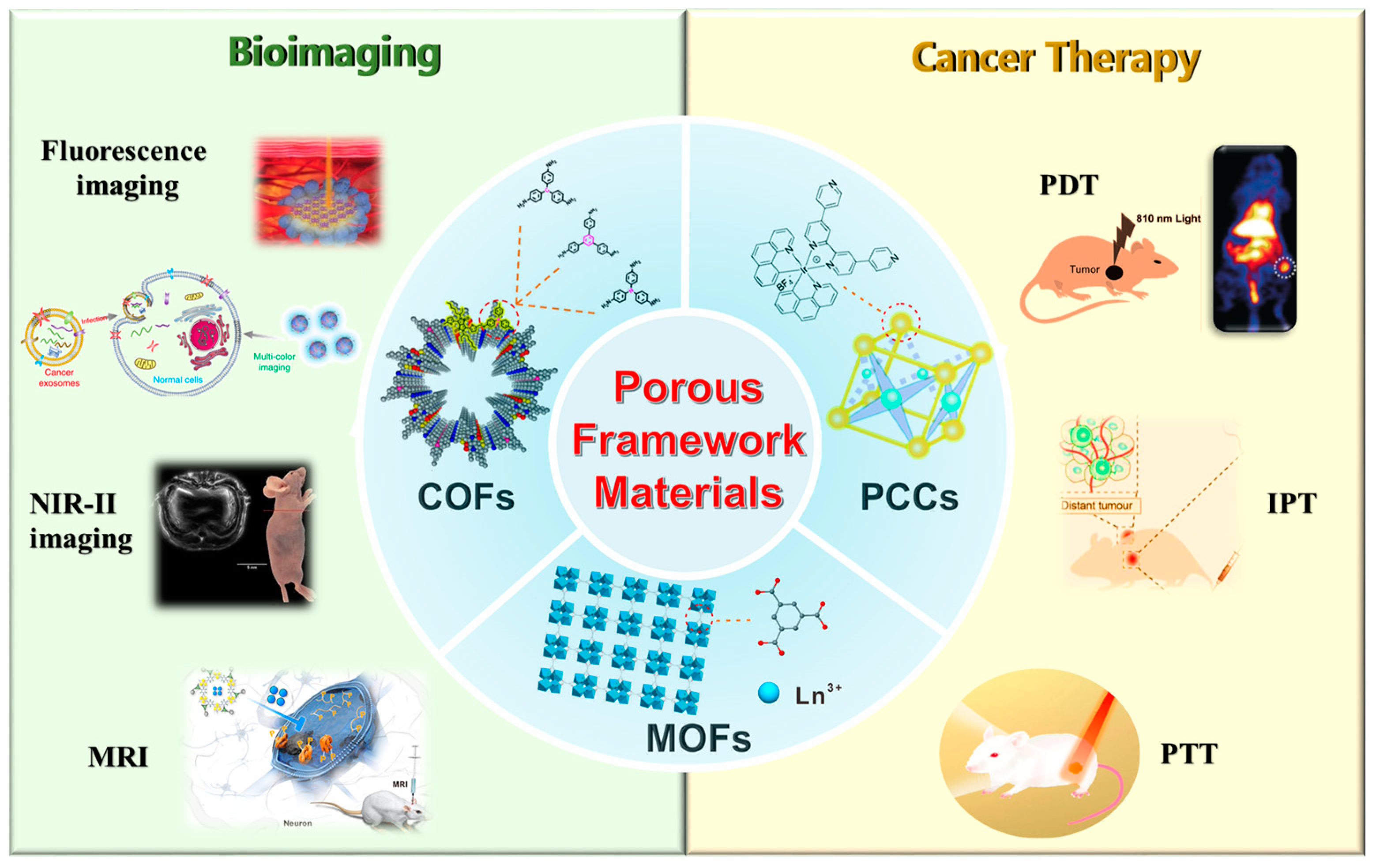
Porous Framework Materials for Bioimaging and Cancer Therapy
Abstract
1. Introduction
2. Porous Framework Materials in Bioimaging
2.1. MOF in Bioimaging
2.2. COF in Bioimaging
2.3. PCC in Bioimaging
3. Porous Framework Materials for Cancer Therapy
3.1. MOF in Cancer Therapy
3.2. COF in Cancer Therapy
3.3. PCC in Cancer Therapy
4. Conclusions, Challenges, and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Zhang, W.; Zhu, G.; Xie, J.; Chen, X. Rethinking cancer nanotheranostics. Nat. Rev. Mater. 2017, 2, 17024. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Zhou, L.L.; Li, W.Y.; Li, Y.A.; Dong, Y.B. Covalent Organic Frameworks (COFs) for Cancer Therapeutics. Chem. Eng. J. 2020, 26, 5583–5591. [Google Scholar]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immun. 2022, 71, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Karimi-Maleh, H.; Taheriazam, A.; Mirzaei, S.; Hashemi, M.; Hushmandi, K.; Makvandi, P.; Nazarzadeh Zare, E.; Sharifi, E.; et al. (Nano)platforms in bladder cancer therapy: Challenges and opportunities. Bioeng. Transl. Med. 2023, 8, e10353. [Google Scholar] [CrossRef]
- Lee, N.-H.; You, S.; Taghizadeh, A.; Taghizadeh, M.; Kim, H.S. Cell Membrane-Cloaked Nanotherapeutics for Targeted Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2223. [Google Scholar] [CrossRef]
- Li, J.-Q.; Zhao, R.-X.; Yang, F.-M.; Qi, X.-T.; Ye, P.-K.; Xie, M. An erythrocyte membrane-camouflaged biomimetic nanoplatform for enhanced chemo-photothermal therapy of breast cancer. J. Mater. Chem. B 2022, 10, 2047–2056. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Ren, Y.; Li, S.; Fu, J.; Shi, J. Study on biomimetic nano tumor targeted delivery system for chemotherapy-laser immunotherapy. Eur. J. Pharm. Biopharm. 2022, 176, 133–152. [Google Scholar] [CrossRef]
- Radhakrishnan, D.; Mohanan, S.; Choi, G.; Choy, J.-H.; Tiburcius, S.; Trinh, H.T.; Bolan, S.; Verrills, N.; Tanwar, P.; Karakoti, A.; et al. The emergence of nanoporous materials in lung cancer therapy. Sci. Technol. Adv. Mat. 2022, 23, 225–274. [Google Scholar] [CrossRef]
- Sondhi, P.; Lingden, D.; Bhattarai, J.K.; Demchenko, A.V.; Stine, K.J. Applications of Nanoporous Gold in Therapy, Drug Delivery, and Diagnostics. Metals 2023, 13, 78. [Google Scholar] [CrossRef]
- Singh, N.; Son, S.; An, J.; Kim, I.; Choi, M.; Kong, N.; Tao, W.; Kim, J.S. Nanoscale porous organic polymers for drug delivery and advanced cancer theranostics. Chem. Soc. Rev. 2021, 50, 12883–12896. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, A.; Deshpande, N.; Kulkarni, A.A. Engineered Multifunctional Nano- and Biological Materials for Cancer Immunotherapy. Adv. Healthc. Mater. 2021, 10, 2001680. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Sharma, N.; Sahi, S.V. Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy. Pharmaceutics 2021, 13, 1174. [Google Scholar] [CrossRef]
- Liu, S.; Pan, X.; Liu, H. Two-Dimensional Nanomaterials for Photothermal Therapy. Angew. Chem. Int. Ed. 2020, 59, 5890–5900. [Google Scholar] [CrossRef]
- Zeng, R.; He, T.; Lu, L.; Li, K.; Luo, Z.; Cai, K. Ultra-thin metal–organic framework nanosheets for chemo-photodynamic synergistic therapy. J. Mater. Chem. B 2021, 9, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Z. Chemiluminescent Nanosystems for Imaging Cancer Chemodynamic Therapy. Chem. Eng. J. 2020, 6, 2127–2129. [Google Scholar] [CrossRef]
- Berraondo, P.; Labiano, S.; Minute, L.; Etxeberria, I.; Vasquez, M.; Sanchez-Arraez, A.; Teijeira, A.; Melero, I. Cellular immunotherapies for cancer. OncoImmunology 2017, 6, e1306619. [Google Scholar] [CrossRef]
- Del Paggio, J.C. Cancer immunotherapy and the value of cure. Nat. Rev. Clin. Oncol. 2018, 15, 268–270. [Google Scholar] [CrossRef]
- Agache, I.; Zemelka-Wiącek, M.; Shamji, M.H.; Jutel, M. Immunotherapy: State-of-the-art review of therapies and theratypes. J. Allergy Clin. 2022, 150, 1279–1288. [Google Scholar] [CrossRef]
- Ng, C.W.; Li, J.; Pu, K. Phototherapy-Synergized Cancer Immunotherapy: Recent Progresses in Phototherapy-Synergized Cancer Immunotherapy. Adv. Funct. Mater. 2018, 28, 1870327. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Wang, H.; Wang, T.; Pan, H.; Ji, W.; Chang, J. A synergistic cancer immunotherapy nano-system for preventing tumor growth. Chem. Eng. J. 2020, 380, 122472. [Google Scholar] [CrossRef]
- Colli, L.M.; Machiela, M.J.; Zhang, H.; Myers, T.A.; Jessop, L.; Delattre, O.; Yu, K.; Chanock, S.J. Landscape of Combination Immunotherapy and Targeted Therapy to Improve Cancer Management. Cancer Res. 2017, 77, 3666–3671. [Google Scholar] [CrossRef] [PubMed]
- Pinto da Silva, L.; Magalhães, C.M.; Núñez-Montenegro, A.; Ferreira, P.J.O.; Duarte, D.; Rodríguez-Borges, J.E.; Vale, N.; Esteves da Silva, J.C.G. Study of the Combination of Self-Activating Photodynamic Therapy and Chemotherapy for Cancer Treatment. Biomolecules 2019, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hua Liu, S.; Yin, J.; Yoon, J. Sonodynamic and chemodynamic therapy based on organic/organometallic sensitizers. Coord. Chem. Rev. 2021, 429, 213610. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Li, X.; Gao, X.; Niu, X.; Wang, W.; Wu, Q.; Yuan, Z. Fluorescence-enhanced covalent organic framework nanosystem for tumor imaging and photothermal therapy. Nanoscale 2019, 11, 10429–10438. [Google Scholar] [CrossRef]
- Ibrahim, J.; Peeters, M.; Van Camp, G.; Op de Beeck, K. Methylation biomarkers for early cancer detection and diagnosis: Current and future perspectives. Eur. J. Cancer 2022, 178, 91–113. [Google Scholar] [CrossRef]
- Hlaváček, A.; Farka, Z.; Mickert, M.J.; Kostiv, U.; Brandmeier, J.C.; Horák, D.; Skládal, P.; Foret, F.; Gorris, H.H. Bioconjugates of photon-upconversion nanoparticles for cancer biomarker detection and imaging. Nat. Protoc. 2022, 17, 1028–1072. [Google Scholar] [CrossRef]
- Nazari, M.; Saljooghi, A.S.; Ramezani, M.; Alibolandi, M.; Mirzaei, M. Current status and future prospects of nanoscale metal–organic frameworks in bioimaging. J. Mater. Chem. B 2022, 10, 8824–8851. [Google Scholar] [CrossRef]
- Shaikh, S.; Wang, Y.; ur Rehman, F.; Jiang, H.; Wang, X. Phosphorescent Ir (III) complexes as cellular staining agents for biomedical molecular imaging. Coord. Chem. Rev. 2020, 416, 213344. [Google Scholar] [CrossRef]
- Shaikh, S.; Younis, M.; Yuan, L. Functionalized DNA nanostructures for bioimaging. Coord. Chem. Rev. 2022, 469, 214648. [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.; Huang, P. Graphene-based nanomaterials for bioimaging. Adv. Drug Deliv. Rev. 2016, 105, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, X.; Liu, X.; Liu, X.; Liu, Z.; Liu, X. Application of photo-responsive metal-organic framework in cancer therapy and bioimaging. Front. Bioeng. Biotechnol. 2022, 10, 31986. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, Y.; Wei, T.; Zhao, X.; Li, F.; Li, Y.; Wang, F.; Cai, Y.; Jin, J. KAT8/MOF-Mediated Anti-Cancer Mechanism of Gemcitabine in Human Bladder Cancer Cells. Biomol. Ther. 2021, 29, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef]
- Saeb, M.R.; Rabiee, N.; Mozafari, M.; Verpoort, F.; Voskressensky, L.G.; Luque, R. Metal-Organic Frameworks (MOFs) for Cancer Therapy. Materials 2021, 14, 7277. [Google Scholar] [CrossRef]
- Wu, M.X.; Yang, Y.W. Metal-Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Y.; Wang, J.; Pei, R. Lanthanide-based MOFs: Synthesis approaches and applications in cancer diagnosis and therapy. J. Mater. Chem. B 2022, 10, 9535–9564. [Google Scholar] [CrossRef]
- Su, J.; Jing, P.; Jiang, K.; Du, J. Recent advances in porous MOFs and their hybrids for photothermal cancer therapy. Dalton Trans. 2022, 51, 8938–8944. [Google Scholar] [CrossRef]
- Hu, X.; Li, R.; Wu, W.; Fang, K.; Zhu, Z.; Wang, Y.; Zhou, L.; Chen, M.; Dong, C.; Shi, S. A Fe(III)-porphyrin-oxaliplatin(IV) nanoplatform for enhanced ferroptosis and combined therapy. J. Control. Release 2022, 348, 660–671. [Google Scholar] [CrossRef]
- Lian, X.; Huang, Y.; Zhu, Y.; Fang, Y.; Zhao, R.; Joseph, E.; Li, J.; Pellois, J.P.; Zhou, H.C. Enzyme-MOF Nanoreactor Activates Nontoxic Paracetamol for Cancer Therapy. Angew. Chem. Int. Ed. 2018, 57, 5725–5730. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Zhang, D.; Chen, Y.-P.; Wang, F.; Li, L.; Liu, T.-F.; Yang, H.; Song, J.; Cao, R. Building Block Symmetry Relegation Induces Mesopore and Abundant Open-Metal Sites in Metal–Organic Frameworks for Cancer Therapy. CCS Chem. 2022, 4, 996–1006. [Google Scholar] [CrossRef]
- Bahari, D.; Babamiri, B.; Moradi, K.; Salimi, A.; Hallaj, R. Graphdiyne nanosheet as a novel sensing platform for self-enhanced electrochemiluminescence of MOF enriched ruthenium (II) in the presence of dual co-reactants for detection of tumor marker. Biosens. Bioelectron. 2022, 195, 113657. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, Q.-L.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Energy Applications. Chem. Eng. J. 2017, 2, 52–80. [Google Scholar] [CrossRef]
- Gao, P.; Wang, M.; Chen, Y.; Pan, W.; Zhou, P.; Wan, X.; Li, N.; Tang, B. A COF-based nanoplatform for highly efficient cancer diagnosis, photodynamic therapy and prognosis. Chem. Sci. 2020, 11, 6882–6888. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Yin, J.; Yan, Q.; Hu, H.; Zheng, T.; Chai, Y.; Pan, W.; Gao, Y.; Li, N.; Tang, B. Sustained-release nanocapsule based on a 3D COF for long-term enzyme prodrug therapy of cancer. Chem. Commun. 2022, 58, 5877–5880. [Google Scholar] [CrossRef]
- Huo, T.; Yang, Y.; Qian, M.; Jiang, H.; Du, Y.; Zhang, X.; Xie, Y.; Huang, R. Versatile hollow COF nanospheres via manipulating transferrin corona for precise glioma-targeted drug delivery. Biomaterials 2020, 260, 120305. [Google Scholar] [CrossRef]
- Gao, P.; Shen, X.; Liu, X.; Chen, Y.; Pan, W.; Li, N.; Tang, B. Nucleic Acid-Gated Covalent Organic Frameworks for Cancer-Specific Imaging and Drug Release. Anal. Chem. 2021, 93, 11751–11757. [Google Scholar] [CrossRef]
- Gao, P.; Shen, X.; Liu, X.; Cui, B.; Wang, M.; Wan, X.; Li, N.; Tang, B. Covalent Organic Framework-Derived Carbonous Nanoprobes for Cancer Cell Imaging. ACS Appl. Mater. Interfaces 2021, 13, 41498–41506. [Google Scholar] [CrossRef]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef]
- Fang, Y.; Lian, X.; Huang, Y.; Fu, G.; Xiao, Z.; Wang, Q.; Nan, B.; Pellois, J.P.; Zhou, H.C. Investigating Subcellular Compartment Targeting Effect of Porous Coordination Cages for Enhancing Cancer Nanotherapy. Small 2018, 14, e1802709. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Pan, M.; Su, C.Y. Metal-Organic Cages for Biomedical Applications. Isr. J. Chem. 2018, 59, 209–219. [Google Scholar] [CrossRef]
- Liang, Y.; Fang, Y.; Cui, Y.; Zhou, H. A stable biocompatible porous coordination cage promotes in vivo liver tumor inhibition. Nano Res. 2021, 14, 3407–3415. [Google Scholar] [CrossRef]
- Liang, T.; Guo, Z.; He, Y.; Wang, Y.; Li, C.; Li, Z.; Liu, Z. Cyanine-Doped Lanthanide Metal-Organic Frameworks for Near-Infrared II Bioimaging. Adv. Sci. 2022, 9, e2104561. [Google Scholar] [CrossRef] [PubMed]
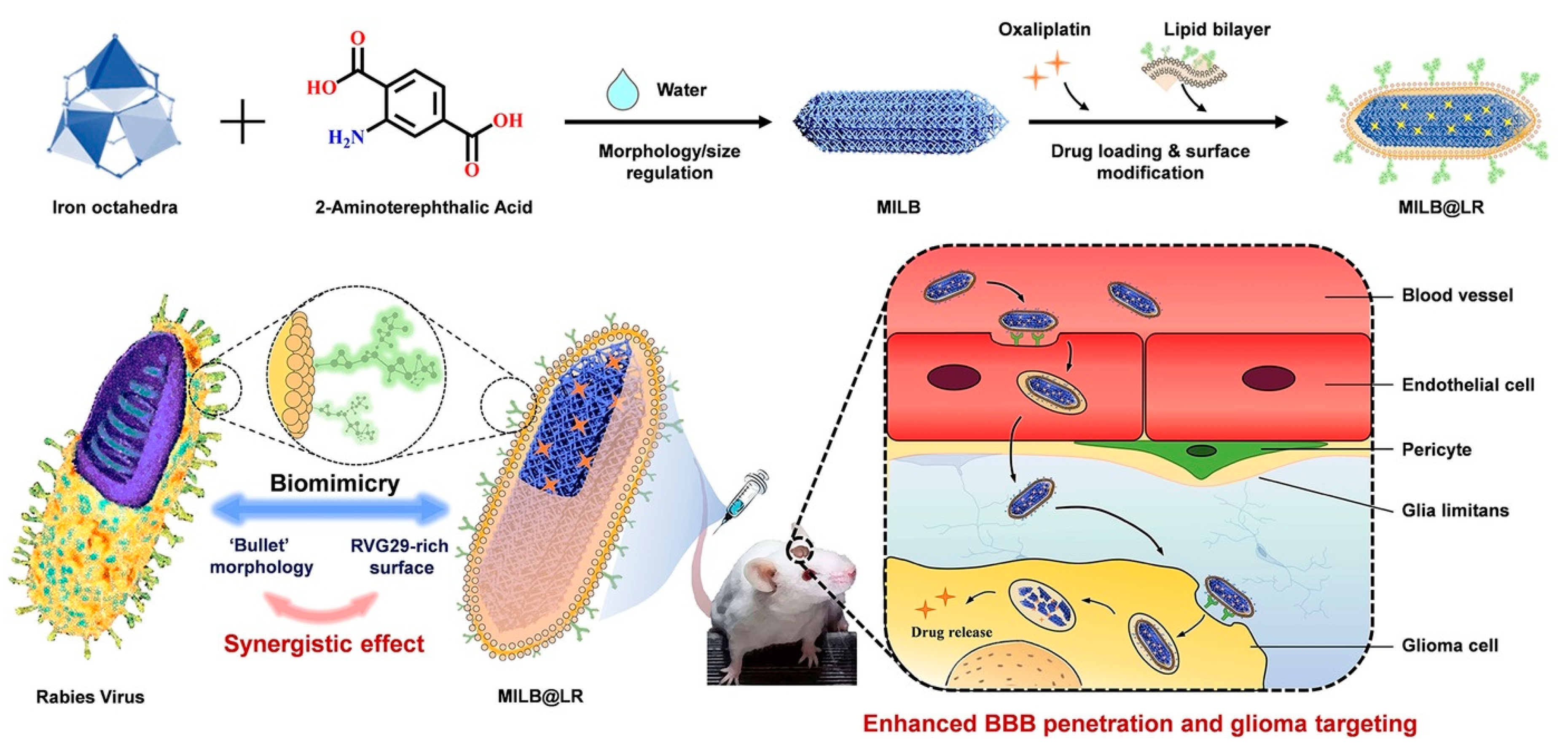
- Qiao, C.; Zhang, R.; Wang, Y.; Jia, Q.; Wang, X.; Yang, Z.; Xue, T.; Ji, R.; Cui, X.; Wang, Z. Rabies Virus-Inspired Metal-Organic Frameworks (MOFs) for Targeted Imaging and Chemotherapy of Glioma. Angew. Chem. Int. Ed. 2020, 59, 16982–16988. [Google Scholar] [CrossRef]
- Li, B.; Lu, X.; Tian, Y.; Li, D. Embedding Multiphoton Active Units within Metal–Organic Frameworks for Turning on High-Order Multiphoton Excited Fluorescence for Bioimaging. Angew. Chem. Int. Ed. 2022, 61, e202206755. [Google Scholar]
- Hamideh, R.A.; Akbari, B.; Fathi, P.; Misra, S.K.; Sutrisno, A.; Lam, F.; Pan, D. Biodegradable MRI Visible Drug Eluting Stent Reinforced by Metal Organic Frameworks. Adv. Healthc. Mater. 2020, 9, 2000136. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, H.; Xue, Y.; Lin, J.; Fu, Y.; Xia, Z.; Pan, D.; Zhang, J.; Qiao, K.; Zhang, Z.; et al. Reversing cold tumors to hot: An immunoadjuvant-functionalized metal-organic framework for multimodal imaging-guided synergistic photo-immunotherapy. Bioact. Mater. 2021, 6, 312–325. [Google Scholar] [CrossRef]
- Zhao, J.; Yin, F.; Ji, L.; Wang, C.; Shi, C.; Liu, X.; Yang, H.; Wang, X.; Kong, L. Development of a Tau-Targeted Drug Delivery System Using a Multifunctional Nanoscale Metal–Organic Framework for Alzheimer’s Disease Therapy. ACS Appl. Mater. Interfaces 2020, 12, 44447–44458. [Google Scholar] [CrossRef]
- Hu, X.; Lu, Y.; Zhou, L.; Chen, L.; Yao, T.; Liang, S.; Han, J.; Dong, C.; Shi, S. Post-synthesis strategy to integrate porphyrinic metal–organic frameworks with CuS NPs for synergistic enhanced photo-therapy. J. Mater. Chem. B 2020, 8, 935–944. [Google Scholar] [CrossRef]
- Zhu, Y.; Xin, N.; Qiao, Z.; Chen, S.; Zeng, L.; Zhang, Y.; Wei, D.; Sun, J.; Fan, H. Bioactive MOFs Based Theranostic Agent for Highly Effective Combination of Multimodal Imaging and Chemo-Phototherapy. Adv. Healthc. Mater. 2020, 9, 2000205. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Wang, X.S.; Xie, B.R.; Li, M.J.; Zhang, X.Z. Covalent Organic Framework for Improving Near-Infrared Light Induced Fluorescence Imaging through Two-Photon Induction. Angew. Chem. Int. Ed. 2020, 59, 10087–10094. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, F.; Zhang, C.; Yin, S.Y.; Teng, L.; Chen, L.; Hu, X.X.; Liu, H.W.; Yin, X.; Zhang, X.B. Ultrathin two-dimensional covalent organic framework nanoprobe for interference-resistant two-photon fluorescence bioimaging. Chem. Sci. 2018, 9, 8402–8408. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Wei, R.; Chen, Y.; Liu, X.; Zhang, J.; Pan, W.; Li, N.; Tang, B. Multicolor Covalent Organic Framework-DNA Nanoprobe for Fluorescence Imaging of Biomarkers with Different Locations in Living Cells. Anal. Chem. 2021, 93, 13734–13741. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Hai, J.; Sun, S.; Lu, S.; Liu, S.; Liu, H.; Chen, F.; Wang, B. Aqueous stable Pd nanoparticles/covalent organic framework nanocomposite: An efficient nanoenzyme for colorimetric detection and multicolor imaging of cancer cells. Nanoscale 2020, 12, 825–831. [Google Scholar] [CrossRef]
- Das, G.; Benyettou, F.; Sharama, S.K.; Prakasam, T.; Gándara, F.; de la Peña-O’Shea, V.A.; Saleh, N.i.; Pasricha, R.; Jagannathan, R.; Olson, M.A.; et al. Covalent organic nanosheets for bioimaging. Chem. Sci. 2018, 9, 8382–8387. [Google Scholar] [CrossRef]
- Dong, J.; Pan, Y.; Yang, K.; Yuan, Y.D.; Wee, V.; Xu, S.; Wang, Y.; Jiang, J.; Liu, B.; Zhao, D. Enhanced Biological Imaging via Aggregation-Induced Emission Active Porous Organic Cages. ACS Nano 2022, 16, 2355–2368. [Google Scholar] [CrossRef] [PubMed]
- Al Kelabi, D.; Dey, A.; Alimi, L.O.; Piwonski, H.; Habuchi, S.; Khashab, N.M. Photostable polymorphic organic cages for targeted live cell imaging. Chem. Sci. 2022, 13, 7341–7346. [Google Scholar] [CrossRef]
- Dong, J.; Pan, Y.; Wang, H.; Yang, K.; Liu, L.; Qiao, Z.; Yuan, Y.D.; Peh, S.B.; Zhang, J.; Shi, L.; et al. Self-Assembly of Highly Stable Zirconium(IV) Coordination Cages with Aggregation Induced Emission Molecular Rotors for Live-Cell Imaging. Angew. Chem. Int. Ed. 2020, 59, 10151–10159. [Google Scholar] [CrossRef]
- Li, B.; Cao, H.; Zheng, J.; Ni, B.; Lu, X.; Tian, X.; Tian, Y.; Li, D. Click Modification of a Metal-Organic Framework for Two-Photon Photodynamic Therapy with Near-Infrared Excitation. ACS Appl. Mater. Interfaces 2021, 13, 9739–9747. [Google Scholar] [CrossRef]
- Luo, T.; Nash, G.T.; Xu, Z.; Jiang, X.; Liu, J.; Lin, W. Nanoscale Metal-Organic Framework Confines Zinc-Phthalocyanine Photosensitizers for Enhanced Photodynamic Therapy. J. Am. Chem. Soc. 2021, 143, 13519–13524. [Google Scholar] [CrossRef]
- Cai, X.; Zhao, Y.; Wang, L.; Hu, M.; Wu, Z.; Liu, L.; Zhu, W.; Pei, R. Synthesis of Au@MOF core–shell hybrids for enhanced photodynamic/photothermal therapy. J. Mater. Chem. B 2021, 9, 6646–6657. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xue, F.; Wang, M.; An, L.; Wu, D.; Tian, Q. 2D Cu-Bipyridine MOF Nanosheet as an Agent for Colon Cancer Therapy: A Three-in-One Approach for Enhancing Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 38604–38616. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, L.; Guan, Y.; Pei, Q.; Jiang, J.; Xie, Z. Integration of metal-organic framework with a photoactive porous-organic polymer for interface enhanced phototherapy. Biomaterials 2020, 235, 119792. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Cai, X.; Ding, P.; Lv, H.; Pei, R. Metal–Organic Frameworks with Enhanced Photodynamic Therapy: Synthesis, Erythrocyte Membrane Camouflage, and Aptamer-Targeted Aggregation. ACS Appl. Mater. Interfaces 2020, 12, 23697–23706. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Q.; Lu, C. Aptamer-Functionalized Iron-Based Metal–Organic Frameworks (MOFs) for Synergistic Cascade Cancer Chemotherapy and Chemodynamic Therapy. Molecules 2022, 27, 4247. [Google Scholar]
- Deng, H.; Zhang, J.; Yang, Y.; Yang, J.; Wei, Y.; Ma, S.; Shen, Q. Chemodynamic and Photothermal Combination Therapy Based on Dual-Modified Metal-Organic Framework for Inducing Tumor Ferroptosis/Pyroptosis. ACS Appl. Mater. Interfaces 2022, 14, 24089–24101. [Google Scholar] [CrossRef]
- Xia, R.; Li, C.; Yuan, X.; Wu, Q.; Jiang, B.; Xie, Z. Facile Preparation of a Thienoisoindigo-Based Nanoscale Covalent Organic Framework with Robust Photothermal Activity for Cancer Therapy. ACS Appl. Mater. Interfaces 2022, 14, 19129–19138. [Google Scholar] [CrossRef]
- Xia, R.; Zheng, X.; Li, C.; Yuan, X.; Wang, J.; Xie, Z.; Jing, X. Nanoscale Covalent Organic Frameworks with Donor-Acceptor Structure for Enhanced Photothermal Ablation of Tumors. ACS Nano 2021, 15, 7638–7648. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, J.; Zuo, K.; Zhang, H.; Hu, H.; Pan, W.; Gao, Y.; Li, N.; Tang, B. A donor-acceptor covalent organic framework as the promising construct for photothermal therapy. Sci. China Mater. 2022. [Google Scholar] [CrossRef]
- Wang, S.-B.; Chen, Z.-X.; Gao, F.; Zhang, C.; Zou, M.-Z.; Ye, J.-J.; Zeng, X.; Zhang, X.-Z. Remodeling extracellular matrix based on functional covalent organic framework to enhance tumor photodynamic therapy. Biomaterials 2020, 234, 119772. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, F.; Guan, K.; Wang, Y.; Fu, X.; Yang, Y.; Yin, X.; Song, G.; Zhang, X.-B.; Tan, W. In vivo therapeutic response monitoring by a self-reporting upconverting covalent organic framework nanoplatform. Chem. Sci. 2020, 11, 1299–1306. [Google Scholar] [CrossRef]
- Gan, S.; Tong, X.; Zhang, Y.; Wu, J.; Hu, Y.; Yuan, A. Covalent Organic Framework-Supported Molecularly Dispersed Near-Infrared Dyes Boost Immunogenic Phototherapy against Tumors. Adv. Funct. Mater. 2019, 29, 1902757. [Google Scholar] [CrossRef]
- Yu, G.; Cen, T.Y.; He, Z.; Wang, S.P.; Wang, Z.; Ying, X.W.; Li, S.; Jacobson, O.; Wang, S.; Wang, L.; et al. Porphyrin Nanocage-Embedded Single-Molecular Nanoparticles for Cancer Nanotheranostics. Angew. Chem. Int. Ed. 2019, 58, 8799–8803. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Yu, H.J.; Wang, Y.P.; Li, C.J.; Wang, X.F.; Ye, C.G.; Yao, H.L.; Pan, M.; Su, C.Y. A photoactive Ir–Pd bimetallic cage with high singlet oxygen yield for efficient one/two-photon activated photodynamic therapy. Mater. Chem. Front. 2022, 6, 948–955. [Google Scholar] [CrossRef]
- Liu, M.; Ren, X.; Meng, X.; Li, H. Metal-Organic Frameworks-Based Fluorescent Nanocomposites for Bioimaging in Living Cells and in vivo. Chin. J. Chem. 2021, 39, 473–487. [Google Scholar] [CrossRef]
- Sava Gallis, D.F.; Butler, K.S.; Rohwer, L.E.S.; McBride, A.A.; Vincent, G.; Chong, C.V.; Pearce, C.J.; Luk, T.S. Biocompatible MOFs with high absolute quantum yield for bioimaging in the second near infrared window. CrystEngComm 2018, 20, 5919–5924. [Google Scholar] [CrossRef]
- Mal, A.; Ding, H.; Li, M.; Li, W.; Wang, C. Covalent Organic Frameworks with Nanopores for Biological Applications: A Review. ACS Appl. Nano Mater. 2022, 5, 13972–13984. [Google Scholar] [CrossRef]
- Sohrabi, H.; Javanbakht, S.; Oroojalian, F.; Rouhani, F.; Shaabani, A.; Majidi, M.R.; Hashemzaei, M.; Hanifehpour, Y.; Mokhtarzadeh, A.; Morsali, A. Nanoscale Metal-Organic Frameworks: Recent developments in synthesis, modifications and bioimaging applications. Chemosphere 2021, 281, 130717. [Google Scholar] [CrossRef]
- Xie, B.; Ding, Y.-F.; Shui, M.; Yue, L.; Gao, C.; Wyman, I.W.; Wang, R. Supramolecular biomaterials for bio-imaging and imaging-guided therapy. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1200–1210. [Google Scholar] [CrossRef]
- Yan, D.; Lou, Y.; Yang, Y.; Chen, Z.; Cai, Y.; Guo, Z.; Zhan, H.; Chen, B. Dye-Modified Metal–Organic Framework as a Recyclable Luminescent Sensor for Nicotine Determination in Urine Solution and Living Cell. ACS Appl. Mater. Interfaces 2019, 11, 47253–47258. [Google Scholar] [CrossRef]
- Gao, X.; Cui, R.; Song, L.; Liu, Z. Hollow structural metal–organic frameworks exhibit high drug loading capacity, targeted delivery and magnetic resonance/optical multimodal imaging. Dalton Trans. 2019, 48, 17291–17297. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J.; Shan, D.; Chen, J.; Zhang, S.; Lu, X. Dual-Emitting Fluorescent Metal–Organic Framework Nanocomposites as a Broad-Range pH Sensor for Fluorescence Imaging. Anal. Chem. 2018, 90, 7056–7063. [Google Scholar] [CrossRef] [PubMed]
- Zebibula, A.; Alifu, N.; Xia, L.; Sun, C.; Yu, X.; Xue, D.; Liu, L.; Li, G.; Qian, J. Ultrastable and Biocompatible NIR-II Quantum Dots for Functional Bioimaging. Adv. Funct. Mater. 2018, 28, 1703451. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Yao, X.; Duan, W.; Zhang, W.; Tian, Y.; Li, D. In Situ Coordination and Confinement of Two-Photon Active Unit Within Metal–Organic Frameworks for High-Order Multiphoton-Excited Fluorescent Performance. Inorg. Chem. 2022, 61, 19282–19288. [Google Scholar] [CrossRef]
- Wang, H.-S. Metal–organic frameworks for biosensing and bioimaging applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- He, L.; Ni, Q.; Mu, J.; Fan, W.; Liu, L.; Wang, Z.; Li, L.; Tang, W.; Liu, Y.; Cheng, Y.; et al. Solvent-Assisted Self-Assembly of a Metal–Organic Framework Based Biocatalyst for Cascade Reaction Driven Photodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 6822–6832. [Google Scholar] [CrossRef] [PubMed]
- Alsaiari, S.K.; Patil, S.; Alyami, M.; Alamoudi, K.O.; Aleisa, F.A.; Merzaban, J.S.; Li, M.; Khashab, N.M. Endosomal Escape and Delivery of CRISPR/Cas9 Genome Editing Machinery Enabled by Nanoscale Zeolitic Imidazolate Framework. J. Am. Chem. Soc. 2018, 140, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, J.; Zhang, L.; Lei, J. Multifunctional metal–organic framework heterostructures for enhanced cancer therapy. Chem. Soc. Rev. 2021, 50, 1188–1218. [Google Scholar] [CrossRef] [PubMed]
- Bessinger, D.; Ascherl, L.; Auras, F.; Bein, T. Spectrally Switchable Photodetection with Near-Infrared-Absorbing Covalent Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 12035–12042. [Google Scholar] [CrossRef]
- Xu, J.; Yang, C.; Bi, S.; Wang, W.; He, Y.; Wu, D.; Liang, Q.; Wang, X.; Zhang, F. Vinylene-Linked Covalent Organic Frameworks (COFs) with Symmetry-Tuned Polarity and Photocatalytic Activity. Angew. Chem. Int. Ed. 2020, 59, 23845–23853. [Google Scholar] [CrossRef]
- Bessinger, D.; Muggli, K.; Beetz, M.; Auras, F.; Bein, T. Fast-Switching Vis–IR Electrochromic Covalent Organic Frameworks. J. Am. Chem. Soc. 2021, 143, 7351–7357. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.; Ju, H.; Um, S.; Oh, D.-C.; Song, J.M. Mitochondria and DNA Targeting of 5,10,15,20-Tetrakis(7-sulfonatobenzo[b]thiophene) Porphyrin-Induced Photodynamic Therapy via Intrinsic and Extrinsic Apoptotic Cell Death. J. Med. Chem. 2015, 58, 6864–6874. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Wang, C.; Cui, L.; Du, J.; Zhou, Z.; Yang, H.; Yang, S. Hollow Bimetallic Complex Nanoparticles for Trimodality Imaging and Photodynamic Therapy In Vivo. ACS Appl. Mater. Interfaces 2020, 12, 37470–37476. [Google Scholar] [CrossRef]
- Sepehrpour, H.; Fu, W.; Sun, Y.; Stang, P.J. Biomedically Relevant Self-Assembled Metallacycles and Metallacages. J. Am. Chem. Soc. 2019, 141, 14005–14020. [Google Scholar] [CrossRef] [PubMed]


| Applications | Porous Framework Materials | Main Functions | Ref. | ||
|---|---|---|---|---|---|
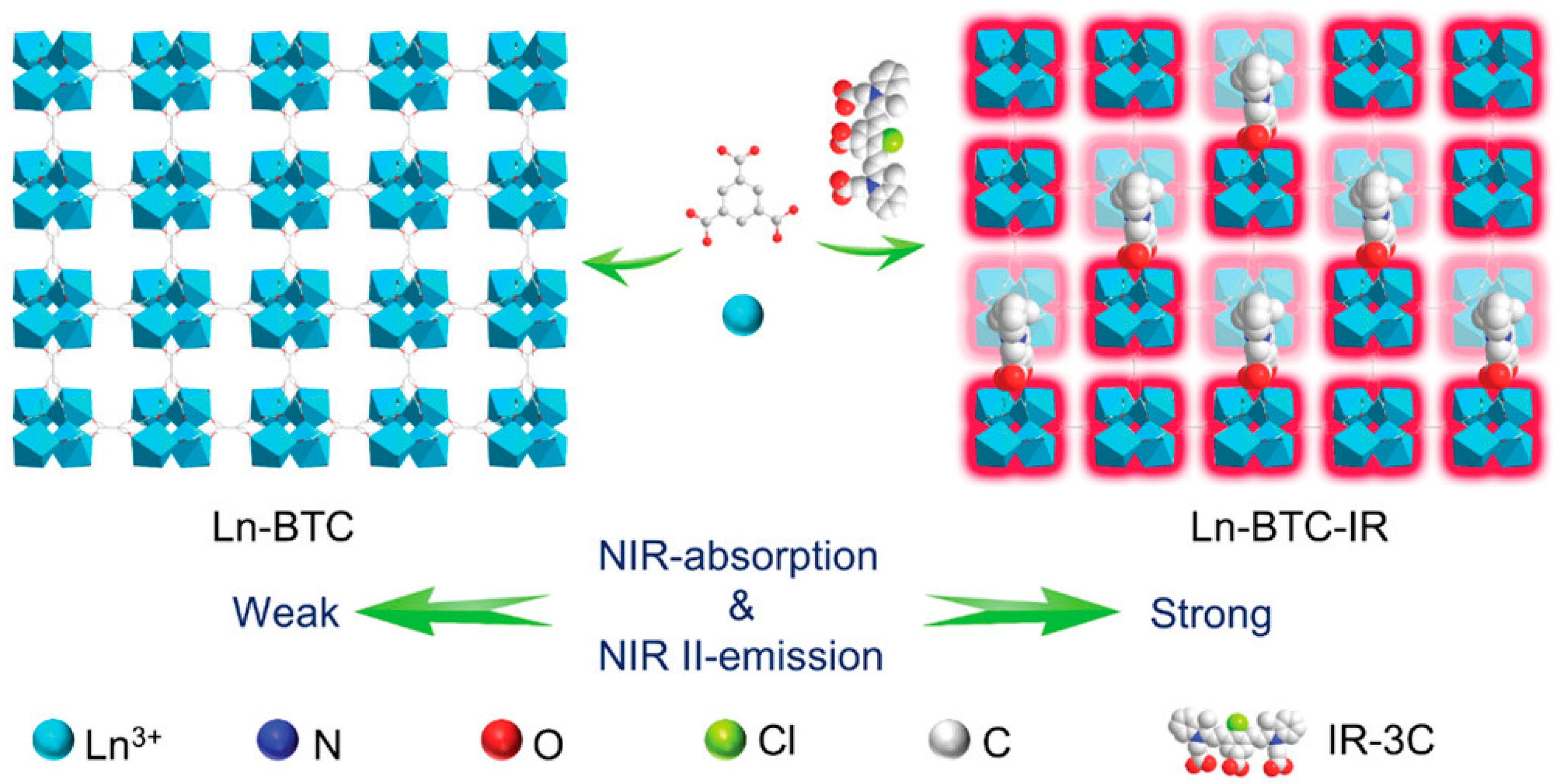
| Bioimaging | NIR-Ⅱ imaging | MOFs | Ln-BTC-MOF (Ln = Yb3+, Nd3+, Er3+) | enhanced the absorption of excitation photons | [53] |
| fluorescence imaging | MIL-Fe | as a contrast agent | [54] | ||
| NIR-Ⅱ imaging | ZrTc; ZrTcl | activated H-MPEF performance | [55] | ||
| magnetic resonance imaging (MRI) | MIL-101(Fe) | high spatial resolution and deep penetration | [56] | ||
| multimode bioimaging | ICG-CpG@MOF | comprehensive diagnostic information | [57] | ||
| MRI | Fe-MIL-88B-NH2-NOTA-DMK6240/MB | enhance tau targeting | [58] | ||
| fluorescence imaging | PCN-CuS-FA-ICG | facile diffusion of 1O2 and prevention of PS self-quenching | [59] | ||
| MRI | FDGI NPs | could effectively accumulate FDG (Fe-DOX@Gd-MOF) NPs at the tumor site | [60] | ||
| two-photon fluorescence imaging | COFs | TPI-COF | improves the delocalization of π electrons, and leads to a high dipole value and fluorescent activity | [61] | |
| two-photon fluorescence imaging | TpASH-NPHS | minimize cellular autofluorescence, reduce tissue injury and increase tissue penetration depth | [62] | ||
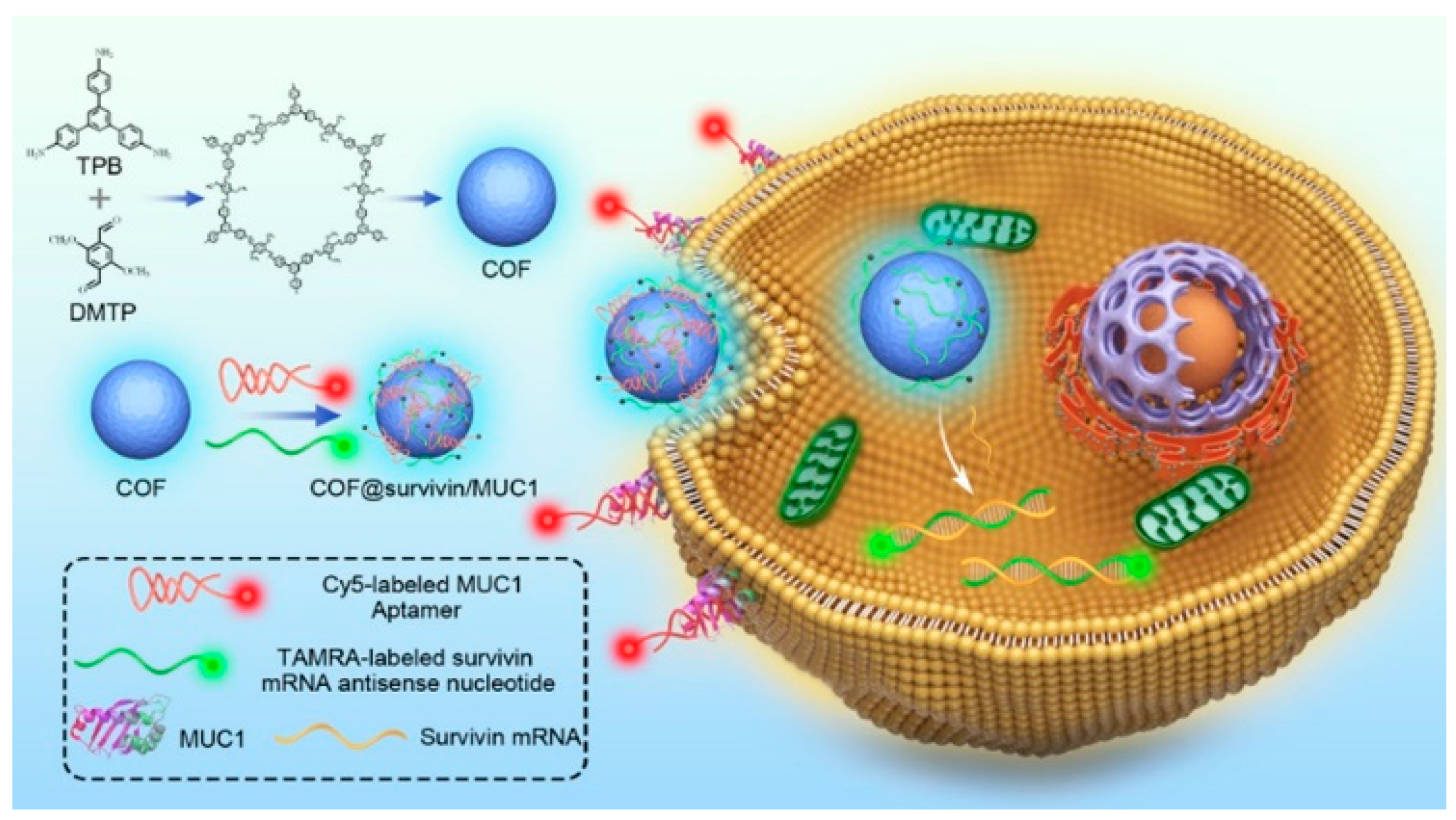
| fluorescence imaging | COF@survivin/MUC1 | employed a freezing method to improve the DNA loading density and ensure detection performance | [63] | ||
| Multicolor imaging | Pd NPs/CMC− COF-LZU1 | produce obvious changes in both color and fluorescence | [64] | ||
| bioimaging | TTA-DFP CONs | possess tunable optical characteristics | [65] | ||
| fluorescence imaging | PCCs | Zr6L3 coordination cage | good aggregation-induced emission (AIE) properties | [66] | |
| fluorescence imaging | TPE-cage | good aggregation-induced emission (AIE) properties | [67] | ||
| fluorescence imaging | POC-OC1 | high biocompatibility, cell permeability, and mitochondrial targetability | [68] | ||
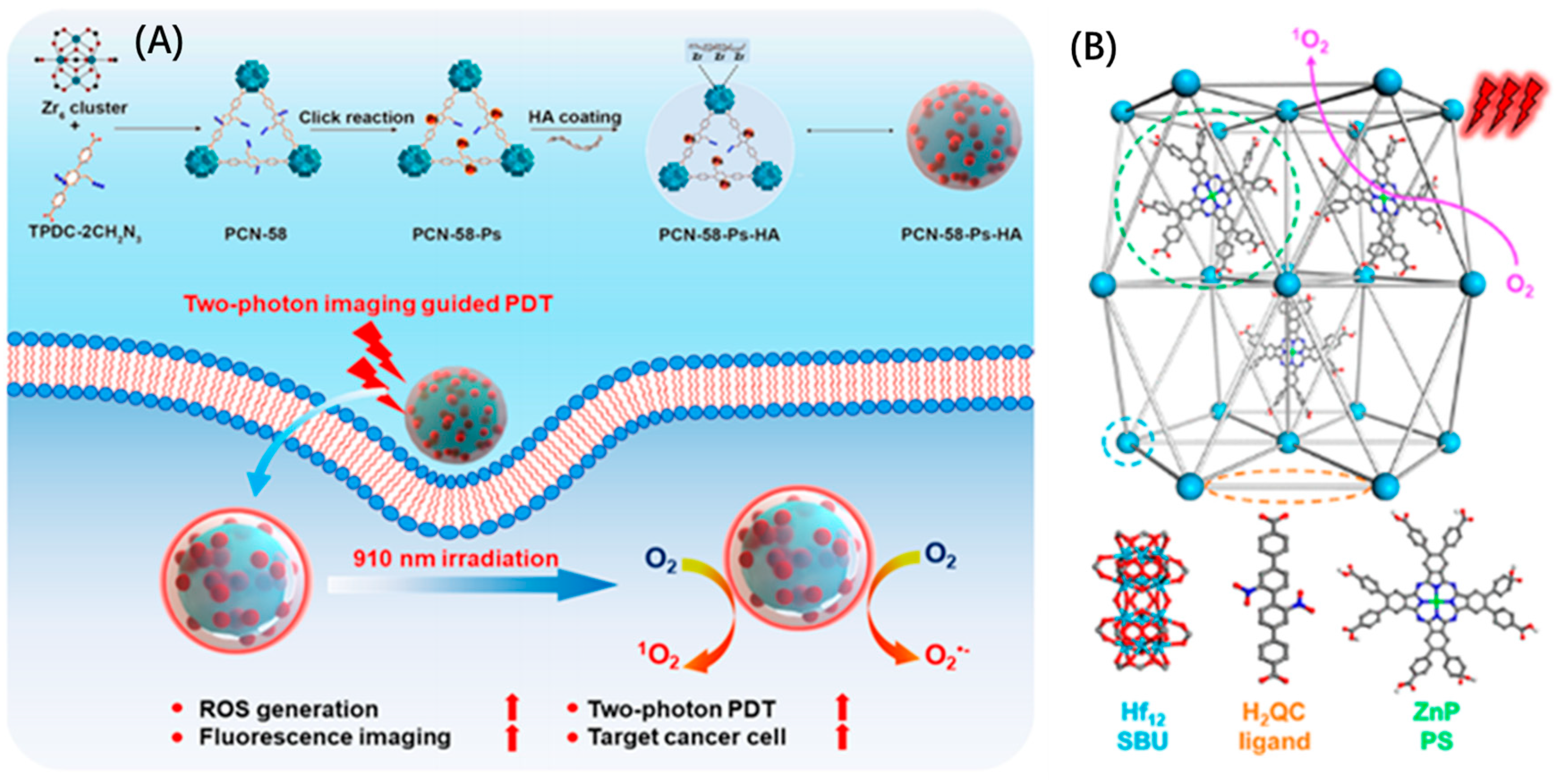
| Cancer therapy | PDT | MOFs | PCN-58-Ps-HA | generates abundant reactive oxygen species (ROS) | [69] |
| PDT | ZnP@Hf-QC | significantly enhances ROS generation upon light irradiation | [70] | ||
| PDT | Au@MOF | leads to ROS production | [71] | ||
| CDT | Cu(bpy)2(OTf)2 | facilitates Fenton-like reactions | [72] | ||
| PTT+PDT | HUC-PEG | leads to ROS production | [73] | ||
| CDT+PDT | FeTCPP/Fe2O3 MOF | leads to ROS and ·OH production | [74] | ||
| Chemotherapy+CDT | TA-MOF | leads to ·OH production and facilitates Fenton reactions | [75] | ||
| CDT+PTT | MP@PI | promote iron death and leads to ROS production | [76] | ||
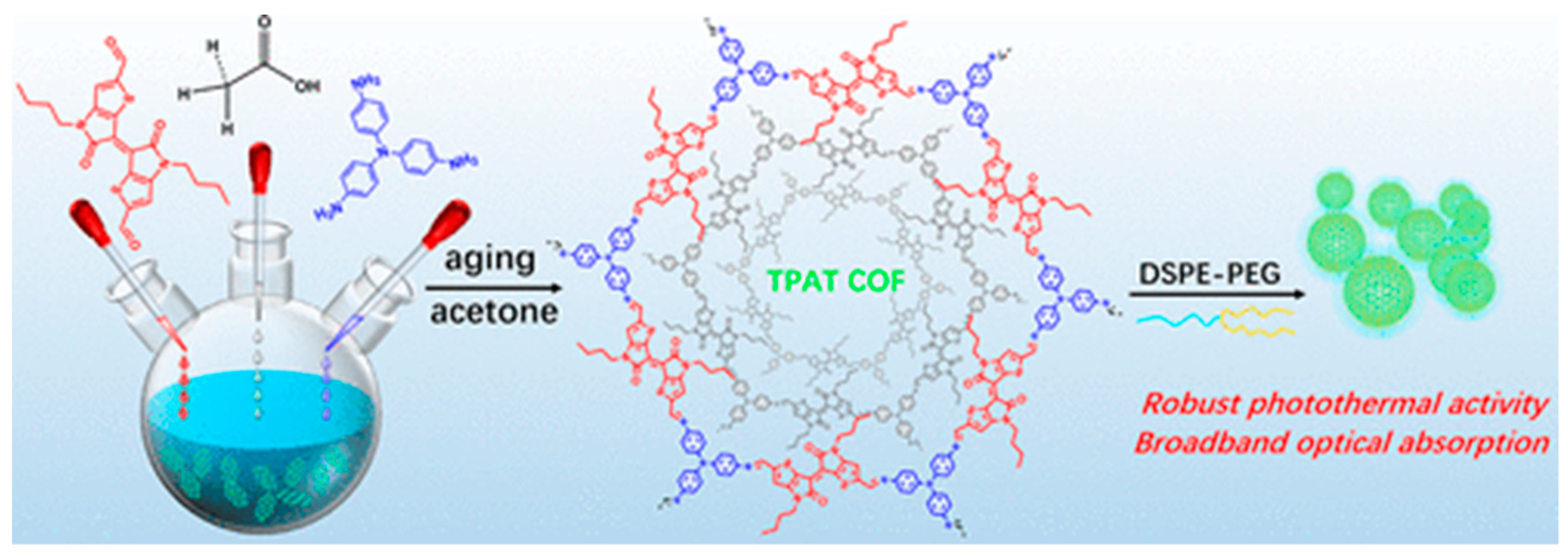
| PTT | COFs | TPAT COF | possesses a high photothermal conversion efficiency (PCE) under 808 nm | [77] | |
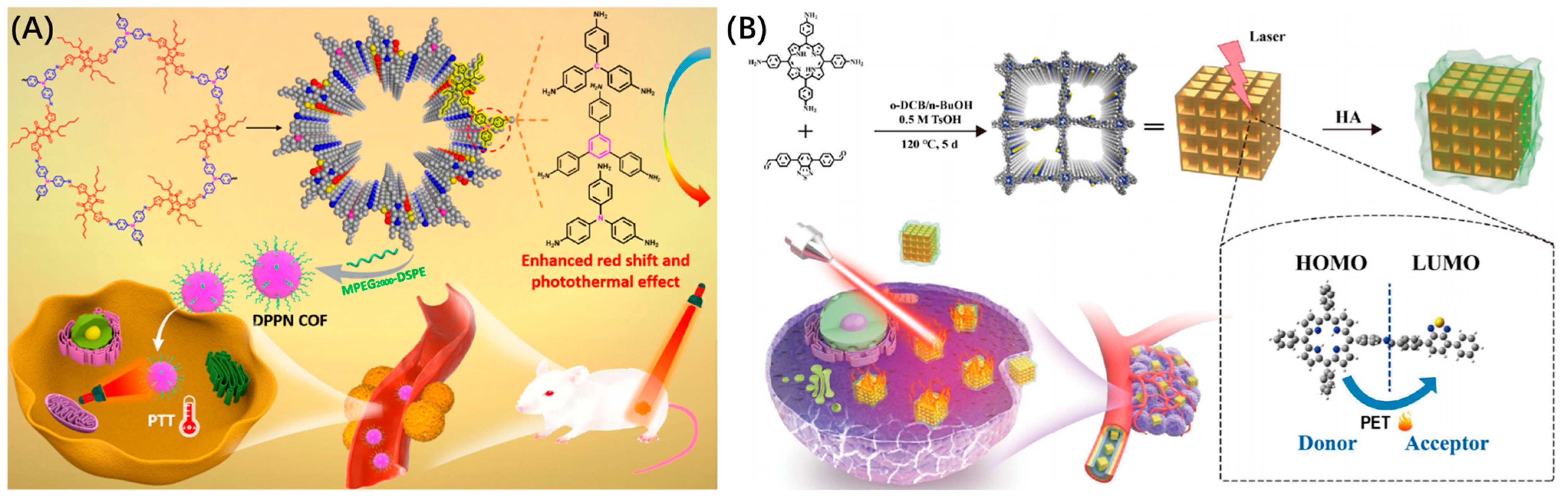
| PTT | DPPN COF | possess potent photothermal activity under laser irradiation | [78] | ||
| PTT | TB-COF | the donor and acceptor under laser irradiation and convert the absorbed light energy into heat energy | [79] | ||
| PDT | PCPP | leads to ROS production | [80] | ||
| PDT | UCCOFs | leads to ROS production | [81] | ||
| IPT (immunogenic photo-therapy) | ICG@COF-1@ PDA | enhanced photodynamic and photothermal therapy | [82] | ||
| PDT | PCCs | porSMNPs | inhibit π–π stacking interactions of photosensitizers and enhance the antitumor performance | [83] | |
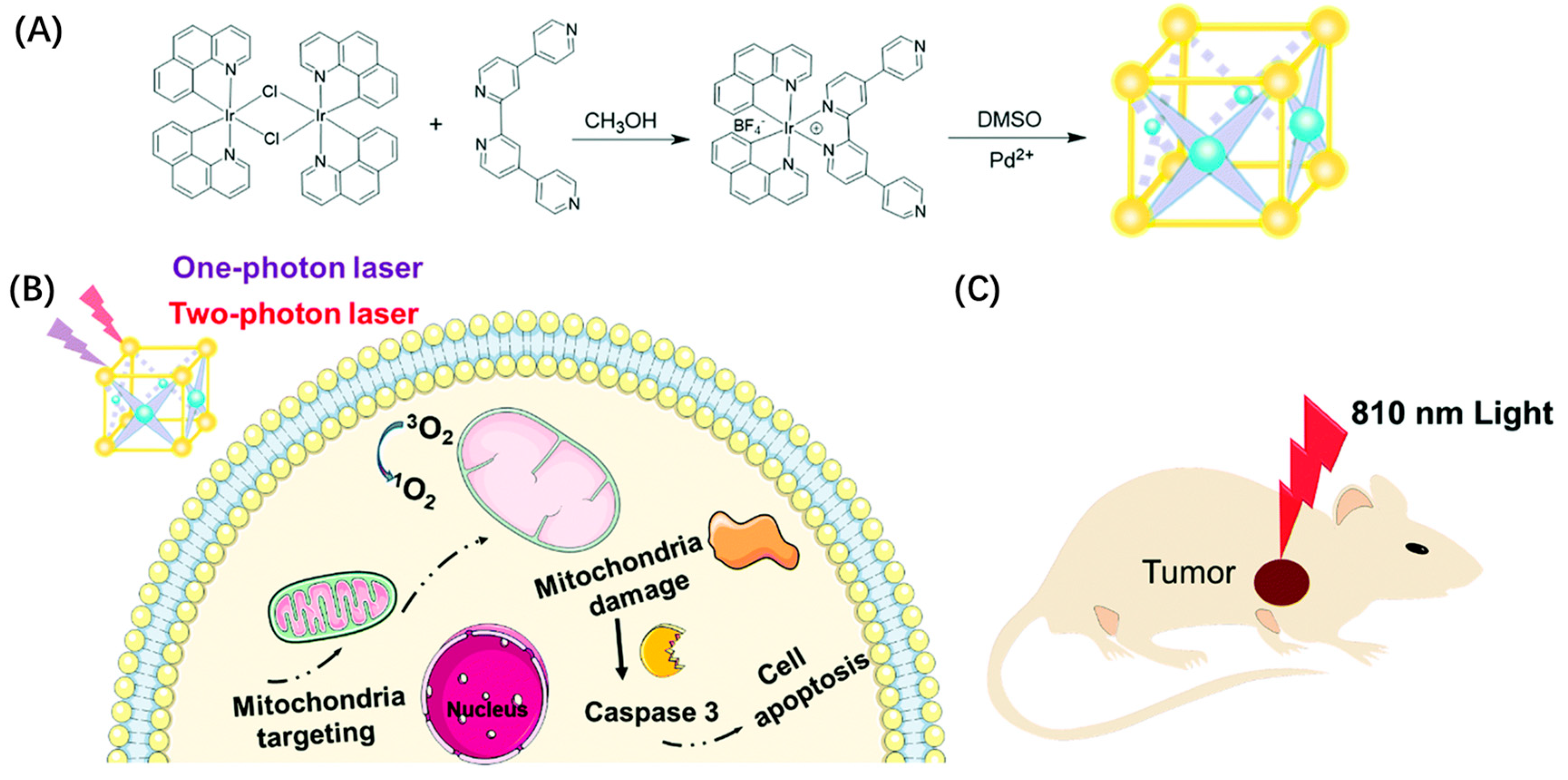
| PDT | MOC-53 | excellent 1O2 production efficiency, cellular uptake ability, and specific mitochondrial targeting capacity | [84] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, M.; Zhao, Y.; Guan, Z.-J.; Fang, Y. Porous Framework Materials for Bioimaging and Cancer Therapy. Molecules 2023, 28, 1360. https://doi.org/10.3390/molecules28031360
Jin M, Zhao Y, Guan Z-J, Fang Y. Porous Framework Materials for Bioimaging and Cancer Therapy. Molecules. 2023; 28(3):1360. https://doi.org/10.3390/molecules28031360
Chicago/Turabian StyleJin, Meng, Yingying Zhao, Zong-Jie Guan, and Yu Fang. 2023. "Porous Framework Materials for Bioimaging and Cancer Therapy" Molecules 28, no. 3: 1360. https://doi.org/10.3390/molecules28031360
APA StyleJin, M., Zhao, Y., Guan, Z.-J., & Fang, Y. (2023). Porous Framework Materials for Bioimaging and Cancer Therapy. Molecules, 28(3), 1360. https://doi.org/10.3390/molecules28031360





