Inhibition of Skin Pathogenic Bacteria, Antioxidant and Anti-Inflammatory Activity of Royal Jelly from Northern Thailand
Abstract
1. Introduction
2. Results
2.1. Antibacterial Activity of Royal Jelly
2.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Royal Jelly
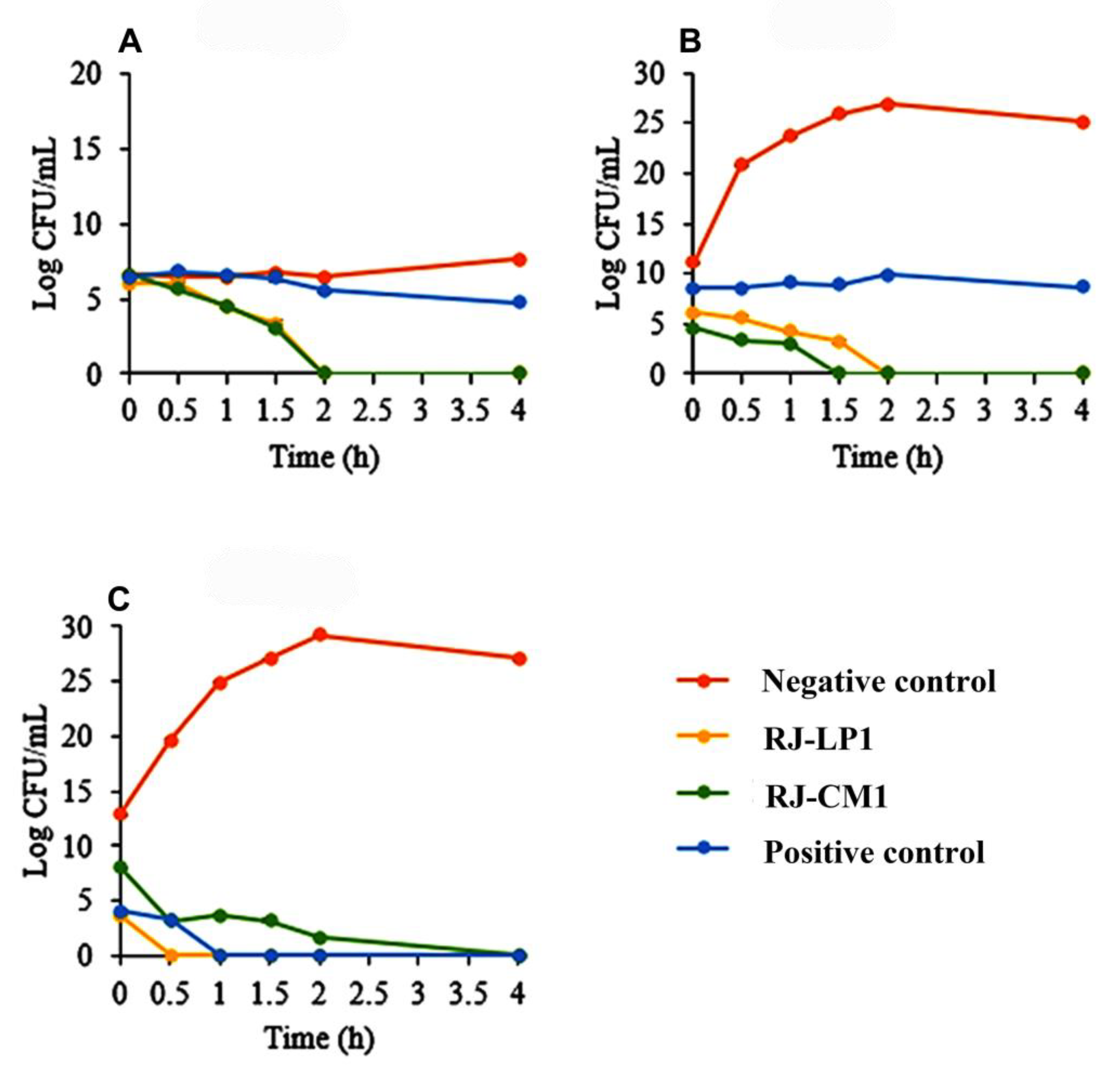
2.3. Time Killing Assay of Royal Jelly
2.4. Anti-Inflammatory Activity
2.4.1. Cytotoxicity of Royal Jelly on RAW264.7 Cells
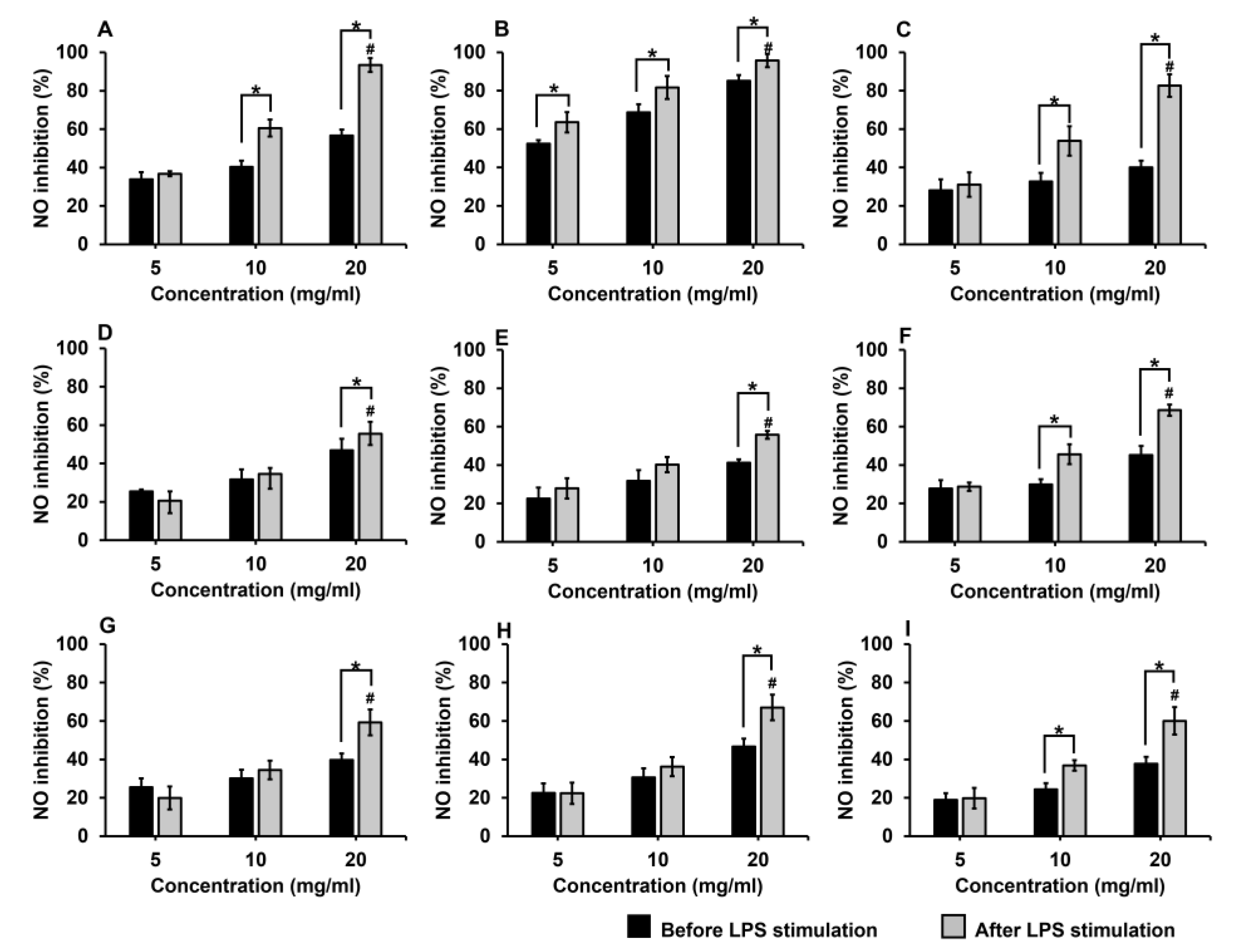
2.4.2. Inhibitory Effects of Royal Jelly on Nitric Oxide (NO) Production
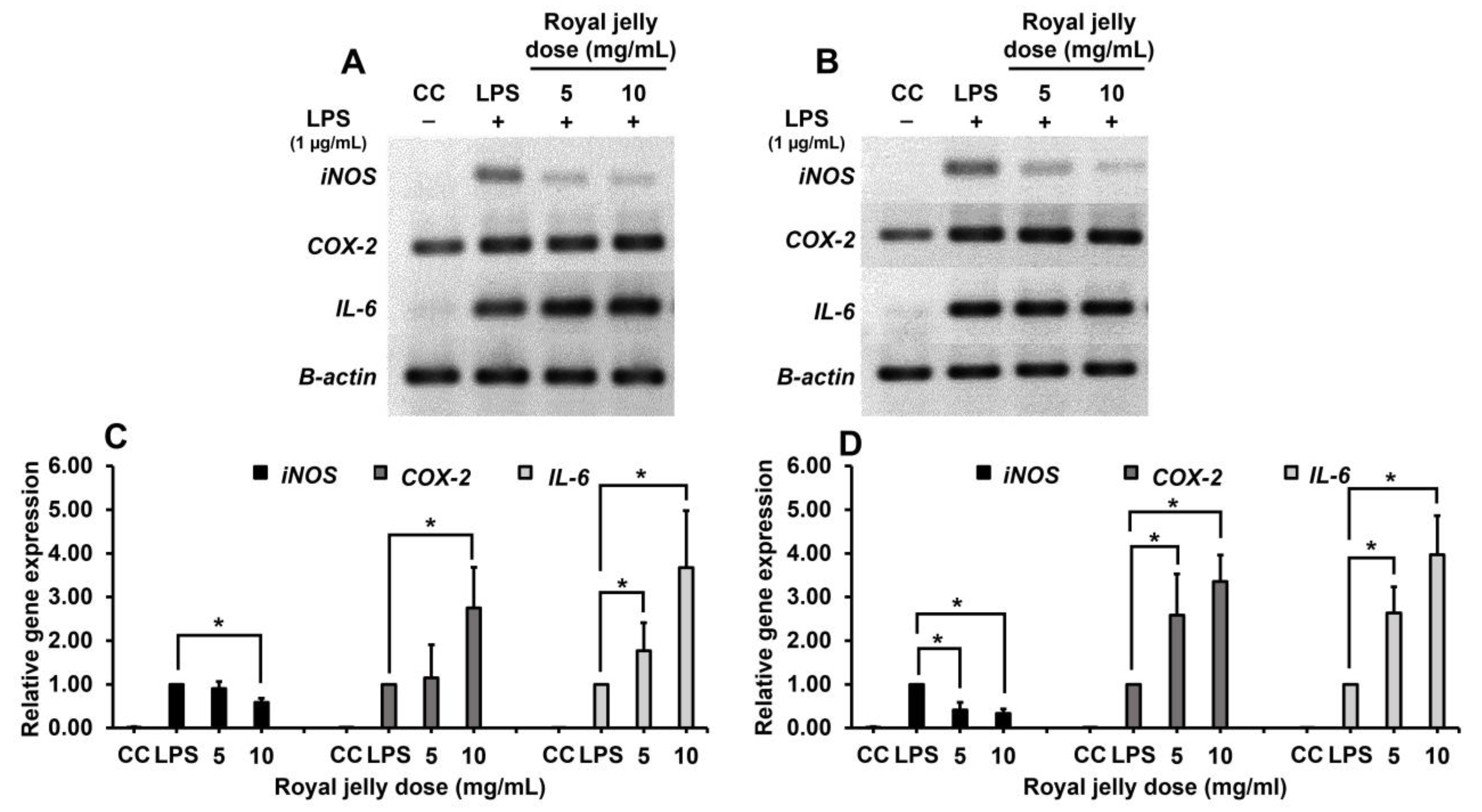
2.4.3. Inhibitory Efficacy of Royal Jelly on Inflammatory Gene Expression
2.5. Antioxidant Activity of Royal Jelly
2.6. Total Phenolic Content of Royal Jelly
2.7. Total Flavonoid Content of Royal Jelly
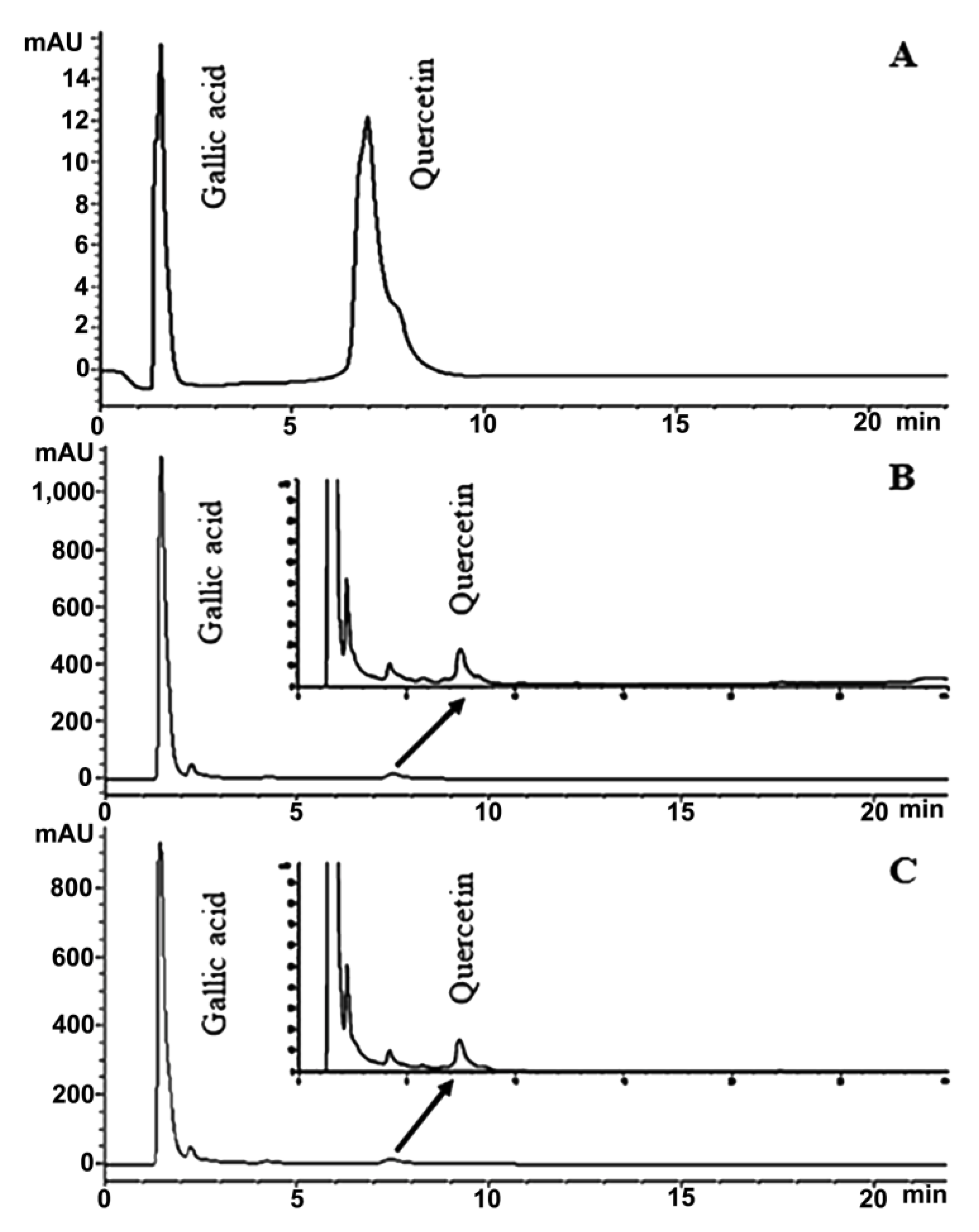
2.8. HPLC Analysis of Phytochemical Content of Royal Jelly
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Royal Jelly Samples
4.3. Bacterial Strains and Culture
4.4. Antibacterial Activity by Agar Well Diffusion Assay
4.5. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Analysis
4.6. Time Killing Assay
4.7. Cytotoxicity Assay of Royal Jelly on RAW264.7 Cells
4.8. Anti-inflammatory Activity of Royal Jelly
4.8.1. Treatment of RAW264.7 Cells by Royal Jelly before Stimulation by Lipopolysaccharide
4.8.2. Treatment of RAW264.7 Cells by Royal Jelly after Stimulation by Lipopolysaccharide
4.8.3. Measurement of Nitric Oxide (NO) Production
4.9. Inflammatory Gene Expression by Quantitative Real-time Polymerase Chain Reaction (qRT-PCR)
4.10. Antioxidant Activity of Royal Jelly by ABTS Radical Cation Decolorization Assay
4.11. Total Phenolic Compound Analysis
4.12. Total Flavonoid Compound Analysis
4.13. High Performance Liquid Chromatography (HPLC) Analysis of Phytochemical Compound Content
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and temporal diversity of the human skin microbiome. Science 2009, 324, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Conlan, S.; Polley, E.C.; Segre, J.A.; Kong, H.H. Shifts in human skin and nares microbiota of healthy children and adults. Genome Med. 2012, 4, 77. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef]
- Brown, R.L.; Clarke, T.B. The regulation of host defences to infection by the microbiota. Immunology 2017, 150, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.Y.; Kang, S.Y.; Kim, K.J. Artemisia princeps inhibits biofilm formation and virulence-factor expression of antibiotic-resistant bacteria. Biomed Res. Int. 2015, 2015, 239519. [Google Scholar] [CrossRef]
- Balkanska, R.; Marghitas, L.A.; Pavel, C.I. Antioxidant activity and total polyphenol content of royal jelly from Bulgaria. Int J Curr. Microbiol. Appl. Sci. 2017, 6, 578–585. [Google Scholar] [CrossRef]
- Ince, S.; Kucukkurt, I.; Demirel, H.H.; Acaroz, D.A.; Akbel, E.; Cigerci, I.H. Protective effects of boron on cyclophosphamide induced lipid peroxidation and genotoxicity in rats. Chemosphere 2014, 108, 197–204. [Google Scholar] [CrossRef]
- Kolayli, S.; Keskin, M. Natural Bee Products and Their Apitherapeutic Applications; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 175–196. [Google Scholar]
- Asma, S.T.; Bobiş, O.; Bonta, V.; Acaroz, U.; Shah, S.R.A.; Istanbullugil, F.R.; Arslan-Acaroz, D. General nutritional profile of bee products and their potential antiviral properties against mammalian viruses. Nutrients 2022, 14, 3579. [Google Scholar] [CrossRef]
- Weis, W.A.; Ripari, N.; Conte, F.L.; da Silva Honorio, M.; Sartori, A.A.; Matucci, R.H.; Sforcin, J.M. An overview about apitherapy and its clinical applications. Phytomedicine Plus 2022, 2, 100239. [Google Scholar] [CrossRef]
- Fujita, T.; Kozuka-Hata, H.; Ao-Kondo, H.; Kunieda, T.; Oyama, M.; Kubo, T. Proteomic analysis of the royal jelly and characterization of the functions of its derivation glands in the honeybee. J. Proteome Res. 2013, 12, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Amoedo, L.H.; Almeida-Muradian, L.B.D. Determination of trans-10-hydroxy-2-decenoic acid (10-HDA) in royal jelly from São Paulo State, Brazil. Food Sci. Technol. 2003, 23, 62–65. [Google Scholar] [CrossRef]
- Mărghitaş, L.A.; Bărnułiu, L.I.; Dezmirean, D.S.; Bobiş, O.; Bonta, V.; Mărgăoan, R.; Gherman, B. Determination of trans-10-hydroxy-2-decenoic acid (10-HDA) in transylvanian royal jelly. Anim. Sci. Biotechnol. 2013, 70, 9–14. [Google Scholar]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Seven, I.; Şįmşek, Ü.G.; Gökçe, Z.; Seven, P.T.; Arslan, A.; Yilmaz, Ö. The effects of royal jelly on performance and fatty acid profiles of different tissues in quail (Coturnix coturnix japonica) reared under high stocking density. Turk. J. Vet. Anim. Sci. 2014, 38, 271–277. [Google Scholar] [CrossRef]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on Royal Jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Bincoletto, C.; Eberlin, S.; Figueiredo, C.A.; Luengo, M.B.; Queiroz, M.L. Effects produced by Royal Jelly on haematopoiesis: Relation with host resistance against Ehrlich ascites tumour challenge. Int. Immunopharmacol. 2005, 5, 679–688. [Google Scholar] [CrossRef]
- Bíliková, K.; Hanes, J.; Nordhoff, E.; Saenger, W.; Klaudiny, J.; Šimúth, J. Apisimin, a new serine–valine-rich peptide from honeybee (Apis mellifera L.) royal jelly: Purification and molecular characterization. FEBS Lett. 2002, 528, 125–129. [Google Scholar] [CrossRef]
- Kolayli, S.; Sahin, H.; Can, Z.; Yildiz, O.; Malkoc, M.; Asadov, A. A member of complementary medicinal food: Anatolian royal jellies, their chemical compositions, and antioxidant properties. J. Evid.-Based Complement. Altern. Med. 2016, 21, 43–48. [Google Scholar] [CrossRef]
- Pavel, C.I.; Mărghitaş, L.A.; Bobiş, O.; Dezmirean, D.S.; Şapcaliu, A.; Radoi, I.; Mădaş, M.N. Biological activities of royal jelly-review. Anim. Sci. Biotechnol. 2011, 44, 108–118. [Google Scholar]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Alreshoodi, F.M.; Sultanbawa, Y. Antimicrobial activity of royal jelly. Anti-Infect. Agents 2015, 13, 50–59. [Google Scholar] [CrossRef]
- Bílikova, K.; Huang, S.C.; Lin, I.P.; Šimuth, J.; Peng, C.C. Structure and antimicrobial activity relationship of royalisin, an antimicrobial peptide from royal jelly of Apis mellifera. Peptides 2015, 68, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Sjaarda, C. Honey glycoproteins containing antimicrobial peptides, Jelleins of the Major Royal Jelly Protein 1, are responsible for the cell wall lytic and bactericidal activities of honey. PLoS ONE 2015, 10, e0120238. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. BSAC standardized disc susceptibility testing method (version 7). J. Antimicrob. Chemother. 2008, 62, 256–278. [Google Scholar] [CrossRef]
- Osés, S.M.; Pascual-Maté, A.; de la Fuente, D.; de Pablo, A.; Fernández-Muiño, M.A.; Sancho, M.T. Comparison of methods to determine antibacterial activity of honeys against Staphylococcus aureus. NJAS-Wagening. J. Life Sci. 2016, 78, 29–33. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Gómez-Romero, M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Advances in the analysis of phenolic compounds in products derived from bees. J. Pharm. Biomed. Anal. 2006, 41, 1220–1234. [Google Scholar] [CrossRef]
- Garcia, M.C.; Finola, M.S.; Marioli, J.M. Bioassay directed identification of royal jelly’s active compounds against the growth of bacteria capable of infecting cutaneous wounds. Adv. Appl. Microbiol. 2013, 3, 138–144. [Google Scholar] [CrossRef]
- Splith, K.; Neundorf, I. Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur. Biophys. J. 2011, 40, 387–397. [Google Scholar] [CrossRef]
- Poole, K. Efflux-mediated multi resistance in Gram-negative bacteria. Clin. Microbiol. Infect. 2004, 10, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Desalegn, A.; Andualem, B. Synergistic antibacterial effect of Sida rhombifolia leaf extracts and Apis mellifera honey against standard and drug resistant clinical isolated pathogenic bacteria. World Appl. Sci. J. 2014, 32, 1600–1610. [Google Scholar]
- Debalke, D.; Birhan, M.; Kinubeh, A.; Yayeh, M. Assessments of antibacterial effects of aqueous-ethanolic extracts of Sida rhombifolia’s aerial part. Sci. World J. 2018, 2018, 8429809. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Ferreres, F.; Garcıa-Viguera, C.; Bankova, V.S.; De Castro, S.L.; Dantas, A.P.; Valente, P.H.M.; Paulino, N. Phenolic compounds from Brazilian propolis with pharmacological activities. J. Ethnopharmacol. 2001, 74, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, M.; Accorsi, A.; Blasa, M.; Diamantini, G.; Piatti, E. Flavonoids from Italian multifloral honeys reduce the extracellular ferricyanide in human red blood cells. J. Agric. Food Chem. 2006, 54, 8328–8334. [Google Scholar] [CrossRef]
- Nagai, T.; Sakai, M.; Inoue, R.; Inoue, H.; Suzuki, N. Antioxidative activities of some commercially honeys, royal jelly, and propolis. Food Chem. 2001, 75, 237–240. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Czyżewska, U.; Jankowska, E.; Bakier, S. Determination of royal jelly acids in honey. Food Chem. 2011, 124, 387–391. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, B.Y.; Park, H.G.; Deng, Y.; Yoon, H.J.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Major royal jelly protein 2 acts as an antimicrobial agent and antioxidant in royal jelly. J. Asia-Pac. Entomol. 2019, 22, 684–689. [Google Scholar] [CrossRef]
- Liu, J.R.; Yang, Y.C.; Shi, L.S.; Peng, C.C. Antioxidant properties of royal jelly associated with larval age and time of harvest. J. Agric. Food Chem. 2008, 56, 11447–11452. [Google Scholar] [CrossRef]
- Čeksteryté, V.; Kurtinaitienė, B.; Venskutonis, P.R.; Pukalskas, A.; Kazernavičiūtė, R.; Balžekas, J. Evaluation of antioxidant activity and flavonoid composition in differently preserved bee products. Czech J. Food Sci. 2016, 34, 133–142. [Google Scholar]
- Park, M.J.; Kim, B.Y.; Deng, Y.; Park, H.G.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Antioxidant capacity of major royal jelly proteins of honeybee (Apis mellifera) royal jelly. J. Asia-Pac. Entomol. 2020, 23, 445–448. [Google Scholar] [CrossRef]
- Inoue, S.I.; Koya-Miyata, S.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal jelly prolongs the life span of C3H/HeJ mice: Correlation with reduced DNA damage. Exp. Gerontol. 2003, 38, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Wang, K.; Zhang, Y.Z.; Zheng, Y.F.; Hu, F.L. In vitro anti-inflammatory effects of three fatty acids from royal jelly. Mediat. Inflamm. 2016, 2016, 3583684. [Google Scholar] [CrossRef]
- Williams, R.O.; Paleolog, E.; Feldmann, M. Cytokine inhibitors in rheumatoid arthritis and other autoimmune diseases. Curr. Opin. Pharmacol. 2007, 7, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Song, I.B.; Han, H.J.; Lee, N.Y.; Cha, J.Y.; Son, Y.K.; Kwon, J. Anti-inflammatory and immune-enhancing effects of enzyme-treated royal jelly. Appl. Biol. Chem. 2018, 61, 227–233. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, propolis, and royal jelly: A comprehensive review of their biological actions and health benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, M.; Wang, L.; Lin, T.; Wang, G.; Peng, J.; Su, S. The In vitro and in vivo wound-healing effects of royal jelly derived from Apis mellifera L. during blossom seasons of Castanea mollissima Bl. and Brassica napus L. in South China exhibited distinct patterns. BMC Complement. Med. Ther. 2020, 20, 357. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Li, Z.G.; Tian, W.L.; Fang, X.M.; Su, S.K.; Peng, W.J. Differential volatile organic compounds in royal jelly associated with different nectar plants. J. Integr. Agric. 2016, 15, 1157–1165. [Google Scholar] [CrossRef]
- Akira, S.; Takeda, K. Toll-like receptor signaling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Ghosh, C.; Hong, B.; Batabyal, S.; Jeon, T.I.; Yang, S.H.; Hwang, S.G. Anti-inflammatory activity of the ethanol extract of Dictamnus dasycarpus leaf in lipopolysaccharide-activated macrophages. BMC Complement. Altern. Med. 2014, 14, 330. [Google Scholar] [CrossRef]
- Abaffy, P.; Tomankova, S.; Naraine, R.; Kubista, M.; Sindelka, R. The role of nitric oxide during embryonic wound healing. BMC Genom. 2019, 20, 815. [Google Scholar] [CrossRef]
- Takahashi, K.; Sugiyama, T.; Tokoro, S.; Neri, P.; Mori, H. Inhibitory effect of 10-hydroxydecanoic acid on lipopolysaccharide-induced nitric oxide production via translational downregulation of interferon regulatory factor-1 in RAW264 murine macrophages. Biomed. Res. 2013, 34, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for In vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Appiah, T.; Boakye, Y.D.; Agyare, C. Antimicrobial activities and time-kill kinetics of extracts of selected Ghanaian mushrooms. J. Evid.-Based Complement. Altern. Med. 2017, 2017, 4534350. [Google Scholar] [CrossRef]
- Maruyama, H.; Sakamoto, T.; Araki, Y.; Hara, H. Anti-inflammatory effect of bee pollen ethanol extract from Cistus sp. of Spanish on carrageenan-induced rat hind paw edema. BMC Complement. Altern. Med. 2010, 10, 30. [Google Scholar] [CrossRef]
- Cho, Y.S.; Lee, S.H.; Kim, S.K.; Ahn, C.B.; Je, J.Y. Aminoethyl-chitosan inhibits LPS-induced inflammatory mediators, iNOS and COX-2 expression in RAW264. 7 mouse macrophages. Process. Biochem. 2011, 46, 465–470. [Google Scholar] [CrossRef]
- Taddesse, Y.; Im, E.J.; Kwak, D.; Lee, Y.C.; Hyun, E.; Hong, M.; Jiao, P.; Jia, Q.; Goo, Y.K.; Hong, S.B.; et al. Stellera chamaejasme methanolic extract attenuates nitric oxide production and enhance heme oxygenase 1 expression in murine macrophages. Chiang Mai J. Sci. 2017, 44, 858–868. [Google Scholar]
- He, J.; Li, J.; Liu, H.; Yang, Z.; Zhou, F.; Wei, T.; Dong, T.; Xue, H.; Tang, L.; Liu, M. Scandoside exerts anti-inflammatory effect via suppressing NF-κB and MAPK signaling pathways in LPS-Induced RAW264.7 macrophages. Int. J. Mol. Sci. 2018, 19, 457. [Google Scholar] [CrossRef]
- Shah, P.; Modi, H.A. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 636–641. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Iadnut, A.; Mamoon, K.; Thammasit, P.; Pawichai, S.; Tima, S.; Preechasuth, K.; Kaewkod, T.; Tragoolpua, Y.; Tragoolpua, K. In vitro antifungal and antivirulence activities of biologically synthesized ethanolic extract of propolis-loaded PLGA nanoparticles against Candida albicans. J. Evid.-Based Complement. Altern. Med. 2019, 2019, 3715481. [Google Scholar] [CrossRef] [PubMed]
| Royal JellySamples | Inhibition Zone Diameter (mm) * | |||||
|---|---|---|---|---|---|---|
| Tested Bacteria | ||||||
| Corynebacterium spp. | C. acnes | MRSA | P. aeruginosa | S. aureus | S. epidermidis | |
| RJ-LP1 | 23.67 ± 3.21 | 12.33 ± 0.58 | 15.00 ± 1.73 | 9.33 ± 0.58 | 13.67 ± 1.15 | 17.00 ± 2.00 |
| RJ-CM1 | 19.33 ± 1.15 | 11.67 ± 0.58 | 13.67 ± 1.15 | 0 | 13.00 ± 1.00 | 14.00 ± 2.65 |
| RJ-CM2 | 27.67 ± 2.31 | 13.33 ± 0.58 | 14.67 ± 0.58 | 10.33 ± 0.58 | 15.33 ± 1.53 | 16.33 ± 2.31 |
| RJ-CM3 | 17.00 ± 1.73 | 14.67 ± 1.53 | 10.67 ± 0.58 | 10.00 ± 1.00 | 11.33 ± 1.15 | 10.33 ± 0.58 |
| RJ-CM4 | 19.67 ± 0.58 | 15.00 ± 1.73 | 13.00 ± 1.00 | 10.33 ± 0.58 | 13.33 ± 1.53 | 11.67 ± 0.58 |
| RJ-CM5 | 17.33 ± 0.58 | 0 | 12.33 ± 0.58 | 0 | 9.67 ± 0.58 | 15.67 ± 1.15 |
| RJ-CM6 | 18.00 ± 1.00 | 0 | 10.33 ± 0.58 | 0 | 10.00 ± 0.00 | 11.67 ± 0.58 |
| RJ-CM7 | 18.67 ± 0.58 | 13.00 ± 1.00 | 11.67 ± 0.58 | 0 | 11.67 ± 0.58 | 11.33 ± 0.58 |
| RJ-CM8 | 17.33 ± 0.58 | 0 | 10.33 ± 0.58 | 0 | 11.67 ± 0.58 | 10.67 ± 0.58 |
| Control | ||||||
| Gentamicin | 34.83 ± 0.29 | 42.17 ± 1.61 | ND | 31.17 ± 0.29 | 31.00 ± 1.32 | 30.67 ± 0.58 |
| Vancomycin | ND | ND | 31.33 ± 0.58 | ND | ND | ND |
| Distilled water | 0 | 0 | 0 | 0 | 0 | 0 |
| Royal JellySamples | MIC and MBC (mg/mL) * | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tested Bacteria | ||||||||||||
| Corynebacterium spp. | C. acnes | MRSA | P. aeruginosa | S. aureus | S. epidermidis | |||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| RJ-LP1 | 18.75 | 18.75 | 18.75 | 37.50 | 37.50 | 37.50 | 75.00 | 75.00 | 37.50 | 75.00 | 75.00 | 75.00 |
| RJ-CM1 | 18.75 | 18.75 | 37.50 | 37.50 | 37.50 | 75.00 | 75.00 | 75.00 | 37.50 | 75.00 | 75.00 | 75.00 |
| RJ-CM2 | 18.75 | 37.50 | 75.00 | 75.00 | 37.50 | 37.50 | 75.00 | 75.00 | 37.50 | 75.00 | 75.00 | 150.00 |
| RJ-CM3 | 18.75 | 37.50 | 75.00 | 150.00 | 37.50 | 75.00 | 75.00 | 150.00 | 37.50 | 150.00 | 75.00 | 150.00 |
| RJ-CM4 | 18.75 | 37.50 | 75.00 | 75.00 | 37.50 | 37.50 | 75.00 | 75.00 | 37.50 | 75.00 | 37.50 | 75.00 |
| RJ-CM5 | 18.75 | 18.75 | 37.50 | 37.50 | 37.50 | 37.50 | 75.00 | 75.00 | 75.00 | 75.00 | 37.50 | 37.50 |
| RJ-CM6 | 18.75 | 18.75 | 75.00 | 75.00 | 75.00 | 150.00 | 150.00 | 150.00 | 75.00 | 75.00 | 75.00 | 75.00 |
| RJ-CM7 | 18.75 | 18.75 | 37.50 | 37.50 | 75.00 | 75.00 | 75.00 | 150.00 | 75.00 | 75.00 | 75.00 | 75.00 |
| RJ-CM8 | 18.75 | 18.75 | 75.00 | 75.00 | 75.00 | 75.00 | 150.00 | 150.00 | 75.00 | 75.00 | 75.00 | 75.00 |
| Control | ||||||||||||
| Gentamicin | 0.0039 | 0.0039 | 0.0039 | 0.0039 | ND | ND | 0.0039 | 0.0039 | 0.0078 | 0.0156 | 0.0039 | 0.0078 |
| Vancomycin | ND | ND | ND | ND | 0.0020 | 0.0020 | ND | ND | ND | ND | ND | ND |
| Royal Jelly Samples | IC50 (mg/mL) * | |
|---|---|---|
| Before Stimulation with LPS | After Stimulation with LPS | |
| RJ-LP1 | 15.99 ± 1.52 | 9.23± 2.67 |
| RJ-CM1 | 5.83 ± 1.49 | 4.46 ± 0.92 |
| RJ-CM2 | >20.00 | 9.37 ± 1.07 |
| RJ-CM3 | >20.00 | 16.67 ± 3.73 |
| RJ-CM4 | >20.00 | 15.19 ± 2.74 |
| RJ-CM5 | >20.00 | 13.31 ± 1.32 |
| RJ-CM6 | >20.00 | 16.37 ± 2.16 |
| RJ-CM7 | >20.00 | 15.08 ± 0.97 |
| RJ-CM8 | >20.00 | 16.35 ± 2.19 |
| Royal Jelly Samples | Antioxidant Activity (mg TEAC/g Royal Jelly) | Total PhenolicContent (mg GAE/g Royal Jelly) | Total FlavonoidContent (mg QUE/g Royal Jelly) |
|---|---|---|---|
| RJ-LP1 | 2.03 ± 0.35 b | 6.64 ± 1.88 b | 4.93 ± 0.45 b |
| RJ-CM1 | 4.13 ± 1.89 a | 8.61 ± 1.57 a | 6.25 ± 0.59 a |
| RJ-CM2 | 3.61 ± 0.47 a | 7.27 ± 2.99 ab | 5.13 ± 0.86 b |
| RJ-CM3 | 1.52 ± 0.08 b | 2.69 ± 0.17 c | 0.70 ± 0.15 c |
| RJ-CM4 | 1.35 ± 0.11 b | 2.53 ± 0.25 c | 0.50 ± 0.11 c |
| RJ-CM5 | 1.25 ± 0.18 b | 2.65 ± 0.42 c | 0.71 ± 0.06 c |
| RJ-CM6 | 0.97 ± 0.23 b | 1.87 ± 0.26 c | 0.29 ± 0.10 c |
| RJ-CM7 | 0.89 ± 0.14 b | 1.82 ± 0.38 c | 0.31 ± 0.04 c |
| RJ-CM8 | 0.94 ± 0.17 b | 2.13 ± 0.56 c | 0.28 ± 0.09 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uthaibutra, V.; Kaewkod, T.; Prapawilai, P.; Pandith, H.; Tragoolpua, Y. Inhibition of Skin Pathogenic Bacteria, Antioxidant and Anti-Inflammatory Activity of Royal Jelly from Northern Thailand. Molecules 2023, 28, 996. https://doi.org/10.3390/molecules28030996
Uthaibutra V, Kaewkod T, Prapawilai P, Pandith H, Tragoolpua Y. Inhibition of Skin Pathogenic Bacteria, Antioxidant and Anti-Inflammatory Activity of Royal Jelly from Northern Thailand. Molecules. 2023; 28(3):996. https://doi.org/10.3390/molecules28030996
Chicago/Turabian StyleUthaibutra, Vitchayaporn, Thida Kaewkod, Pichet Prapawilai, Hataichanok Pandith, and Yingmanee Tragoolpua. 2023. "Inhibition of Skin Pathogenic Bacteria, Antioxidant and Anti-Inflammatory Activity of Royal Jelly from Northern Thailand" Molecules 28, no. 3: 996. https://doi.org/10.3390/molecules28030996
APA StyleUthaibutra, V., Kaewkod, T., Prapawilai, P., Pandith, H., & Tragoolpua, Y. (2023). Inhibition of Skin Pathogenic Bacteria, Antioxidant and Anti-Inflammatory Activity of Royal Jelly from Northern Thailand. Molecules, 28(3), 996. https://doi.org/10.3390/molecules28030996





