Novel Indole-Containing Hybrids Derived from Millepachine: Synthesis, Biological Evaluation and Antitumor Mechanism Study
Abstract
:1. Introduction
2. Results and Discussion
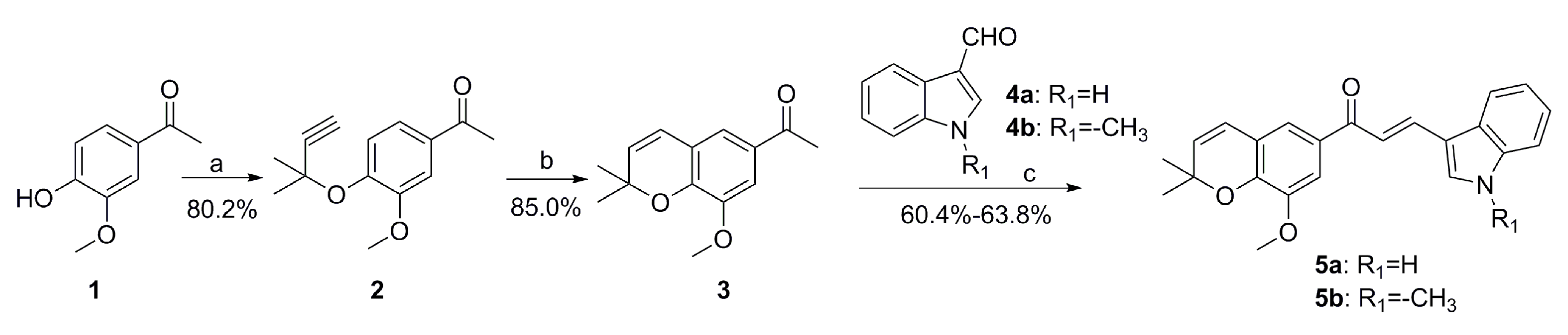
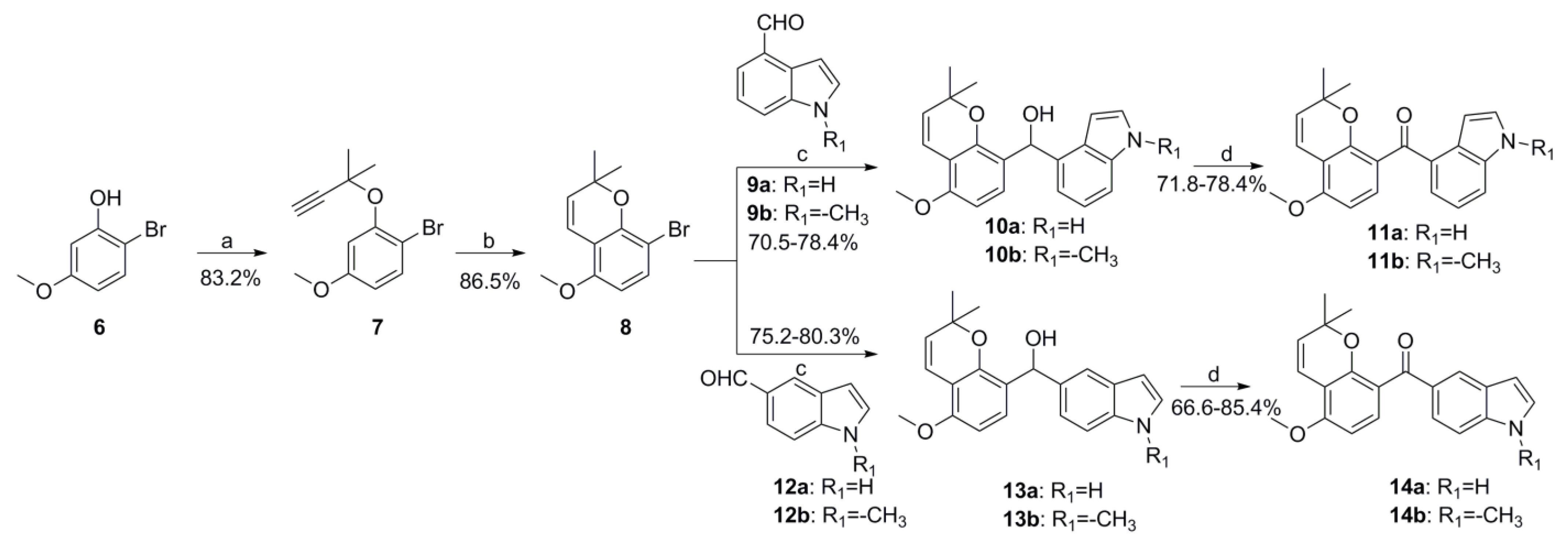
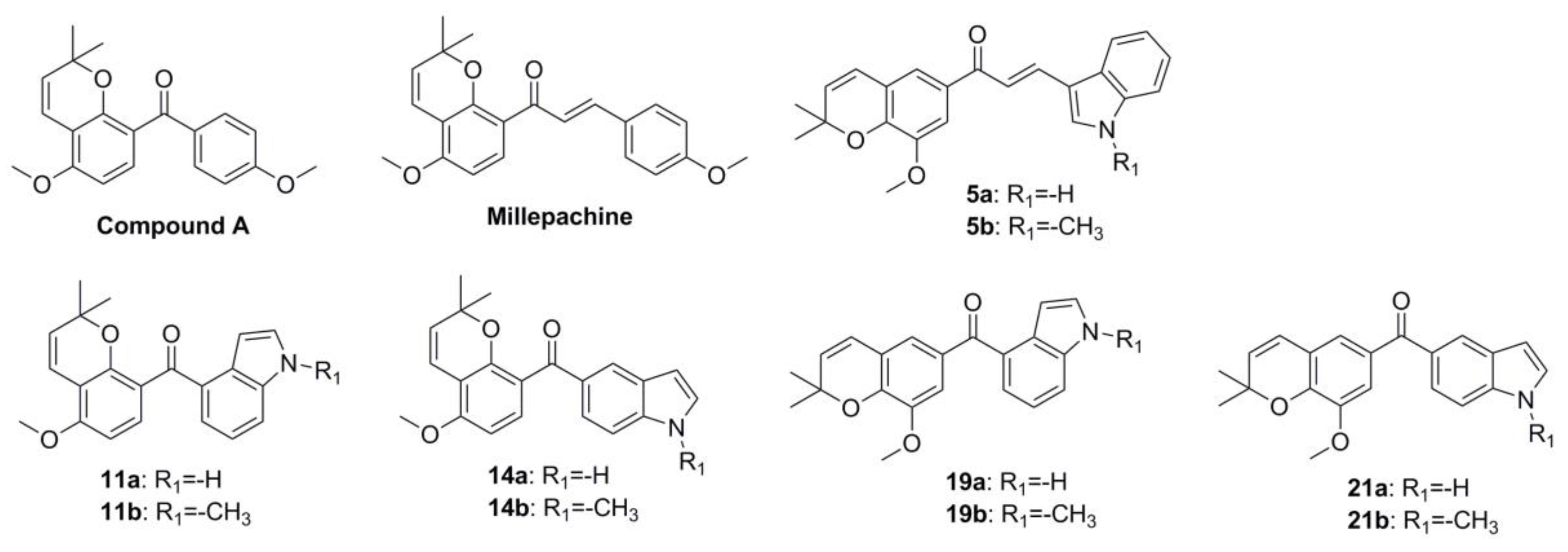
2.1. Chemistry
2.2. In Vitro Antiproliferative Activity Evaluation and SAR Summary
2.3. Cytotoxicity of Compound 14b in Human Normal Cells Activities
2.4. Cytotoxicity of Compound 14b towards Drug-Resistant Cancer Cell Lines
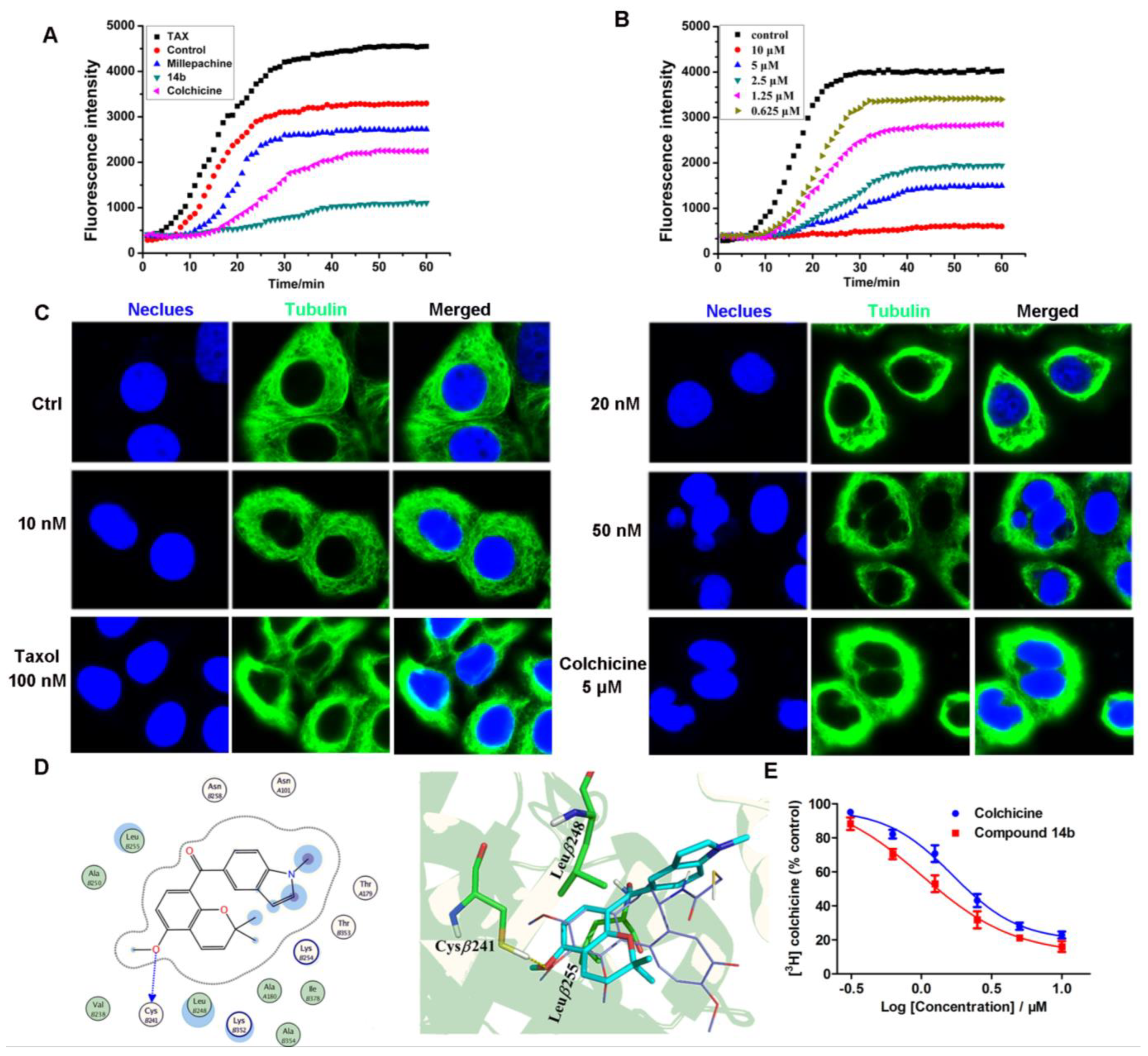
2.5. Compound 14b Inhibited Tubulin Polymerization
2.6. Compound 14b Induced Cell Cycle Arrest at the G2/M Phase
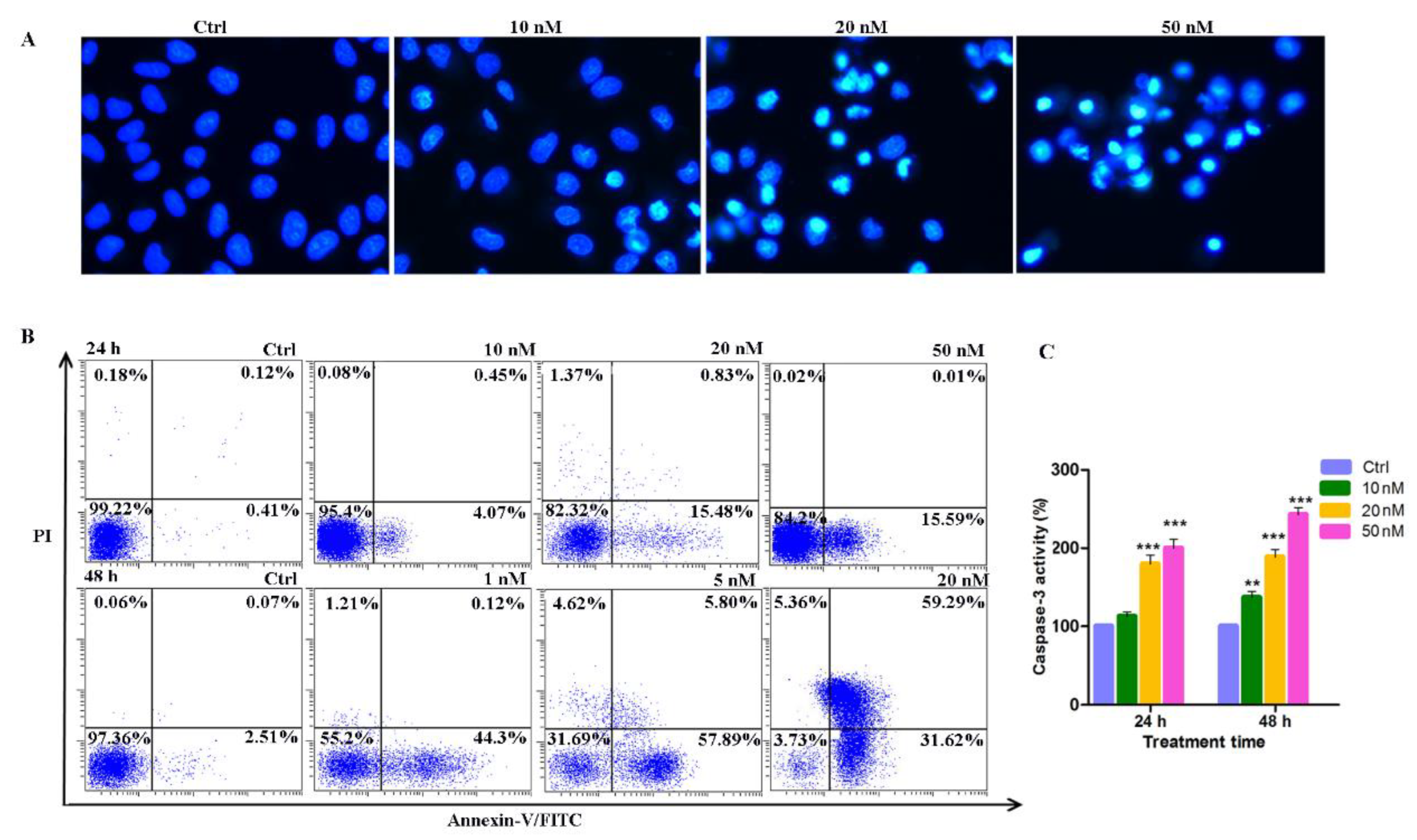
2.7. Induction of Apoptosis by Compound 14b
2.8. Compound 14b Treatment Leads to ROS Accumulation and MMP Decrease
2.9. The Metabolic Stability of Compound 14b
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedures for Known Compounds
3.1.2. General Procedures for Preparation of Compounds 5a-5b
- (E)-3-(1H-indol-3-yl)-1-(8-methoxy-2,2-dimethyl-2H-chromen-6-yl)prop-2-en-1-one (5a)
- (E)-1-(8-methoxy-2,2-dimethyl-2H-chromen-6-yl)-3-(1-methyl-1H-indol-3-yl)prop-2-en-1-one (5b)
3.1.3. General Procedures for Preparation of Compounds 11a-11b, 14a-14b, 19a-19b and 21a-21b
- (1H-indol-4-yl)(5-methoxy-2,2-dimethyl-2H-chromen-8-yl)methanone (11a)
- (5-methoxy-2,2-dimethyl-2H-chromen-8-yl)(1-methyl-1H-indol-4-yl)methanone (11b)
- (1H-indol-5-yl)(5-methoxy-2,2-dimethyl-2H-chromen-8-yl)methanone (14a)
- (5-methoxy-2,2-dimethyl-2H-chromen-8-yl)(1-methyl-1H-indol-5-yl)methanone (14b)
- (1H-indol-4-yl)(8-methoxy-2,2-dimethyl-2H-chromen-6-yl)methanone (19a)
- (8-methoxy-2,2-dimethyl-2H-chromen-6-yl)(1-methyl-1H-indol-4-yl)methanone (19b)
- (1H-indol-5-yl)(8-methoxy-2,2-dimethyl-2H-chromen-6-yl)methanone (21a)
- (8-methoxy-2,2-dimethyl-2H-chromen-6-yl)(1-methyl-1H-indol-5-yl)methanone (21b)
3.2. Cell Lines and Cell Culture
3.3. Biological Evaluation Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Pinto, M.M.; Palmeira, A.; Fernandes, C.; Resende, D.I.; Sousa, E.; Cidade, H.; Tiritan, M.E.; Correia-da-Silva, M.; Cravo, S. From Natural Products to New Synthetic Small Molecules: A Journey through the World of Xanthones. Molecules 2021, 26, 431. [Google Scholar] [CrossRef]
- Ekiert, H.M.; Szopa, A. Biological Activities of Natural Products. Molecules 2020, 25, 5769. [Google Scholar] [CrossRef]
- Arbour, C.A.; Imperiali, B. Uridine natural products: Challenging targets and inspiration for novel small molecule inhibitors. Bioorg. Med. Chem. 2020, 28, 115661. [Google Scholar] [CrossRef]
- Ilan, Y. Microtubules: From understanding their dynamics to using them as potential therapeutic targets. J. Cell Physiol. 2019, 234, 7923–7937. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, Y.; Yang, R.; Wang, Z.; Lu, Y.; Wang, B.; Zhu, H. Tubulin Inhibitors Binding to Colchicine-Site: A Review from 2015 to 2019. Curr. Med. Chem. 2020, 27, 6787–6814. [Google Scholar] [CrossRef]
- Dong, M.; Liu, F.; Zhou, H.; Zhai, S.; Yan, B. Novel Natural Product- and Privileged Scaffold-Based Tubulin Inhibitors Targeting the Colchicine Binding Site. Molecules 2016, 21, 1375. [Google Scholar] [CrossRef]
- Zhou, Y.; Di, B.; Niu, M. Structure-Based Pharmacophore Design and Virtual Screening for Novel Tubulin Inhibitors with Potential Anticancer Activity. Molecules 2019, 24, 3181. [Google Scholar] [CrossRef]
- Ye, H.; Fu, A.; Wu, W.; Li, Y.; Wang, G.; Tang, M.; Li, S.; He, S.; Zhong, S.; Lai, H.; et al. Cytotoxic and apoptotic effects of constituents from Millettia pachycarpa Benth. Fitoterapia 2012, 83, 1402–1408. [Google Scholar] [CrossRef]
- Huang, X.; Wang, M.; Wang, C.; Hu, W.; You, Q.; Ma, T.; Jia, Q.; Yu, C.; Liao, Z.; Wang, H. Synthesis and biological evaluation of novel millepachine derivative containing aminophosphonate ester species as novel anti-tubulin agents. Bioorg. Chem. 2020, 94, 103486. [Google Scholar] [CrossRef]
- Huang, X.; Hua, S.; Huang, R.; Liu, Z.; Gou, S.; Wang, Z.; Liao, Z.; Wang, H. Dual-targeting antitumor hybrids derived from Pt(IV) species and millepachine analogues. Eur. J. Med. Chem. 2018, 148, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wu, W.; Wang, J.; Liu, L.; Li, L.; Yang, J.; Wang, G.; Cao, D.; Zhang, R.; Tang, M.; et al. Synthesis and Biological Evaluation of Novel Millepachine Derivatives as a New Class of Tubulin Polymerization Inhibitors. J. Med. Chem. 2014, 57, 7977–7989. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, W.; Yu, Y.; Wang, Y.; Yang, T.; Xue, L.; Yuan, X.; Long, C.; Liu, Z.; Chen, X.; et al. The compound millepachine and its derivatives inhibit tubulin polymerization by irreversibly binding to the colchicine-binding site in β-tubulin. J. Biol. Chem. 2018, 293, 9461–9472. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, Y.; Ye, H.; Li, Z. Millepachine showed novel antitumor effects in cisplatin-resistant human ovarian cancer through inhibiting drug efflux function of ATP-binding cassette transporters. Phytother. Res. 2018, 32, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef]
- Devi, N.; Kaur, K.; Biharee, A.; Jaitak, V. Recent Development in Indole Derivatives as Anticancer Agent: A Mechanistic Approach. Anti-Cancer Agents Med. Chem. 2021, 21, 1802–1824. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Li, Y.; Yan, C.; Yan, M.; Tang, Z. Indole: A privileged scaffold for the design of anti-cancer agents. Euro. J. Med. Chem. 2019, 183, 111691. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef]
- Tang, S.; Zhou, Z.; Jiang, Z.; Zhu, W.; Qiao, D. Indole-Based Tubulin Inhibitors: Binding Modes and SARs Investigations. Molecules 2022, 27, 1587. [Google Scholar] [CrossRef]
- Naaz, F.; Neha, K.; Haider, M.R.; Shafi, S. Indole derivatives (2010–2020) as versatile tubulin inhibitors: Synthesis and structure–activity relationships. Future Med. Chem. 2021, 13, 1795–1828. [Google Scholar] [CrossRef]
- An, B.; Zhang, S.; Yan, J.; Huang, L.; Li, X. Synthesis, in vitro and in vivo evaluation of new hybrids of millepachine and phenstatin as potent tubulin polymerization inhibitors. Org. Biomol. Chem. 2017, 15, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yan, J.; Hu, J.; Pang, Y.; Huang, L.; Li, X. Synthesis, biological evaluation and mechanism study of chalcone analogues as novel anti-cancer agents. RSC Adv. 2015, 5, 68128–68135. [Google Scholar] [CrossRef]
- Zhang, S.; An, B.; Li, J.; Hu, J.; Huang, L.; Li, X.; Chan, A.S.C. Synthesis and evaluation of selenium-containing indole chalcone and diarylketone derivatives as tubulin polymerization inhibition agents. Org. Biomol. Chem. 2017, 15, 7404–7410. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, J.; Zhang, S.; Hu, J.; Huang, L.; Li, X. Synthesis, Evaluation, and Mechanism Study of Novel Indole-Chalcone Derivatives Exerting Effective Antitumor Activity Through Microtubule Destabilization in Vitro and in Vivo. J. Med. Chem. 2016, 59, 5264–5283. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Pang, Y.; Sheng, J.; Wang, Y.; Chen, J.; Hu, J.; Huang, L.; Li, X. A novel synthetic compound exerts effective anti-tumour activity in vivo via the inhibition of tubulin polymerisation in A549 cells. Biochem. Pharmacol. 2015, 97, 51–61. [Google Scholar] [CrossRef]
- Zhou, J.; Pang, Y.; Zhang, W.; OuYang, F.; Lin, H.; Li, X.; Yan, J. Discovery of a Novel Stilbene Derivative as a Microtubule Targeting Agent Capable of Inducing Cell Ferroptosis. J. Med. Chem. 2022, 65, 4687–4708. [Google Scholar] [CrossRef]
- Pang, Y.; Lin, H.; Ou, C.; Cao, Y.; An, B.; Yan, J.; Li, X. Design, synthesis, and biological evaluation of novel benzodiazepine derivatives as anticancer agents through inhibition of tubulin polymerization in vitro and in vivo. Eur. J. Med. Chem. 2019, 182, 111670. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zheng, L.; Wang, D.; Liang, X.; Gao, F.; Zhou, X. Recent advances in microtubule-stabilizing agents. Euro. J. Med. Chem. 2018, 143, 806–828. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Lim, B. Targeting Apoptosis in Cancer. Curr. Oncol. Rep. 2022, 24, 273–284. [Google Scholar] [CrossRef]
- Zhang, S.; An, B.; Yan, J.; Huang, L.; Li, X. The synthesis and evaluation of new benzophenone derivatives as tubulin polymerization inhibitors. RSC Adv. 2016, 6, 88453–88462. [Google Scholar] [CrossRef]




 | |||||
|---|---|---|---|---|---|
| Compds | IC50, Mean ± SE (μΜ) b | ||||
| A549 | Caski | HepG2 | C42B | MCF-7 | |
| 5a | 0.812 ± 0.022 | 1.929 ± 0.035 | 0.963 ± 0.032 | 1.377 ± 0.021 | 1.553 ± 0.014 |
| 5b | 0.362 ± 0.017 | 0.729 ± 0.033 | 0.461 ± 0.017 | 0.577 ± 0.021 | 0.350 ± 0.011 |
| 11a | 0.631 ± 0.018 | 0.832 ± 0.011 | 0.998 ± 0.034 | 0.911 ± 0.034 | 0.816 ± 0.045 |
| 11b | 0.208 ± 0.009 | 0.516 ± 0.012 | 0.454 ± 0.021 | 0.455 ± 0.015 | 0.651 ± 0.024 |
| 14a | 0.072 ± 0.004 | 0.086 ± 0.009 | 0.142 ± 0.014 | 0.089 ± 0.021 | 0.082 ± 0.011 |
| 14b | 0.022 ± 0.002 | 0.037 ± 0.003 | 0.074 ± 0.013 | 0.051 ± 0.005 | 0.027 ± 0.006 |
| 19a | 1.321 ± 0.016 | 2.311 ± 0.019 | 3.098 ± 0.021 | 2.411 ± 0.042 | 1.775 ± 0.004 |
| 19b | 0.642 ± 0.081 | 1.098 ± 0.032 | 0.898 ± 0.009 | 1.076 ± 0.034 | 1.021 ± 0.015 |
| 21a | 0.105 ± 0.011 | 0.211 ± 0.012 | 0.441 ± 0.071 | 0.209 ± 0.020 | 0.121 ± 0.021 |
| 21b | 0.082 ± 0.010 | 0.106 ± 0.012 | 0.097 ± 0.031 | 0.107 ± 0.006 | 0.099 ± 0.031 |
| Compd. A | 0.226 ± 0.035 | 0.892 ± 0.024 | 0.758 ± 0.017 | 0.526 ± 0.007 | 0.456 ± 0.021 |
| Millepachine | 2.566 ± 0.131 | 6.712 ± 0.233 | 3.881 ± 0.244 | 3.176 ± 0.384 | 4.528 ± 0.338 |
| Cell Lines | IC50, Mean ± SE (μM) b | Selectivity Ratio c |
|---|---|---|
| Hep-G2 | 0.074 ± 0.013 | 16.97 |
| L-02 | 1.256 ± 0.214 | |
| C42B | 0.051 ± 0.005 | 72.51 |
| RWPE-1 | 3.698 ± 0.036 | |
| MCF-7 | 0.027 ± 0.006 | 231.7 |
| MCF-10A | 6.256 ± 0.311 |
| Cell Lines | IC50, Mean ± SE (μM) b | Resistance Index c |
|---|---|---|
| A549 | 0.022 ± 0.002 | 1.59 |
| A549/CDDP d | 0.035 ± 0.021 | |
| C42B | 0.051 ± 0.005 | 0.82 |
| C42B/ENZR e | 0.042 ± 0.011 | |
| MCF-7 | 0.027 ± 0.006 | 1.88 |
| MCF-7/DOX f | 0.051 ± 0.024 | |
| A2780 | 0.036 ± 0.004 | 1.17 |
| A2780/TAX g | 0.042 ± 0.011 | |
| HCT-8 | 0.089 ± 0.015 | 1.37 |
| HCT-8/VCR h | 0.122 ± 0.036 |
| Compd. | Time/min, Remaining% | t1/2, min | ||||||
|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | 150 | 180 | 240 | ||
| 14b | 96.18% | 91.38% | 84.14% | 74.45% | 67.75% | 56.17% | 43.43% | 198 |
| CA-4 | 70.2% | 28.3% | 20.5% | 18.7% | NT a | NT | NT | ND b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, B.; Zou, Q.; Yu, L.; Wang, Y.; Yan, J.; Huang, B. Novel Indole-Containing Hybrids Derived from Millepachine: Synthesis, Biological Evaluation and Antitumor Mechanism Study. Molecules 2023, 28, 1481. https://doi.org/10.3390/molecules28031481
Liang B, Zou Q, Yu L, Wang Y, Yan J, Huang B. Novel Indole-Containing Hybrids Derived from Millepachine: Synthesis, Biological Evaluation and Antitumor Mechanism Study. Molecules. 2023; 28(3):1481. https://doi.org/10.3390/molecules28031481
Chicago/Turabian StyleLiang, Baoxia, Qing Zou, Lintao Yu, Yali Wang, Jun Yan, and Baiqi Huang. 2023. "Novel Indole-Containing Hybrids Derived from Millepachine: Synthesis, Biological Evaluation and Antitumor Mechanism Study" Molecules 28, no. 3: 1481. https://doi.org/10.3390/molecules28031481
APA StyleLiang, B., Zou, Q., Yu, L., Wang, Y., Yan, J., & Huang, B. (2023). Novel Indole-Containing Hybrids Derived from Millepachine: Synthesis, Biological Evaluation and Antitumor Mechanism Study. Molecules, 28(3), 1481. https://doi.org/10.3390/molecules28031481





