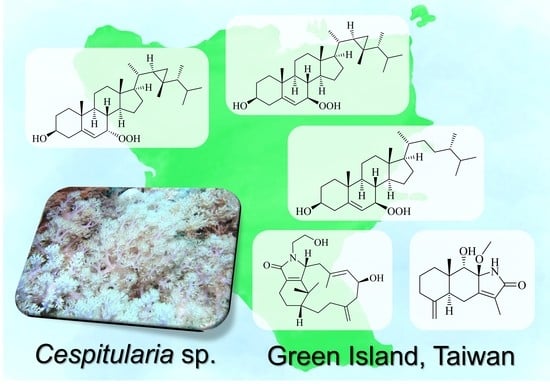
New Verticillene Diterpenoids, Eudesmane Sesquiterpenoids, and Hydroperoxysteroids from the Further Chemical Investigation of a Taiwanese Soft Coral Cespitularia sp.
Abstract
1. Introduction
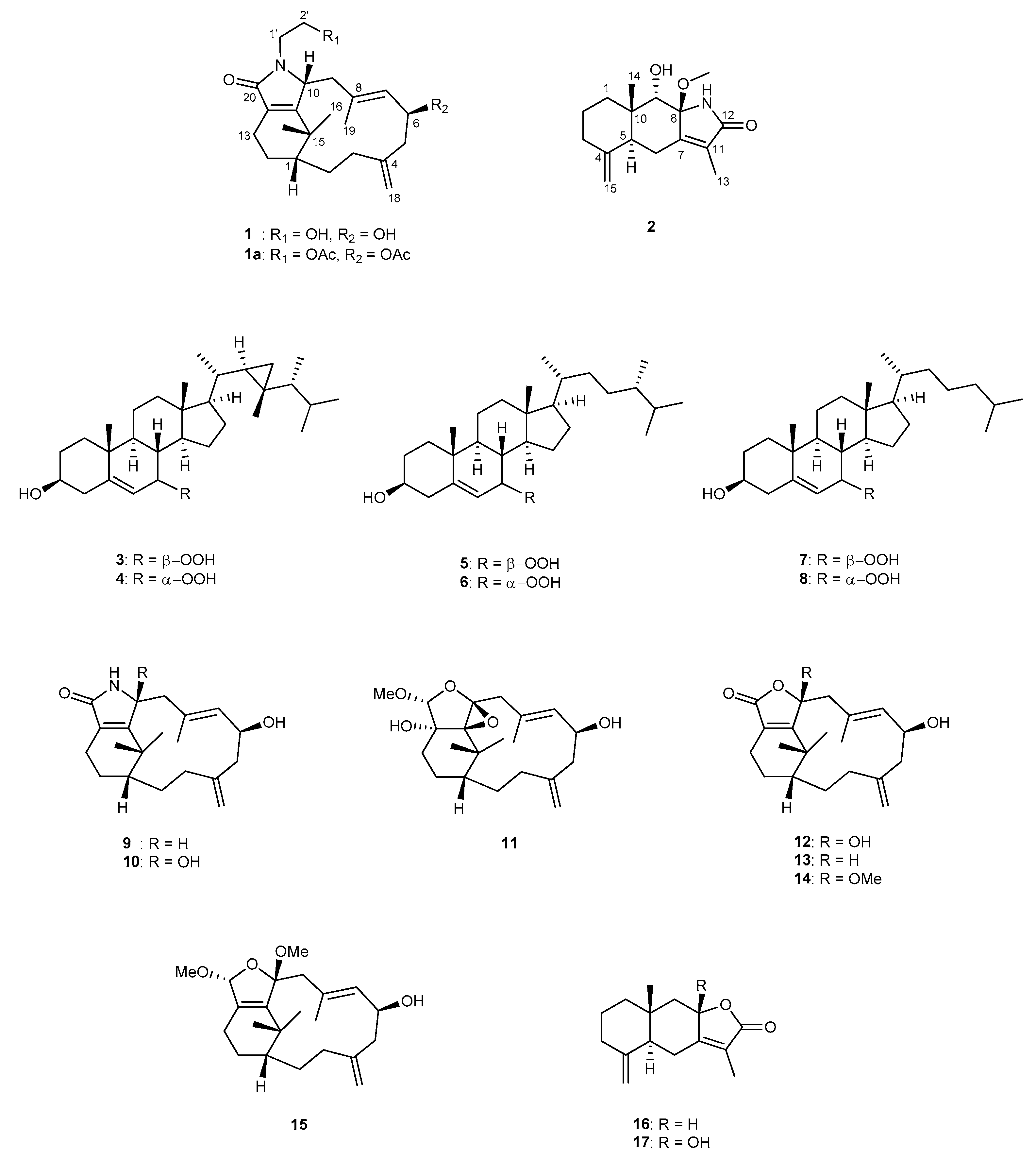
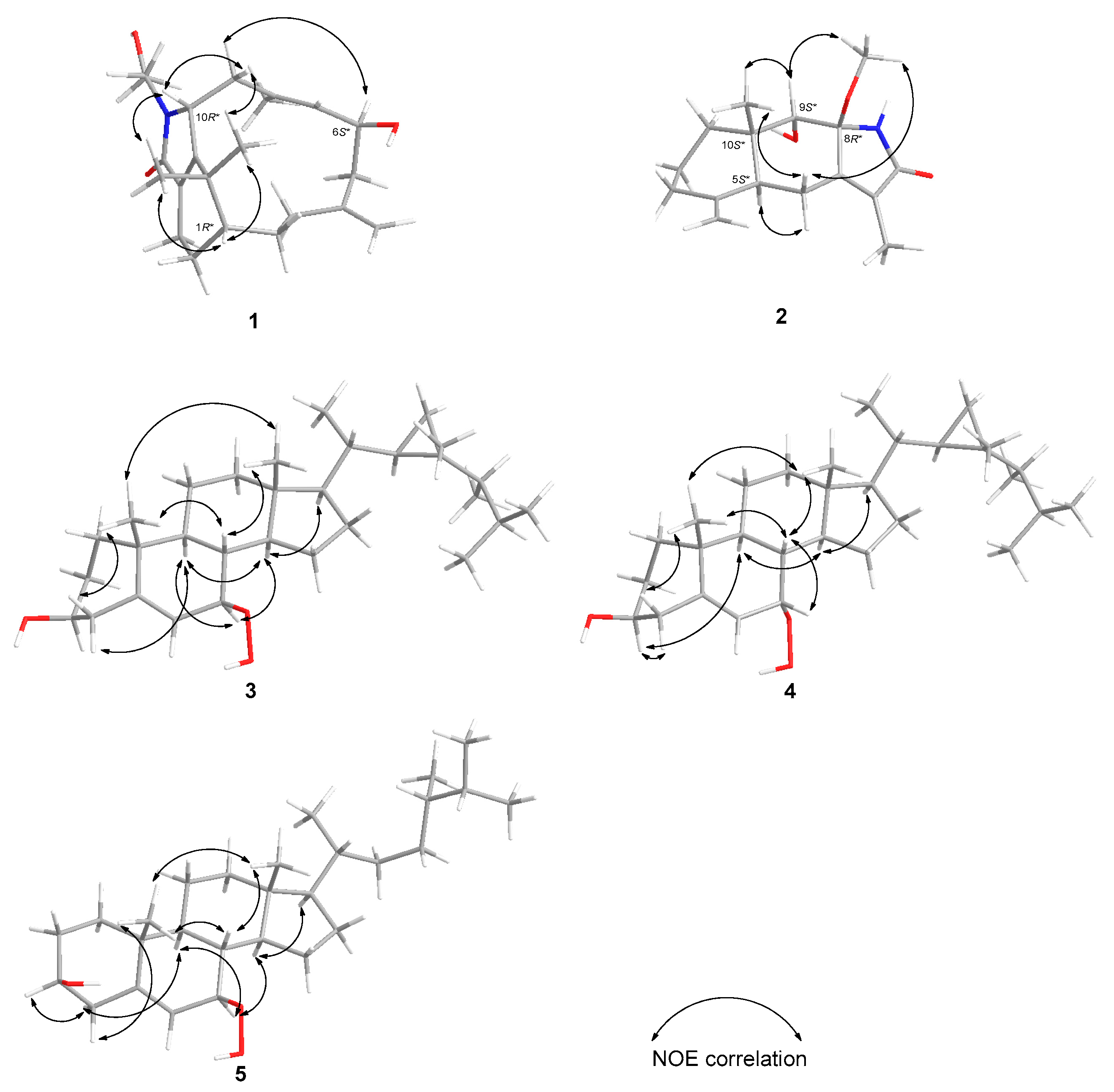
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Cytotoxicity Assay
3.5. In Vitro Antibacterial Assay
3.6. In Vitro Anti-Inflammatory Assay
3.6.1. Measurement of Cytokine Production by Dendritic Cells (DCs)
3.6.2. Measurement of Nitric Oxide (NO) Production by DCs
3.6.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Duh, C.-Y.; El-Gamal, A.A.H.; Wang, S.-K.; Dai, C.-F. Novel Terpenoids from the Formosan Soft Coral Cespitularia hypotentaculata. J. Nat. Prod. 2002, 65, 1429–1433. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Lin, Y.-S.; Kuo, Y.-H.; Cheng, Y.-B. Cespitulactams A, B, and C, Three New Nitrogen-Containing Diterpenes from Cespitularia taeniata May. Tetrahedron Lett. 2005, 46, 7893–7897. [Google Scholar] [CrossRef]
- Cheng, Y.-B.; Chen, C.-Y.; Kuo, Y.-H.; Shen, Y.-C. New Nitrogen-Containing Sesquiterpenoids from the Taiwanese Soft Coral Cespitularia taeniata May. Chem. Biodivers. 2009, 6, 1266–1272. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Lin, E.-H.; Wen, Z.-H.; Chiang, M.Y.-N.; Duh, C.-Y. Two New Verticillane-Type Diterpenoids from the Formosan Soft Coral Cespitularia hypotentaculata. Chem. Pharm. Bull. 2010, 58, 848–851. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Fazary, A.-E.; Lin, Y.-C.; Hwang, T.-L.; Shen, Y.-C. New Verticillane Diterpenoids from Cespitularia taeniata. Chem. Biodivers. 2012, 9, 654–661. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Wang, S.-S.; Chen, C.-H.; Kuo, Y.-H.; Shen, Y.-C. Cespitulones A and B, Cytotoxic Diterpenoids of a New Structure Class from the Soft Coral Cespitularia taeniata. Mar. Drugs 2014, 12, 3477–3486. [Google Scholar] [CrossRef]
- Wang, S.-S.; Cheng, Y.-B.; Lin, Y.-C.; Liaw, C.-C.; Chang, J.-Y.; Kuo, Y.-H.; Shen, Y.-C. Nitrogen-Containing Diterpenoids, Sesquiterpenoids, and Nor-Diterpenoids from Cespitularia taeniata. Mar. Drugs 2015, 13, 5796–5814. [Google Scholar] [CrossRef]
- Duh, C.-Y.; Li, C.-H.; Wang, S.-K.; Dai, C.-F. Diterpenoids, Norditerpenoids, and Secosteroids from the Formosan Soft Coral Cespitularia hypotentaculata. J. Nat. Prod. 2006, 69, 1188–1192. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Lin, J.-J.; Wu, Y.-R.; Chang, J.-Y.; Duh, C.-Y.; Lo, K.-L. New Norditerpenoids from Cespitularia hypotentaculata. Tetrahedron Lett. 2006, 47, 6651–6655. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Ho, C.-J.; Kuo, Y.-H.; Lin, Y.-S. Cespitulactones A and B, New Diterpenoids from Cespitularia taeniata. Bioorganic Med. Chem. Lett. 2006, 16, 2369–2372. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C.; Cheng, Y.-B.; Kobayashi, J.; Kubota, T.; Takahashi, Y.; Mikami, Y.; Ito, J.; Lin, Y.-S. Nitrogen-Containing Verticillene Diterpenoids from the Taiwanese Soft Coral Cespitularia taeniata. J. Nat. Prod. 2007, 70, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C.; Wu, Y.-R.; Lin, J.-J.; Lo, K.-L.; Kuo, Y.-C.; Khalil, A.-T. Eight New Diterpenoids from Soft Coral Cespitularia hypotentaculata. Tetrahedron 2007, 63, 10914–10920. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Lo, K.-L.; Kuo, Y.-H.; Kuo, Y.-C.; Chen, C.-H.; Khalil, A.-T. Cespihypotins Q-V, Verticillene Diterpenoids from Cespitularia hypotentaculata. J. Nat. Prod. 2008, 71, 1993–1997. [Google Scholar] [CrossRef]
- Cheng, Y.-B.; Lo, K.-L.; Chen, C.-Y.; Khalil, A.-T.; Shen, Y.-C. New Verticillane-Type Diterpenoids from the Taiwanese Soft Coral Cespitularia hypotentaculata. Helv. Chim. Acta 2008, 91, 2308–2315. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Abd El-Razek, M.H.; Shen, Y.-C. Verticillane and Norverticillane Diterpenoids from the Formosan Soft Coral Cespitularia hypotentaculata. Helv. Chim. Acta 2009, 92, 2146–2154. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lin, C.-C.; Chu, Y.-C.; Fu, C.-W.; Sheu, J.-H. Bioactive Diterpenes, Norditerpenes, and Sesquiterpenes from a Formosan Soft Coral Cespitularia sp. Pharmaceuticals 2021, 14, 1252. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Abd El-Razek, M.H.; Shen, Y.-C. Verticillane-Type Diterpenoids and an Eudesmanolide-Type Sesquiterpene from the Formosan Soft Coral Cespitularia hypotentaculata. Helv. Chim. Acta 2010, 93, 1238. [Google Scholar] [CrossRef]
- Pinto, F.C.L.; Almeida, J.G.; Silveira, E.R.; Costa, A.M.; Guimarães, L.A.; Wilke, D.V.; Costa-Lotufo, L.V.; Torres, M.C.M.; Pessoa, O.D.L. Steroids from the Brazilian Zoanthids Palythoa caribaeorum and Palythoa variabilis. J. Braz. Chem. Soc. 2017, 28, 485–491. [Google Scholar] [CrossRef]
- Sung, P.-J.; Lin, M.-R.; Chen, J.-J.; Lin, S.-F.; Wu, Y.-C.; Hwang, T.-L.; Fang, L.-S. Hydroperoxysterols from the Tunicate Eudistoma sp. Chem. Pharm. Bull. 2007, 55, 666–668. [Google Scholar] [CrossRef]
- Duan, J.-A.; Wang, L.; Qian, S.; Su, S.; Tang, Y. A New Cytotoxic Prenylated Dihydrobenzofuran Derivative and Other Chemical Constituents from the Rhizomes of Atractylodes lancea DC. Arch. Pharm. Res. 2008, 31, 965–969. [Google Scholar] [CrossRef]
- Thanh, N.V.; Ngoc, N.T.; Anh, H.L.T.; Thung, D.C.; Thao, D.T.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Minh, C.V. Steroid Constituents from the Soft Coral Sinularia microspiculata. J. Asian Nat. Prod. Res. 2016, 18, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Al-Lihaibi, S.S.; Abdel-Lateff, A.; Alarif, W.M.; Alorfi, H.S.; Nogata, Y.; Okino, T. Environmentally Friendly Antifouling Metabolites from Red Sea Organisms. J. Chem. 2019, 2019, 3278394. [Google Scholar] [CrossRef]
- Fan, F.; Li, G.-Q.; Li, Z.-J.; Zhang, J.; Yuan, E.; Wu, L.; Ma, G.-Q.; Bae, Y.-S. Steroidal Compounds from Roots of Cinnamomum camphora. Chem. Nat. Compd. 2020, 56, 177–179. [Google Scholar] [CrossRef]
- Rahelivao, M.P.; Lübken, T.; Gruner, M.; Kataeva, O.; Ralambondrahety, R.; Andriamanantoanina, H.; Checinski, M.P.; Bauer, I.; Knölker, H.J. Isolation and Structure Elucidation of Natural Products of Three Soft Corals and a Sponge from the Coast of Madagascar. Org. Biomol. Chem. 2017, 15, 2593–2608. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue Assay for Cellular Growth and Viability In Vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (Resazurin) Fluorescent Dye for the Assessment of Mammalian Cell Cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chao, C.-H.; Fu, C.-W.; Chiou, S.-F.; Huang, T.-Y.; Yang, Y.-J.; Wu, S.-H.; Chen, S.-L.; Wang, H.-C.; Yu, M.-C.; et al. Computationally Assisted Structure Elucidation of New 2-Guanidinoethanesulfonyl Sesquiterpenoid Alkaloids: Agelasidines G–I from the Marine Sponge Agelas nakamurai. Tetrahedron 2022, 126, 133077. [Google Scholar] [CrossRef]
- Lin, M.-K.; Yu, Y.-L.; Chen, K.-C.; Chang, W.-T.; Lee, M.-S.; Yang, M.-J.; Cheng, H.-C.; Liu, C.-H.; Chen, D.-C.; Chu, C.-L. Kaempferol from Semen cuscutae Attenuates the Immune Function of Dendritic Cells. Immunobiology 2011, 216, 1103–1109. [Google Scholar] [CrossRef]
- Lai, K.-H.; You, W.-J.; Lin, C.-C.; El-Shazly, M.; Liao, Z.-J.; Su, J.-H. Anti-Inflammatory Cembranoids from the Soft Coral Lobophytum crassum. Mar. Drugs 2017, 15, 327. [Google Scholar] [CrossRef]

| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| δHa | δHa | δHa | δHa | δHa | |
| 1 | 1.68 m | 2.00 m 1.31 m | 1.10 td (12.0, 4.2) | 1.84 m 1.14 m | 1.85 m 1.06 m |
| 2 | 2.35 m 2.20 m | 1.64 m | 1.55 m | 1.86 m | 1.87 m 1.55 m |
| 3 | 2.34 m | 2.33 m 1.94 m | 3.56 quint (4.8) | 3.63 quint (5.4) | 3.57 m |
| 4 | 2.40 ddd (13.2, 4.8, 2.4) 2.29 tt (11.4, 2.4) | 2.41 ddd (15.0, 5.4, 1.8) 2.33 tt (11.4, 2.4) | 2.40 ddd (13.2, 4.8, 2.4) 2.29 tt (9.0, 2.4) | ||
| 5 | 2.36 m | 2.28 d (12.0) | |||
| 6 | 4.35 m | 2.54 dd (13.2, 3.0) 2.16 m | 5.58 t (1.8) | 5.73 dd (5.4, 1.8) | 5.58 t (2.4) |
| 7 | 5,41 d (8.4) b | 4.15 dt (8.4, 1.8) | 4.17 td (4.8, 1.8) | 4.15 dt (9.0, 2.4) | |
| 8 | 1.59 m | 1.61 m | 1.65 m | ||
| 9 | 2.74 br d (14.4) 2.68 dd (14.4, 4.2) | 3.51 s | 1.09 m | 1.41 m | 1.09 m |
| 10 | 4.32 br s | ||||
| 11 | 1.55 m 1.46 m | 1.48 m | 1.56 m | ||
| 12 | 2.03 m 1.18 m | 1.98 m 1.18 m | 2.02 dt (12.6, 3.6) 1.14 m | ||
| 13 | 1.57 m | 1.85 s | |||
| 14 | 1.69 m | 1.00 s | 1.10 m | 1.47 m | 1.10 m |
| 15 | 4.86 s 4.59 s | 1.37 m | 1.89 m 1.12 m | 1.77 m 1.35 m | |
| 16 | 1.17 s | 1.36 m | 2.10 m 1.33 m | 1.87 m 1.29 m | |
| 17 | 1.40 s | 1.23 m | 1.31 m | 1.17 m | |
| 18 | 4.82 d (6.0) | 0.67 s | 0.65 s | 0.69 s | |
| 19 | 1.43 s | 1.04 s | 1.00 s | 1.05 s | |
| 20 | 1.60 m | 1.02 m | 1.37 m | ||
| 21 | 1.01 d (4.8) | 1.04 br s | 0.92 d (6.6) | ||
| 22 | 0.18 m | 0.18 m | 1.40 m 0.95 m | ||
| 23 | 1.37 m 0.93 m | ||||
| 24 | 0.24 m | 0.24 m | 1.18 m | ||
| 25 | 1.56 m | 1.54 m | 1.57 m | ||
| 26 | 0.95 d (7.8) | 0.96 d (6.6) | 0.86 d (7.2) | ||
| 27 | 0.85 d (5.4) | 0.85 d (7.2) | 0.79 d (6.6) | ||
| 28 | 0.94 d (7.8) | 0.94 d (7.2) | 0.78 d (6.6) | ||
| 29 | 0.46 dd (9.0, 4.2) −0.12 dd (6.0, 4.2) | 0.46 dd (9.6, 4.2) −0.13 dd (6.0, 4.2) | |||
| 30 | 0.90 s | 0.91 s | |||
| 1′ | 3.92 ddd (15.0, 7.8, 3.0) 3.34 ddd (15.0, 7.8, 3.0) | ||||
| 2′ | 3.85 m | ||||
| 7−OOH | 7.45 s | 7.59 s | 7.48 s | ||
| 8−OMe | 3.09 s |
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| δCa | δCa | δCa | δCa | δCa | |
| 1 | 43.1 (CH) b | 34.8 (CH2) | 36.8 (CH2) | 36.7 (CH2) | 36.8 (CH2) |
| 2 | 18.2 (CH2) | 22.2 (CH2) | 31.6 (CH2) | 31.3 (CH2) | 31.6 (CH2) |
| 3 | 32.5 (CH2) | 36.1 (CH2) | 71.3 (CH) | 71.4 (CH) | 71.3 (CH) |
| 4 | 146.5 (C) | No detected (C) | 41.9 (CH2) | 42.2 (CH2) | 41.8 (CH2) |
| 5 | 43.8 (CH2) | 43.9 (CH) | 146.0 (C) | 148.9 (C) | 146.1 (C) |
| 6 | 68.3 (CH) | 24.3 (CH2) | 121.5 (CH) | 119.9 (CH) | 121.5 (CH) |
| 7 | 134.2 (CH) | 151.8 (C) | 86.6 (CH) | 78.5 (CH) | 86.6 (CH) |
| 8 | 133.4 (C) | 93.1 (C) | 34.6 (CH) | 37.1 (CH) | 34.5 (CH) |
| 9 | 38.5 (CH2) | 78.7 (CH) | 48.7 (CH) | 43.5 (CH) | 48.7 (CH) |
| 10 | 62.4 (CH) | 40.5 (C) | 36.4 (C) | 37.4 (C) | 36.4 (C) |
| 11 | 161.3 (C) | 130.0 (C) | 21.3 (CH2) | 20.9 (CH2) | 21.3 (CH2) |
| 12 | 131.6 (C) | 174.3 (C) | 39.6 (CH2) | 39.1 (CH2) | 39.5 (CH2) |
| 13 | 32.0 (CH2) | 8.1 (CH3) | 43.3 (C) | 42.8 (C) | 42.8 (C) |
| 14 | 24.3 (CH2) | 16.2 (CH3) | 55.8 (CH) | 48.9 (CH) | 55.4 (CH) |
| 15 | 37.1 (C) | 106.6 (CH2) | 26.2 (CH2) | 24.7 (CH2) | 26.0 (CH2) |
| 16 | 35.1 (CH3) | 28.3 (CH2) | 28.2 (CH2) | 28.3 (CH2) | |
| 17 | 25.3 (CH3) | 57.4 (CH) | 57.5 (CH) | 55.9 (CH) | |
| 18 | 113.8 (CH2) | 11.9 (CH3) | 11.3 (CH3) | 11.8 (CH3) | |
| 19 | 17.2 (CH3) | 18.8 (CH3) | 18.2 (CH3) | 18.8 (CH3) | |
| 20 | 172.5 (C) | 35.2 (CH) | 35.4 (CH) | 36.1 (CH) | |
| 21 | 21.2 (CH3) | 21.2 (CH3) | 18.9 (CH3) | ||
| 22 | 32.0 (CH) | 32.0 (CH) | 33.7 (CH2) | ||
| 23 | 25.8 (C) | 25.8 (C) | 30.6 (CH2) | ||
| 24 | 50.8 (CH) | 50.8 (CH) | 39.1 (CH) | ||
| 25 | 32.1 (CH) | 32.2 (CH) | 31.4 (CH) | ||
| 26 | 22.2 (CH3) | 22.2 (CH3) | 20.5 (CH3) | ||
| 27 | 21.5 (CH3) | 21.5 (CH3) | 17.6 (CH3) | ||
| 28 | 15.4 (CH3) | 15.5 (CH3) | 15.4 (CH3) | ||
| 29 | 21.3 (CH2) | 21.3 (CH2) | |||
| 30 | 14.3 (CH3) | 14.3 (CH3) | |||
| 1′ | 44.5 (CH2) | ||||
| 2′ | 62.2 (CH2) | ||||
| 8−OMe | 49.6 (CH3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, C.-W.; Lin, Y.-C.; Chiou, S.-F.; Chen, S.-L.; Lin, C.-C.; Wang, H.-C.; Dai, C.-F.; Sheu, J.-H. New Verticillene Diterpenoids, Eudesmane Sesquiterpenoids, and Hydroperoxysteroids from the Further Chemical Investigation of a Taiwanese Soft Coral Cespitularia sp. Molecules 2023, 28, 1521. https://doi.org/10.3390/molecules28041521
Fu C-W, Lin Y-C, Chiou S-F, Chen S-L, Lin C-C, Wang H-C, Dai C-F, Sheu J-H. New Verticillene Diterpenoids, Eudesmane Sesquiterpenoids, and Hydroperoxysteroids from the Further Chemical Investigation of a Taiwanese Soft Coral Cespitularia sp. Molecules. 2023; 28(4):1521. https://doi.org/10.3390/molecules28041521
Chicago/Turabian StyleFu, Chung-Wei, You-Cheng Lin, Shu-Fen Chiou, Shu-Li Chen, Chi-Chien Lin, Hui-Chun Wang, Chang-Feng Dai, and Jyh-Horng Sheu. 2023. "New Verticillene Diterpenoids, Eudesmane Sesquiterpenoids, and Hydroperoxysteroids from the Further Chemical Investigation of a Taiwanese Soft Coral Cespitularia sp." Molecules 28, no. 4: 1521. https://doi.org/10.3390/molecules28041521
APA StyleFu, C.-W., Lin, Y.-C., Chiou, S.-F., Chen, S.-L., Lin, C.-C., Wang, H.-C., Dai, C.-F., & Sheu, J.-H. (2023). New Verticillene Diterpenoids, Eudesmane Sesquiterpenoids, and Hydroperoxysteroids from the Further Chemical Investigation of a Taiwanese Soft Coral Cespitularia sp. Molecules, 28(4), 1521. https://doi.org/10.3390/molecules28041521