The Cardioprotective Effect of Corosolic Acid in the Diabetic Rats: A Possible Mechanism of the PPAR-γ Pathway
Abstract
1. Introduction
2. Results
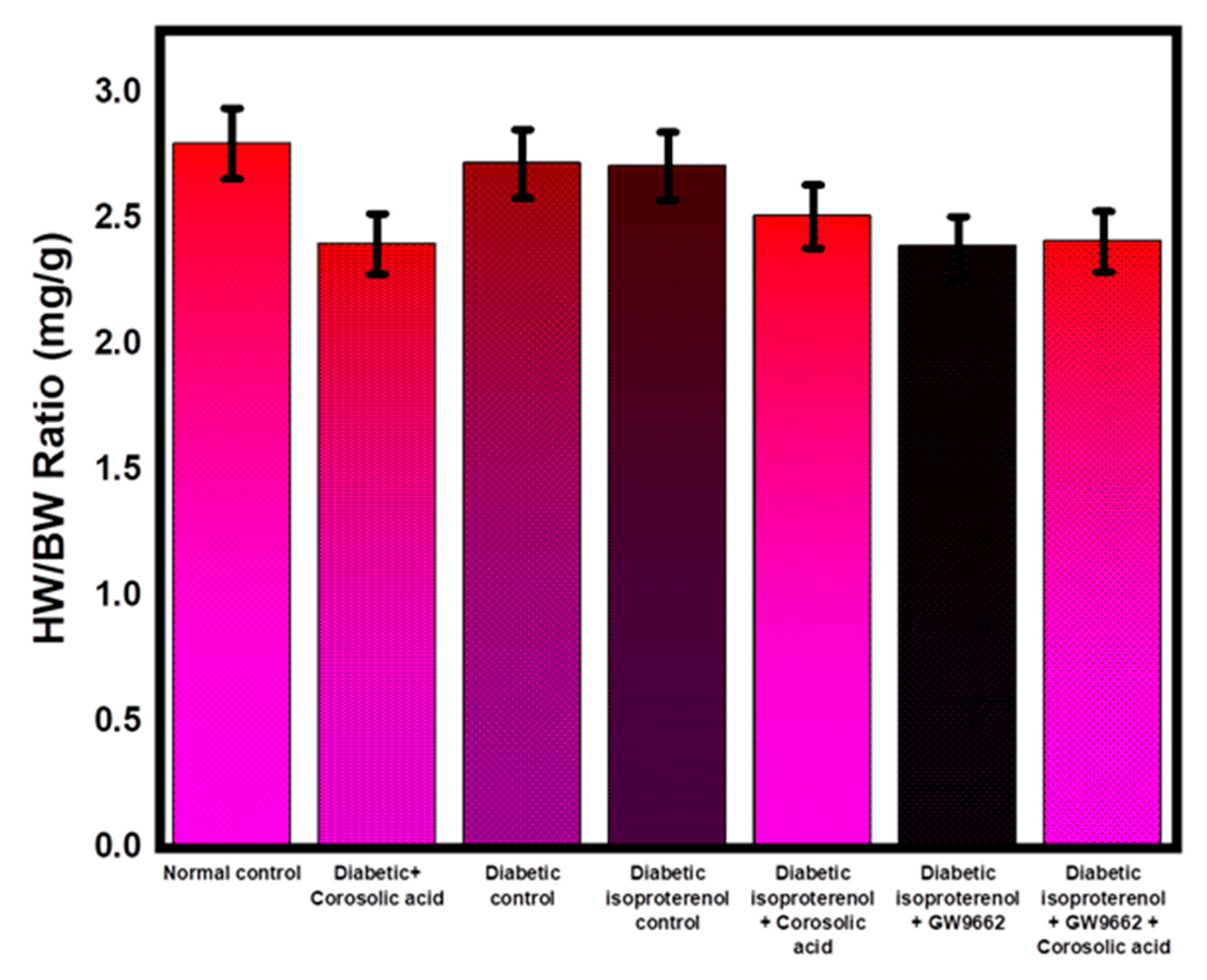
2.1. Effect of Corosolic Acid on Body Weights and Heart Weights
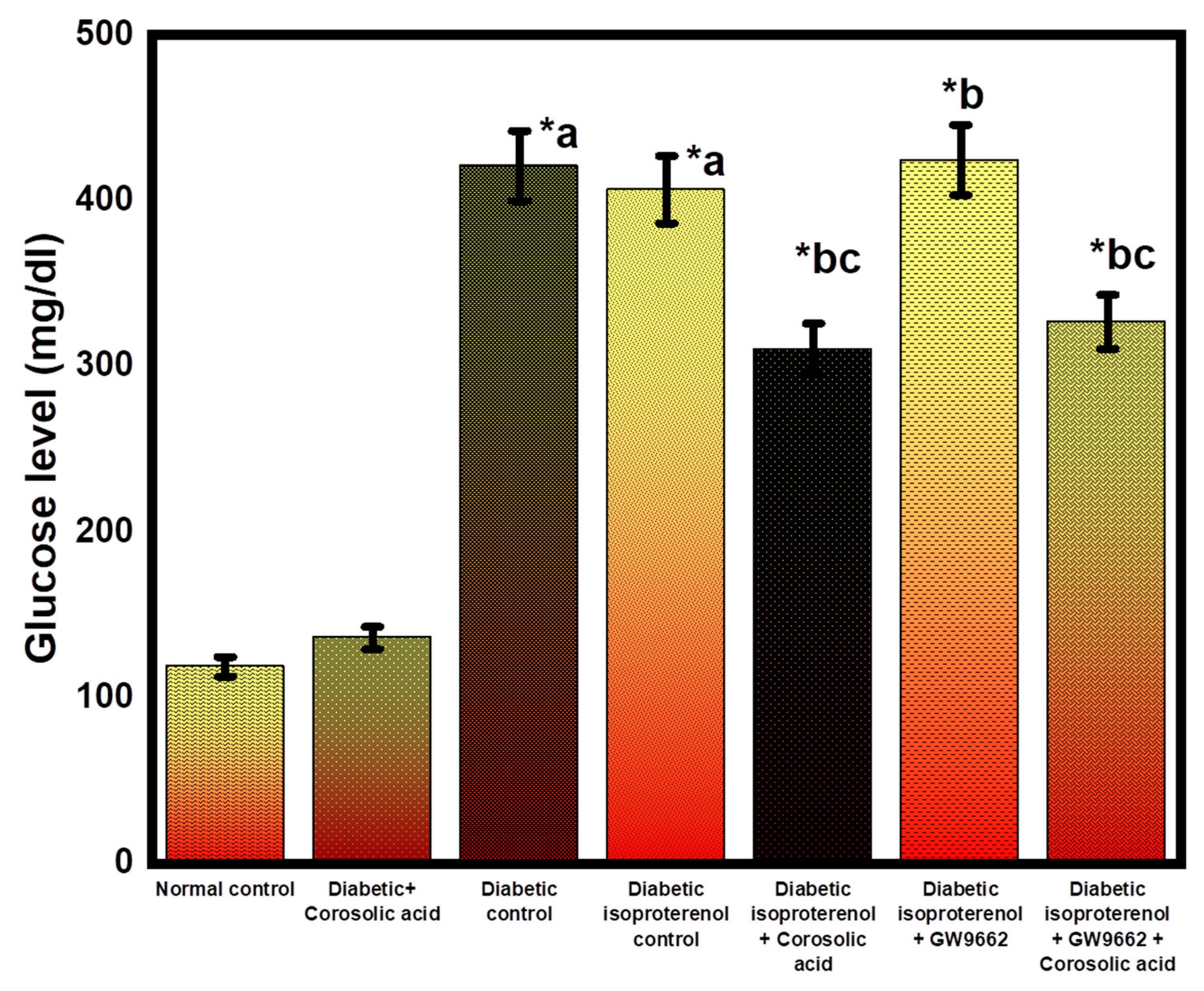
2.2. Effect of Corosolic Acid on Blood Glucose Level
2.3. Effect of Corosolic Acid on the Hemodynamic Parameters and Heart Rate
2.4. Effect of Corosolic Acid on Cardiac Injury Markers
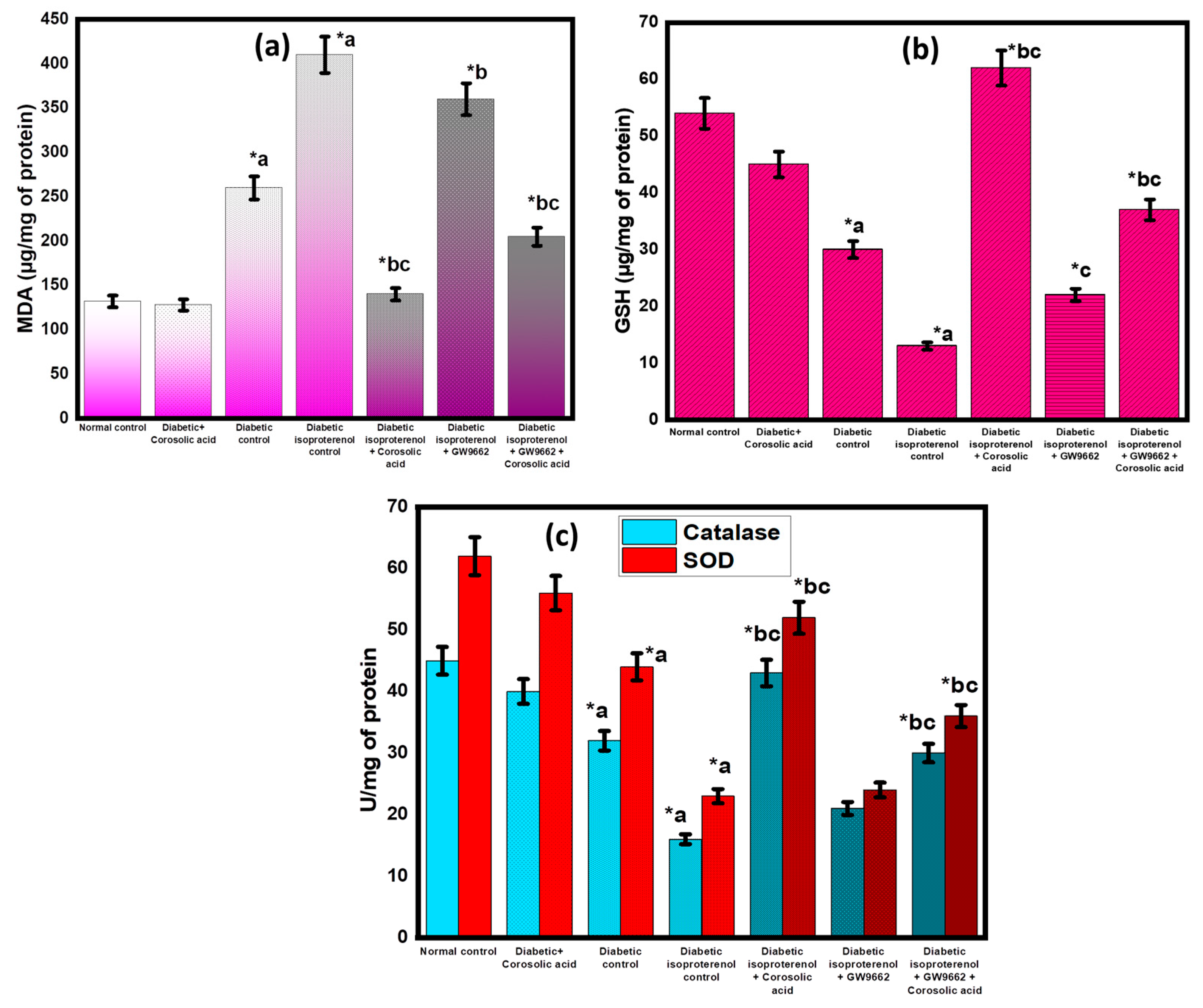
2.5. Effect of Corosolic Acid on Antioxidant Parameters
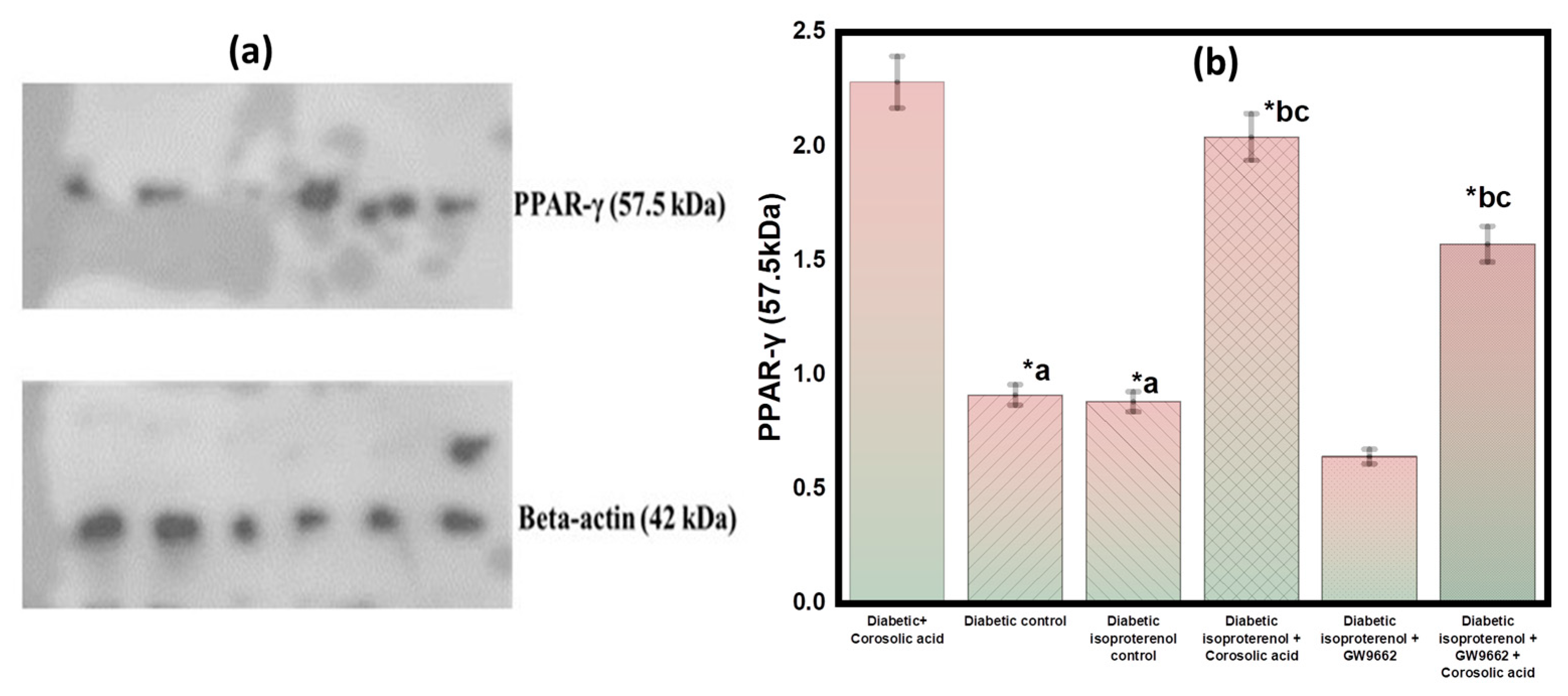
2.6. Effect of Corosolic Acid on PPAR-γ Expression in Different Groups
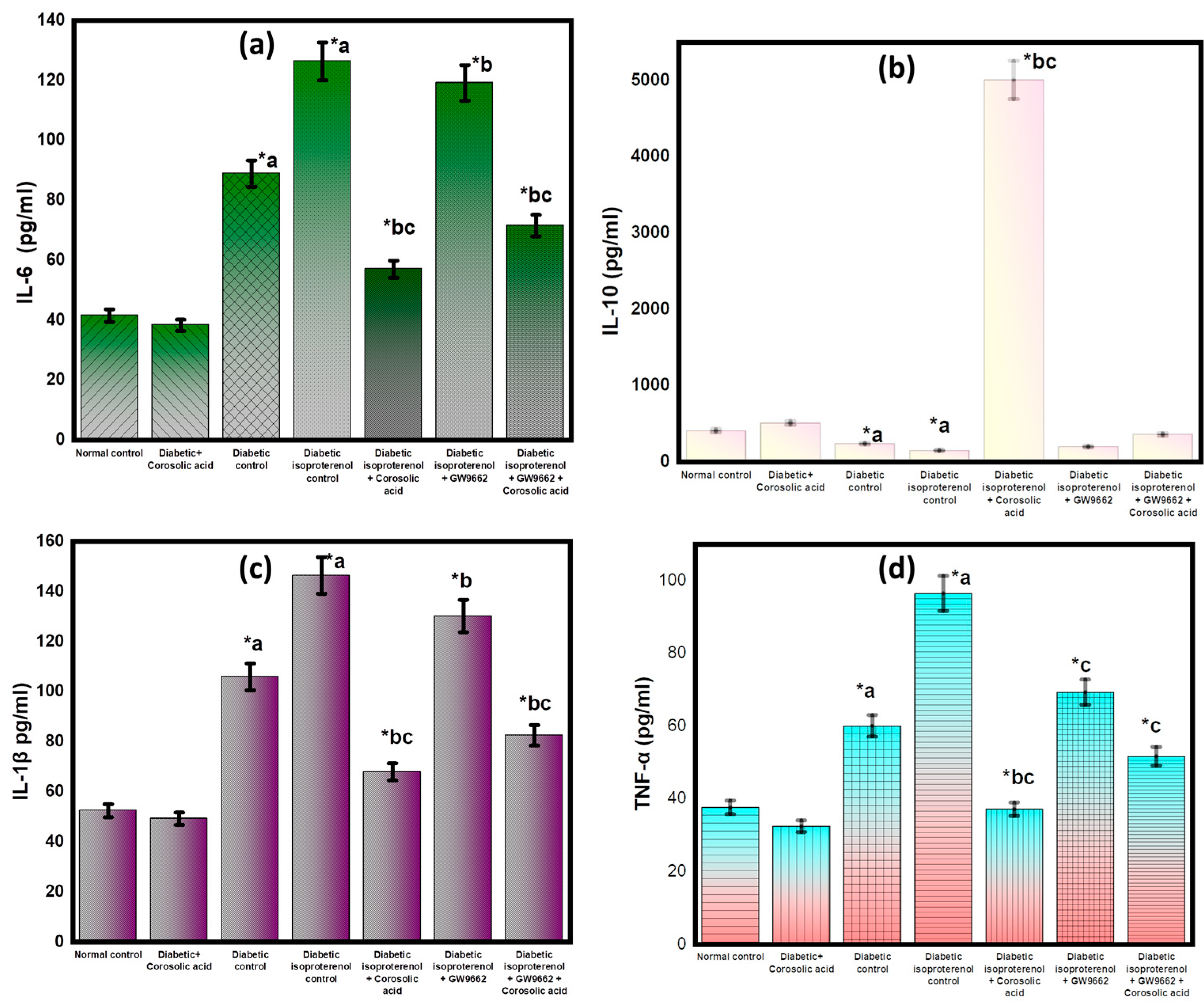
2.7. Effect of Corosolic Acid on Inflammatory Cytokines
2.8. Histopathological Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Experimental Study
4.2.1. Induction of Diabetes
4.2.2. Estimation of Food and Water Consumption
4.2.3. Estimation of Heart Rate and Blood Pressure
4.2.4. Estimation of the Cardiac Injury Markers and Oxidative Stress
4.2.5. Estimation of Lipid Peroxidation (MDA Content)
4.2.6. Estimation of Glutathione Content
4.2.7. Estimation of Catalase Activity
4.2.8. Estimation of Superoxide Dismutase Activity
4.2.9. Western Blot Analysis for PPAR-γ
4.2.10. Measurement of Inflammatory Cytokines by Enzyme-Linked Immunosorbent Assay (ELISA)
4.2.11. Histopathological Analysis
4.2.12. Statistical Analysis
5. Conclusions
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Morrow, D.A. Myocardial Infarction: A Companion to Braunwald’s Heart Disease; e-book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Mathur, M.; Wiegers, S.; Todaro, M.C.; Zito, C.; Carerj, S.; Khandheria, B.K.; Peters, F.; Payvandi, L.A.; Rigolin, V.H.; Quader, N.; et al. Introduction and Echocardiographic Features of Infective Endocarditis. In ASE’s Comprehensive Echocardiography; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Deb, P.; Sharma, S.; Hassan, K. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology 2010, 17, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, S.L.; Lundergan, C.F.; Reiner, J.S.; Greenhouse, S.W.; Thompson, M.A.; Rohrbeck, S.C.; Deychak, Y.; Simoons, M.L.; Califf, R.M.; Topol, E.J.; et al. Angiographic Findings and Outcome in Diabetic Patients Treated With Thrombolytic Therapy for Acute Myocardial Infarction: The GUSTO-I Experience. J. Am. Coll. Cardiol. 1996, 28, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Melendo-Viu, M.; Abu-Assi, E.; Manzano-Fernández, S.; Flores-Blanco, P.J.; Cambronero-Sánchez, F.; Pérez, D.D.; Fernández, M.C.; Galian, M.J.S.; Molina, M.G.; Caneiro-Queija, B.; et al. Incidence, prognosis and predictors of heart failure after acute myocardial infarction. REC CardioClinics 2019, 55, 8–14. [Google Scholar] [CrossRef]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart failure after myocardial infarction: Incidence and predictors. ESC Heart Fail. 2020, 8, 222–237. [Google Scholar] [CrossRef]
- Mátyás, C.; Németh, B.T.; Oláh, A.; Török, M.; Ruppert, M.; Kellermayer, D.; Barta, B.A.; Szabó, G.T.; Kokeny, G.; Horvath, E.M.; et al. Prevention of the development of heart failure with preserved ejection fraction by the phosphodiesterase-5A inhibitor vardenafil in rats with type 2 diabetes. Eur. J. Heart Fail. 2016, 19, 326–336. [Google Scholar] [CrossRef]
- Varma, U.; Koutsifeli, P.; Benson, V.; Mellor, K.; Delbridge, L. Molecular mechanisms of cardiac pathology in diabetes—Experimental insights. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2018, 1864, 1949–1959. [Google Scholar] [CrossRef]
- Santos, J.C.D.F.; Valentim, I.B.; De Araújo, O.R.P.; Ataide, T.D.R.; Goulart, M.O.F. Development of Nonalcoholic Hepatopathy: Contributions of Oxidative Stress and Advanced Glycation End Products. Int. J. Mol. Sci. 2013, 14, 19846. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications review-Article. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef]
- Wu, L.; Guo, C.; Wu, J. Therapeutic potential of PPARγ natural agonists in liver diseases. J. Cells Mol. Med. 2020, 24, 2736–2748. [Google Scholar] [CrossRef]
- Liu, C.; Feng, T.; Zhu, N.; Liu, P.; Han, X.; Chen, M.; Wang, X.; Li, N.; Li, Y.; Xu, Y.; et al. Erratum: Corrigendum: Identification of a novel selective agonist of PPARγ with no promotion of adipogenesis and less inhibition of osteoblastogenesis. Sci. Rep. 2015, 5, 12185. [Google Scholar] [CrossRef]
- Arnold, R.; Neumann, M.; König, W. Peroxisome proliferator-activated receptor-? agonists inhibit respiratory syncytial virus-induced expression of intercellular adhesion molecule-1 in human lung epithelial cells. Immunology 2007, 121, 71–81. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, X.; Li, D. Modulation of Peroxisome Proliferator-Activated Receptor gamma (PPAR γ) by Conjugated Fatty Acid in Obesity and Inflammatory Bowel Disease. J. Agric. Food Chem. 2015, 63, 1883–1895. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Díaz, A.G. Oxidative stress, apoptosis, and mitochon-drial function in diabetic nephropathy. Int. J. Endocrinol. 2018, 2018. [Google Scholar] [CrossRef]
- Dobi, A.; Bravo, S.B.; Veeren, B.; Paradela-Dobarro, B.; Álvarez, E.; Meilhac, O.; Viranaicken, W.; Baret, P.; Devin, A.; Rondeau, P. Advanced glycation end-products disrupt human endothelial cells redox homeostasis: New insights into reactive oxygen species production. Free. Radic. Res. 2019, 53, 150–169. [Google Scholar] [CrossRef]
- Bell, D.S.H. Heart failure: The frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 2003, 26, 2433–2441. [Google Scholar] [CrossRef]
- Garcia-Vallve, S.; Guasch, L.; Mulero, M. Discovery of natural products that modulate the activity of PPARgamma: A source for new antidiabetics. In Foodinformatics: Applications of Chemical Information to Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Qian, X.-P.; Zhang, X.-H.; Sun, L.-N.; Xing, W.-F.; Wang, Y.; Sun, S.-Y.; Ma, M.-Y.; Cheng, Z.-P.; Wu, Z.-D.; Xing, C.; et al. Corosolic acid and its structural analogs: A systematic review of their biological activities and underlying mechanism of action. Phytomedicine 2021, 91, 153696. [Google Scholar] [CrossRef]
- Li, B.-B.; Pang, K.; Hao, L.; Zang, G.-H.; Wang, J.; Wang, X.-T.; Zhang, J.-J.; Cai, L.-J.; Yang, C.-D.; Han, C.-H. Corosolic acid improves erectile function in metabolic syndrome rats by reducing reactive oxygen species generation and increasing nitric oxide bioavailability. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, H.; Yanan, A.N.; Shen, K.; Lu, Y.U. Biological effects of corosolic acid as an anti-inflammatory, anti-metabolic syndrome and anti-neoplasic natural compound (Review). Oncol. Lett. 2020, 21, 84. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Komohara, Y.; Ikeda, T.; Takeya, M. Corosolic acid inhibits glioblastoma cell proliferation by suppressing the activation of signal transducer and activator of transcription-3 and nuclear factor-kappa B in tumor cells and tumor-associated macrophages. Cancer Sci. 2011, 102, 206–211. [Google Scholar]
- Pari, L.; Monisha, P.; Jalaludeen, A.M. Beneficial role of diosgenin on oxidative stress in aorta of streptozotocin induced diabetic rats. Eur. J. Pharmacol. 2012, 691, 143–150. [Google Scholar] [CrossRef]
- Abbas, A.M. Cardioprotective effect of resveratrol analogue isorhapontigenin versus omega-3 fatty acids in isoproterenol-induced myocardial infarction in rats. J. Physiol. Biochem. 2016, 72, 469–484. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Goyal, S.N.; Suchal, K.; Sharma, C.; Patil, C.R.; Ojha, S.K. Pharmacological Properties, Molecular Mechanisms, and Pharmaceutical Development of Asiatic Acid: A Pentacyclic Triterpenoid of Therapeutic Promise. Front. Pharmacol. 2018, 9, 892. [Google Scholar] [CrossRef]
- Dianita, R.; Jantan, I.; Amran, A.Z.; Jalil, J. Protective Effects of Labisia pumila var. alata on Biochemical and Histopathological Alterations of Cardiac Muscle Cells in Isoproterenol-Induced Myocardial Infarction Rats. Molecules 2015, 20, 4746. [Google Scholar] [CrossRef]
- Saxena, P.; Panjwani, D. Cardioprotective potential of hydro-alcoholic fruit extract of Ananas comosus against isoproterenol induced myocardial infraction in Wistar Albino rats. J. Acute Dis. 2014, 3, 228–234. [Google Scholar] [CrossRef]
- Lalitha, G.; Poornima, P.; Archanah, A.; Padma, V.V. Protective Effect of Neferine against Isoproterenol-Induced Cardiac Toxicity. Cardiovasc. Toxicol. 2012, 13, 168–179. [Google Scholar] [CrossRef]
- El-Sayyad, S.M.; Soubh, A.A.; Awad, A.S.; El-Abhar, H.S. Mangiferin protects against intestinal ischemia/reperfusion-induced liver injury: Involvement of PPAR-γ, GSK-3β and Wnt/β-catenin pathway. Eur. J. Pharmacol. 2017, 809, 80–86. [Google Scholar] [CrossRef]
- Rasheed, N.O.A.; Ibrahim, W.W. Telmisartan neuroprotective effects in 3-nitropropionic acid Huntington’s disease model in rats: Cross talk between PPAR-γ and PI3K/Akt/GSK-3β pathway. Life Sci. 2022, 297, 120480. [Google Scholar] [CrossRef]
- Gendy, A.M.; Amin, M.M.; Al-Mokaddem, A.K.; Ellah, M.F.A. Cilostazol mitigates mesenteric ischemia/reperfusion-induced lung lesion: Contribution of PPAR-γ, NF-κB, and STAT3 crosstalk. Life Sci. 2020, 266, 118882. [Google Scholar] [CrossRef]
- Laveti, D.; Kumar, M.; Hemalatha, R.; Sistla, R.; Naidu, V.; Talla, V.; Verma, V.; Kaur, N.; Nagpal, R. Anti-Inflammatory Treatments for Chronic Diseases: A Review. Inflamm. Allergy-Drug Targets 2013, 12, 349–361. [Google Scholar] [CrossRef]
- Hallakou-Bozec, S.; Vial, G.; Kergoat, M.; Fouqueray, P.; Bolze, S.; Borel, A.; Fontaine, E.; Moller, D.E. Mechanism of action of Imeglimin: A novel therapeutic agent for type 2 diabetes. Diabetes Obes. Metab. 2020, 23, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-P.; Che, Y.; Zhou, H.; Meng, Y.-Y.; Wu, H.-M.; Jin, Y.-G.; Wu, Q.-Q.; Wang, S.-S.; Yuan, Y. Corosolic acid attenuates cardiac fibrosis following myocardial infarction in mice. Int. J. Mol. Med. 2020, 45, 1425–1435. [Google Scholar] [CrossRef]
- Higgins, L.S.; DePaoli, A.M. Selective peroxisome proliferator-activated receptor γ (PPARγ) modulation as a strategy for safer therapeutic PPARγ activation. Am. J. Clin. Nutr. 2009, 91, 267S–272S. [Google Scholar] [CrossRef]
- Bhalla, K.; Hwang, B.J.; Choi, J.H.; Dewi, R.; Ou, L.; Mclenithan, J.; Twaddel, W.; Pozharski, E.; Stock, J.; Girnun, G.D. N-Acetylfarnesylcysteine Is a Novel Class of Peroxisome Proliferator-activated Receptor γ Ligand with Partial and Full Agonist Activity in vitro and in vivo. J. Biol. Chem. 2011, 286, 41626–41635. [Google Scholar] [CrossRef]
- Szebeni, G.J.; Vizler, C.; Kitajka, K.; Puskas, L.G. Inflammation and Cancer: Extra- and Intracellular Determinants of Tumor-Associated Macrophages as Tumor Promoters. Mediat. Inflamm. 2017, 2017, 9294018. [Google Scholar] [CrossRef]
- Sivarajah, A.; McDonald, M.C.; Thiemermann, C. The Cardioprotective Effects of Preconditioning with Endotoxin, but Not Ischemia, Are Abolished by a Peroxisome Proliferator-Activated Receptor-γ Antagonist. J. Pharmacol. Exp. Ther. 2005, 313, 896–901. [Google Scholar] [CrossRef]
- Plehm, R.; Barbosa, M.E.; Bader, M. Animal Models for Hypertension/Blood Pressure Recording; Humana Press: Totowa, NJ, USA, 2006; Volume 129, pp. 115–126. [Google Scholar] [CrossRef]
- Suchal, K.; Malik, S.; Gamad, N.; Malhotra, R.K.; Goyal, S.N.; Bhatia, J.; Arya, D.S. Kampeferol protects against oxidative stress and apoptotic damage in experimental model of isoproterenol-induced cardiac toxicity in rats. Phytomedicine 2016, 23, 1401–1408. [Google Scholar] [CrossRef]
- Buwa, C.C.; Mahajan, U.B.; Patil, C.R.; Goyal, S.N. Apigenin Attenuates β-Receptor-Stimulated Myocardial Injury via Safeguarding Cardiac Functions and Escalation of Antioxidant Defence System. Cardiovasc. Toxicol. 2015, 16, 286–297. [Google Scholar] [CrossRef]
- Salbitani, G.; Bottone, C.; Carfagna, S. Determination of Reduced and Total Glutathione Content in Extremophilic Microalga Galdieria phlegrea. Bio-Protocol 2017, 7, e2372. [Google Scholar] [CrossRef]
- Sah, D.; Sah, D.K.; Nagarathana, P.K. Screening of cardioprotective activity of leaves of Andrographis paniculata against isoproterenol induced myocardial infarction in rats. Int. J. Pharmacol. Res. 2016, 6, 23–28. [Google Scholar]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of Superoxide Dismutase (SOD). In Plant-microbe interactions; Humana Press: New York, NY, USA, 2020; pp. 117–118. [Google Scholar]
- Rai, M.; Curley, M.; Coleman, Z.; Nityanandam, A.; Jiao, J.; Graca, F.A.; Hunt, L.C.; Demontis, F. Analysis of proteostasis during aging with western blot of detergent-soluble and insoluble protein fractions. STAR Protoc. 2021, 2, 100628. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.X.; McElhaney, J.E.; Walston, J.D.; Xie, D.; Fedarko, N.S.; Kuchel, G.A. ELISA and Multiplex Technologies for Cytokine Measurement in Inflammation and Aging Research. J. Gerontol. Ser. A 2008, 63, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Kotb, N.; Elfatah, A.; Hasanin, A.H.; Fekry, N.; Hendawy, A. Dose Response Effect of Nitroglycerin on Cardiac Hemodynamic Functions and Myocardial Infarction in a Rat Model of Ischemia Reperfusion. Egypt. J. Hosp. Med. 2018, 71, 3142–3147. [Google Scholar]
- Najar, I.A.; Bhat, M.H.; Qadrie, Z.L.; Amaldoss, M.J.N.; Kushwah, A.S.; Singh, T.G.; Kabra, A.; Khan, N.; Kumar, M. Cardioprotection by Citrus grandis (L.) Peel Ethanolic Extract in Alloxan-Induced Cardiotoxicity in Diabetic Rats. BioMed. Res. Int. 2022, 2022, 2807337. [Google Scholar] [CrossRef]
- Kushwah, A.S.; Mittal, R.; Kumar, M.; Kaur, G.; Goel, P.; Sharma, R.K.; Kabra, A.; Nainwal, L.M. Cardioprotective Activity of Cassia fistula L. Bark Extract in Isoproterenol-Induced Myocardial Infarction Rat Model. Evid. Based Complement. Altern. Med. 2022, 2022, 6874281. [Google Scholar] [CrossRef]
- Khumaedi, A.I.; Purnamasari, D.; Wijaya, I.P.; Soeroso, Y. The relationship of diabetes, periodontitis and cardiovascular disease. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1675–1678. [Google Scholar] [CrossRef]
- Mahajan, U.B.; Chandrayan, G.; Patil, C.R.; Arya, D.S.; Suchal, K.; Agrawal, Y.O.; Ojha, S.; Goyal, S.N. The Protective Effect of Apigenin on Myocardial Injury in Diabetic Rats mediating Activation of the PPAR-γ Pathway. Int. J. Mol. Sci. 2017, 18, 756. [Google Scholar] [CrossRef]


| Groups | Heart Weight (g) | Body Weight (g) |
|---|---|---|
| Normal control | 0.65 ± 0.04 | 243.4 ± 3.23 |
| Diabetic + Corosolic acid | 0.60 ± 0.05 | 238.4 ± 4.50 |
| Diabetic control | 0.62 ± 0.03 | 235 ± 3.61 |
| Diabetic isoproterenol control | 0.59 ± 0.08 | 217 ± 3.32 |
| Diabetic isoproterenol + Corosolic acid | 0.61 ± 0.04 | 239 ± 2.82 |
| Diabetic isoproterenol + GW9662 | 0.56 ± 0.07 | 209 ± 3.41 |
| Diabetic isoproterenol + GW9662 + Corosolic acid | 0.55 ± 0.04 | 228 ± 4.21 |
| Groups | Grade 0 | Grade 1 | Grade 2 | Grade 3 |
|---|---|---|---|---|
| Normal control | 0 | 1 | 0 | 0 |
| Diabetic + Corosolic acid | 0 | 0 | 0 | 0 |
| Diabetic control | 3 | 2 | 2 | 1 |
| Diabetic isoproterenol control | 0 | 2 | 2 | 3 |
| Diabetic isoproterenol + Corosolic acid | 0 | 1 | 1 | 1 |
| Diabetic isoproterenol + GW9662 | 0 | 2 | 2 | 2 |
| Diabetic isoproterenol + GW9662 + Corosolic acid | 1 | 2 | 2 | 1 |
| Groups | Subjects | Treatment Given |
|---|---|---|
| Group I | Normal control | Vehicle (distilled water) for 28 days. |
| Group II | Diabetic rats treated with corosolic acid 50 mg/kg | 50 mg/kg/day of corosolic acid for 28 days by oral route in diabetic rats. |
| Group III | Diabetic control | 28 days of oral administration with distilled water as carrier (1 mL/kg). On days 26 and 27, rats received 0.2 mL saline subcutaneously as vehicle control. |
| Group IV | Diabetic isoproterenol-treated | The above vehicle was administered to the rats in this group for 28 days, and isoproterenol (100 mg/kg) was also administered subcutaneously to the rats on days 26 and 27. |
| Group V | Diabetic isoproterenol-treated rats administered with corosolic acid 50 mg/kg | Rats received 50 mg/kg/day of corosolic acid orally for 28 days. On days 26 and 27, rats were injected subcutaneously with isoproterenol (100 mg/kg). |
| Group VI | Diabetic isoproterenol-treated rats administered with GW9662 | Rats in this group received GW9668 1 mg/kg/day by intraperitoneal (i.p.) injection on day 28, and 100 mg/kg isoproterenol by subcutaneous injection on days 26 and 27. |
| Group VII | Diabetic isoproterenol-treated rats administered with corosolic acid and GW9662 | This group of rats received oral corosolic acid (50 mg/kg) every day. On 28th day, corosolic acid was given just 15 min before GW9662 administration. On the last two days of therapy, 100 mg/kg isoproterenol was administered subcutaneously to the rats in this group [29,41]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkholifi, F.K.; Devi, S.; Yusufoglu, H.S.; Alam, A. The Cardioprotective Effect of Corosolic Acid in the Diabetic Rats: A Possible Mechanism of the PPAR-γ Pathway. Molecules 2023, 28, 929. https://doi.org/10.3390/molecules28030929
Alkholifi FK, Devi S, Yusufoglu HS, Alam A. The Cardioprotective Effect of Corosolic Acid in the Diabetic Rats: A Possible Mechanism of the PPAR-γ Pathway. Molecules. 2023; 28(3):929. https://doi.org/10.3390/molecules28030929
Chicago/Turabian StyleAlkholifi, Faisal K., Sushma Devi, Hasan S. Yusufoglu, and Aftab Alam. 2023. "The Cardioprotective Effect of Corosolic Acid in the Diabetic Rats: A Possible Mechanism of the PPAR-γ Pathway" Molecules 28, no. 3: 929. https://doi.org/10.3390/molecules28030929
APA StyleAlkholifi, F. K., Devi, S., Yusufoglu, H. S., & Alam, A. (2023). The Cardioprotective Effect of Corosolic Acid in the Diabetic Rats: A Possible Mechanism of the PPAR-γ Pathway. Molecules, 28(3), 929. https://doi.org/10.3390/molecules28030929






