Comprehensive Metabolite Profiling of Berdav Propolis Using LC-MS/MS: Determination of Antioxidant, Anticholinergic, Antiglaucoma, and Antidiabetic Effects
Abstract
1. Introduction
2. Results
2.1. Phenolic Contents of Propolis
2.2. Reducing Abilities Results
2.3. Radical Scavenging Results
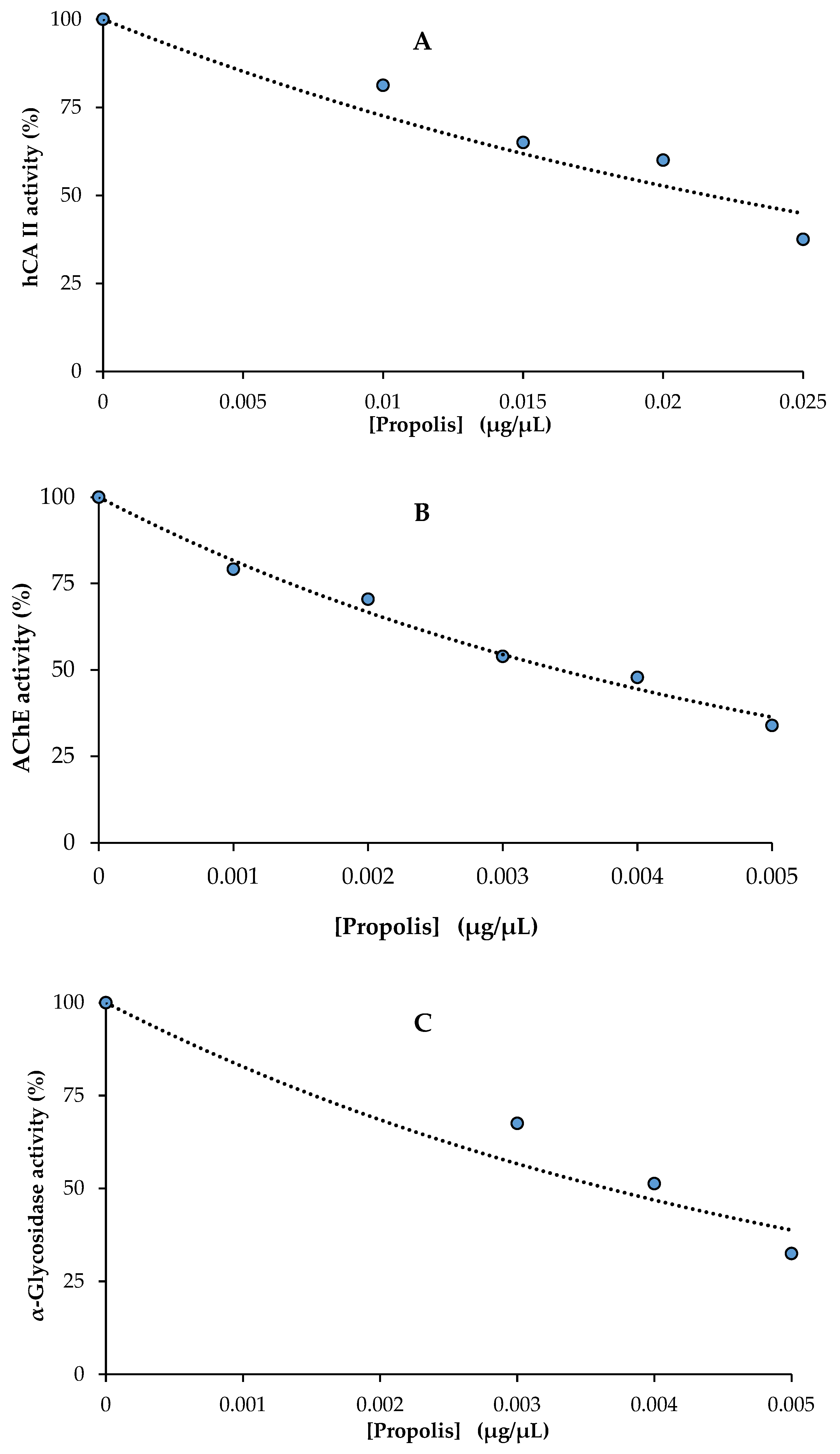
2.4. Enzyme Inhibition Results
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Propolis
4.3. Determination of Total Soluble Phenolic Contents of Propolis
4.4. Determination of Tatal Flavonoid Content of Propolis
4.5. Test Solution for Mass Spectrometer (LC–MS/MS) and Chromatography Conditions
4.6. Ferric Ions (Fe3+) Reducing Assay
4.7. Cupric Ions (Cu2+) Reducing—CUPRAC Assay
4.8. Fe3+-TPTZ Reducing—FRAP Assay
4.9. DPPH• Scavenging Activity
4.10. ABTS•+ Scavenging Activity
4.11. DMPD•+ Scavenging Activity
4.12. Percentage Scavenging and IC50 Determination
4.13. AChE Enzyme Inhibition Assay
4.14. α-Glycosidase Enzyme Inhibition Assay
4.15. hCA II Isoenzyme Inhibition Assay
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shehata, M.G.; Ahmad, F.T.; Badr, A.N.; Masry, S.H.; El-Sohaimy, S.A. Chemical analysis, antioxidant, cytotoxic and antimicrobial properties of propolis from different geographic regions. Ann. Agric. Sci. 2020, 65, 209–217. [Google Scholar] [CrossRef]
- Gulcin, I.; Bursal, E.; Sehitoglu, H.M.; Bilsel, M.; Goren, A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef]
- Kasote, D.; Suleman, T.; Chen, W.; Sandasi, M.; Viljoen, A.; van Vuuren, S. Chemical profiling and chemometric analysis of South African propolis. Biochem. System. Ecol. 2014, 55, 156–163. [Google Scholar] [CrossRef]
- Bilginer, R.; Yildiz, A.A. A facile method to fabricate propolis enriched biomimetic PVA architectures by co-electrospinning. Mater. Lett. 2020, 276, 128191. [Google Scholar] [CrossRef]
- Freitas, A.S.; Cunha, A.; Cardoso, S.M.; Oliveira, R.; Almeida-Aguiar, C. Constancy of the bioactivities of propolis samples collected on the same apiary over four years. Food Res. Int. 2019, 119, 622–633. [Google Scholar] [CrossRef]
- Garzarella, E.U.; Navajas-Porras, B.; Perez-Burillo, S.; Ullah, H.; Esposito, C.; Santarcangelo, C.; Hinojosa-Nogueira, D.; Pastoriza, S.; Zaccaria, V.; Xiao, J.; et al. Evaluating the effects of a standardized polyphenol mixture extracted from poplar-type propolis on healthy and diseased human gut microbiota. Biomed. Pharmacother. 2022, 148, 112759. [Google Scholar] [CrossRef]
- Santos, L.A.; Rosalen, P.L.; Dias, N.A.; Grisolia, J.C.; Gomes, B.J.N.; Blosfeld-Lopes, L.; Ikegaki, M.; de Alencar, S.M.; Burger, E. Brazilian red propolis shows antifungal and immunomodulatory activities against Paracoccidioides brasiliensis. J. Ethnopharmacol. 2021, 277, 114181. [Google Scholar] [CrossRef]
- Abdellatif, M.M.; Elakkad, Y.E.; Elwakeel, A.A.; Allam, R.M.; Mousa, M.R. Formulation and characterization of propolis and tea tree oil nanoemulsion loaded with clindamycin hydrochloride for wound healing: In-vitro and in-vivo wound healing assessment. Saudi Pharm. J. 2021, 29, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Sun, L.; Wang, D.; Chen, L.; Wang, J.; Qi, X.; Zhang, H.; Tang, M.; Wu, G.; Chen, J.; et al. Antiviral properties of propolis ethanol extract against norovirus and its application in fresh juices. LWT 2021, 152, 112169. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Surek, M.; Fachi, M.M.; de Fátima Cobre, A.; de Oliveir, F.F.; Pontarolo, R.; Crisma, A.R.; de Souza, W.M.; Felipe, K.B. Chemical composition, cytotoxicity, and antibacterial activity of propolis from Africanized honeybees and three different Meliponini species. J. Ethnopharmacol. 2021, 269, 113662. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidants and antioxidant methods-An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, L.; Erturk, A.; Akyuz, M.; Polat Kose, L.; Uc, E.M.; Bingol, Z.; Saglamtas, R.; Alwasel, S.; Gulcin, I. Screening of carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase and α-glycosidase enzymes inhibition effects and antioxidant activity of coumestrol. Molecules 2022, 27, 3091. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Bursal, E.; Taslimi, P.; Goren, A.; Gulcin, I. Assessments of anticholinergic, antidiabetic, antioxidant activities and phenolic content of Stachys annua. Biocat. Agric. Biotechnol. 2020, 28, 101711. [Google Scholar] [CrossRef]
- Guzelmeric, E.; Yuksel, P.I.; Yaman, B.K.; Sipahi, H.; Celik, C.; Kırmızıbekmez, H.; Aydın, A.; Yesilada, E. Comparison of antioxidant and anti-inflammatory activity profiles of various chemically characterized Turkish propolis sub-types: Which propolis type is a promising source for pharmaceutical product development? J. Pharm. Biomed. Anal. 2021, 203, 114196. [Google Scholar] [CrossRef]
- Polat Kose, L.; Gulcin, I. Evaluation of the antioxidant and antiradical properties of some phyto and mammalian lignans. Molecules 2021, 26, 7099. [Google Scholar] [CrossRef] [PubMed]
- Taslimi, P.; Koksal, E.; Goren, A.C.; Bursal, E.; Aras, A.; Kılıc, O.; Alwasel, S.; Gulcin, I. Anti-Alzheimer, antidiabetic and antioxidant potential of Satureja cuneifolia and analysis of its phenolic contents by LC-MS/MS. Arab. J. Chem. 2020, 13, 4528–4537. [Google Scholar] [CrossRef]
- Kiziltas, H.; Bingol, Z.; Goren, A.C.; Pınar, S.M.; Alwasel, S.H.; Gulcin, I. LC-HRMS profiling of phytochemicals, antidiabetic, anticholinergic and antioxidant activities of evaporated ethanol extract of Astragalus brachycalyx FISCHER. J. Chem. Metrol. 2021, 15, 135–151. [Google Scholar]
- Bingol, Z.; Kızıltas, H.; Gören, A.C.; Polat Kose, L.; Topal, M.; Durmaz, L.; Alwasel, S.H.; Gulcin, I. Antidiabetic, anticholinergic and antioxidant activities of aerial parts of shaggy bindweed (Convulvulus betonicifolia Miller subsp.)-profiling of phenolic compounds by LC-HRMS. Heliyon 2021, 7, e06986. [Google Scholar] [CrossRef]
- Oztaskin, N.; Kaya, R.; Maras, A.; Sahin, E.; Gulcin, I.; Goksu, S. Synthesis and characterization of novel bromophenols: Determination of their anticholinergic, antidiabetic and antioxidant activities. Bioorg. Chem. 2019, 87, 91–102. [Google Scholar] [CrossRef]
- Eruygur, N.; Atas, M.; Tekin, M.; Taslimi, P.; Kocyigit, U.M.; Gulcin, I. In vitro antioxidant, antimicrobial, anticholinesterase and antidiabetic activities of Turkish endemic Achillea cucullata (Asteraceae) from ethanol extract. S. Afr. J. Bot. 2019, 120, 141–145. [Google Scholar] [CrossRef]
- Gulcin, I.; Buyukokuroglu, M.E.; Kufrevioglu, O.I. Metal chelating and hydrogen peroxide scavenging effects of melatonin. J. Pineal Res. 2003, 34, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Maharramova, G.; Taslimi, P.; Sujayev, A.; Farzaliyev, F.; Durmaz, L.; Gulcin, I. Synthesis, characterization, antioxidant, antidiabetic, anticholinergic, and antiepileptic properties of novel N-substituted tetrahydropyrimidines based on phenylthiourea. J. Biochem. Mol. Toxicol. 2018, 32, e22221. [Google Scholar] [CrossRef]
- Takım, K.; Yigin, A.; Koyuncu, I.; Kaya, R.; Gulcin, I. Anticancer, anticholinesterase and antidiabetic activities of Tunceli garlic (Allium tuncelianum)-Determining its phytochemical content by LC-MS/MS analysis. J. Food Meas. Charact. 2021, 15, 3323–3335. [Google Scholar] [CrossRef]
- Taslimi, P.; Aslan, H.E.; Demir, Y.; Oztaskın, N.; Maras, A.; Gulcin, I.; Beydemir, S.; Goksu, S. Diarilmethanon, bromophenols and diarilmetan compounds: Discovery of potent aldose reductase, α-amylase and α-glycosidase inhibitors as new therapeutic approach in diabetes and functional hyperglycemia. Int. J. Biol. Macromol. 2018, 119, 857–863. [Google Scholar] [CrossRef]
- Gulcin, I.; Taslimi, P.; Aygun, A.; Sadeghian, N.; Bastem, E.; Kufrevioglu, O.I.; Turkan, F.; Sen, F. Antidiabetic and antiparasitic potentials: Inhibition effects of some natural antioxidant compounds on α-glycosidase, α-amylase and human glutathione S-transferase enzymes. Int. J. Biol. Macromol. 2018, 119, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Burmaoglu, S.; Yılmaz, A.O.; Polat, M.F.; Kaya, R.; Gulcin, I.; Algul, O. Synthesis and biological evaluation of novel tris-chalcones as potent carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase, and α-glycosidase inhibitors. Bioorg. Chem. 2019, 85, 191–197. [Google Scholar] [CrossRef]
- Cetin Cakmak, K.; Gulcin, I. Anticholinergic and antioxidant activities of USNIC acid-An activity-structure insight. Toxicol. Rep. 2019, 6, 1273–1280. [Google Scholar] [CrossRef]
- Oztaskin, N.; Taslimi, P.; Maras, A.; Goksu, S.; Gulcin, I. Novel antioxidant bromophenols with acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase inhibitory actions. Bioorg. Chem. 2017, 74, 104–114. [Google Scholar] [CrossRef]
- Burmaoglu, S.; Kazancioglu, E.A.; Kazancioglu, M.Z.; Saglamtas, R.; Yalçın, G.; Gulcin, I.; Algul, O. Synthesis, molecular docking and some metabolic enzyme inhibition properties of biphenyl-substituted chalcone derivatives. J. Mol. Struct. 2022, 1254, 132358. [Google Scholar] [CrossRef]
- Kaya, Y.; Ercag, A.; Zorlu, Y.; Demir, Y.; Gulcin, I. New Pd(II) complexes of the bisthiocarbohydrazones derived from isatin and disubstituted salicylaldehydes: Synthesis, characterization, crystal structures and inhibitory properties against some metabolic enzymes. J. Biol. Inorg. Chem. 2022, 27, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Taslimi, P.; Caglayan, C.; Gulcin, I. The impact of some natural phenolic compounds on carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase, and α-glycosidase enzymes: An antidiabetic, anticholinergic, and antiepileptic study. J. Biochem. Mol. Toxicol. 2017, 31, e21995. [Google Scholar] [CrossRef] [PubMed]
- Kızıltas, H.; Bingol, Z.; Goren, A.C.; Alwasel, S.H.; Gulcin, I. Anticholinergic, antidiabetic and antioxidant activities of Ferula orientalis L.-Analysis of its polyphenol contents by LC-HRMS. Rec. Nat. Prod. 2021, 15, 513–528. [Google Scholar] [CrossRef]
- Kose, L.P.; Gulcin, I. Inhibition effects of some lignans on carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase enzymes. Rec. Nat. Prod. 2017, 11, 558–561. [Google Scholar] [CrossRef]
- Zahedi, N.A.; Mohammadi-Khanaposhtani, M.; Rezaei, P.; Askarzadeh, M.; Alikhani, M.; Adib, M.; Mahdavi, M.; Larijani, B.; Niakan, S.; Tehrani, M.B.; et al. Dual functional cholinesterase and carbonic anhydrase inhibitors for the treatment of Alzheimer’s disease: Design, synthesis, in vitro, and in silico evaluations of coumarin-dihydropyridine derivatives. J. Mol. Struct. 2023, 1276, 134767. [Google Scholar] [CrossRef]
- Yiğit, B.; Taslimi, P.; Celepci, D.B.; Taskin-Tok, T.; Yiğit, M.; Aygün, M.; Ozdemir, I.; Gulcin, I. Novel PEPPSI-type N-heterocyclic carbene palladium(II) complexes: Synthesis, characterization, in silico studies and enzyme inhibitory properties against some metabolic enzymes. Inorg. Chim. Acta 2023, 544, 121239. [Google Scholar] [CrossRef]
- Oztaskın, N.; Goksu, S.; Demir, Y.; Maras, A.; Gulcin, I. Synthesis of novel bromophenol including diaryl Methanes-Determination of their inhibition effects on carbonic anhydrase and acetylcholinesterase. Molecules 2022, 27, 7426. [Google Scholar] [CrossRef]
- Erdemir, F.; Celepci, D.B.; Aktas, A.; Taslimi, P.; Gok, Y.; Karabıyık, H.; Gulcin, I. 2-Hydroxyethyl substituted NHC precursors: Synthesis, characterization, crystal structure and carbonic anhydrase, α-glycosidase, butyrylcholinesterase, and acetylcholinesterase inhibitory properties. J. Mol. Struc. 2018, 1155, 797–806. [Google Scholar] [CrossRef]
- Arabaci, B.; Gulcin, I.; Alwasel, S. Capsaicin: A potent inhibitor of carbonic anhydrase isoenzymes. Molecules 2014, 19, 10103–10114. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Gulcin, I.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. Antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(β-D-glucopyranosyl)-hederagenin. Phytother. Res. 2006, 20, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Bursal, E.; Aras, A.; Dogru, M.; Kilic, O. Phenolic content, antioxidant potentials of Saponaria prostrata endemi phenolic content, antioxidant potentials of Saponaria prostrata endemic plant. Int. J. Life Sci. Biotechnol. 2021, 5, 1–8. [Google Scholar]
- Tohma, H.; Koksal, E.; Kilic, O.; Alan, Y.; Yılmaz, M.A.; Gulcin, I.; Bursal, E.; Alwasel, S.H. RP-HPLC/MS/MS analysis of the phenolic compounds, antioxidant and antimicrobial activities of Salvia L. species. Antioxidants 2016, 5, 38. [Google Scholar] [CrossRef]
- Gulcin, I.; Tel, A.Z.; Kirecci, E. Antioxidant, antimicrobial, antifungal and antiradical activities of Cyclotrichium niveum (Boiss.) Manden and Scheng. Int. J. Food Prop. 2008, 11, 450–471. [Google Scholar] [CrossRef]
- Sarıkahya, N.N.; Goren, A.C.; Sumer Okkalı, G.; Coven, F.O.; Orman, B.; Kırcıf, D.; Yucel, B.; Kısla, D.; Demirci, B.; Altun, M.; et al. Chemical composition and biological activities of propolis samples from different geographical regions of Turkey. Phytochem. Lett. 2021, 44, 129–136. [Google Scholar] [CrossRef]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial activities of European propolis collected from various geographic origins alone and in combination with antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef]
- Jiang, X.; Tao, L.; Li, C.; You, M.; Li, G.Q.; Zhang, C.; Hu, F. Grouping, spectrum-effect relationship and antioxidant compounds of Chinese propolis from different regions using multivariate analyses and off-line anti-DPPH assay. Molecules. 2020, 25, 3243. [Google Scholar] [CrossRef]
- Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Bakour, M.; Ousaaid, D.; Teixeira, J.A.; Lyoussi, B. Unraveling the chemical composition, antioxidant, α-amylase and α-glucosidase inhibition of Moroccan propolis. Food Biosci. 2021, 42, 101160. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Cheng, Y.; Wang, Y. Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed. Chromatogr. 2013, 27, 148–155. [Google Scholar] [CrossRef]
- Kucukoglu, K.; Gul, H.I.; Taslimi, P.; Gulcin, I.; Supuran, C.T. Investigation of inhibitory properties of some hydrazone compounds on hCA I, hCA II and AChE enzymes. Bioorg. Chem. 2019, 86, 316–321. [Google Scholar] [CrossRef]
- Polat Kose, L.; Gulcin, I.; Goren, A.C.; Namiesnik, J.; Martinez-Ayala, A.L.; Gorinstein, S. LC-MS/MS analysis, antioxidant and anticholinergic properties of galanga (Alpinia officinarum Hance) rhizomes. Ind. Crops Prod. 2015, 74, 712–721. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant properties of resveratrol: A structure-activity insight. Innov. Food Sci. Emerg. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of eugenol-a structure and activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Koksal, E.; Gulcin, I. Antioxidant activity of cauliflower (Brassica oleracea L.). Turk. J. Agric. For. 2008, 32, 65–78. [Google Scholar]
- Ekinci Akdemir, F.N.; Albayrak, M.; Çalik, M.; Bayir, Y.; Gulcin, I. The protective effects of p-coumaric acid on acute liver and kidney damages induced by cisplatin. Biomedicines 2017, 5, 18. [Google Scholar] [CrossRef]
- Ekinci Akdemir, F.N.; Gulcin, I.; GUrsul, C.; Alwasel, S.H.; Bayir, Y. Effect of p-coumaric acid against oxidative stress induced by cisplatin in brain tissue of rats. J. Anim. Plant Sci. 2017, 27, 1560–1564. [Google Scholar]
- Apak, R.; Calokerinos, A.; Gorinstein, S.; Segundo, M.A.; Hibbert, D.B.; Gulcin, I.; Demirci Cekic, S.; Guclu, K.; Ozyurek, M.; Esin Çelik, S.; et al. Methods to evaluate the scavenging activity of antioxidants toward reactive oxygen and nitrogen species. Pure Appl. Chem. 2022, 94, 87–144. [Google Scholar] [CrossRef]
- Kızıltas, H.; Bingol, Z.; Goren, A.C.; Polat Kose, L.; Durmaz, L.; Topal, F.; Alwasel, S.H.; Gulcin, I. LC-HRMS profiling, antidiabetic, anticholinergic and anti-oxidant activities of aerial parts of kınkor (Ferulago stelleta). Molecules 2021, 26, 2469. [Google Scholar] [CrossRef] [PubMed]
- Oleggrio, L.S.; Andrade, J.K.S.; Andrade, G.R.S.; Andrade, G.R.S.; Denadai, M.; Cavalcanti, R.L.; da Silva, M.A.A.P.; Narain, N. Chemical characterization of four Brazilian brown propolis: An insight in tracking of its geographical location of production and quality control. Food Res. Int. 2019, 123, 481–502. [Google Scholar] [CrossRef] [PubMed]
- Baltas, N.; Yildiz, O.; Kolayli, S. Inhibition properties of propolis extracts to some clinically important enzymes. J. Enzyme Inhib. Med. Chem. 2016, 31 (Suppl. S1), 52–55. [Google Scholar] [CrossRef]
- Aygul, I.; Yaylaci Karahalil, F.; Supuran, C.T. Investigation of the inhibitory properties of some phenolic standards and bee products against human carbonic anhydrase I and II. J. Enzyme Inhib. Med. Chem. 2016, 31 (Suppl. S4), 119–124. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int. J. Food Sci. Nutr. 2005, 56, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Artunc, T.; Menzek, A.; Taslimi, P.; Gulcin, I.; Kazaz, C.; Sahin, E. Synthesis and antioxidant activities of phenol derivatives from 1,6-bis(dimethoxyphenyl)hexane-1,6-dione. Bioorg. Chem. 2020, 100, 103884. [Google Scholar] [CrossRef]
- Balaydın, H.T.; Gulcin, I.; Menzek, A.; Goksu, S.; Sahin, E. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J. Enzyme Inhib. Med. Chem. 2010, 25, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Oktay, M.; Koksal, E.; Serbetci, H.; Beydemir, S.; Kufrevioglu, Ö.I. Antioxidant and radical scavenging activities of uric acid. Asian J. Chem. 2008, 20, 2079–2090. [Google Scholar]
- Gulcin, I.; Topal, F.; Cakmakcı, R.; Goren, A.C.; Bilsel, M.; Erdogan, U. Pomological features, nutritional quality, polyphenol content analysis and antioxidant properties of domesticated and three wild ecotype forms of raspberries (Rubus idaeus L.). J. Food Sci. 2011, 76, C585–C593. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Goren, A.C.; Taslimi, P.; Alwasel, S.H.; Kilic, O.; Bursal, E. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium)-Analysis of its polyphenol contents by LC-MS/MS. Biocat. Agric. Biotechnol. 2020, 23, 101441. [Google Scholar] [CrossRef]
- Kiziltas, H.; GOren, A.C.; Alwasel, S.; Gulcin, I. Sahlep (Dactylorhiza osmanica): Phytochemical analyses by LC-HRMS, molecular docking, antioxidant activity and enzyme inhibition profiles. Molecules 2022, 27, 6907. [Google Scholar] [CrossRef]
- Turkan, F.; Atalar, M.N.; Aras, A.; Gulcin, I.; Bursal, E. ICP-MS and HPLC analyses, enzyme inhibition and antioxidant potential of Achillea schischkinii Sosn. Bioorg. Chem. 2020, 94, 103333. [Google Scholar] [CrossRef]
- Talaz, O.; Gulcin, I.; Goksu, S.; Saracoglu, N. Antioxidant activity of 5,10-dihydroindeno[1,2-b]indoles containing substituents on dihydroindeno part. Bioorg. Med. Chem. 2009, 17, 6583–6589. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Kufrevioglu, O.I.; Oktay, M.; Buyukokuroglu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef]
- Han, H.; Yılmaz, H.; Gulcin, I. Antioxidant activity of flaxseed (Linum usitatissimum L.) shell and analysis of its polyphenol contents by LC-MS/MS. Rec. Nat. Prod. 2018, 12, 397–402. [Google Scholar] [CrossRef]
- Bursal, E.; Aras, A.; Kilic, O.; Taslimi, P.; Goren, A.C.; Gulcin, I. Phytochemical content, antioxidant activity and enzyme inhibition effect of Salvia eriophora Boiss. & Kotschy against acetylcholinesterase, α-amylase, butyrylcholinesterase and α-glycosidase enzymes. J. Food Biochem. 2019, 43, e12776. [Google Scholar]
- Hamad, H.O.; Alma, M.H.; Gulcin, I.; Yılmaz, M.A.; Karaogul, E. Evaluation of phenolic contents and bioactivity of root and nutgall extracts from Iraqian Quercus infectoria Olivier. Rec. Nat. Prod. 2017, 11, 205–210. [Google Scholar]
- Bayrak, C.; Uc, E.M.; Rezaei, M.; Gulcin, I.; Menzek, A. Synthesis and antioxidant activities of benzylic bromophenols inclusive of natural products. Turk. J. Chem. 2022, 46, 1405–1416. [Google Scholar] [CrossRef]
- Popova, M.; Lyoussi, B.; Aazza, S.; Antunes, D.; Bankova, V.; Miguel, G. Antioxidant and α-glucosidase inhibitory properties and chemical profiles of Moroccan propolis. Nat. Prod. Commun. 2015, 10, 1961–1964. [Google Scholar] [CrossRef] [PubMed]
- Aktas, A.; Yakalı, G.; Demir, Y.; Gulcin, I.; Aygun, M.; Gok, Y. The palladium-based complexes bearing 1,3-dibenzylbenzimidazolium with morpholine, triphenylphosphine, and pyridine derivate ligands: Synthesis, characterization, structure and enzyme inhibitions. Heliyon 2022, 8, e10625. [Google Scholar] [CrossRef]
- Bora, R.E.; Bilgicli, H.G.; Uc, E.M.; Alagoz, M.A.; Zengin, M.; Gulcin, I. Synthesis, characterization, evaluation of metabolic enzyme inhibitors and in silico studies of thymol based 2-amino thiol and sulfonic acid compounds. Chem. Biol. Interact. 2022, 366, 110134. [Google Scholar] [CrossRef]
- Aktas Anıl, D.; Polat, M.F.; Saglamtas, R.; Tarikogulları, A.H.; Alagöz, M.A.; Gulcin, I.; Algul, O.; Burmaoglu, S. Exploring enzyme inhibition profiles of novel halogenated chalcone derivatives on some metabolic enzymes: Synthesis, characterization and molecular modeling studies. Comp. Biol. Chem. 2022, 100, 107748. [Google Scholar] [CrossRef]
- Gulcin, I.; Scozzafava, A.; Supuran, C.T.; Akıncıoglu, H.; Koksal, Z.; Turkan, F.; Alwasel, S. The effect of caffeic acid phenethyl ester (CAPE) on metabolic enzymes including acetylcholinesterase, butyrylcholinesterase, glutathione S-transferase, lactoperoxidase, and carbonic anhydrase isoenzymes I., II, IX, and XII. J. Enzyme Inhib. Med. Chem. 2016, 31, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Topal, M.; Gulcin, I. Evaluation of the in vitro antioxidant, antidiabetic and anticholinergic properties of rosmarinic acid from rosemary (Rosmarinus officinalis L.). Biocat. Agric. Biotechnol. 2022, 43, 102417. [Google Scholar] [CrossRef]
- Gulcin, I.; Petrova, O.V.; Taslimi, P.; Malysheva, S.F.; Schmidt, E.Y.; Sobenina, L.N.; Gusarova, N.K.; Trofimov, B.A.; Tuzun, B.; Farzaliyev, V.M.; et al. Synthesis, characterization, molecular docking, acetylcholinesterase and α-glycosidase inhibition profiles of nitrogen-based novel heterocyclic compounds. ChemistrySelect 2022, 7, e20220037. [Google Scholar] [CrossRef]
- Gumus, M.; Babacan, Ş.N.; Demir, Y.; Sert, Y.; Koca, I.; Gulcin, I. Discovery of sulfadrug-pyrrole conjugates as carbonic anhydrase and acetylcholinesterase inhibitors. Arch. Pharm. 2022, 355, e2100242. [Google Scholar] [CrossRef]
- Yigit, M.; Celepci, D.B.; Taslimi, P.; Yigit, B.; Cetinkaya, B.; Ozdemir, I.; Aygun, M.; Gulcin, I. Selenourea and thiourea derivatives of chiral and achiral enetetramines: Synthesis, characterization and enzyme inhibitory properties. Bioorg. Chem. 2022, 120, 105566. [Google Scholar] [CrossRef]
- Topal, F.; Aksu, K.; Gulcin, I.; Tumer, F.; Goksu, S. Inhibition profiles of some symmetric sulfamides derived from phenethylamines on human carbonic anhydrase I., and II isoenzymes. Chem. Biodiver. 2021, 18, e2100422. [Google Scholar] [CrossRef]
- Gulcin, I.; Buyukokuroglu, M.E.; Oktay, M.; Kufrevioglu, O.I. Antioxidant and analgesic activities of turpentine of Pinus nigra Arn. Subsp. pallsiana (Lamb.) Holmboe. J. Ethnopharmacol. 2003, 86, 51–58. [Google Scholar] [CrossRef]
- Gulcin, I.; Kaya, R.; Gören, A.C.; Akıncıoğlu, H.; Topal, M.; Bingöl, Z.; Cetin Cakmak, K.; Ozturk Sarikaya, S.B.; Durmaz, L.; Alwasel, S. Anticholinergic, antidiabetic and antioxidant activities of cinnamon (Cinnamomum verum) bark extracts: Polyphenol contents analysis by LC-MS/MS. Int. J. Food Prop. 2019, 22, 1511–1526. [Google Scholar] [CrossRef]
- Koksal, E.; Bursal, E.; Gulcin, I.; Korkmaz, M.; Caglayan, C.; Goren, A.C.; Alwasel, S.H. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by LC-MS/MS. Int. J. Food Prop. 2017, 20, 514–525. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Gulcin, I.; Beydemir, Ş.; Sat, I.G.; Kufrevioglu, O.I. Evaluation of antioxidant activity of cornelian cherry (Cornus mas L.). Acta Aliment. Hung. 2005, 34, 193–202. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Cakmakci, S.; Topdas, E.F.; Kalin, P.; Han, H.; Sekerci, P.; Polat Kose, L.; Gulcin, I. Antioxidant capacity and functionality of oleaster (Elaeagnus angustifolia L.) flour and crust in a new kind of fruity ice cream. Int. J. Food Sci. Technol. 2015, 50, 472–481. [Google Scholar] [CrossRef]
- Gulcin, I.; Tel, A.Z.; Goren, A.C.; Taslimi, P.; Alwasel, S. Sage (Salvia pilifera): Determination its polyphenol contents, anticholinergic, antidiabetic and antioxidant activities. J. Food Meas. Charact. 2019, 13, 2062–2074. [Google Scholar] [CrossRef]
- Kalin, P.; Gulcin, I.; Goren, A.C. Antioxidant activity and polyphenol content of cranberries (Vaccinium macrocarpon). Rec. Nat. Prod. 2015, 9, 496–502. [Google Scholar]
- Yilmaz, M.A. Simultaneous quantitative screening of 53 phytochemicals in 33 species of medicinal and aromatic plants: A detailed, robust and comprehensive LC–MS/MS method validation. Ind. Crops Prod. 2020, 149, 112347. [Google Scholar] [CrossRef]
- Elmastas, M.; Turkekul, I.; Ozturk, L.; Gulcin, I.; İsıldak, O.; Aboul-Enein, H.Y. The antioxidant activity of two wild edible mushrooms (Morchella vulgaris and Morchella esculanta). Comb. Chem. High Throughput Screen. 2006, 9, 443–448. [Google Scholar] [CrossRef]
- Cetinkaya, Y.; Gocer, H.; Menzek, A.; Gulcin, I. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch. Pharm. 2012, 345, 323–334. [Google Scholar] [CrossRef]
- Taslimi, P.; Gulcin, I. Antioxidant and anticholinergic properties of olivetol. J. Food Biochem. 2018, 42, e12516. [Google Scholar] [CrossRef]
- Rezai, M.; Bayrak, C.; Taslimi, P.; Gulcin, I.; Menzek, A. The first synthesis, antioxidant and anticholinergic activities of 1-(4,5-dihydroxybenzyl)pyrrolidin-2-one derivative bromophenols including natural products. Turk. J. Chem. 2018, 42, 808–825. [Google Scholar]
- Gulcin, I.; Elias, R.; Gepdiremen, A.; Boyer, L.; Koksal, E. A comparative study on the antioxidant activity of fringe tree (Chionanthus virginicus L.) extracts. Afr. J. Biotechnol. 2007, 6, 410–418. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 26, 1199–1200. [Google Scholar] [CrossRef]
- Huyut, Z.; Beydemir, S.; Gulcin, I. Antioxidant and antiradical properties of some flavonoids and phenolic compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Tohma, H.; Gulcin, I.; Bursal, E.; Goren, A.C.; Alwasel, S.H.; Koksal, E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J. Food Meas. Charact. 2017, 11, 556–566. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Beydemir, S.; Topal, F.; Gagua, N.; Bakuridze, A.; Bayram, R.; Gepdiremen, A. Apoptotic, antioxidant and antiradical effects of majdine and isomajdine from Vinca herbacea Waldst. and kit. J. Enzyme Inhib. Med. Chem. 2012, 27, 587–594. [Google Scholar] [CrossRef]
- Gulcin, I.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.R.M.F. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–90. [Google Scholar] [CrossRef]
- Verpoorte, J.A.; Mehta, S.; Edsall, J.T. Esterase activities of human carbonic anhydrases B and C. J. Biol. Chem. 1967, 242, 4221–4229. [Google Scholar] [CrossRef]
- Mutlu, M.; Bingol, Z.; Uç, E.M.; Koksal, E.; Goren, A.C.; Alwasel, S.H.; Gulcin, I. Comprehensive metabolite profiling of cinnamon (Cinnamomum zeylanicum) leaf oil using LC-HR/MS, GC/MS, and GC-FID: Determination of antiglaucoma, antioxidant, anticholinergic, and antidiabetic profiles. Life 2023, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Gocer, H.; Akincioglu, A.; Goksu, S.; Gulcin, I. Carbonic anhydrase inhibitory properties of phenolic sulfonamides derived from dopamine related compounds. Arab. J. Chem. 2017, 10, 398–402. [Google Scholar] [CrossRef]
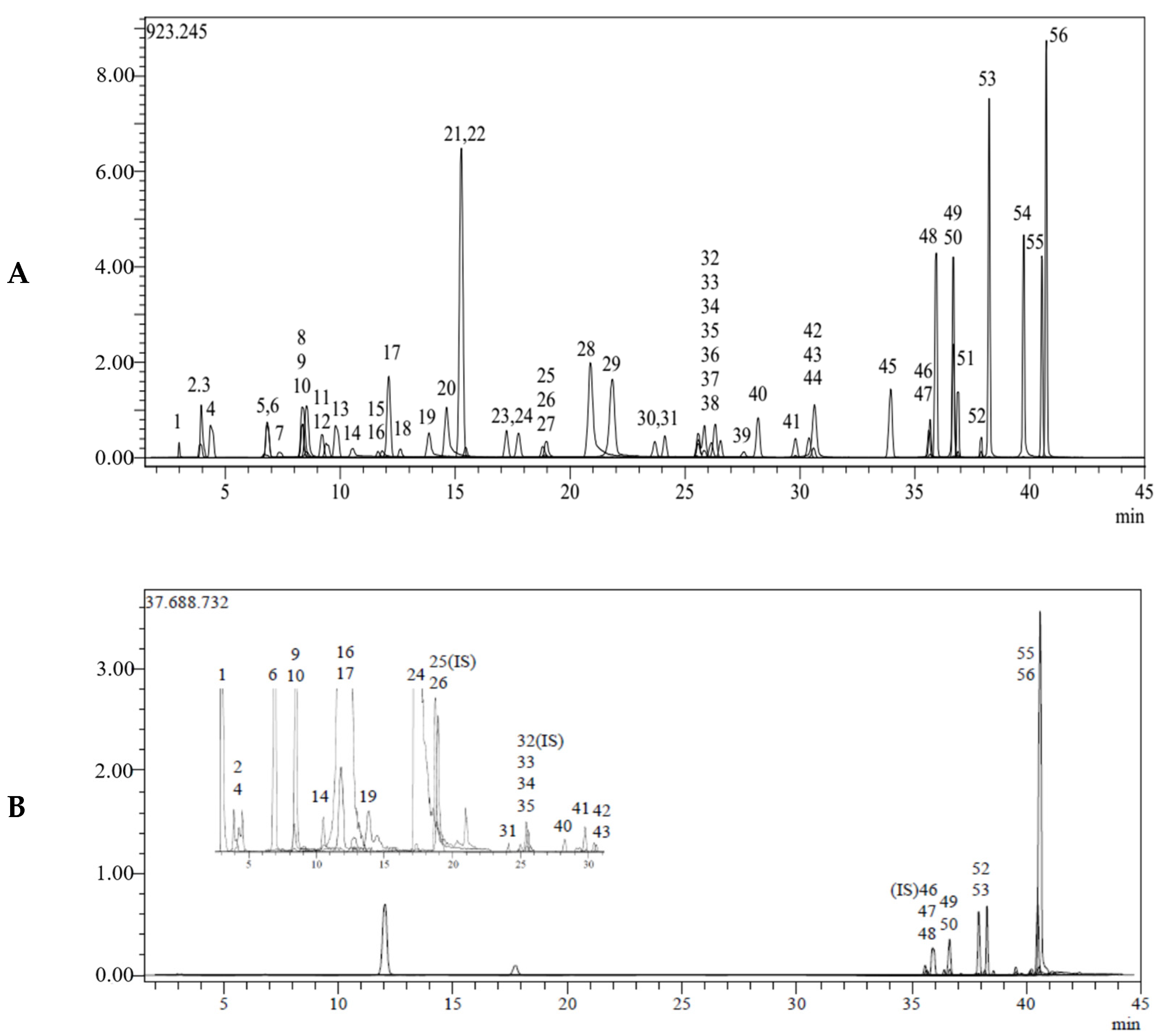
| No. | Analytes | RT a | M.I. (m/z) b | F.I. (m/z) c | Ion. Mode | Equation | R2 d | RSD% e | Linearity | LOD/LOQ (µg/L) f | Recovery (%) | U g | Gr. No. i | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interday | Intraday | Interday | Intraday | ||||||||||||
| 1 | Quinic acid | 3.0 | 190.8 | 93.0 | Negative | y = −0.0129989 + 2.97989x | 0.996 | 0.69 | 0.51 | 0.1–5 | 25.7/33.3 | 1.0011 | 1.0083 | 0.0372 | 1 |
| 2 | Fumaric aid | 3.9 | 115.2 | 40.9 | Negative | y = −0.0817862 + 1.03467x | 0.995 | 1.05 | 1.02 | 1–50 | 135.7/167.9 | 0.9963 | 1.0016 | 0.0091 | 1 |
| 3 | Aconitic acid | 4.0 | 172.8 | 129.0 | Negative | y = −0.7014530 + 32.9994x | 0.971 | 2.07 | 0.93 | 0.1–5 | 16.4/31.4 | 0.9968 | 1.0068 | 0.0247 | 1 |
| 4 | Gallic acid | 4.4 | 168.8 | 79.0 | Negative | y = 0.0547697 + 20.8152x | 0.999 | 1.60 | 0.81 | 0.1–5 | 13.2/17.0 | 1.0010 | 0.9947 | 0.0112 | 1 |
| 5 | Epigallocatechin | 6.7 | 304.8 | 219.0 | Negative | y = −0.00494986 + 0.0483704x | 0.998 | 1.22 | 0.73 | 1–50 | 237.5/265.9 | 0.9969 | 1.0040 | 0.0184 | 3 |
| 6 | Protocatechuic acid | 6.8 | 152.8 | 108.0 | Negative | y = 0.211373 + 12.8622x | 0.957 | 1.43 | 0.76 | 0.1–5 | 21.9/38.6 | 0.9972 | 1.0055 | 0.0350 | 1 |
| 7 | Catechin | 7.4 | 288.8 | 203.1 | Negative | y = −0.00370053 + 0.431369x | 0.999 | 2.14 | 1.08 | 0.2–10 | 55.0/78.0 | 1.0024 | 1.0045 | 0.0221 | 3 |
| 8 | Gentisic acid | 8.3 | 152.8 | 109.0 | Negative | y = −0.0238983 + 12.1494x | 0.997 | 1.81 | 1.22 | 0.1–5 | 18.5/28.2 | 0.9963 | 1.0077 | 0.0167 | 1 |
| 9 | Chlorogenic acid | 8.4 | 353.0 | 85.0 | Negative | y = 0.289983 + 36.3926x | 0.995 | 2.15 | 1.52 | 0.1–5 | 13.1/17.6 | 1.0000 | 1.0023 | 0.0213 | 1 |
| 10 | Protocatechuic aldehyde | 8.5 | 137.2 | 92.0 | Negative | y = 0.257085 + 25.4657x | 0.996 | 2.08 | 0.57 | 0.1–5 | 15.4/22.2 | 1.0002 | 0.9988 | 0.0396 | 1 |
| 11 | Tannic acid | 9.2 | 182.8 | 78.0 | Negative | y = 0.0126307 + 26.9263x | 0.999 | 2.40 | 1.16 | 0.05–2.5 | 15.3/22.7 | 0.9970 | 0.9950 | 0.0190 | 1 |
| 12 | Epigallocatechin gallate | 9.4 | 457.0 | 305.1 | Negative | y = −0.0380744 + 1.61233x | 0.999 | 1.30 | 0.63 | 0.2–10 | 61.0/86.0 | 0.9981 | 1.0079 | 0.0147 | 3 |
| 13 | 1,5-Dicaffeoylquinic acid | 9.8 | 515.0 | 191.0 | Negative | y = −0.0164044 + 16.6535x | 0.999 | 2.42 | 1.48 | 0.1–5 | 5.8/9.4 | 0.9983 | 0.9997 | 0.0306 | 1 |
| 14 | 4-OH Benzoic acid | 10.5 | 137.2 | 65.0 | Negative | y = −0.0240747 + 5.06492x | 0.999 | 1.24 | 0.97 | 0.2–10 | 68.4/88.1 | 1.0032 | 1.0068 | 0.0237 | 1 |
| 15 | Epicatechin | 11.6 | 289.0 | 203.0 | Negative | y = −0.0172078 + 0.0833424x | 0.996 | 1.47 | 0.62 | 1–50 | 139.6/161.6 | 1.0013 | 1.0012 | 0.0221 | 3 |
| 16 | Vanillic acid | 11.8 | 166.8 | 108.0 | Negative | y = −0.0480183 + 0.779564x | 0.999 | 1.92 | 0.76 | 1–50 | 141.9/164.9 | 1.0022 | 0.9998 | 0.0145 | 1 |
| 17 | Caffeic acid | 12.1 | 179.0 | 134.0 | Negative | y = 0.120319 + 95.4610x | 0.999 | 1.11 | 1.25 | 0.05–2.5 | 7.7/9.5 | 1.0015 | 1.0042 | 0.0152 | 1 |
| 18 | Syringic acid | 12.6 | 196.8 | 166.9 | Negative | y = −0.0458599 + 0.663948x | 0.998 | 1.18 | 1.09 | 1–50 | 82.3/104.5 | 1.0006 | 1.0072 | 0.0129 | 1 |
| 19 | Vanillin | 13.9 | 153.1 | 125.0 | Positive | y = 0.00185898 + 20.7382x | 0.996 | 1.10 | 0.85 | 0.1–5 | 24.5/30.4 | 1.0009 | 0.9967 | 0.0122 | 1 |
| 20 | Syringic aldehyde | 14.6 | 181.0 | 151.1 | Negative | y = −0.0128684 + 7.90153x | 0.999 | 2.51 | 0.77 | 0.4–20 | 19.7/28.0 | 1.0001 | 0.9964 | 0.0215 | 1 |
| 21 | Daidzin | 15.2 | 417.1 | 199.0 | Positive | y = 9.45747 + 152.338x | 0.996 | 2.25 | 1.32 | 0.05–2.5 | 7.0/9.5 | 0.9955 | 1.0017 | 0.0202 | 2 |
| 22 | Epicatechin gallate | 15.5 | 441.0 | 289.0 | Negative | y = −0.0142216 + 1.06768x | 0.997 | 1.63 | 1.28 | 0.1–5 | 19.5/28.5 | 0.9984 | 0.9946 | 0.0229 | 3 |
| 23 | Piceid | 17.2 | 391.0 | 135.0/106.9 | Positive | y = 0.00772525 + 25.4181x | 0.999 | 1.94 | 1.16 | 0.05–2.5 | 13.8/17.8 | 1.0042 | 0.9979 | 0.0199 | 1 |
| 24 | p-Coumaric acid | 17.8 | 163.0 | 93.0 | Negative | y = 0.0249034 + 18.5180x | 0.999 | 1.92 | 1.43 | 0.1–5 | 25.9/34.9 | 1.0049 | 1.0001 | 0.0194 | 1 |
| 25 | Ferulic acid-D3-IS h | 18.8 | 196.2 | 152.1 | Negative | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | 0.0170 | 1 |
| 26 | Ferulic acid | 18.8 | 192.8 | 149.0 | Negative | y = −0.0735254 + 1.34476x | 0.999 | 1.44 | 0.53 | 1–50 | 11.8/15.6 | 0.9951 | 0.9976 | 0.0181 | 1 |
| 27 | Sinapic acid | 18.9 | 222.8 | 193.0 | Negative | y = −0.0929932 + 0.836324x | 0.999 | 1.45 | 0.52 | 0.2–10 | 65.2/82.3 | 1.0031 | 1.0037 | 0.0317 | 1 |
| 28 | Coumarin | 20.9 | 146.9 | 103.1 | Positive | y = 0.0633397 + 136.508x | 0.999 | 2.11 | 1.54 | 0.05–2.5 | 214.2/247.3 | 0.9950 | 0.9958 | 0.0383 | 1 |
| 29 | Salicylic acid | 21.8 | 137.2 | 65.0 | Negative | y = 0.239287 + 153.659x | 0.999 | 1.48 | 1.18 | 0.05–2.5 | 6.0/8.3 | 0.9950 | 0.9998 | 0.0158 | 1 |
| 30 | Cynaroside | 23.7 | 447.0 | 284.0 | Negative | y = 0.280246 + 6.13360x | 0.997 | 1.56 | 1.12 | 0.05–2.5 | 12.1/16.0 | 1.0072 | 1.0002 | 0.0366 | 2 |
| 31 | Miquelianin | 24.1 | 477.0 | 150.9 | Negative | y = −0.00991585 + 5.50334x | 0.999 | 1.31 | 0.95 | 0.1–5 | 10.6/14.7 | 0.9934 | 0.9965 | 0.0220 | 2 |
| 33 | Rutin | 25.6 | 608.9 | 301.0 | Negative | y = −0.0771907 + 2.89868x | 0.999 | 1.38 | 1.09 | 0.1–5 | 15.7/22.7 | 0.9977 | 1.0033 | 0.0247 | 2 |
| 34 | Isoquercitrin | 25.6 | 463.0 | 271.0 | Negative | y = −0.111120 + 4.10546x | 0.998 | 2.13 | 0.78 | 0.1–5 | 8.7/13.5 | 1.0057 | 0.9963 | 0.0220 | 2 |
| 35 | Hesperidin | 25.8 | 611.2 | 449.0 | Positive | y = 0.139055 + 13.2785x | 0.999 | 1.84 | 1.35 | 0.1–5 | 19.0/26.0 | 0.9967 | 1.0043 | 0.0335 | 2 |
| 36 | o-Coumaric acid | 26.1 | 162.8 | 93.0 | Negative | y = 0.00837193 + 11.2147x | 0.999 | 2.11 | 1.46 | 0.1–5 | 31.8/40.4 | 1.0044 | 0.9986 | 0.0147 | 1 |
| 37 | Genistin | 26.3 | 431.0 | 239.0 | Negative | y = 1.65808 + 7.57459x | 0.991 | 2.01 | 1.28 | 0.1–5 | 14.9/21.7 | 1.0062 | 1.0047 | 0.0083 | 2 |
| 38 | Rosmarinic acid | 26.6 | 359.0 | 197.0 | Negative | y = −0.0117238 + 8.04377x | 0.999 | 1.24 | 0.86 | 0.1–5 | 16.2/21.2 | 1.0056 | 1.0002 | 0.0130 | 1 |
| 39 | Ellagic acid | 27.6 | 301.0 | 284.0 | Negative | y = 0.00877034 + 0.663741x | 0.999 | 1.57 | 1.23 | 0.4–20 | 56.9/71.0 | 1.0005 | 1.0048 | 0.0364 | 1 |
| 40 | Cosmosiin | 28.2 | 431.0 | 269.0 | Negative | y = −0.708662 + 8.62498x | 0.998 | 1.65 | 1.30 | 0.1–5 | 6.3/9.2 | 0.9940 | 0.9973 | 0.0083 | 2 |
| 41 | Quercitrin | 29.8 | 447.0 | 301.0 | Negative | y = −0.00153274 + 3.20368x | 0.999 | 2.24 | 1.16 | 0.1–5 | 4.8/6.4 | 0.9960 | 0.9978 | 0.0268 | 2 |
| 42 | Astragalin | 30.4 | 447.0 | 255.0 | Negative | y = 0.00825333 + 3.51189x | 0.999 | 2.08 | 1.72 | 0.1–5 | 6.6/8.2 | 0.9968 | 0.9957 | 0.0114 | 2 |
| 43 | Nicotiflorin | 30.6 | 592.9 | 255.0/284.0 | Negative | y = 0.00499333 + 2.62351x | 0.999 | 1.48 | 1.23 | 0.05–2.5 | 11.9/16.7 | 0.9954 | 1.0044 | 0.0108 | 2 |
| 44 | Fisetin | 30.6 | 285.0 | 163.0 | Negative | y = 0.0365705 + 8.09472x | 0.999 | 1.75 | 1.19 | 0.1–5 | 10.1/12.7 | 0.9980 | 1.0042 | 0.0231 | 3 |
| 45 | Daidzein | 34.0 | 253.0 | 223.0 | Negative | y = −0.0329252 + 6.23004x | 0.999 | 2.18 | 1.73 | 0.1–5 | 9.8/11.6 | 0.9926 | 0.9963 | 0.0370 | 3 |
| 47 | Quercetin | 35.7 | 301.0 | 272.9 | Negative | y = +0.00597342 + 3.39417x | 0.999 | 1.89 | 1.38 | 0.1–5 | 15.5/19.0 | 0.9967 | 0.9971 | 0.0175 | 3 |
| 48 | Naringenin | 35.9 | 270.9 | 119.0 | Negative | y = −0.00393403 + 14.6424x | 0.999 | 2.34 | 1.69 | 0.1–5 | 2.6/3.9 | 1.0062 | 1.0020 | 0.0392 | 3 |
| 49 | Hesperetin | 36.7 | 301.0 | 136.0/286.0 | Negative | y = +0.0442350 + 6.07160x | 0.999 | 2.47 | 2.13 | 0.1–5 | 7.1/9.1 | 0.9998 | 0.9963 | 0.0321 | 3 |
| 50 | Luteolin | 36.7 | 284.8 | 151.0/175.0 | Negative | y = −0.0541723 + 30.7422x | 0.999 | 1.67 | 1.28 | 0.05–2.5 | 2.6/4.1 | 0.9952 | 1.0029 | 0.0313 | 3 |
| 51 | Genistein | 36.9 | 269.0 | 135.0 | Negative | y = −0.00507501 + 12.1933x | 0.999 | 1.48 | 1.19 | 0.05–2.5 | 3.7/5.3 | 1.0069 | 1.0012 | 0.0337 | 3 |
| 52 | Kaempferol | 37.9 | 285.0 | 239.0 | Negative | y = −0.00459557 + 3.13754x | 0.999 | 1.49 | 1.26 | 0.05–2.5 | 10.2/15.4 | 0.9992 | 0.9990 | 0.0212 | 3 |
| 53 | Apigenin | 38.2 | 268.8 | 151.0/149.0 | Negative | y = 0.119018 + 34.8730x | 0.998 | 1.17 | 0.96 | 0.05–2.5 | 1.3/2.0 | 0.9985 | 1.0003 | 0.0178 | 3 |
| 54 | Amentoflavone | 39.7 | 537.0 | 417.0 | Negative | y = 0.727280 + 33.3658x | 0.992 | 1.35 | 1.12 | 0.05–2.5 | 2.8/5.1 | 0.9991 | 1.0044 | 0.0340 | 3 |
| 55 | Chrysin | 40.5 | 252.8 | 145.0/119.0 | Negative | y = −0.0777300 + 18.8873x | 0.999 | 1.46 | 1.21 | 0.05–2.5 | 1.5/2.8 | 0.9922 | 1.0050 | 0.0323 | 3 |
| 56 | Acacetin | 40.7 | 283.0 | 239.0 | Negative | y = −0.559818 + 163.062x | 0.997 | 1.67 | 1.28 | 0.02–1 | 1.5/2.5 | 0.9949 | 1.0011 | 0.0363 | 3 |
| Compounds | Analyte | Phenolics (mg Analyte/g Propolis) |
|---|---|---|
| 1 | Quinic acid | 7.285 |
| 2 | Fumaric aid | 0.134 |
| 3 | Aconitic acid | N.D. |
| 4 | Gallic acid | 0.150 |
| 5 | Epigallocatechin | N.D. |
| 6 | Protocatechuic acid | 1,158 |
| 7 | Catechin | N.D. |
| 8 | Gentisic acid | N.D. |
| 9 | Chlorogenic acid | 0.018 |
| 10 | Protocatechuic aldehyde | 0.338 |
| 11 | Tannic acid | N.D. |
| 12 | Epigallocatechin gallate | N.D. |
| 13 | 1,5-Dicaffeoylquinic acid | N.D. |
| 14 | 4-OH Benzoic acid | 0.306 |
| 15 | Epicatechin | N.D. |
| 16 | Vanillic acid | 1.647 |
| 17 | Caffeic acid | 21.358 |
| 18 | Syringic acid | N.D. |
| 19 | Vanillin | 0.116 |
| 20 | Syringic aldehyde | N.D. |
| 21 | Daidzin | N.D. |
| 22 | Epicatechin gallate | N.D. |
| 23 | Piceid | N.D. |
| 24 | p-Coumaric acid | 16.911 |
| 25 | Ferulic acid-D3-IS | N.D. |
| 26 | Ferulic acid | 5.110 |
| 27 | Sinapic acid | N.D. |
| 28 | Coumarin | N.D. |
| 29 | Salicylic acid | N.D. |
| 30 | Cyranoside | N.D. |
| 31 | Miquelianin | 0.020 |
| 32 | Rutin-D3-IS | N.D. |
| 33 | Rutin | 0.035 |
| 34 | Isoquercitrin | 0.045 |
| 35 | Hesperidin | 0.045 |
| 36 | O-Coumaric acid | N.D. |
| 37 | Genistin | N.D. |
| 38 | Rosmarinic acid | N.D. |
| 39 | Ellagic acid | N.D. |
| 40 | Cosmosiin | 0.026 |
| 41 | Quercitrin | 0.087 |
| 42 | Astragalin | 0.030 |
| 43 | Nicotiflorin | 0.024 |
| 44 | Fisetin | N.D. |
| 45 | Daidzein | N.D. |
| 46 | Quercetin-D3-IS | N.D. |
| 47 | Quercetin | 6.223 |
| 48 | Naringenin | 11.340 |
| 49 | Hesperetin | 2.089 |
| 50 | Luteolin | 4.394 |
| 51 | Genistein | N.D. |
| 52 | Kaempferol | 4.043 |
| 53 | Apigenin | 4.686 |
| 54 | Amentoflavone | N.D. |
| 55 | Chrysin | 9.860 |
| 56 | Acacetin | 76.359 |
| Antioxidants | Fe3+-Reducing | Cu2+-Reducing | FRAP-Reducing | |||
|---|---|---|---|---|---|---|
| λ700 | r2 | λ700 | r2 | λ700 | r2 | |
| BHA | 1.257 ± 0.088 | 0.9523 | 1.800 ± 0.156 | 0.9742 | 0.884 ± 0.116 | 0.9899 |
| BHT | 2.018 ± 0.029 | 0.9466 | 2.912 ± 0.012 | 0.9969 | 2.089 ± 0.027 | 0.9581 |
| α-Tocopherol | 1.895 ± 0.008 | 0.9402 | 1.139 ± 0.096 | 0.9967 | 1.995 ± 0.016 | 0.9807 |
| Trolox | 1.545 ± 0.019 | 0.9966 | 2.323 ± 0.049 | 0.9980 | 1.755 ± 0.093 | 0.9990 |
| Propolis | 0.894 ± 0.020 | 0.9953 | 0.778 ± 0.054 | 0.9986 | 1.114 ± 0.045 | 0.9970 |
| Antioxidants | DPPH• Scavenging | ABTS•+ Scavenging | DMPD•+ Scavenging | |||
|---|---|---|---|---|---|---|
| IC50 | r2 | IC50 | r2 | IC50 | r2 | |
| BHA | 9.00 | 0.9399 | 7.71 | 0.9330 | 31.43 | 0.9993 |
| BHT | 21.00 | 0.9668 | 7.71 | 0.9330 | - | - |
| α-Tocopherol | 5.92 | 0.9770 | 7.71 | 0.9330 | 14.38 | 0.9349 |
| Trolox | 9.63 | 0.9947 | 8.10 | 0.9550 | - | - |
| Propolis | 20.55 | 0.9989 | 8.157 | 0.9985 | 86.64 | 0.9855 |
| Antioxidants | hCA II | AChE | α-Glycosidase | |||
|---|---|---|---|---|---|---|
| IC50 | r2 | IC50 | r2 | IC50 | r2 | |
| Propolis | 19.6 | 0.9327 | 3.4 | 0.9869 | 3.7 | 0.9362 |
| Acetazolamide * | 8.37 | 0.9825 | - | - | - | - |
| Tacrine ** | - | - | 5.97 | 0.9706 | - | - |
| Acarbose *** | - | - | - | - | 22,800 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karagecili, H.; Yılmaz, M.A.; Ertürk, A.; Kiziltas, H.; Güven, L.; Alwasel, S.H.; Gulcin, İ. Comprehensive Metabolite Profiling of Berdav Propolis Using LC-MS/MS: Determination of Antioxidant, Anticholinergic, Antiglaucoma, and Antidiabetic Effects. Molecules 2023, 28, 1739. https://doi.org/10.3390/molecules28041739
Karagecili H, Yılmaz MA, Ertürk A, Kiziltas H, Güven L, Alwasel SH, Gulcin İ. Comprehensive Metabolite Profiling of Berdav Propolis Using LC-MS/MS: Determination of Antioxidant, Anticholinergic, Antiglaucoma, and Antidiabetic Effects. Molecules. 2023; 28(4):1739. https://doi.org/10.3390/molecules28041739
Chicago/Turabian StyleKaragecili, Hasan, Mustafa Abdullah Yılmaz, Adem Ertürk, Hatice Kiziltas, Leyla Güven, Saleh H. Alwasel, and İlhami Gulcin. 2023. "Comprehensive Metabolite Profiling of Berdav Propolis Using LC-MS/MS: Determination of Antioxidant, Anticholinergic, Antiglaucoma, and Antidiabetic Effects" Molecules 28, no. 4: 1739. https://doi.org/10.3390/molecules28041739
APA StyleKaragecili, H., Yılmaz, M. A., Ertürk, A., Kiziltas, H., Güven, L., Alwasel, S. H., & Gulcin, İ. (2023). Comprehensive Metabolite Profiling of Berdav Propolis Using LC-MS/MS: Determination of Antioxidant, Anticholinergic, Antiglaucoma, and Antidiabetic Effects. Molecules, 28(4), 1739. https://doi.org/10.3390/molecules28041739









