Development of Solid Lipid Nanoparticles as Dry Powder: Characterization and Formulation Considerations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influence of Spray-Drying Parameters on Yield Value
Characterization of Spray-Dried Nanoparticles
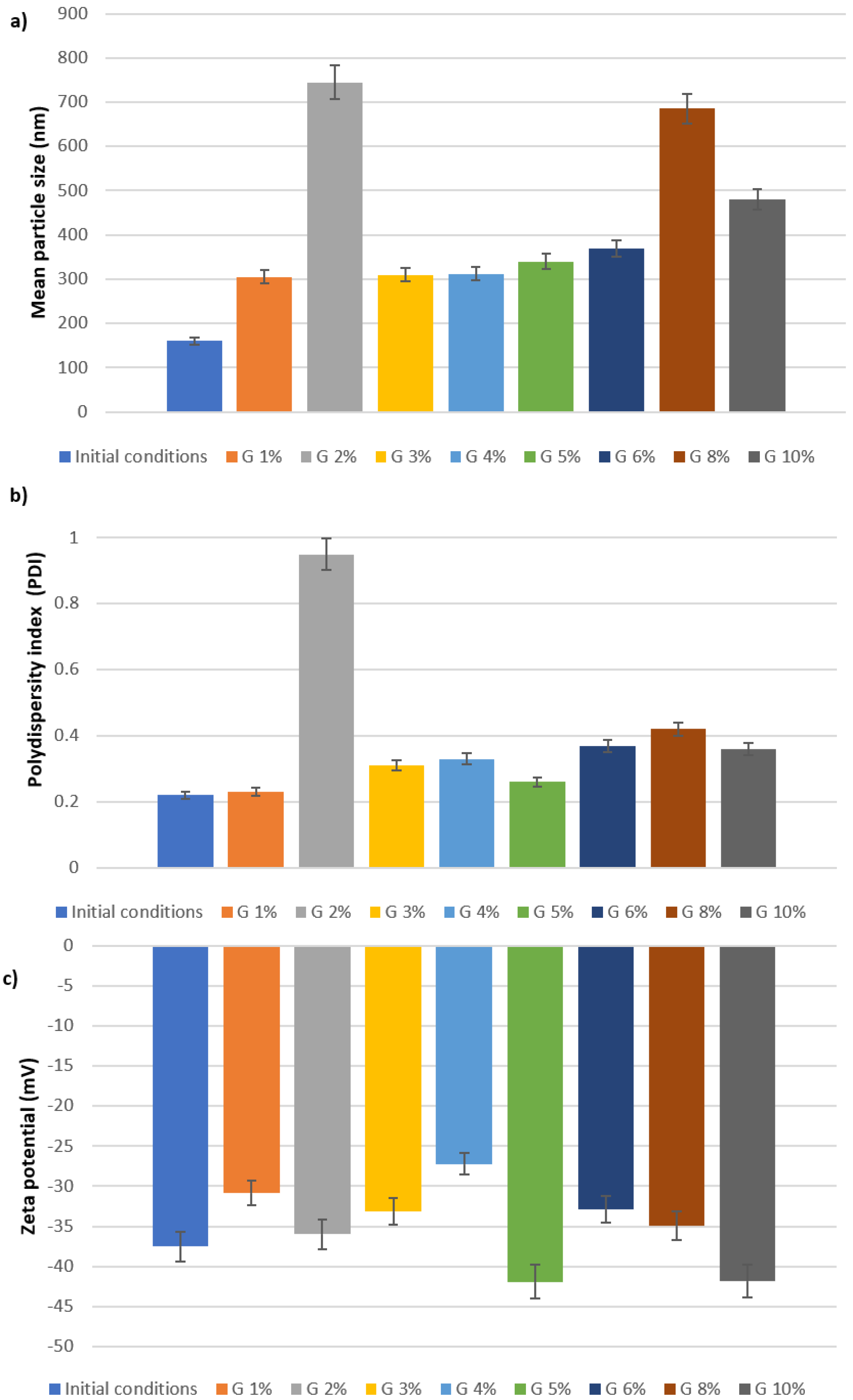
2.2. Characterization of Lyophilized Nanoparticles
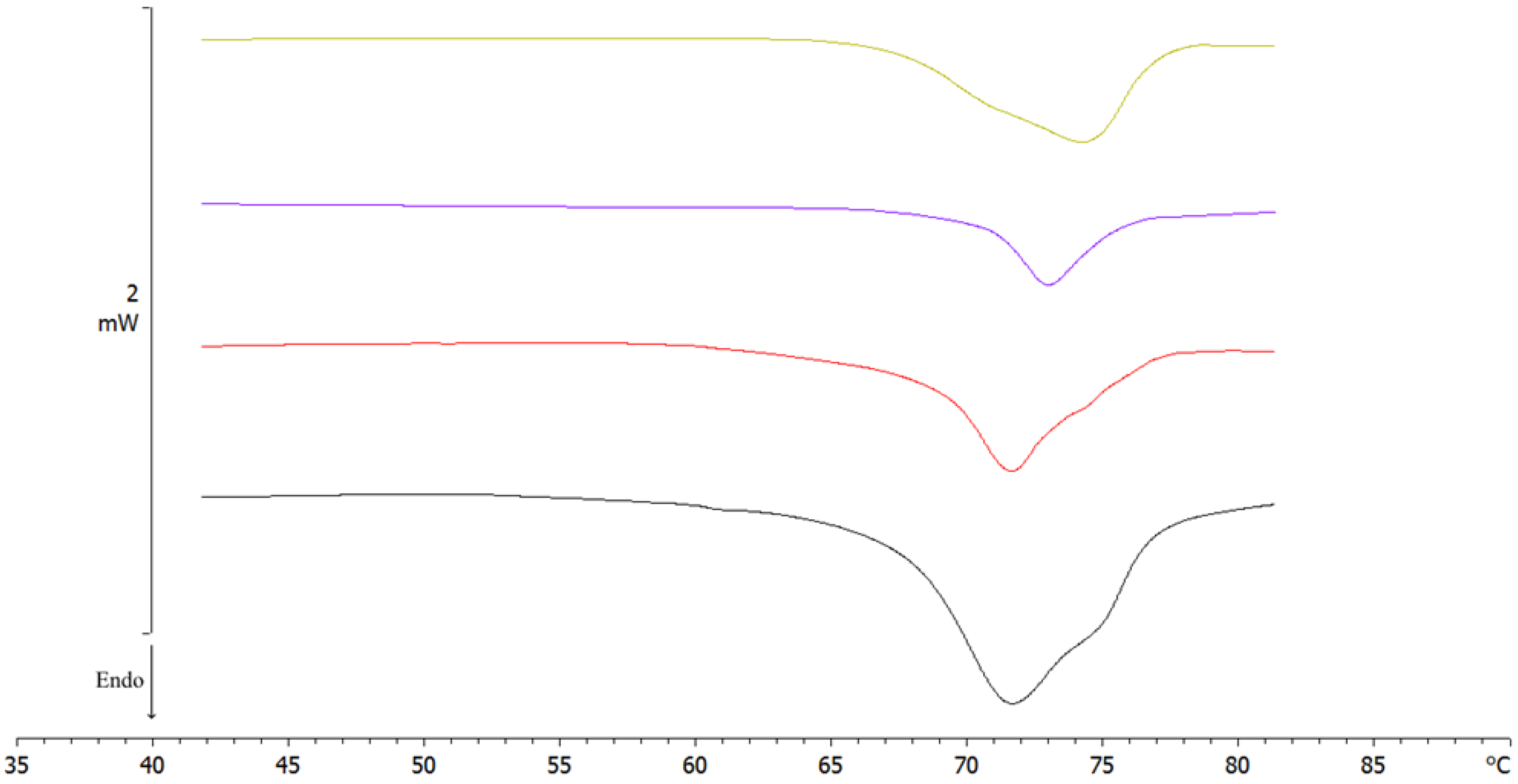
Differential Scanning Calorimetry (DSC)
3. Materials and Methods
3.1. Materials
3.2. Preparation of SLNs
3.3. Characterization and Morphology of SLNs
3.4. Spray-Drying of Nanoparticles
3.5. Freeze-Drying of Nanoparticles
3.6. Differential Scanning Calorimetry (DSC)
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liparulo, A.; Esposito, R.; Santonocito, D.; Muñoz-Ramírez, A.; Spaziano, G.; Bruno, F.; Xiao, J.; Puglia, C.; Filosa, R.; Berrino, L. Formulation and Characterization of Solid Lipid Nanoparticles Loading Rf22-c, a Potent and Selective 5-Lo Inhibitor, in a Monocrotaline-Induced Model of Pulmonary Hypertension. Front. Pharmacol. 2020, 11, 83. [Google Scholar] [CrossRef]
- Müller, R.H.; Mehnert, W.; Lucks, J.-S.; Schwarz, C.; Zur Mühlen, A.; Meyhers, H. Solid Lipid Nanoparticles (SLN): An Alternative Colloidal Carrier System for Controlled Drug Delivery. Eur. J. Pharm. Biopharm. 1995, 41, 62–69. [Google Scholar]
- Santonocito, D.; Raciti, G.; Campisi, A.; Sposito, G.; Panico, A.; Siciliano, E.A.; Sarpietro, M.G.; Damiani, E.; Puglia, C. Astaxanthin-Loaded Stealth Lipid Nanoparticles (AST-SSLN) as Potential Carriers for the Treatment of Alzheimer’s Disease: Formulation Development and Optimization. Nanomaterials 2021, 11, 391. [Google Scholar] [CrossRef]
- Puglia, C.; Lauro, M.R.; Tirendi, G.G.; Fassari, G.E.; Carbone, C.; Bonina, F.; Puglisi, G. Modern Drug Delivery Strategies Applied to Natural Active Compounds. Expert Opin. Drug Deliv. 2017, 14, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Pignatello, R.; Fuochi, V.; Furneri, P.M.; Lauro, M.R.; Santonocito, D.; Cortesi, R.; Esposito, E. Lipid Nanoparticles and Active Natural Compounds: A Perfect Combination for Pharmaceutical Applications. Curr. Med. Chem. 2019, 26, 4681–4696. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Santonocito, D.; Romeo, G.; Intagliata, S.; Romano, G.L.; Strettoi, E.; Novelli, E.; Ostacolo, C.; Campiglia, P.; Sommella, E.M. Lipid Nanoparticles Traverse Non-Corneal Path to Reach the Posterior Eye Segment: In Vivo Evidence. Molecules 2021, 26, 4673. [Google Scholar] [CrossRef]
- Frasca, G.; Cardile, V.; Puglia, C.; Bonina, C.; Bonina, F. Gelatin Tannate Reduces the Proinflammatory Effects of Lipopolysaccharide in Human Intestinal Epithelial Cells. Clin. Exp. Gastroenterol. 2012, 5, 61. [Google Scholar] [PubMed]
- Santonocito, D.; Puglia, C. Applications of Lipid-Based Nanocarriers for Parenteral Drug Delivery. Curr. Med. Chem. 2022, 29, 4152–4169. [Google Scholar] [CrossRef]
- Esposito, E.; Drechsler, M.; Mariani, P.; Panico, A.M.; Cardile, V.; Crascì, L.; Carducci, F.; Graziano, A.C.E.; Cortesi, R.; Puglia, C. Nanostructured Lipid Dispersions for Topical Administration of Crocin, a Potent Antioxidant from Saffron (Crocus Sativus L.). Mater. Sci. Eng. C 2017, 71, 669–677. [Google Scholar] [CrossRef]
- Puglia, C.; Santonocito, D.; Bonaccorso, A.; Musumeci, T.; Ruozi, B.; Pignatello, R.; Carbone, C.; Parenti, C.; Chiechio, S. Lipid Nanoparticle Inclusion Prevents Capsaicin-Induced TRPV1 Defunctionalization. Pharmaceutics 2020, 12, 339. [Google Scholar] [CrossRef]
- Freitas, C.; Lucks, J.S.; Müller, R.H. P238 Effect of Storage Conditions on Long-Term Stability of “Solid Lipid Nanoparticles” (SLN) in Aqueous Dispersion. Eur. J. Pharm. Sci. 1994, 2, 178. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Correlation between Long-Term Stability of Solid Lipid Nanoparticles (SLNTM) and Crystallinity of the Lipid Phase. Eur. J. Pharm. Biopharm. 1999, 47, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Coffin, M.D.; McGinity, J.W. Biodegradable Pseudolatexes: The Chemical Stability of Poly (D, L-Lactide) and Poly (ε-Caprolactone) Nanoparticles in Aqueous Media. Pharm. Res. 1992, 9, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, D.; Sarpietro, M.G.; Carbone, C.; Panico, A.; Campisi, A.; Siciliano, E.A.; Sposito, G.; Castelli, F.; Puglia, C. Curcumin Containing PEGylated Solid Lipid Nanoparticles for Systemic Administration: A Preliminary Study. Molecules 2020, 25, 2991. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, D.; Puglia, C. Nanotechnological Systems and Lung: A Perfect Combination for Lung Pharmaceutical Applications. Curr. Med. Chem. 2022, 30, 725–743. [Google Scholar] [CrossRef]
- Ali, M.E.; Lamprecht, A. Spray Freeze Drying as an Alternative Technique for Lyophilization of Polymeric and Lipid-Based Nanoparticles. Int. J. Pharm. 2017, 516, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Seremeta, K.P. Advantages and Challenges of the Spray-Drying Technology for the Production of Pure Drug Particles and Drug-Loaded Polymeric Carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray Drying of Pharmaceuticals and Biopharmaceuticals: Critical Parameters and Experimental Process Optimization Approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef]
- Zimmermann, C.M.; Baldassi, D.; Chan, K.; Adams, N.B.; Neumann, A.; Porras-Gonzalez, D.L.; Wei, X.; Kneidinger, N.; Stoleriu, M.G.; Burgstaller, G. Spray Drying SiRNA-Lipid Nanoparticles for Dry Powder Pulmonary Delivery. J. Control. Release 2022, 351, 137–150. [Google Scholar] [CrossRef]
- Steiner, D.; Schumann, L.V.; Bunjes, H. Processing of Lipid Nanodispersions into Solid Powders by Spray Drying. Pharmaceutics 2022, 14, 2464. [Google Scholar] [CrossRef]
- Salminen, H.; Ankenbrand, J.; Zeeb, B.; Badolato Bönisch, G.; Schäfer, C.; Kohlus, R.; Weiss, J. Influence of spray drying on the stability of food-grade solid lipid nanoparticles. Food Res. Int. 2019, 119, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Müller, R.H. Spray-drying of solid lipid nanoparticles (SLNTM). Eur. J. Pharm. Biopharm. 1998, 46, 145–151. [Google Scholar] [CrossRef]
- Heiati, H.; Tawashi, R.; Phillips, N.C. Drug Retention and Stability of Solid Lipid Nanoparticles Containing Azidothymidine Palmitate after Autoclaving, Storage and Lyophilization. J. Microencapsul. 1998, 15, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, A.; Pellitteri, R.; Ruozi, B.; Puglia, C.; Santonocito, D.; Pignatello, R.; Musumeci, T. Curcumin Loaded Polymeric vs. Lipid Nanoparticles: Antioxidant Effect on Normal and Hypoxic Olfactory Ensheathing Cells. Nanomaterials 2021, 11, 159. [Google Scholar] [CrossRef]
- Luo, W.-C.; Beringhs, A.O.; Kim, R.; Zhang, W.; Patel, S.M.; Bogner, R.H.; Lu, X. Impact of Formulation on the Quality and Stability of Freeze-Dried Nanoparticles. Eur. J. Pharm. Biopharm. 2021, 169, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Fonte, P.; Costa, A.; Andrade, J.; Seabra, V.; Ferreira, D.; Reis, S.; Sarmento, B. Effect of Freeze-Drying, Cryoprotectants and Storage Conditions on the Stability of Secondary Structure of Insulin-Loaded Solid Lipid Nanoparticles. Int. J. Pharm. 2013, 456, 370–381. [Google Scholar] [CrossRef]
- Abdelwahed, W. Lyophilization of Solid Lipid Nanoparticles for Brain Targeting. Int. J. Pharm. Pharm. Sci. 2015, 7, 381–385. [Google Scholar]
- Pikal, M.J.; Shah, S. The Collapse Temperature in Freeze Drying: Dependence on Measurement Methodology and Rate of Water Removal from the Glassy Phase. Int. J. Pharm. 1990, 62, 165–186. [Google Scholar] [CrossRef]
- Strojewski, D.; Krupa, A. Spray drying and nano spray drying as manufacturing methods of drug-loaded polymeric particles. Polim. Med. 2022, 52, 101–111. [Google Scholar] [CrossRef]
- Marante, T.; Viegas, C.; Duarte, I.; Macedo, A.S.; Fonte, P. An Overview on Spray-Drying of Protein-Loaded Polymeric Nanoparticles for Dry Powder Inhalation. Pharmaceutics 2020, 12, 1032. [Google Scholar] [CrossRef]
- Samantha, S.C.; Bruna, A.S.M.; Adriana, R.M.; Fabio, B.; Sandro, A.R.; Aline, R.C.A. Drying by Spray Drying in the Food Industry: Micro-Encapsulation, Process Parameters and Main Carriers Used. Afr. J. Food Sci. 2015, 9, 462–470. [Google Scholar] [CrossRef]
- Kecht-Wyrsch, P. Hochdisperse Glycerid-Mikropartikel Als Perorales Arzneiträgersystem. Ph.D. Thesis, ETH Zurich, Zürich, Switzerland, 1987. [Google Scholar]
- Rao, H.; Ahmad, S.; Madni, A.; Rao, I.; Ghazwani, M.; Hani, U.; Umair, M.; Ahmad, I.; Rai, N.; Ahmed, M.; et al. Compritol-Based Alprazolam Solid Lipid Nanoparticles for Sustained Release of Alprazolam: Preparation by Hot Melt Encapsulation. Molecules 2022, 27, 8894. [Google Scholar] [CrossRef]
- Broadhead, J.; Edmond Rouan, S.K.; Rhodes, C.T. The Spray Drying of Pharmaceuticals. Drug Dev. Ind. Pharm. 1992, 18, 1169–1206. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Cheng, P.; Yang, P.; Shi, P.; Kong, L.; Liu, H. Preparation of Curcumin Solid Lipid Nanoparticles Loaded with Flower-Shaped Lactose for Lung Inhalation and Preliminary Evaluation of Cytotoxicity In Vitro. Evid. Based Complement. Altern. Med. 2021, 2021, 4828169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kho, K.; Cheow, W.S.; Hadinoto, K. A Comparison between Spray Drying and Spray Freeze Drying for Dry Powder Inhaler Formulation of Drug-Loaded Lipid–Polymer Hybrid Nanoparticles. Int. J. Pharm. 2012, 424, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Chen, M.-L.; Chen, H.-Y.; Liu, S.-C. Effects of Temperature and Sugar Concentration on the Colour Development, 5-Hydroxymethoxylfurfural Production, and Antioxidative Activity Development in the Caramelisation of Acidic Glucose Solution. Int. J. Food Eng. 2012, 8, 1–17. [Google Scholar] [CrossRef]
- Batch, G.L.; Macosko, C.W. DSC Sample Temperature Control While Measuring Reaction Kinetics. Thermochim. Acta 1991, 188, 1–15. [Google Scholar] [CrossRef]
- Venir, E.; Spaziani, M.; Maltini, E. Crystallization in “Tarassaco” Italian Honey Studied by DSC. Food Chem. 2010, 122, 410–415. [Google Scholar] [CrossRef]
- Demetzos, C. Differential Scanning Calorimetry (DSC): A Tool to Study the Thermal Behavior of Lipid Bilayers and Liposomal Stability. J. Liposome Res. 2008, 18, 159–173. [Google Scholar] [CrossRef]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, L.; Qian, Y.; Chen, Y. The effects of cryoprotectants on the freeze-drying of ibuprofen-loaded solid lipid microparticles (SLM). Eur. J. Pharm. Biopharm. 2008, 69, 750–759. [Google Scholar] [CrossRef]
- Chacón, M.; Molpeceres, J.; Berges, L.; Guzmán, M.; Aberturas, M.R. Stability and Freeze-Drying of Cyclosporine Loaded Poly(d,l Lactide–Glycolide) Carriers. Eur. J. Pharm. Sci. 1999, 8, 99–107. [Google Scholar] [CrossRef]
- Schwarz, C.; Mehnert, W. Freeze-Drying of Drug-Free and Drug-Loaded Solid Lipid Nanoparticles (SLN). Int. J. Pharm. 1997, 157, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Offerta, A.; Rizza, L.; Zingale, G.; Bonina, F.; Ronsisvalle, S. Optimization of Curcumin Loaded Lipid Nanoparticles Formulated Using High Shear Homogenization (HSH) and Ultrasonication (US) Methods. J. Nanosci. Nanotechnol. 2013, 13, 6888–6893. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Müller, R.H. Solid Lipid Nanoparticles for Parenteral Drug Delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, D.; Vivero-Lopez, M.; Lauro, M.R.; Torrisi, C.; Castelli, F.; Sarpietro, M.G.; Puglia, C. Design of Nanotechnological Carriers for Ocular Delivery of Mangiferin: Preformulation Study. Molecules 2022, 27, 1328. [Google Scholar] [CrossRef]
- Montenegro, L.; Castelli, F.; Sarpietro, M.G. Differential Scanning Calorimetry Analyses of Idebenone-Loaded Solid Lipid Nanoparticles Interactions with a Model of Bio-Membrane: A Comparison with In Vitro Skin Permeation Data. Pharmaceuticals 2018, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, C.; Cardullo, N.; Russo, S.; La Mantia, A.; Acquaviva, R.; Muccilli, V.; Castelli, F.; Sarpietro, M.G. Benzo[k,l]Xanthene Lignan-Loaded Solid Lipid Nanoparticles for Topical Application: A Preliminary Study. Molecules 2022, 27, 5887. [Google Scholar] [CrossRef]






| Batch | Feed (% w/w) EtOH:H2O | V (mL) | d (µm) | P (atm) | Tin (°C) | Tout (°C) | ASP (%) | Ψ (mL/min) | Rp (%) ± SD |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 60:40 | 100 | 700 | 6 | 60 | 29–30 | 70 | 3 | 45.0 ± 0.5 |
| A2 | 60:40 | 100 | 700 | 6 | 60 | 25–26 | 50 | 3 | 58.33 ± 0.9 |
| B1 | 50:50 | 100 | 700 | 6 | 60 | 32–33 | 70 | 3 | 43.71 ± 0.2 |
| B2 | 50:50 | 100 | 700 | 6 | 60 | 34–35 | 50 | 3 | 56.82 ± 0.2 |
| C1 | 40:60 | 100 | 700 | 6 | 60 | 34–35 | 70 | 3 | 38.16 ± 0.2 |
| C2 | 40:60 | 100 | 700 | 6 | 60 | 34–35 | 50 | 3 | 44.31 ± 0.1 |
| D1 | 0:100 | 100 | 700 | 6 | 110 | 69–70 | 70 | 3 | 21.0 ± 0.1 |
| D2 | 0:100 | 100 | 700 | 6 | 110 | 69–70 | 50 | 3 | 24.84 ± 0.2 |
| Batches | Average Size (nm) | PDI | ZP (mV) |
|---|---|---|---|
| Initial conditions | |||
| A1 | 228.2 | 0.278 | −28.2 |
| A2 | 220.0 | 0.250 | −31.5 |
| B1 | 234.5 | 0.264 | −28.4 |
| B2 | 237.1 | 0.283 | −28.3 |
| C1 | 209.5 | 0.252 | −32.1 |
| C2 | 221.9 | 0.300 | −36.71 |
| D1 | 159.1 | 0.268 | −37.8 |
| D2 | 167 | 0.253 | −38.2 |
| After spray-drying | |||
| A1 | 1000 | 0.362 | +48.0 |
| A2 | 1000 | 0.266 | +22.2 |
| B1 | 1000 | 0.368 | +46.3 |
| B2 | 1000 | 0.282 | +39.3 |
| C1 | 1000 | 0.280 | +62.8 |
| C2 | 1000 | 0.351 | +42.2 |
| D1 | 1000 | 0.343 | +43.0 |
| D2 | 1000 | 0.301 | +50.8 |
| Sample | ΔH (J/g) Compritol 888 ATO | ΔH (J/g) Lutrol F68 | ΔH (J/g) Glucose | Peak T (°C) Compritol 888 ATO | Peak T (°C) Lutrol F68 | Peak T (°C) Glucose |
|---|---|---|---|---|---|---|
| Pierced lid scan 2 | −113.63 | −49.71 | ---- | 71.16 | 50.99 | ---- |
| Pierced lid scan 1 | −108.98 | −108.98 | −119.71; −22.88 | 71.51 | 50.05 | 141.73; 146.31 |
| Inverted lid scan 2 | −120.22 | ---- | ---- | 69.60 | ---- | ---- |
| Inverted lid scan 1 | −98.52 | −140.29 | −76.29 | 71.07 | 47.42 | 80.32 |
| Sample | ΔH (J/g) Lutrol F68 | ΔH (J/g) Compritol 888 ATO | Peak T (°C) Lutrol F68 | Peak T (°C) Compritol 888 ATO |
|---|---|---|---|---|
| Lutrol F68 | −120.80 | ---- | 52.49 | ---- |
| SLN G4 | −24.58 | −106.62 | 51.06 | 70.82 |
| SLN G3 | −24.89 | −105.73 | 51.05 | 70.79 |
| SLN G1 | −50.02 | −113.62 | 51.02 | 71.26 |
| SLN G0 | −62.64 | −114.64 | 50.06 | 71.49 |
| Compritol 888 ATO | ---- | −127.67 | ---- | 71.64 |
| Sample | ΔH (J/g) | Peak T (°C) |
|---|---|---|
| SLN G4 | −24.58 | 74.74 |
| SLN G3 | −21.74 | 74.35 |
| SLN G1 | −23.66 | 72.86 |
| SLN G0 | −25.82 | 74.00 |
| SLN pre-lyo | −100.86 | 71.68 |
| Sample | ΔH (J/g) | Peak T (°C) |
|---|---|---|
| SLN G1 vortexed | −51.58 | 74.22 |
| SLN G1 manually shaken | −23.66 | 72.86 |
| SLN G1 ultrasonicated | −59.83 | 71.64 |
| SLN pre-lyo | −100.86 | 71.68 |
| Segment | Start Temperature (°C) | End Temperature (°C) | Heating Rate (°C/min) | N2 Flow (mL/min) |
|---|---|---|---|---|
| 1 | 25 | 170 | 2 | 70 |
| 2 | 170 | 25 | −4 | 70 |
| Segment | Start Temperature (°C) | End Temperature (°C) | Heating Rate (°C/min) | N2 Flow (mL/min) |
|---|---|---|---|---|
| 1 | 25 | 85 | 2 | 70 |
| 2 | 85 | 25 | −4 | 70 |
| Segment | Start Temperature (°C) | End Temperature (°C) | Heating Rate (°C/min) | N2 Flow (mL/min) |
|---|---|---|---|---|
| 1 | 25 | 85 | 2 | 70 |
| 2 | 85 | 25 | −4 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santonocito, D.; Sarpietro, M.G.; Castelli, F.; Lauro, M.R.; Torrisi, C.; Russo, S.; Puglia, C. Development of Solid Lipid Nanoparticles as Dry Powder: Characterization and Formulation Considerations. Molecules 2023, 28, 1545. https://doi.org/10.3390/molecules28041545
Santonocito D, Sarpietro MG, Castelli F, Lauro MR, Torrisi C, Russo S, Puglia C. Development of Solid Lipid Nanoparticles as Dry Powder: Characterization and Formulation Considerations. Molecules. 2023; 28(4):1545. https://doi.org/10.3390/molecules28041545
Chicago/Turabian StyleSantonocito, Debora, Maria Grazia Sarpietro, Francesco Castelli, Maria Rosaria Lauro, Cristina Torrisi, Stefano Russo, and Carmelo Puglia. 2023. "Development of Solid Lipid Nanoparticles as Dry Powder: Characterization and Formulation Considerations" Molecules 28, no. 4: 1545. https://doi.org/10.3390/molecules28041545
APA StyleSantonocito, D., Sarpietro, M. G., Castelli, F., Lauro, M. R., Torrisi, C., Russo, S., & Puglia, C. (2023). Development of Solid Lipid Nanoparticles as Dry Powder: Characterization and Formulation Considerations. Molecules, 28(4), 1545. https://doi.org/10.3390/molecules28041545








