Acute Toxicity Evaluation of Phosphatidylcholine Nanoliposomes Containing Nisin in Caenorhabditis elegans
Abstract
1. Introduction
2. Results
2.1. Liposome Encapsulation of Nisin
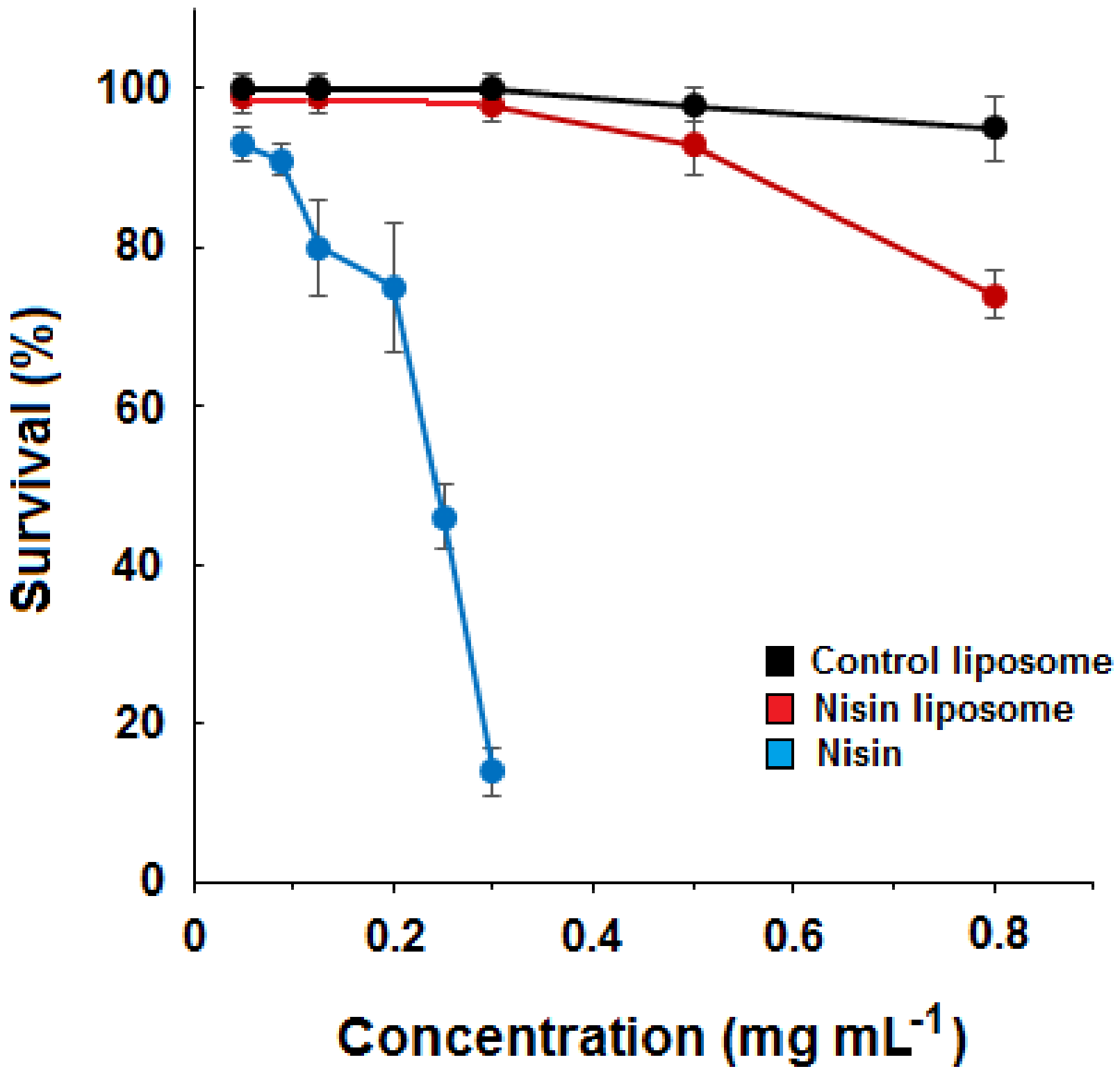
2.2. Dose–Response Curves for Liposomes and Free Nisin
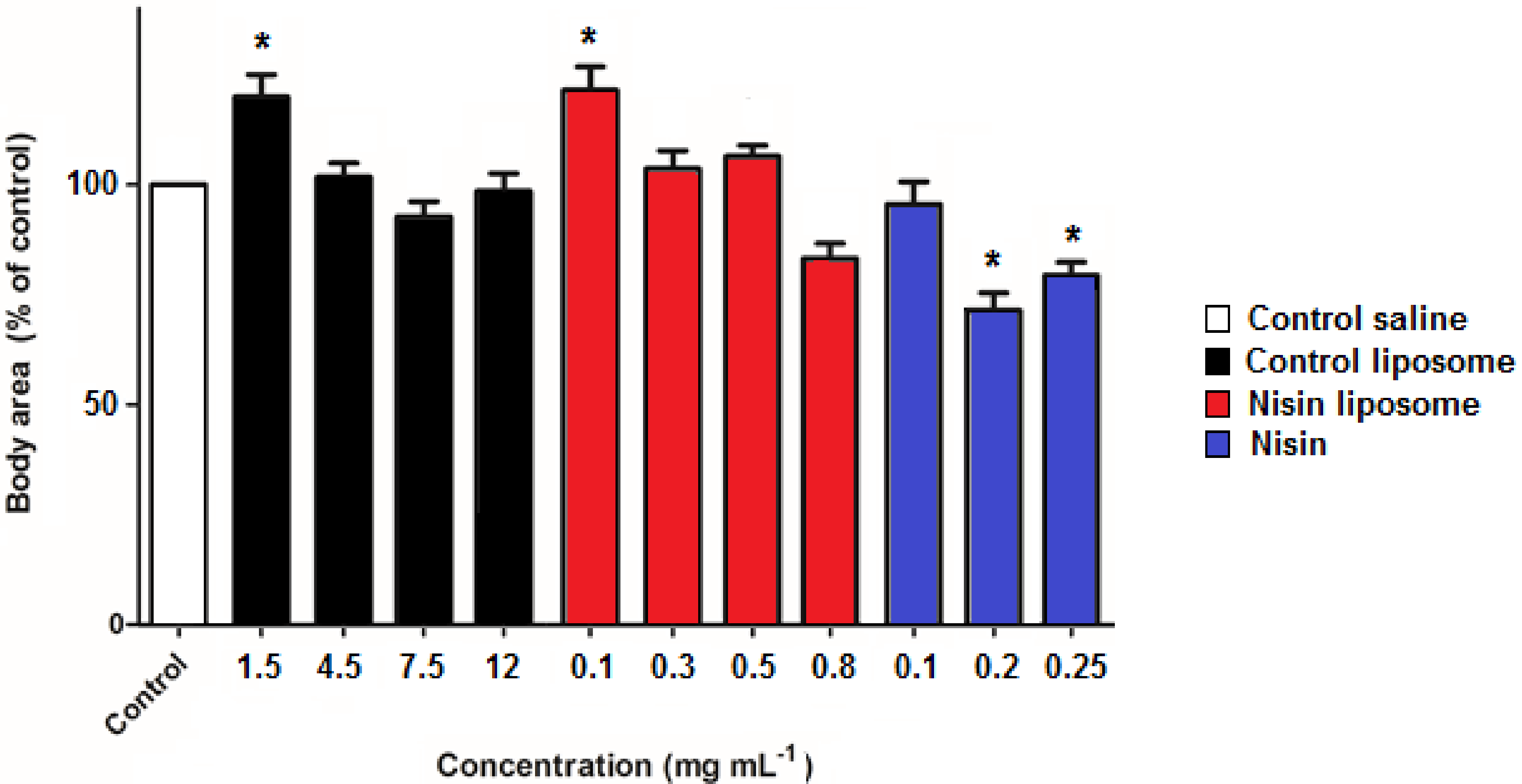
2.3. Development of Nematodes
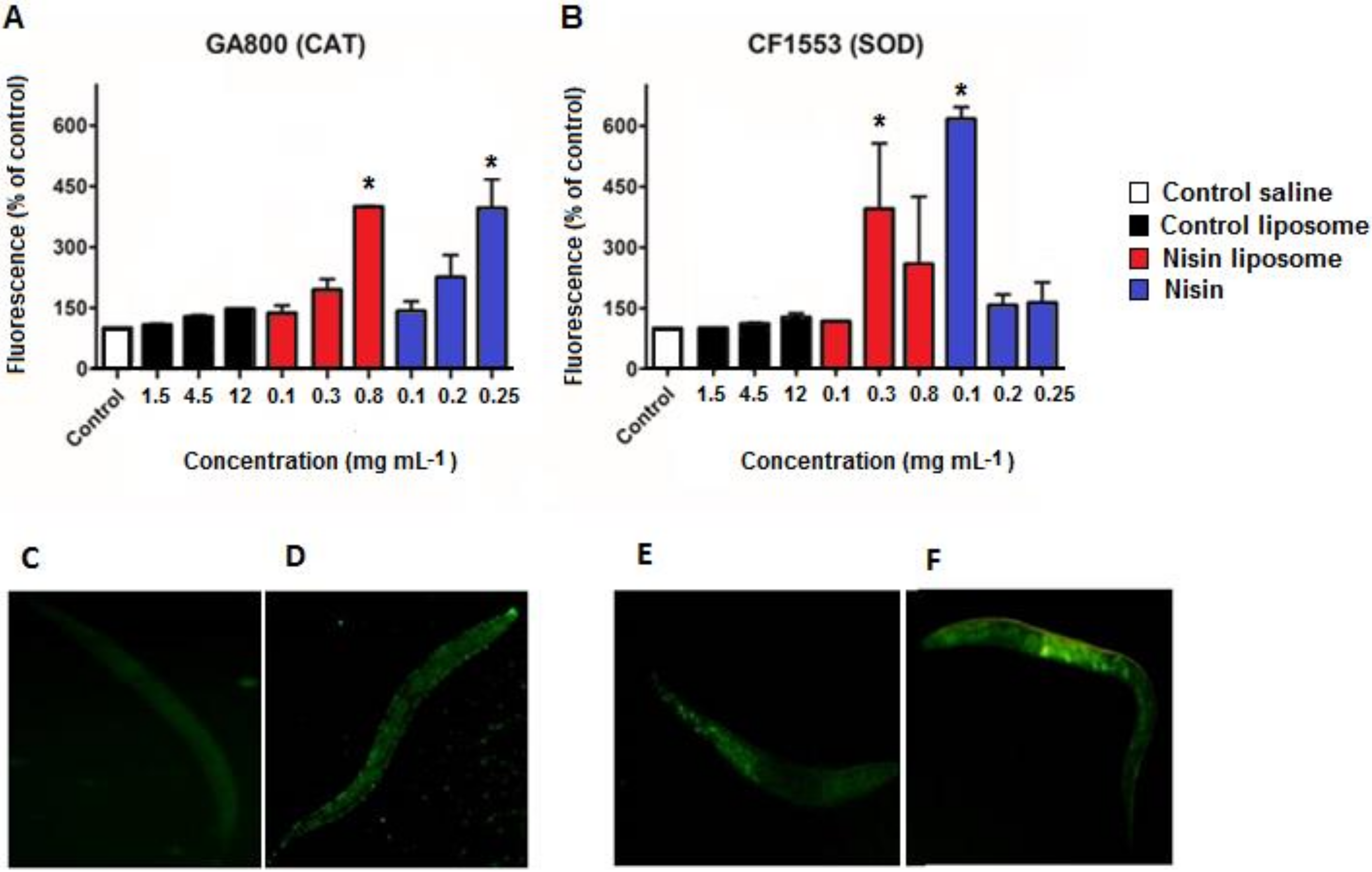
2.4. ROS Levels
2.5. Fluorescence Quantification of Antioxidant Enzymes
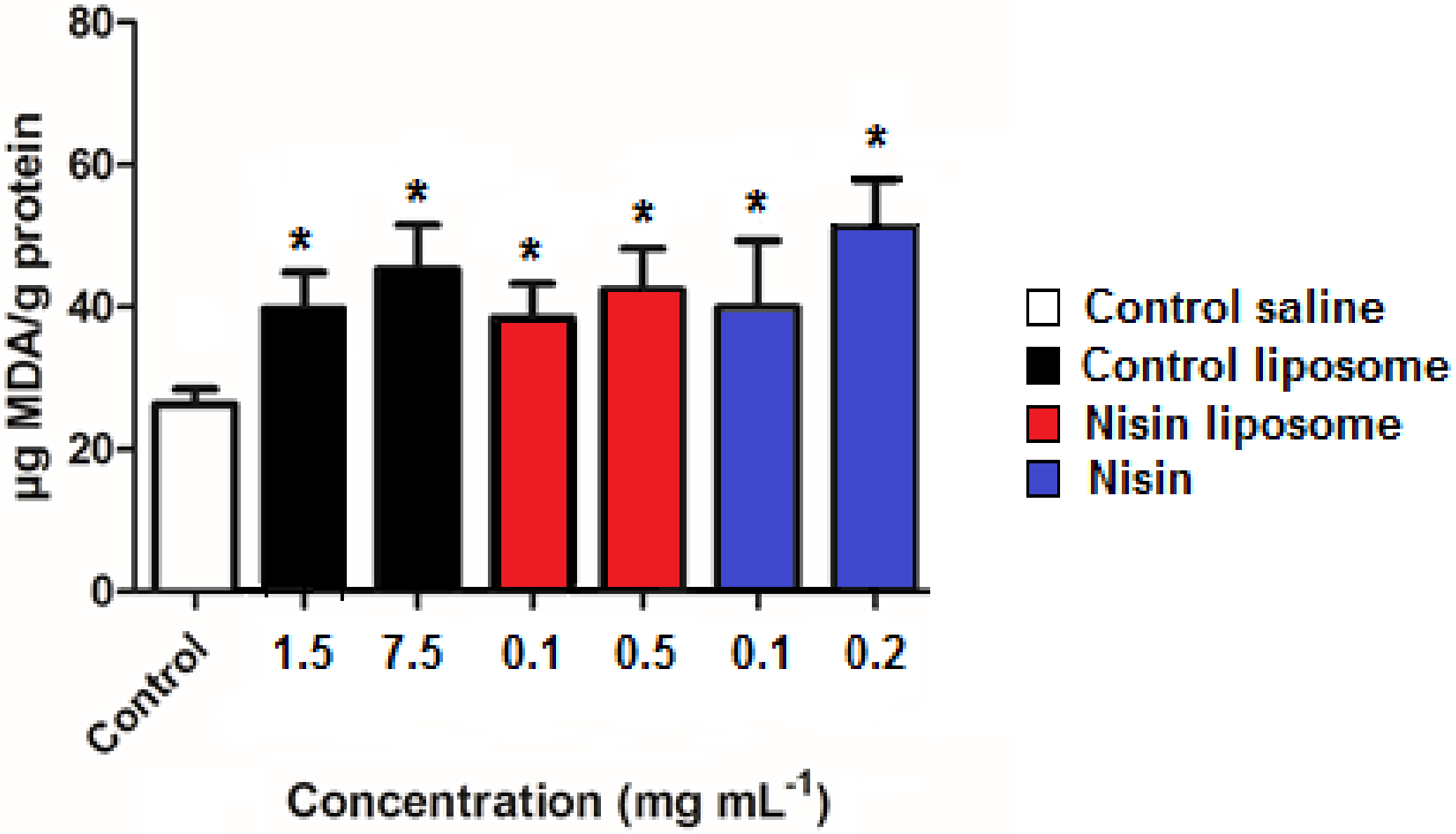
2.6. Lipid Peroxidation
2.7. Correlation of Data
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Liposome Preparation and Characterization
4.3. Strains, Culture, and Synchronization of C. elegans
4.4. Exposure to Liposomes with Nisin, Control Liposomes, and Free Nisin
4.5. LD50 Determination
4.6. Development of Nematodes
4.7. Measurement of Reactive Oxygen Species (ROS)
4.8. Fluorescence Quantification
4.9. Fluorescence Microscopy
4.10. Lipid Peroxidation
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Delgado, G.C. Economics and governance of nanomaterials: Potential and risks. Technol. Soc. 2010, 32, 137–144. [Google Scholar] [CrossRef]
- Dan, D. Nanotechnology, nanoparticles and nanoscience: A new approach in chemistry and life sciences. Soft Nanosci. Lett. 2020, 10, 17–26. [Google Scholar] [CrossRef]
- Dhawan, A.; Shanker, R.; Das, M.; Gupta, K.C. Guidance for safe handling of nanomaterials. J. Biomed. Nanotechnol. 2011, 7, 218–224. [Google Scholar] [CrossRef]
- Lee, B.; Yun, Y.; Park, K. Smart nanoparticles for drug delivery: Boundaries and opportunities. Chem. Eng. Sci. 2015, 125, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A. Nanostructures as promising tools for delivery of antimicrobial peptides. Mini-Rev. Med. Chem. 2012, 12, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A.; Pinilla, C.M.B.; Lopes, N.A. Nanoliposomes as a plataform for delivery of antimicrobials. In Nanotechnology Applied to Pharmaceutical Technology; Rai, M., Santos, C.A., Eds.; Springer: Cham, Switzerland, 2017; pp. 55–90. [Google Scholar]
- Jesorka, A.; Orwar, O. Liposomes: Technologies and analytical applications. Annu. Rev. Anal. Chem. 2008, 1, 801–832. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A review of liposomes as a drug delivery system: Current status of approved products, regulatory, environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Lopes, N.A.; Brandelli, A. Nanostructures for delivery of natural antimicrobials in food. Crit. Rev. Food Sci. Nutr. 2018, 58, 2202–2212. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef]
- Alves, O.L.; Moraes, A.C.M.; Simões, M.B.; Fonseca, L.C.; Nascimento, R.O.; Holtz, R.D.; Faria, A.F. Nanomaterials. In Nanotoxicology—Materials, Methodologies and Assessments; Durán, N., Guterres, S.S., Alves, O.L., Eds.; Springer: New York, NY, USA, 2014; pp. 1–29. [Google Scholar]
- Brandelli, A. The interaction of nanostructured antimicrobials with biological systems: Cellular uptake, trafficking and potential toxicity. Food Sci. Hum. Wellness 2020, 9, 8–20. [Google Scholar] [CrossRef]
- Festing, S.; Wilkinson, R. The ethics of animal research. Talking point on the use of animals in scientific research. EMBO Rep. 2007, 9, 526–530. [Google Scholar] [CrossRef]
- Cazarin, K.C.C.; Corrêa, C.L.; Zambrone, F.A.D. Reduction, refinement and replacement of animal use in toxicity testing: An overview. Braz. J. Pharm. Sci. 2004, 40, 289–299. [Google Scholar]
- Moyson, S.; Town, R.M.; Vissenberg, K.; Blust, R. The effect of metal mixture composition on toxicity to C. elegans at individual and population levels. PLoS One 2019, 14, e0218929. [Google Scholar] [CrossRef]
- Tang, B.; Tong, P.; Xue, K.S.; Williams, P.L.; Wang, J.S.; Tang, L. High-throughput assessment of toxic effects of metal mixtures of cadmium(Cd), lead(Pb), and manganese(Mn) in nematode Caenorhabditis elegans. Chemosphere 2019, 234, 232–241. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Li, H.; Ma, R.; Yu, Z.; Li, L.; Xiang, M.; Chen, X.; Hua, X.; Yu, Y. A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans. J. Environ. Manag. 2019, 237, 519–525. [Google Scholar] [CrossRef]
- Huang, P.; Liu, S.S.; Xu, Y.Q.; Wang, Y.; Wang, Z.J. Combined lethal toxicities of pesticides with similar structures to Caenorhabditis elegans are not necessarily concentration additives. Environ. Pollut. 2021, 286, 117207. [Google Scholar] [CrossRef]
- Charão, M.F.; Souto, C.; Brucjer, N.; Barth, A.; Jornada, D.S.; Fagundez, D.; Ávila, D.S.; Eifler-Lima, V.L.; Guterres, S.S.; Pohlmann, A.R.; et al. Caenorhabditis elegans as an alternative in vivo model to determine oral uptake, nanotoxicity, and efficacy of melatonin-loaded lipid-core nanocapsules on paraquat damage. Int. J. Nanomed. 2015, 10, 5093–5106. [Google Scholar] [CrossRef]
- Wu, T.; Xu, H.; Liang, X.; Tang, M. Caenorhabditis elegans as a complete model organism for biosafety assessments of nanoparticles. Chemosphere 2019, 221, 708–726. [Google Scholar] [CrossRef]
- Peterson, R.T.; Nass, R.; Boyd, W.A.; Freedman, J.H.; Dong, K.; Narahashi, T. Use of non-mammalian alternative models for neurotoxicological study. Neurotoxicology 2008, 29, 546–555. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Ennis, A.C.; Polli, J.R.; Xiao, P.; Zhang, B.; Stellwag, E.J.; Overton, A.; Pan, X. Chemical dispersant potentiates crude oil impacts on growth, reproduction, and gene expression in Caenorhabditis elegans. Arch. Toxicol. 2013, 87, 371–382. [Google Scholar] [CrossRef]
- Jorgensen, E.; Mango, S. The art and design of genetic screens: Caenorhabditis elegans. Nat. Rev. Genet. 2002, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Helmcke, K.J.; Avila, D.S.; Aschner, M. Utility of Caenorhabditis elegans in high throughput neurotoxicological research. Neurotoxicol. Teratol. 2010, 32, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.M.; Gaysinsky, S.; Davidson, P.M.; Bruce, B.D.; Weiss, J. Characterization of antimicrobial-bearing liposomes by ζ-potential, vesicle size, and encapsulation efficiency. Food Biophys. 2007, 2, 1–9. [Google Scholar] [CrossRef]
- Angius, F.; Floris, A. Liposomes and MTT cell viability assay: An incompatible affair. Toxicol. In Vitro 2015, 29, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Uccelletti, D.; Zanni, E.; Marcellini, L.; Palleschi, C.; Barra, D.; Mangoni, M.L. Anti-Pseudomonas activity of frog skin antimicrobial peptides in Caenorhabditis elegans infection model: A plausible mode of action in vitro and in vivo. Antimicrob. Ag. Chemother. 2010, 54, 3853–3860. [Google Scholar] [CrossRef]
- Xie, H.; Chen, X.; Zeng, Q.; Zhan, Y.; Xu, X.; Chen, D.; Liang, J. Antimicrobial peptide brevinin-2isb, new drug candidates enhance the innate immune response and cured Caenorhabditis elegans with methicillin-resistant Staphylococcus aureus (MRSA). Biomed. J. Sci. Tech. Res. 2019, 13, 10181–10184. [Google Scholar]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef]
- Gupta, S.M.; Aranha, C.C.; Reddy, K.V.R. Evaluation of developmental toxicity of microbiocide nisin in rats. Food Chem. Toxicol. 2008, 46, 598–603. [Google Scholar] [CrossRef]
- Hagiwara, A.; Imai, N.; Nakashima, H.; Toda, Y.; Kawabe, M.; Furukawa, F.; Delves-Broughton, J.; Yasuhara, K.; Hayashi, S. A 90-day oral toxicity study of nisin A, an anti-microbial peptide derived from Lactococcus lactis subsp. lactis, in F344 rats. Food Chem. Toxicol. 2010, 48, 2421–2428. [Google Scholar] [CrossRef]
- Vaucher, R.A.; Gewehr, C.C.V.; Corrêa, A.P.F.; Sant’Anna, V.; Ferreira, J.; Brandelli, A. Evaluation of the immunogenicity and in vivo toxicity of the antimicrobial peptide P34. Int. J. Pharm. 2011, 421, 94–98. [Google Scholar] [CrossRef]
- Paranpje, M.; Neuhaus, V.; Braun, A.; Müller-Goymann, C.C. Toxicity testing of sildenafil base-loaded liposomes in in vitro and ex vivo models for pulmonary application. Eur. J. Lipid Sci. Technol. 2016, 116, 1129–1136. [Google Scholar]
- Knudsen, K.B.; Northeved, H.; Kumar, P.E.; Permin, A.; Gjetting, T.; Andresen, T.L.; Larsen, S.; Wegener, K.M.; Lykkesfeldt, J.; Jantzen, K.; et al. In vivo toxicity of cationic micelles and liposomes. Nanomedicine 2015, 11, 467–477. [Google Scholar] [CrossRef]
- Shibamura, A.; Ikeda, T.; Nishikawa, Y. A method for oral administration of hydrophilic substances to Caenorhabditis elegans: Effects of oral supplementation with antioxidants on the nematode lifespan. Mech. Ageing Dev. 2009, 130, 652–655. [Google Scholar] [CrossRef]
- Miyako, E.; Chechtka, S.A.; Doi, M.; Yuba, E.; Kono, K. In vivo remote control of reactions in Caenorhabditis elegans by using supramolecular nanohybrids of carbon nanotubes and liposomes. Angew. Chem. Int. 2015, 54, 9903–9906. [Google Scholar] [CrossRef]
- Chen, J.; Yan, G.; Hu, R.; Gu, Q.; Chen, M.; Gu, W.; Chen, Z.; Cai, B. Improved pharmacokinetics and reduced toxicity of brucine after encapsulation into stealth liposomes: Role of phosphatidylcholine. Int. J. Nanomed. 2012, 7, 3567–3577. [Google Scholar] [CrossRef]
- Charão, M.F.; Göethel, G.; Brucker, N.; Paese, K.; Eifler-Lima, V.L.; Pohlmann, A.R.; Guterres, S.S.; Garcia, S.C. Melatonin-loaded lipid-core nanocapsules protect against lipid peroxidation caused by paraquat through increased SOD expression in Caenorhabditis elegans. BMC Pharmacol. Toxicol. 2019, 20, 80. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the safety of nisin (E 234) as a food additive in the light of new toxicological data and the proposed extension of use. EFSA J. 2017, 15, 5063. [Google Scholar]
- Wu, Q.; Nouara, A.; Li, Y.; Zhang, M.; Wang, W.; Tang, M.; Ye, B.; Ding, J.; Wang, D. Comparison of toxicities from three metal oxide nanoparticles at environmental relevant concentrations in nematode Caenorhabditis elegans. Chemosphere 2013, 90, 1123–1131. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, B.K.; Park, S.; Park, S.K. Phosphatidylcholine extends lifespan via DAF-16 and reduces amyloid-beta-induced toxicity in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2019, 2019, 2860642. [Google Scholar] [CrossRef]
- Wu, D.; Cysper, J.R.; Yashin, A.; Johnson, T.E. Multiple mild heat-shocks decrease the Gompertz component of mortality in Caenorhabditis elegans. Exp. Gerontol. 2009, 44, 607–612. [Google Scholar] [CrossRef][Green Version]
- Zhou, K.I.; Pincus, Z.; Slack, F.J. Longevity and stress in Caenorhabditis elegans. Aging 2011, 3, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Qu, X.; Aleman-Meza, B.; Wang, T.; Riepe, C.; Liu, Z.; Li, Q.; Zhong, W. Multi-endpoint, high-throughput study of nanomaterial toxicity in Caenorhabditis elegans. Environ. Sci. Technol. 2015, 49, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Oliván, L.M.; Galar-Martínez, M.; Islas-Flores, H.; García-Medina, S.; San Juan-Reyes, N. DNA damage and oxidative stress induced by salicylic acid in Daphnia magna. Comp. Biochem. Physiol. C 2011, 164, 21–26. [Google Scholar]
- Zhao, Y.L.; Wang, D.Y. Formation and regulation of adaptive response in nematode Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2012, 2012, 164093. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Van Raamsdonk, J.M.; Hekimi, S. Superoxide dismutase is dispensable for normal animal lifespan. PNAS 2012, 109, 5785–5790. [Google Scholar] [CrossRef]
- Miranda-Vizuete, A.; Veal, E.A. Caenorhabditis elegans as a model for understanding ROS function in physiology and disease. Redox Biol. 2017, 11, 708–714. [Google Scholar] [CrossRef]
- Gems, D.; Doonan, R. Antioxidant defense and aging in C. elegans. Is the oxidative damage theory of aging wrong? Cell Cycle 2009, 8, 1–7. [Google Scholar] [CrossRef]
- Tao, J.; Wu, Q.Y.; Ma, Y.C.; Chen, Y.L.; Zou, C.G. Antioxidant response is a protective mechanism against nutrient deprivation in C. elegans. Sci. Rep. 2017, 7, 43547. [Google Scholar] [CrossRef]
- Jensen, C.; Li, H.; Vestergaard, M.; Frees, D.; Leisner, J.J. Nisin damages the septal membrane and triggers DNA condensation in methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 1007. [Google Scholar] [CrossRef]
- Malheiros, P.S.; Micheletto, Y.M.S.; Silveira, N.P.; Brandelli, A. Development and characterization of phosphatidylcholine nanovesicles containing the antimicrobial peptide nisin. Food Res. Int. 2010, 43, 1198–1203. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Brandelli, A. Antimicrobial activity of nanoliposomes co-encapsulating nisin and garlic extract against Gram-positive and Gram-negative bacteria in milk. Innov. Food Sci. Emerg. Technol. 2016, 36, 287–293. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 71, 248–254. [Google Scholar] [CrossRef]
| Sample | Dose (mg mL−1) | Relative Fluorescence (%) 1 |
|---|---|---|
| Free nisin | 0.1 | 61 ± 10 * |
| 0.2 | 161 ± 25 * | |
| 0.25 | 321 ± 191 * | |
| Nisin liposome | 0.1 | 136 ± 76 |
| 0.3 | 131 ± 75 | |
| 0.5 | 144 ± 65 | |
| 0.8 | 514 ± 251 * | |
| Control liposome 2 | 1.5 | 176 ± 73 |
| 4.5 | 162 ± 56 | |
| 7.5 | 338 ± 250 * | |
| 12 | 577 ± 203 * |
| Treatment | Correlation | r | p Value |
|---|---|---|---|
| Free nisin | Death rate x ROS | 0.7667 | 0.0214 |
| Nisin-loaded liposome | Death rate x ROS | 0.7486 | 0.0255 |
| Death rate x Size | −0.8398 | 0.0061 | |
| Control liposome | Death rate x ROS | 0.7886 | 0.0172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boelter, J.F.; Garcia, S.C.; Göethel, G.; Charão, M.F.; de Melo, L.M.; Brandelli, A. Acute Toxicity Evaluation of Phosphatidylcholine Nanoliposomes Containing Nisin in Caenorhabditis elegans. Molecules 2023, 28, 563. https://doi.org/10.3390/molecules28020563
Boelter JF, Garcia SC, Göethel G, Charão MF, de Melo LM, Brandelli A. Acute Toxicity Evaluation of Phosphatidylcholine Nanoliposomes Containing Nisin in Caenorhabditis elegans. Molecules. 2023; 28(2):563. https://doi.org/10.3390/molecules28020563
Chicago/Turabian StyleBoelter, Juliana Ferreira, Solange Cristina Garcia, Gabriela Göethel, Mariele Feiffer Charão, Livia Marchi de Melo, and Adriano Brandelli. 2023. "Acute Toxicity Evaluation of Phosphatidylcholine Nanoliposomes Containing Nisin in Caenorhabditis elegans" Molecules 28, no. 2: 563. https://doi.org/10.3390/molecules28020563
APA StyleBoelter, J. F., Garcia, S. C., Göethel, G., Charão, M. F., de Melo, L. M., & Brandelli, A. (2023). Acute Toxicity Evaluation of Phosphatidylcholine Nanoliposomes Containing Nisin in Caenorhabditis elegans. Molecules, 28(2), 563. https://doi.org/10.3390/molecules28020563






