Development of Meat Substitutes from Filamentous Fungi Cultivated on Residual Water of Tempeh Factories
Abstract
1. Introduction
2. Results and Discussion
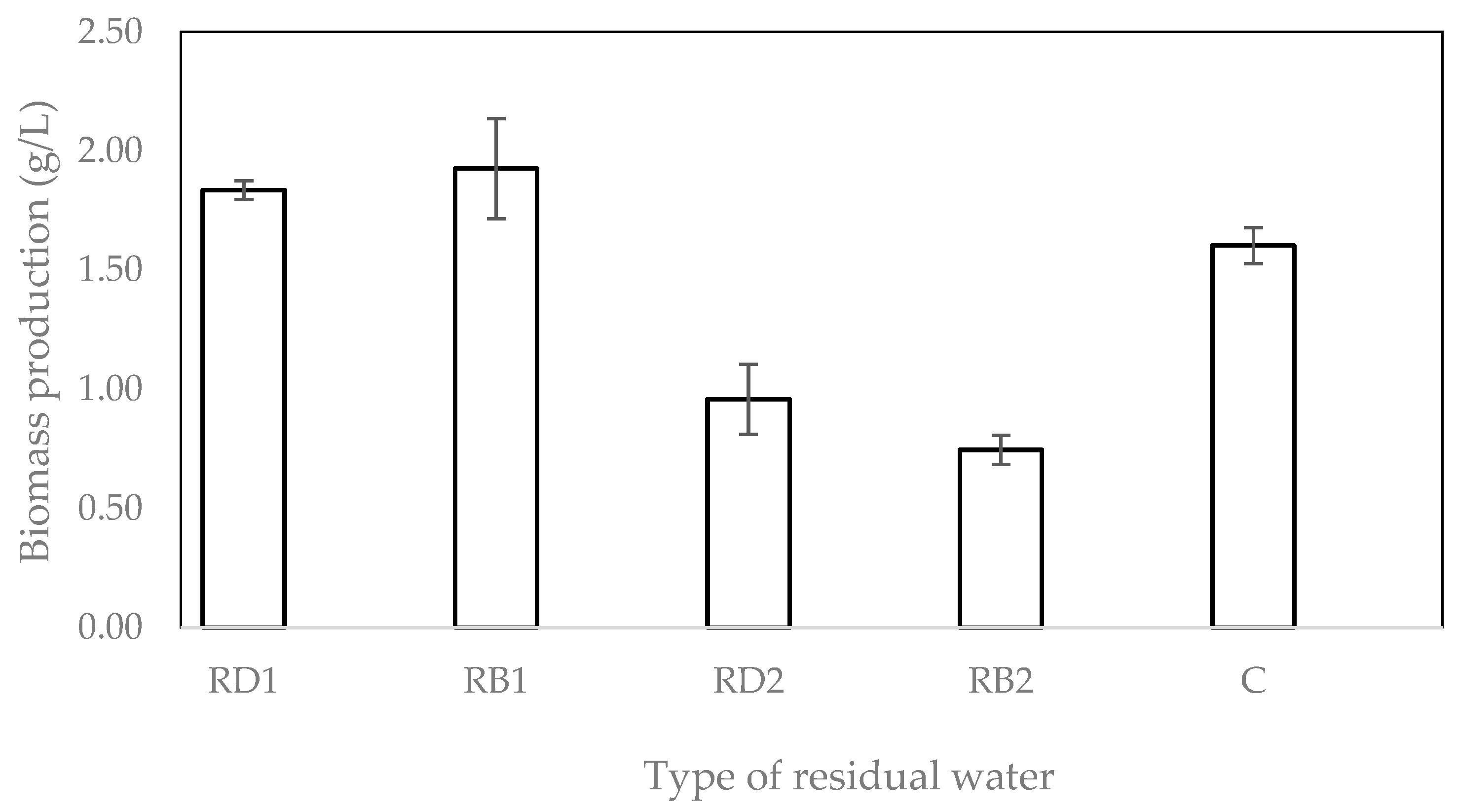
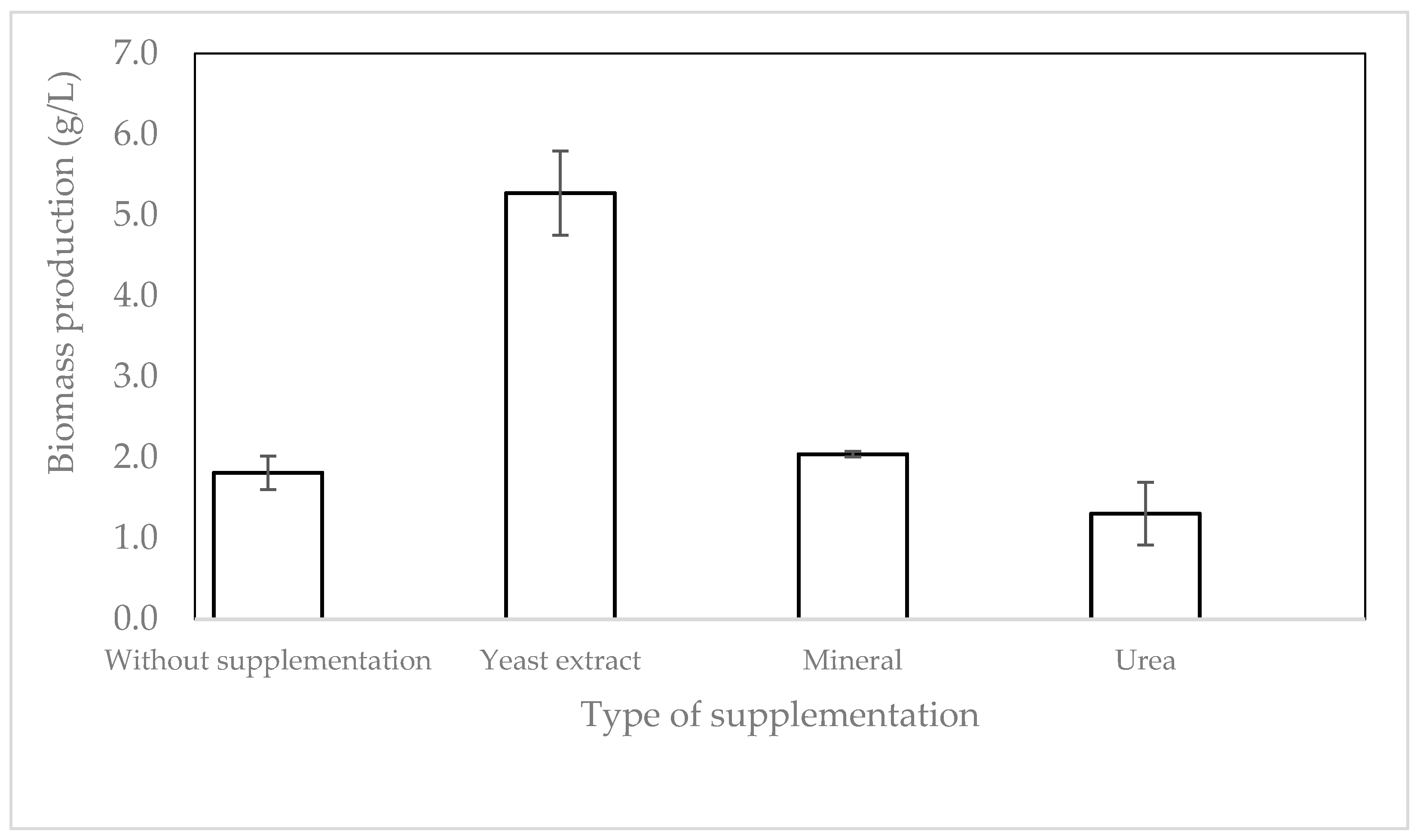
2.1. Effect of Media Composition on Biomass Production
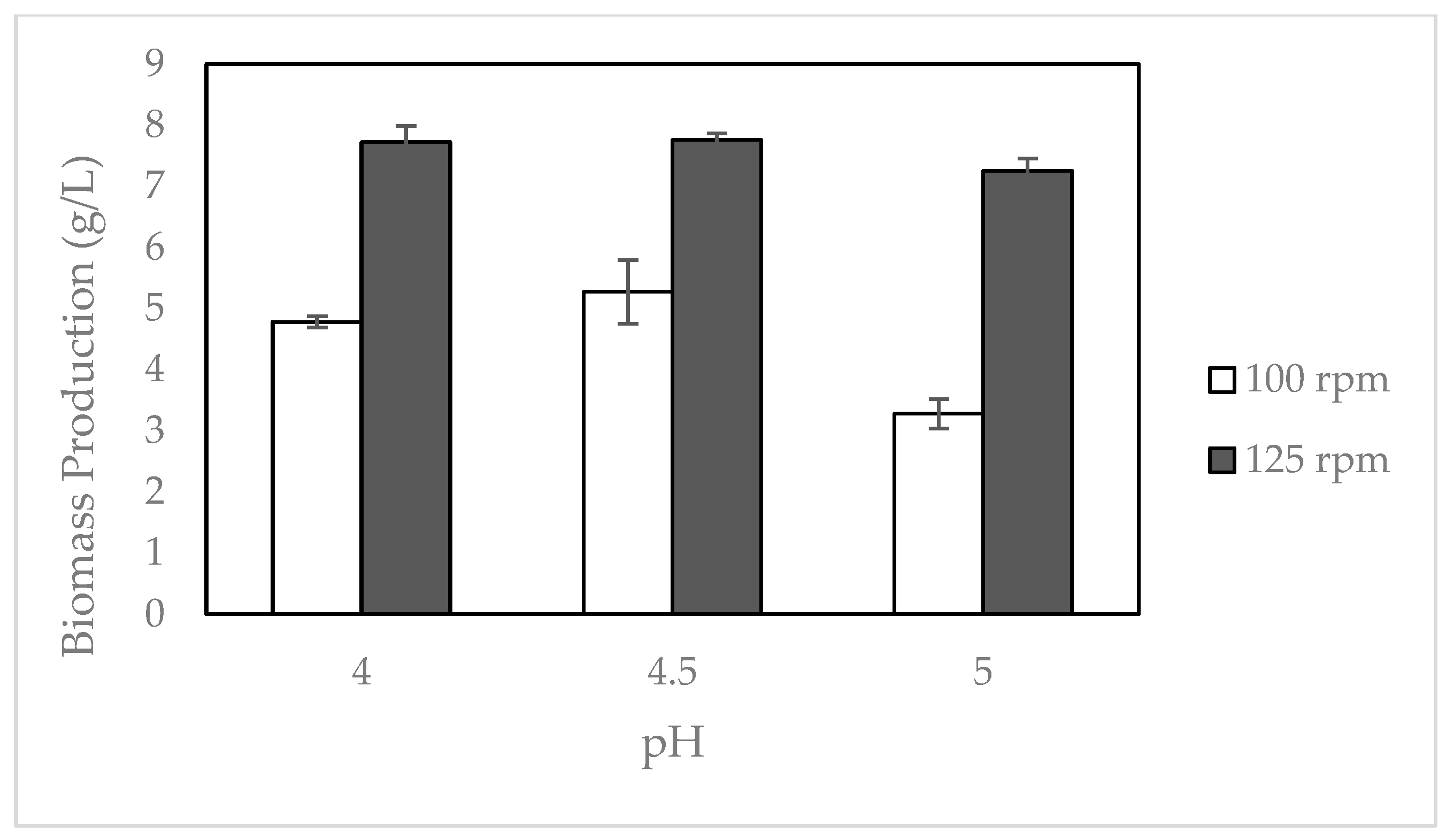
2.2. Effect of Cultivation Conditions on Biomass Production
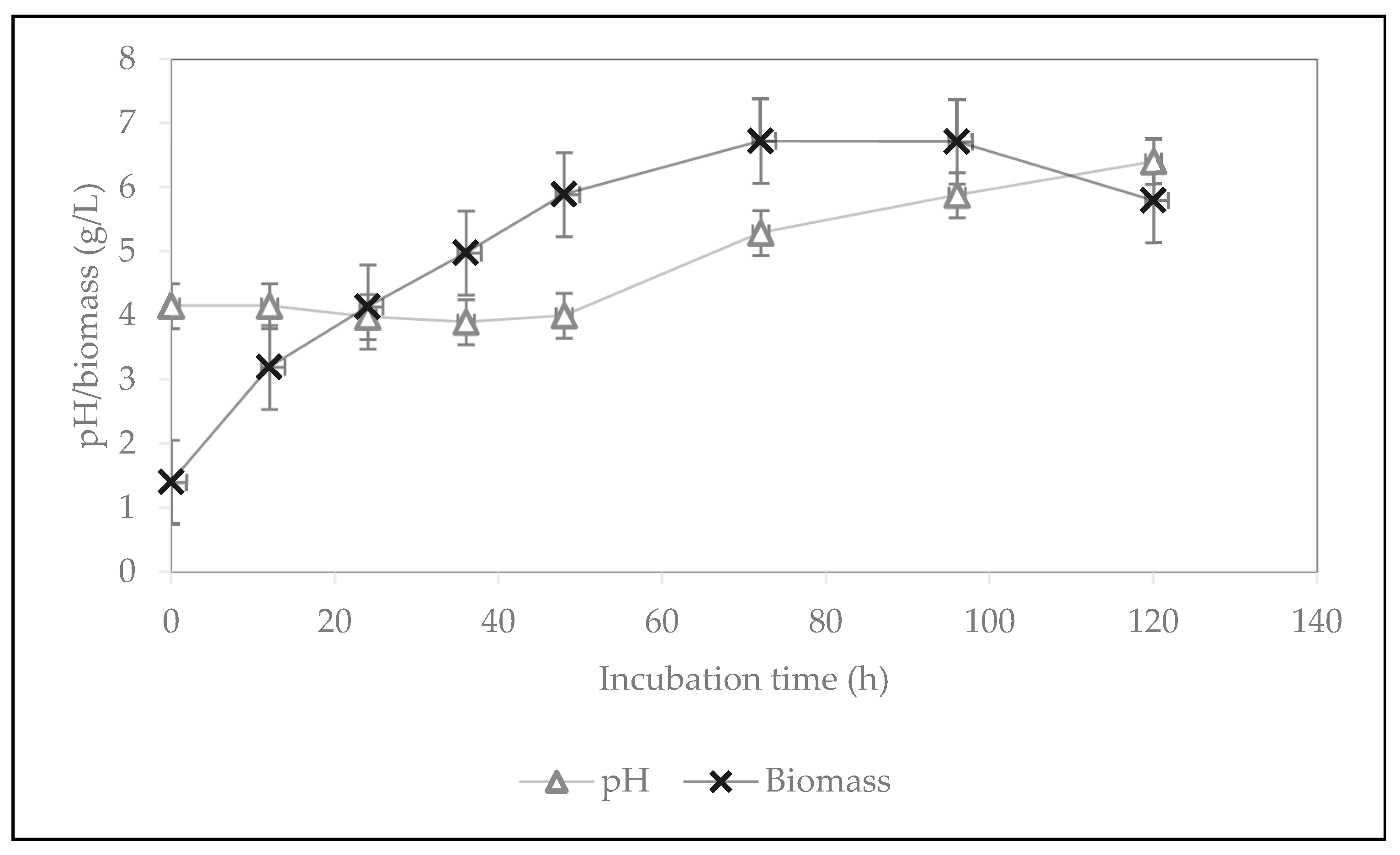
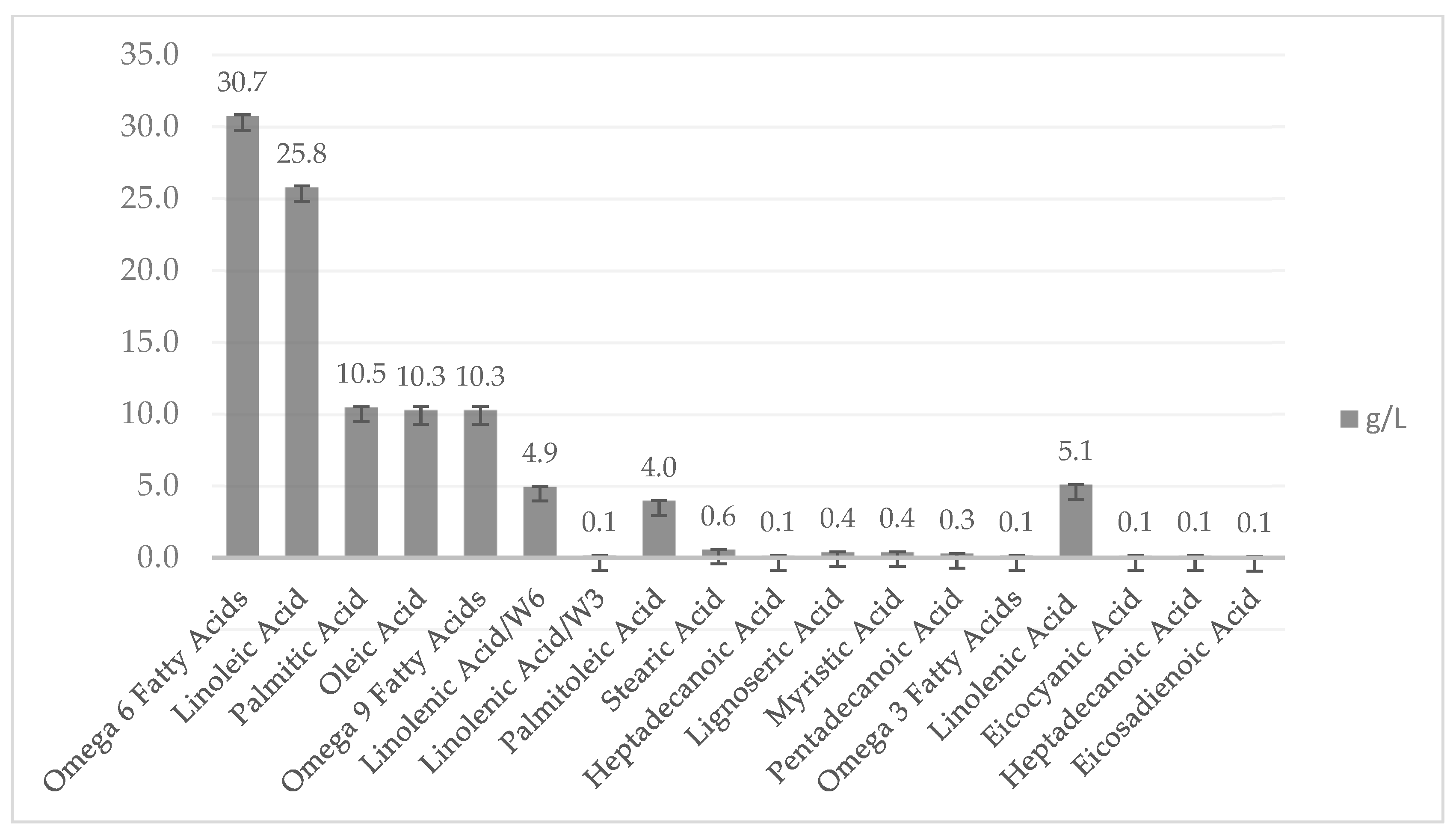
2.3. Nutritional Quality of Fungal Biomass Cultivated on Residual Water of First Boiling in Tempeh Processing
3. Materials and Methods
3.1. Microorganisms
3.2. Residual Water from Tempeh Processing
3.3. Microorganism Cultivation of Filamentous Fungi in Residual Waters
3.4. Determination of Residual Water Composition
3.5. Determination of Biomass Concentration
3.6. Proximate Analysis of the Mycoprotein
3.7. Determination of Protein Digestibility
3.8. Determination of Fiber Content
3.9. Determination of the Fatty Acid Profile
3.10. Determination of the Amino Acid Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global Nutrition Transition and the Pandemic of Obesity in Developing Countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Henchion, M.; Hayes, M.; Mullen, A.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Clark, M. Global Diets Link Environmental Sustainability and Human Health. Nature 2014, 515, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Van Zanten, H.H.E.; Mollenhorst, H.; Klootwijk, C.W.; van Middelaar, C.E.; de Boer, I.J.M. Global Food Supply: Land Use Efficiency of Livestock Systems. Int. J. Life Cycle Assess. 2016, 21, 747–758. [Google Scholar] [CrossRef]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of Red Meat and Processed Meat and Cancer Incidence: A Systematic Review and Meta-Analysis of Prospective Studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef]
- Sadler, M.J. Mycoprotein. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4072–4079. [Google Scholar]
- USDA. USDA FoodData Central; USDA: Nevada, IA, USA, 2021. [Google Scholar]
- Finnigan, T.; Needham, L.; Abbott, C. Mycoprotein: A healthy new protein with a low environmental impact. In Sustainable Protein Sources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 305–325. [Google Scholar]
- Elzerman, J.E.; Hoek, A.C.; van Boekel, M.A.J.S.; Luning, P.A. Consumer Acceptance and Appropriateness of Meat Substitutes in a Meal Context. Food Qual. Prefer. 2011, 22, 233–240. [Google Scholar] [CrossRef]
- Finnigan, T.J.A. Mycoprotein: Origins, Production and Properties; Woodhead Publishing Limited: Sawston, UK, 2011. [Google Scholar]
- Derbyshire, E.J.; Finnigan, T.J.A. Chapter 16—Mycoprotein: A futuristic portrayal. In Future Foods: Global Trends, Opportunities, and Sustainability Challenges; Bhat, R.B.T.-F.F., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 287–303. ISBN 978-0-323-91001-9. [Google Scholar]
- Finnigan, T.J.A.; Wall, B.T.; Wilde, P.J.; Stephens, F.B.; Taylor, S.L.; Freedman, M.R. Mycoprotein: The Future of Nutritious Nonmeat Protein, a Symposium Review. Curr. Dev. Nutr. 2019, 3, nzz021. [Google Scholar] [CrossRef]
- Badan Standardisasi Nasional. Tempe: Persembahan Indonesia Untuk Dunia; Badan Standardisasi Nasional: Jakarta Pusat, Indonesia, 2012. [Google Scholar]
- Andriawan, S.; Zubaidah, A.; Setiani, F.S.; Wananda, A.Z.J.; Baihaqi, A.U.; Calvin, D. Tempe Liquid Waste as Hydroponic Fertilizer in Sanan Village, Malang. J. Dedik. 2021, 18, 12–19. [Google Scholar]
- Hikma, N.; Alwi, M.; Umrah, U. Potensi Limbah Cair Tempe Secara Mikrobiologis Sebagai Alternatif Penghasil Biogas. Biocelebes 2014, 8, 54–59. [Google Scholar]
- Chaerun, S.K. Tempeh Waste as a Natural, Economical Carbon and Nutrient Source: ED-XRF and NCS Study. HAYATI J. Biosci. 2009, 16, 120–122. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, A. Soluble soybean polysaccharide. In Handbook of Hydrocolloids; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 693–709. [Google Scholar]
- Moretti, A.; Arias, C.L.; Mozzoni, L.A.; Chen, P.; Mcneece, B.T.; Mian, M.A.R.; Mchale, L.K.; Alonso, A.P. Workflow for the Quantification of Soluble and Insoluble Carbohydrates in Soybean Seed. Molecules 2020, 25, 3806. [Google Scholar] [CrossRef] [PubMed]
- Dzurendova, S.; Zimmermann, B.; Tafintseva, V.; Kohler, A.; Ekeberg, D.; Shapaval, V. The Influence of Phosphorus Source and the Nature of Nitrogen Substrate on the Biomass Production and Lipid Accumulation in Oleaginous Mucoromycota Fungi. Appl. Microbiol. Biotechnol. 2020, 104, 8065–8076. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, D.; Patel, A.; Rova, U.; Christakopoulos, P.; Katapodis, P.; Matsakas, L. Heterotrophic Cultivation of the Cyanobacterium Pseudanabaena sp. on Forest Biomass Hydrolysates toward Sustainable Biodiesel Production. Microorganisms 2022, 10, 1756. [Google Scholar] [CrossRef]
- Jin, B.; Yin, P.; Ma, Y.; Zhao, L. Production of Lactic Acid and Fungal Biomass by Rhizopus Fungi from Food Processing Waste Streams. J. Ind. Microbiol. Biotechnol. 2005, 32, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Rousta, N.; Ferreira, J.A.; Taherzadeh, M.J. Production of L-Carnitine-Enriched Edible Filamentous Fungal Biomass through Submerged Cultivation. Bioengineered 2021, 12, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Diego, J.; Cobos, V.; Rodríguez-grimón, R.O.; Grijalva-endara, A. Production and Characterization of Biomass and Exopolysaccharides Obtained in Submerged Culture under Different Initial PHs Used in the Cultivation of Colletotrichum Gloeosporioides and Rhizopus Stolonifer. Emir. J. Food Agric. 2020, 32, 628–632. [Google Scholar] [CrossRef]
- Kim, S.W.; Hwang, H.J.; Xu, C.P.; Choi, J.W.; Yun, J.W. Effect of Aeration and Agitation on the Production of Mycelial Biomass and Exopolysaccharides in an Enthomopathogenic Fungus Paecilomyces Sinclairii. Lett. Appl. Microbiol. 2003, 36, 321–326. [Google Scholar] [CrossRef]
- Nursiwi, A.; Dwikiputra, B.I.; Ishartani, D.; Sari, A.M. Changes on Microbial Growth during Mlanding Tempeh (Leucaena Leucocephala) over Fermentation. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Yogyakarta, Indonesia, 14–15 November 2018; IOP Publishing: Bristol, UK, 2019; Volume 379, p. 12001. [Google Scholar]
- Ahmad, M.I.; Farooq, S.; Alhamoud, Y.; Li, C.; Zhang, H. A Review on Mycoprotein: History, Nutritional Composition, Production Methods, and Health Benefits. Trends Food Sci. Technol. 2022, 121, 14–29. [Google Scholar] [CrossRef]
- Bowman, S.M.; Free, S.J. The Structure and Synthesis of the Fungal Cell Wall. Bioessays 2006, 28, 799–808. [Google Scholar] [CrossRef]
- Free, S.J. Fungal Cell Wall Organization and Biosynthesis. Adv. Genet. 2013, 81, 33–82. [Google Scholar]
- Krogdahl, Å.; Hemre, G.; Mommsen, T.P. Carbohydrates in Fish Nutrition: Digestion and Absorption in Postlarval Stages. Aquac. Nutr. 2005, 11, 103–122. [Google Scholar] [CrossRef]
- Gilani, G.S.; Lee, N. Sources of Food-grade Protein. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 4873–4879. ISBN 9780122270550. [Google Scholar]
- Baer, D.J.; Rumpler, W.V.; Miles, C.W.; Fahey, G.C., Jr. Dietary Fiber Decreases the Metabolizable Energy Content and Nutrient Digestibility of Mixed Diets Fed to Humans. J. Nutr. 1997, 127, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.T.; Mahboubi, A.; Ferreira, J.A. Production of Fi Lamentous Fungal Biomass with Increased Oil Content Using Olive Oil as a Carbon Source. J. Chem. Technol. Biotechnol. 2022, 97, 2626–2635. [Google Scholar] [CrossRef]
- Maurya, N.K.; Kushwaha, R. Novel Protein Foods: Alternative Sources of Protein for Human Consumption; Akinik Publication: New Delhi, India, 2019. [Google Scholar] [CrossRef]
- Wikandari, R.; Kinanti, D.A.; Permatasari, R.D.; Rahmaningtyas, N.L.; Chairunisa, N.R.; Hellwig, C.; Taherzadeh, M.J. Correlations between the Chemical, Microbiological Characteristics and Sensory Profile of Fungal Fermented Food. Fermentation 2021, 7, 261. [Google Scholar] [CrossRef]
- Kudełka, W.; Kowalska, M.; Popis, M. Quality of Soybean Products in Terms of Essential Amino Acids Composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Cross, H.R.; Gehring, K.B.; Savell, J.W.; Arnold, A.N.; Mcneill, S.H. Composition of Free and Peptide-Bound Amino Acids in Beef Chuck, Loin, and Round Cuts. J. Anim. Sci. 2016, 94, 2603–2613. [Google Scholar] [CrossRef]
- Young, V.R.; El-khoury, A.E. Human Amino Acid Requirements: A Re-Evaluation. Food Nutr. Bull. 1996, 17, 1–15. [Google Scholar] [CrossRef]
- Tanugraha, D.R. Produksi Biomassa Mikoprotein Dengan Jamur Rhizopus Oligosporus Pada Media Limbah Cair Industri Tempe; Gadjah Mada University: Yogyakarta, Indonesia, 2022. [Google Scholar]
- Wood, I.P.; Elliston, A.; Ryden, P.; Bancroft, I.; Roberts, I.N.; Waldron, K.W. Rapid Quantification of Reducing Sugars in Biomass Hydrolysates: Improving the Speed and Precision of the Dinitrosalicylic Acid Assay. Biomass Bioenergy 2012, 44, 117–121. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the AOAC, 18th ed.Association of Official Analytical Chemist: Washington, DC, USA, 2002. [Google Scholar]
- Ketnawa, S.; Ogawa, Y. In Vitro Protein Digestibility and Biochemical Characteristics of Soaked, Boiled and Fermented Soybeans. Sci. Rep. 2021, 11, 14257. [Google Scholar] [CrossRef]
- Cheng, A.; Yan, H.; Han, C.; Chen, X.; Wang, W.; Xie, C.; Qu, J.; Gong, Z.; Shi, X. Acid and Alkaline Hydrolysis Extraction of Non-Extractable Polyphenols in Blueberries: Optimisation by Response Surface Methodology. Czech J. Food Sci. 2014, 32, 218–225. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, D.; Li, X.; Meng, L. Chromatographic Method for Determination of the Free Amino Acid Content of Chamomile Flowers. Pharmacogn. Mag. 2015, 11, 176–179. [Google Scholar] [CrossRef] [PubMed]
| Substrate * | Protein (g/L) | Reducing Sugar (g/L) |
|---|---|---|
| RD1 | 1.11 | 0.461 |
| RB1 | 2.62 | 0.508 |
| RD2 | 0.41 | 0.08 |
| RB2 | 0.7 | 0.099 |
| C | 1.19 | 0.055 |
| Proximate Content | (%) |
|---|---|
| Protein (wb) | 19.44 |
| Protein digestibility | 45.48 |
| Lipid (db) | 1.56 |
| Crude fiber (db) | 8.51 |
| Amino Acids | Mycoprotein Obtained in This Study (g/100 g) | Amino Acids in Soybean g/100 g [36] | Amino Acids in Beef g/100 g [37] | Requirements mg/kg [38] |
|---|---|---|---|---|
| L-Phenylalanine | 1.95 | 1.929 | 3.09 | 14 |
| L-Valine | 2.16 | 1.734 | 4.48 | 13 |
| L-Tryptophan | 0.56 | 0.45 | 0.934 | 3.5–6 |
| L-Threonine | 2.45 | 1.382 | 3.43 | 9 |
| L-Isoleucine | 1.62 | 1.709 | 3.84 | 10 |
| L-Methionine | 0.36 | 0 | 2.37 | 13 |
| L-Leucine | 2.58 | 2.841 | 6.18 | 14 |
| L-Histidine | 2.53 | 1.151 | 2.94 | 8–12 |
| L-Lysine | 3.05 | 2.363 | 6.66 | 12 |
| L-Serine | 1.77 | 1.35 | 3.2 | - |
| L-Glutamic Acid | 4.21 | 5.31 | 6.89 | - |
| L-Alanine | 2.96 | 1.23 | 4.22 | - |
| L-Arginine | 2.66 | 1.92 | 4.79 | - |
| Glycine | 2.11 | 1.3 | 3.1 | - |
| L-Aspartic Acid | 2.99 | 3.2 | 3.73 | - |
| L-Tyrosine | 1.83 | 0.96 | 2.71 | - |
| L-Proline | 1.36 | 1.29 | 3 | - |
| L-Cysteine | 1.66 | 0.64 | 1.01 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wikandari, R.; Tanugraha, D.R.; Yastanto, A.J.; Manikharda; Gmoser, R.; Teixeira, J.A. Development of Meat Substitutes from Filamentous Fungi Cultivated on Residual Water of Tempeh Factories. Molecules 2023, 28, 997. https://doi.org/10.3390/molecules28030997
Wikandari R, Tanugraha DR, Yastanto AJ, Manikharda, Gmoser R, Teixeira JA. Development of Meat Substitutes from Filamentous Fungi Cultivated on Residual Water of Tempeh Factories. Molecules. 2023; 28(3):997. https://doi.org/10.3390/molecules28030997
Chicago/Turabian StyleWikandari, Rachma, Daniel Reinhart Tanugraha, Anang Juni Yastanto, Manikharda, Rebecca Gmoser, and José António Teixeira. 2023. "Development of Meat Substitutes from Filamentous Fungi Cultivated on Residual Water of Tempeh Factories" Molecules 28, no. 3: 997. https://doi.org/10.3390/molecules28030997
APA StyleWikandari, R., Tanugraha, D. R., Yastanto, A. J., Manikharda, Gmoser, R., & Teixeira, J. A. (2023). Development of Meat Substitutes from Filamentous Fungi Cultivated on Residual Water of Tempeh Factories. Molecules, 28(3), 997. https://doi.org/10.3390/molecules28030997








