Anti-Dengue Activity of Lipophilic Fraction of Ocimum basilicum L. Stem
Abstract
:1. Introduction
2. Results
2.1. Chemical Constituents of LFOB
2.2. Effect of LFOB Treatment on Proliferation of Vero Cells (MTT Assay)
2.3. Primary Screening of LFOB against DENV and CHIKV Replication
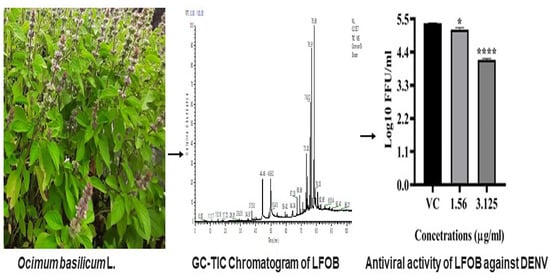
2.4. Antiviral Activity of LFOB against DENV
2.5. In-Silico Interaction Studies of Compounds with DENV Protein Targets
3. Discussion
4. Materials and Methods
4.1. Collection and Identification of Plant Material
4.2. Preparation of Extract
4.3. Analysis of the Extract
4.4. In Vitro Antiviral Activity
4.4.1. Cell Culture and Virus Stock
4.4.2. Cytotoxicity and Cytopathic Effect Inhibition Assay
4.4.3. Antiviral Activity of the LFOB in the In Vitro System
4.4.4. FFU Assay
4.5. Docking Studies Using Viral Proteins and Molecular Modeling of Chemical Compositions
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chavez-Gonzalez, M.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Essential Oils: A Natural Alternative to Combat Antibiotics Resistance. In Antibiotic Resistance; Elsevier: Amsterdam, The Netherlands, 2016; pp. 227–237. [Google Scholar]
- Dzoyem, J.P.; McGaw, L.J.; Kuete, V.; Bakowsky, U. Anti-Inflammatory and Anti-Nociceptive Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 239–270. [Google Scholar]
- Bora, K.S.; Arora, S.; Shri, R. Role of Ocimum basilicum L. in Prevention of Ischemia and Reperfusion-Induced Cerebral Damage, and Motor Dysfunctions in Mice Brain. J. Ethnopharmacol. 2011, 137, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Shams-Ardekani, M.R.; Abdollahi, M. A Review of the Efficacy of Traditional Iranian Medicine for Inflammatory Bowel Disease. World J. Gastroenterol. 2010, 16, 4504–4514. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S. Ocimum basilicum L. (Lamiaceae): (Syns.: O. Americanum Jacq.; O. Barrelieri Roth; O. Bullatum Lam.; O. Thyrsiflorum L.; Plectranthus Barrelieri (Roth) Spreng.). In Handbook of 200 Medicinal Plants; Springer International Publishing: Cham, Switzerland, 2020; pp. 1313–1326. [Google Scholar]
- Saganuwan, A. Some Medicinal Plants of Arabian Pennisula. J. Med. Plant Res. 2010, 4, 767–789. [Google Scholar]
- Ullah, R.; Alqahtani, A.S.; Noman, O.M.A.; Alqahtani, A.M.; Ibenmoussa, S.; Bourhia, M. A Review on Ethno-Medicinal Plants Used in Traditional Medicine in the Kingdom of Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 2706–2718. [Google Scholar] [CrossRef]
- Amrani, S.; Harnafi, H.; Bouanani, N.E.H.; Aziz, M.; Caid, H.S.; Manfredini, S.; Besco, E.; Napolitano, M.; Bravo, E. Hypolipidaemic Activity of Aqueous Ocimum basilicum Extract in Acute Hyperlipidaemia Induced by Triton WR-1339 in Rats and Its Antioxidant Property. Phytother. Res. 2006, 20, 1040–1045. [Google Scholar] [CrossRef]
- Umar, A.; Zhou, W.; Abdusalam, E.; Tursun, A.; Reyim, N.; Tohti, I.; Moore, N. Effect of Ocimum basilicum L. on Cyclo-Oxygenase Isoforms and Prostaglandins Involved in Thrombosis. J. Ethnopharmacol. 2014, 152, 151–155. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; García-Barriuso, M.; Gordaliza, M.; Amich, F. Traditional Plant-Based Remedies to Control Insect Vectors of Disease in the Arribes Del Duero (Western Spain): An Ethnobotanical Study. J. Ethnopharmacol. 2011, 138, 595–601. [Google Scholar] [CrossRef]
- Ntonifor, N.N.; Ngufor, C.A.; Kimbi, H.K.; Oben, B.O. Traditional Use of Indigenous Mosquito-Repellents to Protect Humans against Mosquitoes and Other Insect Bites in a Rural Community of Cameroon. East Afr. Med. J. 2006, 83, 553–558. [Google Scholar]
- Filip, S. Basil (Ocimum basilicum L.) a Source of Valuable Phytonutrients. Int. J. Clin. Nutr. Diet. 2017, 3, 118. [Google Scholar] [CrossRef]
- Marwat, S.K.; Khan, M.S.; Ghulam, S.; Anwar, N.; Mustafa, G.; Usman, K. Phytochemical Constituents and Pharmacological Activities of Sweet Basil-Ocimum basilicum L. (Lamiaceae). Asian J. Chem. 2011, 23, 3773–3782. [Google Scholar]
- Sestili, P.; Ismail, T.; Calcabrini, C.; Guescini, M.; Catanzaro, E.; Turrini, E.; Layla, A.; Akhtar, S.; Fimognari, C. The Potential Effects of Ocimum basilicum on Health: A Review of Pharmacological and Toxicological Studies. Expert Opin. Drug Metab. Toxicol. 2018, 14, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Al Abbasy, D.W.; Pathare, N.; Al-Sabahi, J.N.; Khan, S.A. Chemical Composition and Antibacterial Activity of Essential Oil Isolated from Omani Basil (Ocimum basilicum Linn.). Asian Pac. J. Trop. Dis. 2015, 5, 645–649. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical Composition and Antimicrobial Activity of the Essential Oil of Ocimum basilicum L. (Sweet Basil) from Western Ghats of North West Karnataka, India. Anc. Sci. Life 2014, 33, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil. Plants 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J.; Cakic, M.D. Chemical Composition, Antioxidant and Antimicrobial Activity of Basil (Ocimum basilicum L.) Essential Oil. J. Essent. Oil-Bear. Plants 2017, 20, 1557–1569. [Google Scholar] [CrossRef]
- Chowdhury, T.; Mandal, A.; Roy, S.C.; De Sarker, D. Diversity of the Genus Ocimum (Lamiaceae) through Morpho-Molecular (RAPD) and Chemical (GC–MS) Analysis. J. Genet. Eng. Biotechnol. 2017, 15, 275–286. [Google Scholar] [CrossRef]
- Yucharoen, R.; Anuchapreeda, S.; Tragoolpua, Y. Anti-Herpes Simplex Virus Activity of Extracts from the Culinary Herbs Ocimum sanctum L., Ocimum basilicum L. and Ocimum americanum L. Afr. J. Biotechnol. 2011, 10, 860–866. [Google Scholar]
- Behbahani, M.; Mohabatkar, H.; Soltani, M. Anti-HIV-1 Activities of Aerial Parts of Ocimum basilicum and Its Parasite Cuscuta Campestris. J. Antivir. Antiretrovir. 2013, 5, 57–61. [Google Scholar] [CrossRef]
- Chiang, L.C.; Ng, L.T.; Cheng, P.W.; Chiang, W.; Lin, C.C. Antiviral Activities of Extracts and Selected Pure Constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef]
- Singh, P.; Chakraborty, P.; He, D.-H.; Mergia, A. Extract Prepared from the Leaves of Ocimum basilicum Inhibits the Entry of Zika Virus. Acta Virol. 2019, 63, 316–321. [Google Scholar] [CrossRef]
- Joshi, R.K.; Agarwal, S.; Patil, P.; Alagarasu, K.; Panda, K.; Parashar, C.; Kakade, M.; Sai, D.K.; Cherian, S.; Parashar, D.; et al. Effect of Sauropus Androgynus L. Merr. on Dengue Virus-2: An in Vitro and in Silico Study. J. Ethnopharmacol. 2022, 304, 116044. [Google Scholar] [CrossRef] [PubMed]
- Pirintsos, S.; Panagiotopoulos, A.; Bariotakis, M.; Daskalakis, V.; Lionis, C.; Sourvinos, G.; Karakasiliotis, I.; Kampa, M.; Castanas, E. From Traditional Ethnopharmacology to Modern Natural Drug Discovery: A Methodology Discussion and Specific Examples. Molecules 2022, 27, 4060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.D.; Cheng, J.X.; Zhang, C.F.; Bai, Y.D.; Liu, W.Y.; Li, W.; Koike, K.; Akihisa, T.; Feng, F.; Zhang, J. Sauropus Androgynus L. Merr.-A Phytochemical, Pharmacological and Toxicological Review. J. Ethnopharmacol. 2020, 257, 112778. [Google Scholar] [CrossRef] [PubMed]
- Ramphan, S.; Suksathan, S.; Wikan, N.; Ounjai, P.; Boonthaworn, K.; Rimthong, P.; Kanjanapruthipong, T.; Worawichawong, S.; Jongkaewwattana, A.; Wongsiriroj, N.; et al. Oleic Acid Enhances Dengue Virus but Not Dengue Virus-like Particle Production from Mammalian Cells. Mol. Biotechnol. 2017, 59, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, M.C.; Hong, P.P.; Zhao, X.F.; Wang, J.X. Metabolomic Profiles in the Intestine of Shrimp Infected by White Spot Syndrome Virus and Antiviral Function of the Metabolite Linoleic Acid in Shrimp. J. Immunol. 2021, 206, 2075–2087. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Hohmann, J.; El-Shazly, M.; Chang, L.K.; Dankó, B.; Kúsz, N.; Hsieh, C.T.; Hunyadi, A.; Chang, F.R. Bioactive Constituents of Lindernia Crustacea and Its Anti-EBV Effect via Rta Expression Inhibition in the Viral Lytic Cycle. J. Ethnopharmacol. 2020, 250, 112493. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Salehi, B.; Schnitzler, P.; Ayatollahi, S.A.; Kobarfard, F.; Fathi, M.; Eisazadeh, M.; Sharifi-Rad, M. Susceptibility of Herpes Simplex Virus Type 1 to Monoterpenes Thymol, Carvacrol, p-Cymene and Essential Oils of Sinapis arvensis L., Lallemantia Royleana Benth. and Pulicaria Vulgaris Gaertn. Cell. Mol. Biol. 2017, 63, 42–47. [Google Scholar] [CrossRef]
- Zhou, B.X.; Li, J.; Liang, X.L.; Pan, X.P.; Hao, Y.B.; Xie, P.F.; Jiang, H.M.; Yang, Z.F.; Zhong, N.S. β-Sitosterol Ameliorates Influenza A Virus-Induced Proinflammatory Response and Acute Lung Injury in Mice by Disrupting the Cross-Talk between RIG-I and IFN/STAT Signaling. Acta Pharmacol. Sin. 2020, 41, 1178–1196. [Google Scholar] [CrossRef]
- Chen, C.; Shen, J.-L.; Liang, C.-S.; Sun, Z.-C.; Jiang, H.-F. First Discovery of Beta-Sitosterol as a Novel Antiviral Agent against White Spot Syndrome Virus. Int. J. Mol. Sci. 2022, 23, 10448. [Google Scholar] [CrossRef]
- Mir, A.; Ismatullah, H.; Rauf, S.; Niazi, U.H.K. Identification of Bioflavonoid as Fusion Inhibitor of Dengue Virus Using Molecular Docking Approach. Inform. Med. Unlocked 2016, 3, 1–6. [Google Scholar] [CrossRef]
- Panda, K.; Alagarasu, K.; Patil, P.; Agrawal, M.; More, A.; Kumar, N.V.; Mainkar, P.S.; Parashar, D.; Cherian, S. In Vitro Antiviral Activity of α-Mangostin against Dengue Virus Serotype-2 (DENV-2). Molecules 2021, 26, 3016. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.L.; Xu, T.; Chen, Y.-L.; Malet, H.; Egloff, M.-P.; Canard, B.; Vasudevan, S.G.; Lescar, J. Crystal Structure of the Dengue Virus RNA-Dependent RNA Polymerase Catalytic Domain at 1.85-Angstrom Resolution. J. Virol. 2007, 81, 4753–4765. [Google Scholar] [CrossRef] [PubMed]
- Bouazzi, S.; El Mokni, R.; Nakbi, H.; Dhaouadi, H.; Joshi, R.K.; Hammami, S. Chemical Composition and Antioxidant Activity of Essential Oils and Hexane Extract of Onopordum Arenarium from Tunisia. J. Chromatogr. Sci. 2020, 58, 287–293. [Google Scholar] [CrossRef]
- Joshi, R.K. Antioxidant Activity Influenced by Seasonal Variation of Essential Oil Constituents of Ocimum Gratissimum L. ACS Food Sci. Technol. 2021, 1, 1661–1669. [Google Scholar] [CrossRef]
- Joshi, R.K. Terpenoid Constituents of the Roots of a Traditional Herb, Blumea Paniculata, from India. Chem. Nat. Compd. 2022, 58, 152–153. [Google Scholar] [CrossRef]
- Joshi, R.K.; Sharma, A.K. Determination of Seasonal Variation of Volatile Organic Constituents of the Leaves of Traditional Herb Ocimum Sanctum Linn. Indian J. Pharm. Sci. 2021, 83, 750–757. [Google Scholar] [CrossRef]
- Joshi, R.K. GC-MS and GC-FID Analysis of Volatile Secondary Metabolites of the Root of Anaphalis Contorta Hook F. from India. Bull. Chem. Soc. Ethiop. 2022, 36, 235–240. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Business Media: Carol Stream, IL, USA, 2007. [Google Scholar]
- Alagarasu, K.; Patil, P.; Kaushik, M.; Chowdhury, D.; Joshi, R.K.; Hegde, H.V.; Kakade, M.B.; Hoti, S.L.; Cherian, S.; Parashar, D. In Vitro Antiviral Activity of Potential Medicinal Plant Extracts against Dengue and Chikungunya Viruses. Front. Cell. Infect. Microbiol. 2022, 12, 866452. [Google Scholar] [CrossRef]
- Patil, P.; Agrawal, M.; Almelkar, S.; Jeengar, M.K.; More, A.; Alagarasu, K.; Kumar, N.V.; Mainkar, P.S.; Parashar, D.; Cherian, S. In Vitro and in Vivo Studies Reveal α-Mangostin, a Xanthonoid from Garcinia Mangostana, as a Promising Natural Antiviral Compound against Chikungunya Virus. Virol. J. 2021, 18, 47. [Google Scholar] [CrossRef]




| Compound | RI | % Content | Identification |
|---|---|---|---|
| Terpinene-4-ol | 1173 | t | RI, MS, CI |
| α-Terpineol | 1191 | 0.1 | RI, MS, CI |
| Methyl chavicol | 1198 | 0.5 | RI, MS, CI |
| Chavicol | 1262 | 0.2 | RI, MS |
| (E)-Anethole | 1288 | t | RI, MS, CI |
| Eugenol | 1361 | 0.1 | RI, MS, CI |
| β-Caryophyllene | 1421 | t | RI, MS, CI |
| n-Hexadecane | 1600 | 0.2 | RI, MS, CI |
| epi-α-Bisabolol | 1686 | 0.1 | RI, MS |
| Benzyl benzoate | 1766 | 1.5 | RI, MS |
| Hexadecanoic acid | 1976 | 7.5 | RI, MS, CI |
| Methyl linoleate | 2114 | 0.5 | RI, MS |
| Linolenic acid | 2154 | 6.8 | RI, MS |
| n-Tricosane | 2300 | 0.5 | RI, MS, CI |
| n-Pentacosane | 2500 | 0.7 | RI, MS, CI |
| n-Heptacosane | 2700 | 1.5 | RI, MS, CI |
| Squalene | 2831 | 1.5 | RI, MS |
| n-Nonacosane | 2900 | 1.9 | RI, MS, CI |
| n-Triacontane | 3000 | 1.3 | RI, MS, CI |
| n-Untriacontane | 3100 | 5.7 | RI, MS |
| Vitamin E | 3131 | 3.9 | RI, MS, CI |
| Dotriacontane | 3200 | 1.9 | RI, MS |
| Campesterol | 3219 | 12.9 | RI, MS, CI |
| Stigmasterol | 3256 | 18.7 | RI, MS, CI |
| n-Tritriacontane | 3300 | 4.5 | RI, MS |
| β-Sitosterol | 3319 | 22.9 | RI, MS, CI |
| Oxygenated monoterpenes | 0.1 | ||
| Sesquiterpene hydrocarbon | t | ||
| Oxygenated sesquiterpene | 0.1 | ||
| Phenylpropanoids | 2.3 | ||
| Long chain hydrocarbons | 18.2 | ||
| Long chain oxygenated hydrocarbons | 14.8 | ||
| Triterpenoids | 56.0 | ||
| Chromane terpenoid | 3.9 | ||
| Total identified | 95.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, R.K.; Agarwal, S.; Patil, P.; Alagarasu, K.; Panda, K.; Cherian, S.; Parashar, D.; Roy, S. Anti-Dengue Activity of Lipophilic Fraction of Ocimum basilicum L. Stem. Molecules 2023, 28, 1446. https://doi.org/10.3390/molecules28031446
Joshi RK, Agarwal S, Patil P, Alagarasu K, Panda K, Cherian S, Parashar D, Roy S. Anti-Dengue Activity of Lipophilic Fraction of Ocimum basilicum L. Stem. Molecules. 2023; 28(3):1446. https://doi.org/10.3390/molecules28031446
Chicago/Turabian StyleJoshi, Rajesh Kumar, Shivankar Agarwal, Poonam Patil, Kalichamy Alagarasu, Kingshuk Panda, Sarah Cherian, Deepti Parashar, and Subarna Roy. 2023. "Anti-Dengue Activity of Lipophilic Fraction of Ocimum basilicum L. Stem" Molecules 28, no. 3: 1446. https://doi.org/10.3390/molecules28031446
APA StyleJoshi, R. K., Agarwal, S., Patil, P., Alagarasu, K., Panda, K., Cherian, S., Parashar, D., & Roy, S. (2023). Anti-Dengue Activity of Lipophilic Fraction of Ocimum basilicum L. Stem. Molecules, 28(3), 1446. https://doi.org/10.3390/molecules28031446