Added-Value Compounds in Cork By-Products: Methods for Extraction, Identification, and Quantification of Compounds with Pharmaceutical and Cosmetic Interest
Abstract
1. Introduction
2. Cork—Chemical Properties
2.1. Suberin
2.2. Lignin
2.3. Polysaccharides
2.4. Extractives
3. Cork Processing
3.1. Cork Harvesting and Industrial Transformation
3.2. Sub-Products and Added-Value Compounds
4. Extraction, Purification and Analytical Methods for Added-Value Compounds in Cork
4.1. Methods for Extraction
4.2. Methods for Purification
4.3. Analytical Methods for Identification and Quantification
5. Biological Activity Evaluation
5.1. Antioxidant Capacity
5.2. Antimicrobial Activity
5.3. Other Assays
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pereira, H. Cork: Biology, Production and Uses; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Gil, L.; Varela, M. Technical Guidelines for Genetic Conservation of Cork Oak (Quercus suber); Bioversity International: Rome, Italy, 2008. [Google Scholar]
- APCOR Sobreiro. Available online: https://www.apcor.pt/montado/sobreiro/ (accessed on 12 April 2022).
- Silva, S.; Sabino, M.; Fernandes, E.; Correlo, V.; Boesel, L.; Reis, R. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef]
- Amorim The Cork Oak and Natural Cork Play an Instrumental Role in the Fight against Climate Changes. Available online: https://www.amorim.com/en/media/news/the-cork-oak-and-natural-cork-play-an-instrumental-role-in-the-fight-against-climate-changes/1371/ (accessed on 16 April 2022).
- Pereira, J.S.; Bugalho, M.N.; Caldeira, M.d.C. From the Cork Oak to Cork—A Sustainable System; Portuguese Cork Association: Santa Maria de Lamas, Portugal, 2008. [Google Scholar]
- WWF. Cork Screwed? Environmental and Economic Impacts of the Cork Stoppers Market; WWF Mediterranean Programme Office: Rome, Italy, 2006. [Google Scholar]
- APCOR. Cork—Environmental Importance; Associação Portuguesa da Cortiça (APCOR): Santa Maria de Lamas, Portugal, 2015. [Google Scholar]
- Pereira, H.; Tomé, M. Cork oak. In Encyclopedia of Forest Sciences; Elsevier: Oxford, UK, 2004; pp. 613–620. [Google Scholar]
- Gil, L. Cortiça: Produção, Tecnologia e Aplicação; Instituto Nacional de Engenharia e Tecnologia Industrial: Lisboa, Portugal, 1998. [Google Scholar]
- Mestre, A.; Gil, L. Cork for sustainable product design. Ciência E Tecnol. Dos Mater. 2011, 23, 52–63. [Google Scholar]
- Aronson, J.; Pereira, J.S.; Pausas, J.G. Cork Oak Woodlands on the Edge: Ecology, Adaptive Management, and Restoration; Island Press: Washington, DC, USA, 2012. [Google Scholar]
- Gil, L. Cork: Sustainability and new applications. Front. Mater. 2015, 1, 38. [Google Scholar] [CrossRef]
- Silva, J.M.; Devezas, T.C.; Silva, A.; Gil, L.; Nunes, C.; Franco, N. Exploring the Use of Cork Based Composites for Aerospace Applications; Materials Science Forum; Trans Tech Publications, Ltd.: Wollerau, Switzerland, 2010; pp. 260–265. [Google Scholar]
- Gil, L. Cork as a Building Material. Technical Manual; APCOR—Portuguese Cork Association: Santa Maria de Lamas, Portugal, 2007. [Google Scholar]
- Duarte, A.P.; Bordado, J.C. Cork—A Renewable Raw Material: Forecast of Industrial Potential and Development Priorities. Front. Mater. 2015, 2, 2. [Google Scholar] [CrossRef]
- Knapic, S.; Oliveira, V.; Machado, J.S.; Pereira, H. Cork as a building material: A review. Eur. J. Wood Wood Prod. 2016, 74, 775–791. [Google Scholar] [CrossRef]
- Karade, S.R.; Irle, M.; Maher, K. Influence of granule properties and concentration on cork-cement compatibility. Holz Als Roh. Und Werkst. 2006, 64, 281–286. [Google Scholar] [CrossRef]
- Brundland, G. World Commission on Environment and Development. Our Common Future; University Press: Oxford, UK, 1987. [Google Scholar]
- Carriço, C.; Ribeiro, H.; Marto, J. Converting cork by-products to ecofriendly cork bioactive ingredients: Novel pharmaceutical and cosmetics applications. Ind. Crops Prod. 2018, 125, 72–84. [Google Scholar] [CrossRef]
- Mota, S.; Pinto, C.; Cravo, S.; Rocha e Silva, J.; Afonso, C.; Sousa Lobo, J.M.; Tiritan, M.E.; Cidade, H.; Almeida, I.F. Quercus suber: A Promising Sustainable Raw Material for Cosmetic Application. Appl. Sci. 2022, 12, 4604. [Google Scholar] [CrossRef]
- Pereira, H. Chemical composition and variability of cork from Quercus suber L. Wood Sci. Technol. 1988, 22, 211–218. [Google Scholar] [CrossRef]
- Conde, E.; Cadahía, E.; García-Vallejo, M.C.; Fernández de Simón, B. Polyphenolic composition of Quercus suber cork from different Spanish provenances. J. Agric. Food Chem. 1998, 46, 3166–3171. [Google Scholar] [CrossRef]
- Pereira, H. Variability of the chemical composition of cork. BioResources 2013, 8, 2246–2256. [Google Scholar] [CrossRef]
- Branco, D.G.; Campos, J.R.; Cabrita, L.; Evtuguin, D.V. Structural features of macromolecular components of cork from Quercus suber L. Holzforschung 2020, 74, 625–633. [Google Scholar] [CrossRef]
- Costa, R.; Lourenço, A.; Oliveira, V.; Pereira, H. Chemical characterization of cork, phloem and wood from different Quercus suber provenances and trees. Heliyon 2019, 5, e02910. [Google Scholar] [CrossRef] [PubMed]
- Jové, P.; Olivella, À.; Cano, L. Study of the variability in chemical composition of bark layers of Quercus suber L. from different production areas. BioResources 2011, 6, 1806–1815. [Google Scholar]
- Bernards, M.A. Demystifying suberin. Can. J. Bot. 2002, 80, 227–240. [Google Scholar] [CrossRef]
- Kolattukudy, P.E. Polyesters in higher plants. In Biopolyesters; Springer: Berlin/Heidelberg, Germany, 2001; pp. 1–49. [Google Scholar]
- Pollard, M.; Beisson, F.; Li, Y.; Ohlrogge, J.B. Building lipid barriers: Biosynthesis of cutin and suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef]
- Marques, A.V.; Pereira, H. On the determination of suberin and other structural components in cork from Quercus suber L. An. Do Inst. Super. De Agron. 1987, 42, 321. [Google Scholar]
- Graça, J.; Pereira, H. Methanolysis of bark suberins: Analysis of glycerol and acid monomers. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2000, 11, 45–51. [Google Scholar] [CrossRef]
- Holloway, P. The composition of suberin from the corks of Quercus suber L. and Betula pendula Roth. Chem. Phys. Lipids 1972, 9, 158–170. [Google Scholar] [CrossRef]
- Kolattukudy, P.; Espelie, K. Chemistry, biochemistry, and function of suberin and associated waxes. In Natural Products of Woody Plants; Springer: Berlin/Heidelberg, Germany, 1989; pp. 304–367. [Google Scholar]
- Silvestre, A.J.; Neto, C.P.; Gandini, A. Cork and suberins: Major sources, properties and applications. In Monomers, Polymers and COMPOSITES from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 305–320. [Google Scholar]
- Graça, J. Hydroxycinnamates in suberin formation. Phytochem. Rev. 2010, 9, 85–91. [Google Scholar] [CrossRef]
- Ankita, N.; Rashmi, K. Suberin-A potential source of renewable chemicals for industrial applications. Res. J. Biotechnol. 2022, 17, 3. [Google Scholar]
- Gandini, A.; Neto, C.P.; Silvestre, A.J. Suberin: A promising renewable resource for novel macromolecular materials. Prog. Polym. Sci. 2006, 31, 878–892. [Google Scholar] [CrossRef]
- Cordeiro, N.; Blayo, A.; Belgacem, N.; Gandini, A.; Neto, C.P.; LeNest, J.-F. Cork suberin as an additive in offset lithographic printing inks. Ind. Crops Prod. 2000, 11, 63–71. [Google Scholar] [CrossRef]
- Sousa, A.F.; Gandini, A.; Silvestre, A.J.; Neto, C.P.; Cruz Pinto, J.J.; Eckerman, C.; Holmbom, B. Novel suberin-based biopolyesters: From synthesis to properties. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2281–2291. [Google Scholar] [CrossRef]
- Coquet, C.; Bauza, E.; Oberto, G.; Berghi, A.; Farnet, A.; Ferré, E.; Peyronel, D.; Dal Farra, C.; Domloge, N. Quercus suber cork extract displays a tensor and smoothing effect on human skin: An in vivo study. Drugs Under Exp. Clin. Res. 2005, 31, 89–99. [Google Scholar]
- Churchward, C.P.; Alany, R.G.; Snyder, L.A. Alternative antimicrobials: The properties of fatty acids and monoglycerides. Crit. Rev. Microbiol. 2018, 44, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Tamm, I.; Heinämäki, J.; Laidmäe, I.; Rammo, L.; Paaver, U.; Ingebrigtsen, S.G.; Škalko-Basnet, N.; Halenius, A.; Yliruusi, J.; Pitkänen, P. Development of suberin fatty acids and chloramphenicol-loaded antimicrobial electrospun nanofibrous mats intended for wound therapy. J. Pharm. Sci. 2016, 105, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.L.; Menager, C.; Guigo, N.; Holban, A.M.; Iordache, F.; Pignatelli, F.; Grumezescu, A.M.; Mazzolai, B.; Sbirrazzuoli, N. Suberin/trans-Cinnamaldehyde Oil Nanoparticles with Antimicrobial Activity and Anticancer Properties When Loaded with Paclitaxel. ACS Appl. Bio Mater. 2019, 2, 3484–3497. [Google Scholar] [CrossRef]
- Križková, L.; Lopes, M.H.; Polónyi, J.; Belicová, A.; Dobias, J.; Ebringer, L. Antimutagenicity of a suberin extract from Quercus suber cork. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 446, 225–230. [Google Scholar] [CrossRef]
- Pinto, P.C.; Sousa, A.F.; Silvestre, A.J.; Neto, C.P.; Gandini, A.; Eckerman, C.; Holmbom, B. Quercus suber and Betula pendula outer barks as renewable sources of oleochemicals: A comparative study. Ind. Crops Prod. 2009, 29, 126–132. [Google Scholar] [CrossRef]
- Marques, A.; Pereira, H.; Meier, D.; Faix, O. Quantitative Analysis of Cork (Quercus suber L.) and Milled Cork Lignin by FTIR Spectroscopy, Analytical Pyrolysis, and Total Hydrolysis. Holzforschung 1994, 48, 43–50. [Google Scholar]
- Marques, A.; Pereira, H.; Meier, D.; Faix, O. Structural Characterization of Cork Lignin by Thioacidolysis and Permanganate Oxidation. Holzforschung 1999, 53, 167–174. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Kolattukudy, P. Biopolyester membranes of plants: Cutin and suberin. Science 1980, 208, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.; Lourenço, A.; Barreiros, S.; Paiva, A.; Simões, P. Valorization of cork using subcritical water. Molecules 2020, 25, 4695. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Lignin as a UV light blocker—A review. Polymers 2020, 12, 1134. [Google Scholar] [CrossRef]
- Tran, M.H.; Phan, D.-P.; Lee, E.Y. Review on lignin modifications toward natural UV protection ingredient for lignin-based sunscreens. Green Chem. 2021, 23, 4633–4646. [Google Scholar] [CrossRef]
- Asensio, A.; Seoane, E. Polysaccharides from the cork of Quercus suber, I. Holocellulose and cellulose. J. Nat. Prod. 1987, 50, 811–814. [Google Scholar] [CrossRef]
- Asensio, A. Polysaccharides from the Cork of Quercus suber, II. Hemicellulose. J. Nat. Prod. 1988, 51, 488–491. [Google Scholar] [CrossRef]
- Aroso, I.M.; Araujo, A.R.; Pires, R.A.; Reis, R.L. Cork: Current technological developments and future perspectives for this natural, renewable, and sustainable material. ACS Sustain. Chem. Eng. 2017, 5, 11130–11146. [Google Scholar] [CrossRef]
- Touati, R.; Santos, S.A.; Rocha, S.M.; Belhamel, K.; Silvestre, A.J. The potential of cork from Quercus suber L. grown in Algeria as a source of bioactive lipophilic and phenolic compounds. Ind. Crops Prod. 2015, 76, 936–945. [Google Scholar] [CrossRef]
- Castola, V.; Marongiu, B.; Bighelli, A.; Floris, C.; Laï, A.; Casanova, J. Extractives of cork (Quercus suber L.): Chemical composition of dichloromethane and supercritical CO2 extracts. Ind. Crops Prod. 2005, 21, 65–69. [Google Scholar] [CrossRef]
- Castola, V.; Bighelli, A.; Rezzi, S.; Melloni, G.; Gladiali, S.; Desjobert, J.-M.; Casanova, J. Composition and chemical variability of the triterpene fraction of dichloromethane extracts of cork (Quercus suber L.). Ind. Crops Prod. 2002, 15, 15–22. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, C.L.; Silva, G.F.; Dias, P.C.; Possenti, A.; de Carvalho, J.E. Evaluation of the antiulcerogenic activity of friedelan-3β-ol and friedelin isolated from Maytenus ilicifolia (Celastraceae). J. Ethnopharmacol. 2000, 72, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Antonisamy, P.; Duraipandiyan, V.; Aravinthan, A.; Al-Dhabi, N.A.; Ignacimuthu, S.; Choi, K.C.; Kim, J.H. Protective effects of friedelin isolated from Azima tetracantha Lam. against ethanol-induced gastric ulcer in rats and possible underlying mechanisms. Eur J Pharm. 2015, 750, 167–175. [Google Scholar] [CrossRef]
- Duraipandiyan, V.; Al-Dhabi, N.A.; Irudayaraj, S.S.; Sunil, C. Hypolipidemic activity of friedelin isolated from Azima tetracantha in hyperlipidemic rats. Rev. Bras. Farmacogn. 2016, 26, 89–93. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-6/omega-3 essential fatty acids: Biological effects. World Rev. Nutr. Diet 2009, 99, 1–16. [Google Scholar]
- Fernandes, A.; Fernandes, I.; Cruz, L.; Mateus, N.; Cabral, M.; de Freitas, V. Antioxidant and biological properties of bioactive phenolic compounds from Quercus suber L. J. Agric. Food Chem. 2009, 57, 11154–11160. [Google Scholar] [CrossRef]
- Santos, S.A.; Pinto, P.C.; Silvestre, A.J.; Neto, C.P. Chemical composition and antioxidant activity of phenolic extracts of cork from Quercus suber L. Ind. Crops Prod. 2010, 31, 521–526. [Google Scholar] [CrossRef]
- Conde, E.; Cadahía, E.; García-Vallejo, M.C.; Fernández de Simón, B.; González Adrados, J.R. Low molecular weight polyphenols in cork of Quercus suber. J. Agric. Food Chem. 1997, 45, 2695–2700. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; He, Y.; Luo, C.; Feng, B.; Ran, F.; Xu, H.; Ci, Z.; Xu, R.; Han, L.; Zhang, D. New progress in the pharmacology of protocatechuic acid: A compound ingested in daily foods and herbs frequently and heavily. Pharmacol. Res. 2020, 161, 105109. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Mahmood, T.; Menaa, F.; Shahzad, Y.; Yousaf, A.M.; Hussain, T.; Ray, S.D. New perspectives on the efficacy of gallic acid in cosmetics & nanocosmeceuticals. Curr. Pharm. Des. 2018, 24, 5181–5187. [Google Scholar] [PubMed]
- Cadahía, E.; Conde, E.; Fernández de Simón, B.; García-Vallejo, M.C. Changes in tannic composition of reproduction cork Quercus suber throughout industrial processing. J. Agric. Food Chem. 1998, 46, 2332–2336. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef]
- Muccilli, V.; Cardullo, N.; Spatafora, C.; Cunsolo, V.; Tringali, C. α-Glucosidase inhibition and antioxidant activity of an oenological commercial tannin. Extraction, fractionation and analysis by HPLC/ESI-MS/MS and 1H NMR. Food Chem. 2017, 215, 50–60. [Google Scholar] [CrossRef]
- Horvathova, M.; Orszaghova, Z.; Laubertova, L.; Vavakova, M.; Sabaka, P.; Rohdewald, P.; Durackova, Z.; Muchova, J. Effect of the French oak wood extract Robuvit on markers of oxidative stress and activity of antioxidant enzymes in healthy volunteers: A pilot study. Oxidative Med. Cell. Longev. 2014, 2014, 639868. [Google Scholar] [CrossRef]
- Sargin, S.A.; Selvi, S.; López, V. Ethnomedicinal plants of sarigöl district (manisa), Turkey. J. Ethnopharmacol. 2015, 171, 64–84. [Google Scholar] [CrossRef]
- Fialho, C.; Lopes, F.; Pereira, H. The effect of cork removal on the radial growth and phenology of young cork oak trees. For. Ecol. Manag. 2001, 141, 251–258. [Google Scholar] [CrossRef]
- AMORIM Curiosities. Available online: https://www.amorim.com/en/cork/curiosities/ (accessed on 22 April 2022).
- APCOR Boletim Estatístico, Anuário da APCOR. Available online: https://www.apcor.pt/portfolio-posts/boletim-estatistico-2020/ (accessed on 25 June 2022).
- Reis, S.F.; Lopes, P.; Roseira, I.; Cabral, M.; Mateus, N.; Freitas, V. Recovery of added value compounds from cork industry by-products. Ind. Crops Prod. 2019, 140, 111599. [Google Scholar] [CrossRef]
- Santos, S.A.; Villaverde, J.J.; Sousa, A.F.; Coelho, J.F.; Neto, C.P.; Silvestre, A.J. Phenolic composition and antioxidant activity of industrial cork by-products. Ind. Crops Prod. 2013, 47, 262–269. [Google Scholar] [CrossRef]
- Ferreira, R.; Pereira, D.; Gago, A.; Proença, J. Experimental characterisation of cork agglomerate core sandwich panels for wall assemblies in buildings. J. Build. Eng. 2016, 5, 194–210. [Google Scholar] [CrossRef]
- Sousa, A.F.; Pinto, P.C.; Silvestre, A.J.; Pascoal Neto, C. Triterpenic and other lipophilic components from industrial cork byproducts. J. Agric. Food Chem. 2006, 54, 6888–6893. [Google Scholar] [CrossRef]
- Gil, L. Cork composites: A review. Materials 2009, 2, 776–789. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, R.A.; Aroso, I.; Fernandes, E.M.; Pinto, M.L.; Reis, R.L.; Andrade, M.A.; Pires, J.; Silva, S.P.; Carvalho, A.P. Activated carbons prepared from industrial pre-treated cork: Sustainable adsorbents for pharmaceutical compounds removal. Chem. Eng. J. 2014, 253, 408–417. [Google Scholar] [CrossRef]
- Gil, L. Cork powder waste: An overview. Biomass Bioenergy 1997, 13, 59–61. [Google Scholar] [CrossRef]
- Montero, I.; Miranda, T.; Sepúlveda, F.J.; Arranz, J.I.; Nogales, S. Analysis of pelletizing of granulometric separation powder from cork industries. Materials 2014, 7, 6686–6700. [Google Scholar] [CrossRef]
- Pintor, A.M.; Ferreira, C.I.; Pereira, J.C.; Correia, P.; Silva, S.P.; Vilar, V.J.; Botelho, C.M.; Boaventura, R.A. Use of cork powder and granules for the adsorption of pollutants: A review. Water Res. 2012, 46, 3152–3166. [Google Scholar] [CrossRef]
- Chubar, N.; Carvalho, J.R.; Correia, M.J.N. Cork biomass as biosorbent for Cu (II), Zn (II) and Ni (II). Colloids Surf. A Physicochem. Eng. Asp. 2003, 230, 57–65. [Google Scholar] [CrossRef]
- Madureira, J.; Pimenta, A.I.; Popescu, L.; Besleaga, A.; Dias, M.I.; Santos, P.M.; Melo, R.; Ferreira, I.C.; Verde, S.C.; Margaça, F.M. Effects of gamma radiation on cork wastewater: Antioxidant activity and toxicity. Chemosphere 2017, 169, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Dias-Machado, M.; Madeira, L.M.; Nogales, B.; Nunes, O.C.; Manaia, C.M. Treatment of cork boiling wastewater using chemical oxidation and biodegradation. Chemosphere 2006, 64, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Robles, L.; Miralles-Cuevas, S.; Oller, I.; Agüera, A.; Trinidad-Lozano, M.; Yuste, F.; Malato, S. Cork boiling wastewater treatment and reuse through combination of advanced oxidation technologies. Environ. Sci. Pollut. Res. 2017, 24, 6317–6328. [Google Scholar] [CrossRef]
- Minhalma, M.; de Pinho, M.N. Tannic-membrane interactions on ultrafiltration of cork processing wastewaters. Sep. Purif. Technol. 2001, 22, 479–488. [Google Scholar] [CrossRef]
- Barbero, G.F. Extraction and Analysis of Natural Product in Plant. Agronomy 2021, 11, 415. [Google Scholar] [CrossRef]
- Chuo, S.C.; Nasir, H.M.; Mohd-Setapar, S.H.; Mohamed, S.F.; Ahmad, A.; Wani, W.A.; Muddassir, M.; Alarifi, A. A Glimpse into the extraction methods of active compounds from plants. Crit. Rev. Anal. Chem. 2020, 52, 667–696. [Google Scholar] [CrossRef]
- Stéphane, F.F.Y.; Jules, B.K.J.; Batiha, G.E.-S.; Ali, I.; Bruno, L.N. Extraction of Bioactive Compounds from Medicinal Plants and Herbs. In Natural Medicinal Plant; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- López-Bascón, M.; De Castro, M.L. Soxhlet extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 327–354. [Google Scholar]
- De Castro, M.L.; Garcıa-Ayuso, L. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Fernandes, A.; Sousa, A.; Mateus, N.; Cabral, M.; de Freitas, V. Analysis of phenolic compounds in cork from Quercus suber L. by HPLC–DAD/ESI–MS. Food Chem. 2011, 125, 1398–1405. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Asl, A.H.; Khajenoori, M. Subcritical water extraction. In Mass Transfer—Advances in Sustainable Energy and Environment Oriented Numerical Modeling; IntechOpen: London, UK, 2013; pp. 459–487. [Google Scholar]
- Bouras, M.; Chadni, M.; Barba, F.J.; Grimi, N.; Bals, O.; Vorobiev, E. Optimization of microwave-assisted extraction of polyphenols from Quercus bark. Ind. Crops Prod. 2015, 77, 590–601. [Google Scholar] [CrossRef]
- Feng, W.; Li, M.; Hao, Z.; Zhang, J. Analytical methods of isolation and identification. In Phytochemicals in Human Health; IntechOpen: London, UK, 2019. [Google Scholar]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef]
- de Freitas, V.A.; Glories, Y.; Bourgeois, G.; Vitry, C. Characterisation of oligomeric and polymeric procyanidins from grape seeds by liquid secondary ion mass spectrometry. Phytochemistry 1998, 49, 1435–1441. [Google Scholar] [CrossRef]
- Scott, R.P.W. Tandem Techniques; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Poole, C. Gas Chromatography; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Snyder, L.R.; Kirkland, J.J.; Dolan, J.W. Introduction to Modern Liquid Chromatography; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Papadoyannis, I.; Gika, H. Peak purity determination with a diode array detector. J. Liq. Chromatogr. Relat. Technol. 2004, 27, 1083–1092. [Google Scholar] [CrossRef]
- Croxatto, A.; Prod’Hom, G.; Greub, G. Applications of MALDI-TOF mass spectrometry in clinical diagnostic microbiology. FEMS Microbiol. Rev. 2012, 36, 380–407. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. MALDI-TOF MS for the Diagnosis of Infectious Diseases. Clin. Chem. 2015, 61, 100–111. [Google Scholar] [CrossRef]
- Castola, V.; Bighelli, A.; Casanova, J. Direct qualitative and quantitative analysis of triterpenes using 13C NMR spectroscopy exemplified by dichloromethanic extracts of cork. Appl. Spectrosc. 1999, 53, 344–350. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-W.; Cai, L.; Xing, Y.; Yu, J.; Ding, Z.-T. Re-evaluation of ABTS•+ assay for total antioxidant capacity of natural products. Nat. Prod. Commun. 2015, 10, 1934578X1501001239. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.-F.; Tsimidou, M.; Zhang, H.-Y. Estimation of scavenging activity of phenolic compounds using the ABTS•+ assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Apak, R.; Capanoglu, E.; Shahidi, F. Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Barreto, M.; Simões, N. Determination of Biological Activities. A Laboratory Manual; Universidade dos Açores: Ponta Delgada, Portugal, 2012. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.Y.; Choi, J.S.; Kang, S.W.; Lee, Y.J.; Park, J.; Kang, Y.H. Dietary compound ellagic acid alleviates skin wrinkle and inflammation induced by UV-B irradiation. Exp. Dermatol. 2010, 19, e182–e190. [Google Scholar] [CrossRef]
- Mansauda Karlah Lifie, R.; Effionora, A.; Tati, N. Antioxidant and Anti-Collagenase Activity of Sargassum Plagyophyllum Extract as an Anti-Wrinkle Cosmetic Ingredient. Pharmacogn. J. 2018, 10, 5. [Google Scholar]
- Pientaweeratch, S.; Panapisal, V.; Tansirikongkol, A. Antioxidant, anti-collagenase and anti-elastase activities of Phyllanthus emblica, Manilkara zapota and silymarin: An in vitro comparative study for anti-aging applications. Pharm. Biol. 2016, 54, 1865–1872. [Google Scholar] [CrossRef]
- Sugimoto, K.; Nakagawa, K.; Hayashi, S.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Yamaji, R.; Nakano, Y.; Inui, H. Hydrolyzable Tannins as Antioxidants in the Leaf Extract of Eucalyptus globulus Possessing Tyrosinase and Hyaluronidase Inhibitory Activities. Food Sci. Technol. Res. 2009, 15, 331–336. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Aldesouki, H.M.; Badria, F.A. Effect of phenolic compounds from the leaves of Psidium guajava on the activity of three metabolism-related enzymes. Biotechnol. Appl. Biochem. 2021, 68, 497–512. [Google Scholar] [CrossRef]
- Duckworth, C.; Stutts, J.; Clatterbuck, K.; Nosoudi, N. Effect of ellagic acid and retinoic acid on collagen and elastin production by human dermal fibroblasts. Bio-Med. Mater. Eng. 2023, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Lee, Y.I.; Almurayshid, A.; Jung, J.Y.; Lee, J.H. Effect of a topical antioxidant serum containing vitamin C, vitamin E, and ferulic acid after Q-switched 1064-nm Nd: YAG laser for treatment of environment-induced skin pigmentation. J. Cosmet. Dermatol. 2020, 19, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
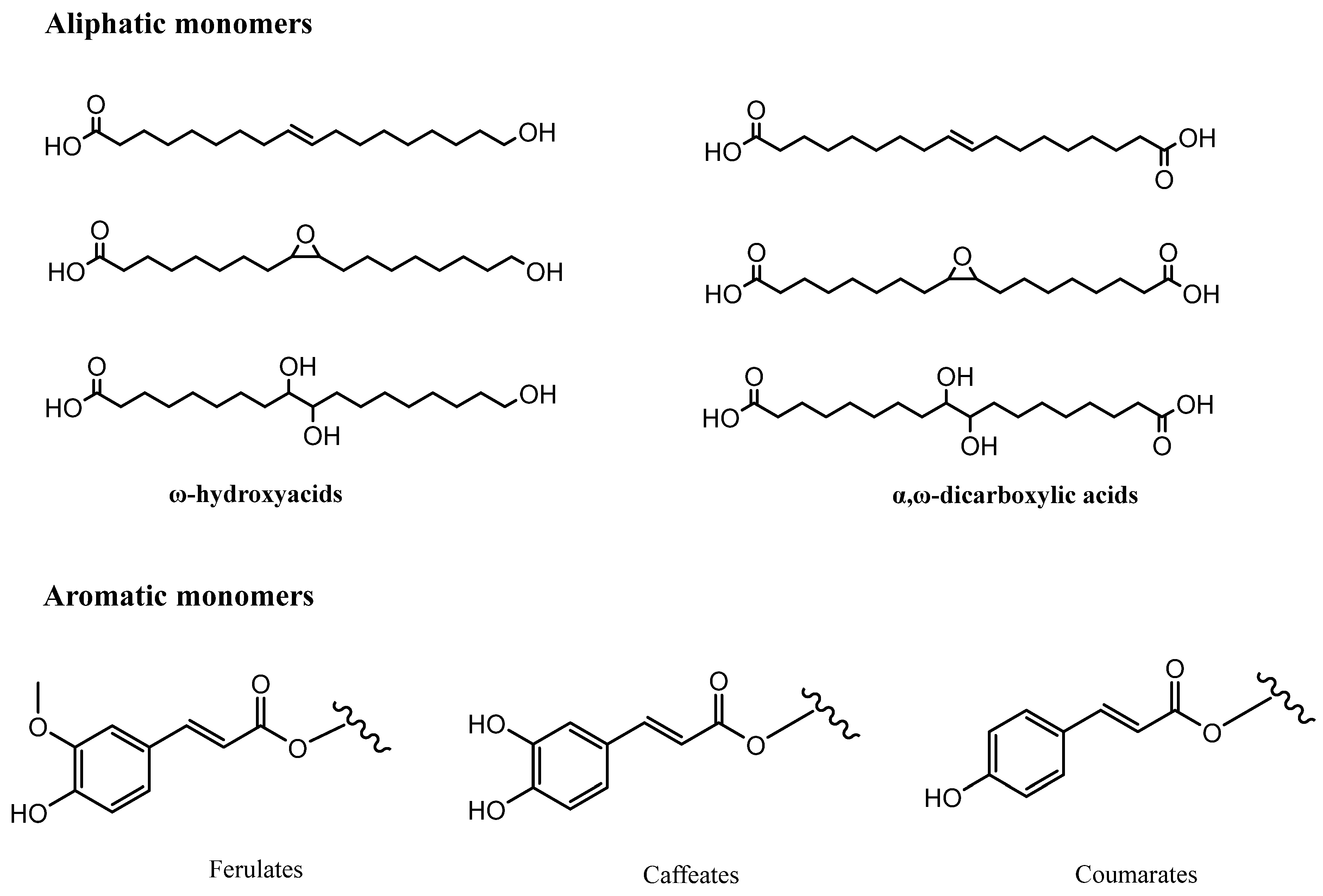
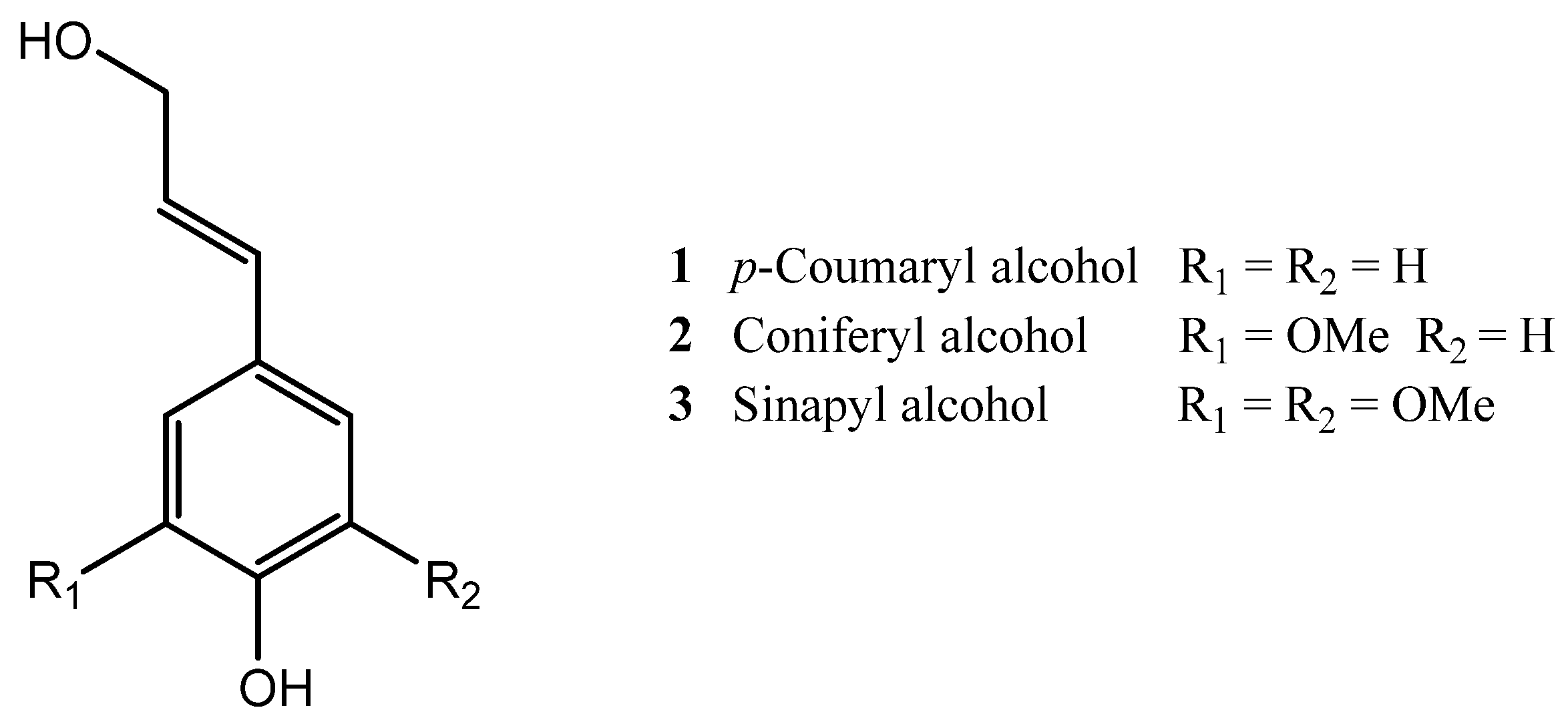

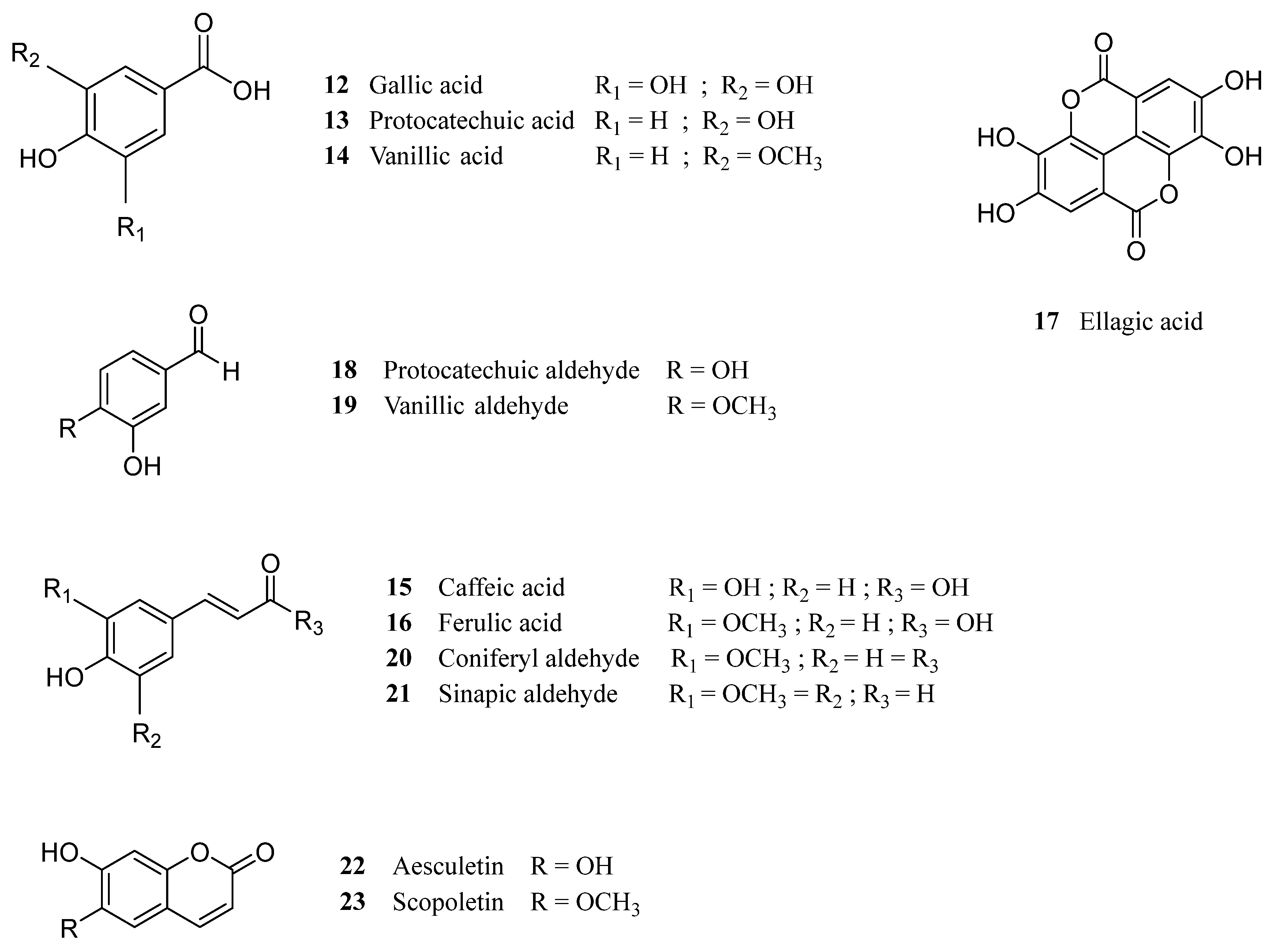
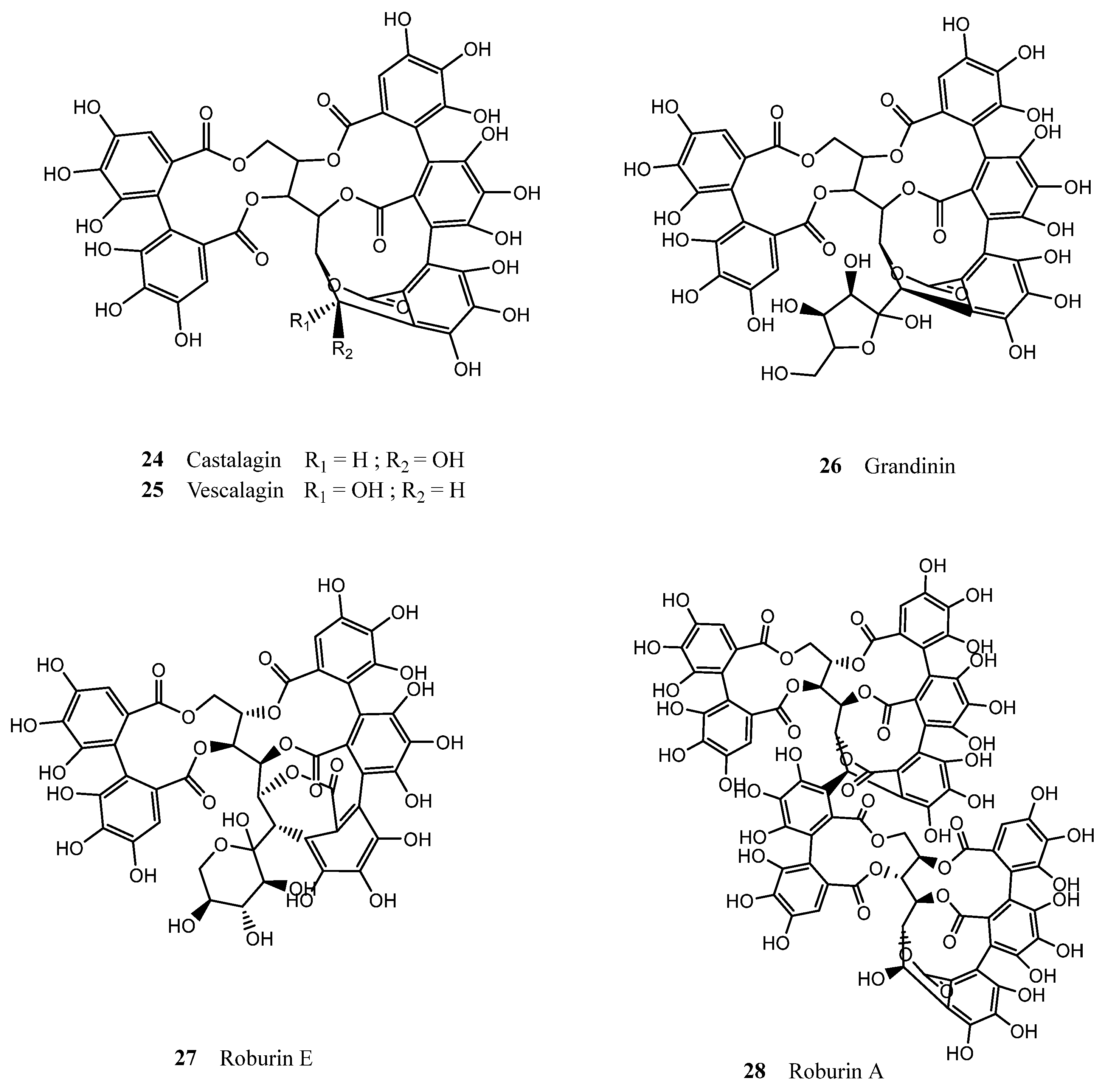
| Cork By-Products | |||
|---|---|---|---|
| Granulates | Agglomerates | Powder | Cooking Wastewaters |
| Ellagic acid (17) | Friedelin (4) | Betulinic acid (7) | Ellagic acid (17) |
| Gallic acid (12) | β-sitosterol (10) | Cerin (5) | Gallic acid (12) |
| Protocatechuic acid (13) | Betulin (6) | Friedelin (4) | Protocatechuic acid (13) |
| Cerin (5) | Betulinic acid (7) | Ellagic acid (17) | Ferulic acid (16) |
| Friedelin (4) | Ferulic acid (16) | Betulin (6) | Vanillic acid (14) |
| Betulinic acid (7) | Coniferyl aldehyde (20) | Castalagin (24) | Syringic acid |
| Betulin (6) | Aesculetin (22) | Tergallic-c-Glucose | |
| By-Product Compound(s) | Yield (mg of Compounds/kg Dry Cork By-Product) | Sample Preparation Extraction and Purification | Identification and Quantification | Biological Assays | Ref. |
|---|---|---|---|---|---|
| Cork granulate Ellagic acid (17) Protocatechuic acid (13) Vanillic acid (14) Gallic acid (12) Vanillin (19) Scopoletin (23) Caffeic acid (15) Coniferaldehyde (20) Ferulic acid (16) Protocatechuic aldehyde (18) Aesculetin (22) Sinapaldehyde (21) | 228.4 48.8 27.4 18.3 16.1 12.7 12.1 11.2 10.7 8.1 7.5 4.5 | Grinding and sieving of cork material; Extraction with MeOH:H2O (80:20, v/v), for 24 h, at room temperature; Filtration of the suspension, removal of MeOH by vacuum distillation; Aqueous solution extracted with Et2O. | HPLC-UV (Identification) GC-MS Folin-Ciocalteu Method | - | [67] |
| Cork powder Betulinic acid (7) Cerin (5) Friedelin (4) Betulin (6) β-Sitosterol (10) Ursolic acid (8) Lupeol Ellagic acid (17) | 11,719 2060 2009 875 254 104 60 1347 | Sequential Soxhlet extraction with DCM, MeOH and H2O for 10 h with each solvent. | GC-MS | - | [82] |
| Black condensate Friedelin (4) β-Sitosterol (10) Betulin (6) Betulinic acid (7) Ferulic acid (16) Vanilylpropanoic acid Benzoic acid | 95,290 20,930 13,130 12,080 10,960 5360 3480 | Soxhlet extraction with DCM for 10 h; Alkaline hydrolysis with 0.5 M NaOH; Extraction with DCM 3x. | GC-MS | [82] | |
| Cork granulate Friedelin (4) Betulinic acid (7) Sitost-4-en-3-one (11) Betulin (6) | 21.0 * 7.3 * 2.9 * 2.7 * | Soxhlet extraction with DCM for 8 h. | 13C NMR (identification and quantification) | [58] | |
| Cork granulate Friedelin (4) Sitost-4-en-3-one (11) β-Sitosterol (10) Betulinic acid (7) Betulin (6) | 20.4 * 15.0 * 2.9 * 2.1 * 1.4 * | Supercritical CO2 extraction at 50 °C and 220 bar for 6 h. | 13C NMR (identification and quantification) | [58] | |
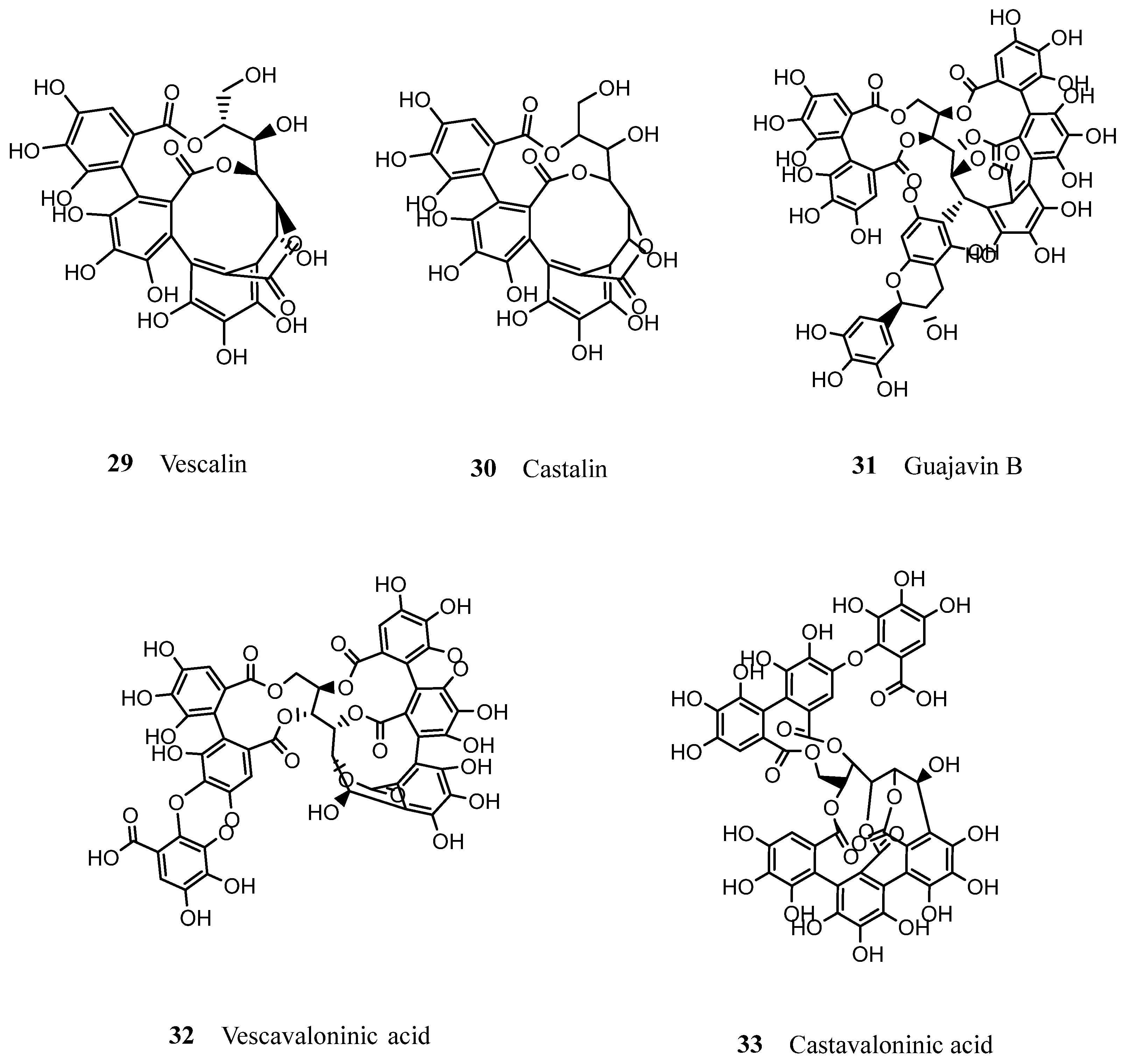
| Cork granulate Castalagin (24) Tergallic-C-Glc Roburin D Vescalagin (25) Vescavaloninic acid (32) Roburin A (28) Roburin E (27) Castavaloninic acid (33) Grandinin (26) | 424 264 149 130 118 103 77 67 58 | Steam treatment of the cork granulates for removal of volatile compounds; Defatting with chloroform in a Soxhlet system for 8 h; Maceration of cork granulates with an aqueous solution of acetone (90%), for 150 h at 25 °C; Precipitation of supernatant with 4 volumes of ethanol (96%); Centrifugation at 10,000 rpm for 10 min at 4 °C; Liquid-liquid extraction with ethyl acetate; Purification of aqueous phase by solid-phase extraction; Elution of tannins fraction with a mixture of methanol/acetone/water (3:1:1, v/v/v). | HPLC-DAD MALDI-TOF | [79] | |
| Cork powder Castalagin (24) Tergallic-C-Glc Vescalagin (25) Roburin D Roburin A (28) Vescavaloninic acid (32) Grandinin (26) Roburin E (27) Castavaloninic acid (33) | 842 440 346 324 258 257 193 190 185 | Defatting with chloroform in a Soxhlet system for 8 h; Maceration of cork powder with an aqueous solution of acetone (60%), for 9 h 30 min at room temperature; Precipitation of supernatant with 4 volumes of ethanol (96%); Centrifugation at 10,000 rpm for 10 min at 4 °C; Liquid-liquid extraction with ethyl acetate; Purification of aqueous phase by solid-phase extraction; Elution of tannins with a mixture of methanol/acetone/water (3:1:1, v/v/v). | HPLC-DAD MALDI-TOF | [79] | |
| Cork granulate Di-HHDP-glc HHDP-di-galloyl-glc Di-HHDP-galloyl-glc HHDP-tri-galloyl-glc Castalagin (24) Mongolicain B | NA | Grinding and sieving of cork to obtain 0.5–1 mm particles; Extraction with 12% ethanol, 5 g/L tartaric acid (pH 3.2) for 72 h at room temperature (wine-model solution); Filtration by gravity, removal of the ethanol by vacuum distillation; Spray drying of the aqueous residue, and extraction of the obtained powder with ethyl acetate 3 times; Isolation and purification of phenolic compounds by preparative HPLC, with two solvents: (1) H2O/CH3COOH (92:2, v/v) and (2) CH3COOH/CH3CN/H2O (2:20:78, v/v/v) | HPLC-DAD LC-DAD/ESI-MS | DPPH scavenging assay (Avg ~25 μM equiv. Trolox); FRAP method; SRB Assay (Avg ~20 μM equiv. Trolox). | [65] |
| Cork granulate Protocatechuic aldehyde (18) Vanillin (19) Protocatechuic acid (13) Gallic acid (12) Coniferaldehyde (20) Caffeic acid (15) Ferulic acid (16) Ellagic acid (17) Ellagic acid-pentose Ellagic-acid deoxyhexose Ellagic acid-hexose Valoneic acid dilactone HHDP-glc Valoneic acid Tergallic-C-glucoside HHDP-galloyl-glc Trigalloyl-glc Di-HHDP-glc HHDP-digalloyl-glc Tetragalloyl-glc Castalagin/Vescalagin (24/25) Trigalloyl-HHDP-glc Mongolicain A/B | NA | Grinding and sieving of cork to obtain 0.5–1 mm particles; Extraction with 12% ethanol, 5 g/L tartaric acid (pH 3.2) for 72 h at room temperature (wine-model solution); Filtration on a Büchner funnel, removal of the ethanol by vacuum distillation; Spray drying of the aqueous residue, and extraction of the obtained powder with ethyl acetate 3 times; Fractionation of phenolic compounds by column chromatography, using MeOH as eluent. | HPLC-DAD LC-DAD/ESI-MS | [98] | |
| Cork granulate Ellagic acid (17) Gallic acid (12) Protocatechuic acid (13) Caffeic acid (15) Aesculetin (22) Vanillin (19) Vanillic acid (14) Ferulic acid (16) Coumaric acid Salicylic acid Eriodictyol Naringenin | 2031.5 30.6 17.5 57.6 4.9 14.3 Trace Trace Trace 32.7 27.4 2.6 | Milling of the cork planks to obtain cork granulate; Soxhlet extraction with DCM for 6 h; Suspension in MeOH:H2O (80:20, v/v) at room temperature, for 24 h; Filtration and removal of the solvent by low-pressure evaporation; Extraction with diethyl ether 3 times. | HPLC-MS Folin-Ciocalteu method | DPPH scavenging assay (IC50 = 2.79 ± 0.15 μg of extract/mL) | [66] |
| Cork granulate Ellagic acid (17) Gallic acid (12) Protocatechuic acid (13) Caffeic acid (15) p-Hydroxybenzoic acid | 526.5 241.6 118.3 12.9 1.0 | Milling of the cork planks to obtain cork granulate; Soxhlet extraction with DCM for 6 h; Extraction with MeOH for 6 h; Removal of solvent by low-pressure evaporation; Reflux with water for 6 h. | |||
| Cork granulate Gallic acid (12) Ferulic acid (16) Caffeic acid (15) | 61.2 7.5 6.5 | Semi-continuous subcritical water extraction, with a pressure of 100 bar and temperature range of 50–120 °C. | HPLC-DAD Folin-Ciocalteu method | DPPH scavenging assay (EC50 = 0.253 ± 0.001 mg extract/mg DPPH) | [51] |
| Cork granulate Ellagic acid (17) Ellagic acid-pentoside Gallic acid (12) Aesculetin (22) Quinic acid Methyl gallate Brevifolin-carboxylic acid Protocatechuic acid (13) Ferulic acid (16) Coniferyl aldehyde (20) p-Hydroxyphenyllactic acid Valoneic acid dilactone Caffeic acid isoprenyl ester Isorhamnetin-rhamnoside Eriodictyol Isorhamnetin | 1246.46 770.16 736.48 391.59 372.86 251.43 102.03 79.26 Trace Trace Trace 168.01 127.98 Trace Trace Trace | Soxhlet extraction with DCM for 6 h; Extraction with MeOH:H2O (50:50, v/v), for 24 h, at room temperature. | HPLC-UV (separation) ESI-MS (analysis) Folin-Ciocalteu | DPPH scavenging assay (IC50 = 4.77 ± 0.02 μg of extract/mL) | [80] |
| Black condensate Coniferyl aldehyde (20) Aesculetin (22) Gallic acid (12) Quinic acid Ellagic acid (17) p-Hydroxyphenyllactic acid p-Coumaric acid Vanillin (19) Caffeic acid (15) Protocatechuic acid (13) Ferulic acid (16) Eriodictyol | 194.34 125.28 118.46 117.17 52.52 49.36 35.76 32.47 17.68 9.97 Trace Trace | Soxhlet extraction with DCM for 6 h; Extraction with MeOH:H2O (50:50, v/v), for 24 h, at room temperature. | HPLC-UV (separation) ESI-MS (analysis) Folin-Ciocalteu | DPPH scavenging assay (IC50 = 1.57 ± 0.01 μg of extract/mL) | [80] |
| Cork powder Ellagic acid (17) Gallic acid (12) Aesculetin (22) Quinic acid Methyl gallate Ellagic acid-pentoside Valoneic acid dilactone Protocatechuic acid (13) Ferulic acid (16) Coniferyl aldehyde (20) Caffeic acid isoprenyl ester Brevifolin-carboxylic acid Isorhamnetin-rhamnoside Isorhamnetin | 527.59 263.04 176.80 137.02 96.93 46.18 46.05 16.44 14.77 Trace 82.47 53.72 Trace Trace | Soxhlet extraction with DCM for 6 h; Extraction with MeOH:H2O (50:50, v/v), for 24 h, at room temperature. | HPLC-UV (separation) ESI-MS (analysis) Folin-Ciocalteu | DPPH scavenging assay (IC50 = 3.33 ± 0.02 μg of extract/mL) | [80] |
| Cork granulate Cerine (5) Friedelin (4) Betulinic acid (7) Betulin (6) Sitost-4-en-3-one (11) β-Sitosterol (10) Ursolic acid (8) Docosanoic acid Hexadecanoic acid Octadecenoic acid Tetracosanoic acid Cis-9-octadecenoic acid 9,12-Octadecadienoic acid Nonanedioic acid Docosan-1-ol Tetracosan-1-ol Eicosan-1-ol | 7278.7 4702.3 2798.2 1651.2 1175.2 514.6 435.7 338.9 294.6 181.3 160.2 149.1 129.2 125.5 1409.4 412.0 295.9 | Soxhlet extraction with DCM for 6 h; Alkaline hydrolysis with 0.5 M NaOH; Extraction with DCM 3 times. | GC-MS | - | [57] |
| Cork granulate Protocatechuic acid (13) Ellagic acid (17) Gallic acid (12) HHDP-glc Castalagin/vescalagin (24/25) Methyl gallate Brevifolin-carboxylic acid Syringaldehyde Valoneic acid dilactone Caffeic acid (15) Ellagic acid-pentoside Chlorogenic acid Vanillic acid (14) Caffeic acid isoprenyl ester Ellagic acid-rhamnoside Vanillin (19) Isorhamnetin-rhamnoside | 1414.41 1060.47 931.01 678.88 490.89 330.02 231.19 213.92 124.34 112.09 78.82 69.45 67.74 64.89 60.06 42.30 26.18 | The residues obtained after DCM extraction (described above) were suspended in MeOH:H2O (50:50, v/v) for 24 h, at room temperature. | UHPLC-MS | DPPH assay (IC50 = 5.60 ± 0.020 μg of extract/mL); ABTS assay (IC50 = 27.55 ± 0.70 μg of extract/mL); Reducing power (dose-dependent); Mueller-Hinton agar diffusion test | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carriço, C.M.; Tiritan, M.E.; Cidade, H.; Afonso, C.; Silva, J.R.e.; Almeida, I.F. Added-Value Compounds in Cork By-Products: Methods for Extraction, Identification, and Quantification of Compounds with Pharmaceutical and Cosmetic Interest. Molecules 2023, 28, 3465. https://doi.org/10.3390/molecules28083465
Carriço CM, Tiritan ME, Cidade H, Afonso C, Silva JRe, Almeida IF. Added-Value Compounds in Cork By-Products: Methods for Extraction, Identification, and Quantification of Compounds with Pharmaceutical and Cosmetic Interest. Molecules. 2023; 28(8):3465. https://doi.org/10.3390/molecules28083465
Chicago/Turabian StyleCarriço, Carolina Morais, Maria Elizabeth Tiritan, Honorina Cidade, Carlos Afonso, Joana Rocha e Silva, and Isabel F. Almeida. 2023. "Added-Value Compounds in Cork By-Products: Methods for Extraction, Identification, and Quantification of Compounds with Pharmaceutical and Cosmetic Interest" Molecules 28, no. 8: 3465. https://doi.org/10.3390/molecules28083465
APA StyleCarriço, C. M., Tiritan, M. E., Cidade, H., Afonso, C., Silva, J. R. e., & Almeida, I. F. (2023). Added-Value Compounds in Cork By-Products: Methods for Extraction, Identification, and Quantification of Compounds with Pharmaceutical and Cosmetic Interest. Molecules, 28(8), 3465. https://doi.org/10.3390/molecules28083465











