Synthesis and Antimicrobial Activity Evaluation of Homodrimane Sesquiterpenoids with a Benzimidazole Unit
Abstract
1. Introduction
2. Results and Discussion
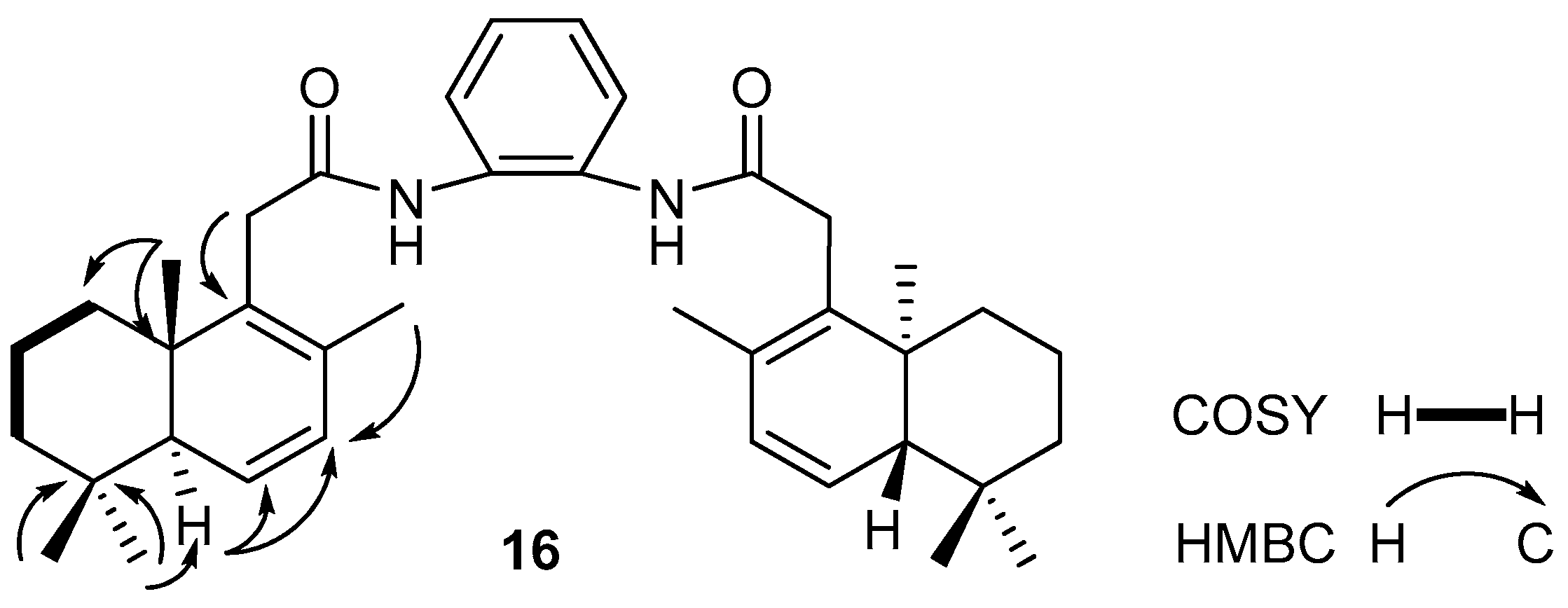
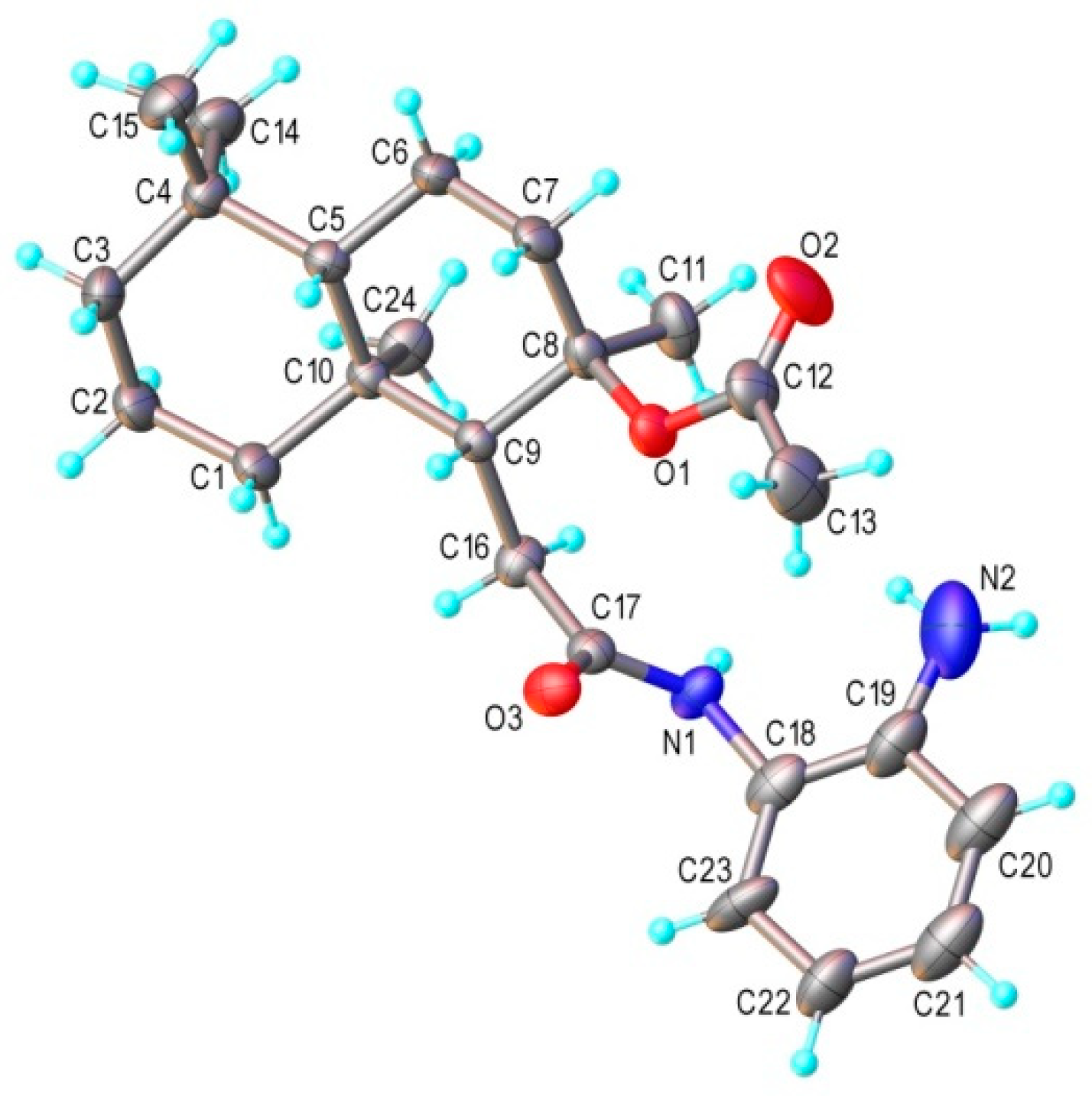
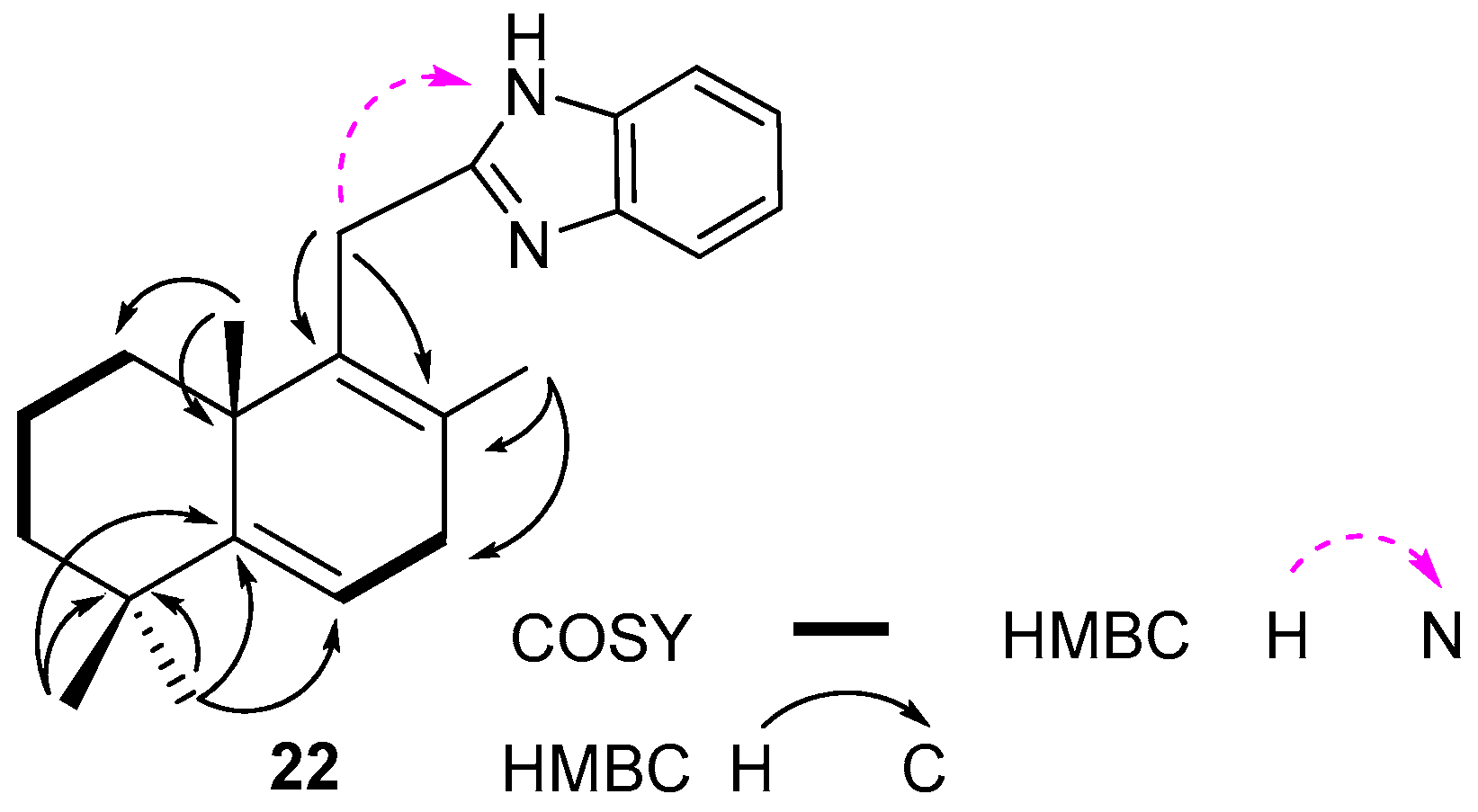
2.1. Synthesis and Characterization
2.2. Antimicrobial Activity
3. Materials and Methods
3.1. Synthesis and Characterization
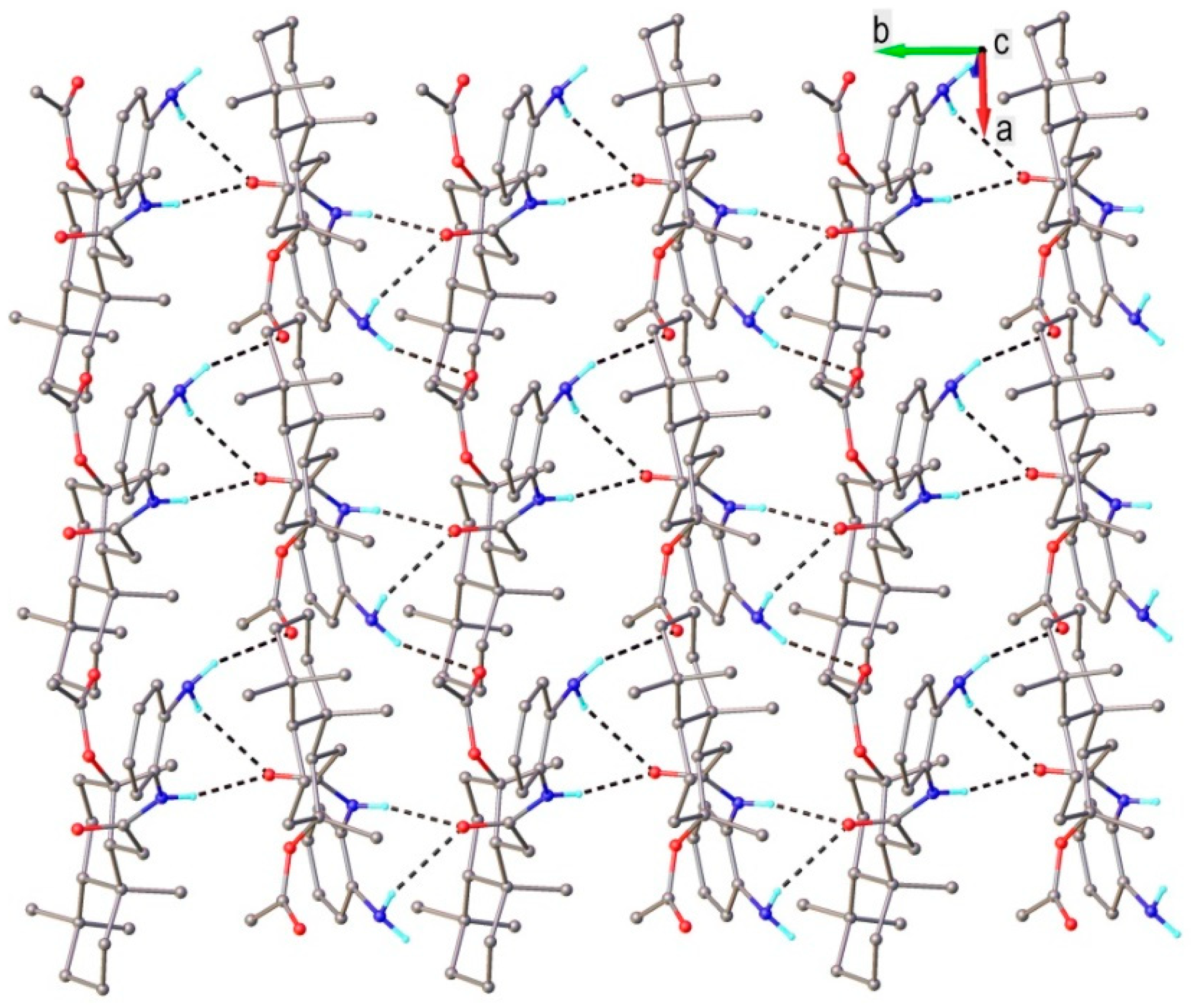
3.2. X-ray Crystallography
3.3. Antifungal and Antibacterial Activity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grahman, P.L. An Introduction to Medicinal Chemistry, 2nd ed.; Oxford University Press: New York, NY, USA, 2001; p. 646. ISBN 9780198505334/978-0198505334. [Google Scholar]
- Greenwood, D.; Finch, R.; Davey, P.; Wilcox, M. Antimicrobial Chemotherapy, 5th ed.; Oxford University Press: New York, NY, USA, 2007; p. 504. ISBN 0198570163/978-0198570165. [Google Scholar]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2013, 30, 1226–1264. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.J.M.; De Groot, A. Occurrence, biological activity and synthesis of drimane sesquiterpenoids. Nat. Prod. Rep. 2004, 21, 449–477. [Google Scholar] [CrossRef] [PubMed]
- Allouche, N.; Apel, C.; Martin, M.-T.; Dumontet, V.; Guéritte, F.; Litaudon, M. Cytotoxic sesquiterpenoids from Winteraceae of Caledonian rainforest. Phytochemistry 2009, 70, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhu, T.; Cai, S.; Gu, Q.; Li, D. Drimane sesquiterpenoids from the mangrove-derived Fungus Aspergillus ustus. Chem. Pharm. Bull. 2011, 59, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Lhinhatrakool, T.; Prabpai, S.; Kongsaeree, P.; Sutthivaiyakit, S. Antiplasmodial sesquiterpene alkaloids from the roots of Maytenus mekongensis. J. Nat. Prod. 2011, 74, 1386–1391. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Li, X.; Chen, W.; Xi, Z.; Sun, L. Three new sesquiterpenes from Tithonia diversifolia and their anti-hyperglycemic activity. Fitoterapia 2012, 83, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Komala, I.; Ito, T.; Nagashima, F.; Yagi, Y.; Asakawa, Y. Cytotoxic, radical scavenging and antimicrobial activities of sesquiterpenoids from the Tahitian liverwort Mastigophora diclados (Brid.) Nees (Mastigophoraceae). J. Nat. Med. 2010, 64, 417–422. [Google Scholar] [CrossRef]
- Pathare, B.; Bansode, T. Review—Biological active benzimidazole derivatives. Results Chem. 2021, 3, 100200. [Google Scholar] [CrossRef]
- Kuchkova, K.; Aricu, A.; Barba, A.; Ungur, N.; Tuchilus, C.; Shova, S.; Zbancioc, G.; Mangalagiu, I.I. An Efficient and Straightforward Method to New Organic Compounds: Homodrimane Sesquiterpenoids with Diazine Units. Synlett 2013, 24, 697–700. [Google Scholar]
- Kuchkova, K.; Aricu, A.; Secara, E.; Barba, A.; Vlad, P.; Ungur, N.; Tuchilus, C.; Shova, S.; Zbancioc, G.; Mangalagiu, I.I. Design, Synthesis, and Antimicrobial Activity of Some Novel Homodrimane Sesquiterpenoids with Diazine Skeleton. Med. Chem. Res. 2014, 23, 1559–1568. [Google Scholar] [CrossRef]
- Kuchkova, K.I.; Arycu, A.N.; Sekara, E.S.; Barba, A.N.; Vlad, P.F.; Makaev, F.Z.; Mel’nik, E.; Kravtsov, V.K. Synthesis and Structure of Homodrimane Sesquiterpenoids Containing 1,2,4-Triazole and Carbazole Rings. Chem. Nat. Compd. 2015, 51, 684–688. [Google Scholar] [CrossRef]
- Duca, G.; Aricu, A.; Lungu, L.; Tenu, N.; Ciocarlan, A.; Gutu, Y.; Dragalin, I.; Barba, A. Synthesis of New Homodrimane Sesquiterpenoids Containing Diazine, 1,2,4-Triazole and Carbazole Rings. Chem. J. Mold. 2018, 13, 69–73. [Google Scholar] [CrossRef]
- Aricu, A.; Ciocarlan, A.; Lungu, L.; Barba, A.; Shova, S.; Zbancioc, G.; Mangalagiu, I.I.; D’Ambrosio, M.; Vornicu, N. Synthesis, Antibacterial, and Antifungal Activities of New Drimane Sesquiterpenoids with Azaheterocyclic Units. Med. Chem. Res. 2016, 25, 2316–2323. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Aricu, A.; Lungu, L.; Edu, C.; Barba, A.; Shova, S.; Mangalagiu, I.I.; D’Ambrosio, M.; Nicolescu, A.; Deleanu, C.; et al. Synthesis of Novel Tetranorlabdane Derivatives with Unprecedented Carbon Skeleton. Synlett 2017, 28, 565–571. [Google Scholar] [CrossRef]
- Lungu, L.; Ciocarlan, A.; Barba, A.; Shova, S.; Pogrebnoi, S.; Mangalagiu, I.I.; Moldoveanu, C.; Vornicu, N.; D’Ambrosio, M.; Babak, M.V.; et al. Synthesis and Evaluation of Biological Activity of Homodrimane Sesquiterpenoids Bearing Hydrazinecarbothioamideor 1,2,4-Triazole Unit. Chem. Heterocycl. Compd. 2019, 55, 716–724. [Google Scholar] [CrossRef]
- Lungu, L.; Ciocarlan, A.; Smigon, C.; Ozer, I.; Shova, S.; Gutu, I.; Vornicu, N.; Mangalagiu, I.I.; D’Ambrosio, M.; Aricu, A. Synthesis and Evaluation of Biological Activity of Homodrimane Sesquiterpenoids Bearing 1,3,4-Oxadiazole or 1,3,4-Thiadiazole Units. Chem. Heterocycl. Compd. 2020, 56, 578–585. [Google Scholar] [CrossRef]
- Blaja, S.P.; Lungu, L.V.; Kuchkova, K.I.; Ciocarlan, A.G.; Barba, A.N.; Vornicu, N.; Aricu, A.N. Norlabdane Compounds Containing Thiosemicarbazone or 1,3-Thiazole Fragments: Synthesis and Antimicrobial Activity. Chem. Nat. Compd. 2021, 57, 101–110. [Google Scholar] [CrossRef]
- Lungu, L.; Cucicova, C.; Blaja, S.; Ciocarlan, A.; Dragalin, I.; Barba, A.; Vornicu, N.; Geana, E.-I.; Mangalagiu, I.I.; Aricu, A. Synthesis of Homodrimane Sesquiterpenoids Bearing1,3-Benzothiazole Unit and Their Antimicrobial Activity Evaluation. Molecules 2022, 27, 5082. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Lungu, L.; Blaja, S.; Dragalin, I.; Aricu, A. The Use of Some Non-Conventional Methods in Chemistry of Bicyclohomofarnesenic Methyl Esters. Chem. J. Mold. 2020, 15, 69–77. [Google Scholar] [CrossRef]
- Aricu, A.N.; Kuchkova, K.I.; Barba, A.N.; Dragalin, I.P.; Shova, S.G.; Vornicu, N.; Gorincioi, E.K.; Secara, E.S.; Lungu, L.V.; Niculaua, M.; et al. Synthesis from Norambreinolide, Structure, and Antimicrobial Activity of Dihomodrimane Sesquiterpenoids with Azine, Hydrazide, and Dihydrazide Fragments. Chem. Nat. Compd. 2016, 52, 1029–1036. [Google Scholar] [CrossRef]
- Barrero, A.F.; Alvarez-Manzaneda, E.J.; Chahboun, R.; González Díaz, C.C. New Routes Toward Drimanes and nor-Drimanes from (-)-Sclareol. Synlett 2000, 11, 1561–1564. [Google Scholar]
- Mariappan, G.; Hazarika, R.; Alam, F.; Karki, R.; Patangia, U.; Nath, S. Synthesis and Biological Evaluation of 2-Substituted Benzimidazole Derivatives. Arab. J. Chem. 2015, 8, 715–719. [Google Scholar] [CrossRef]
- Leonard, J.T.; Jeyaseeli, L.; Shivakumar, R.; Gunasekaran, V. Synthesis, Antiimflamatory and Antibacterial Activities of 2-Hydroxyphenyl Benzimidazoles. Asian J. Chem. 2005, 17, 2674–2678. [Google Scholar]
- Sharma, M.C.; Kohli, D.V.; Sharma, S.; Sharma, A.D. Synthesis and Antihypertensive Activity of Some New Benzimidazolederivatives of 4’-(6-Methoxy-2-Substituted-Benzimidazole-1-ylmethyl)-biphenyl-2-carboxylic Acid in the Presences of BF3·OEt2. Pharm. Sinica 2010, 1, 104–115. [Google Scholar]
- Ge, F.; Wang, Z.; Wan, W.; Lua, W.; Hao, J. One-Pot Synthesis of 2-Trifluoromethyl and 2-Difluoromethyl Substituted Benzo-1,3-diazoles. Tetrahedron Lett. 2007, 48, 3251–3254. [Google Scholar] [CrossRef]
- Charton, J.; Girault-Mizzi, S.; Sergheraert, C. Conversion of Sterically Hindered Diacylated 1,2-Phenylenediamines into 2-Substituted Benzimidazoles. Chem. Pharm. Bull. 2005, 53, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Susceptibility Testing (AST) Standards M07 and M100; National Committee on Clinical Laboratory Standards: Wayne, IL, USA, 2003.
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.41.64; Rigaku Corporation: Oxford, UK, 2015. [Google Scholar]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]



| Entry | Compound | MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Aspergillus niger | Fusarium solani | Penicillium chrysogenum | Penicillium frequentans | Alternaria alternata | Bacillus sp. | Pseudomonas aeruginosa | ||
| 1 | 4 | 1.60 * | 1.60 * | 1.60 * | 1.60 * | 1.60 * | 4.0 * | 4.0 * |
| 2 | 7 | 0.064 ** | 0.064 ** | 0.064 ** | 0.064 ** | 0.064 ** | 0.5 ** | 0.5 ** |
| 3 | 10 ** | 0.8 ** | 0.8 ** | 0.8 ** | 0.8 *** | 0.8 *** | 3.9 *** | 3.9 *** |
| 4 | 14 *4 | 1.04 *4 | 1.04 *4 | 1.04 *4 | 1.04 *4 | 1.04 *4 | 4.0 *4 | 4.0 *4 |
| 5 | 19 *5 | 1.16 *5 | 1.16 *5 | 1.16 *5 | 1.16 *5 | 1.16 *5 | 6.0 *5 | 6.0 *5 |
| 6 | 20 *6 | 0.05 *6 | 0.05 *6 | 0.05 *6 | 0.05 *6 | 0.05 *6 | 0.032 *6 | 0.032 *6 |
| 7 | Caspofungin | 0.32 | 0.32 | 0.32 | 0.32 | 0.32 | - | - |
| 8 | Kanamycin | - | - | - | - | - | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lungu, L.; Blaja, S.; Cucicova, C.; Ciocarlan, A.; Barba, A.; Kulcițki, V.; Shova, S.; Vornicu, N.; Geana, E.-I.; Mangalagiu, I.I.; et al. Synthesis and Antimicrobial Activity Evaluation of Homodrimane Sesquiterpenoids with a Benzimidazole Unit. Molecules 2023, 28, 933. https://doi.org/10.3390/molecules28030933
Lungu L, Blaja S, Cucicova C, Ciocarlan A, Barba A, Kulcițki V, Shova S, Vornicu N, Geana E-I, Mangalagiu II, et al. Synthesis and Antimicrobial Activity Evaluation of Homodrimane Sesquiterpenoids with a Benzimidazole Unit. Molecules. 2023; 28(3):933. https://doi.org/10.3390/molecules28030933
Chicago/Turabian StyleLungu, Lidia, Svetlana Blaja, Caleria Cucicova, Alexandru Ciocarlan, Alic Barba, Veaceslav Kulcițki, Sergiu Shova, Nicoleta Vornicu, Elisabeta-Irina Geana, Ionel I. Mangalagiu, and et al. 2023. "Synthesis and Antimicrobial Activity Evaluation of Homodrimane Sesquiterpenoids with a Benzimidazole Unit" Molecules 28, no. 3: 933. https://doi.org/10.3390/molecules28030933
APA StyleLungu, L., Blaja, S., Cucicova, C., Ciocarlan, A., Barba, A., Kulcițki, V., Shova, S., Vornicu, N., Geana, E.-I., Mangalagiu, I. I., & Aricu, A. (2023). Synthesis and Antimicrobial Activity Evaluation of Homodrimane Sesquiterpenoids with a Benzimidazole Unit. Molecules, 28(3), 933. https://doi.org/10.3390/molecules28030933








