2-Phenylpyridine Derivatives: Synthesis and Insecticidal Activity against Mythimna separata, Aphis craccivora, and Tetranychus cinnabarinus
Abstract
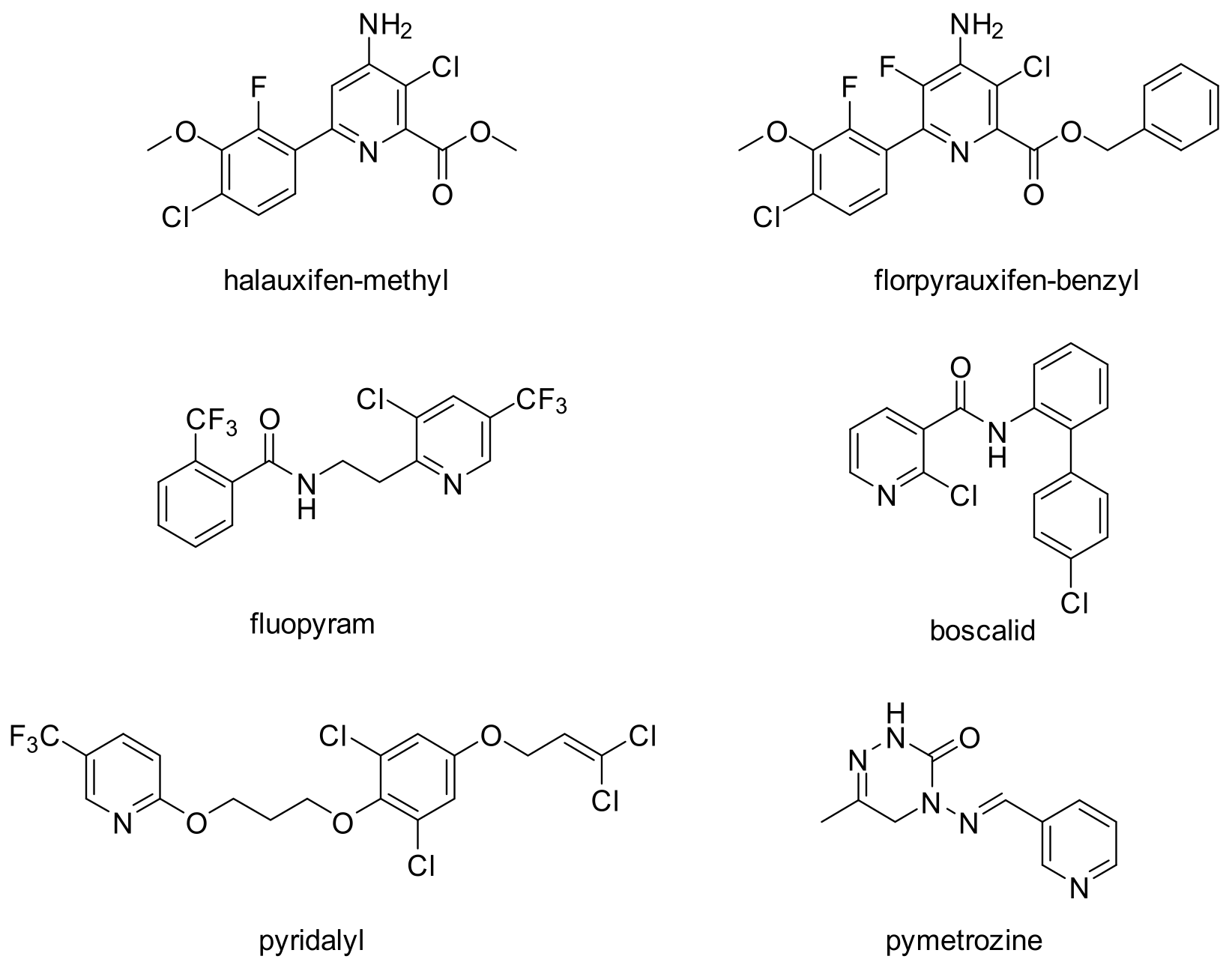
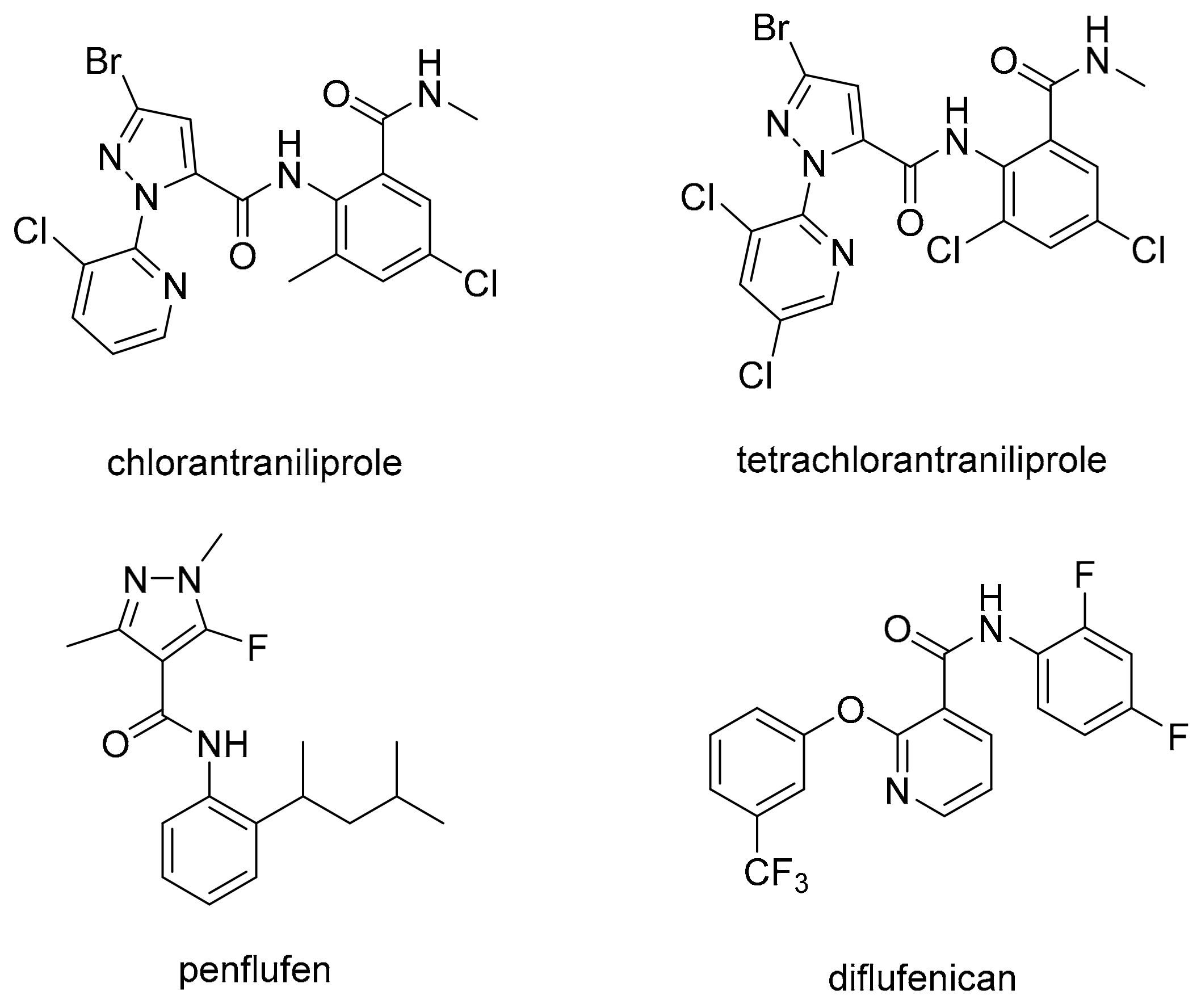
:1. Introduction
2. Results and Discussion
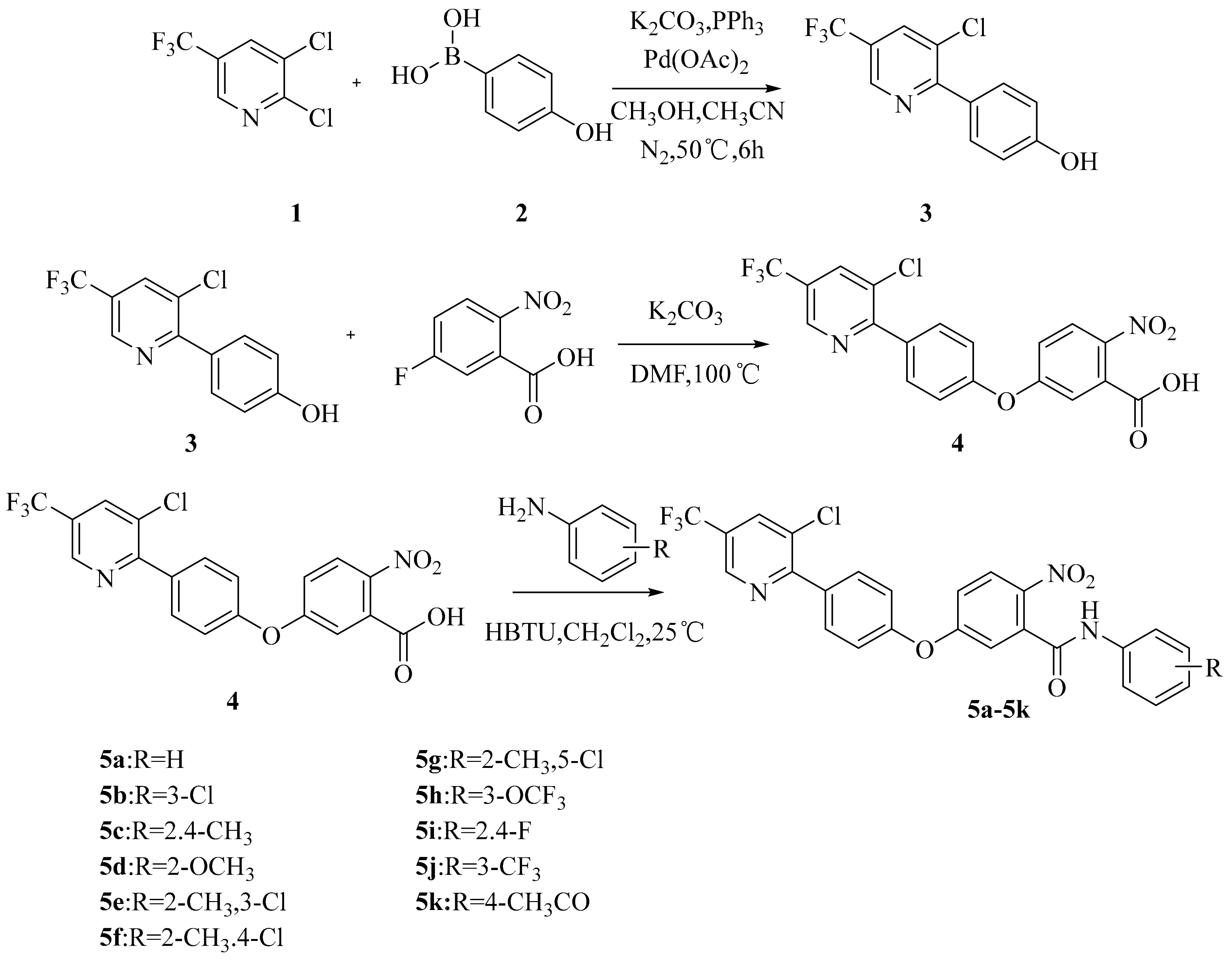
2.1. Chemistry
2.2. Greenhouse Insecticidal Activity Assays
3. Materials and Methods
3.1. Instrumentation
3.2. Synthesis
3.2.1. General Approach to the Synthesis of 3
3.2.2. General Approach to the Synthesis of 4
3.2.3. General Approach to the Synthesis of 5a–5k
3.3. Insecticidal Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Q.-H.; Chen, L.-Y.; Li, L.-J.; Li, H.-Y.; Zhang, L.-Z.; Zhao, N.-X.; Xu, Y.-G. Preparation Method of Fluorochloropyridine Ester. CN Patent CN 113603637, 5 November 2021. [Google Scholar]
- Jason, S.K.; David, J.C.; Abraham, D.S.; Megan, E.D.; Brian, M.; Ronald, B.L. Method of Preparing Benzyl 4-amino-3-chloro-5-fluoro-6-(4-chloro-2-fluoro-3-methoxyphenyl)picolinate. U.S. Patent US 20180162814, 14 June 2018. [Google Scholar]
- Yoshinobu, J.; Akihiro, T. Herbicidal Agent Composition and Weed Control Method. U.S. Patent US 20220322666, 4 March 2021. [Google Scholar]
- Mansfield, A.J.; Cooke, T.; Thomas, P.S.; Coqueron, P.Y.; Vors, J.P.; Briggs, G.G.; Lachaise, H.; Rieck, H.; Desbordes, P.; Grosjean, M.C. Novel 2-pyridylethylbebzamide Derivative. WO Patent WO 2004026088, 26 February 2004. [Google Scholar]
- Bristow, J.T. Process for Preparing Boscalid. WO Patent WO 2018024145, 7 February 2018. [Google Scholar]
- Yang, C.-H.; Geng, L.-W.; Zhou, D.-F.; Peng, L.; Zhang, H.; Cui, D.-L.; Li, Z.-N.; Wang, L.-Q.; Zang, S.-G.; Zhang, Z.-Y. N-(2-substituted phenyl)-N-methoxy carbamates and their preparation and use thereof. EP Patent EP 1845086, 10 August 2006. [Google Scholar]
- Yang, G.-F.; Li, J. Preparation of Dichloroallyl ether Compound and Their Application as the Insecticide Agents. CN Patent CN 104974083, 14 October 2015. [Google Scholar]
- Ke, S.-Y.; Yang, Z.-W.; Zhang, Z.-G.; Wang, K.-M.; Liang, Y. Preparation of Bisamide Structure-Containing Heterocyclic Imine Derivatives Useful as Pesticides. CN Patent CN 105622534, 1 June 2016. [Google Scholar]
- Jeschke, P.; Velten, R.; Schenke, T.; Schallner, O.; Beck, M.; Pontzen, R.; Malsam, O.; Reckmann, U.; Nauen, R.; Gorgens, U. Preparation of 4-[(pyridin-3-ylmethyl)amino]-5H-furan-2-ones as Insecticides. DE Patent DE 102006015467, 4 October 2007. [Google Scholar]
- Robert, H.; Christine, G.; Robert, S. N-phenyl-N’-(pyridinyl-N-oxide)urea Plant Regulators. U.S. Patent US 4787931, 29 November 1988. [Google Scholar]
- Yang, S.-X.; Chen, R.-Y.; Chen, J.-L. Preparation of 3-(2-pyridyl) Propanol. CN Patent CN 85102587, 10 February 1986. [Google Scholar]
- Cai, Z.-F.; Cao, Y.-Y.; Du, X.-H. Synthesis of Novel a-Trifluoroanisole Derivatives Containing Phenylpyridine Moieties with Herbicidal Activity. Int. J. Mol. Sci. 2022, 23, 11083. [Google Scholar] [CrossRef]
- Cao, Y.-Y.; Mao, D.-J.; Wang, W.-W.; Du, X.-H. Kresoxim-methyl derivatives: Synthesis and herbicidal activities of (pyridinylphenoxymethylene)phenyl methoxyiminoacetates. J. Agric. Food Chem. 2017, 65, 6114–6121. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-Y.; Cai, Z.-F.; Zhang, W.-L.; Du, X.-H. Synthesis and herbicidal activity of novel β-methoxyacrylate derivatives containing a substituted phenylpyridine moiety. Chem. Res. Chin. Univ. 2019, 35, 1008–1011. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.-T.; Du, X.-H. Design, synthesis, and fungicidal activity of novel N-substituted piperazine-containing phenylpyridines against cucumber downy mildew. Pest Manag. Sci. 2022, 78, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Xie, Y.; Song, Y.-Q.; Xu, Y.; Li, K.-K.; Guan, A.-Y.; Yang, J.-C.; Yang, F. Ayrl Pyridine(pyrimidine) Compound and Use Thereof. WO Patent WO 2015032280, 18 March 2015. [Google Scholar]
- Jeanguenat, A.; Emery, D.; Hall, R.G.; Iosub, V.A.; Lechapelain, C.; Gagnepain, J.D.H.; Kilaru, J.P.; Phadte, M.; Pitterna, T. Pesticidally Active Azole-Amide Compounds. WO Patent WO 2021122645, 24 June 2021. [Google Scholar]
- Lahm, G.P.; Mccann, S.F.; Patel, K.M.; Selby, T.P.; Stevenson, T.M. Method for Controlling Particular Inset Pests by Applying Anthranilamide Compounds. WO Patent WO 03015518, 27 February 2003. [Google Scholar]
- Li, B.; Yang, H.-B.; Wang, J.-F.; Yu, H.-B.; Zhang, H.; Li, Z.-N.; Song, Q. 1-Substituted Pyridyl-Pyrazolyl Amide Compousnds and Uses Thereof. US Patent US 8492409, 31 December 2008. [Google Scholar]
- Elbe, H.L.; Rieck, H.; Dunkel, R.; Qin, Z.-O.; Mauler-Machnik, A.; Wachendorff-Neumann, U.; Kuck, K.H. Pyrazolylcarboxanilides as Fungicides. WO Patent WO 0300149, 6 February 2003. [Google Scholar]
- Munro, D.; Patel, B. Preparation of Benzoic Acid-2′,4′-difluoroanilide Herbicidal Compounds. EP Patent EP 0467473, 22 January 1992. [Google Scholar]
- Hao, S.-L.; Cai, Z.-F.; Cao, Y.-Y.; Du, X.-H. Design, synthesis, and acaricidal activity of phenyl methoxyacrylates containing 2-alkenylthiopyrimidine. Molecules 2020, 25, 3379–3390. [Google Scholar] [PubMed]
- Panchal, D.M.; Desai, J.K. Process for preparation of insecticidal anthranilamides. WO Patent WO 2022149098, 15 July 2022. [Google Scholar]
- Zhao, Y.; Xiong, L.-X.; Xu, L.-P.; Wang, H.-X.; Xu, H.; Li, H.-B.; Tong, J.; Li, Z.-M. Synthesis, crystal structure and biological activity of a novel anthranilic diamide insecticide containing allyl ether. Res. Chem. Intermed. 2013, 39, 3071–3088. [Google Scholar] [CrossRef]
- Jeffrey, T.M.; Yong-Jae, K.; Mike, L.; Chen, X.-Q.; Jeff, D.; Malgorzata, W.; Yu, M.; Fu, J.-S.; Chen, X.; Zhang, A.; et al. Discovery of a new class of ghrelin receptor antagonists. Bioorg. Med. Chem. Lett. 2012, 22, 2046–2051. [Google Scholar]
- Wang, J.-K.; Liu, S.-J.; Lu, Y.-Q. Method for Synthesizing Flubendiamide. CN Patent CN 1094855, 19 March 2019. [Google Scholar]
- Ying, J.-W.; Luo, H.; Song, Y.-Q.; Li, B.; Yang, H.-B. Synthesis and insecticidal activity of cyclaniliprole. Mod. Agrochem. 2016, 15, 28–31. [Google Scholar]
- Li, R.; Cheng, S.-H.; Liang, P.-Z.; Chen, Z.-B.; Zhang, Y.-J.; Liang, P.; Zhang, L.; Guo, X.-W. Status of the Resistance of Aphis gossypii Glover, 1877 (Hemiptera: Aphididae) to Afidopyropen Originating from Microbial Secondary Metabolites in China. Toxins 2022, 14, 750. [Google Scholar] [CrossRef] [PubMed]
- Moores, G.D.; Gao, X.W.; Denholm, I.; Devonshire, A.L. Characterisation of insensitive acetylcholinesterase in insecticide-resistant cotton aphids, Aphis gossypii glover (Homoptera: Aphididae). Pestic. Biochem. Physiol. 1996, 56, 102–110. [Google Scholar] [CrossRef]
- Chen, S.-L.; Zhang, Y.; Liu, Y.-X.; Wang, Q.-M. Highly Efficient Synthesis and Acaricidal and Insecticidal Activities of Novel Oxazolines with N-Heterocyclic Substituents. J. Agric. Food Chem. 2021, 69, 3601–3606. [Google Scholar] [CrossRef] [PubMed]

| Compound | Chemical Structure | Dosage/ (mg/L) | Pest a | ||
|---|---|---|---|---|---|
| R | MS | AC | TC | ||
| 5a | H | 500 | 80 | 0 | 0 |
| 5b | 3-Cl | 500 | 100 | 70 | 0 |
| 5c | 2.4-CH3 | 500 | 0 | 0 | 0 |
| 5d | 2-OCH3 | 500 | 100 | 0 | 0 |
| 5e | 2-CH3,3-Cl | 500 | 70 | 0 | 0 |
| 5f | 2-CH3,4-Cl | 500 | 90 | 0 | 0 |
| 5g | 2-CH3,5-Cl | 500 | 100 | 0 | 0 |
| 5h | 3-OCF3 | 500 | 100 | 0 | 0 |
| 5i | 2.4-F | 500 | 80 | 0 | 0 |
| 5j | 3-CF3 | 500 | 100 | 0 | 0 |
| 5k | 4-CH3CO | 500 | 90 | 0 | 0 |
| chlorantraniliprole | / | 500 | 100 | 100 | 100 |
| CK | / | / | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Chen, J.; Du, X. 2-Phenylpyridine Derivatives: Synthesis and Insecticidal Activity against Mythimna separata, Aphis craccivora, and Tetranychus cinnabarinus. Molecules 2023, 28, 1567. https://doi.org/10.3390/molecules28041567
Zhang W, Chen J, Du X. 2-Phenylpyridine Derivatives: Synthesis and Insecticidal Activity against Mythimna separata, Aphis craccivora, and Tetranychus cinnabarinus. Molecules. 2023; 28(4):1567. https://doi.org/10.3390/molecules28041567
Chicago/Turabian StyleZhang, Wenliang, Jingjing Chen, and Xiaohua Du. 2023. "2-Phenylpyridine Derivatives: Synthesis and Insecticidal Activity against Mythimna separata, Aphis craccivora, and Tetranychus cinnabarinus" Molecules 28, no. 4: 1567. https://doi.org/10.3390/molecules28041567
APA StyleZhang, W., Chen, J., & Du, X. (2023). 2-Phenylpyridine Derivatives: Synthesis and Insecticidal Activity against Mythimna separata, Aphis craccivora, and Tetranychus cinnabarinus. Molecules, 28(4), 1567. https://doi.org/10.3390/molecules28041567






