Anti-Photoaging Effect of Phaseolus angularis L. Extract on UVB-Exposed HaCaT Keratinocytes and Possibilities as Cosmetic Materials
Abstract
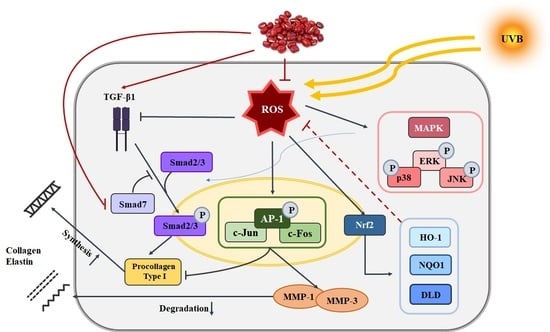
1. Introduction
2. Results
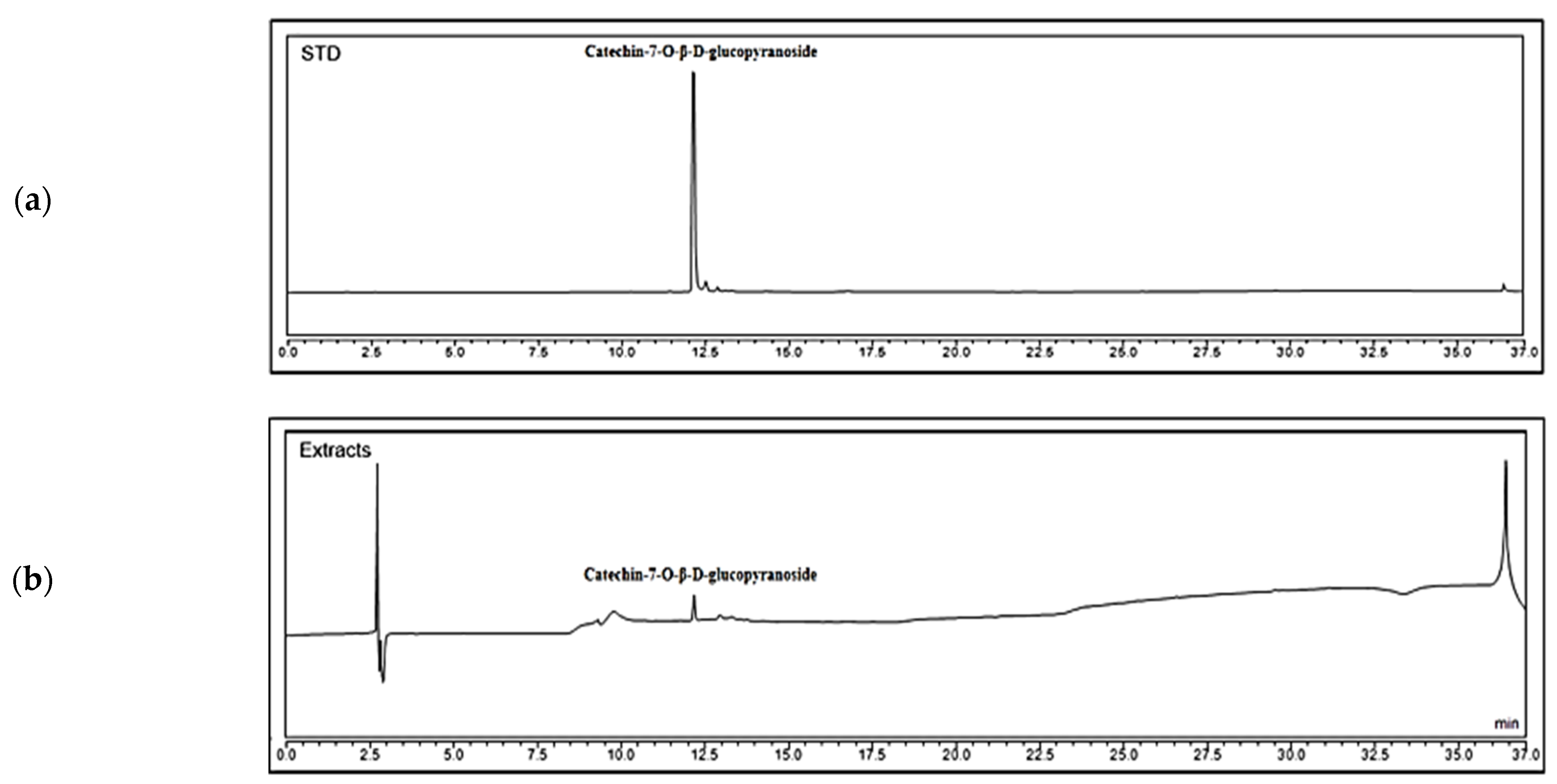
2.1. Analysis of Chemical Contents of PASE
2.2. Total Phenolic and Flavonoid Contents of PASE
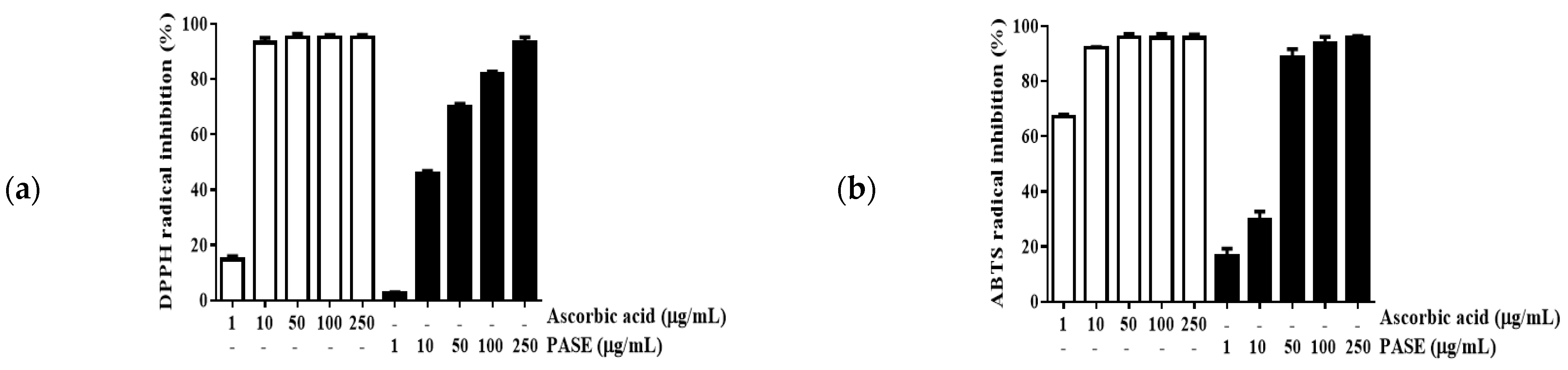
2.3. Antioxidative Activity of PASE
2.4. Toxicity and Cytoprotective Effects of PASE
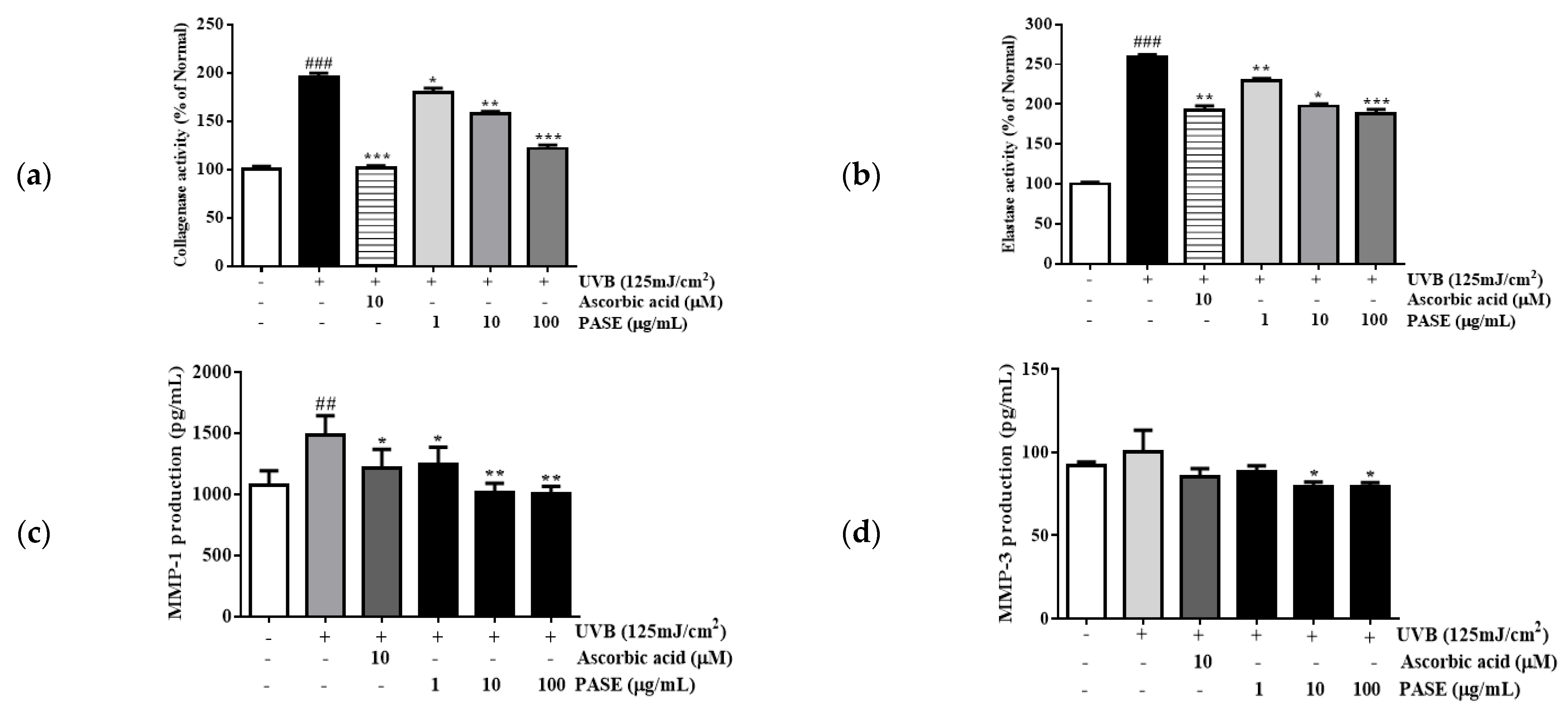
2.5. Effect of PASE on Collagenase and Elastase Inhibition in UVB-Exposed HaCaT Cells
2.6. Effect of PASE on MMP-1 and MMP-3 Secretion in UVB-Exposed HaCaT Cells
2.7. Effect of PASE on MMP-1, TGF-β1, and Procollagen Type I mRNA Expression in UVB-Exposed HaCaT Cells
2.8. Effect of PASE on TGF-β1/Smad7 Activation in UVB-Exposed HaCaT Cells
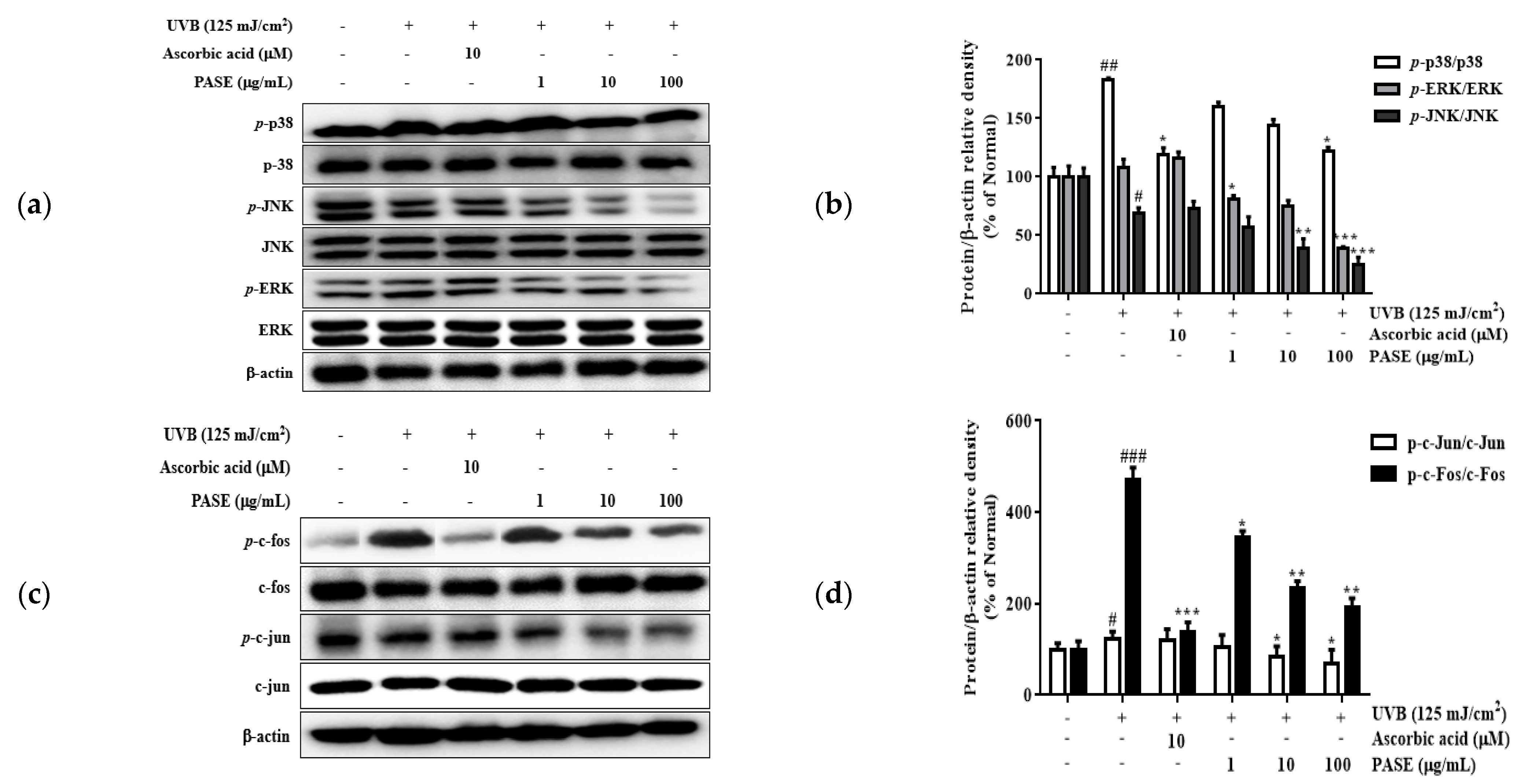
2.9. Effect of PASE on MAPK/AP-1 Phosphorylation in UVB-Exposed HaCaT Cells
2.10. Effect of PASE on Nrf2/ARE Translocation in UVB-Exposed HaCaT Cells
3. Discussion
4. Materials and Methods
4.1. Sample Preparations
4.2. High-Performance Liquid Chromatography (HPLC) Analysis
4.3. Total Phenolic and Flavonoid Contents
4.4. 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) and 2,2′-Azino-Bis-(3-Ethylbenzothiazoline)-6-Sulfonic Acid (ABTS) Radical Scavenging Activity
4.5. Cell Culture and Treatment
4.6. Cytotoxicity
4.7. Reactive Oxygen Species (ROS)
4.8. Collagenase and Elastase Inhibition Assay
4.9. Enzyme-Linked Immunosorbent Assay (ELISA)
4.10. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) Analysis
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Wlaschek, M.; Tantcheva-Poór, I.; Naderi, L.; Ma, W.; Schneider, L.A.; Razi-Wolf, Z.; Schüller, J.; Scharffetter-Kochanek, K. Solar UV irradiation and dermal photoaging. J. Photochem. Photobiol. B Biol. 2001, 63, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-R.; Noh, E.-M.; Han, J.-H.; Kim, J.-M.; Hwang, J.-K.; Hwang, B.-M.; Chung, E.-Y.; Kim, B.-S.; Lee, S.-H.; Lee, S.J.; et al. Brazilin inhibits UVB-induced MMP-1/3 expressions and secretions by suppressing the NF-κB pathway in human dermal fibroblasts. Eur. J. Pharmacol. 2012, 674, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Molavipordanjani, S.; Hosseinimehr, S.J. The Role of NF-κB Inhibitors in Cell Response to Radiation. Curr. Med. Chem. 2016, 23, 3951–3963. [Google Scholar] [CrossRef]
- Wu, T.-J.; Lin, C.-Y.; Tsai, C.-H.; Huang, Y.-L.; Tang, C.-H. Glucose suppresses IL-1β-induced MMP-1 expression through the FAK, MEK, ERK, and AP-1 signaling pathways. Environ. Toxicol. 2018, 33, 1061–1068. [Google Scholar] [CrossRef]
- Muscella, A.; Vetrugno, C.; Cossa, L.G.; Marsigliante, S. TGF-β1 activates RSC96 Schwann cells migration and invasion through MMP-2 and MMP-9 activities. J. Neurochem. 2020, 153, 525–538. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Panich, U. Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2. Front. Pharmacol. 2022, 13, 823881. [Google Scholar] [CrossRef]
- Hwang, E.; Ngo, H.T.; A Seo, S.; Park, B.; Zhang, M.; Yi, T.-H. Protective effect of dietary Alchemilla mollis on UVB-irradiated premature skin aging through regulation of transcription factor NFATc1 and Nrf2/ARE pathways. Phytomedicine 2018, 39, 125–136. [Google Scholar] [CrossRef]
- Choi, J.M.; Lee, S.I.; Cho, E.J. Effect of Vigna angularis on High-Fat Diet-Induced Memory and Cognitive Impairments. J. Med. Food 2020, 23, 1155–1162. [Google Scholar] [CrossRef]
- Li, H.; Zou, L.; Li, X.; Wu, D.; Liu, H.; Li, H.; Gan, R. Adzuki bean (Vigna angularis): Chemical compositions, physicochemical properties, health benefits, and food applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2335–2362. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.-M.; Wei, Q.-M.; Deng, W.-L.; Zheng, Y.-L.; Chen, X.-Y.; Huang, Q.; Ou-Yang, C.; Peng, Y.-Y. Anti-melanogenesis properties of condensed tannins from Vigna angularis seeds with potent antioxidant and DNA damage protection activities. Food Funct. 2019, 10, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Mukai, Y.; Yamate, J.; Kato, J.; Kurasaki, M.; Hatai, A.; Sagai, M. Effect of polyphenol-containing azuki bean (Vigna angularis) extract on blood pressure elevation and macrophage infiltration in the heart and kidney of spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2008, 35, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Kobayashi, M.; Horio, F.; Furuichi, Y. Hypoglycemic effect of hot-water extract of adzuki (Vigna angularis) in spontaneously diabetic KK-A(y) mice. Nutrition 2009, 25, 134–141. [Google Scholar] [CrossRef]
- Kawahara, S.-I.; Ishihara, C.; Matsumoto, K.; Senga, S.; Kawaguchi, K.; Yamamoto, A.; Suwannachot, J.; Hamauzu, Y.; Makabe, H.; Fujii, H. Identification and characterization of oligomeric proanthocyanidins with significant anti-cancer activity in adzuki beans (Vigna angularis). Heliyon 2019, 5, e02610. [Google Scholar] [CrossRef]
- Mukai, Y.; Sato, S. Polyphenol-containing azuki bean (Vigna angularis) seed coats attenuate vascular oxidative stress and inflammation in spontaneously hypertensive rats. J. Nutr. Biochem. 2011, 22, 16–21. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-Degrading Metalloproteinases in Photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef]
- Salma, U.; Xue, M.; Sheikh, S.A.; Guan, X.; Xu, B.; Zhang, A.; Huang, L.; Xu, D. Role of Transforming Growth Factor-β1 and Smads Signaling Pathway in Intrauterine Adhesion. Mediat. Inflamm. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Hwang, E.; Ngo, H.T.T.; Perumalsamy, H.; Kim, Y.J.; Li, L.; Yi, T.-H. Protective Effects of Euphrasia officinalis Extract against Ultraviolet B-Induced Photoaging in Normal Human Dermal Fibroblasts. Int. J. Mol. Sci. 2018, 19, 3327. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Park, S.Y.; Hwang, E.; Zhang, M.; Seo, S.A.; Lin, P.; Yi, T. Thymus vulgaris alleviates UVB irradiation induced skin damage via inhibition of MAPK/AP-1 and activation of Nrf2-ARE antioxidant system. J. Cell. Mol. Med. 2017, 21, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ha, L.-K.; Oh, S.; Fang, M.; Zheng, S.; Bellere, A.D.; Jeong, J.; Yi, T.-H. Antiphotoaging Effects of Damiana (Turnera diffusa) Leaves Extract via Regulation AP-1 and Nrf2/ARE Signaling Pathways. Plants 2022, 11, 1486. [Google Scholar] [CrossRef]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, J.-H.; Tu, X.-L.; Zhang, C.; Zhao, M.; Zhang, Q.-W.; Gao, F.-H. Tiron Inhibits UVB-Induced AP-1 Binding Sites Transcriptional Activation on MMP-1 and MMP-3 Promoters by MAPK Signaling Pathway in Human Dermal Fibroblasts. PLoS ONE 2016, 11, e0159998. [Google Scholar] [CrossRef]
- Benbow, U.; Brinckerhoff, C.E. The AP-1 site and MMP gene regulation: What is all the fuss about? Matrix Biol. 1997, 15, 519–526. [Google Scholar] [CrossRef]
- Plotnikov, A.; Zehorai, E.; Procaccia, S.; Seger, R. The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim. Biophys. Acta 2011, 1813, 1619–1633. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Cho, J.-G.; Hwang, E.-S.; Yang, J.-E.; Gao, W.; Fang, M.-Z.; Zheng, S.-D.; Yi, T.-H. Enhancement of Protective Effects of Radix Scutellariae on UVB-induced Photo Damage in Human HaCaT Keratinocytes. Appl. Biochem. Biotechnol. 2017, 184, 1073–1093. [Google Scholar] [CrossRef]
- Johnston, E.F.; Gillis, T.E. Transforming growth factor beta-1 (TGF-β1) stimulates collagen synthesis in cultured rainbow trout cardiac fibroblasts. J. Exp. Biol. 2017, 220 Pt 14, 2645–2653. [Google Scholar] [CrossRef]
- Choi, S.-I.; Han, H.-S.; Kim, J.-M.; Park, G.; Jang, Y.-P.; Shin, Y.-K.; Ahn, H.-S.; Lee, S.-H.; Lee, K.-T. Eisenia bicyclis Extract Repairs UVB-Induced Skin Photoaging In Vitro and In Vivo: Photoprotective Effects. Mar. Drugs 2021, 19, 693. [Google Scholar] [CrossRef] [PubMed]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, S.; Zheng, S.; Fang, M.; Kim, M.; Bellere, A.D.; Jeong, J.; Yi, T.-H. Anti-Photoaging Effect of Phaseolus angularis L. Extract on UVB-Exposed HaCaT Keratinocytes and Possibilities as Cosmetic Materials. Molecules 2023, 28, 1407. https://doi.org/10.3390/molecules28031407
Oh S, Zheng S, Fang M, Kim M, Bellere AD, Jeong J, Yi T-H. Anti-Photoaging Effect of Phaseolus angularis L. Extract on UVB-Exposed HaCaT Keratinocytes and Possibilities as Cosmetic Materials. Molecules. 2023; 28(3):1407. https://doi.org/10.3390/molecules28031407
Chicago/Turabian StyleOh, Sarang, Shengdao Zheng, Minzhe Fang, Myeongju Kim, Arce Defeo Bellere, Jeehaeng Jeong, and Tae-Hoo Yi. 2023. "Anti-Photoaging Effect of Phaseolus angularis L. Extract on UVB-Exposed HaCaT Keratinocytes and Possibilities as Cosmetic Materials" Molecules 28, no. 3: 1407. https://doi.org/10.3390/molecules28031407
APA StyleOh, S., Zheng, S., Fang, M., Kim, M., Bellere, A. D., Jeong, J., & Yi, T.-H. (2023). Anti-Photoaging Effect of Phaseolus angularis L. Extract on UVB-Exposed HaCaT Keratinocytes and Possibilities as Cosmetic Materials. Molecules, 28(3), 1407. https://doi.org/10.3390/molecules28031407