Byproducts of Globe Artichoke and Cauliflower Production as a New Source of Bioactive Compounds in the Green Economy Perspective: An NMR Study
Abstract
1. Introduction
2. Results
2.1. Globe Artichoke Byproducts Metabolite Profiles
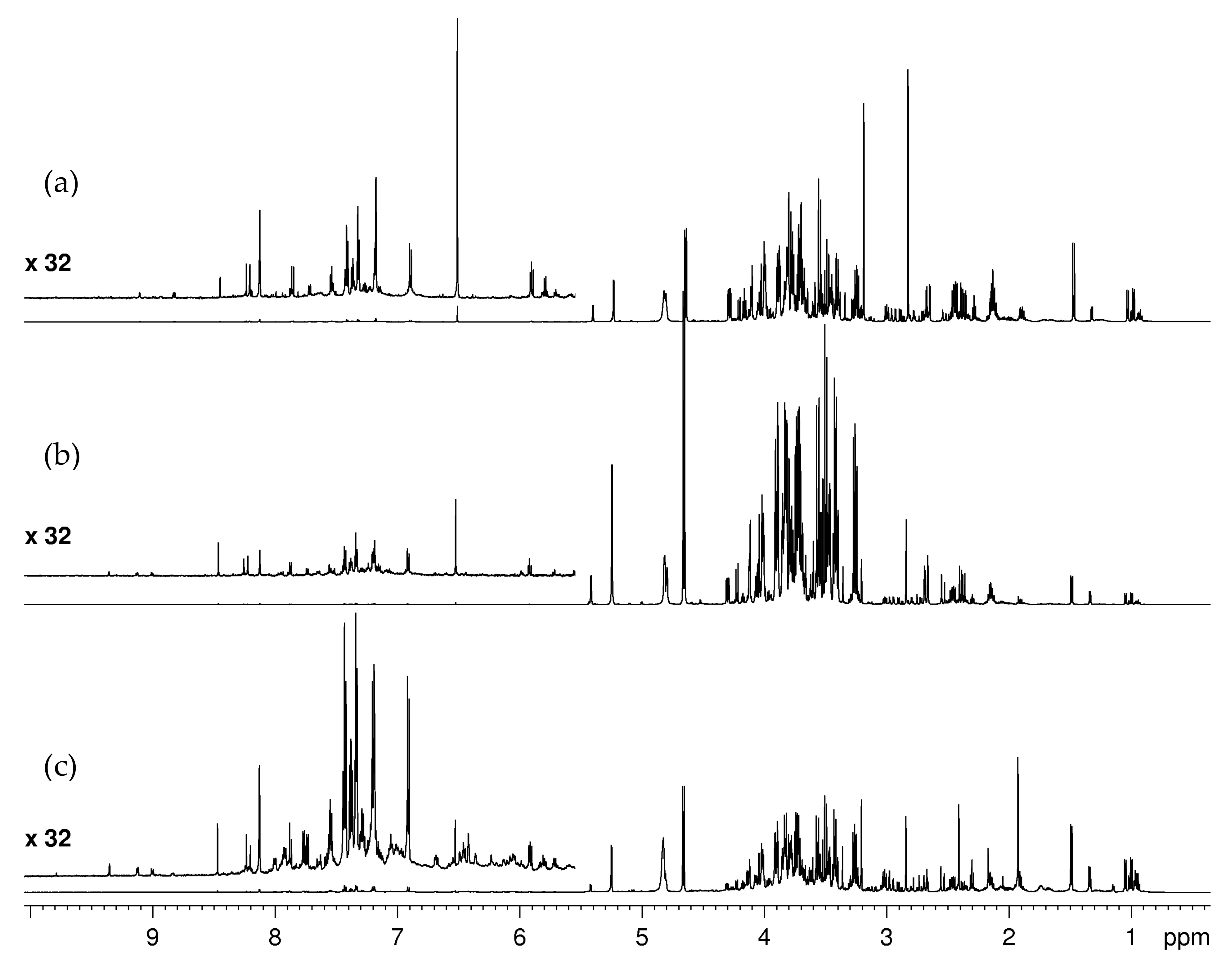
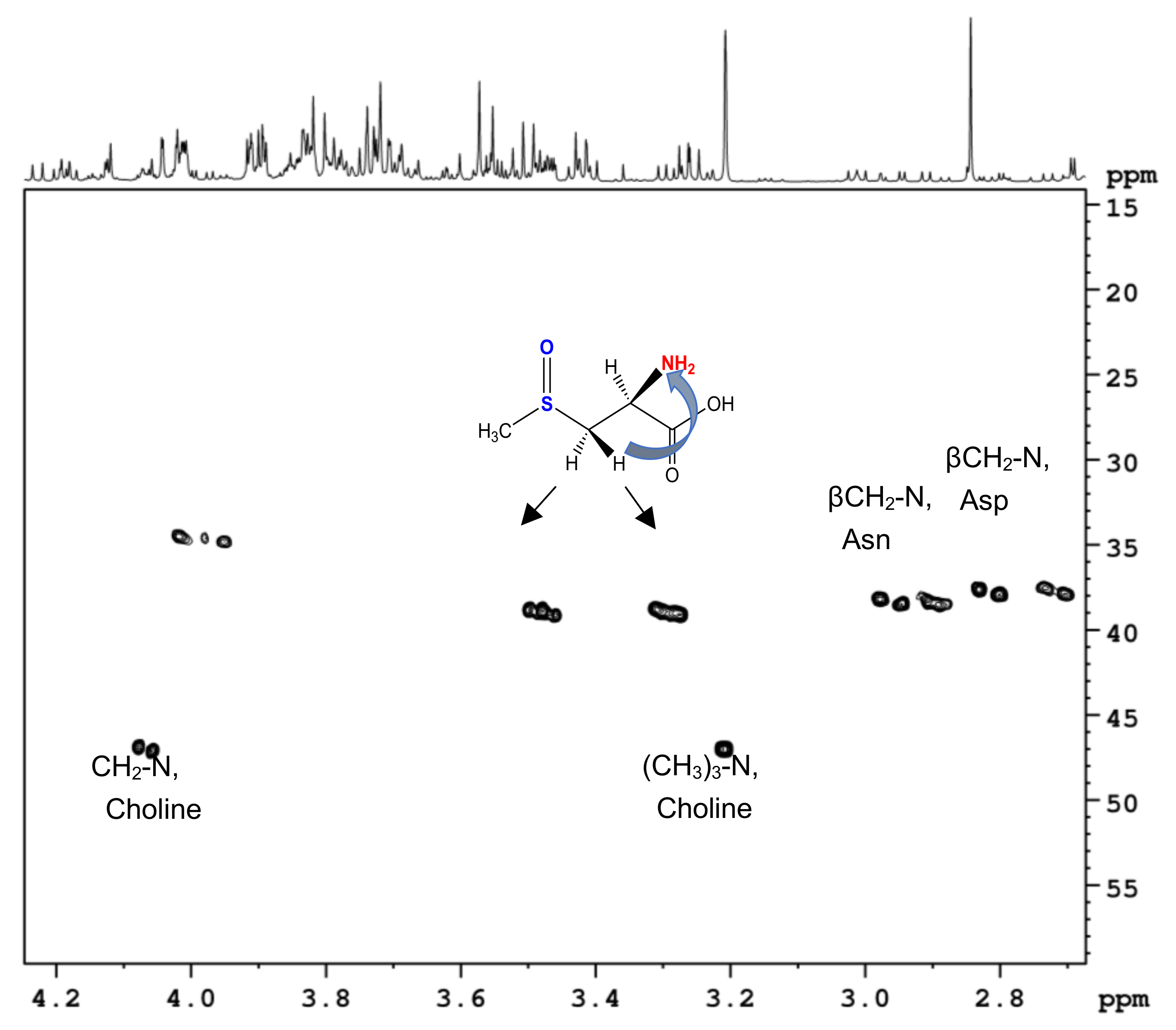
2.1.1. Water-Soluble Metabolites: Assignment of NMR Spectra and Metabolite Identification
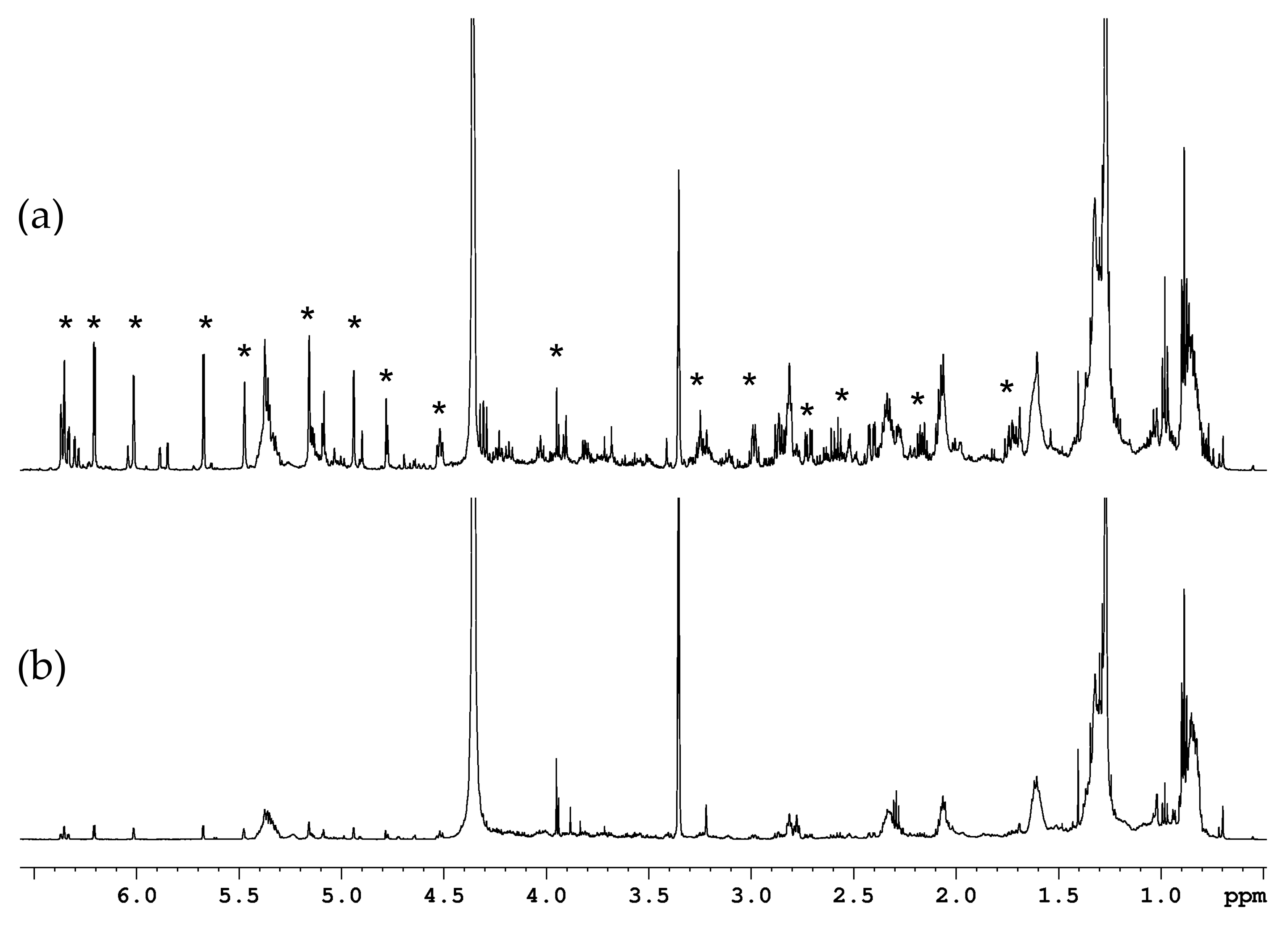
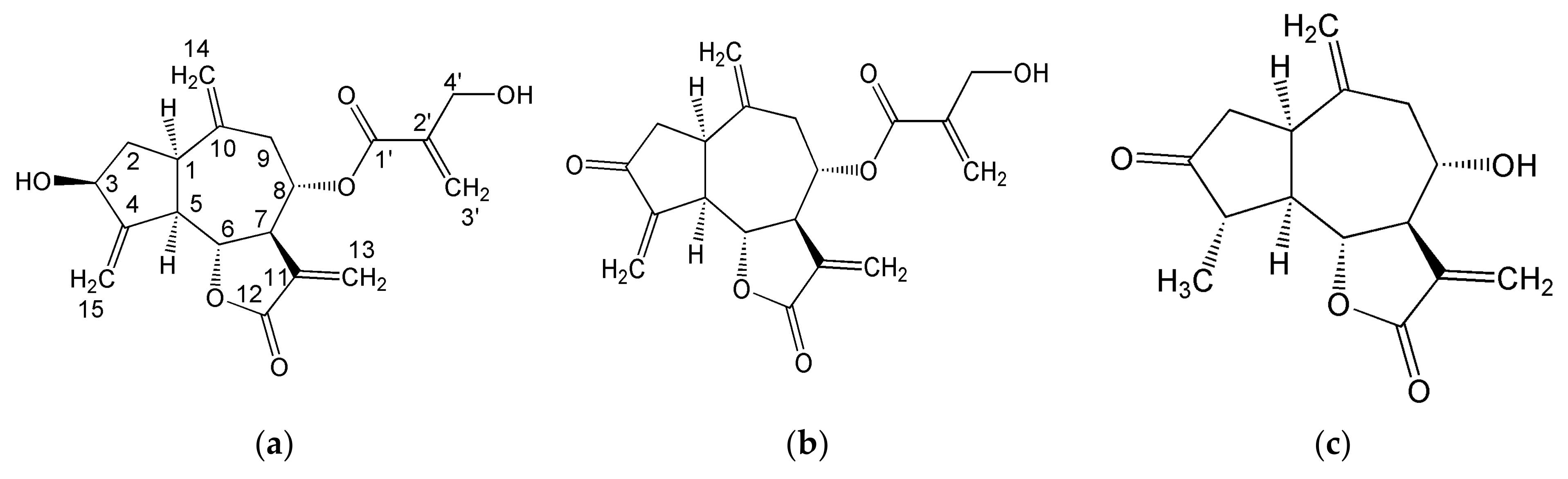
2.1.2. Organic Fraction: Sesquiterpene Lactones and Other Metabolites
2.1.3. Metabolite Quantification
2.2. Cauliflower Byproducts Metabolite Profile
2.2.1. Assignment of S-Methyl-L-Cysteine-Sulfoxide (Methiin) in Aqueous Extracts
2.2.2. Glucosinolates
2.2.3. Other Metabolites in Aqueous Extracts
2.2.4. Liposoluble Metabolites
2.2.5. Metabolite Quantification in Cauliflower Byproducts
3. Discussion
3.1. Globe Artichoke Byproducts
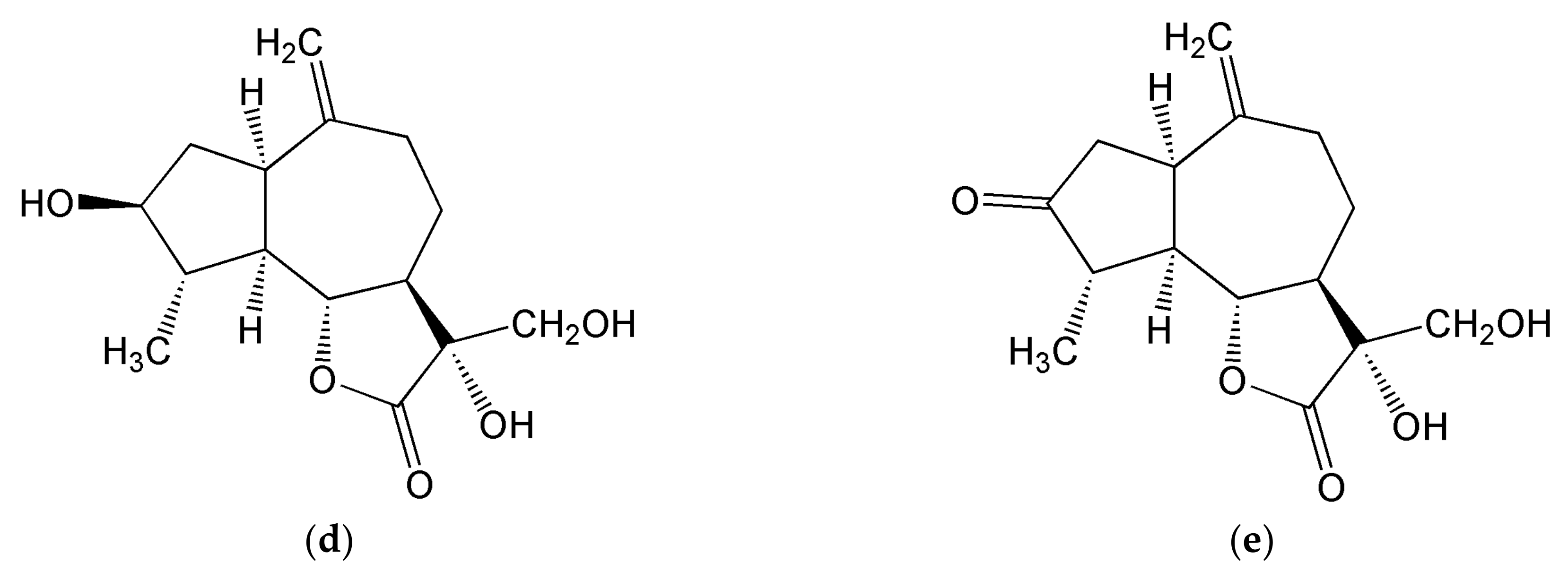
3.1.1. Inositols
3.1.2. Sesquiterpene Lactones
3.1.3. Caffeoylquinic Acids
3.1.4. Inulins
3.1.5. Other Metabolites
3.2. Cauliflower ByProducts
- (1)
- Close similarity of qualitative composition of the most abundant metabolites (including sugars, amino acids, organic acids, amines, etc.), in all samples studied. For example, 17 out of 19 identified amino acids were observed in both crops’ byproducts (Table 1). Among sugars, only minor components (such as inulin in artichoke and raffinose in cauliflower) were crop-specific;
- (2)
- The presence of crop-specific secondary metabolites. In the case of globe artichoke byproducts, the crop-specific metabolites included sesquiterpene lactones, stigmasterol, inositol isomers (chiro- and scyllo-inositols), inulin, chlorogenic and neochlorogenic acids, and glycine betaine, whereas only cauliflower’s byproducts contained methiin, glycine, glucosinolates, pyruvic acid, and raffinose;
- (3)
- The drastic variation of metabolite levels occurs in crop- and tissue-specific manner. For example, dehydrocynaropicrin present in globe artichoke leaves was absent in stalks, whereas glucosinolates were found only in cauliflower leaves. For both crops, leaves were generally richer in amino acids and amines and poorer in sugars with respect to stalks. Florets (in the case of cauliflower) were even richer in amino acids and amines than leaves;
- (4)
- The detailed analysis of metabolite profiling of a given byproduct can indicate the best strategies for the recovery and the reutilization of its constituents. Both water-soluble and apolar metabolite fractions contain bioactive compounds that can be extracted and reutilized in food supplements or as components of functional food. In the case of globe artichoke, there is a remarkable potential for new product development from byproducts as sources of inositol isomers, caffeoylquinic acids and inulin in water-soluble fraction and sesquiterpene lactones in organic fraction. Cauliflower byproducts can be utilized for the extraction of bioactive sulfur-containing compounds including S-methyl-L-cysteine S-oxide and glucosinolates.
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. Extraction Procedures
4.4. NMR Analysis
4.4.1. NMR Samples Preparation
4.4.2. NMR Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO The State of Food and Agriculture. Moving forward on Food Loss and Waste Reduction. Licence: CC BYNC-SA 3.0 IGO. Rome. 2019. Available online: https://www.fao.org/3/ca6030en/ca6030en.pdf (accessed on 20 December 2022).
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef] [PubMed]
- United Nations Department of Economic and Social Affairs. Goal 12: Ensure Sustainable Consumption and Production Patterns. Available online: https://sdgs.un.org/goals/goal12 (accessed on 16 November 2022).
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.Q.; Sabran, M.R.; Shafie, S.R. Utilization of vegetable and fruit byproducts as functional ingredient and food. Front. Nutr. 2021, 8, 661693. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, B.; Luo, H.; Meng, K.; Wang, Y.; Liu, M.; Bai, Y.; Yao, B.; Tu, T. Production pectin oligosaccharides using Humicola insolens Y1-derived unusual pectate lyase. J. Biosci. Bioeng. 2020, 129, 16–22. [Google Scholar] [CrossRef]
- Basri, M.S.M.; Shah, N.N.A.K.; Sulaiman, A.; Tawakkal, I.S.M.A.; Nor, M.Z.M.; Ariffin, S.H.; Ghani, N.H.A.; Salleh, F.S.M. Progress in the valorization of fruit and vegetable wastes: Active packaging, biocomposites, byproducts, and innovative technologies used for bioactive compound extraction. Polymers 2021, 13, 3503. [Google Scholar] [CrossRef]
- Arquelau, P.B.F.; de Silva, V.D.M.; Garcia, M.A.V.T.; de Araújo, R.L.B.; Fante, C.A. Characterization of edible coatings based on ripe “Prata” banana peel flour. Food. Hydrocolloid. 2019, 89, 570–578. [Google Scholar] [CrossRef]
- González-García, E.; Marina, M.L.; García, M.C. Plum (Prunus Domestica L.) byproduct as a new and cheap source of bioactive peptides: Extraction method and peptides characterization. J. Funct. Foods 2014, 11, 428–437. [Google Scholar] [CrossRef]
- Xu, Y.; Bao, T.; Han, W.; Chen, W.; Zheng, X.; Wang, J. Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from cauliflower byproducts protein hydrolysate. Process. Biochem. 2016, 51, 1299–1305. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database—Crops and Livestock Products. FAO, 2020. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 20 December 2022).
- Francavilla, M.; Marone, M.; Marasco, P.; Contillo, F.; Monteleone, M. Artichoke biorefinery: From food to advanced technological applications. Foods 2021, 10, 112. [Google Scholar] [CrossRef]
- Khedkar, M.A.; Nimbalkar, P.R.; Chavan, P.V.; Chendake, Y.J.; Bankar, S.B. Cauliflower waste utilization for sustainable biobutanol production: Revelation of drying kinetics and bioprocess development. Bioprocess. Biosyst. Eng. 2017, 40, 1493–1506. [Google Scholar] [CrossRef]
- Zayed, A.; Farag, M.A. Valorization, extraction optimization and technology advancements of artichoke biowastes: Food and non-food applications. Food. Sci. Technol. 2020, 132, 109883. [Google Scholar] [CrossRef]
- de Falco, B.; Incerti, G.; Amato, M.; Lanzotti, V. Artichoke: Botanical, agronomical, phytochemical, and pharmacological overview. Phytochem. Rev. 2015, 14, 993–1018. [Google Scholar] [CrossRef]
- Zeaiter, Z.; Regonesi, M.E.; Cavini, S.; Labra, M.; Sello, G.; Di Gennaro, P. Extraction and characterization of inulin-type fructans from artichoke wastes and their effect on the growth of intestinal bacteria associated with health. Biomed. Res. Int. 2019, 2019, 1083952. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Aceituno, L.; García-Sarrió, M.J.; Alonso-Rodriguez, B.; Ramos, L.; Sanz, M.L. Extraction of bioactive carbohydrates from artichoke (Cynara scolymus L.) external bracts using microwave assisted extraction and pressurized liquid extraction. Food Chem. 2016, 196, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Bernardi, J.; Cardarelli, M.; Bernardo, L.; Kane, D.; Colla, G.; Lucini, L. Phenolic compounds and sesquiterpene lactones profile in leaves of nineteen artichoke cultivars. J. Agric. Food. Chem. 2016, 64, 8540–8548. [Google Scholar] [CrossRef]
- Órbenes, G.; Rodríguez-Seoane, P.; Torres, M.D.; Chamy, R.; Zúñiga, M.E.; Domínguez, H. Valorization of artichoke industrial byproducts using green extraction technologies: Formulation of hydrogels in combination with paulownia extracts. Molecules 2021, 26, 4386. [Google Scholar] [CrossRef]
- Mena-García, A.; Rodríguez-Sánchez, S.; Ruiz-Matute, A.I.; Sanz, M.L. Exploitation of artichoke byproducts to obtain bioactive extracts enriched in inositols and caffeoylquinic acids by microwave assisted extraction. J. Chromatogr. A 2020, 1613, 460703. [Google Scholar] [CrossRef]
- Mabeau, S.; Baty-Julien, C.; Chodosas, O.; Surbled, M.; Metra, P.; Durand, D.; Morice, G.; Chesné, C.; Mekideche, K. Antioxidant activity of artichoke extracts and byproducts. Acta Hortic. 2007, 744, 431–443. [Google Scholar] [CrossRef]
- De Menna, F.; Malagnino, R.A.; Vittuari, M.; Molari, G.; Seddaiu, G.; Deligios, P.A.; Solinas, S.; Ledda, L. Potential biogas production from artichoke byproducts in Sardinia, Italy. Energies 2016, 9, 92. [Google Scholar] [CrossRef]
- Llorach, R.; Espín, J.C.; Tomás-Barberán, F.A.; Ferreres, F. Valorization of cauliflower (Brassica oleracea L. var. botrytis) byproducts as a source of antioxidant phenolics. J. Agric. Food Chem. 2003, 51, 2181–2187. [Google Scholar] [CrossRef]
- Collado-González, J.; Piñero, M.C.; Del Amor, F.M.; Otálora, G.; López-Marín, J. The effect of foliar putrescine application, ammonium exposure, and heat stress on antioxidant compounds in cauliflower waste. Antioxidants 2021, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.A.; Ali, R.F.M. Bioactive compounds and antioxidant activity of fresh and processed white cauliflower. Biomed. Res. Int. 2013, 2013, 367819. [Google Scholar] [CrossRef]
- Femenia, A.; Robertson, J.A.; Waldron, K.W.; Selvendran, R.R.; Cauliflower (Brassica oleracea, L. Cauliflower (Brassica oleracea L.), globe artichoke (Cynara scolymus) and chicory witloof (Cichorium intybus) processing byproducts as sources of dietary fibre. J. Sci. Food. Agric. 1998, 77, 511–518. [Google Scholar] [CrossRef]
- Caliceti, C.; Capriotti, A.L.; Calabria, D.; Bonvicini, F.; Zenezini Chiozzi, R.; Montone, C.M.; Piovesana, S.; Zangheri, M.; Mirasoli, M.; Simoni, P.; et al. Peptides from cauliflower byproducts, obtained by an efficient, ecosustainable, and semi-industrial method, exert protective effects on endothelial function. Oxid. Med. Cell Longev. 2019, 2019, 1046504. [Google Scholar] [CrossRef] [PubMed]
- Mannina, L.; Sobolev, A.P.; Viel, S. Liquid state 1H high field NMR in food analysis. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 66, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Deborde, C.; Fontaine, J.X.; Jacob, D.; Botana, A.; Nicaise, V.; Richard-Forget, F.; Lecomte, S.; Decourtil, C.; Hamade, K.; Mesnard, F.; et al. Optimizing 1D 1H-NMR profiling of plant samples for high throughput analysis: Extract preparation, standardization, automation and spectra processing. Metabolomics 2019, 15, 28. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Brosio, E.; Gianferri, R.; Segre, A.L. Metabolic profile of lettuce leaves by high-field NMR spectra. Magn. Reson. Chem. 2005, 43, 625–638. [Google Scholar] [CrossRef]
- Wedeking, R.; Maucourt, M.; Deborde, C.; Moing, A.; Gibon, Y.; Goldbach, H.E.; Wimmer, M.A. 1H-NMR metabolomic profiling reveals a distinct metabolic recovery response in shoots and roots of temporarily drought-stressed sugar beets. PLoS ONE 2018, 13, e0196102. [Google Scholar] [CrossRef]
- Ingallina, C.; Sobolev, A.P.; Circi, S.; Spano, M.; Fraschetti, C.; Filippi, A.; Di Sotto, A.; Di Giacomo, S.; Mazzoccanti, G.; Gasparrini, F.; et al. Cannabis sativa, L. inflorescences from monoecious cultivars grown in central Italy: An untargeted chemical characterization from early flowering to ripening. Molecules 2020, 25, 1908. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.I.; Radziszewska, A.; Neumann, M.; Marchev, A.; Alipieva, K.; Ludwig-Müller, J.; Metabolic alterations of Verbascum nigrum, L. Metabolic alterations of Verbascum nigrum L. plants and SAArT transformed roots as revealed by NMR-based metabolomics. Plant. Cell Tissue Organ. Cult. 2015, 123, 349–356. [Google Scholar] [CrossRef]
- Ingallina, C.; Spano, M.; Sobolev, A.P.; Esposito, C.; Santarcangelo, C.; Baldi, A.; Daglia, M.; Mannina, L. Characterization of local products for their industrial use: The case of italian potato cultivars analyzed by untargeted and targeted methodologies. Foods 2020, 9, 1216. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, A.P.; Mannina, L.; Proietti, N.; Carradori, S.; Daglia, M.; Giusti, A.M.; Antiochia, R.; Capitani, D. Untargeted NMR-based methodology in the study of fruit metabolites. Molecules 2015, 20, 4088–4108. [Google Scholar] [CrossRef] [PubMed]
- de Falco, B.; Incerti, G.; Pepe, R.; Amato, M.; Lanzotti, V. Metabolomic fingerprinting of Romaneschi globe artichokes by NMR spectroscopy and multivariate data analysis. Phytochem. Anal. 2016, 27, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Albergamo, A.; Rotondo, A.; Salvo, A.; Pellizzeri, V.; Bua, D.G.; Maggio, A.; Cicero, N.; Dugo, G. Metabolite and mineral profiling of “Violetto di Niscemi” and “Spinoso di Menfi” globe artichokes by 1H-NMR and ICP-MS. Nat. Prod. Res. 2016, 31, 990–999. [Google Scholar] [CrossRef]
- Blanco, E.; Musio, B.; Todisco, S.; Mastrorilli, P.; Gallo, V.; Sonnante, G. Non-targeted NMR approach to unveil and promote the biodiversity of globe artichoke in the Mediterranean area. J. Food Compos. Anal. 2022, 110, 104539. [Google Scholar] [CrossRef]
- Lucarini, M.; Di Cocco, M.E.; Raguso, V.; Milanetti, F.; Durazzo, A.; Lombardi-Boccia, G.; Santini, A.; Delfini, M.; Sciubba, F. NMR-based metabolomic comparison of Brassica oleracea (var. italica): Organic and conventional farming. Foods 2020, 9, 945. [Google Scholar] [CrossRef]
- Ulrich, E.L.; Akutsu, H.; Doreleijers, J.F.; Harano, Y.; Ioannidis, Y.E.; Lin, J.; Livny, M.; Mading, S.; Maziuk, D.; Miller, Z.; et al. BioMagResBank. Nucleic Acids Res. 2008, 36, D402–D408. [Google Scholar] [CrossRef]
- Angyal, S.J.; Odier, L. The effect of O-methylation on chemical shifts in the 1H- and 13C-nm.r. spectra of cyclic polyols. Carbohydr. Res. 1983, 123, 23–29. [Google Scholar] [CrossRef]
- Angyal, S.J.; Odier, L. The 13C-nm.r. spectra of inositols and cyclohexanepentols: The validity of rules correlating chemical shifts with configuration. Carbohydr. Res. 1982, 100, 43–54. [Google Scholar] [CrossRef]
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef]
- Pauli, G.F.; Kuczkowiak, U.; Nahrstedt, A. Solvent effects in the structure dereplication of caffeoyl quinic acids. Magn. Reson. Chem. 1999, 37, 827–836. [Google Scholar] [CrossRef]
- Tastan, P.; Hajdú, Z.; Kúsz, N.; Zupkó, I.; Sinka, I.; Kivcak, B.; Hohmann, J. Sesquiterpene lactones and flavonoids from Psephellus pyrrhoblepharus with antiproliferative activity on human gynecological cancer cell lines. Molecules 2019, 24, 3165. [Google Scholar] [CrossRef] [PubMed]
- Hussien, T.A.; Mohamed, T.A.; Elshamy, A.I.; Moustafa, M.F.; El-Seedi, H.R.; Pare, P.W.; Hegazy, M.E.F. Guaianolide sesquiterpene lactones from Centaurothamnus maximus. Molecules 2021, 26, 2055. [Google Scholar] [CrossRef]
- Samek, Z.; Holub, M.; Drozdz, B.; Iommi, G.; Corbella, A.; Gariboldi, P.; Sesquiterpenic lactones of the Cynara scolymus, L. Sesquiterpenic lactones of the Cynara scolymus L. species. Tetrahedron Lett. 1971, 50, 4775–4778. [Google Scholar] [CrossRef]
- Barbetti, P.; Chiappini, I.; Fardella, G.; Grandolini, G. Grosulfeimin and new related guaianolides from Cynara scolymusl. Nat. Prod. Lett. 1993, 3, 21–30. [Google Scholar] [CrossRef]
- Adekenov, S.; Shaimerdenova, Z.R.; Adekenova, K.S.; Kishkentayeva, A.S. Synthesis and biological evaluation of new derivatives of grossheimin. Fitoterapia 2022, 158, 105154. [Google Scholar] [CrossRef] [PubMed]
- Ingallina, C.; Capitani, D.; Mannina, L.; Carradori, S.; Locatelli, M.; Di Sotto, A.; Di Giacomo, S.; Toniolo, C.; Pasqua, G.; Valletta, A.; et al. Phytochemical and biological characterization of Italian “sedano bianco di Sperlonga” Protected Geographical Indication celery ecotype: A multimethodological approach. Food. Chem. 2020, 309, 125649. [Google Scholar] [CrossRef]
- Salzano, A.M.; Sobolev, A.; Carbone, V.; Petriccione, M.; Renzone, G.; Capitani, D.; Vitale, M.; Minasi, P.; Pasquariello, M.S.; Novi, G.; et al. A proteometabolomic study of Actinidia deliciosa fruit development. J. Proteomics 2018, 172, 11–24. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce β-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef]
- Hibi, M.; Kawashima, T.; Yajima, H.; Smirnov, S.V.; Kodera, T.; Sugiyama, M.; Shimizu, S.; Yokozeki, K.; Ogawa, J. Enzymatic synthesis of chiral amino acid sulfoxides by Fe(II)/α-ketoglutarate-dependent dioxygenase. Tetrahedron Asymmetry 2013, 24, 990–994. [Google Scholar] [CrossRef]
- Marks, H.S.; Hilson, J.A.; Leichtweis, H.C.; Stoewsand, G.S. S-Methylcysteine sulfoxide in Brassica vegetables and formation of methyl methanethiosulfinate from brussels sprouts. J. Agric. Food Chem. 1992, 40, 2098–2101. [Google Scholar] [CrossRef]
- Edmands, W.M.B.; Beckonert, O.P.; Stella, C.; Campbell, A.; Lake, B.G.; Lindon, J.C.; Holmes, E.; Gooderham, N.J. Identification of human urinary biomarkers of cruciferous vegetable consumption by metabonomic profiling. J. Proteome Res. 2011, 10, 4513–4521. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.M.; Park, B.; Dang, Y.M.; Kim, S.Y.; Seo, H.Y. Simultaneous direct determination of 15 glucosinolates in eight Brassica species by UHPLC-Q-Orbitrap-MS. Food. Chem. 2019, 282, 127–133. [Google Scholar] [CrossRef]
- Jaki, B.; Sticher, O.; Veit, M.; Fröhlich, R.; Pauli, G.F. Evaluation of glucoiberin reference material from Iberis amara by spectroscopic fingerprinting. J. Nat. Prod. 2002, 65, 517–522. [Google Scholar] [CrossRef]
- Vo, Q.V.; Trenerry, C.; Rochfort, S.; Wadeson, J.; Leyton, C.; Hughes, A.B. Synthesis and anti-inflammatory activity of indole glucosinolates. Bioorgan. Med. Chem. 2014, 22, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Globe artichoke leaves and floral stems as a source of bioactive compounds. Ind. Crop. Prod. 2013, 44, 44–49. [Google Scholar] [CrossRef]
- Venezia, V.; Pota, G.; Silvestri, B.; Vitiello, G.; Di Donato, P.; Landi, G.; Mollo, V.; Verrillo, M.; Cangemi, S.; Piccolo, A.; et al. A study on structural evolution of hybrid humic acids-SiO2 nanostructures in pure water: Effects on physico-chemical and functional properties. Chemosphere 2022, 287, 131985. [Google Scholar] [CrossRef]
- Verrillo, M.; Salzano, M.; Savy, D.; Di Meo, V.; Valentini, M.; Cozzolino, V.; Piccolo, A. Antibacterial and antioxidant properties of humic substances from composted agricultural biomasses. Chem. Biol. Technol. Agric. 2022, 9, 28. [Google Scholar] [CrossRef]
- Kubec, R.; Dadáková, E. Quantitative determination of S-alk(en)ylcysteine-S-oxides by micellar electrokinetic capillary chromatography. J. Chromatogr. A 2008, 1212, 154–157. [Google Scholar] [CrossRef]
- Mancini, M.; Andreassi, A.; Salvioni, M.; Pelliccione, F.; Mantellassi, G.; Banderali, G. myo-Inositol and D-chiro-inositol in improving insulin resistance in obese male children: Preliminary Data. Int. J. Endocrinol. 2016, 2016, 8720342. [Google Scholar] [CrossRef]
- Croze, M.L.; Vella, R.E.; Pillon, N.J.; Soula, H.A.; Hadji, L.; Guichardant, M.; Soulage, C.O. Chronic treatment with myo-inositol reduces white adipose tissue accretion and improves insulin sensitivity in female mice. J. Nutr. Biochem. 2013, 24, 457–466. [Google Scholar] [CrossRef]
- Okazaki, Y.; Katayama, T. Effects of dietary carbohydrate and myo-inositol on metabolic changes in rats fed 1,1,1-trichloro-2,2-bis (p-chlorophenyl) ethane (DDT). J. Nutr. Biochem. 2003, 14, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Elsebai, M.F.; Mocan, A.; Atanasov, A.G. Cynaropicrin: A comprehensive research review and therapeutic potential as an anti-hepatitis C virus agent. Front. Pharmacol. 2016, 7, 472. [Google Scholar] [CrossRef] [PubMed]
- Mena-García, A.; Ruiz-Matute, A.I.; Soria, A.C.; Sanz, M.L. A multi-analytical strategy for evaluation of quality and authenticity of artichoke food supplements for overweight control. J. Chromatogr. A 2021, 1647, 462102. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Introducing inulin-type fructans. Br. J. Nutr. 2005, 93, S13–S25. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Augusti, K.T. Lipid lowering effect of S-methyl cysteine sulfoxide from Allium cepa Linn in high cholesterol diet fed rats. J. Ethnopharmacol. 2007, 109, 367–371. [Google Scholar] [CrossRef]
- Castro, V.M.D.D.; Medeiros, K.C.D.P.; Lemos, L.I.C.D.; Pedrosa, L.D.F.C.; Ladd, F.V.L.; Carvalho, T.G.D.; Araújo Júnior, R.F.D.; Abreu, B.J.; Farias, N.B.D.S. S-methyl cysteine sulfoxide ameliorates duodenal morphological alterations in streptozotocin-induced diabetic rats. Tissue Cell 2021, 69, 101483. [Google Scholar] [CrossRef]
- Almushayti, A.Y.; Brandt, K.; Carroll, M.A.; Scotter, M.J. Current analytical methods for determination of glucosinolates in vegetables and human tissues. J. Chromatogr. A 2021, 1643, 462060. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Mannina, L.; Capitani, D.; Sanzò, G.; Ingallina, C.; Botta, B.; Fornarini, S.; Crestoni, M.E.; Chiavarino, B.; Carradori, S.; et al. A multi-methodological approach in the study of Italian PDO “Cornetto di Pontecorvo” red sweet pepper. Food Chem. 2018, 255, 120–131. [Google Scholar] [CrossRef]
- Wishart, D.S.; Bigama, C.G.; Yao, J.; Abildgaard, F.; Dyson, H.J.; Oldfield, E.; Markley, J.L.; Sykes, B.D. 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J. Biomol. NMR 1995, 6, 135–140. [Google Scholar] [CrossRef] [PubMed]
| Metabolite | Assignment | δ 1H (ppm) | Multiplicity (JH-H, Hz) | δ 13C (ppm) | Globe Artichoke | Cauliflower |
|---|---|---|---|---|---|---|
| Amino acids | ||||||
| Alanine (Ala) | β-CH3 | 1.49 * | d (7.2) | 17.2 | L, S | L, S, F |
| α-CH | 3.80 | 51.5 | ||||
| Arginine (Arg) | α-CH | 3.78 | 55.1 | L | L, S, F | |
| β-CH2 | 1.93 | 28.6 | ||||
| γ, γ′-CH2 | 1.74; 1.67 * | 24.9 | ||||
| δ-CH2 | 3.24 | 41.5 | ||||
| Asparagine (Asn) | β-CH | 2.89 | dd (16.9; 7.2) | 35.6 | L, S | L, S, F |
| β′-CH | 2.96 * | dd (16.9; 4.5) | 35.6 | |||
| α-CH | 4.01 | 52.2 | ||||
| Aspartate (Asp) | β-CH | 2.71 | dd (17.4; 8.1) | 37.6 | L, S | L, S, F |
| β′-CH | 2.80 * | dd (17.4; 3.8) | 37.6 | |||
| α-CH | 3.91 | 53.2 | ||||
| γ-Aminobutyric acid (GABA) | β-CH2 | 1.91 | 24.7 | L, S | L, S, F | |
| α-CH2 | 2.30 * | t (7.4) | 35.4 | |||
| γ-CH2 | 3.02 | t (7.6) | 40.2 | |||
| Glycine (Gly) | α-CH2 | 3.57 | s | 42.5 | L, F | |
| Glutamate (Glu) | β, β′-CH2 | 2.13; 2.08 | m | 28.0 | L, S | L, S, F |
| γ-CH2 | 2.35 * | m | 34.4 | |||
| α-CH | 3.77 | 55.5 | ||||
| Glutamine (Gln) | β-CH2 | 2.15 | m | 27.3 | L, S | L, S, F |
| γ-CH2 | 2.46 * | m | 31.8 | |||
| α-CH | 3.78 | 55.2 | ||||
| Histidine (His) | β, β′-CH2 | 3.30; 3.24 | 28.2 | L | L, S, F | |
| α-CH | 4.02 | 55.4 | ||||
| CH-5 | 7.18 | 118.3 | ||||
| CH-2 | 8.13 * | |||||
| Isoleucine (Ile) | δ-CH3 | 0.94 | t (7.4) | 12.1 | L, S | L, S, F |
| γ-CH3 | 1.01 * | d (7.0) | 15.7 | |||
| γ, γ′-CH2 | 1.48; 1.27 | 25.5 | ||||
| β-CH | 1.98 | 36.8 | ||||
| α-CH | 3.68 | 60.5 | ||||
| Leucine (Leu) | δ-CH3 | 0.96 * | d (6.2) | 22.1 | L, S | L, S, F |
| δ′-CH3 | 0.97 * | d (6.0) | 23.0 | |||
| β-CH2 | 1.73 | 40.9 | ||||
| α-CH | 3.74 | 54.4 | ||||
| γ-CH | 1.70 | 25.2 | ||||
| Lysine (Lys) | α-CH | 3.77 | 55.3 | L | L, F | |
| β-CH2 | 1.92 | 30.9 | ||||
| γ-CH2 | 1.49 | 22.5 | ||||
| δ-CH2 | 1.74 | 27.4 | ||||
| ε-CH2 | 3.04 * | t (7.6) | 40.0 | |||
| S-Methyl-L-cysteine-S-oxide (Methiin) | α-CH | 4.18 | t (6.8) | 51.8 | L, S, F | |
| β-CH2 | 3.47; 3.28 | dd (14.0; 7.2) | 54.5 | |||
| γ-CH3 | 2.84 * | s | 39.1 | |||
| COOH | 173.1 | |||||
| Phenylalanine (Phe) | β, β′-CH2 | 3.27; 3.16 | 37.4 | L, S | L, S, F | |
| α-CH | 4.00 | 56.9 | ||||
| CH-2,6 | 7.34 | d (7.3) | 130.5 | |||
| CH-4 | 7.38 | t (7.0) | 128.7 | |||
| CH-3,5 | 7.43 * | t (7.3) | 130.2 | |||
| Pyroglutamic acid | β, β’-CH2 | 2.04; 2.51 | 26.3 | L | L, F | |
| γ-CH2 | 2.40 | 30.7 | ||||
| α-CH | 4.18 | 59.3 | ||||
| Threonine (Thr) | γ-CH3 | 1.34 * | d (6.6) | 20.5 | L, S | L, S, F |
| α-CH | 3.61 | 61.4 | ||||
| β-CH | 4.26 | qd (6.6; 4.9) | 66.9 | |||
| Tryptophan (Trp) | CH-4 | 7.73 * | d (7.9) | 119.5 | L | L, S, F |
| CH-7 | 7.55 | d (7.4) | 113.0 | |||
| CH-6 | 7.29 | t (7.4) | 123.2 | |||
| CH-5 | 7.20 | 120.4 | ||||
| CH-2 | 7.34 | s | 126.2 | |||
| α-CH | 4.06 | 55.9 | ||||
| β, β′-CH2 | 3.48; 3.32 | 27.4 | ||||
| Tyrosine (Tyr) | CH-2,6 | 7.20 | d (8.5) | 131.7 | L | L, S, F |
| CH-3,5 | 6.91 * | d (8.5) | 116.9 | |||
| α-CH | 3.95 | 57.1 | ||||
| β, β′-CH2 | 3.19; 3.07 | 36.5 | ||||
| Valine (Val) | γ-CH3 | 1.00 | d (7.0) | 17.7 | L, S | L, S, F |
| γ′-CH3 | 1.05 * | d (7.0) | 19.0 | |||
| β-CH | 2.28 | 30.1 | ||||
| α-CH | 3.62 | 61.3 | ||||
| Organic acids | ||||||
| Acetic acid (AA) | αCH3 | 1.93 * | s | 24.4 | L, S | L, S, F |
| Citric acid (CA) | α, γ-CH | 2.54 *; 2.68 | d (15.5) | 46.5 | L, S | L, S, F |
| Formic acid (FA) | HCOO- | 8.47 * | s | 173.8 | L, S | L, S, F |
| Fumaric acid (FumA) | α, β-HC=CH | 6.53 * | s | 136.5 | L, S | L, S, F |
| Lactic acid (LA) | β-CH3 | 1.33 * | d (6.9) | 21.1 | S | L |
| α-CH | 4.12 | 69.6 | ||||
| Malic acid (MA) | β-CH | 2.68 | dd (15.4; 3.2) | 43.6 | L, S | L, S, F |
| β′-CH | 2.39 | dd (15.4; 10.0) | 43.6 | |||
| α-CH | 4.30 * | dd (10.0; 3.2) | 71.4 | |||
| Pyruvic acid (PA) | CH3 | 2.36 | s | 30.0 | L, F | |
| Quinic acid (QA) | C(OH)COOH | 78.1 | L, S | L | ||
| 2,2′-CH2 | 2.04; 1.97 | 38.5 | ||||
| 6,6′-CH2 | 2.08; 1.88 * | 41.8 | ||||
| CH-3 | 4.16 | 71.5 | ||||
| CH-4 | 3.56 | 76.3 | ||||
| CH-5 | 4.03 | 68.0 | ||||
| Succinic acid (SA) | α, β-CH2 | 2.41 * | s | 35.1 | L, S | L, S, F |
| Carbohydrates, polyols | ||||||
| α-Glucose (α-Glc) | CH-1 | 5.25 * | d (3.8) | 93.1 | L, S | L, S, F |
| CH-2 | 3.55 | 72.4 | ||||
| CH-3 | 3.72 | 73.8 | ||||
| CH-4 | 3.42 | 70.7 | ||||
| CH-5 | 3.84 | 72.5 | ||||
| CH2-6 | 3.84; 3.78 | 61.6 | ||||
| β-Glucose (β-Glc) | CH-1 | 4.65 * | d (8.0) | 96.9 | L, S | L, S, F |
| CH-2 | 3.26 | 75.2 | ||||
| CH-3 | 3.50 | 76.7 | ||||
| CH-4 | 3.42 | 70.7 | ||||
| CH-5 | 3.47 | 76.9 | ||||
| CH2-6 | 3.90; 3.74 | 61.7 | ||||
| α-Fructofuranose | CH-3 | 4.13 * | 82.9 | L, S | L, S, F | |
| CH-5 | 4.07 | 82.4 | ||||
| β-Fructofuranose | CH2-1,1′ | 3.60; 3.57 | 63.8 | L, S | L, S, F | |
| CH-3 | 4.12 * | 76.4 | ||||
| CH-4 | 4.12 * | 75.4 | ||||
| CH-5 | 3.83 | 81.6 | ||||
| CH2-6,6′ | 3.81; 3.68 | 63.3 | ||||
| β-Fructopyranose | CH2-1, 1′ | 3.72; 3.56 | 64.8 | L, S | L, S, F | |
| CH-3 | 3.81 | 68.5 | ||||
| CH-4 | 3.90 | 70.6 | ||||
| CH-5 | 4.00 | 70.2 | ||||
| CH2-6,6′ | 4.03; 3.71 | 64.4 | ||||
| Sucrose (Suc) | CH-1 | 5.42 * | d (3.8) | 93.2 | L, S | L, S, F |
| CH-2 | 3.56 | 72.0 | ||||
| CH-3 | 3.77 | 73.5 | ||||
| CH-4 | 3.48 | 70.2 | ||||
| CH-5 | 3.85 | 73.4 | ||||
| CH2-6 | 3.82 | 61.2 | ||||
| CH2-1′ | 3.69 | 62.4 | ||||
| C-2 | / | 104.8 | ||||
| CH-3′ | 4.22 | 77.4 | ||||
| CH-4′ | 4.06 | 75.0 | ||||
| CH-5′ | 3.90 | 82.4 | ||||
| CH-6′ | 3.82 | 63.4 | ||||
| Raffinose | CH-1 (Gal) | 5.00 * | d (3.8) | 99.4 | L, S | |
| CH-1(Glc) | 5.44 | d (3.8) | 93.2 | |||
| Inulin | CH-1 (Glc) | 5.44 * | 93.5 | L, S | ||
| CH-3 (Fru) | 4.27 | 77.8 | ||||
| CH-4 (Fru) | 4.10 | 75.3 | ||||
| chiro-Inositol | CH-1,6 | 4.05 | 72.7 | L, S | ||
| CH-2,5 | 3.76 | 71.4 | ||||
| CH-3,4 | 3.59 * | 73.8 | ||||
| myo-Inositol | CH-2,5 | 3.54 | 72.4 | L, S | L, S, F | |
| CH-1 | 4.08 | 73.2 | ||||
| CH-3,6 | 3.63 | 73.5 | ||||
| CH-4 | 3.29 * | 75.3 | ||||
| scyllo-Inositol | CH-1,2,3,4,5,6 | 3.36 * | s | 74.6 | L, S | |
| Glucosinolates | ||||||
| Glucoiberin | CH-1′ (Gluc) | 5.08 * | d (9.8) | 82.7 | L | |
| CH-2′ | 3.47 | 72.9 | ||||
| CH-3′ | 3.58 | 78.1 | ||||
| CH-4′ | 3.47 | 70.2 | ||||
| CH-5′ | 3.60 | 81.2 | ||||
| CH2-6a′,6b′ | 3.92; 3.73 | 61.7 | ||||
| S-CH3 | 2.74 | s | 37.7 | |||
| α-CH2 | 3.05; 2.99 | 52.5 | ||||
| β-CH2 | 2.21 | 20.7 | ||||
| γ-CH2 | 2.95 | 31.9 | ||||
| C=N | - | 163.6 | ||||
| Glucobrassicin | CH-1′ (Gluc) | 4.84 | 82.4 | L | ||
| CH-2′ | 3.31 | 72.8 | ||||
| CH-3′ | 3.23 | 77.9 | ||||
| CH-4′ | 3.38 | 69.6 | ||||
| CH-5′ | 2.96 | 80.9 | ||||
| CH2-6′ | 3.59 | 61.2 | ||||
| CH2-1a, 1b | 4.28; 4.22 | 30.4 | ||||
| CH-2″ (Ind) | 7.37 | 125.2 | ||||
| CH-4″ | 7.76 * | d (8.0) | 119.5 | |||
| CH-5″ | 7.21 | 120.8 | ||||
| CH-6″ | 7.28 | 123.2 | ||||
| CH-7″ | 7.56 | 113.1 | ||||
| Glucoraphanin | CH-1′ (Gluc) | 5.05 | d (9.8) | L | ||
| S-CH3 | 2.72 | s | ||||
| Sinigrin | CH-1′ (Gluc) | 5.07 | d (9.8) | L | ||
| Miscellaneous | ||||||
| Chlorogenic acid (5-caffeoylquinic acid) | 2,2′-CH2 | 2.19; 2.02 | 39.5 | L, S | ||
| 6,6′-CH2 | 2.14; 2.06 | 38.4 | ||||
| CH-3 | 4.26 | 71.7 | ||||
| CH-4 | 3.88 | 73.9 | ||||
| CH-5 | 5.32 * | 72.2 | ||||
| CH-2′ | 7.18 | d (1.6) | 116.1 | |||
| CH-5′ | 6.94 | d (8.3) | 117.5 | |||
| CH-6′ | 7.09 | dd (8.3; 1.6) | 123.7 | |||
| CH-7′ | 7.60 | d (15.9) | 147.2 | |||
| CH-8′ | 6.37 | d (15.9) | 115.6 | |||
| Neochlorogenic acid (3-caffeoylquinic acid) | 2,2′-CH2 | 2.21; 2.09 | S | |||
| 6,6′-CH2 | 2.11; 1.93 | |||||
| CH-3 | 5.40 | 74.0 | ||||
| CH-4 | 3.76 | |||||
| CH-5 | 4.17 | |||||
| CH-2′ | 7.23 | 116.1 | ||||
| CH-5′ | 6.97 | 117.5 | ||||
| CH-6′ | 7.14 | 123.7 | ||||
| CH-7′ | 7.66 | d (16.0) | 147.2 | |||
| CH-8′ | 6.44 | d (16.0) | 115.6 | |||
| Glycine betaine | N(CH3)3 | 3.27 * | 54.4 | L | ||
| CH2 | 3.91 | 67.3 | ||||
| Ethanolamine | CH2-NH2 | 3.15 * | 42.2 | L | L, S, F | |
| CH2OH | 3.83 | 58.6 | ||||
| Choline | N(CH3)3 | 3.21 * | s | 54.9 | L, S | L, S, F |
| CH2OH | 4.06 | 56.6 | ||||
| CH2N | 3.52 | 68.4 | ||||
| Phosphorylcholine | N(CH3)3 | 3.23 * | s | 55.0 | L, S | L, S, F |
| CH2OPO3 | 4.15 | 62.2 | ||||
| CH2N | 3.61 | 67.4 | ||||
| Glycerophosphorylcholine | N(CH3)3 | 3.24 * | s | 55.1 | L | L, S, F |
| CH2N | 3.68 | 67.0 | ||||
| CH2OP | 4.33 | 60.5 | ||||
| CH2OP | 3.94; 3.89 | 67.5 | ||||
| CHOH | 3.92 | 71.6 | ||||
| CH2OH | 3.67; 3.6 | 63.1 | ||||
| Trigonelline | CH3 | 4.44 | s | 49.3 | L, S | L, F |
| CH-6 | 9.12 | |||||
| CH-4,2 | 8.84 * | |||||
| CH-3 | 8.09 | 128.8 | ||||
| Uridine | CH-6 | 7.87 | d (8.2) | 143.0 | L, S | L, S, F |
| CH-5 | 5.92 * | d (8.2) | 103.4 | |||
| CH-1′ (rib) | 5.93 * | d (4.6) | 90.3 | |||
| CH-2′ (rib) | 4.36 | 74.6 | ||||
| CH-3′ (rib) | 4.24 | 70.5 | ||||
| CH-4′ (rib) | 4.14 | 85.3 | ||||
| Position | Cynaropicrin | Dehydrocynaropicrin | Grosheimin | |||
|---|---|---|---|---|---|---|
| 1H | 13C | 1H | 13C | 1H | 13C | |
| 1 | 2.99 ddd (10.2; 9.8; 7.2) | 45.4 | 3.30 | 40.9 | 3.21 | 40.3 |
| 2a | 2.17 dt (12.9; 7.1) | 39.1 | 2.64 dd (18.6; 8.4) | 43.7 | 2.58 | 43.7 |
| 2b | 1.74 ddd (12.8;11.6; 8.6) | 39.1 | 2.58 | 43.7 | 2.52 | 43.7 |
| 3 | 4.52 ddt (8.5; 7.3; 2.2) | 73.3 | - | 205.2 | - | 220.8 |
| 4 | - | 152.3 | - | 2.34 | 47.5 | |
| 5 | 2.87 | 51.3 | 3.35 | 49.4 | 2.35 | 51.3 |
| 6 | 4.31 dd (10.6; 9.0) | 79.4 | 4.18 dd (9.9; 8.9) | 80.4 | 4.03 t (9.0) | 83.6 |
| 7 | 3.25 | 47.7 | 3.50 | 46.9 | 3.11 | 49.6 |
| 8 | 5.14 dd (5.1; 3.5) | 74.5 | 5.13 | 74.5 | 3.80 dd (10.0; 6.1) | 73.1 |
| 9a | 2.72 dd (14.8; 5.2) | 37.0 | 2.92 dd( 13.4; 5.7) | 41.5 | 2.85 | 48.5 |
| 9b | 2.41 dd (14.8; 3.6) | 37.0 | 2.38 | 41.5 | 2.29 | 48.5 |
| 10 | - | 142.2 | - | - | 144.2 | |
| 12 | - | 170.2 | - | 170.0 | - | 171.1 |
| 13a | 6.20 d (3.5) | 122.9 | 6.30 d (3.3) | 125.0 | 6.37 dd (2.8; 1.2) | 126.0 |
| 13b | 5.67 * d (3.3) | 122.9 | 5.85 * d (3.0) | 125.0 | 6.33 * dd (3.3; 1.2) | 126.0 |
| 14a | 5.16 | 118.2 | 5.09 | 115.2 | 5.07 | 115.2 |
| 14b | 4.94 d (1.5) | 118.2 | 4.77 | 115.2 | 4.78 | 115.2 |
| 15a | 5.47 t (1.8) | 113.0 | 6.28 dd (2.4; 0.6) | 123.6 | 1.26 | 14.9 |
| 15b | 5.38 t (2.3) | 113.0 | 5.89 dd (2.2; 0.6) | 123.6 | ||
| 1′ | - | 165.7 | - | |||
| 2′ | - | 140.2 | - | |||
| 3′a | 6.35 q (1.2) | 126.0 | 6.37 | 126.3 | ||
| 3′b | 6.01 q (1.6) | 126.0 | 6.04 | 126.3 | ||
| 4′ | 4.35 t (1.3) | 61.0 | 4.36 | 61.0 | ||
| Metabolite | Assignment | δ 1H (ppm) | Multiplicity | δ 13C (ppm) | Globe Artichoke | Cauliflower |
|---|---|---|---|---|---|---|
| Pheophytin a | CH-10 | 9.54 | s | 105.0 | L | L |
| CH-5 | 9.39 * | s | 97.8 | |||
| CH-20 | 8.59 | s | 93.7 | |||
| CH-31 | 8.01 | dd (17.7; 11.5) | 129.3 | |||
| CH2-32 | 6.32; 6.22 | dd (17.7; 1.1) dd (11.5; 1.2) | 123.5 | |||
| CH-P2 | 4.89 | 118.0 | ||||
| CH-18 | 4.48 | 50.5 | ||||
| CH-17 | 4.12 | 52.0 | ||||
| CH3-134 | 3.91 | s | 53.1 | |||
| CH2-81 | 3.70 | 19.7 | ||||
| CH3-181 | 1.82 | d (7.6) | 23.4 | |||
| CH3-82 | 1.71 | t (7.9) | 17.6 | |||
| Pheophytin b | CH-71 | 11.19 | s | L | L | |
| CH-5 | 9.98 * | s | ||||
| CH-10 | 9.65 | s | ||||
| CH-31 | 7.93 | dd (17.8; 11.5) | ||||
| CH2-32 | 6.24; 6.02 | |||||
| Squalene | CH3 -a | 1.69 | 25.3 | L, S | L, F | |
| CH3 -b | 1.61 | 16.2 | ||||
| CH -c | 5.12 * | 124.6 | ||||
| CH2-d | 1.99 | 40.2 | ||||
| CH2-e | 2.07 | 26.8 | ||||
| Linolenic acid chains | CH2-2 | 2.32 | 34.6 | L, S | L, S, F | |
| CH2-3 | 1.63 | 25.3 | ||||
| CH2-4-7 | 1.32 | |||||
| CH2-8 | 2.06 | 27.5 | ||||
| CH-9 | 5.38 | 130.3 | ||||
| CH2-11,14 | 2.81 * | t (6.1) | 25.9 | |||
| CH-10, 12,13 | 5.36 | 128.5 | ||||
| CH-15 | 5.31 | 127.4 | ||||
| CH-16 | 5.39 | 132.2 | ||||
| CH2-17 | 2.09 | 20.9 | ||||
| CH3-18 | 0.98 | t (7.6) | 14.5 | |||
| Linoleic acid chains | CH2-2 | 2.32 | 34.6 | L, S | L, S, F | |
| CH2-3 | 1.63 | 25.3 | ||||
| CH2-4–7 | 1.32 | |||||
| CH2-8,14 | 2.06 | 27.5 | ||||
| CH-9,13 | 5.37 | 130.5 | ||||
| CH-10,12 | 5.35 | 128.4 | ||||
| CH2-11 | 2.78 * | t (6.7) | 25.8 | |||
| CH3-18 | 0.90 | t (7.6) | 14.3 | |||
| Phosphatidylcholine | (CH3)3N | 3.23 | 54.5 | L, S | L, F, S | |
| CH2OP | 4.44 | 61.9 | ||||
| CH2 sn1 | 4.39; 4.17 | 63.1 | ||||
| CH sn2 | 5.26 | 70.8 | ||||
| CH2 sn3 | 4.14 | 65.4 | ||||
| Phosphatidylethanolamine | CH2NH2 | 3.16 | 40.8 | S, F | ||
| CH2OP | 4.10 | 62.1 | ||||
| Digalactosyldiacylglycerol | CH-1′ | 4.23 | 104.3 | L, S | L, F, S | |
| CH-2′ | 3.53 | 71.7 | ||||
| CH-3′ | 3.51 | 73.8 | ||||
| CH-4′ | 3.92 | 68.5 | ||||
| CH-1″ | 4.91 * | 99.7 | ||||
| CH-3″,5″ | 3.74 | 70.6 | ||||
| CH-4″ | 3.96 | 70.2 | ||||
| CH2-6″ | 3.82; 3.74 | 61.8 | ||||
| CH2 sn3 | 3.95; 3.72 | 68.5 | ||||
| CH2 sn1 | 4.39; 4.17 | 63.1 | ||||
| β-Sitosterol | CH2-1 | 1.85; 1.07 | 37.6 | L, S | L, F, S | |
| CH2-2 | 1.82 | 31.4 | ||||
| CH-3 | 3.54 | 71.6 | ||||
| CH2-4 | 2.25 | 42.1 | ||||
| CH-6 | 5.34 | 121.8 | ||||
| CH-8 | 1.47 | 32.2 | ||||
| CH-9 | 0.94 | 50.6 | ||||
| CH2-11 | 1.51 | 21.4 | ||||
| CH2-12 | 2.00; 1.17 | 40.0 | ||||
| CH-14 | 1.01 | 57.1 | ||||
| CH2-16 | 1.86 | 28.4 | ||||
| CH-17 | 1.13 | 56.4 | ||||
| CH3-18 | 0.70 * | 12.1 | ||||
| CH3-19 | 1.02 | 19.5 | ||||
| CH-20 | 1.35 | 36.5 | ||||
| CH3-21 | 0.94 | 18.9 | ||||
| CH2-23 | 1.19 | 26.4 | ||||
| CH-24 | 0.95 | 46.1 | ||||
| Campesterol | CH3-18 | 0.70 * | 12.0 | L, S | L, F, S | |
| Stigmasterol | CH3-18 | 0.72 * | 11.9 | L, S |
| Metabolite | Globe Artichoke | Cauliflower | |||
|---|---|---|---|---|---|
| L | S | L | S | F | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Amino acids | |||||
| Alanine | 0.30 ± 0.017 a | 0.29 ± 0.015 a | 4.72 ± 0.22 a | 1.96 ± 0.02 b | 7.01 ± 0.05 c |
| Arginine | 5.23 ± 0.22 | 3.90 ± 0.45 | |||
| Asparagine | 14.48 ± 1.45 a | 2.59 ± 0.43 b | 4.04 ± 0.27 a | 2.78 ± 0.05 b | 6.83 ± 0.13 c |
| Aspartate | 1.11 ± 0.19 a | 0.28 ± 0.036 b | 2.41 ± 0.23 a | 2.66 ± 0.30 a | 6.03 ± 0.14 b |
| GABA | 4.27 ± 0.14 a | 1.18 ± 0.12 b | 4.68 ± 0.20 c | ||
| Glutamate | 1.41 ± 0.12 a | 0.55 ± 0.058 b | 2.76 ± 0.09 a | 1.97 ± 0.17 b | 3.86 ± 0.07 c |
| Glutamine | 1.52 ± 0.065 a | 0.70 ± 0.264 b | 9.78 ± 0.64 a | 12.19 ± 0.45 b | 37.63 ± 0.54 c |
| Histidine | 0.27 ± 0.093 a | 0.039 ± 0.011 b | 1.06 ± 0.02 a | 0.21 ± 0.019 b | 1.09 ± 0.01 a |
| Isoleucine | 0.12 ± 0.013 a | 0.058 ± 0.0061 b | 1.23 ± 0.04 a | 0.32 ± 0.024 b | 1.17 ± 0.03 a |
| Leucine | 0.20 ± 0.009 a | 0.064 ± 0.0092 b | 1.46 ± 0.08 a | 0.19 ± 0.008 b | 0.58 ± 0.005 c |
| Lysine | 1.41 ± 0.08 | 0.41 ± 0.013 | |||
| Methiin | 4.83 ± 0.04 a | 4.95 ± 0.27 a | 20.71 ± 0.24 b | ||
| Phenylalanine | 1.32 ± 0.071 a | 0.17 ± 0.013 b | 2.24 ± 0.10 a | 0.23 ± 0.012 b | 0.84 ± 0.016 c |
| Threonine | 0.49 ± 0.030 a | 0.14 ± 0.012 b | 2.37 ± 0.11 a | 1.19 ± 0.09 b | 1.76 ± 0.09 c |
| Tryptophan | 0.56 ± 0.028 | 0.66 ± 0.05 a | 0.12 ± 0.012 b | 0.26 ± 0.004 c | |
| Tyrosine | 1.20 ± 0.08 a | 0.14 ± 0.010 b | 0.37 ± 0.007 c | ||
| Valine | 0.50 ± 0.018 a | 0.15 ± 0.020 b | 3.09 ± 0.01 a | 1.01 ± 0.021 b | 3.94 ± 0.03 c |
| Organic acids | |||||
| Acetic acid | 0.14 ± 0.049 a | 0.061 ± 0.011 a | 2.68 ± 0.209 a | 0.10 ± 0.031 b | 0.36 ± 0.032 b |
| Citric acid | 5.30 ± 0.192 a | 0.58 ± 0.026 b | 5.80 ± 0.03 a | 6.67 ± 0.06 b | 5.95 ± 0.27 a |
| Formic acid | 0.034 ± 0.009 a | 0.032 ± 0.006 a | 0.051 ± 0.006 a | 0.029 ± 0.011 b | 0.027 ± 0.001 b |
| Fumaric acid | 0.082 ± 0.011 a | 0.051 ± 0.010 b | 0.045 ± 0.002 a | 0.082 ± 0.024 b | 1.04 ± 0.005 c |
| Lactic acid | 0.34 ± 0.085 | 0.73 ± 0.086 a | 0.040 ± 0.009 b | 0.065 ± 0.011 b | |
| Malic acid | 7.48 ± 0.54 a | 8.32 ± 0.77 a | 3.32 ± 0.24 a | 12.90 ± 0.18 b | 24.72 ± 0.14 c |
| Quinic acid | 6.51 ± 0.57 a | 7.61 ± 0.26 b | |||
| Succinic acid | 1.97 ± 0.05 a | 1.15 ± 0.12 b | 2.87 ± 0.381 | 0.505 ± 0.014 | |
| Carbohydrates, polyols | |||||
| Fructose | 5.15 ± 1.07 a | 30.2 ± 0.35 b | 37.90 ± 4.33 a | 83.08 ± 2.23 b | 68.33 ± 0.39 c |
| Glucose | 24.8 ± 5.00 a | 203.5 ± 4.02 b | 61.07 ± 1.66 a | 196.8 ± 6.88 b | 70.72 ± 0.43 a |
| Inulin | 0.44 ± 0.026 a | 4.14 ± 0.65 b | |||
| Raffinose | 0.69 ± 0.032 | 3.09 ± 0.27 | |||
| Sucrose | 5.87 ± 1.56 a | 29.47 ± 3.10 b | 6.68 ± 0.62 a | 27.32 ± 0.47 b | 21.22 ± 0.44 c |
| chiro-Inositol | 10.07 ± 2.69 a | 5.05 ± 0.33 b | |||
| myo-Inositol | 0.27 ± 0.013 a | 1.95 ± 0.15 b | 3.06 ± 0.20 | 2.61 ± 0.38 | |
| scyllo-Inositol | 1.78 ± 0.05 a | 0.57 ± 0.034 b | |||
| Miscellaneous | |||||
| Glucoiberin | 2.09 ± 0.138 | ||||
| Glucobrassicin | 1.43 ± 0.078 | ||||
| Chlorogenic acid | 7.53 ± 0.41 a | 2.32 ± 0.26 b | |||
| Glycine betaine | 0.30 ± 0.029 a | ||||
| Choline | 1.01 ± 0.09 a | 0.35 ± 0.005 b | 1.57 ± 0.077 a | 0.73 ± 0.022 b | 4.73 ± 0.06 c |
| Ethanolamine | 0.066 ± 0.010 a | 0.11 ± 0.010 b | 0.31 ± 0.017 a | 0.22 ± 0.015 b | 0.79 ± 0.012 c |
| Glycerophosphorylcholine | 0.090 ± 0.007 a | 0.42 ± 0.059 | |||
| Phosphorylcholine | 0.28 ± 0.039 a | 0.12 ± 0.005 b | 0.11 ± 0.016 a | 0.25 ± 0.024 b | 0.58 ± 0.029 c |
| Trigonelline | 0.30 ± 0.012 a | 0.064 ± 0.003 b | 0.063 ± 0.003 | ||
| Uridine | 0.13 ± 0.010 a | 0.057 ± 0.010 b | 0.373 ± 0.040 a | 0.14 ± 0.005 b | 0.55 ± 0.006 c |
| Metabolite | Globe Artichoke | Cauliflower | |||
|---|---|---|---|---|---|
| L | S | L | S | F | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Sterols | |||||
| β-Sitosterol + Campesterol | 7.53 ± 0.68 b | 4.43 ± 0.27 a | 9.07 ± 1.26 a | 7.14 ± 1.91 a | 25.53 ± 2.13 b |
| Stigmasterol | 2.56 ± 0.25 b | 1.26 ± 0.10 a | |||
| Fatty acids | |||||
| Saturated + monounsaturated fatty chains | 65.1 ± 14.8 a | 73.3 ± 11.2 a | 61.5 ± 15.5 a | 52.5 ± 21.8 a | 109.3 ± 1.8 b |
| Linoleic fatty chains | 18.9 ± 1.5 b | 12.9 ± 0.4 a | 22.2 ± 3.6 b | 8.0 ± 1.5 a | 29.3 ± 2.4 c |
| Linolenic fatty chains | 58.0 ± 4.3 b | 8.2 ± 0.5 a | 76.7 ± 10.8 b | 17.2 ± 0.6 a | 104.7 ± 8.1 c |
| Miscellaneous | |||||
| Digalactosyldiacylglycerol | 7.55 ± 0.63 b | 1.52 ± 0.14 a | 5.41 ± 0.72 c | 0.80 ± 0.19 a | 2.36 ± 0.26 b |
| Squalene | 9.07 ± 2.24 b | 1.35 ± 0.27 a | 6.83 ± 0.93 | 1.61 ± 0.15 | |
| Pheophytin a | 4.76 ± 0.97 | 4.68 ± 1.53 | |||
| Pheophytin b | 1.30 ± 0.24 | 0.86 ± 0.14 | |||
| Sesquiterpene lactones | |||||
| Cynaropicrin | 27.5 ± 2.7 b | 2.47 ± 0.21 a | |||
| Dehydrocynaropicrin | 7.22 ± 0.92 | ||||
| Grosheimin | 10.8 ± 1.7 b | 1.00 ± 0.10 a | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ingallina, C.; Di Matteo, G.; Spano, M.; Acciaro, E.; Campiglia, E.; Mannina, L.; Sobolev, A.P. Byproducts of Globe Artichoke and Cauliflower Production as a New Source of Bioactive Compounds in the Green Economy Perspective: An NMR Study. Molecules 2023, 28, 1363. https://doi.org/10.3390/molecules28031363
Ingallina C, Di Matteo G, Spano M, Acciaro E, Campiglia E, Mannina L, Sobolev AP. Byproducts of Globe Artichoke and Cauliflower Production as a New Source of Bioactive Compounds in the Green Economy Perspective: An NMR Study. Molecules. 2023; 28(3):1363. https://doi.org/10.3390/molecules28031363
Chicago/Turabian StyleIngallina, Cinzia, Giacomo Di Matteo, Mattia Spano, Erica Acciaro, Enio Campiglia, Luisa Mannina, and Anatoly Petrovich Sobolev. 2023. "Byproducts of Globe Artichoke and Cauliflower Production as a New Source of Bioactive Compounds in the Green Economy Perspective: An NMR Study" Molecules 28, no. 3: 1363. https://doi.org/10.3390/molecules28031363
APA StyleIngallina, C., Di Matteo, G., Spano, M., Acciaro, E., Campiglia, E., Mannina, L., & Sobolev, A. P. (2023). Byproducts of Globe Artichoke and Cauliflower Production as a New Source of Bioactive Compounds in the Green Economy Perspective: An NMR Study. Molecules, 28(3), 1363. https://doi.org/10.3390/molecules28031363










