Olive Mill Wastewater Fermented with Microbial Pools as a New Potential Functional Beverage
Abstract
1. Introduction
2. Results
2.1. Chemico-Physical Characterization of Sample of Different Trials
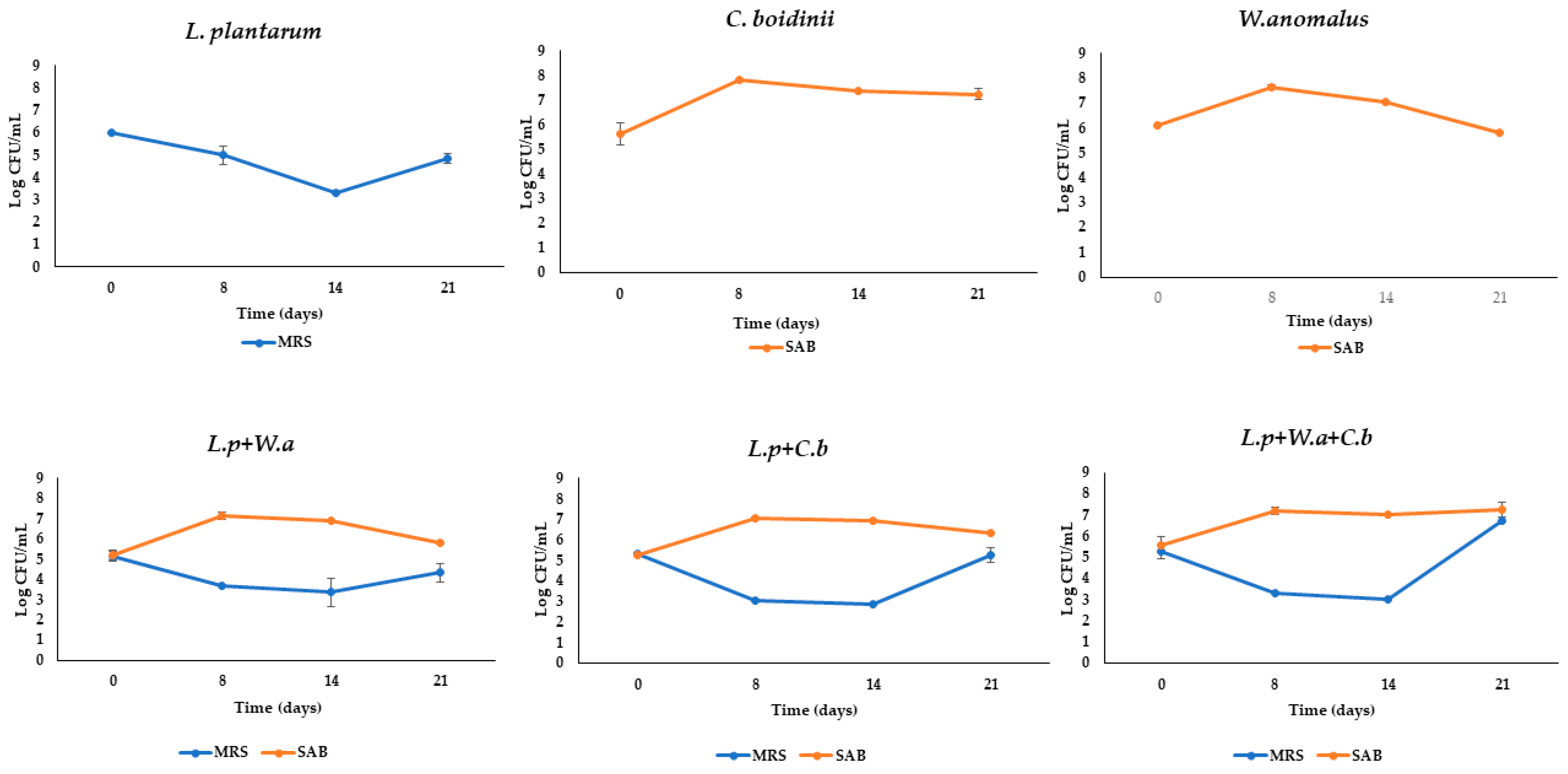
2.2. Microbiological Analyses
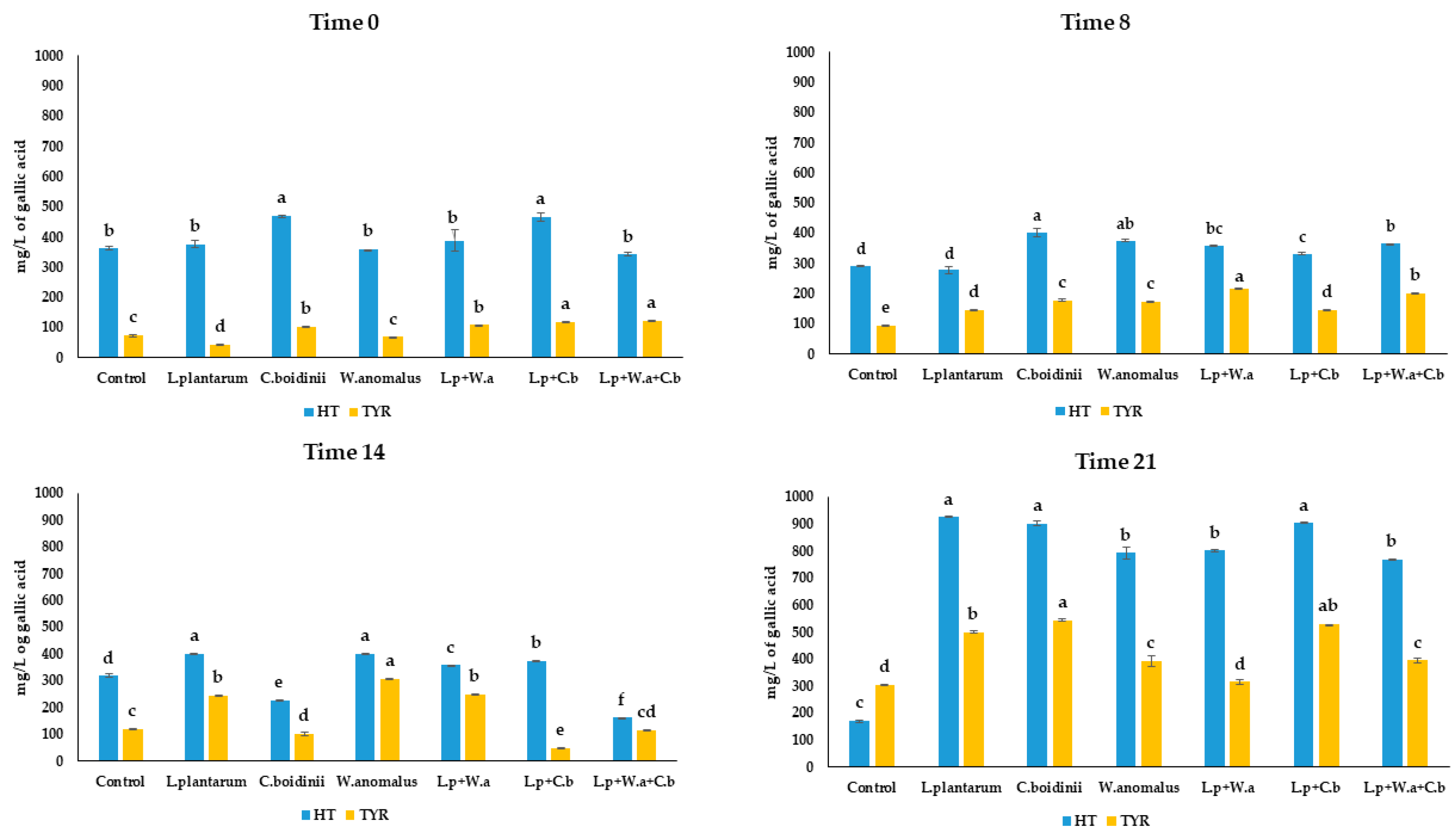
2.3. Phenol and Organic Acid Detection
2.4. Biological Assay
2.4.1. Transepithelial Transport through Caco-2 Cell Monolayers
2.4.2. Evaluation Activity on COX-1 and COX-2 Isoenzymes
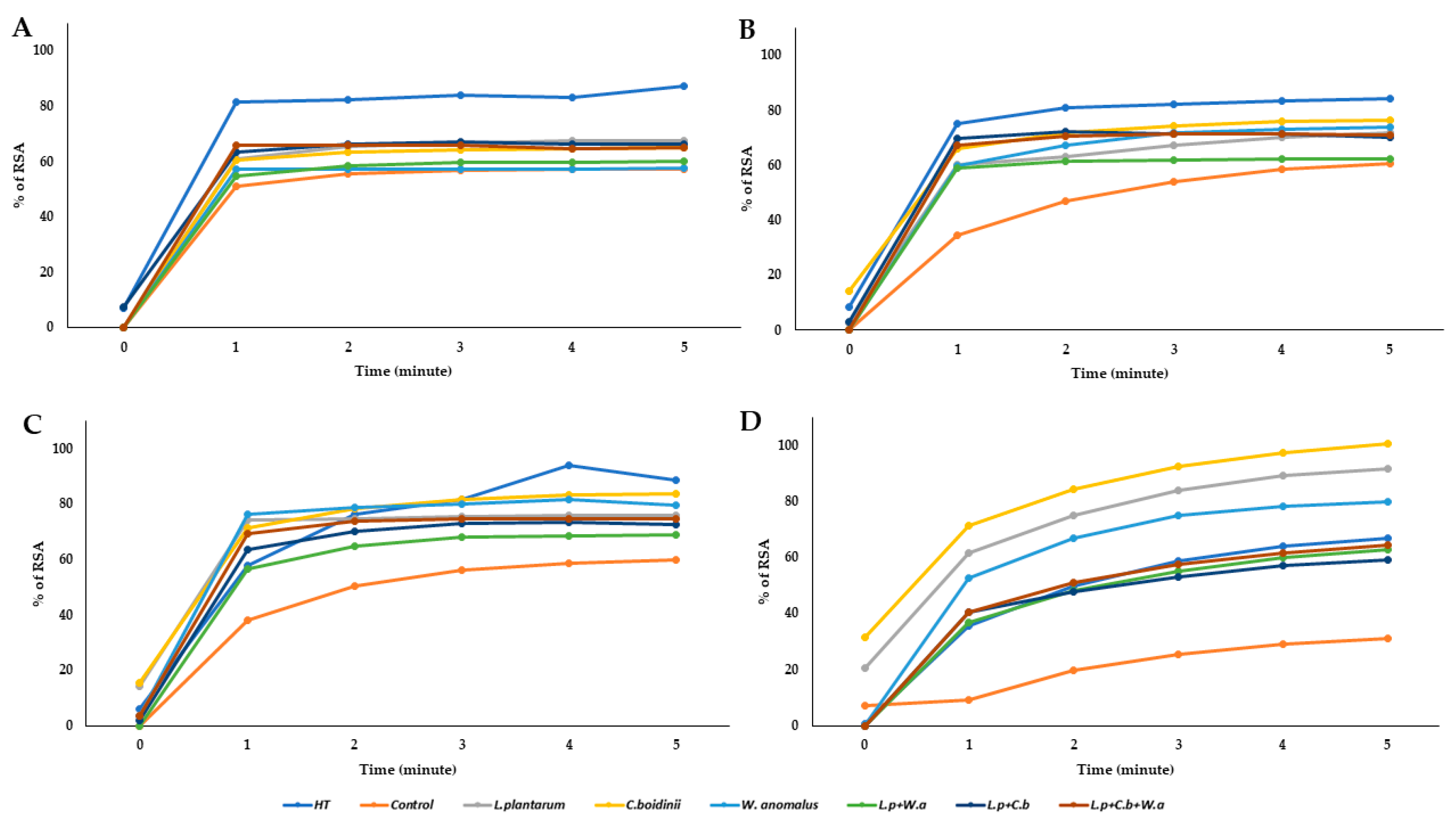
2.4.3. Antioxidant Activity
3. Discussion
4. Materials and Methods
4.1. OMWW Sampling
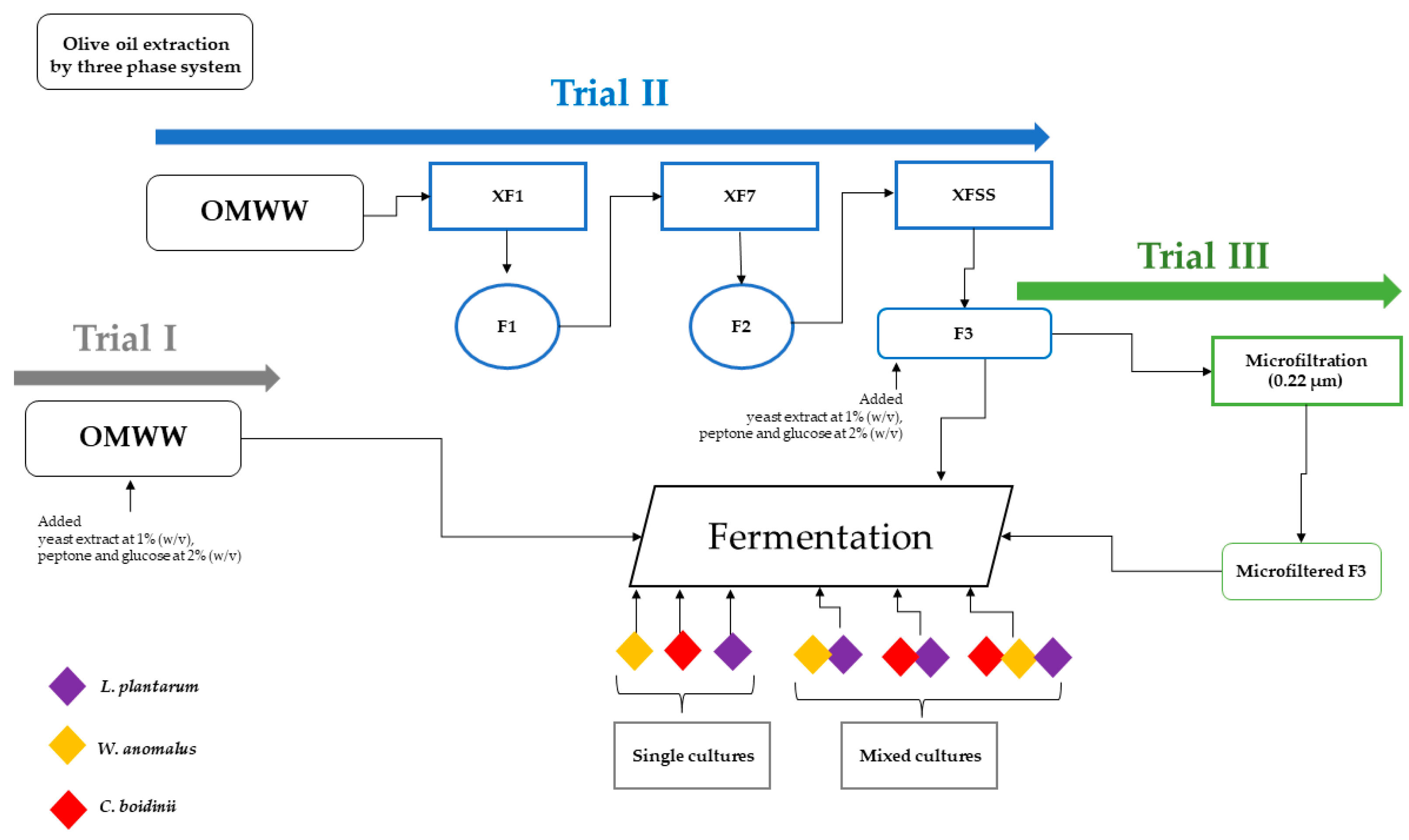
4.2. Set–Up of Fermentation Process
4.3. Chemical Analyses
4.4. Microbiological Analyses
4.5. HPLC Analysis
4.5.1. Detection of Phenols
4.5.2. Detection of Organic Acids
4.6. Biological Assays
4.6.1. Cell Culture and Cytotoxicity
4.6.2. Transport Caco-2 Monolayer
4.6.3. Cyclooxygenase Activity Inhibition
4.6.4. Antioxidant Activity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gullon, P.; Gullon, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef] [PubMed]
- Flamminii, F.; Di Mattia, C.D.; Difonzo, G.; Neri, L.; Faieta, M.; Caponio, F.; Pittia, P. From by-product to food ingredient: Evaluation of compositional and technological properties of olive-leaf phenolic extracts. J. Sci. Food Agric. 2019, 99, 6620–6627. [Google Scholar] [CrossRef] [PubMed]
- Lama-Muñoz, A.; del Mar Contreras, M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Extraction of oleuropein and luteolin-7-O-glucoside from olive leaves: Optimization of technique and operating conditions. Food Chem. 2019, 293, 161–168. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process. Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Sordini, B.; Veneziani, G.; Urbani, S. L’acqua di Vegetazione dei Frantoi Oleari: Una Risorsa da Valorizzare; Accademia dei Georgofili: Florence, Italy, 2021; pp. 317–337. [Google Scholar]
- Foti, P.; Occhipinti, P.S.; Romeo, F.V.; Timpanaro, N.; Musumeci, T.; Randazzo, C.L.; Caggia, C. Phenols recovered from olive mill wastewater as natural booster to fortify blood orange juice. Food Chem. 2022, 393, 133428. [Google Scholar] [CrossRef]
- Cedola, A.; Cardinali, A.; D’Antuono, I.; Conte, A.; Del Nobile, M.A. Cereal foods fortified with by-products from the olive oil industry. Food Biosci. 2019, 33, 100490. [Google Scholar] [CrossRef]
- Hadj Saadoun, J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of Agri-Food Waste: A Promising Route for the Production of Aroma Compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef]
- Aponte, M.; Ungaro, F.; d’Angelo, I.; De Caro, C.; Russo, R.; Blaiotta, G.; Dal Piaz, F.; Calignano, A.; Miro, A. Improving in vivo conversion of oleuropein into hydroxytyrosol by oral granules containing probiotic Lactobacillus plantarum 299v and an Olea europaea standardized extract. Int. J. Pharmaceut. 2018, 543, 73–82. [Google Scholar] [CrossRef]
- Romeo, F.V.; Granuzzo, G.; Foti, P.; Ballistreri, G.; Caggia, C.; Rapisarda, P. Microbial application to improve olive mill wastewater phenolic extracts. Molecules 2021, 26, 1944. [Google Scholar] [CrossRef]
- Ruiz Rodríguez, L.G.; Zamora Gasga, V.M.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Sánchez Burgos, J.A. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Hao, H.; Yan, R.; Wang, X.; Wang, B.; Sun, J.; Li, Z.; Zhang, Y.; Sun, B. Individualization of Chinese alcoholic beverages: Feasibility towards a regulation of organic acids. LWT 2022, 172, 114168. [Google Scholar] [CrossRef]
- Starzyńska-Janiszewska, A.; Fernández-Fernández, C.; Martín-García, B.; Verardo, V.; Gómez-Caravaca, A.M. Solid state fermentation of olive leaves as a promising technology to obtain hydroxytyrosol and elenolic acid derivatives enriched extracts. Antioxidants 2022, 11, 1693. [Google Scholar] [CrossRef]
- Benítez-Cabello, A.; Calero-Delgado, B.; Rodríguez-Gómez, F.; Garrido-Fernández, A.; Jiménez-Díaz, R.; Arroyo-López, F.N. Biodiversity and multifunctional features of lactic acid bacteria isolated from table olive biofilms. Front. Microbiol. 2019, 10, 836. [Google Scholar] [CrossRef]
- Lanza, B.; Di Marco, S.; Bacceli, M.; Di Serio, M.G.; Di Loreto, G.; Cellini, M.; Simone, N. Lactiplantibacillus plantarum used as single, multiple, and mixed starter combined with Candida boidinii for table olive fermentations: Chemical, textural, and sensorial characterization of final products. Fermentation 2021, 7, 239. [Google Scholar] [CrossRef]
- Zullo, B.A.; Ciafardini, G. Evaluation of physiological properties of yeast strains isolated from olive oil and their in vitro probiotic trait. Food Microbiol. 2019, 78, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Guerra, F.; Souto, S.B.; Souto, E.B.; Santini, A.I. Nutraceuticals and functional beverages: Focus on Prebiotics and Probiotics active beverages. In Future Foods; Academic Press: Cambridge, MA, USA, 2022; pp. 251–258. [Google Scholar]
- Parra-Perez, A.M.; Pérez-Jiménez, A.; Gris-Cárdenas, I.; Bonel-Pérez, G.C.; Carrasco-Díaz, L.M.; Mokhtari, K.; García-Salguero, L.; Lupiáñez, J.A.; Rufino-Palomares, E.E. Involvement of the PI3K/AKT intracellular signaling pathway in the anticancer activity of hydroxytyrosol, a polyphenol from Olea europaea, in hematological cells and implication of HSP60 levels in its anti-inflammatory activity. Int. J. Mol. Sci. 2022, 23, 7053. [Google Scholar] [CrossRef]
- Perrone, M.G.; Vitale, P.; Ferorelli, S.; Boccarelli, A.; Coluccia, M.; Pannunzio, A.; Campanella, F.; Di Mauro, G.; Bonaccorso, C.; Fortuna, C.G.; et al. Effect of mofezolac-galactose distance in conjugates targeting cyclooxygenase (COX)-1 and CNS GLUT-1 carrier. Eur. J. Med. Chem. 2017, 141, 404–416. [Google Scholar] [CrossRef]
- Di Mauro, M.D.; Fava, G.; Spampinato, M.; Aleo, D.; Melilli, B.; Saita, M.G.; Centonze, G.; Maggiore, R.; D’Antona, N. Polyphenolic fraction from olive mill wastewater: Scale-up and in vitro studies for ophthalmic nutraceutical applications. Antioxidants 2019, 8, 462. [Google Scholar] [CrossRef]
- Ricci, A.; Bernini, V.; Maoloni, A.; Cirlini, M.; Galaverna, G.; Neviani, E.; Lazzi, C. Vegetable by-product lacto-fermentation as a new source of antimicrobial compounds. Microorganisms 2019, 7, 607. [Google Scholar] [CrossRef]
- Alfano, A.; Corsuto, L.; Finamore, R.; Savarese, M.; Ferrara, F.; Falco, S.; Santabarbara, G.; De Rosa, M.; Schiraldi, C. Valorization of olive mill wastewater by membrane processes to recover natural antioxidant compounds for cosmeceutical and nutraceutical applications or functional foods. Antioxidants 2018, 7, 72. [Google Scholar] [CrossRef]
- Arienzo, A.; Murgia, L.; Fraudentali, I.; Gallo, V.; Angelini, R.; Antonini, G. Microbiological quality of ready-to-eat leafy green salads during shelf-life and home-refrigeration. Foods 2020, 9, 1421. [Google Scholar] [CrossRef]
- France, T.C.; Kelly, A.L.; Crowley, S.V.; O’Mahony, J.A. Cold microfiltration as an enabler of sustainable dairy protein ingredient innovation. Foods 2021, 10, 2091. [Google Scholar] [CrossRef]
- Allouche, N.; Fki, I.; Sayadi, S. Toward a high yield recovery of antioxidants and purified hydroxytyrosol from olive mill wastewaters. J. Agric. Food Chem. 2004, 52, 267–273. [Google Scholar] [CrossRef]
- Vaccalluzzo, A.; Pino, A.; De Angelis, M.; Bautista-Gallego, J.; Romeo, F.V.; Foti, P.; Caggia, C.; Randazzo, C.L. Effects of different stress parameters on growth and on oleuropein-degrading abilities of Lactiplantibacillus plantarum strains selected as tailored starter cultures for naturally table olives. Microorganisms 2020, 8, 1607. [Google Scholar] [CrossRef] [PubMed]
- Reverón, I.; Plaza-Vinuesa, L.; Santamaría, L.; Oliveros, J.C.; de Las Rivas, B.; Muñoz, R.; López de Felipe, F. Transcriptomic evidence of molecular mechanisms underlying the response of Lactobacillus plantarum WCFS1 to hydroxytyrosol. Antioxidants 2020, 9, 442. [Google Scholar] [CrossRef] [PubMed]
- De Melo Pereira, G.V.; Maske, B.L.; de Carvalho Neto, D.P.; Karp, S.G.; De Dea Lindner, J.; Martin, J.G.P.; de Oliveira Hosken, B.; Soccol, C.R. What is Candida doing in my food? A review and safety alert on its use as starter cultures in fermented foods. Microorganisms 2022, 10, 1855. [Google Scholar] [CrossRef] [PubMed]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A. Comparison between different D-dime cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control. 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Bangar, S.P.; Suri, S.; Trif, M.; Ozogul, F. Organic acids production from lactic acid bacteria: A preservation approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Okoye, C.O.; Dong, K.; Wang, Y.; Gao, L.; Li, X.; Wu, Y.; Jiang, J. Comparative genomics reveals the organic acid biosynthesis metabolic pathways among five lactic acid bacterial species isolated from fermented vegetables. New Biotechnol. 2022, 70, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, T.; Strigun, A.; Verlohner, A.; Huener, H.A.; Peter, E.; Herold, M.; Bordag, N.; Mellert, W.; Walk, T.; Spitzer, M.; et al. Prediction of liver toxicity and mode of action using metabolomics in vitro in HepG2 cells. Arch. Toxicol. 2018, 92, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Colabufo, N.A.; Contino, M.; Cantore, M.; Capparelli, E.; Grazia, M.; Cassano, G.; Gasparre, G.; Leopoldo, M.; Berardi, F.; Perrone, R. Naphthalenyl derivatives for hitting P-gp/MRP1/BCRP transporters. Bioorg. Med. Chem. 2013, 21, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Riganti, C.; Contino, M.; Guglielmo, S.; Perrone, M.G.; Salaroglio, I.C.; Milosevic, V.; Giampietro, R.; Leonetti, F.; Rolando, B.; Lazzarato, L.; et al. Design, biological evaluation, and molecular modeling of tetrahydroisoquinoline derivatives: Discovery of a potent P-glycoprotein ligand overcoming multidrug resistance in cancer stem cells. J. Med. Chem. 2019, 62, 974–986. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Soler, A.; Romero, M.P.; Macia, A.; Saha, S.; Furniss, C.S.M.; Kroon, P.A.; Motilva, M.J. Digestion stability and evaluation of the metabolism and transport of olive oil phenols in the human small-intestinal epithelial Caco 2/TC7 cell line. Food Chem. 2010, 119, 703–714. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Extra virgin olive oil polyphenols: Modulation of cellular pathways related to oxidant species and inflammation in aging. Cells 2020, 9, 478. [Google Scholar] [CrossRef]
- Bartolomei, M.; Bollati, C.; Bellumori, M.; Cecchi, L.; Cruz-Chamorro, I.; Santos-Sánchez, G.; Ranaldi, G.; Ferruzza, S.; Sambuy, Y.; Arnoldi, A.; et al. Extra virgin olive oil phenolic extract on human hepatic HepG2 and intestinal Caco-2 Cells: Assessment of the antioxidant activity and intestinal trans-epithelial transport. Antioxidants 2021, 10, 118. [Google Scholar] [CrossRef]
- Abdallah, M.; Marzocco, S.; Adesso, S.; Zarrouk, M.; Guerfel, M. Olive oil polyphenols extracts inhibit inflammatory markers in J774A.1 murine macrophages and scavenge free radicals. Acta Histochem. 2018, 120, 1–10. [Google Scholar] [CrossRef]
- Scoditti, E.; Nestola, A.; Massaro, M.; Calabriso, N.; Storelli, C.; De Caterina, R.; Carluccio, M.A. Hydroxytyrosol suppresses MMP-9 and COX-2 activity and expression in activated human monocytes via PKCα and PKCβ1 inhibition. Atherosclerosis 2014, 232, 17–24. [Google Scholar] [CrossRef]
- Perrone, M.G.; Scilimati, A.; Simone, L.; Vitale, P. Selective COX-1 inhibition: A therapeutic target to be reconsidered. Curr. Med. Chem. 2010, 17, 3769–3805. [Google Scholar] [CrossRef] [PubMed]
- Calvello, R.; Lofrumento, D.D.; Perrone, M.G.; Cianciulli, A.; Salvatore, R.; Vitale, P.; De Nuccio, F.; Giannotti, L.; Nicolardi, G.; Panaro, M.A.; et al. Highly selective cyclooxygenase-1 inhibitors P6 and mofezolac counteract inflammatory state both in vitro and in vivo models of neuroinflammation. Front Neurol. 2017, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Pati, M.L.; Vitale, P.; Ferorelli, S.; Iaselli, M.; Miciaccia, M.; Boccarelli, A.; Di Mauro, G.D.; Fortuna, C.G.; Souza Domingos, T.F.; Rodrigues Pereira da Silva, L.C.; et al. Translational impact of novel widely pharmacological characterized mofezolac-derived COX-1 inhibitors combined with bortezomib on human multiple myeloma cell lines viability. Eur. J. Med. Chem. 2019, 164, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Vitale, P.; Miciaccia, M.; Ferorelli, S.; Centonze, A.; Solidoro, R.; Munzone, C.; Bonaccorso, C.; Fortuna, C.G.; Kleinmanns, K.; et al. Fluorochrome selection for imaging intraoperative ovarian cancer probes. Pharmaceuticals 2022, 15, 668. [Google Scholar] [CrossRef]
- Rosignoli, P.; Fuccelli, R.; Sepporta, M.V.; Fabiani, R. In vitro chemo-preventive activities of hydroxytyrosol: The main phenolic compound present in extra-virgin olive oil. Food Funct. 2016, 7, 301–307. [Google Scholar] [CrossRef]
- El-Azem, N.; Pulido-Moran, M.; Ramirez-Tortosa, C.L.; Quiles, J.L.; Cara, F.E.; Sanchez-Rovira, P.; Granados-Principal, S.; Ramirez-Tortosa, M.C. Modulation by hydroxytyrosol of oxidative stress and antitumor activities of paclitaxel in breast cancer. Eur. J. Nutr. 2019, 58, 1203–1211. [Google Scholar] [CrossRef]
- Ramirez-Tortosa, C.; Sanchez, A.; Perez-Ramirez, C.; Quiles, J.L.; Robles-Almazan, M.; Pulido-Moran, M.; Sanchez-Rovira, P.; Ramirez-Tortosa, M. Hydroxytyrosol supplementation modifies plasma levels of tissue inhibitor of metallopeptidase 1 in women with breast cancer. Antioxidants 2019, 8, 393. [Google Scholar] [CrossRef]
- Sanlier, N.; Gokcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Pino, A.; Vaccalluzzo, A.; Solieri, L.; Romeo, F.V.; Todaro, A.; Caggia, C.; Arroyo-López, F.N.; Bautista-Gallego, J.; Randazzo, C.L. Effect of sequential inoculum of beta-glucosidase positive and probiotic strains on brine fermentation to obtain low salt Sicilian table olives. Front. Microbiol. 2019, 10, 174. [Google Scholar] [CrossRef]
- Palmeri, R.; Siracusa, L.; Carrubba, M.; Parafati, L.; Proetto, I.; Pesce, F.; Fallico, B. Olive leaves, a promising byproduct of olive oil industry: Assessment of metabolic profiles and antioxidant capacity as a function of cultivar and seasonal change. Agronomy 2022, 12, 2007. [Google Scholar] [CrossRef]
| Sample | Time | pH | TSS (°Brix) | Total Phenol (mg/L) |
|---|---|---|---|---|
| Control | 0 | 5.18 ± 0.01 | 8.30 ± 0.77 | 3627.4 ± 0.54 c |
| L. plantarum | 0 | 5.16 ± 0.01 | 10.60 ± 0.78 | 3711.2 ± 4.89 b |
| C. boidinii | 0 | 5.18 ± 0.01 | 10.28 ± 0.70 | 3539.8 ± 0.54 d |
| W. anomalus | 0 | 5.12 ± 0.08 | 9.76 ± 1.53 | 3172.9 ± 1.63 f |
| L.p + W.a | 0 | 5.13 ± 0.06 | 8.56 ± 0.80 | 4135.0 ± 4.89 a |
| L.p + C.b | 0 | 5.19 ± 0.02 | 10.04 ± 1.13 | 3474.7 ± 1.09 e |
| L.p + W.a + C.b | 0 | 5.18 ± 0.01 | 8.88 ± 1.39 | 2967.1 ± 2.18 g |
| n.s. | n.s. | ** | ||
| Control | 8 | 5.17 ± 0.01 a | 8.30 ± 0.78 | 1985.8 ± 3.26 g |
| L. plantarum | 8 | 5.04 ± 0.03 b | 10.30 ± 0.98 | 3032.5 ± 2.18 b |
| C. boidinii | 8 | 4.97 ± 0. 01 bcd | 8.88 ± 2.18 | 3020.6 ± 0.54 c |
| W. anomalus | 8 | 4.87 ± 0.02 e | 9.14 ± 1.77 | 2395.3 ± 1.63 f |
| L.p + W.a | 8 | 4.88 ± 0.04 de | 7.52 ± 1.17 | 2897.2 ± 0.01 e |
| L.p + C.b | 8 | 5.00 ± 0.01 bc | 9.50 ± 0.32 | 2991.0 ± 5.44 d |
| L.p + W.a + C.b | 8 | 4.94 ± 0.01 cde | 8.24 ± 1.29 | 3268.2 ± 1.63 a |
| ** | n.s. | ** | ||
| Control | 14 | 5.18 ± 0.02 a | 8.30 ± 0.78 | 1809.5 ± 0.54 f |
| L. plantarum | 14 | 4.68 ± 0.01 cd | 9.90 ± 0.99 | 3282.9 ± 0.54 c |
| C. boidinii | 14 | 4.77 ± 0.01 b | 7.99 ± 2.82 | 2443.8 ± 1.63 d |
| W. anomalus | 14 | 4.67 ± 0.04 cd | 8.28 ± 1.44 | 3539.7 ± 0.54 b |
| L.p + W.a | 14 | 4.62 ± 0.09 d | 6.64 ± 1.52 | 2199.2 ± 3.81 e |
| L.p + C.b | 14 | 4.89 ± 0.01 b | 9.03 ± 1.12 | 3545.1 ± 53.84 b |
| L.p + W.a + C.b | 14 | 4.82 ± 0.02 bc | 7.62 ± 1.36 | 4015.0 ± 2.72 a |
| ** | n.s. | ** | ||
| Control | 21 | 5.19 ± 0.01 a | 8.20 ± 0.61 | 1009.4 ± 0.54 f |
| L. plantarum | 21 | 4.65 ± 0.03 c | 8.15 ± 1.20 | 3392.1 ± 0.54 a |
| C. boidinii | 21 | 4.60 ± 0.01 d | 5.60 ± 0.57 | 3005.2 ± 0.54 b |
| W. anomalus | 21 | 4.54 ± 0.04 e | 6.36 ± 0.37 | 2394.2 ± 1.09 c |
| L.p + W.a | 21 | 4.49 ± 0.01 de | 5.32 ± 0.04 | 1914.6 ± 1.63 d |
| L.p + C.b | 21 | 4.84 ±0.02 b | 8.00 ± 1.41 | 3403.2 ± 10.88 a |
| L.p + W.a + C.b | 21 | 4.82 ± 0.01 b | 5.99 ± 0.01 | 1543.6 ± 1.09 e |
| ** | n.s. | ** |
| Sample | Time (Days) | Citric Acid | Lactic Acid | Acetic Acid | Propionic Acid | Isobutyric Acid | Butyric Acid |
|---|---|---|---|---|---|---|---|
| Control | 0 | 4172.9 ± 96.54 | 1606.6 ± 99.00 | 416.8 ± 97.31 | 3865.9 ± 268.47 | 3136.9 ± 188.31 | 0.00 ± 0.00 |
| Control | 21 | 4529.3 ± 100.00 de | 1219.2 ± 18.03 f | 326.3 ± 78.89 e | 3743.1 ± 34.21 g | 1654.9 ± 15.21 d | 566.4 ±48.79 d |
| L. plantarum | 21 | 7033.4 ± 15.76 a | 4512.6 ± 18.07 a | 7212.8 ± 82.59 a | 9802.4 ± 12.82 a | 3235.3 ± 5.51 a | 4666.4 ± 103.03 a |
| C. boidinii | 21 | 6624.4 ± 87.69 b | 4123.3 ± 20.03 b | 4568.4 ± 58.78 c | 9153.8 ± 19.41 b | 3202.7 ± 27.72 a | 4393.3 ± 44.23 a |
| W. anomalus | 21 | 5214.4 ± 121.00 c | 3774.5 ± 99.00 c | 6214.7 ± 168.83 b | 8219.2 ± 41.95 c | 2072.0 ± 77.52 b | 4239.4 ± 176.96 a |
| L.p + W.a | 21 | 4126.9 ± 106.79 f | 2846.0 ± 35.53 e | 6188.4 ± 85.52 b | 6831.2 ± 10.08 f | 1626.7 ± 1.41 e | 3682.8 ± 26.00 b |
| L.p + C.b | 21 | 4744.1 ± 16.31 d | 3167.5 ± 33.49 d | 4366.7 ± 132.82 c | 7913.0 ± 24.25 d | 2096.8 ± 16.35 b | 2995.9 ± 54.51 c |
| L.p + W.a + C.b | 21 | 4381.7 ± 20.88 ef | 3075.3 ± 31.95 d | 3334.0 ± 8.10 d | 7342.0 ± 116.15 e | 1810.2 ± 5.28 c | 3550.1 ± 25.58 b |
| Samples | Concentration of HT (mg/L) | Papp BA (nm/s) Passive Transport | Papp AB (nm/s) Active Transport | BA/AB | λ (nm) | ε |
|---|---|---|---|---|---|---|
| Control | 1.70 | 2581 | 457 | 4.22 | 275 | 0.80 |
| L. planturum | 9.50 | 4015 | 1014 | 3.95 | 285 | 0.20 |
| C. boidinii | 9.00 | 2540 | 575 | 4.41 | 275 | 0.09 |
| W. anomalus | 7.90 | 1958 | 367 | 5.34 | 285 | 0.22 |
| L.p + W.a | 8.00 | 1905 | 335 | 5.67 | 284 | 0.19 |
| L.p + C.b | 9.00 | 2912 | 1125 | 2.58 | 275 | 0.19 |
| L.p + W.a + C.b | 7.70 | 2587 | 522 | 4.95 | 283 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, P.; Occhipinti, P.S.; Russo, N.; Scilimati, A.; Miciaccia, M.; Caggia, C.; Perrone, M.G.; Randazzo, C.L.; Romeo, F.V. Olive Mill Wastewater Fermented with Microbial Pools as a New Potential Functional Beverage. Molecules 2023, 28, 646. https://doi.org/10.3390/molecules28020646
Foti P, Occhipinti PS, Russo N, Scilimati A, Miciaccia M, Caggia C, Perrone MG, Randazzo CL, Romeo FV. Olive Mill Wastewater Fermented with Microbial Pools as a New Potential Functional Beverage. Molecules. 2023; 28(2):646. https://doi.org/10.3390/molecules28020646
Chicago/Turabian StyleFoti, Paola, Paride S. Occhipinti, Nunziatina Russo, Antonio Scilimati, Morena Miciaccia, Cinzia Caggia, Maria Grazia Perrone, Cinzia L. Randazzo, and Flora V. Romeo. 2023. "Olive Mill Wastewater Fermented with Microbial Pools as a New Potential Functional Beverage" Molecules 28, no. 2: 646. https://doi.org/10.3390/molecules28020646
APA StyleFoti, P., Occhipinti, P. S., Russo, N., Scilimati, A., Miciaccia, M., Caggia, C., Perrone, M. G., Randazzo, C. L., & Romeo, F. V. (2023). Olive Mill Wastewater Fermented with Microbial Pools as a New Potential Functional Beverage. Molecules, 28(2), 646. https://doi.org/10.3390/molecules28020646













