In Vitro Neurotrophic Properties and Structural Characterization of a New Polysaccharide LTC-1 from Pyrola corbieri Levl (Luticao)
Abstract
1. Introduction
2. Results
2.1. Extraction and Purification of LTC-1
2.2. Physicochemical Properties of LTC-1
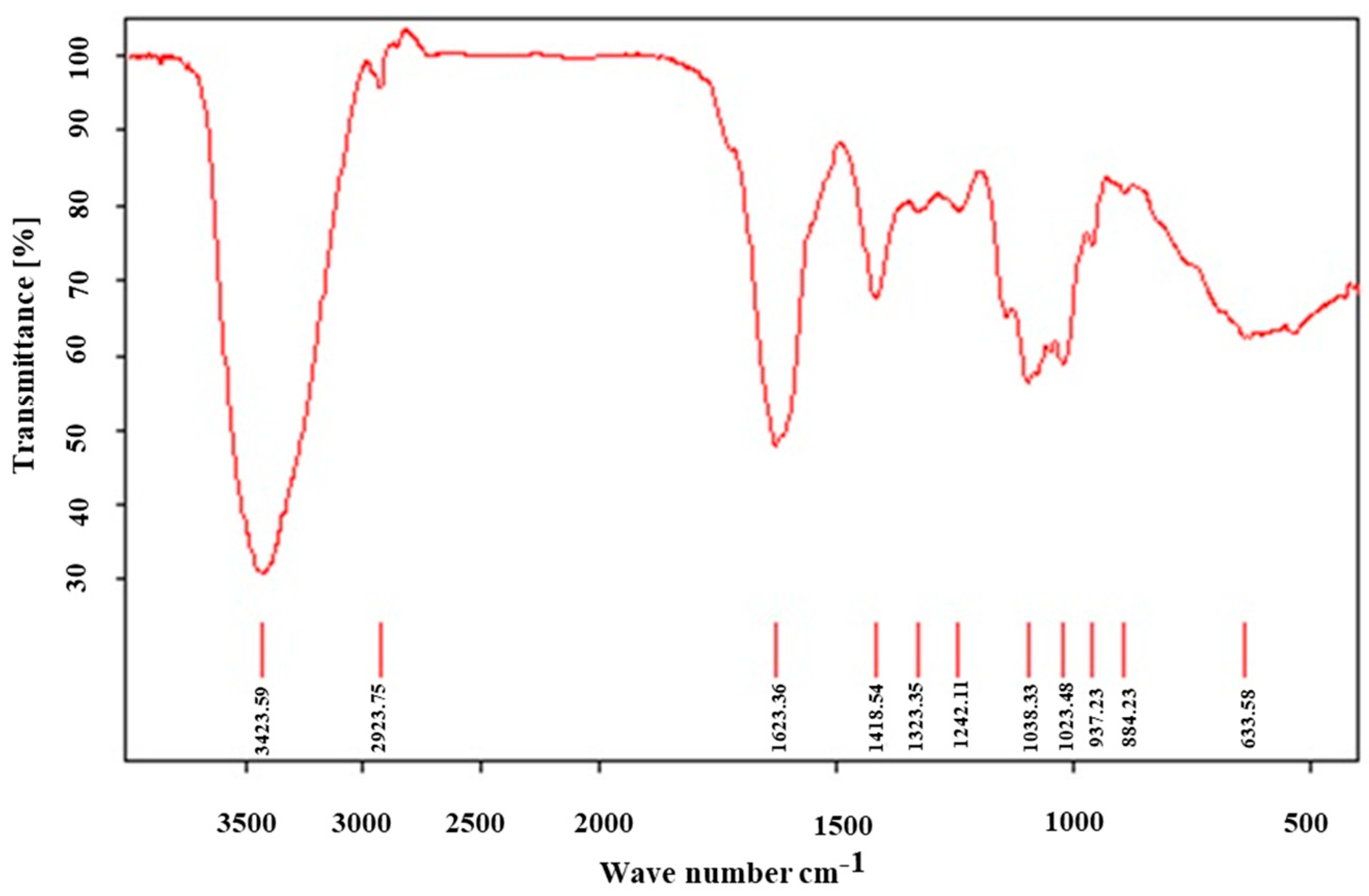
2.3. Structural Characterization of LTC-1
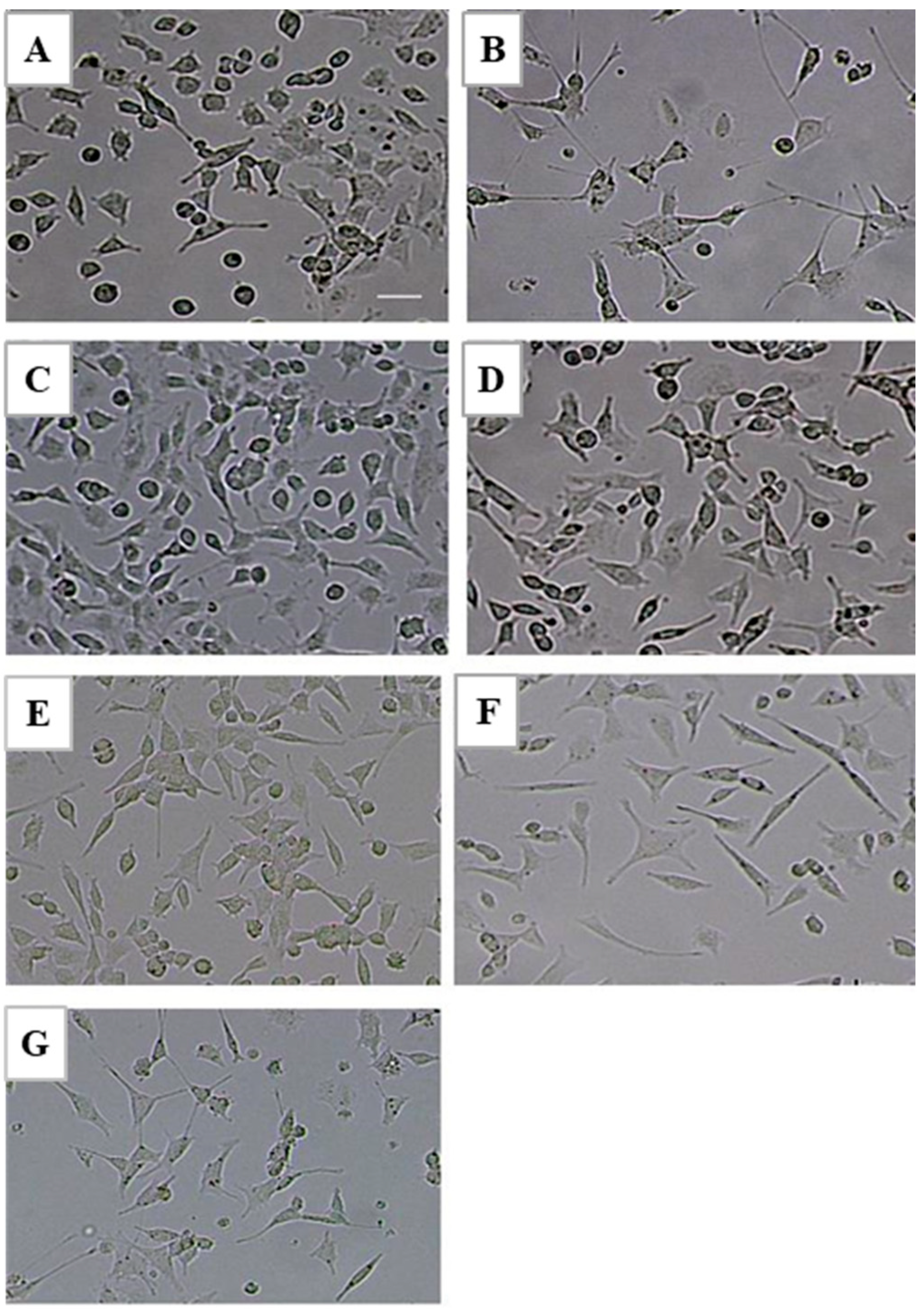
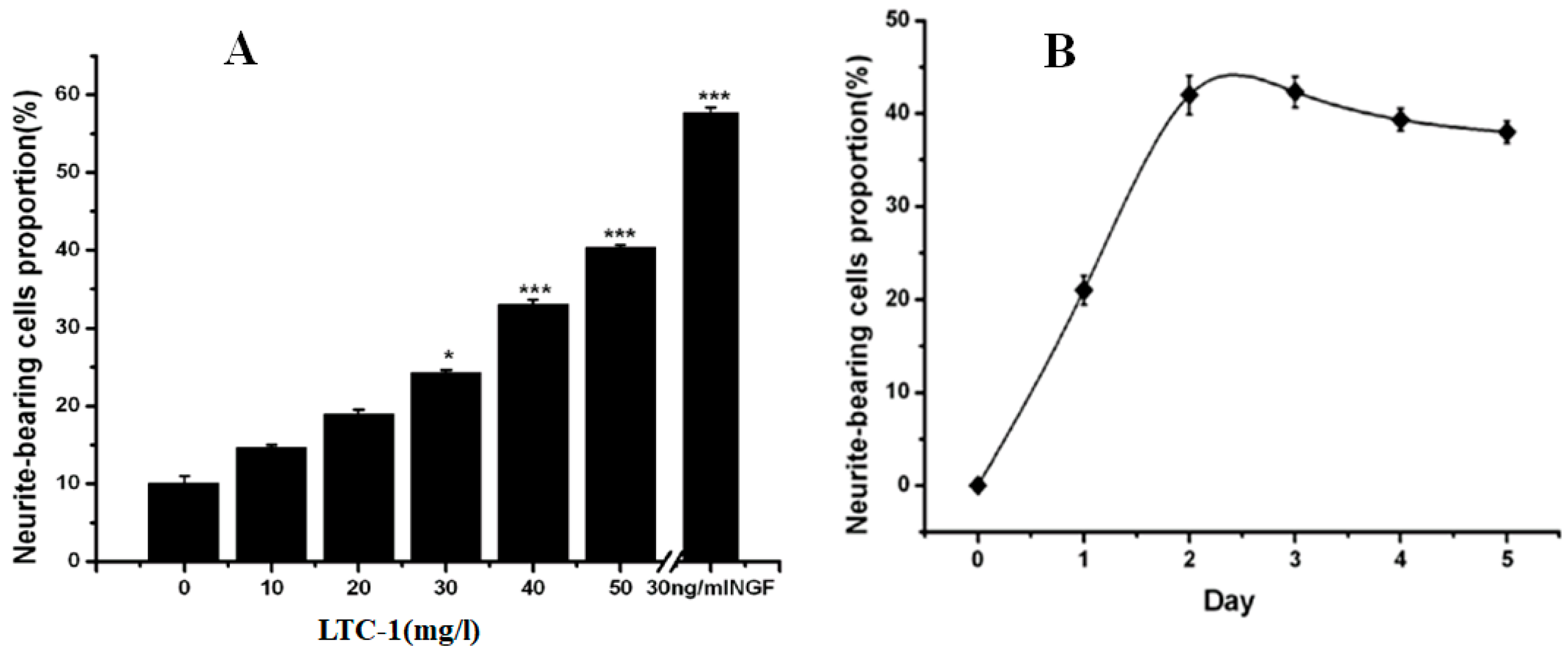
2.4. Effect of LTC-1 on Neurite Outgrowth in PC12 Cells
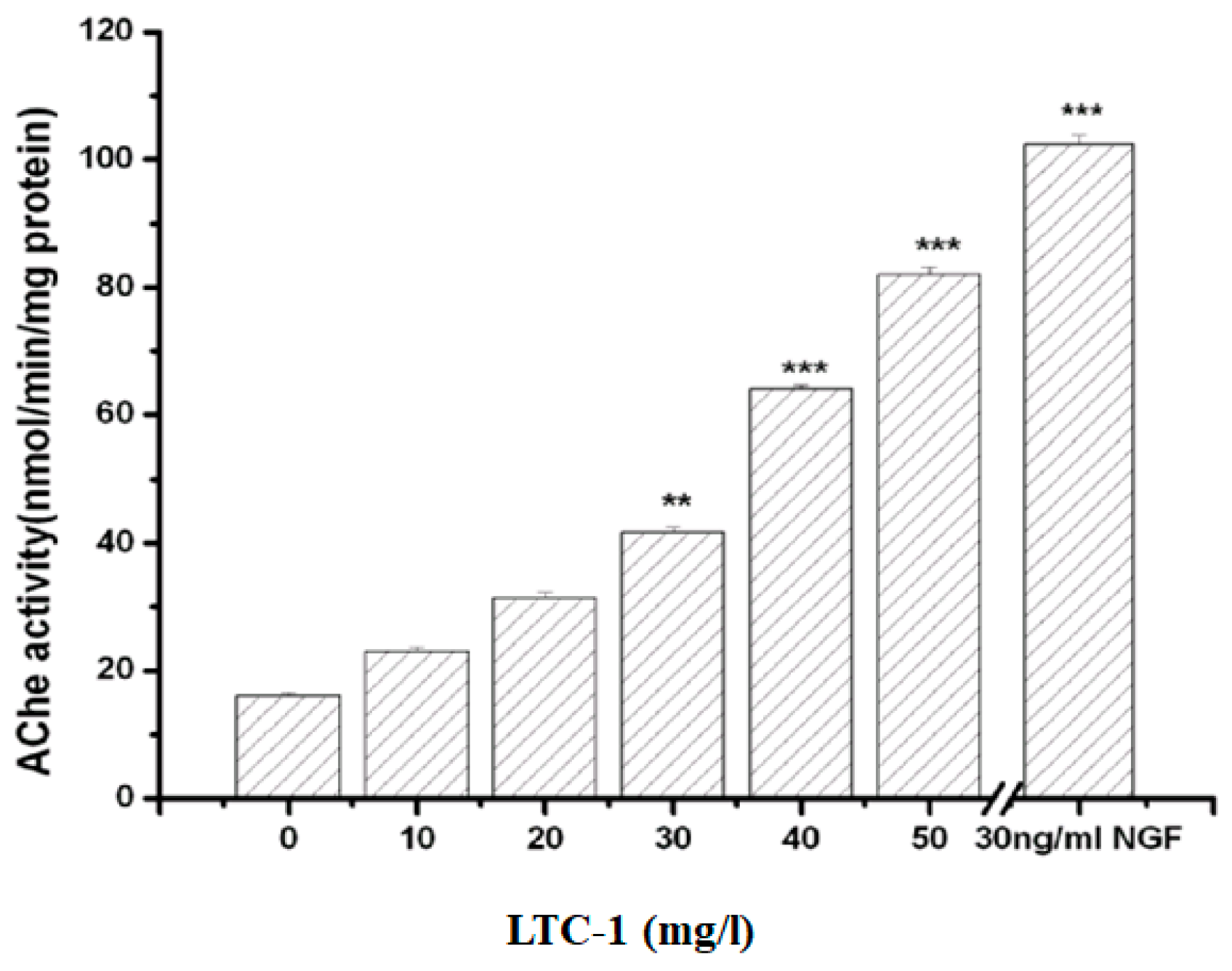
2.5. Effect of LTC-1 on Acetylcholinesterase Activity in PC12 Cells
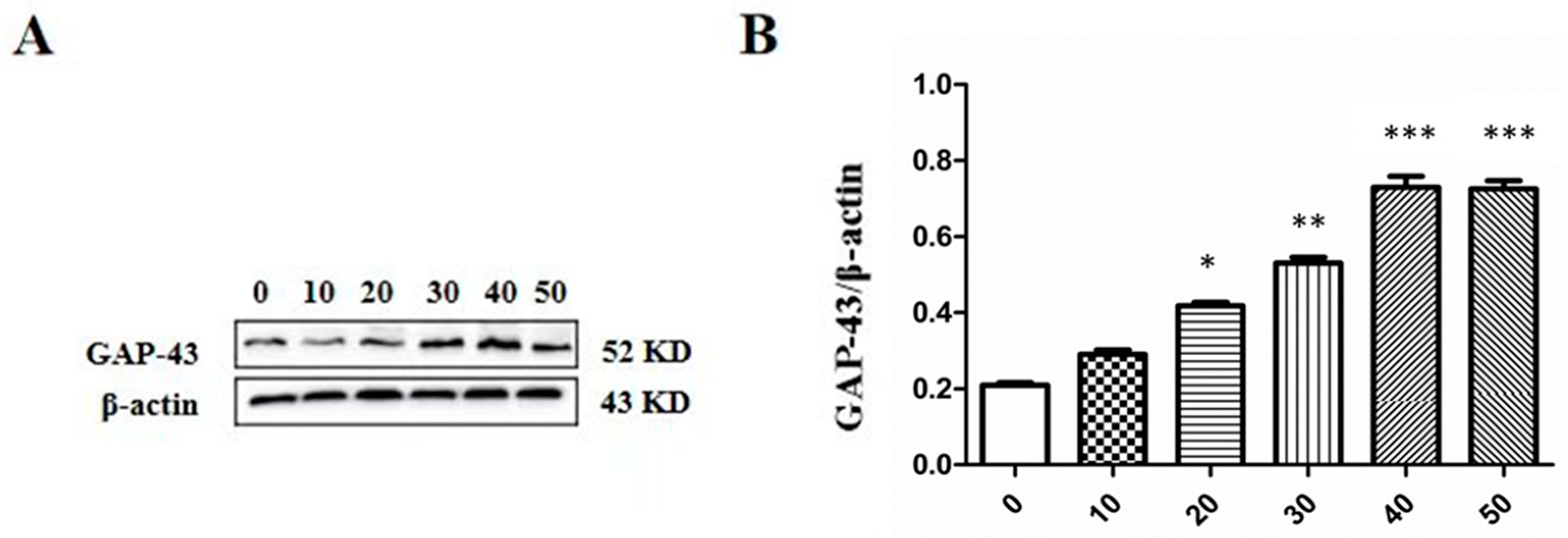
2.6. Effect of LTC-1 on GAP-43 Protein Expression in PC12 Cells
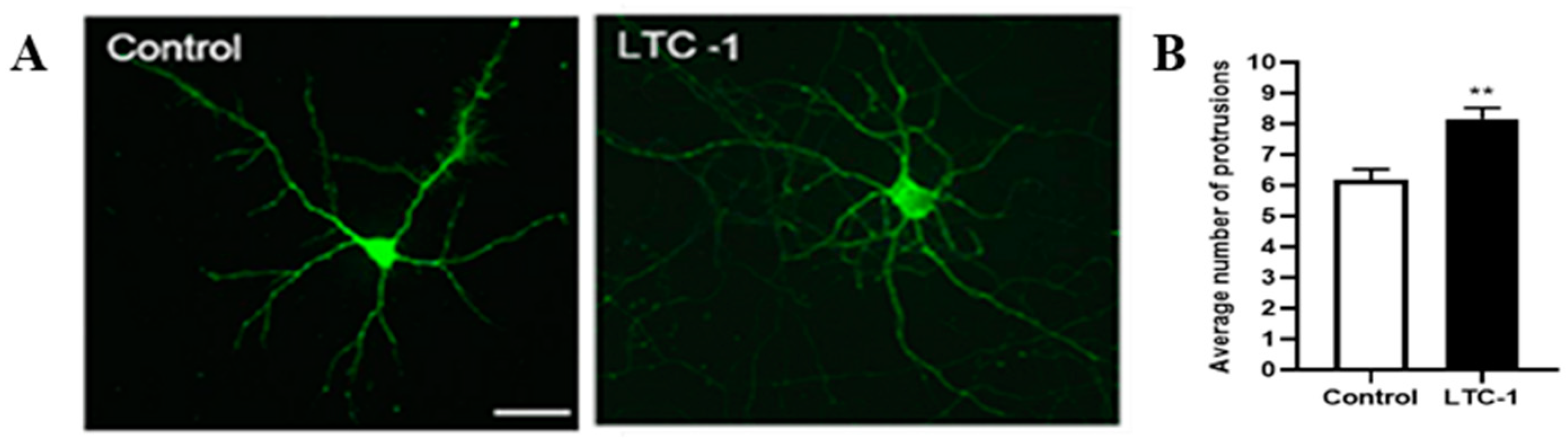
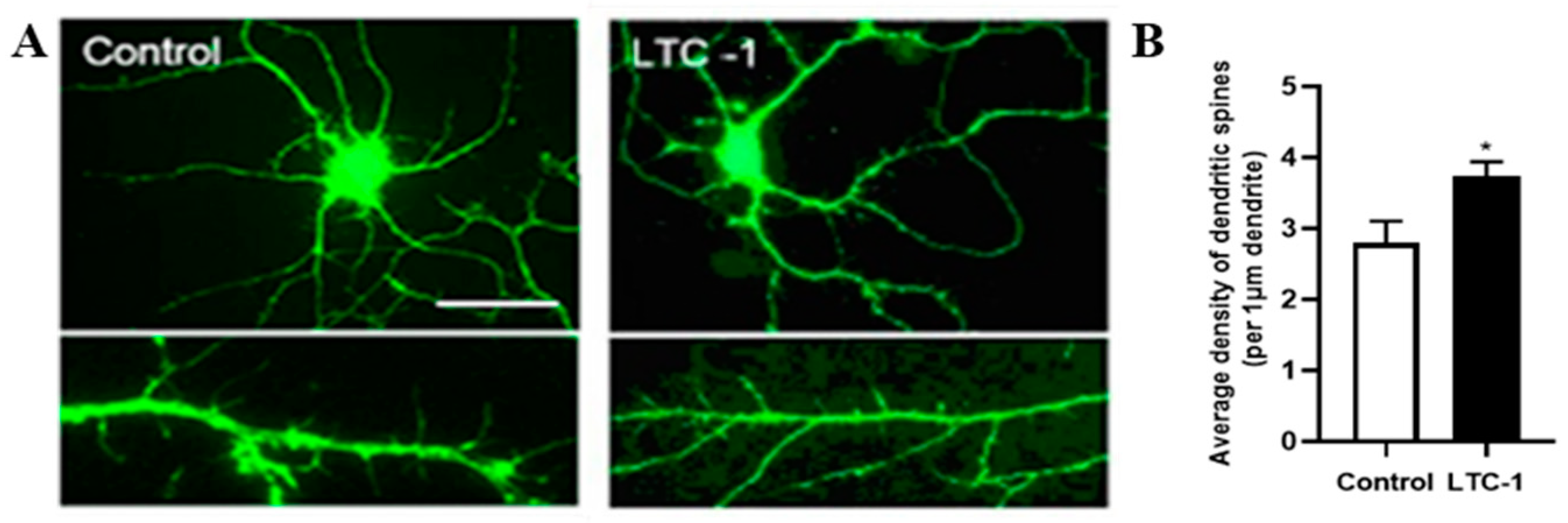
2.7. Effect of LTC-1 on the Length, Number and Density of Dendritic Dpines in Hippocampal Neurons
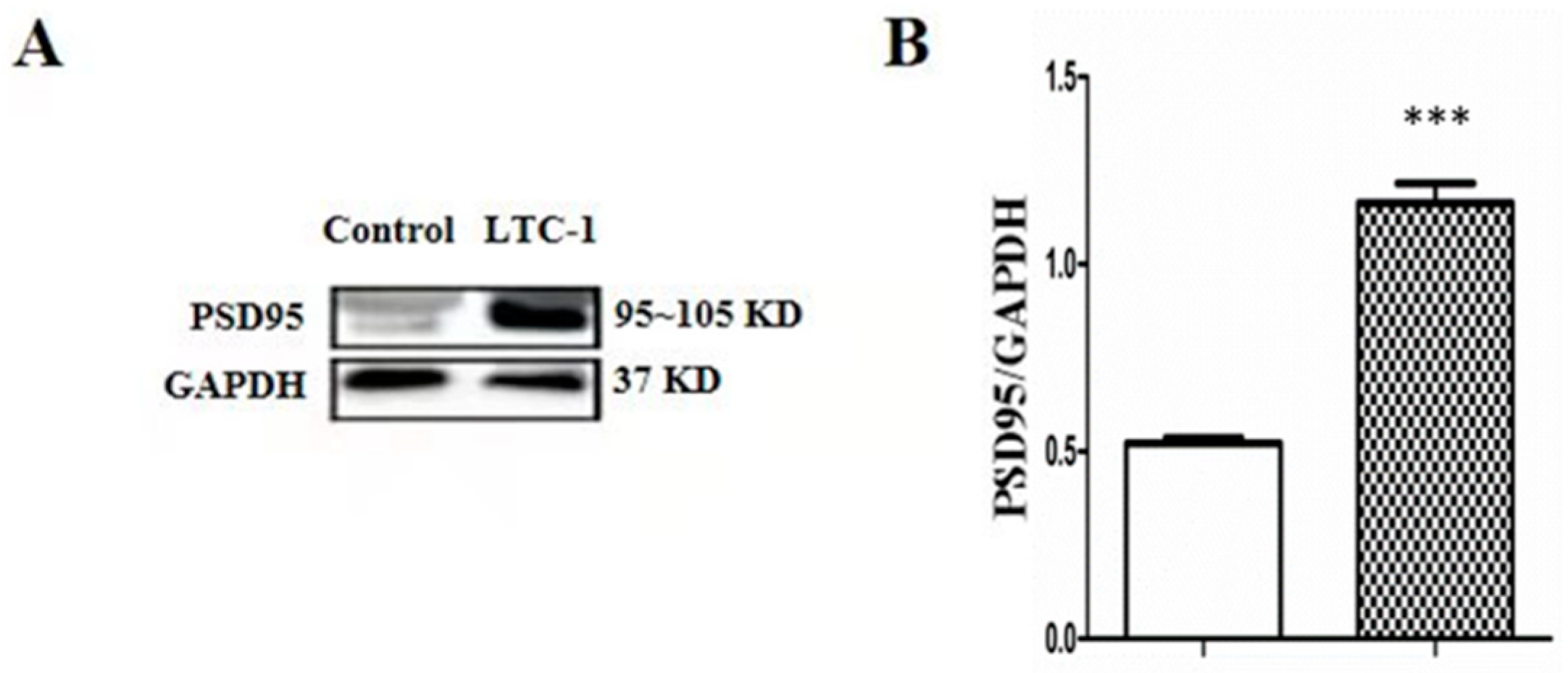
2.8. Effect of LTC-1 on the Expression of the Synapse Protein PSD-95
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. General Analysis Methods
4.3. Extraction and Purification of Polysaccharides
4.4. Structural Characterization of Polysaccharides [40]
4.4.1. Homogeneity and Molecular Weight Determination
4.4.2. Monosaccharide Composition Analysis
4.4.3. Periodate Oxidation-Smith Degradation
4.4.4. Partial Acid Hydrolysis
4.4.5. Methylation Analysis
4.5. In Vitro Neurotrophic Properties [43]
4.5.1. PC12 Cell Culture and Administration Treatment
4.5.2. Morphological Observation and Neurite Growth Analysis of PC12 Cells
4.5.3. Measurement of Acetylcholinesterase Activity
4.5.4. GFP Transgenic Mouse Hippocampal Neuron Culture and Administration Treatment
4.5.5. Long-Term Recording of Living Cells
4.5.6. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Lo, J.; Liu, C.C.; Li, Y.S.; Lee, P.Y.; Liu, P.L.; Wu, P.C.; Lin, T.C.; Chen, C.S.; Chiu, C.C.; Lai, Y.H.; et al. Punicalagin attenuates LPS-induced inflammation and ROS production in microglia by inhibiting the MAPK/NF-κB signaling pathway and NLRP3 inflammasome activation. J. Inflamm. Res. 2022, 15, 5347–5359. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Alsalahi, A.; Imam, M.U.; Ooi, D.J.H.; Khaza’ai, M.A.; Aljaberi, M.N.; Shamsudin, Z. IdrusSafety and neuroprotective efficacy of palm oil and tocotrienol-rich fraction from palm oil: A systematic review. Nutrients 2020, 12, 521. [Google Scholar] [CrossRef]
- Losenkov, I.S.; Mulder, N.J.V.; Levchuk, L.A.; Vyalova, N.M.; Loonen, A.J.M.; Bosker, F.J.; Simutkin, G.G.; Boiko, A.S.; Bokhan, N.A.; Wilffert, B.; et al. Association between BDNF gene variant Rs6265 and the severity of depression in antidepressant treatment-free depressed patients. Front. Psychiatry 2020, 11, 38. [Google Scholar] [CrossRef]
- Andrea, M.; Margrate, A.; Marinella, C.; Giulia, A.; Sara, A.B.; Gregorio, P.; Emanuela, T.; Mariachiara, P.; Giovanni, R.; Erika, O.; et al. The bright side of psychedelics: Latest advances and challenges in neuropharmacology. Int. J. Mol. Sci. 2023, 24, 1329. [Google Scholar] [CrossRef]
- Kim, A.; Jung, H.G.; Kim, Y.E.; Kim, S.C.; Park, J.Y.; Lee, S.G.; Hwang, E.M. The knockdown of TREK-1 in hippocampal neurons attenuate lipopolysaccharide-induced depressive-like behavior in mice. Int. J. Mol. Sci. 2019, 20, 5902. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.K.; Zhou, Y.; Fan, L.Y.; Sun, F.G.; Xue, M. The antidepressant-like effects of Danggui Buxue Decoction in GK rats by activating CREB/BDNF/TrkB signaling pathway. Phytomedicine 2021, 89, 153600. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F.B.; Abdolelah, J.; Mohammad, H.A.; Abdallah, Y.N. Smart pellets for controlled delivery of 5-fluorouracil. Molecules 2023, 28, 306. [Google Scholar] [CrossRef]
- Raut, M.P.; Asare, E.; Mohamed, S.M.D.S.; Amadi, E.N.; Roy, I. Bacterial cellulose-based blends and composites: Versatile biomaterials for tissue engineering applications. Int. J. Mol. Sci. 2023, 24, 986. [Google Scholar] [CrossRef]
- Alaofi, A.; Kiptoo, N.O.; Williams, P.T.D.; Miller, D.W.; Siahaan, T.J. Comparison of linear and cyclic His-Ala-Val peptides in modulating the blood–brain barrier permeability: Impact on delivery of molecules to the brain. J. Pharm. Sci. 2015, 105, 797–807. [Google Scholar] [CrossRef]
- Shu, G.W.; He, Y.X.N.; Lei, J.L.; Cao, H.; Chen, L. Cellulase-assisted extraction of polysaccharides from white hyacinth bean: Characterization of antioxidant activity and promotion for probiotics proliferation. Molecules 2017, 22, 1764. [Google Scholar] [CrossRef]
- Yang, J.; Yang, F.M.; Sun, Q.Y. Study on isolation and neurotrophic activity of polysaccharides from Rosa roxburghii. Chin. Pharm. J. 2006, 41, 980–982. [Google Scholar]
- Gao, Q.H.; Fu, X.Y.; Zhang, R.; Wang, Z.S.; Guo, M.Z. Neuroprotective effects of plant polysaccharides: A review of the mechanisms. Int. J. Biol. Macromol. 2018, 106, 749–754. [Google Scholar] [CrossRef]
- Nanjing University of Chinese Medicine. Dictionary of Traditional Chinese Medicine, 2nd ed.; Shanghai Science and Technology Publishing House: Shanghai, China, 2006; pp. 3120–3122. [Google Scholar]
- He, S.Z.; Xu, W.F. Study on the Resources of Chinese Herbal Medicine in Guizhou; Guizhou Science and Technology Press: Guiyang, China, 2007; pp. 508–509. [Google Scholar]
- Yao, X.H.; Zhang, D.Y.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Gu, C.B.; Li, C.Y.; Mu, F.S.; Efferth, T. Free radical scavenging capability, antioxidant activity and chemical constituents of Pyrola incarnata Fisch leaves. Ind. Crop. Prod. 2013, 49, 247–255. [Google Scholar] [CrossRef]
- Lee, M.H.; Ko, N.Y.; Lim, B.O.; Lee, S.H.; Mun, S.H.; Choi, W.S.; Lee, J.M.; Jun, S.H.; Choi, D.K.; Kim, N.W.; et al. The anti-inflammatory effects of Pyrolae herba extract through the inhibition of the expression of inducible nitric oxide synthase (iNOS) and NO production. J. Ethnopharmacol. 2007, 112, 49–54. [Google Scholar] [CrossRef]
- Wang, F.; Wu, L.H.; Li, L.F.; Chen, S.Y. Monotropein exerts protective effects against IL-1β-induced apoptosis and catabolic responses on osteoarthritis chondrocytes. Int. Immunopharmacol. 2014, 23, 575–580. [Google Scholar] [CrossRef]
- Mo, Z.C.; Wu, L.F.; Yang, J.; Wang, D.P. Studies on separation, purification and structure characteristics of a polysaccharide LTC-II from Pyrola corbieri. Chin. J. Chin. Mater. Med. 2011, 36, 1633–1636. [Google Scholar]
- Kim, J.S.; Shim, S.H.; Xu, Y.N.; Kang, S.S.; Son, K.H.; Chang, H.W.; Kim, H.P.; Bae, K. Phenolic glycosides from Pyrola japonica. Chem. Pharm. Bull. 2004, 52, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Marston, A.; Antus, S.; Gauthier, R.; Hostettmann, K. Flavonoids from Pyrola elliptica. Phytochemistry 1998, 49, 233–236. [Google Scholar] [CrossRef]
- Burnett, A.R.; Thomson, R.H. Naturally occurring quinones. Part XIV. The quinonoid constituents of Pyrola media Sw. (Pyrolaceae). J. Chem. Soc. C Org. 1968, 7, 857–860. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Halliez, M.C.M.; Buret, A.G. Gastrointestinal parasites and the neural control of gut functions. Front. Cell. Neurosci. 2015, 9, 452–472. [Google Scholar] [CrossRef]
- Cole, J.D.; Espinueva, D.F.; Seib, D.R.; Ash, A.M.; Cooke, M.B.; Cahill, S.P.; O’Leary, T.P.; Kwan, S.S.; Snyder, J.S. Adult-born hippocampal neurons undergo extended development and are morphologically distinct from neonatally born neurons. J. Neurosci. 2020, 40, 5740–5756. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Aoyama, T.; Shibata, S.; Sone, T.; Miyoshi, H.; Watanabe, H.; Nakamura, M.; Morota, S.; Uchino, H.; Yoo, A.S.; et al. miRNA-based rapid differentiation of purified neurons from hPSCs advancestoward quick. Cells 2020, 9, 532. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S.; Zhang, W.W.; Gao, Y.; Rong, C.B.; Wang, H.X.; Liu, Y.; Wong, J.H.; Ng, T.B. First demonstration of protective effects of purified mushroom polysaccharide-peptides against fatty liver injury and the mechanisms involved. Sci. Rep. 2019, 9, 13725. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Namekata, K.; Guo, X.L.; Harada, C.; Harada, T. Neuroprotection, growth factors and BDNF-TrkB signaling in retinal degeneration. Int. J. Mol. Sci. 2016, 17, 1584. [Google Scholar] [CrossRef] [PubMed]
- Szelenberger, R.; Kostka, J.; Saluk-Bijak, J.; Miller, E. Pharmacological interventions and rehabilitation approach for enhancing brain self-repair and stroke recovery. Curr. Neuropharmacol. 2020, 18, 51–64. [Google Scholar] [CrossRef]
- Xu, B.; Xu, X.; Zhang, C.Z.; Zhang, Y.Z.; Wu, G.R.; Yan, M.M.; Jia, M.L.; Xie, T.X.; Jia, X.H.; Wang, P.L.; et al. Synthesis and protective effect of new ligustrazine-vanillic acid derivatives against CoCl2-induced neurotoxicity in differentiated PC12 cells. Chem. Cent. J. 2017, 11, 20–29. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, X.; Guo, S.; Cao, F.N.; Zhang, X.F.; Wang, Y.Q.; Liu, J.Z.; Qian, B.W.; Yan, Y.C.; Chen, P.D.; et al. An acidic heteropolysaccharide from Lycii fructus: Purification, characterization, neurotrophic and neuroprotective activities in vitro. Carbohydr. Polym. 2020, 249, 116894. [Google Scholar] [CrossRef]
- Yu, M.S.; Lai, S.W.; Lin, K.F.; Fang, J.N.; Yuen, W.H.; Chang, R.C. Characterization of polysaccharides from the flowers of Nerium indicum and their neuroprotective effects. Int. J. Mol. Med. 2004, 14, 917–924. [Google Scholar] [CrossRef]
- Carriba, P.; Davies, A.M. CD40 is a major regulator of dendrite growth from developing excitatory and inhibitory neurons. eLife 2017, 6, e30442. [Google Scholar] [CrossRef]
- Druckenbrod, N.R.; Hale, E.B.; Olukoya, O.O.; Shatzer, W.E.; Goodrich, L.V. Neuronal processes and glial precursors form a scaffold for wiring the developing mouse cochlea. Nat. Commun. 2020, 11, 5866. [Google Scholar] [CrossRef]
- Rosenbohm, A.; Nagel, G.; Peter, R.S.; Brehme, T.; Koenig, W.; Rothenbacher, L.; Dupuis, D.; Ludolph, A.C. Association of serum retinol-binding protein 4 concentration with risk for and prognosis of amyotrophic lateral sclerosis. JAMA Neurol. 2018, 75, 600–607. [Google Scholar] [CrossRef]
- Zheng, L.P.; Feng, Z.Y.; Hu, H.H.; Wang, Z.X.; Yuan, Y.X.; Wei, F. The appearance order of varying intervals introduces extra modulation effects on neuronal firing through nonlinear dynamics of sodium channels during high-frequency stimulations. Front. Neurosci. 2020, 14, 397. [Google Scholar] [CrossRef] [PubMed]
- Duangjan, C.; Rangsinth, P.; Zhang, S.X.; Wink, M.; Tencomnao, T.; Anacardium Occidentale, L. Leaf extracts protect against glutamate/H2O2-induced oxidative toxicity and induce neurite outgrowth: The involvement of SIRT1/Nrf2 signaling pathway and teneurin 4 transmembrane protein. Front. Pharmacol. 2021, 12, 627738. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, D.Y.; Yang, Y.J.; Lei, W.F. Effects and mechanism of dexmedetomidine on neuronal cell injury induced by hypoxia-ischemia. BMC Anesthesiol. 2017, 17, 117. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W.; Qu, Y.H.; Chen, J.Y.; Chen, S.; Sun, L.; Zhou, Y.F.; Fan, Y.Y. Bee pollen polysaccharide from Rosa rugosa Thunb. (Rosaceae) promotes pancreatic β-cell proliferation and insulin secretion. Front. Pharmacol. 2021, 12, 688073. [Google Scholar] [CrossRef]
- Parkar, S.G.; Frost, J.K.T.; Rosendale, D.; Stoklosinski, H.M.; Jobsis, C.M.H.; Hedderley, D.I.; Gopal, P. The sugar composition of the fibre in selected plant foods modulates weaning infants’ gut microbiome composition and fermentation metabolites in vitro. Sci. Rep. 2021, 11, 9292. [Google Scholar] [CrossRef]
- Guan, H.; Ling, X.; Xu, J.; Zhu, Y.Q.; Zhang, J.Y.; Liu, X.Y. Structural characterization of polysaccharide derived from gastrodia elata and its immunostimulatory effect on RAW264.7 Cells. Molecules 2022, 27, 59. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Tan, C.H.; Tan, J.J.; Qu, S.J.; Wang, H.B.; Zhang, Q.; Jiang, S.H.; Zhu, D.Y. Phenolic and triterpenoid glycosides from Pyrola calliantha. Helv. Chim. Acta 2008, 90, 2421–2426. [Google Scholar] [CrossRef]
- Needs, P.W.; Swlvendran, R.R. Avoiding oxidative degradation during sodium hydroxide/methyl oidine-mediated carbohydrate methylation in dimethyl sulfoxide. Carbohydr. Res. 1993, 245, 1–10. [Google Scholar] [CrossRef]
- Sultan, N.; Amin, L.E.; Zaher, A.R.; Grawish, M.E.; Scheven, B.A. Dental pulp stem cells stimulate neuronal differentiation of PC12 cells. Neural Regen. Res. 2021, 16, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Dhutia, H.; Malhotra, A.; Finocchiaro, G.; Parpia, S.; Bhatia, R.; D’Silva, A.; Gati, S.; Mellor, G.; Narain, R.; Chandra, N.; et al. Diagnostic yield and financial implications of a nationwide electrocardiographic screening programme to detect cardiac disease in the young. Europace 2021, 23, 1295–1301. [Google Scholar] [CrossRef] [PubMed]


| Retention Time (Min) | Methylation Position | Linked Type | Molar Ratio | Characteristics of Fragment Ions |
|---|---|---|---|---|
| 19.10 | 2,3-Ara | 1→5 | 2.00 | 101, 117, 161 |
| 20.52 | 2,4,6-Glu | 1→3 | 2.05 | 87, 101, 117, 129, 161, 189 |
| 21.05 | 2,3,4,6-Glu | 1→ | 11.20 | 71, 87, 101, 117, 129, 145, 161 |
| 21.34 | 2,3,4,6-Gal | 1→ | 4.27 | 71, 87, 101, 117, 129, 145, 161, 205 |
| 22.43 | 2,3,6-Glu | 1→4 | 28.74 | 71, 87, 101, 117, 129, 233 |
| 22.53 | 2,4,6-Gal | 1→3 | 1.00 | 71, 87, 101, 117, 129, 233 |
| 22.66 | 2,3,4-Glu | 1→6 | 2.13 | 71, 87, 101, 117, 129, 161, 189, 233 |
| 23.05 | 2,3,4-Gal | 1→6 | 1.01 | 71, 87, 101, 117, 129, 161, 189, 233 |
| 24.16 | 2,4-Man | 1→3,6 | 1.90 | 58, 87, 129, 159, 173, 189 |
| Methylation before Reduction | Methylated after Reduction | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Methylation Position | Linked Type | Molar Ratio | Total Moles | Ratio | Ratio | Total Moles | Molar Ratio | Linked Type | Methylation Position |
| 2,3-Ara | 1→5 | 1.78 | 1.78 | 4% | 5% | 1.59 | 1.59 | 1→5 | 2,3-Ara |
| 2,3,4,6-Gal | 1→ | 3.55 | 5.53 | 12% | 10% | 3.12 | 1.59 | 1→ | 2,3,4,6-Gal |
| 2,3,4-Gal | 1→6 | 0.98 | 0.53 | 1→6 | 2,3,4-Gal | ||||
| 2,4,6-Gal | 1→3 | 1.00 | 1.00 | 1→3 | 2,4,6-Gal | ||||
| 2,3,4,6-Glc | 1→ | 7.74 | 39.58 | 81% | 75% | 22.79 | 3.12 | 1→ | 2,3,4,6-Glc |
| 2,3,6-Glc | 1→4 | 27.49 | 18.23 | 1→4 | 2,3,6-Glc | ||||
| 2,3,4-Glc | 1→6 | 1.58 | 1.44 | 1→6 | 2,3,4-Glc | ||||
| 2,4,6-Glc | 1→3 | 2.77 | |||||||
| 2,4-Man | 1→3,6 | 0.94 | 0.94 | 3% | 8% | 2.60 | 2.60 | 1→5 | 2,4-Man |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Yu, K.; Mo, Z.; Yang, K.; Chen, F.; Yang, J. In Vitro Neurotrophic Properties and Structural Characterization of a New Polysaccharide LTC-1 from Pyrola corbieri Levl (Luticao). Molecules 2023, 28, 1544. https://doi.org/10.3390/molecules28041544
Li L, Yu K, Mo Z, Yang K, Chen F, Yang J. In Vitro Neurotrophic Properties and Structural Characterization of a New Polysaccharide LTC-1 from Pyrola corbieri Levl (Luticao). Molecules. 2023; 28(4):1544. https://doi.org/10.3390/molecules28041544
Chicago/Turabian StyleLi, Liangqun, Kangkang Yu, Zhengchang Mo, Keling Yang, Fuxue Chen, and Juan Yang. 2023. "In Vitro Neurotrophic Properties and Structural Characterization of a New Polysaccharide LTC-1 from Pyrola corbieri Levl (Luticao)" Molecules 28, no. 4: 1544. https://doi.org/10.3390/molecules28041544
APA StyleLi, L., Yu, K., Mo, Z., Yang, K., Chen, F., & Yang, J. (2023). In Vitro Neurotrophic Properties and Structural Characterization of a New Polysaccharide LTC-1 from Pyrola corbieri Levl (Luticao). Molecules, 28(4), 1544. https://doi.org/10.3390/molecules28041544






