Optimization of HS-SPME-GC/MS Analysis of Wine Volatiles Supported by Chemometrics for the Aroma Profiling of Trebbiano d’Abruzzo and Pecorino White Wines Produced in Abruzzo (Italy)
Abstract
:1. Introduction
2. Results
2.1. Qualitative Composition of Wine Aroma by HS-SPME-GC/MS
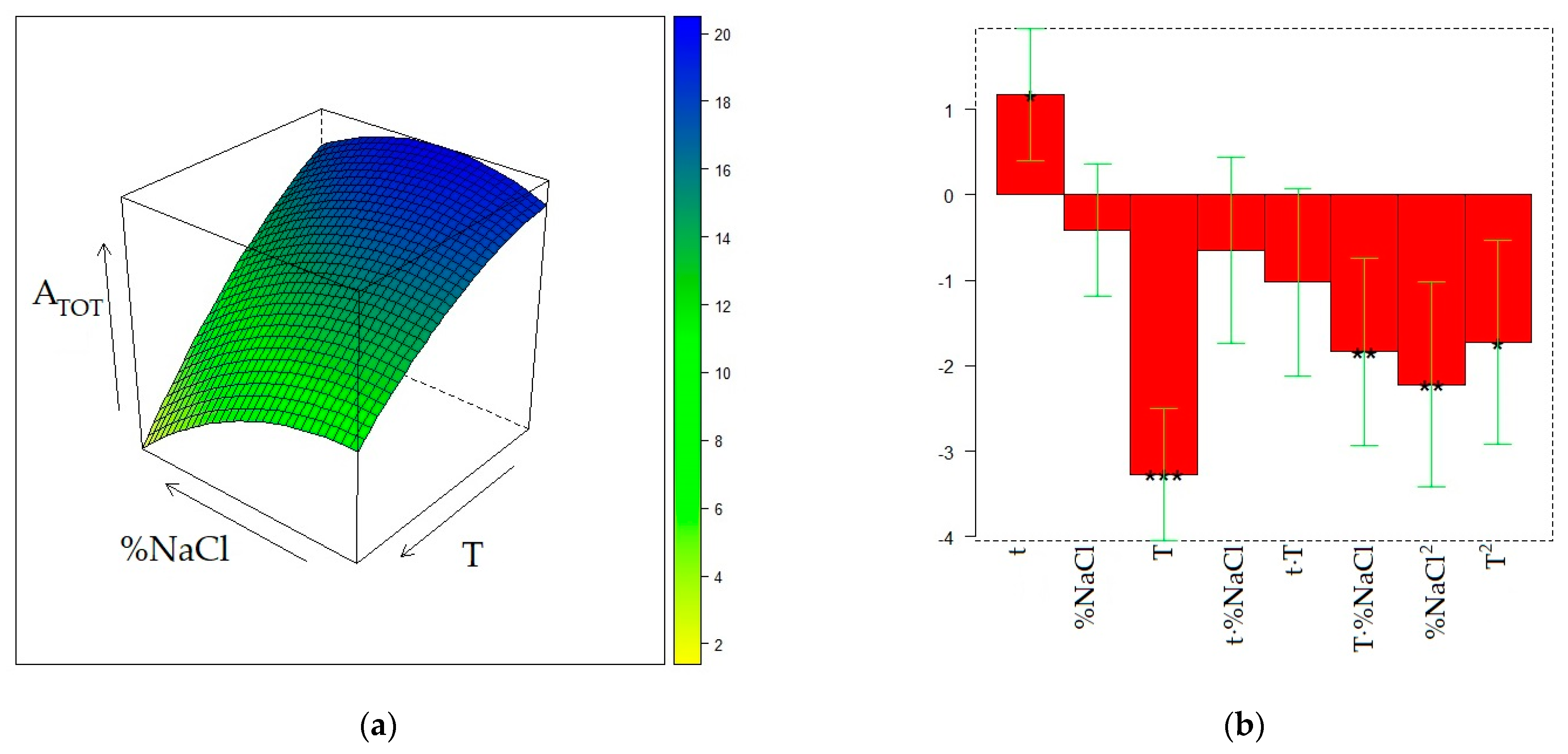
2.2. Optimization of HS-SPME on the PDMS Fiber
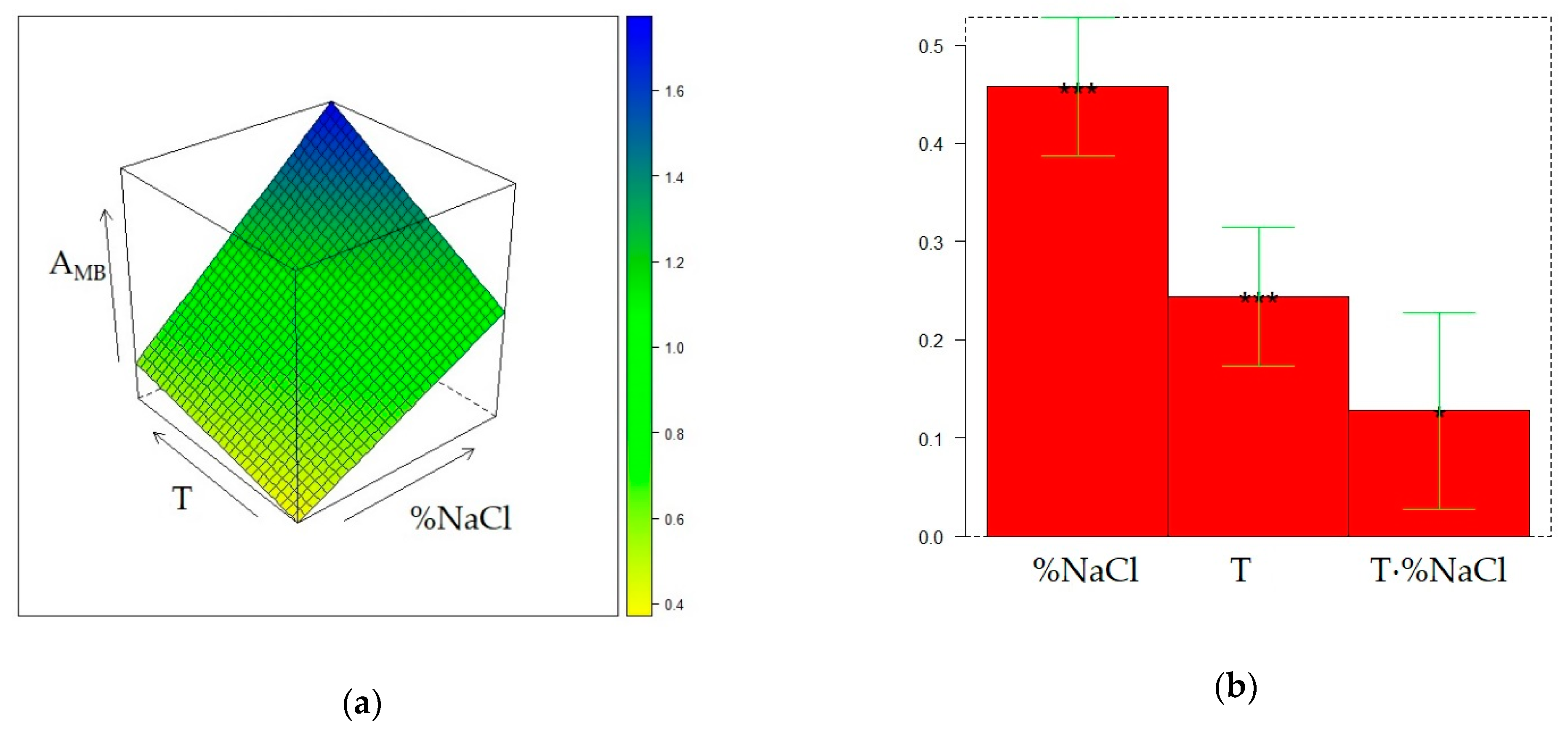
2.3. Optimization of HS-SPME on the CW/DVB Fiber
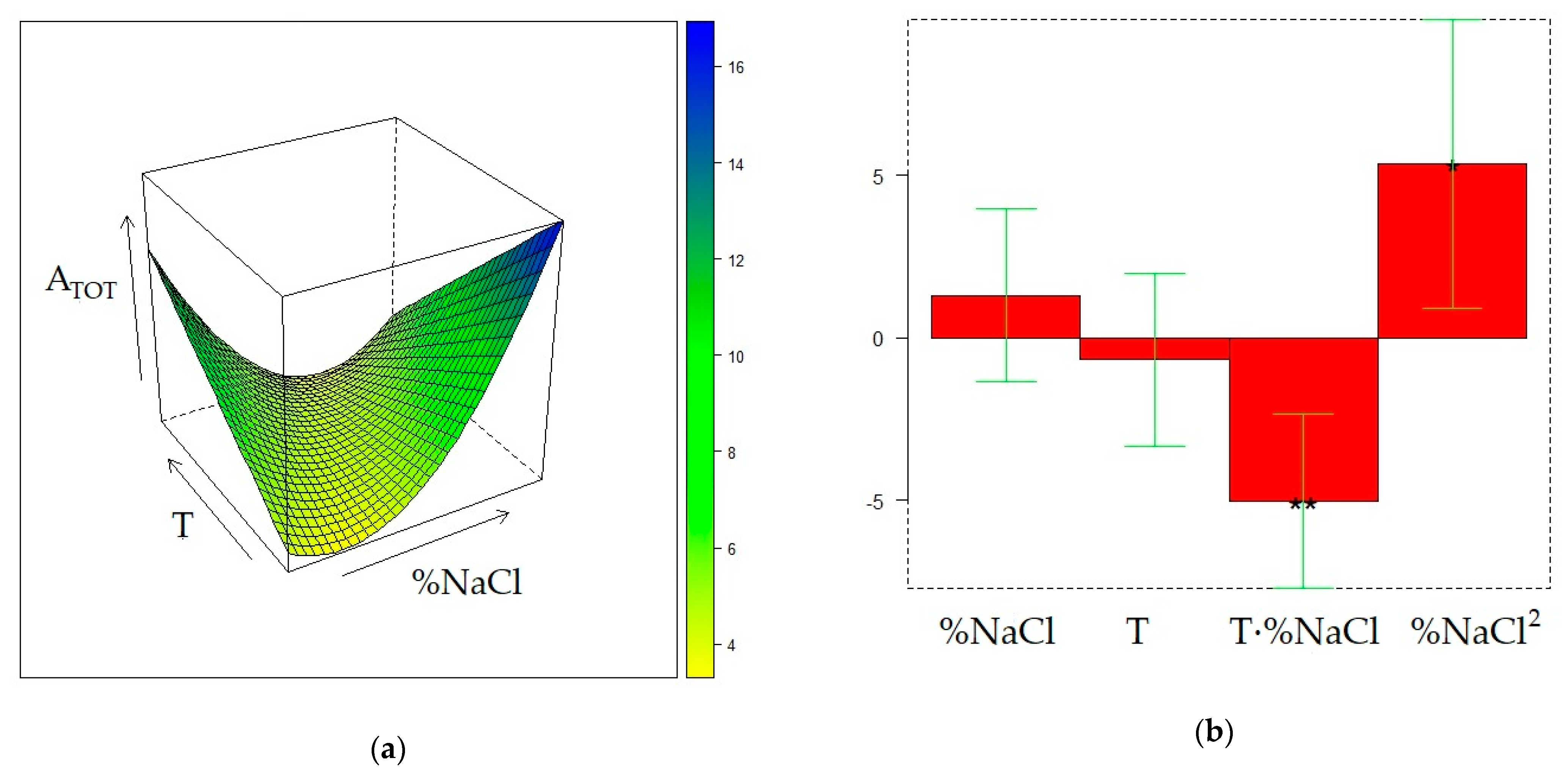
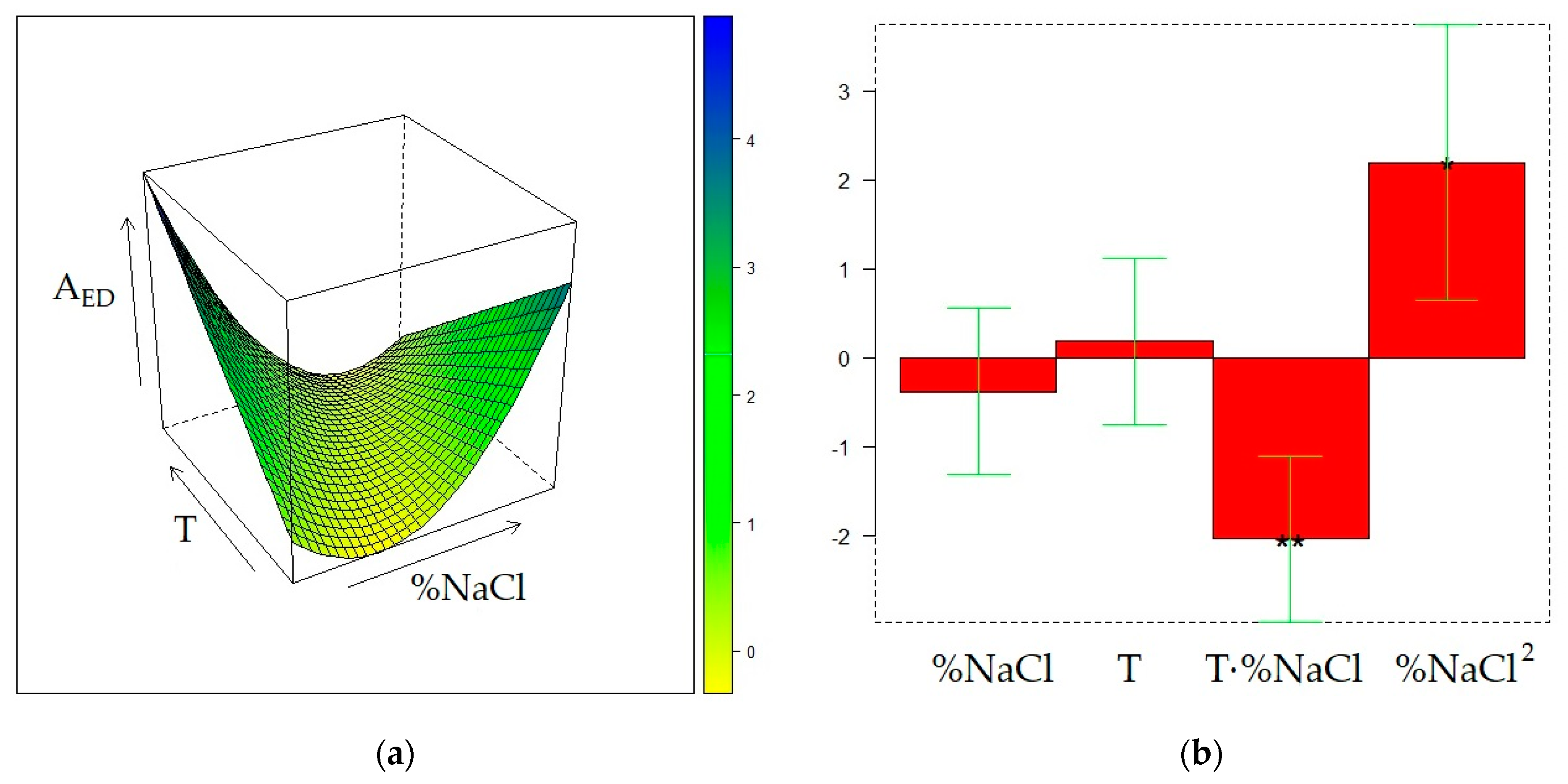
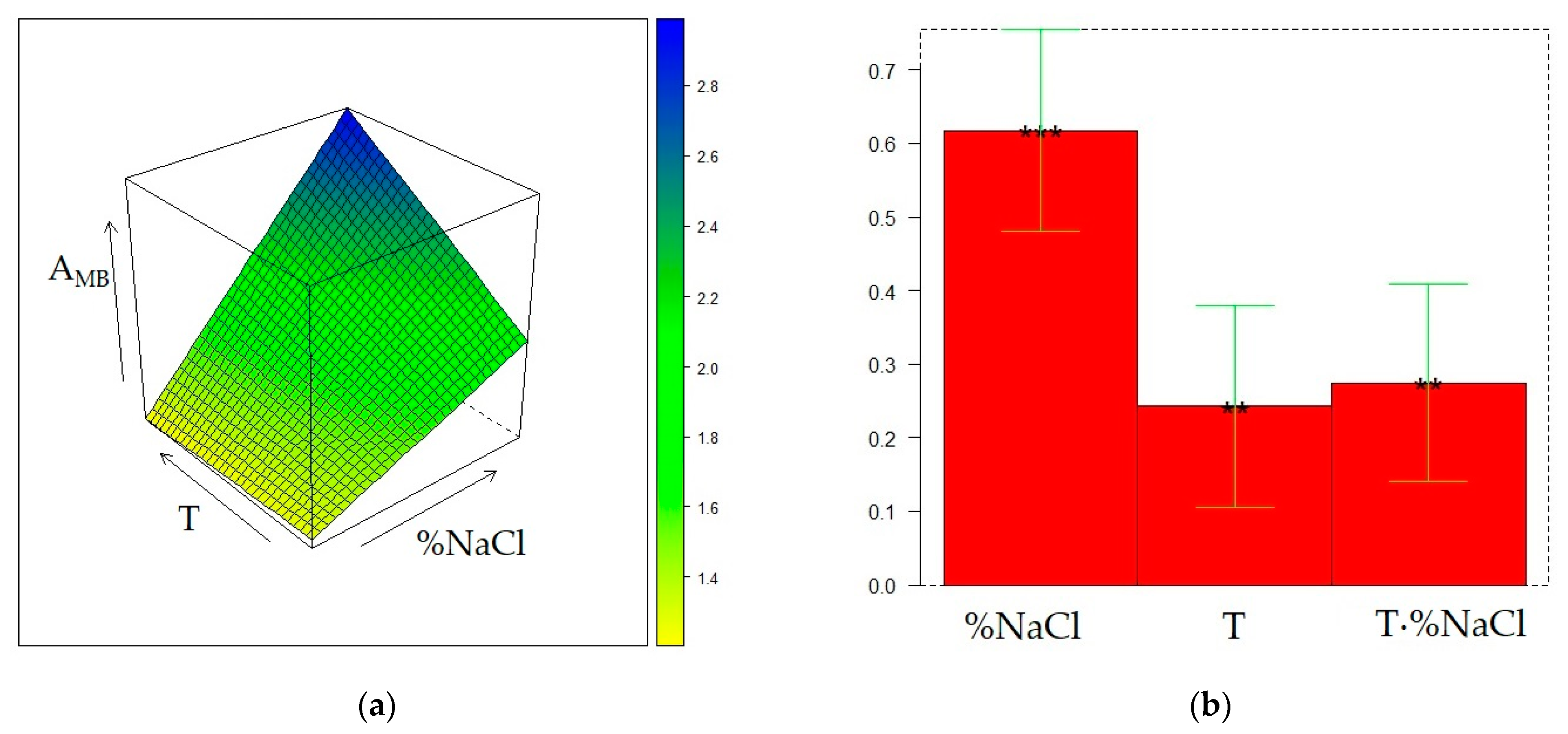
2.4. Optimization of HS-SPME on the DVB/CAR/PDMS fiber
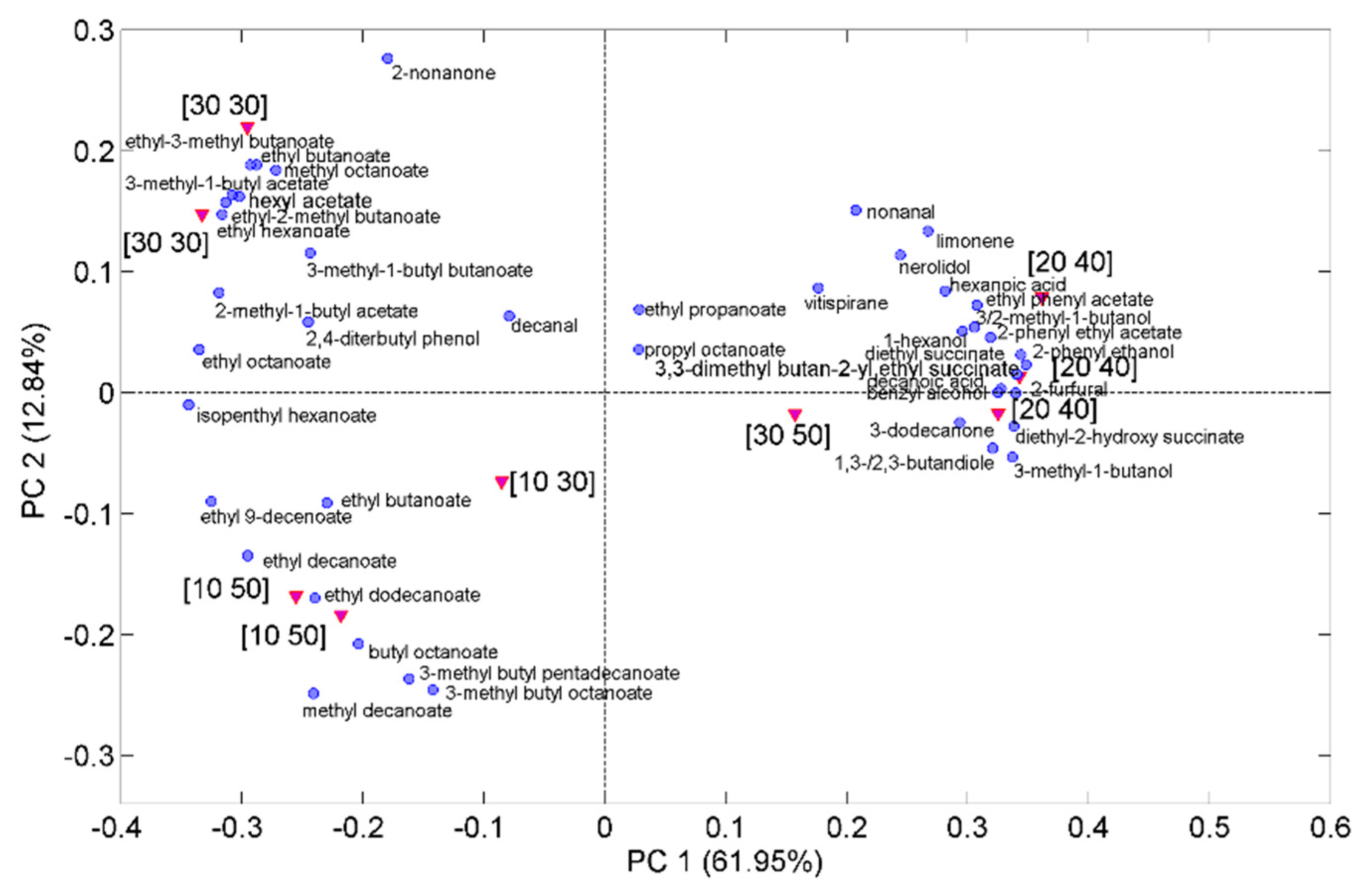
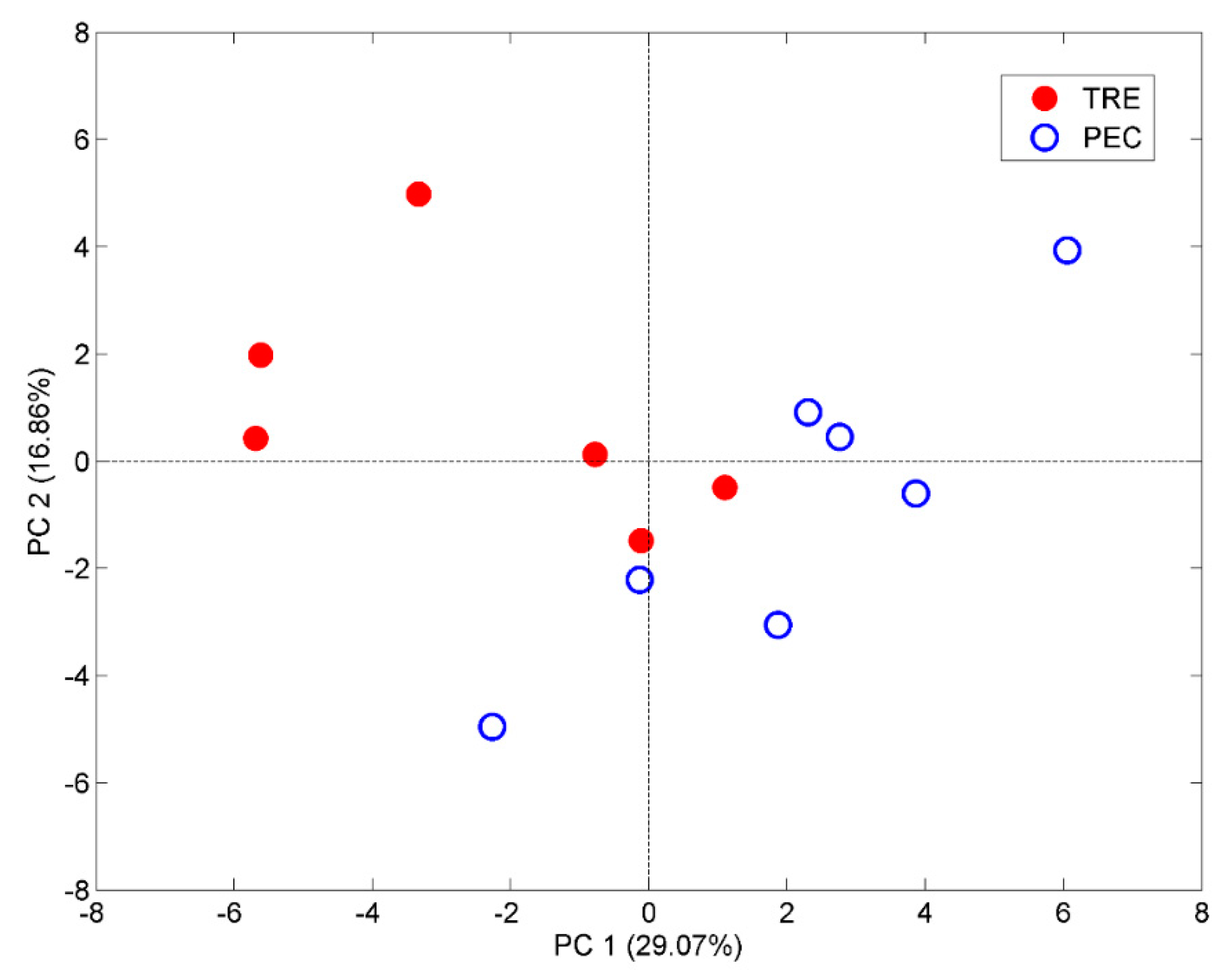
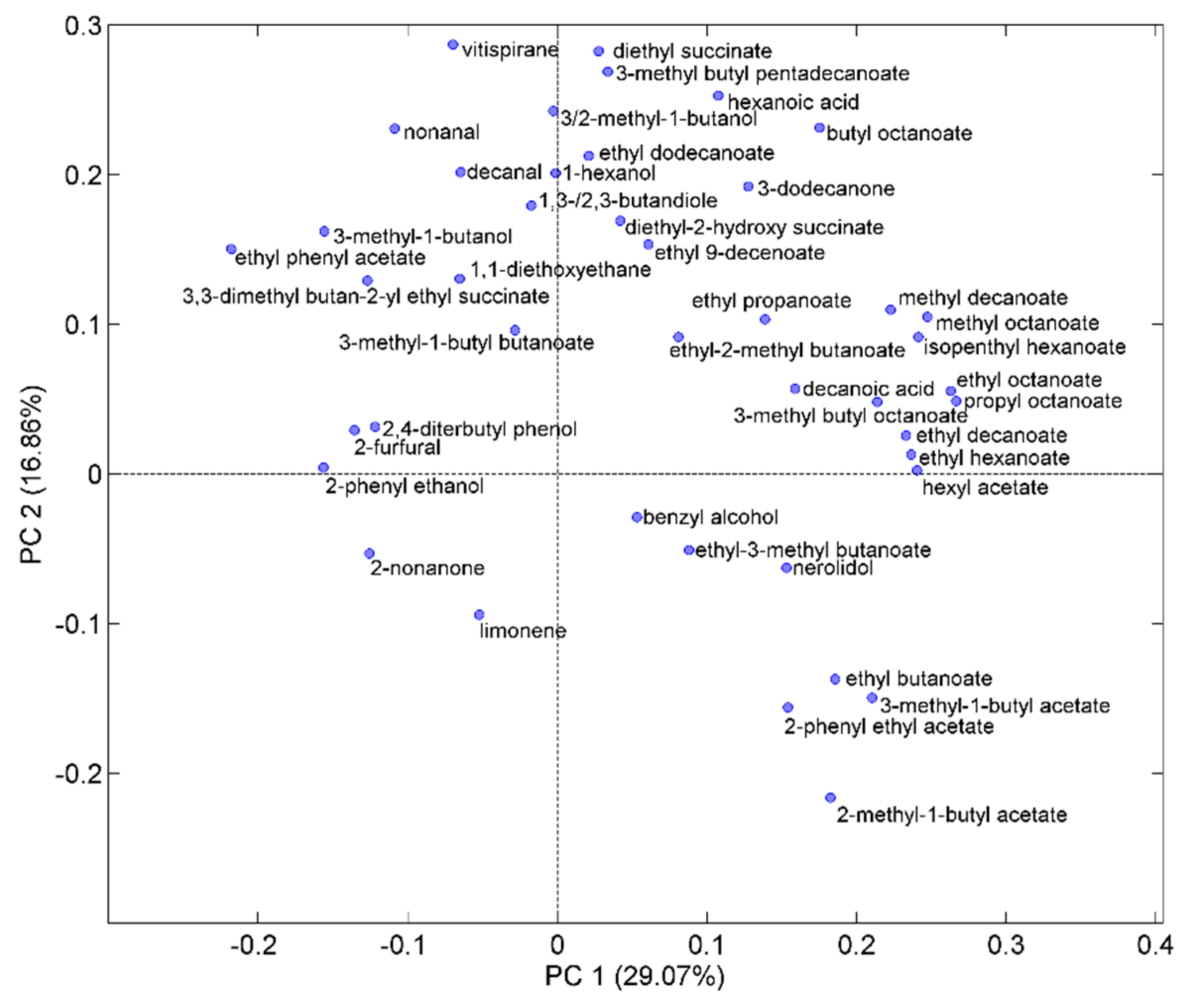
2.5. Aroma Profiles of Trebbiano d’Abruzzo and Pecorino White Wines
3. Materials and Methods
3.1. Wine Samples and Chemicals
3.2. Headspace Solid-Phase Extraction of Volatiles
3.3. Chromatographic Analysis
3.4. Design of the Experiments and Response Surface Methodology
3.5. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research progress of wine aroma components: A critical review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef] [PubMed]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors influencing the aroma composition of chardonnay wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef]
- Welke, J.E.; Nicolli, K.P.; Hernandes, K.C.; Biasoto, A.C.T.; Zini, C.A. Adaptation of an olfactometric system in a GC-FID in combination with GCxGC/MS to evaluate odor-active compounds of wine. Food Chem. 2022, 370, 131004. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Ziółkowska, A.; Wąsowicz, E.; Jeleń, H.H. Differentiation of wines according to grape variety and geographical origin based on volatiles profiling using SPME-MS and SPME-GC/MS methods. Food Chem. 2016, 213, 714–720. [Google Scholar] [CrossRef]
- Karimali, D.; Kosma, I.; Badeka, A. Varietal classification of red wine samples from four native Greek grape varieties based on volatile compound analysis, color parameters and phenolic composition. Eur. Food Res. Technol. 2020, 246, 41–53. [Google Scholar] [CrossRef]
- Wang, J.; Capone, D.L.; Wilkinson, K.L.; Jeffery, D.W. Chemical and sensory profiles of rosé wines from Australia. Food Chem. 2016, 196, 682–693. [Google Scholar] [CrossRef]
- Bouzas-Cid, Y.; Falqué, E.; Orriols, I.; Mirás-Avalos, J.M. Effects of irrigation over three years on the amino acid composition of Treixadura (Vitis vinifera L.) musts and wines, and on the aromatic composition and sensory profiles of its wines. Food Chem. 2018, 240, 707–716. [Google Scholar] [CrossRef]
- Martínez-Gil, A.M.; del Alamo-Sanza, M.; Gutiérrez-Gamboa, G.; Moreno-Simunovic, Y.; Nevares, I. Volatile composition and sensory characteristics of Carménère wines macerating with Colombian (Quercus humboldtii) oak chips compared to wines macerated with American (Q. alba) and European (Q. petraea) oak chips. Food Chem. 2018, 266, 90–100. [Google Scholar] [CrossRef]
- González-Álvarez, M.; Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Sensory description of sweet wines obtained by the winemaking procedures of raisining, botrytisation and fortification. Food Chem. 2014, 145, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Noguerol-Pato, R.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Quantitative determination and characterisation of the main odourants of Mencía monovarietal red wines. Food Chem. 2009, 117, 473–484. [Google Scholar] [CrossRef]
- Lenti, L.; Scortichini, S.; Pacetti, D.; Cespi, M.; Fiorini, D. Polydimethylsiloxane/divinylbenzene overcoated fiber and its application to extract and analyse wine volatile compounds by solid-phase microextraction and gas chromatography coupled to mass spectrometry: Direct immersion, headspace or both? Food Res. Int. 2021, 148, 110632. [Google Scholar] [CrossRef] [PubMed]
- Marinaki, M.; Sampsonidis, I.; Lioupi, A.; Arapitsas, P.; Thomaidis, N.; Zinoviadou, K.; Theodoridis, G. Development of two-level Design of Experiments for the optimization of a HS-SPME-GC-MS method to study Greek monovarietal PDO and PGI wines. Talanta 2023, 253, 123987. [Google Scholar] [CrossRef]
- Pawliszyn, J. Theory of Solid-Phase Microextraction. In Handbook of Solid Phase Microextraction; Elsevier: Amsterdam, The Netherlands, 2012; pp. 13–59. ISBN 9780124160170. [Google Scholar]
- Pati, S.; Tufariello, M.; Crupi, P.; Coletta, A.; Grieco, F.; Losito, I. Quantification of volatile compounds in wines by HS-SPME-GC/MS: Critical issues and use of multivariate statistics in method optimization. Processes 2021, 9, 662. [Google Scholar] [CrossRef]
- Marrubini, G.; Dugheri, S.; Cappelli, G.; Arcangeli, G.; Mucci, N.; Appelblad, P.; Melzi, C.; Speltini, A. Experimental designs for solid-phase microextraction method development in bioanalysis: A review. Anal. Chim. Acta 2020, 1119, 77–100. [Google Scholar] [CrossRef]
- Reis, M.S.; Pereira, A.C.; Leça, J.M.; Rodrigues, P.M.; Marques, J.C. Multiresponse and multiobjective latent variable optimization of modern analytical instrumentation for the quantification of chemically related families of compounds: Case study—Solid-phase microextraction (SPME) applied to the quantification of analytes. J. Chemom. 2019, 33, 1–12. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Paula, E.; Moreira, N.; Elias, G.; Gomes, S.; Leite, F.; Moraes, C.; Guedes, P.; Pinho, D. Talanta Development and validation of automatic HS-SPME with a gas chromatography-ion trap / mass spectrometry method for analysis of volatiles in wines. Talanta 2012, 101, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.; Watkins, P.; Smith, J.; Allen, M.; Marriott, P. Analysis of methoxypyrazines in wine using headspace solid phase microextraction with isotope dilution and comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2005, 28, 1075–1082. [Google Scholar] [CrossRef]
- Burin, V.M.; Marchand, S.; De Revel, G.; Bordignon-Luiz, M.T. Development and validation of method for heterocyclic compounds in wine: Optimization of HS-SPME conditions applying a response surface methodology. Talanta 2013, 117, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Sadoughi, N.; Schmidtke, L.M.; Antalick, G.; Blackman, J.W.; Steel, C.C. Gas chromatography-mass spectrometry method optimized using response surface modeling for the quantitation of fungal off-flavors in grapes and wine. J. Agric. Food Chem. 2015, 63, 2877–2885. [Google Scholar] [CrossRef]
- Martínez-Uruñuela, A.; González-Sáiz, J.M.; Pizarro, C. Optimisation of a headspace solid-phase microextraction method for the direct determination of chloroanisoles related to cork taint in red wine. J. Chromatogr. A 2004, 1056, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Ravelo-Pérez, L.M.; Hernández-Borges, J.; Borges-Miguel, T.M.; Rodríguez-Delgado, M.Á. Multiple pesticide analysis in wine by MEKC combined with solid-phase microextraction and sample stacking. Electrophoresis 2007, 28, 4072–4081. [Google Scholar] [CrossRef] [PubMed]
- Pietrangeli, F.; Odoardi, M. L’Abruzzo Della Vite e del Vino; Menabò srl: Ortona, Italy, 2017. [Google Scholar]
- Biancolillo, A.; D’Archivio, A.A.; Pietrangeli, F.; Cesarone, G.; Ruggieri, F.; Foschi, M.; Reale, S.; Rossi, L.; Crucianelli, M. Varietal Discrimination of Trebbiano d’Abruzzo, Pecorino and Passerina White Wines Produced in Abruzzo (Italy) by Sensory Analysis and Multi-Block Classification Based on Volatiles, Organic Acids, Polyphenols, and Major Elements. Appl. Sci. 2022, 12, 9794. [Google Scholar] [CrossRef]
- NIST14: Mass Spectral Database; NIST, National Institute of Standards and Technology: Gaithersburg, MD, USA, 2014.
- Rocha, S.; Ramalheira, V.; Barros, A.; Delgadillo, I.; Coimbra, M.A. Headspace solid phase microextraction (SPME) analysis of flavor compounds in wines. Effect of the matrix volatile composition in the relative response factors in a wine model. J. Agric. Food Chem. 2001, 49, 5142–5151. [Google Scholar] [CrossRef]
- Plutowska, B.; Wardencki, W. Determination of volatile fatty acid ethyl esters in raw spirits using solid phase microextraction and gas chromatography. Anal. Chim. Acta 2008, 613, 64–73. [Google Scholar] [CrossRef]
- Ferreira, V.; Herrero, P.; Zapata, J.; Escudero, A. Coping with matrix effects in headspace solid phase microextraction gas chromatography using multivariate calibration strategies. J. Chromatogr. A 2015, 1407, 30–41. [Google Scholar] [CrossRef]
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Tool) 2019. Available online: http://gruppochemiometria.it/index.php/software (accessed on 20 December 2022).
- Wold, S. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]

| Compound | SPME Fiber | ||
|---|---|---|---|
| PDMS | CW/DVB | DVB/CAR/PDMS | |
| ethyl acetate | x | x | - |
| 2-methyl-1-propanol | x | x | x |
| butanol | - | - | x |
| ethyl propanoate | x | x | x |
| 1,1-diethoxyethane | - | - | x |
| 3-methyl-1-butanol | x | x | x |
| 2-methyl-1-butanol | x | x | x |
| ethyl 2-methylpropanoate | x | x | x |
| 1,3-butandiole/2,3-butandiole | x | x | x |
| 1,3-butandiole/2,3-butandiole | x | x | x |
| ethyl butanoate | x | x | x |
| ethyl lactate | x | x | x |
| 2-furfural | - | - | x |
| 3-methylbutanoic acid | - | - | x |
| 4-methyl-1-pentanol | - | x | x |
| ethyl 2-methylbutanoate | x | - | x |
| ethyl 3-methylbutanoate | x | x | x |
| 1-hexanol | x | x | x |
| 3-methyl-1-butyl-acetate | x | x | x |
| 2-methyl-1-butyl acetate | - | - | x |
| 2-methyl 2,3-pentandiol | - | - | x |
| 4-methyldihydrofurane-2(3H)-one/butyrolactone | - | - | x |
| anisole | - | - | x |
| benzaldehyde | - | - | x |
| hexanoic acid | x | x | x |
| ethyl hexanoate | x | x | x |
| hexyl acetate | x | x | x |
| limonene | - | x | - |
| 2-ethyl hexanol | - | - | x |
| benzyl alcohol | - | - | x |
| (E)-2-hexenoic acid | x | - | x |
| 3-methyl-1-butylbutanoate/pentyl 2-methylpropanoate | - | - | x |
| 1-octanol | - | - | x |
| guaiacol (o-methoxyphenol) | - | - | x |
| 2-nonanone | - | - | x |
| ethyl eptanoate | x | x | - |
| lynalol | x | x | x |
| 2-nonen-1-ol/nonanal | - | - | x |
| 2-phenyl ethanol | x | x | x |
| methyl octanoate | x | x | x |
| octanoic acid | x | x | - |
| diethyl succinate | x | x | x |
| ethyl octanoate | x | x | x |
| decanal | - | - | x |
| ethyl phenylacetate | x | x | x |
| isopenthyl hexanoate | x | x | x |
| 2-phenylethyl acetate | x | x | x |
| diethyl 2-hydrosuccinate | - | - | x |
| 1-decanol | - | - | x |
| vitispirane | x | x | x |
| propyl octanoate | - | - | x |
| phthalic anhydride | - | x | x |
| methyl decanoate | - | x | x |
| butyl octanoate | x | x | x |
| 1,1,6-trimethyl-1,2-dihydronaphtalene | x | x | x |
| decanoic acid | x | - | x |
| 3-dodecanone | - | x | x |
| ethyl 9-decenoate | x | x | x |
| ethyl decanoate | x | x | x |
| 3,3-dimethylbutan-2-yl ethyl succinate | x | x | x |
| 3-methylbutyl octanoate | x | x | x |
| 2,5-di-tert-butylcyclohexa-2,5-diene-1,4-dione | - | - | x |
| 2-dodecenale (E)/dodecanol | - | - | x |
| 2,5-diterbutyl phenol | - | - | x |
| nerolidol | - | x | x |
| ethyl dodecanoate | x | x | x |
| isoamyl decanoate | x | x | - |
| 3-methylbutyl pentadecanoate | - | - | x |
| galaxolide | - | x | - |
| 2-methylpropyl phthalate | - | x | - |
| t(min) | NaCl (%) | T(°C) | ATOT 10−6 | AED∙10−6 | AMB∙10−6 | |||
|---|---|---|---|---|---|---|---|---|
| exp | calc | exp | calc | exp | calc | |||
| 10 | 10 | 40 | 9.16 | 8.73 | 2.66 | 2.61 | 0.46 | 0.49 |
| 30 | 10 | 40 | 13.18 | 12.37 | 4.98 | 4.54 | 0.60 | 0.49 |
| 10 | 30 | 40 | 9.04 | 9.20 | 1.47 | 1.68 | 1.43 | 1.40 |
| 30 | 30 | 40 | 10.46 | 10.24 | 2.24 | 2.06 | 1.60 | 1.40 |
| 10 | 20 | 30 | 12.06 | 11.07 | 2.99 | 2.56 | 0.72 | 0.70 |
| 30 | 20 | 50 | 7.80 | 7.51 | 2.46 | 2.66 | 1.13 | 1.19 |
| 10 | 20 | 50 | 7.88 | 7.21 | 2.37 | 2.18 | 1.13 | 1.19 |
| 30 | 20 | 30 | 16.07 | 16.09 | 4.42 | 4.38 | 0.66 | 0.70 |
| 20 | 30 | 50 | 2.70 | 2.89 | 0.16 | 0.02 | 1.74 | 1.77 |
| 20 | 10 | 30 | 9.80 | 10.26 | 2.41 | 2.78 | 0.36 | 0.37 |
| 20 | 10 | 50 | 6.61 | 7.39 | 3.34 | 3.47 | 0.64 | 0.60 |
| 20 | 30 | 30 | 13.22 | 13.09 | 2.71 | 2.81 | 0.95 | 1.03 |
| 20 | 20 | 40 | 12.12 | 12.36 | 3.18 | 3.40 | 0.85 | 0.95 |
| 20 | 20 | 40 | 11.29 | 12.36 | 3.15 | 3.40 | 0.96 | 0.95 |
| NaCl (%) | T(°C) | ATOT∙10−6 | AED∙10−6 | AMB∙10−6 |
|---|---|---|---|---|
| 10 | 30 | 4.30 | 1.50 | 0.88 |
| 10 | 50 | 4.63 | 1.88 | 0.94 |
| 30 | 50 | 9.24 | 0.38 | 4.98 |
| 30 | 30 | 3.95 | 0.22 | 2.23 |
| 20 | 40 | 2.69 | 0.08 | 1.19 |
| 20 | 40 | 4.58 | 0.28 | 2.34 |
| 20 | 40 | 3.86 | 0.12 | 2.04 |
| NaCl (%) | T (°C) | ATOT∙10−6 | AED∙10−6 | AMB∙10−6 | |||
|---|---|---|---|---|---|---|---|
| exp | calc | exp | calc | exp | calc | ||
| 10 | 30 | 4.29 | 4.29 | 0.52 | 0.52 | 1.31 | 1.27 |
| 10 | 50 | 12.01 | 13.02 | 4.59 | 4.96 | 1.30 | 1.21 |
| 10 | 50 | 14.02 | 13.02 | 5.33 | 4.96 | 1.15 | 1.21 |
| 30 | 30 | 19.89 | 16.94 | 4.87 | 3.84 | 1.90 | 1.96 |
| 30 | 30 | 13.99 | 16.94 | 2.81 | 3.84 | 2.05 | 1.96 |
| 30 | 50 | 5.57 | 5.57 | 0.13 | 0.13 | 3.03 | 2.99 |
| 20 | 40 | 4.83 | 4.60 | 0.15 | 0.17 | 1.95 | 1.85 |
| 20 | 40 | 4.74 | 4.60 | 0.20 | 0.17 | 1.78 | 1.85 |
| 20 | 40 | 4.24 | 4.60 | 0.15 | 0.17 | 1.67 | 1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, L.; Foschi, M.; Biancolillo, A.; Maggi, M.A.; D’Archivio, A.A. Optimization of HS-SPME-GC/MS Analysis of Wine Volatiles Supported by Chemometrics for the Aroma Profiling of Trebbiano d’Abruzzo and Pecorino White Wines Produced in Abruzzo (Italy). Molecules 2023, 28, 1534. https://doi.org/10.3390/molecules28041534
Rossi L, Foschi M, Biancolillo A, Maggi MA, D’Archivio AA. Optimization of HS-SPME-GC/MS Analysis of Wine Volatiles Supported by Chemometrics for the Aroma Profiling of Trebbiano d’Abruzzo and Pecorino White Wines Produced in Abruzzo (Italy). Molecules. 2023; 28(4):1534. https://doi.org/10.3390/molecules28041534
Chicago/Turabian StyleRossi, Leucio, Martina Foschi, Alessandra Biancolillo, Maria Anna Maggi, and Angelo Antonio D’Archivio. 2023. "Optimization of HS-SPME-GC/MS Analysis of Wine Volatiles Supported by Chemometrics for the Aroma Profiling of Trebbiano d’Abruzzo and Pecorino White Wines Produced in Abruzzo (Italy)" Molecules 28, no. 4: 1534. https://doi.org/10.3390/molecules28041534
APA StyleRossi, L., Foschi, M., Biancolillo, A., Maggi, M. A., & D’Archivio, A. A. (2023). Optimization of HS-SPME-GC/MS Analysis of Wine Volatiles Supported by Chemometrics for the Aroma Profiling of Trebbiano d’Abruzzo and Pecorino White Wines Produced in Abruzzo (Italy). Molecules, 28(4), 1534. https://doi.org/10.3390/molecules28041534









