P. thellungiana | [63] |
| 306 | 5-O-trans-caffeoylquinic acid | C16H18O9 | 354 | P. thellungiana P. brachycarpa | [63] [82] |
| 307 | 4-O-trans-caffeoylquinic acid | C16H18O9 | 354 | P. thellungiana P. brachycarpa | [63] [82] |
| 308 | 3,5-O-trans-dicaffeoylquinic acid | C25H24O12 | 516 | P. thellungiana P. brachycarpa | [63] [82] |
| 309 | 3,4-O-trans-dicaffeoylquinic acid | C25H24O12 | 516 | P. thellungiana P. brachycarpa | [63] [82] |
| 310 | 4,5-O-trans-dicaffeoylquinic acid | C25H24O12 | 516 | P. thellungiana P. brachycarpa | [63] [82] |
| 311 | 4-O-feruloylquinic acid | C17H20O9 | 368 | P. thellungiana | [81] |
| 312 | 1-O-feruloylquinic acid | C17H20O9 | 368 | P. thellungiana | [81] |
| 313 | 5-O-trans-coumaroylquinic acid | C16H18O8 | 338 | P. thellungiana P. brachycarpa | [81] [82] |
| 314 | 3-O-trans-caffeoyl-5-feruloylquinic acid | C26H26O12 | 530 | P. thellungiana | [81] |
| 315 | 4-O-trans-caffeoyl-5-feruloylquinic acid | C26H26O12 | 530 | P. thellungiana | [81] |
| 316 | 1-O-trans-caffeoyl-5-O-trans-coumaroylquinicacid. | C25H24O11 | 500 | P. brachycarpa | [82] |
| 317 | 1-O-trans-caffeoyl-5-O-7, 8-dihydro-7α-methoxycaffeoylquinic acid | C26H28O13 | 548 | P. brachycarpa | [82] |
| 318 | 1-O-7, 8-dihydro-7α-methoxycaffeoyl-5-O-trans-caffeoylquinic acid | C26H28O13 | 548 | P. brachycarpa | [82] |
| 319 | 1-O-trans-coumaroyl-5-O-cis-coumaroylquinic acid | C25H24O10 | 484 | P. brachycarpa | [82] |
| 320 | 1,5-di-O-cis-coumaroylquinic acid | C25H24O10 | 484 | P. brachycarpa | [82] |
| 321 | 1,5-O-trans-dicaffeoylquinic acid | C25H24O12 | 516 | P. brachycarpa | [82] |
| 322 | 5-O-cis-caffeoylquinic acid | C16H18O9 | 354 | P. brachycarpa | [82] |
| 323 | 4-O-trans-coumaroylquinic acid | C16H18O8 | 338 | P. brachycarpa | [82] |
| 324 | 5-O-cis-coumaroylquinic acid | C16H18O8 | 338 | P. brachycarpa | [82] |
| 325 | 5-hydroxyferulic acid | C10H10O5 | 210 | P. anisum | [66] |
| 326 | ferulic acid | C10H10O4 | 196 | P. anisum | [66] |
| 327 | sinapinic acid | C11H12O5 | 224 | P. anisum | [66] |
| 328 | caffeic acid | C9H8O4 | 180 | P. anisum | [66] |
| 329 | p-coumaric acid | C9H8O3 | 164 | P. anisum | [66] |
| 330 | trans-cinnamic acid | C9H8O2 | 148 | P. anisum | [66] |
| 331 | rosmarinic acid | C18H16O8 | 360 | P. anisum | [66] |
| 332 | 3-hydroxybenzoic acid | C7H6O3 | 138 | P. anisum | [66] |
| 333 | salicylic acid | C7H6O3 | 138 | P. anisum | [66] |
| 334 | 4-hydroxybenzoic acid | C7H6O3 | 138 | P. anisum | [66] |
| 335 | vanillic acid | C8H8O4 | 168 | P. anisum | [66] |
| 336 | syringic acid | C9H10O5 | 198 | P. anisum | [66] |
| 337 | 3-phenyllactic acid | C9H10O3 | 166 | P. anthriscoides | [65] |
| 338 | citric acid | C6H8O7 | 192 | P. anthriscoides | [65] |
| 339 | tetradecanoic acid | C14H28O2 | 228 | P. diversifolia | [53] |
| 340 | linoleic acid | C18H32O2 | 280 | P. diversifolia | [53] |
| 341 | stearic acid | C18H36O2 | 284 | P. diversifolia | [53] |
| 342 | dodecanoic acid | C12H24O2 | 200 | P. aurea | [36] |
| 343 | pentadecanoic acid | C15H30O2 | 242 | P. aurea | [36] |
| Pharmaceutical Activity | Part | Extract/ Compound | Experimental Model | Species | Reference |
|---|---|---|---|---|---|
| Antioxidant | Seed | Essential oil (in vivo) | Favism rats | P. anisum | [7] |
| Seed | Ethanol extract (in vivo) | GN induced nephrotoxicity in rats | P. anisum | [84] | |
| Seed | Essential oil | DPPH radical scavenging activity | P. anisum | [50] [85] [86] | |
| Seed | Nanostructuredessential oil | DPPH and ABTS scavenging activity | P. anisum | [87] | |
| Seed | Water extract | DPPH and ABTS scavenging activity; FRAP | P. anisum | [56] | |
| Seed | N-hexane extract | DPPH and ABTS scavenging activity; FRAP and β-carotene bleaching tests | P. anisum | [11] | |
| Seed | Polysaccharide | DPPH radical scavenging activity | P. anisum | [9] | |
| Seed | Fatty acids and phenolic compounds | DPPH and ABTS scavenging activity | P. anisum | [66] [78] | |
| Aerial part | Flavonoid glycosides (230–237) | DPPH radical scavenging activity; FRAP | P. cappadocica | [16] | |
| Aerial part | Flavonoid glycosides (238–239) | DPPH radical scavenging activity; FRAP | P. rhodantha | [17] | |
| Fruit | Essential oil | DPPH radical scavenging activity; β-carotene bleaching inhibition | P. enguezekensis | [51] | |
| Aerial part | Essential oil | DPPH and ABTS scavenging activity; phosphomolybdenum and FRAP | P. anthriscoides | [65] | |
| Aerial part | Ethyl acetate extract | DPPH scavenging activity (IC50 = 53.07µg/mL) | P. alpina | [88] | |
| Aerial part | Ethyl acetate extract | DPPH radical scavenging activity (IC50 = 74.9 µg/mL) | P. affinis | [89] | |
| Seed | 3% essential oil | DPPH radical scavenging activity (IC50 =6.81 µg/mL), β-carotene bleaching inhibition (IC50 = 206 µg/mL), FRAP (EC50 =35.20 µg/mL) | P. saxifraga | [46] [90] | |
| Antibacterial | Seed | Polysaccharide | E. coli, P. aeruginosa, B. cerus, and S. aureus (50 mg/mL) | P. anisum | [9] |
| Fruit | Essential oil | P. aeruginosa | P. anisum | [90] | |
| Seed | Essential oil | P. aeruginosa | P. anisum | [8] | |
| Seed | Essential oil | S. aureus and E. coli biofilms | P. anisum | [91] | |
| Seed | Essential oil | E. coli, P. aeruginosa, K. pneumonia, S. epidermidis, E. faecalis, S. pyogenes, B.cerus and S. aureus | P. anisum | [92] | |
| Seed | Essential oil | E amylovora with MIC of 31.25 μg/ml | P. anisum | [93] | |
| Seed | Oil-based hydrogel | C. albicans, C.glabrata and C. Parapsilosis. | P. anisum | [94] | |
| Aerial part | Essential oil | F.solani, S.brevicaulis, A spp., A. fumigatus and F. oxysporum (MIC = 50–490 μg/mL) | P. anisum | [95] | |
| Seed | Combination oil with terbinafine | T. rubrum and T. mentagrophytes | P. anisum | [96] | |
| Seed | Essential oils | T. rubrum | P. anisum | [97] | |
| Seed | Essential oil | A. niger, A. oryzae, M. pusillus and F. oxysporum | P. anisum | [98] | |
| Seed | Essential oil | C. perfringens with MIC of 10 μg/ml | P. anisum | [99] | |
| Seed | Essential oil | A. niger, A. oryzae, and A. ochraceus | P. anisum | [100] | |
| Seed | Essential oil | A. carbonarius | P. anisum | [101] | |
| Seed | Oil-based PLA films | L. monocytogenes and V. parahaemolyticus | P. anisum | [102] | |
| Seed | Nanostructured oil | Y. enterocolitica, B. cereus, E. coli, and L. monocytogenes | P. anisum | [103] [104] | |
| Seed | Nanostructured oil | 14 food infesting fungi: A. sydowii, A. repens, A. fumigatus, A. niger, A. candidus, A. luchuensis, F. oxysporum, C. herbarum, F. poae, M. sterilia, C. lunata, A. humicola and A. alternata at its MIC dose (0.08–0.5μL/mL) | P. anisum | [105] | |
| Seed | Nanostructured oil | S. aureus, E. coli, C. albicans, and A. niger | P. anisum | [87] | |
| Seed | Essential oil | S. aureus and E. coli | P. alpine | [88] | |
| Aerialpart | Essential oil | B.cereus, S. typhimurium, M. luteus | P. saxifraga | [46] | |
| Fruit | Essential oil | A. lwoffii, E. coli, K. pneumonia, B. cereus, C. perfringens, S. pneumonia, C. krusei and C. albicans (MIC: 5–75 mg/mL) | P. enguezekensis | [51] | |
| Aerial part | Essential oil | S. parasitica (MIC: 2 μg/mL, MFC: 4 μg/mL) | P. affinis | [52] | |
| Aerial part | Essential oil | S. aureus, L. monocytogenes, B. cereus, S. typhimurium, P. aeruginosa, E. cloacae, E. coli, A. fumigatus, A. ochraceus, A. niger, A. versicolor, T. viride, P. funiculosum, P. ochrochloron, and P. verrucosum | P. anthriscoides | [65] | |
| Anti-inflammatory activity | Seed | Combination oil with terbinafine | LPS stimulated neutrophils | P. anisum | [96] |
| Fruit | Anethole (40) | Croton oil-induced ear edema and carrageenan-induced pleurisy model | P. anisum | [106] | |
| Fruit | 0.3% essential oil | LPS-treated HBEpC and HTEpC cells | P. anisum | [107] | |
| Fruit | Anethole (40) | PM2.5 induced BEAS-2B and HepG2 | P. anisum | [108] | |
| Seed | BLAB tea | Oval bumin-induced allergic rhinitis model mice | P. anisum | [109] | |
| Seed | Polysaccharide | Model of foot swelling induced by carrageenan in mice | P. anisum | [9] | |
| Antitumor activity | Seed | Essential oil | Cytotoxicity against Hep G2 cells | P. anisum | [110] |
| Seed | Agnps containing aqueous extract | Cytotoxicity against human neonatal skin stromal cells and HT115 cells | P. anisum | [111] | |
| Seed | Agnps containing aqueous extract | Cytotoxicity against colorectal cancer cell lines | P. anisum | [112] | |
| Fruit | Pimpinelol (205) | Cytotoxicity against MCF-7 cell, IC50: 1.06 μM | P. haussknechtii | [10] | |
| Hypoglycemic activity | Seed | Aqueous extract | Inhibitory activity against α-amylase and α-glucosidase, (IC50=692.6±5.2 and 73.9±2.2 μg/mL) | P. anisum | [11] |
| Seed | Aqueous extract | Against pancreatic damage in STZ-induced diabetic rats | P. anisum | [113] | |
| Seed | Methanolic extract | Wound healing activity in STZ-induced diabetic rats | P. anisum | [114] | |
| Hypotensive activity | Herb | Ethyl acetate and ethanol extract | Inhibition effect on angiotensin-converting enzyme (0.5–10mg/mL) | P. brachycarpa | [115] |
| Seed | Aqueous extract | Calcium channel antagonist | P. anisum | [116] | |
| Insecticidal activity | Seed | Essential oil | Insecticidal effect against C. quinquefasciatus (LC50 = 25.4 μL/L) and S. littoralis (LD50 = 57.3 μg/larva) | P. anisum | [117] |
| Seed | Essential oil | Insecticidal effect against L. dispar | P. anisum | [118] | |
| Seed | Essential oil | Insecticidal effect against P.truncatus and T. granarium | P. anisum | [119] | |
| Seed | Essential oil | Insecticidal effect against L. decemlineata | P. anisum | [120] | |
| Seed | Essential oil | Insecticidal effect against B. aeneus | P. anisum | [121] | |
| Seed | Essential oil | Insecticidal effect against T. castaneum and P. interpunctella | P. anisum | [122] | |
| Seed | Essential oil | Insecticidal effect against M. incognita | P. anisum | [123] | |
| Seed | Essential oil | Insecticidal effect against aphids | P. anisum | [124] | |
| Seed | Essential oil | Insecticidal effect against Ipstypographu | P. anisum | [125] | |
| Seed | Essential oil | Insecticidal effect against P. citri | P. anisum | [126] | |
| Seed | Essential oil | Insecticidal effect against A. obtectus | P. anisum | [127] | |
| Seed | Essential oil | Acaricidal and reduce AchEin two-spotted spider mite | P. anisum | [86] | |
| Seed | Essential oil nano-emulsions | Insecticidal effect against S. oryzae and T. castaneum | P. anisum | [128] | |
| Seed | Essential oil nano-emulsions | Insecticidal effect against B. oleae | P. anisum | [129] | |
| Seed | Essential oil nano-emulsions | Insecticidal effect against M. persicae | P. anisum | [130] | |
| Seed | Essential oil nano-emulsions | Insecticidal effect against T. confusum | P. anisum | [131] | |
| Seed | Essential oil nano-emulsions | Insecticidal effect against T. castaneum | P. anisum | [132] [133] | |
| Seed | Essential oil | Insecticidal effect against Aedes aegypti | P. anisum | [134] | |
| Seed | Essential oil | Insecticidal effect against Musca domestica | P. anisum | [135] | |
| Seed | Essential oil | Insecticidal effect against Ixodes ricinus | P. anisum | [136] | |
| Seed | Essential oil | Insecticidal effect against Trypanosoma brucei | P. anisum | [46] | |
| Seed | Essential oil | Insecticidal effect against Culexquinquefasciatus | P. anisum | [137] [138] | |
| Seed | P-anisaldehyde (47) | Insecticidal effect against horn fly, H.irritansirritans | P. anisum | [139] | |
| Seed | P-anisaldehyde (47) | Insecticidal effect against lone star tick, A. americanum | P. anisum | [140] | |
| Seed | Essential oil-loaded zein nanocapsules | Insecticidal effect against mosquito | P. anisum | [141] | |
| Enzymes inhibitory activity | Aerial part | 95% ethanol extract | Inhibitory effects on CYP1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, and 3A4 in human liver microsomes | P. brachycarpa | [142] |
| Aerial part | Essential oil | Tyrosinase, α-amylase, α-glucosidase, AChE and BChE | P. anthriscoides | [65] | |
| Seed | Water extract | Inhibitory effects on GSTA1–1(IC50 = 3.40 ± 0.83μg/mL) | P. anisum | [143] | |
| Seed | Essential oil | Inhibitory effects on xanthine oxidase (IC50 = 2.37 ± 0.23 μg/mL) | P. anisum | [144] | |
| Seed | Ethanol extract | Selective modulators of RALDHs | P. anisum | [145] | |
| Root | Bergapten (256) isopimpinellin (274) methoxsalen (275) | Inhibitory effects on CYP 1A2 | P. anisum | [70] | |
| Seed | Phenolic extract | Inhibitory effects on AChE and BChE (IC50 = 0.07 and 0.34 μg/mL) | P. anisum | [56] [66] | |
| Anti depressant activity | Seed | 70% ethanol total extract (100 mg/kg) | Antidepressant and anxiolytic effects on Swiss Albino mice | P. anisum | [145] |
| Seed | Essential oil | Memory impairment, anxiety, and depression in scopolamine-induced rats | P. peregrina | [18] | |
| Seed | Essential oil | Inhibition of brain cerebral cortex and hippocampus inflammation | P. anisum | [146] | |
| Seed | Essential oil | Inhibition of brain cerebral cortex and hippocampus antioxidant effects | P. anisum | [147] | |
| Herb | Extract | Clinical treatment of depression in patients with IBS | P. anisum | [148] | |
| Uterine relaxant activity | Seed | 50%hidroalcoholic extract | Uterine contraction induced by oxytocin, Bay K8644, carbachol, or generated spontaneously | P. anisum | [140] |
| Wound healing effect | Seed | Polysaccharide | Reparation of laser burn wounds in mice | P. anisum | [9] |
| Migraine headache | Herb | Essential oil | Clinical treatment of migraine | P. anisum | [141] |
| Premenstrual syndrome | Herb | Extract | Clinical treatment of premenstrual syndrome | P. anisum | [142] |
| Skin whitening effect | Herb | Umbelliprenin (276) | Melan-a cells of mice | P. anisum | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Cao, Z.; Hassan, S.S.u.; Zhang, H.; Ishaq, M.; Yu, X.; Yan, S.; Xiao, X.; Jin, H.-Z. Emerging Biopharmaceuticals from Pimpinella Genus. Molecules 2023, 28, 1571. https://doi.org/10.3390/molecules28041571
Wu J, Cao Z, Hassan SSu, Zhang H, Ishaq M, Yu X, Yan S, Xiao X, Jin H-Z. Emerging Biopharmaceuticals from Pimpinella Genus. Molecules. 2023; 28(4):1571. https://doi.org/10.3390/molecules28041571
Chicago/Turabian StyleWu, Jiajia, Zhen Cao, Syed Shams ul Hassan, Haozhen Zhang, Muhammad Ishaq, Xu Yu, Shikai Yan, Xue Xiao, and Hui-Zi Jin. 2023. "Emerging Biopharmaceuticals from Pimpinella Genus" Molecules 28, no. 4: 1571. https://doi.org/10.3390/molecules28041571
APA StyleWu, J., Cao, Z., Hassan, S. S. u., Zhang, H., Ishaq, M., Yu, X., Yan, S., Xiao, X., & Jin, H.-Z. (2023). Emerging Biopharmaceuticals from Pimpinella Genus. Molecules, 28(4), 1571. https://doi.org/10.3390/molecules28041571







