Investigation of the Protective Effect for GcMAF by a Glycosidase Inhibitor and the Glycan Structure of Gc Protein
Abstract
1. Introduction
2. Results and Discussion
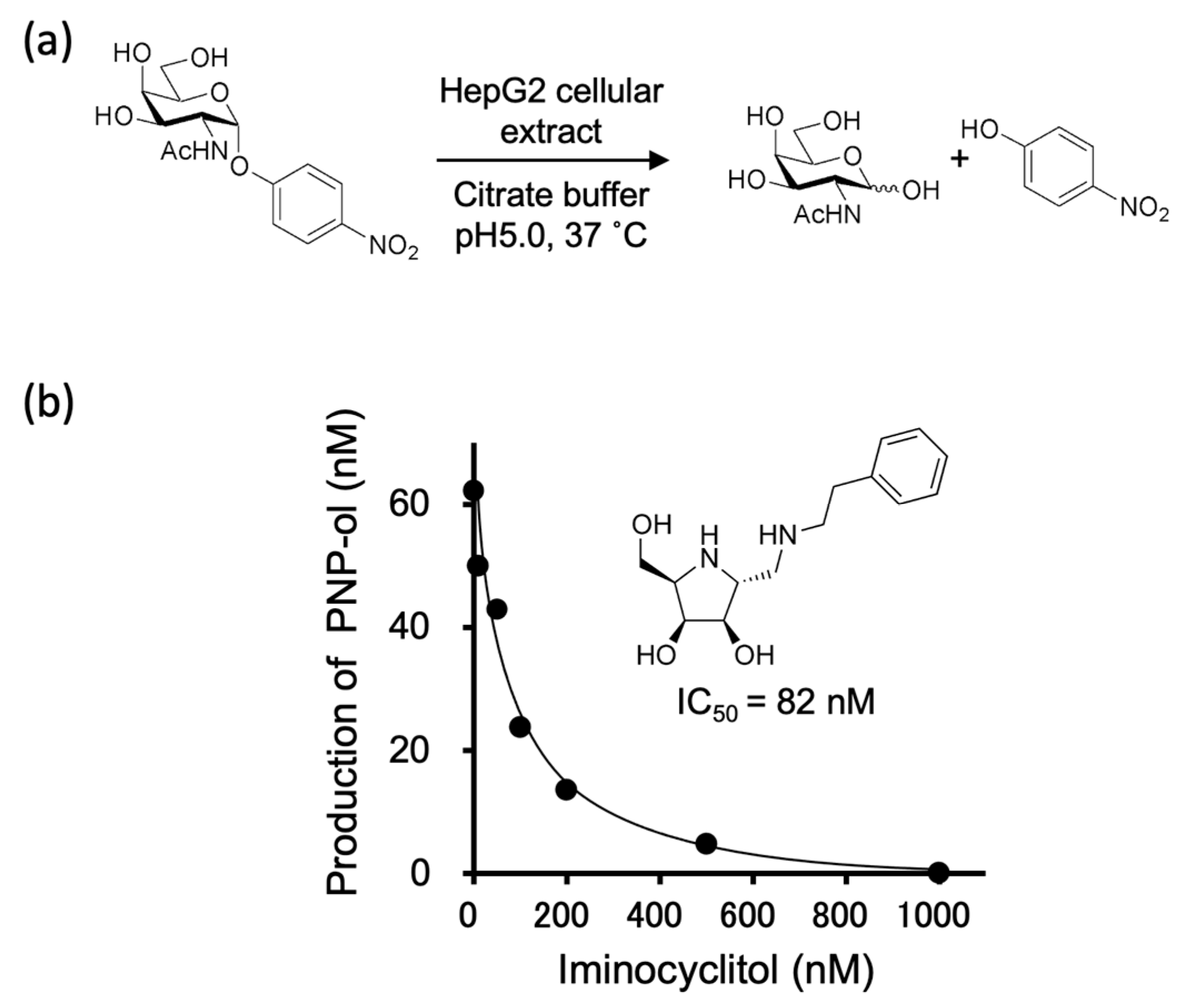
2.1. Glycosidase Activities of a Cellular Extract from HepG2 Cells and Their Inhibition by an Iminocyclitol
2.2. Analysis of O-Glycans in Gc Protein
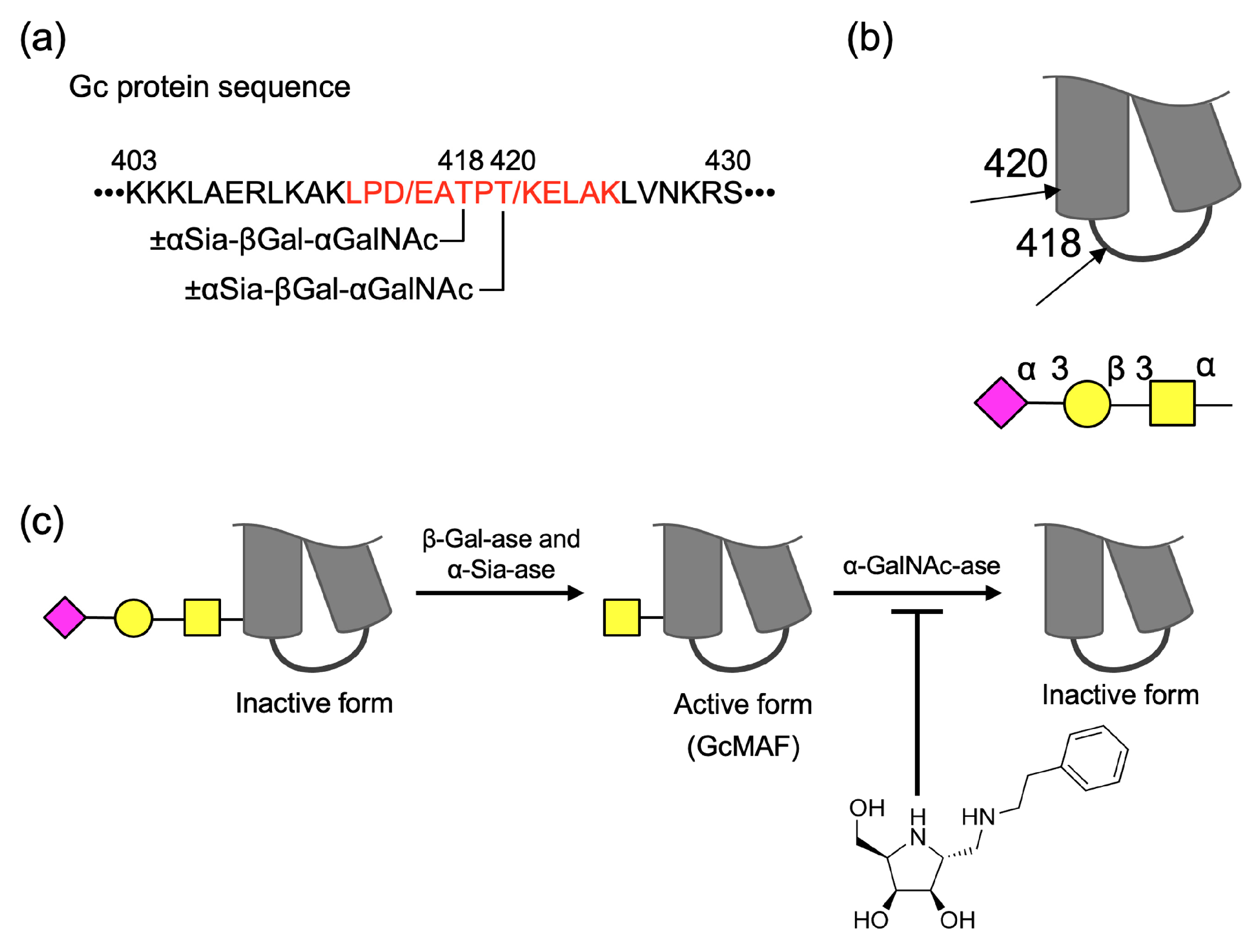
2.3. Protective Effect of Iminocyclitol for GcMAF
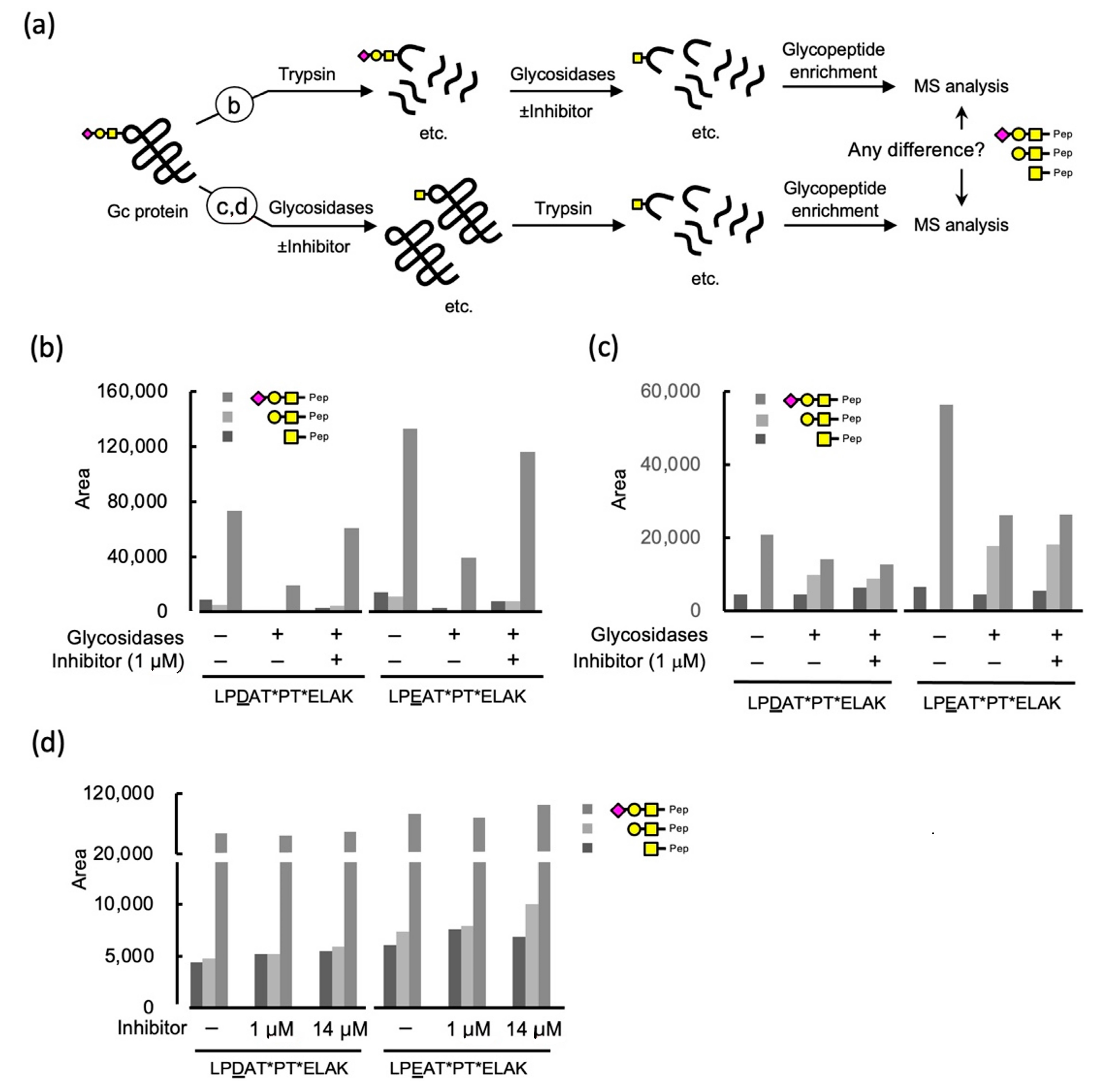
2.3.1. Glycosidase Treatment of the Tryptic Digests of Gc Protein and the Effect of an Iminocyclitol
2.3.2. Inhibition of Glycosidases by an Iminocyclitol Using Gc Protein as a Substrate
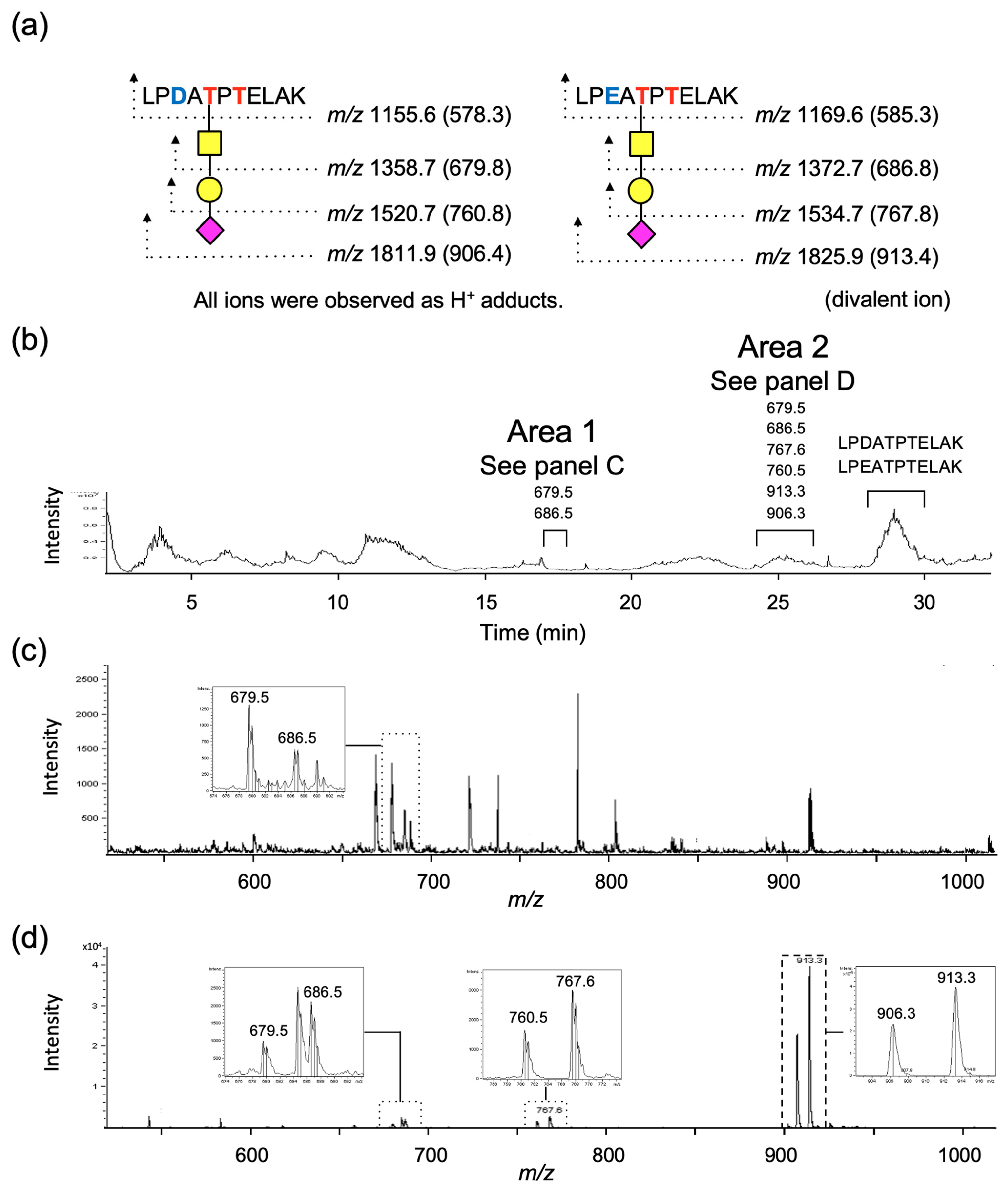
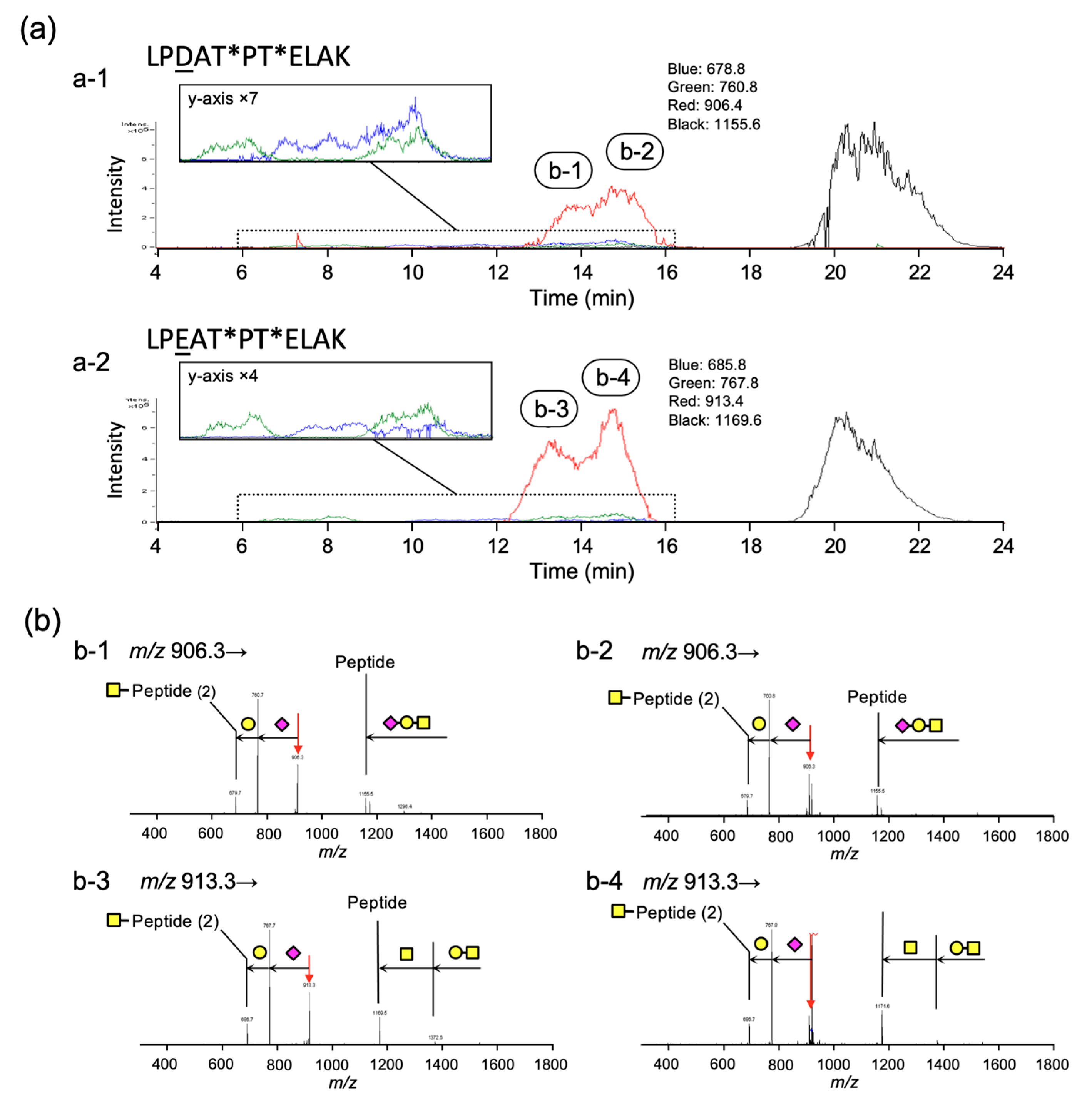
2.4. Analyses of Structures of O-Glycans and Their Site in Gc-Protein
3. Materials and Methods
3.1. Chemicals
3.2. LC-MS Analysis of Glycopeptides
3.3. Cell Culture and Preparation of Cellular Extract
3.4. Glycosidase Activity in the Cellular Extract from HepG2
3.5. Preparation of Glycopeptides from Gc Protein
3.6. The Inhibition Study of an Iminocyclitol against Digestion of Glycans by α-Sia-ase and HepG2 Cellular Extract Using Glycopeptides
3.7. Treatment of Gc Protein with Glycosidases and Evaluation of the Inhibitory Activity of Iminocyclitol
3.8. β- Elimination of O-Glycan from Glycopeptides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Viau, M.; Constans, J.; Debray, H.; Montreuil, J. Isolation and characterization of the O-glycan chain of the human vitamin-D binding protein. Biochem. Biophys. Res. Commun. 1983, 117, 324–331. [Google Scholar] [CrossRef] [PubMed]
- White, P.; Cooke, N. The multifunctional properties and characteristics of vitamin D-binding protein. Trends Endocrinol. Metab. 2000, 11, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, H.; Uto, Y.; Sasaki, H.; Okamura, N.; Murakami, A.; Kubo, S.; Kirt, K.; Hori, H. Gc protein (vitamin D-binding protein): Gc Genotyping and GcMAF precursor activity. Anticancer Res. 2005, 25, 3689–3696. [Google Scholar] [PubMed]
- Borges, C.R.; Jarvis, J.W.; Oran, P.E.; Nelson, R.W. Population studies of vitamin D binding protein microheterogeneity by mass spectrometry lead to characterization of its genotype-dependent O-glycosylation patterns. J. Prot. Res. 2008, 7, 4143–4153. [Google Scholar] [CrossRef]
- Ravnsborg, T.; Olsen, D.T.; Thysen, A.H.; Christiansen, M.; Houen, G.; Højrup, P. The glycosylation and characterization of the candidate Gc macrophage activating factor. Biochim. Biophys. Acta 2010, 1804, 909–917. [Google Scholar] [CrossRef]
- Albracht, S.P. Immunotherapy with GcMAF revised-A critical overview of the research of Nobuto Yamamoto. Cancer Treat. Res. Commun. 2022, 31, 100537. [Google Scholar] [CrossRef]
- Meier, U.; Gressner, O.; Lammert, F.; Gressner, A.M. Gc-Globulin: Roles in response to injury. Clin. Chem. 2006, 52, 1247–1253. [Google Scholar] [CrossRef]
- Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Behind the scenes of vitamin D binding protein: More than vitamin D binding. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 773–786. [Google Scholar] [CrossRef]
- Christiansen, M.; Jørgensen, C.S.; Laursen, I.; Hirschberg, D.; Højrup, P.; Houen, G. Protein chemical characterization of Gc globulin (vitamin D-binding protein) isoforms; Gc-1f, Gc-1s and Gc-2. Biochim. Biophys. Acta 2007, 1774, 481–492. [Google Scholar] [CrossRef]
- Yamamoto, N. Structural definition of a potent macrophage activating factor derived from vitamin D3-binding protein with adjuvant activity for antibody production. Mol. Immunol. 1996, 33, 1157–1164. [Google Scholar] [CrossRef]
- Yamamoto, N.; Naraparaju, V.R.; Urabe, M. Prognostic utility of serum alpha-N-acetylgalactosaminidase and immunosuppression resulted from deglycosylation of serum Gc protein in oral cancer patients. Cancer Res. 1997, 57, 295–299. [Google Scholar] [PubMed]
- Mohamad, S.B.; Nagasawa, H.; Uto, Y.; Hori, H. Tumor cell alpha-N-acetylgalactosaminidase activity and its involvement in GcMAF-related macrophage activation. Comp. Biochem. Physiol. Part A 2002, 132, 1–8. [Google Scholar] [CrossRef]
- Matsuura, T.; Uematsu, T.; Yamaoka, M.; Furusawa, K. Effect of salivary gland adenocarcinoma cell-derived α-N-acetylgalactosaminidase on the bioactivity of macrophage activating factor. Int. J. Oncol. 2004, 24, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Reddi, A.L.; Sankaranarayanan, K.; Arulraj, H.S.; Devaraj, N.; Devaraj, H. Serum α-N-acetylgalactosaminidase is associated with diagnosis/prognosis of patients with squamous cell carcinoma of the uterine cervix. Cancer Lett. 2000, 158, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Rehder, D.S.; Nelson, R.W.; Borges, C.R. Glycosylation status of vitamin D binding protein in cancer patients. Protein Sci. 2009, 18, 2036–2042. [Google Scholar] [CrossRef]
- Asano, N.; Nash, R.J.; Molyneux, R.J.; Fleet, G.W. Sugar-mimic glycosidase inhibitors: Natural occurrence, biological activity and prospects for therapeutic application. Tetrahedron Asymmetry 2000, 11, 1645–1680. [Google Scholar] [CrossRef]
- Watson, A.A.; Fleet, G.W.; Asano, N.; Molyneux, R.J.; Nash, R.J. Polyhydroxylated alkaloids-natural occurrence and therapeutic applications. Phytochemistry 2001, 56, 265–295. [Google Scholar] [CrossRef]
- Winchester, B.G. Iminosugars: From botanical curiosities to licensed drugs. Tetrahedron Asymmetry 2009, 20, 645–651. [Google Scholar] [CrossRef]
- Nash, R.J.; Kato, A.; Yu, C.Y.; Fleet, G.W. Iminosugars as therapeutic agents: Recent advances and promising trends. Future Med. Chem. 2011, 3, 1513–1521. [Google Scholar] [CrossRef]
- Fleet, G.W.; Nicholas, S.J.; Smith, P.W.; Evans, S.V.; Fellows, L.E.; Nash, R.J. Potent competitive inhibition of α-galactosidase and α-glucosidase activity by 1,4-dideoxy-1,4-iminopentitols: Syntheses of 1,4-dideoxy-1,4-imino-d-lyxitol and of both enantiomers of 1,4-dideoxy-1,4-iminoarabinitol. Tetrahedron Lett. 1985, 26, 3127–3130. [Google Scholar] [CrossRef]
- Kajimoto, T.; Liu, K.K.C.; Pederson, R.L.; Zhong, Z.; Ichikawa, Y.; Porco, J.A., Jr.; Wong, C.H. Enzyme-catalyzed aldol condensation for asymmetric synthesis of azasugars: Synthesis, evaluation, and modeling of glycosidase inhibitors. J. Am. Chem. Soc. 1991, 113, 6187–6196. [Google Scholar] [CrossRef]
- Winchester, B.; Al Daher, S.; Carpenter, N.C.; Cenci Di Bello, I.; Choi, S.S.; Fairbanks, A.J.; Fleet, G.W. The structural basis of the inhibition of human α-mannosidases by azafuranose analogues of mannose. Biochem. J. 1993, 290, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Adachi, I.; Miyauchi, M.; Ikeda, K.; Komae, T.; Kizu, H.; Kameda, Y.; Watson, A.A.; Nash, R.J.; Wormald, M.R.; et al. Polyhydroxylated pyrrolidine and pyrrolizidine alkaloids from Hyacinthoides non-scripta and Scilla campanulata. Carbohydr. Res. 1999, 316, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Takebayashi, M.; Hiranuma, S.; Kanie, Y.; Kajimoto, T.; Kanie, O.; Wong, C.H. A versatile synthetic strategy for the preparation and discovery of new iminocyclitols as inhibitors of glycosidases. J. Org. Chem. 1999, 64, 5280–5291. [Google Scholar] [CrossRef]
- Saotome, C.; Kanie, Y.; Kanie, O.; Wong, C.H. Synthesis and enzymatic evaluation of five-membered iminocyclitols and pseudodisaccharide. Bioorg. Med. Chem. 2000, 8, 2249–2261. [Google Scholar] [CrossRef]
- Saotome, C.; Wong, C.H.; Kanie, O. Combinatorial library of five-membered iminocyclitol and the inhibitory activities against glyco-enzymes. Chem. Biol. 2001, 8, 1061–1070. [Google Scholar] [CrossRef]
- Suzuki, K.; Nakahara, T.; Kanie, O. 3,4-Dihydroxypyrrolidine as glycosidase inhibitor. Curr. Top. Med. Chem. 2009, 9, 34–57. [Google Scholar] [CrossRef]
- Packer, N.H.; Lawson, M.A.; Jardine, D.R.; Redmond, J.W. A general approach to desalting oligosaccharides released from glycoproteins. Glycoconj. J. 1998, 15, 737–747. [Google Scholar] [CrossRef]
- An, H.J.; Peavy, T.R.; Hedrick, J.L.; Lebrilla, C.B. Determination of N-glycosylation sites and site heterogeneity in glycoproteins. Anal. Chem. 2003, 75, 5628–5637. [Google Scholar] [CrossRef]
- Nwosu, C.C.; Seipert, R.R.; Strum, J.S.; Hua, S.S.; An, H.J.; Zivkovic, A.M.; German, B.J.; Lebrilla, C.B. Simultaneous and extensive site-specific N-and O-glycosylation analysis in protein mixtures. J. Proteome Res. 2011, 10, 2612–2624. [Google Scholar] [CrossRef]
- Ohkuma, S.; Poole, B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Nat. Acad. Sci. USA 1978, 75, 3327–3331. [Google Scholar] [CrossRef] [PubMed]
- Chokhawala, H.A.; Yu, H.; Chen, X. High throughput substrate specificity studies of sialidases by using chemoenzymatically synthesized sialoside libraries. ChemBioChem 2007, 8, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Ena, J.M.; Esteban, C.; Perez, M.D.; Uriel, J.; Calvo, M. Fatty acids bound to vitamin D-binding protein (DBP) from human and bovine sera. Biochem. Int. 1989, 19, 1–7. [Google Scholar]
- Yamamoto, N.; Naraparaju, V.R. Vitamin D3-binding protein as a precursor for macrophage activating factor in the inflammation-primed macrophage activation cascade in rats. Cell. Immunol. 1996, 170, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Kanie, Y.; Kanie, O. Addressing the glycan complexity by using mass spectrometry: In the pursuit of decoding glycologic. Biochem. Compd. 2017, 5, 3. [Google Scholar] [CrossRef]
- Takeda, T.; Sugiura, Y.; Ogihara, Y.; Shibata, S. Synthesis of N-(l-glutam-5-oyl)-α-D-glucopyranosylamine and O-α-D-glucopyranosyl-(1→6)-O-β-D-glucopyranosyl-(1→6)-N-(l-glutam-5-oyl)-α-D-glucopyranosylamine. Carbohydr. Res. 1982, 105, 271–275. [Google Scholar] [CrossRef]
- Dorman, D.E.; Bovey, F.A. Carbon-13 magnetic resonance spectroscopy. Spectrum of proline in oligopeptides. J. Org. Chem. 1973, 38, 2379–2383. [Google Scholar] [CrossRef]
- Thomas, W.A.; Williams, M.K. 13C nuclear magnetic resonance spectroscopy and cis/trans isomerism in dipeptides containing proline. J. Chem. Soc. Chem. Commun. 1972, 994. [Google Scholar] [CrossRef]
- Zheng, Y.; Guo, Z.; Cai, Z. Combination of β-elimination and liquid chromatography/quadrupole time-of-flight mass spectrometry for the determination of O-glycosylation sites. Talanta 2009, 78, 358–363. [Google Scholar] [CrossRef]

| pH | |||
|---|---|---|---|
| 5.0 | 6.0 | 6.9 | |
| α-GalNAc-ase | 1.07 | 0.21 | 0.31 |
| β-Gal-ase | 2.00 | 0.25 | 0.36 |
| α-Sia-ase | <0.01 | <0.01 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanie, Y.; Maegawa, Y.; Wei, Y.; Kanie, O. Investigation of the Protective Effect for GcMAF by a Glycosidase Inhibitor and the Glycan Structure of Gc Protein. Molecules 2023, 28, 1570. https://doi.org/10.3390/molecules28041570
Kanie Y, Maegawa Y, Wei Y, Kanie O. Investigation of the Protective Effect for GcMAF by a Glycosidase Inhibitor and the Glycan Structure of Gc Protein. Molecules. 2023; 28(4):1570. https://doi.org/10.3390/molecules28041570
Chicago/Turabian StyleKanie, Yoshimi, Yuya Maegawa, Yi Wei, and Osamu Kanie. 2023. "Investigation of the Protective Effect for GcMAF by a Glycosidase Inhibitor and the Glycan Structure of Gc Protein" Molecules 28, no. 4: 1570. https://doi.org/10.3390/molecules28041570
APA StyleKanie, Y., Maegawa, Y., Wei, Y., & Kanie, O. (2023). Investigation of the Protective Effect for GcMAF by a Glycosidase Inhibitor and the Glycan Structure of Gc Protein. Molecules, 28(4), 1570. https://doi.org/10.3390/molecules28041570





