The Effect of Nonthermal Pretreatment on the Drying Kinetics and Quality of Black Garlic
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Properties of Black Garlic
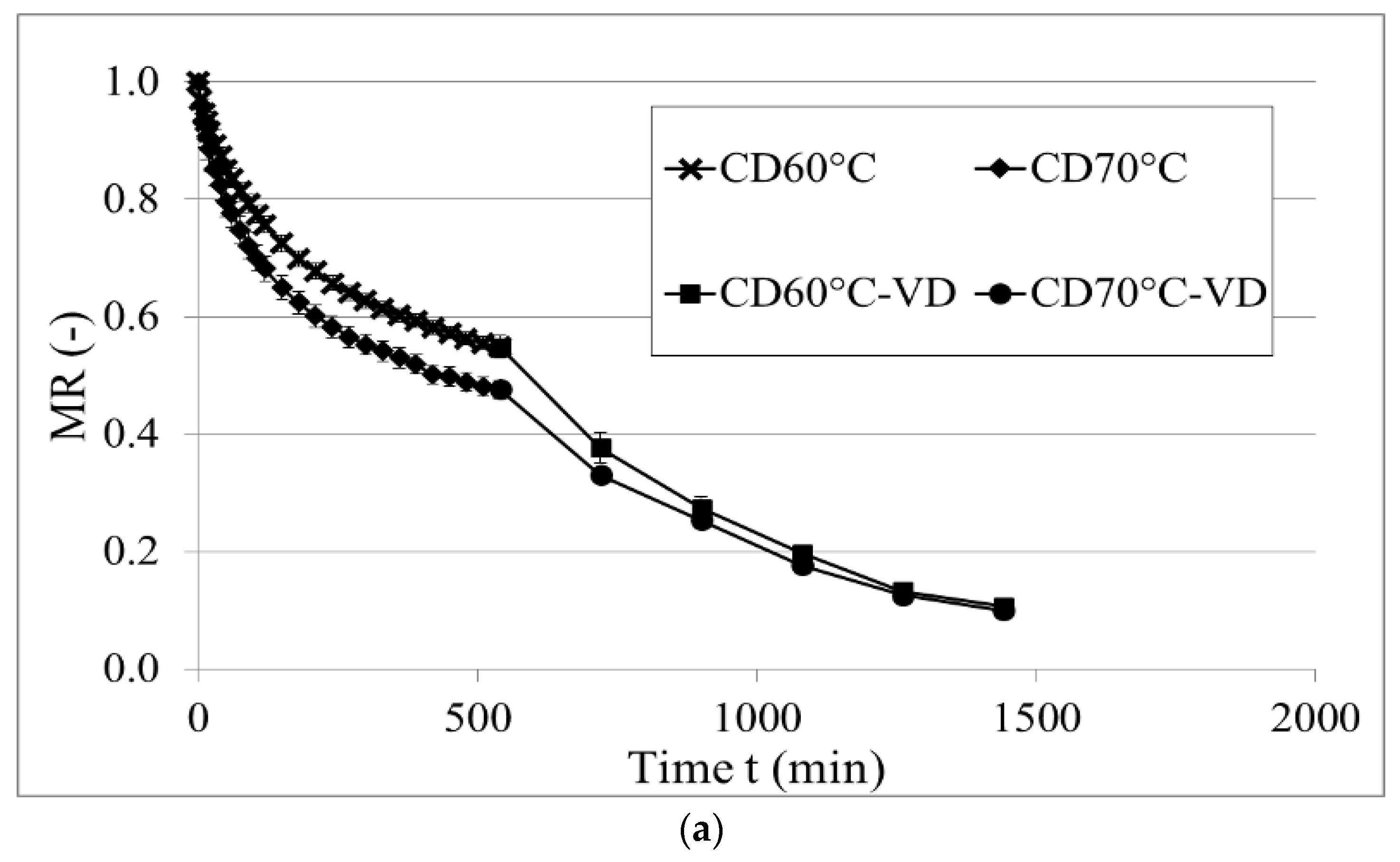
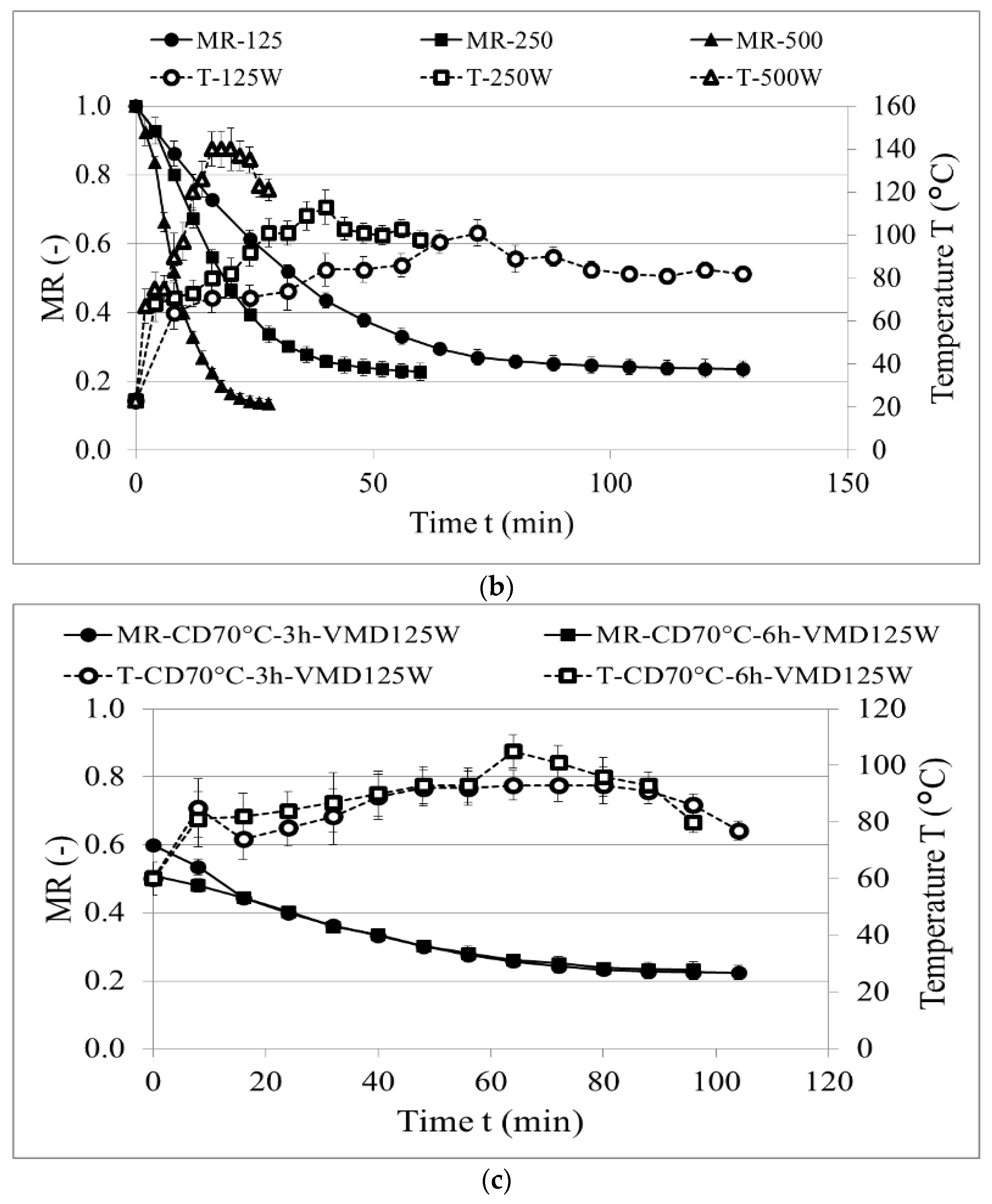
2.2. Drying Kinetics
2.3. Chemical Analysis
Bioactive Compounds
2.4. In Vitro Pro-Health Potency and Antioxidant Capacity
Antioxidant Capacity
2.5. α-Amylase, and α-Glucosidase Inhibitory Effect
3. Materials and Methods
3.1. Material
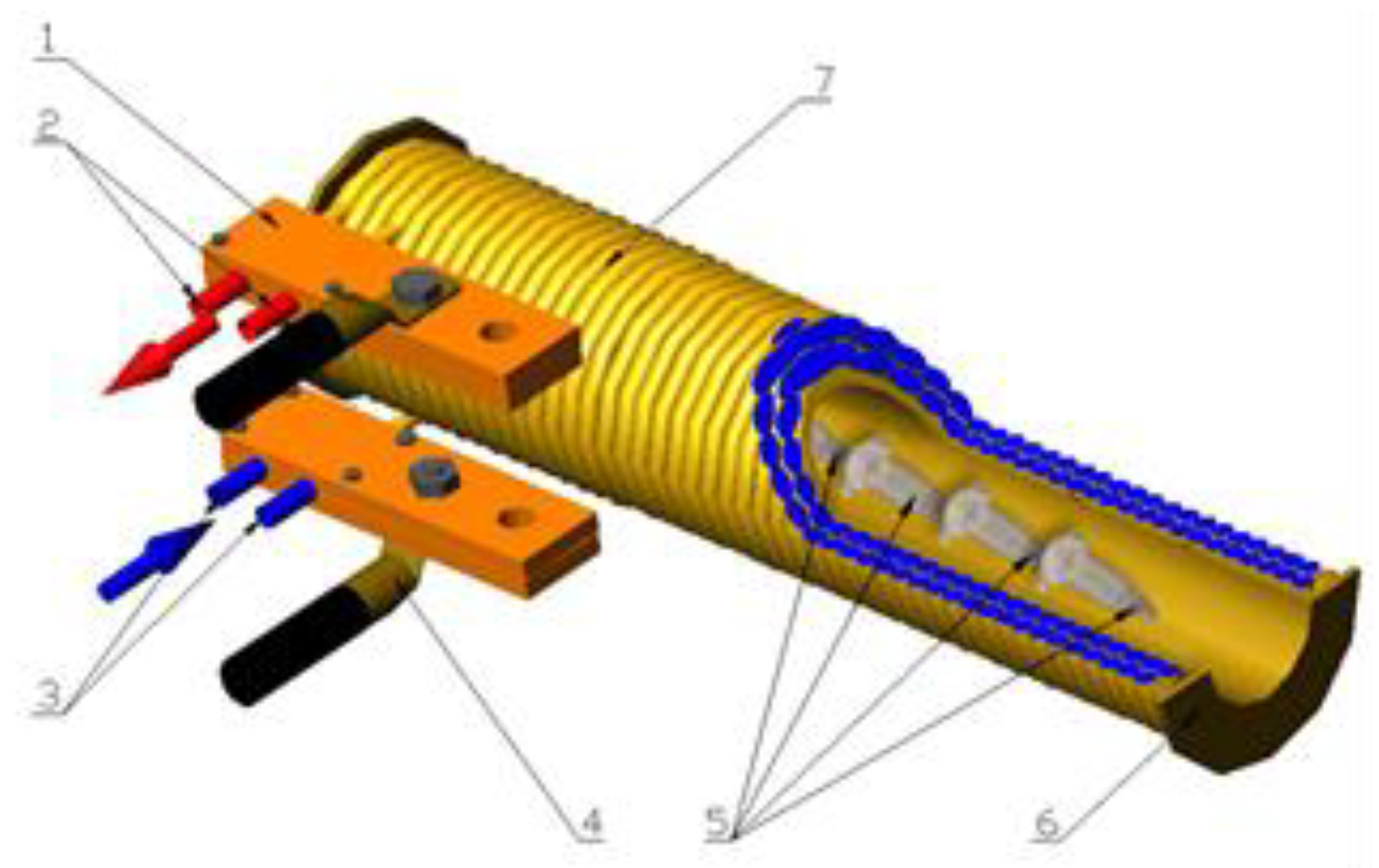
3.2. Pretreatment Methods
3.3. Drying Methods
3.4. Physical Properties
3.4.1. The Moisture Content
3.4.2. Water Activity
3.4.3. Color
3.5. Chemical Analysis
3.5.1. Determination of Phenolic Compounds, including Polymeric Proanthocyanidins by UPLC
3.5.2. Analysis of Health-Promoting Properties by In Vitro Methods
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of Temperature on the Quality of Black Garlic. J. Sci. Food Agric. 2016, 96, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Tung, Y.-C.; Pan, M.-H.; Su, N.-W.; Lai, Y.-J.; Cheng, K.-C. Black Garlic: A Critical Review of Its Production, Bioactivity, and Application. J. Food Drug Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. The Comparison of the Contents of Sugar, Amadori, and Heyns Compounds in Fresh and Black Garlic. J. Food Sci. 2016, 81, C1662–C1668. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Zheng, Z.; Zhang, B.; Sun-Waterhouse, D.; Qiao, X. Formation, Nutritional Value, and Enhancement of Characteristic Components in Black Garlic: A Review for Maximizing the Goodness to Humans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 801–834. [Google Scholar] [CrossRef]
- Kim, D.; Kang, M.J.; Hong, S.S.; Choi, Y.-H.; Shin, J.H. Antiinflammatory Effects of Functionally Active Compounds Isolated from Aged Black Garlic. Phytother. Res. 2017, 31, 53–61. [Google Scholar] [CrossRef]
- Mirzavandi, F.; Mollahosseini, M.; Salehi-Abargouei, A.; Makiabadi, E.; Mozaffari-Khosravi, H. Effects of Garlic Supplementation on Serum Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1153–1161. [Google Scholar] [CrossRef]
- Thomson, M.; Al-Qattan, K.K.; JS, D.; Ali, M. Anti-Diabetic and Anti-Oxidant Potential of Aged Garlic Extract (AGE) in Streptozotocin-Induced Diabetic Rats. BMC Complement. Altern. Med. 2016, 16, 17. [Google Scholar] [CrossRef]
- Jung, Y.-M.; Lee, S.-H.; Lee, D.-S.; You, M.-J.; Chung, I.K.; Cheon, W.H.; Kwon, Y.-S.; Lee, Y.-J.; Ku, S.-K. Fermented Garlic Protects Diabetic, Obese Mice When Fed a High-Fat Diet by Antioxidant Effects. Nutr. Res. 2011, 31, 387–396. [Google Scholar] [CrossRef]
- Amor, S.; González-Hedström, D.; Martín-Carro, B.; Inarejos-García, A.M.; Almodóvar, P.; Prodanov, M.; García-Villalón, A.L.; Granado, M. Beneficial Effects of an Aged Black Garlic Extract in the Metabolic and Vascular Alterations Induced by a High Fat/Sucrose Diet in Male Rats. Nutrients 2019, 11, 153. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, F.; Wang, Q.-W.; Wang, J.; Yang, K.; Hu, R.-R.; Liu, H.-C.; Wang, H.-Y.; Wang, Y.-S. Aged Black Garlic Extract Induces Inhibition of Gastric Cancer Cell Growth in Vitro and in Vivo. Mol. Med. Rep. 2012, 5, 66–72. [Google Scholar] [CrossRef]
- Agbana, Y.L.; Ni, Y.; Zhou, M.; Zhang, Q.; Kassegne, K.; Karou, S.D.; Kuang, Y.; Zhu, Y. Garlic-Derived Bioactive Compound S-Allylcysteine Inhibits Cancer Progression through Diverse Molecular Mechanisms. Nutr. Res. 2020, 73, 1–14. [Google Scholar] [CrossRef]
- Asdaq, S.M.; Inamdar, M.N. Potential of Garlic and Its Active Constituent, S-Allyl Cysteine, as Antihypertensive and Cardioprotective in Presence of Captopril. Phytomedicine 2010, 17, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Quesada, I.; de Paola, M.; Torres-Palazzolo, C.; Camargo, A.; Ferder, L.; Manucha, W.; Castro, C. Effect of Garlic’s Active Constituents in Inflammation, Obesity and Cardiovascular Disease. Curr. Hypertens. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, S.H.; Rico, C.W.; Kang, M.Y. A Comparative Study on the Antioxidative and Anti-Allergic Activities of Fresh and Aged Black Garlic Extracts. Int. J. Food Sci. Technol. 2012, 47, 1176–1182. [Google Scholar] [CrossRef]
- Ahmed, T.; Wang, C.-K. Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules 2021, 26, 5028. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Figiel, A.; Wojdyło, A.; Szarycz, M.; Carbonell-Barrachina, Á.A. Drying of Garlic Slices Using Convective Pre-Drying and Vacuum-Microwave Finishing Drying: Kinetics, Energy Consumption, and Quality Studies. Food Bioprocess Technol. 2014, 7, 398–408. [Google Scholar] [CrossRef]
- Lyczko, J.; Masztalerz, K.; Lipan, L.; Lech, K.; Carbonell-Barrachina, A.; Szumny, A. Chemical Determinants of Dried Thai Basil (O. Basilicum Var. Thyrsiflora) Aroma Quality. Ind. Crop. Prod. 2020, 155, 112769. [Google Scholar] [CrossRef]
- Kwaśnica, A.; Pachura, N.; Masztalerz, K.; Figiel, A.; Zimmer, A.; Kupczyński, R.; Wujcikowska, K.; Carbonell-Barrachina, A.A.; Szumny, A.; Różański, H. Volatile Composition and Sensory Properties as Quality Attributes of Fresh and Dried Hemp Flowers (Cannabis Sativa L.). Foods 2020, 9, 1118. [Google Scholar] [CrossRef]
- Bialik, M.; Wiktor, A.; Rybak, K.; Witrowa-Rajchert, D.; Latocha, P.; Gondek, E. The Impact of Vacuum and Convective Drying Parameters on Kinetics, Total Phenolic Content, Carotenoid Content and Antioxidant Capacity of Kiwiberry (Actinidia Arguta). Appl. Sci. 2020, 10, 6914. [Google Scholar] [CrossRef]
- Łyczko, J.; Jałoszyński, K.; Surma, M.; Masztalerz, K.; Szumny, A. HS-SPME Analysis of True Lavender (Lavandula Angustifolia Mill.) Leaves Treated by Various Drying Methods. Molecules 2019, 24, 764. [Google Scholar] [CrossRef]
- Chua, L.Y.W.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdyło, A.; Szumny, A.; Lech, K. Characterisation of the Convective Hot-Air Drying and Vacuum Microwave Drying of Cassia Alata: Antioxidant Activity, Essential Oil Volatile Composition and Quality Studies. Molecules 2019, 24, 1625. [Google Scholar] [CrossRef] [PubMed]
- Masztalerz, K.; Łyczko, J.; Lech, K. Effect of Filtrated Osmotic Solution Based on Concentrated Chokeberry Juice and Mint Extract on the Drying Kinetics, Energy Consumption and Physicochemical Properties of Dried Apples. Molecules 2021, 26, 3274. [Google Scholar] [CrossRef] [PubMed]
- Tylewicz, U.; Castagnini, J.M.; Tappi, S.; Romani, S.; Rocculi, P.; Rosa, M.D. Current Validation of NTP Technologies and Overview of Their Current and Potential Implementation in the Production Chain Including Agri-Food Wastes. In Nonthermal Processing in Agri-Food-Bio Sciences: Sustainability and Future Goals; Režek Jambrak, A., Ed.; Food Engineering Series; Springer International Publishing: Cham, Switzerland, 2022; pp. 567–594. ISBN 978-3-030-92415-7. [Google Scholar]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The Application of Unconventional Technologies as Pulsed Electric Field, Ultrasound and Microwave-Vacuum Drying in the Production of Dried Cranberry Snacks. Ultrason. Sonochem. 2019, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.R.; Lyng, J.G.; Frontuto, D.; Marra, F.; Cinquanta, L. Effect of Pulsed Electric Field Pretreatment on Drying Kinetics, Color, and Texture of Parsnip and Carrot. J. Food Sci. 2018, 83, 2159–2166. [Google Scholar] [CrossRef]
- Ostermeier, R.; Giersemehl, P.; Siemer, C.; Töpfl, S.; Jäger, H. Influence of Pulsed Electric Field (PEF) Pre-Treatment on the Convective Drying Kinetics of Onions. J. Food Eng. 2018, 237, 110–117. [Google Scholar] [CrossRef]
- Liu, C.; Pirozzi, A.; Ferrari, G.; Vorobiev, E.; Grimi, N. Impact of Pulsed Electric Fields on Vacuum Drying Kinetics and Physicochemical Properties of Carrot. Food Res. Int. 2020, 137, 109658. [Google Scholar] [CrossRef]
- Rybak, K.; Parniakov, O.; Samborska, K.; Wiktor, A.; Witrowa-Rajchert, D.; Nowacka, M. Energy and Quality Aspects of Freeze-Drying Preceded by Traditional and Novel Pre-Treatment Methods as Exemplified by Red Bell Pepper. Sustainability 2021, 13, 2035. [Google Scholar] [CrossRef]
- Dziadek, K.; Kopeć, A.; Dróżdż, T.; Kiełbasa, P.; Ostafin, M.; Bulski, K.; Oziembłowski, M. Effect of Pulsed Electric Field Treatment on Shelf Life and Nutritional Value of Apple Juice. J. Food Sci. Technol. 2019, 56, 1184–1191. [Google Scholar] [CrossRef]
- Yu, Y.; Yuanshan, Y.; Jin, T.Z.; Fan, X.; Jijun, W.; Jijun, W. Biochemical Degradation and Physical Migration of Polyphenolic Compounds in Osmotic Dehydrated Blueberries with Pulsed Electric Field and Thermal Pretreatments. Food Chem. 2018, 239, 1219–1225. [Google Scholar] [CrossRef]
- Miernik, A.; Kiełbasa, P.; Findura, P.; Byrska, K. Influence of the Constant Electric Field on the Photon Emission Characteristics of Selected Utility Cultivars of the Camellia Plant. J. Phys. Conf. Ser. 2021, 1782, 12021. [Google Scholar] [CrossRef]
- Miernik, A.; Juliszewski, T.; Popardowski, E.; Trzyniec, K.; Kovalyshyn, S.; Wiśniowski, B. Influence of Constant Electric Field Impact on the Intensity and Structure of the Secondary Luminescence of Selected Industrial Cannabis Products. J. Phys. Conf. Ser. 2021, 1782, 12022. [Google Scholar] [CrossRef]
- Vashisth, A.; Nagarajan, S. Effect on Germination and Early Growth Characteristics in Sunflower (Helianthus Annuus) Seeds Exposed to Static Magnetic Field. J. Plant Physiol. 2010, 167, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kumar, M. An Innovation in Magnetic Field Assisted Freezing of Perishable Fruits and Vegetables: A Review. Food Rev. Int. 2020, 36, 761–780. [Google Scholar] [CrossRef]
- Mujumdar, A.S.; Woo, M.W. Effects of Electric and Magnetic Field on Freezing. In Drying Technologies for Biotechnology and Pharmaceutical Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 283–301. ISBN 978-3-527-80210-4. [Google Scholar]
- Memmedov, A.; Kelbaliyev, G.I.; Alisoy, G.T. Solution of an Inverse Problem for Mass Transfer in a Drying Process in a Magnetic Field. Inverse Probl. Sci. Eng. 2010, 18, 723–736. [Google Scholar] [CrossRef]
- Ostermeier, R.; Parniakov, O.; Töpfl, S.; Jäger, H. Applicability of Pulsed Electric Field (PEF) Pre-Treatment for a Convective Two-Step Drying Process. Foods 2020, 9, 512. [Google Scholar] [CrossRef]
- Rahaman, A.; Zeng, X.-A.; Kumari, A.; Farooq, M.A.; Muhammad Adil Farooq; Muhammad Adil Farooq; Siddeeg, A.; Manzoor, M.F. Combined Effect of Pulsed Electric Fields and Ultrasound on Mass Energy Transfer and Diffusion Coefficient of Plum. Heat Mass Transf. 2021, 57, 1087–1095. [Google Scholar] [CrossRef]
- Figiel, A. Drying Kinetics and Quality of Vacuum-Microwave Dehydrated Garlic Cloves and Slices. J. Food Eng. 2009, 94, 98–104. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.; Balasa, A.; Knorr, D.; Martín-Belloso, O. Effects of Pulsed Electric Fields on Bioactive Compounds in Foods: A Review. Trends Food Sci. Technol. 2009, 20, 544–556. [Google Scholar] [CrossRef]
- Tylewicz, U.; Tappi, S.; Mannozzi, C.; Romani, S.; Dellarosa, N.; Laghi, L.; Ragni, L.; Rocculi, P.; Rosa, M.D. Effect of Pulsed Electric Field (PEF) Pre-Treatment Coupled with Osmotic Dehydration on Physico-Chemical Characteristics of Organic Strawberries. J. Food Eng. 2017, 213, 2–9. [Google Scholar] [CrossRef]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of Water Activity (a w) on Microbial Stability as a Hurdle in Food Preservation. In Water Activity in Foods; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 323–355. ISBN 978-1-118-76598-2. [Google Scholar]
- Rasooli Sharabiani, V.; Kaveh, M.; Abdi, R.; Szymanek, M.; Tanaś, W. Estimation of Moisture Ratio for Apple Drying by Convective and Microwave Methods Using Artificial Neural Network Modeling. Sci. Rep. 2021, 11, 9155. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Lipan, L.; Cano-Lamadrid, M.; Kharaghani, A.; Masztalerz, K.; Carbonell-Barrachina Á, A.; Figiel, A. Comparison of Traditional and Novel Drying Techniques and Its Effect on Quality of Fruits, Vegetables and Aromatic Herbs TI2. Foods 2020, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Figiel, A.; Lech, K.; Nowicka, P.; Oszmiański, J. Effect of Convective and Vacuum–Microwave Drying on the Bioactive Compounds, Color, and Antioxidant Capacity of Sour Cherries. Food Bioprocess Technol. 2014, 7, 829–841. [Google Scholar] [CrossRef]
- Taskin, O.; Polat, A.; Izli, N.; Asik, B.B. Intermittent Microwave-Vacuum Drying Effects on Pears. Pol. J. Food Nutr. Sci. 2019, 69, 101–108. [Google Scholar] [CrossRef]
- Ríos-Ríos, K.L.; Montilla, A.; Olano, A.; Villamiel, M. Physicochemical Changes and Sensorial Properties during Black Garlic Elaboration: A Review. Trends Food Sci. Technol. 2019, 88, 459–467. [Google Scholar] [CrossRef]
- Castillo-Gironés, S.; Masztalerz, K.; Lech, K.; Issa-Issa, H.; Figiel, A.; Carbonell-Barrachina, A.A. Impact of Osmotic Dehydration and Different Drying Methods on the Texture and Sensory Characteristic of Sweet Corn Kernels. J. Food Process. Preserv. 2021, 45, e15383. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, M.; Mujumdar, A.S.; Xiao, G.; Jin-cai, S. Drying of Edamames by Hot Air and Vacuum Microwave Combination. J. Food Eng. 2006, 77, 977–982. [Google Scholar] [CrossRef]
- Babalis, S.J.; Papanicolaou, E.; Kyriakis, N.; Belessiotis, V.G. Evaluation of Thin-Layer Drying Models for Describing Drying Kinetics of Figs (Ficus Carica). J. Food Eng. 2006, 75, 205–214. [Google Scholar] [CrossRef]
- Nguyen, T.V.L.; Nguyen, M.D.; Nguyen, D.C.; Bach, L.G.; Lam, T.D. Model for Thin Layer Drying of Lemongrass (Cymbopogon Citratus) by Hot Air. Processes 2019, 7, 21. [Google Scholar] [CrossRef]
- Tzempelikos, D.A.; Vouros, A.P.; Bardakas, A.V.; Filios, A.E.; Margaris, D.P. Experimental Study on Convective Drying of Quince Slices and Evaluation of Thin-Layer Drying Models. Eng. Agric. Environ. Food 2015, 8, 169–177. [Google Scholar] [CrossRef]
- Karaaslan, S.; Ekinci, K.; Akbolat, D. Drying Characteristics for Sultana Grape Fruit in Microwave Dryer. Infrastrukt. I Ekol. Teren. Wiej./Infrastruct. Ecol. Rural. Areas 2017, 4.1, 1317–1327. [Google Scholar] [CrossRef]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Polyphenolics from Peach (Prunus Persica Var. Rich Lady) Inhibit Tumor Growth and Metastasis of MDA-MB-435 Breast Cancer Cells in Vivo. J. Nutr. Biochem. 2014, 25, 796–800. [Google Scholar] [CrossRef]
- Noratto, G.; Martino, H.S.D.; Simbo, S.; Byrne, D.; Mertens-Talcott, S.U. Consumption of Polyphenol-Rich Peach and Plum Juice Prevents Risk Factors for Obesity-Related Metabolic Disorders and Cardiovascular Disease in Zucker Rats. J. Nutr. Biochem. 2015, 26, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Kang, O.-J.; Gweon, O.-C. Comparison of Phenolic Acids and Flavonoids in Black Garlic at Different Thermal Processing Steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Choi, I.S.; Cha, H.S.; Lee, Y.S. Physicochemical and Antioxidant Properties of Black Garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Lech, K.; Figiel, A. Chemical Composition, Antioxidant Capacity, and Sensory Quality of Dried Sour Cherry Fruits Pre-Dehydrated in Fruit Concentrates. Food Bioprocess Technol. 2015, 8, 2076–2095. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Legua, P.; Lech, K.; Carbonell-Barrachina, Á.A.; Hernández, F. Chemical Composition, Antioxidant Capacity, and Sensory Quality of Dried Jujube Fruits as Affected by Cultivar and Drying Method. Food Chem. 2016, 207, 170–179. [Google Scholar] [CrossRef]
- Poovitha, S.; Parani, M. In Vitro and in Vivo α-Amylase and α-Glucosidase Inhibiting Activities of the Protein Extracts from Two Varieties of Bitter Gourd (Momordica Charantia L.). BMC Complement. Altern. Med. 2016, 16, 185. [Google Scholar] [CrossRef]
- Wesołowski, M.; Nęcka, K.; Dróżdż, T.; Kiełbasa, P. Koncepcja modelowania wyładowania pulsacyjnego pola elektrycznego (PEF) w produktach przemysłu rolno—Spożywczego. Przegląd Elektrotechniczny 2018, 1, 121–125. [Google Scholar] [CrossRef]
- Tulej, W.; Głowacki, S. Modeling of the Drying Process of Apple Pomace. Appl. Sci. 2022, 12, 1434. [Google Scholar] [CrossRef]
- Lech, K.; Figiel, A.; Wojdyło, A.; Korzeniowska, M.; Serowik, M.; Szarycz, M. Drying Kinetics and Bioactivity of Beetroot Slices Pretreated in Concentrated Chokeberry Juice and Dried with Vacuum Microwaves. Dry. Technol. 2015, 33, 1644–1653. [Google Scholar] [CrossRef]
- Subhashree, S.N.; Sunoj, S.; Xue, J.; Bora, G.C. Quantification of Browning in Apples Using Colour and Textural Features by Image Analysis. Food Qual. Saf. 2017, 1, 221–226. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Content of Bioactive Compounds in the Peach Kernels and Their Antioxidant, Anti-Hyperglycemic, Anti-Aging Properties. Eur. Food Res. Technol. 2019, 245, 1123–1136. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of Proanthocyanidin Cleavage Products Following Acid-Catalysis in the Presence of Excess Phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of Phytochemicals, Antioxidant Capacity, and Antidiabetic Activity of Novel Smoothies from Selected Prunus Fruits. J. Funct. Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]




| Method | Mc (%) | aw (-) | Color | |||
|---|---|---|---|---|---|---|
| L*(D65) | a*(D65) | b*(D65) | BI | |||
| VMD 500W | 8.16 ± 0.4 ab* | 0.2172 ± 0.0054 b | 35.35 ± 0.11 f | 6.21 ± 0.28 a | 8.72 ± 0.68 a | 40.8 ± 3.2 d |
| VMD 250W | 13.06 ± 0.63 c | 0.229 ± 0.0048 b | 38.01 ± 0.08 d | 6.9 ± 0.11 b | 12.2 ± 0.44 f | 51.5 ± 2.0 c |
| VMD 125W | 13.42 ± 0.65 c | 0.2891 ± 0.0071 a | 37.76 ± 0.17 d | 7.71 ± 0.09 h | 13.61 ± 0.28 g | 59.0 ± 1.6 b |
| CD70-3h/125W | 12.95 ± 0.63 c | 0.2936 ± 0.003 a | 38.89 ± 0.11 e | 8.34 ± 0.09 i | 14.8 ± 0.18 c | 62.8 ± 1.1 ab |
| CD70-6h/125W | 13.44 ± 0.65 c | 0.2994 ± 0.0034 a | 39.70 ± 0.30 e | 9.06 ± 0.27 j | 14.93 ± 0.37 c | 63.1 ± 1.5 a |
| CD60-9h/VD60 | 8.89 ± 0.43 a | 0.3957 ± 0.0058 d | 29.08 ± 0.32 b | 2.31 ± 0.1 d | 2.7 ± 0.12 e | 15.3 ± 0.6 g |
| CD70-9h/VD60 | 8.57 ± 0.41 ab | 0.3479 ± 0.0054 c | 29.16 ± 0.48 b | 1.66 ± 0.14 c | 1.84 ± 0.03 d | 10.5 ± 0.5 h |
| PEF + H2O/CD70-3h/125W | 10.51 ± 0.51 e | 0.4694 ± 0.0133 g | 31.00 ± 0.52 a | 4.65 ± 0.02 g | 4.52 ± 0.1 a | 26.3 ± 0.7 e |
| CEF + H2O/CD70-3h/125W | 8.89 ± 0.43 a | 0.3891 ± 0.0079 d | 31.23 ± 0.45 a | 4.04 ± 0.04 f | 4.53 ± 0.04 a | 24.8 ± 0.6 ef |
| CEF/CD70-3h/125W | 8.14 ± 0.39 ab | 0.3691 ± 0.0044 f | 30.92 ± 0.11 a | 3.19 ± 0.05 e | 4.26 ± 0.1 a | 22.0 ± 0.6 f |
| MF + H2O/CD70-3h/125W | 7.26 ± 0.35 bd | 0.3485 ± 0.0022 c | 33.57 ± 0.18 c | 5.9 ± 0.02 a | 7.99 ± 0.04 b | 39.6 ± 0.4 d |
| MF/CD70-3h/125W | 6.41 ± 0.31 d | 0.3211 ± 0.0054 e | 34.28 ± 0.1 c | 6.72 ± 0.03 b | 8.57 ± 0.05 b | 42.6 ± 0.3 d |
| Pretreatment | Drying | Constants | Statistics | ||||
|---|---|---|---|---|---|---|---|
| a | b | k | n | RMSE | R2 | ||
| - | CD60 °C | 0.460 | −0.540 | 0.0180 | 0.728 | 0.0014 | 0.9999 |
| CD70 °C | 0.416 | −0.589 | 0.0290 | 0.692 | 0.0033 | 0.9996 | |
| CD60 °C-VD | −0.001 | −0.550 | 0.0028 | 0.939 | 0.0076 | 0.9959 | |
| CD70 °C-VD | −0.029 | −0.506 | 0.0030 | 0.901 | 0.0091 | 0.9918 | |
| VMD125W | 0.230 | −0.765 | 0.0134 | 1.245 | 0.0057 | 0.9994 | |
| VMD250W | 0.230 | −0.773 | 0.0136 | 1.490 | 0.0023 | 0.9999 | |
| VMD500W | 0.138 | −0.864 | 0.0267 | 1.630 | 0.0123 | 0.9982 | |
| CD70 °C-3h-VMD125W | 0.205 | −0.395 | 0.0236 | 1.060 | 0.0068 | 0.9965 | |
| CD70 °C-6h-VMD125W | 0.221 | −0.287 | 0.0047 | 1.450 | 0.0030 | 0.9989 | |
| PEF + H2O | CD70 °C-3h | 0.171 | −0.837 | 0.0230 | 0.866 | 0.0071 | 0.9990 |
| CEF + H2O | CD70 °C-3h | 0.104 | −0.900 | 0.0220 | 0.786 | 0.0044 | 0.9995 |
| CEF | CD70 °C-3h | 0.443 | −0.559 | 0.0170 | 0.858 | 0.0014 | 0.9999 |
| MF + H2O | CD70 °C-3h | 0.139 | −0.869 | 0.0150 | 0.933 | 0.0058 | 0.9993 |
| MF | CD70 °C-3h | 0.374 | −0.631 | 0.0180 | 0.834 | 0.0052 | 0.9986 |
| PEF + H2O | VMD125W | −0.810 | −1.080 | 0.0075 | 0.764 | 0.0037 | 0.9958 |
| CEF + H2O | VMD125W | 0.079 | −0.272 | 0.0180 | 1.300 | 0.0036 | 0.9982 |
| CEF | VMD125W | 0.0156 | −0.410 | 0.0120 | 1.270 | 0.0015 | 0.9998 |
| MF + H2O | VMD125W | 0.041 | −0.228 | 0.0460 | 0.957 | 0.0054 | 0.9930 |
| MF | VMD125W | 0.139 | −0.393 | 0.0220 | 1.160 | 0.0043 | 0.9986 |
| Method | Phenolic Acids | Flavan-3-ols | Polymeric Proanthocyanidins | Total Polyphenols |
|---|---|---|---|---|
| [mg/100 g dm] | ||||
| Fresh | 11.26 ± 0.01 a* | 488.24 ± 2.48 i | 43.41 ± 0.23 a | 543.92 ± 2.72 i |
| VMD 500 W | 2.19 ± 0.04 k | 1088.86 ± 9.32 a | 21.25 ± 0.53 h | 1112.31 ± 9.86 a |
| VMD 250 W | 1.98 ± 0.03 l | 967.67 ± 3.81 c | 24.94 ± 0.82 f | 994.59 ± 4.66 c |
| VMD 125 W | 10.02 ± 0.38 c | 1083.67 ± 15.09 a | 29.86 ± 0.05 b | 1123.54 ± 15.52 a |
| CD70 °C-3h/125W | 10.14 ± 0.21 c | 1086.46 ± 18.13 a | 28.76 ± 0.13 d | 1125.36 ± 18.47 a |
| CD70 °C-6h/125W | 2.99 ± 0.02 i | 1023.53 ± 13.05 b | 22.37 ± 0.09 g | 1048.89 ± 13.16 b |
| CD60 °C-9h/VD60 | 9.46 ± 0.15 d | 627.13 ± 9.00 g | 17.61 ± 0.25 i | 654.20 ± 9.40 g |
| CD70 °C-9h/VD60 | 9.07 ± 0.24 e | 709.64 ± 4.04 f | 24.10 ± 0.03 f | 742.81 ± 4.31 f |
| PEF + H2O/CD70-3h/125W | 6.55 ± 0.05 g | 366.55 ± 4.07 j | 29.30 ± 0.09 c | 402.40 ± 4.21 j |
| CEF + H2O/CD70-3h/125W | 4.09 ± 0.08 h | 576.37 ± 2.13 h | 11.97 ± 0.19 k | 592.42 ± 2.40 h |
| CEF/CD70-3h/125W | 7.54 ± 0.05 f | 612.78 ± 9.26 g | 25.57 ± 0.15 e | 645.89 ± 9.46 f |
| MF + H2O/CD70-3h/125W | 10.57 ± 0.24 bc | 821.67 ± 10.38 d | 25.79 ± 0.15 e | 858.04 ± 10.57 d |
| MF/CD70-3h/125W | 2.66 ± 0.01 j | 776.50 ± 8.82 e | 21.73 ± 0.43 h | 800.90 ± 9.26 e |
| Method | ABTS | FRAP | ORAC | Inhibition of α-Amylase | Inhibition of α-Glucosidase |
|---|---|---|---|---|---|
| mmol Trolox/100 g dm | IC50 [mg/mL] | ||||
| Fresh | 2.60 ± 0.24 e* | 1.35 ± 0.04 f | 6.67 ± 0.03 c | 186.56 h | 211.24 g |
| VMD 500 W | 6.05 ± 0.31 a | 3.73 ± 0.10 a | 7.90 ± 0.05 a | 61.73 a | 63.11 c |
| VMD 250 W | 5.18 ± 0.51 b | 3.08 ± 0.04 b | 7.85 ± 0.03 a | 135.04 e | 208.59 g |
| VMD 125 W | 3.50 ± 0.20 c | 1.92 ± 0.08 d | 6.82 ± 0.03 b | 140.87 f | 186.71 f |
| CD70 °C-3h/125W | 3.76 ± 0.31 c | 2.17 ± 0.06 c | 5.50 ± 0.02 g | 111.98 d | 152.50 e |
| CD70 °C-6h/125W | 5.06 ± 0.16 b | 3.07 ± 0.02 b | 6.80 ± 0.03 b | 83.03 b | 51.21 a |
| CD60 °C-9h/VD60 | 3.06 ± 0.17 d | 1.80 ± 0.07 d | 5.51 ± 0.02 g | 134.71 e | 227.79 hi |
| CD70 °C-9h/VD60 | 2.87 ± 0.12 de | 1.57 ± 0.02 e | 5.89 ± 0.03 d | 229.46 j | 155.59 e |
| PEF + H2O/CD70-3h/125W | 2.03 ± 0.19 f | 1.09 ± 0.05 g | 4.38 ± 0.03 i | 142.86 f | 223.77 h |
| CEF + H2O/CD70-3h/125W | 2.71 ± 0.19 d | 1.18 ± 0.02 g | 5.60 ± 0.01 f | 99.00 c | 56.00 b |
| CEF/CD70-3h/125W | 2.49 ± 0.17 e | 1.41 ± 0.05 f | 5.18 ± 0.04 h | 188.39 h | 149.64 e |
| MF + H2O/CD70-3h/125W | 2.38 ± 0.23 ef | 1.37 ± 0.06 f | 5.76 ± 0.02 e | 153.09 g | 231.85 i |
| MF/CD70-3h/125W | 2.72 ± 0.08 e | 1.54 ± 0.02 e | 6.75 ± 0.02 b | 213.62 i | 109.37 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masztalerz, K.; Dróżdż, T.; Nowicka, P.; Wojdyło, A.; Kiełbasa, P.; Lech, K. The Effect of Nonthermal Pretreatment on the Drying Kinetics and Quality of Black Garlic. Molecules 2023, 28, 962. https://doi.org/10.3390/molecules28030962
Masztalerz K, Dróżdż T, Nowicka P, Wojdyło A, Kiełbasa P, Lech K. The Effect of Nonthermal Pretreatment on the Drying Kinetics and Quality of Black Garlic. Molecules. 2023; 28(3):962. https://doi.org/10.3390/molecules28030962
Chicago/Turabian StyleMasztalerz, Klaudia, Tomasz Dróżdż, Paulina Nowicka, Aneta Wojdyło, Paweł Kiełbasa, and Krzysztof Lech. 2023. "The Effect of Nonthermal Pretreatment on the Drying Kinetics and Quality of Black Garlic" Molecules 28, no. 3: 962. https://doi.org/10.3390/molecules28030962
APA StyleMasztalerz, K., Dróżdż, T., Nowicka, P., Wojdyło, A., Kiełbasa, P., & Lech, K. (2023). The Effect of Nonthermal Pretreatment on the Drying Kinetics and Quality of Black Garlic. Molecules, 28(3), 962. https://doi.org/10.3390/molecules28030962









