The Impact of Diclofenac Gel on Ion Transport in the Rabbit (Oryctolagus cuniculus) Skin: An In Vitro Study
Abstract
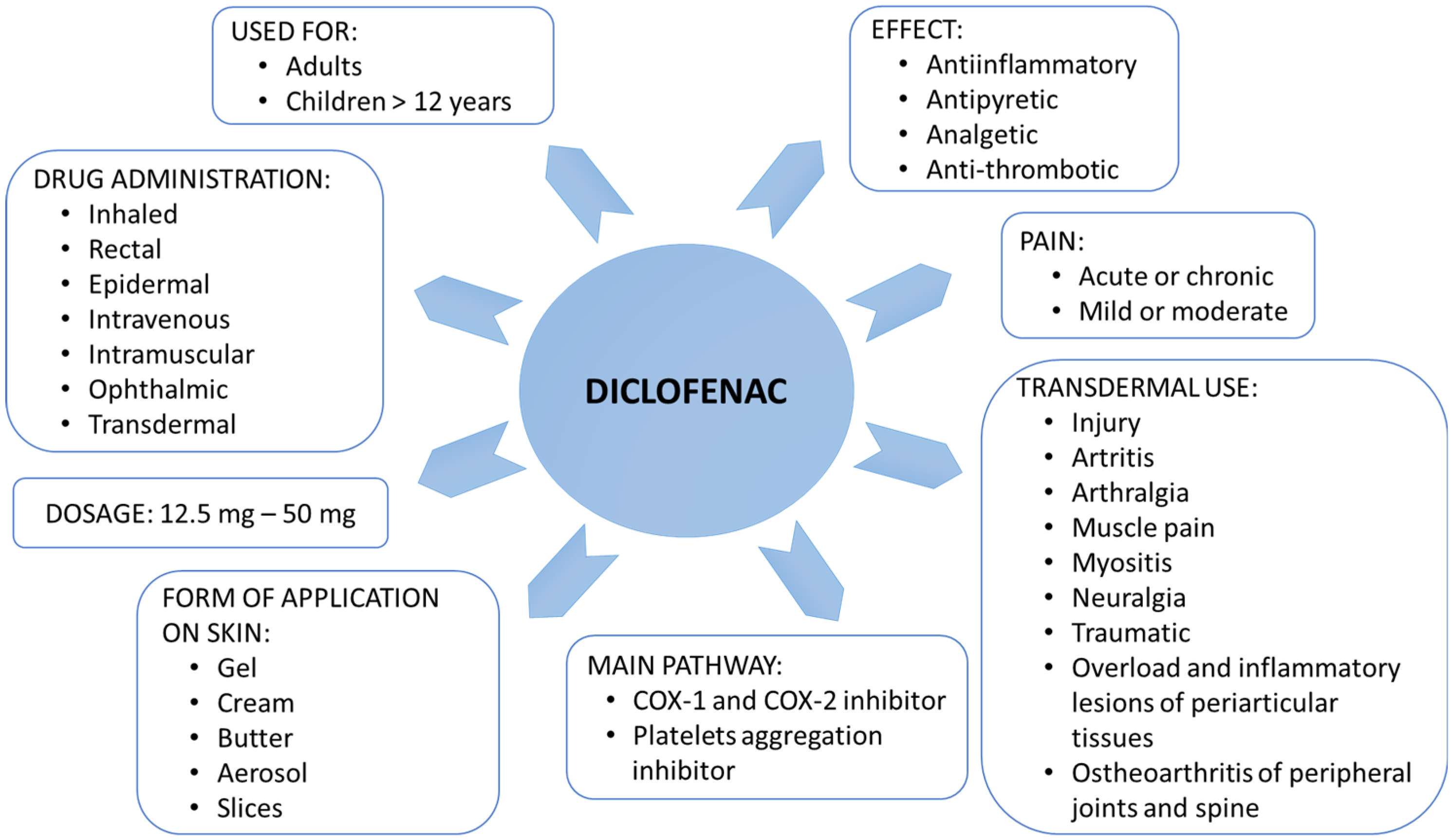
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solutions
- RH—iso-osmotic Ringer solution: K+ 4.0 mM; Na+ 147.2 mM; Ca2+ 2.2 mM; Mg2+ 2.6 mM; Cl− 160.8 mM (Avantor Performance Materials, Gliwice, Poland); pH = 7.4, used as a basic solution;
- B—bumetanide, 3-butylamino-4-phenoxy-5-sulfamoylbenzoic acid, 0.1 mM, 364.42 g/mol (Sigma-Aldrich, St. Louis, MO, USA), used as an inhibitor of transepithelial chloride transport pathways.
- A—amiloride, 3,5-diamino-6-chloro-2-carboxylic acid, 0.1 mM, 266.09 g/mol (Sigma-Aldrich, St. Louis, MO, USA), used as an inhibitor of transepithelial sodium transport pathways.
- AB—a solution of amiloride (A, 0.1 mM) and bumetanide (B, 0.1 mM).
- Diclofenac—a gel containing diclofenacum natricum, 10 mg/g (Perrigo, Poland).
4.2. Experimental Procedure
4.3. Measurement of Electrophysiological Parameters
- -
- R—transepithelial electrical resistance was recorded while the tissue sample was exposed to a current with a stimulus intensity of ±10 μA; after measuring the voltage change, calculations were made according to Ohm’s law (Ω/cm2).
- -
- PD—changes in transepithelial electric potential difference measured in stationary conditions, i.e., without a stimulation, recorded continuously (mV).
- -
- PDmax and PDmin—minimal and maximal transepithelial electric potential difference measured during a 15-s stimulation (mV).
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gan, T.J. Diclofenac: An update on its mechanism of action and safety profile. Curr. Med. Res. Opin. 2010, 26, 1715–1731. [Google Scholar] [CrossRef] [PubMed]
- Paulose-Ram, R.; Hirsch, R.; Dillon, C.; Gu, Q. Frequent monthly use of selected non-prescription and prescription non-narcotic analgesics among U.S. adults. Pharmacoepidemiol. Drug. Saf. 2005, 14, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Todd, P.A.; Sorkin, E.M. Diclofenac Sodium: A Reappraisal of Its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Efficacy. Drugs 1988, 35, 244–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Mukhtar, H.; Bickers, D.R.; Kopelovich, L.; Athara, M. Cyclooxygenases in the skin: Pharmacological and toxicological implications. Toxicol. Appl. Pharmacol. 2003, 192, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.G.; Crawford, G.M.; Young, D.; Corson, S.; Brown, C. Comparison of diclofenac gel, ibuprofen gel, and ibuprofen gel with levomenthol for the topical treatment of pain associated with musculoskeletal injuries. J. Int. Med. Res. 2019, 47, 4454–4468. [Google Scholar] [CrossRef]
- Singh, G.; Fort, J.G.; Goldstein, J.L.; Levy, R.A.; Hanrahan, P.S.; Bello, A.E.; Andrade-Ortega, L.; Wallemark, C.; Agrawal, N.M.; Eisen, G.M. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I study. Am. J. Med. 2006, 119, 255–266. [Google Scholar] [CrossRef]
- Atzeni, F.; Masala, I.F.; Sarzi-Puttini, P. A Review of Chronic Musculoskeletal Pain: Central and Peripheral Effects of Diclofenac. Pain Ther. 2018, 7, 163–177. [Google Scholar] [CrossRef]
- Camlibel, M.; Erdur, B.; Yilmaz, A.; Ozen, M.; Uyanik, A. Comparison of the Effects of Piroxicam and Diclofenac Sodium as Treatments for Primary Dysmenorrhea. Med. Sci. Monit. 2019, 6, 157–164. [Google Scholar] [CrossRef]
- Gasparini, L.; Ongini, E.; Wenk, G. Non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer’s disease: Old and new mechanisms ofaction. J. Neurochem. 2004, 91, 521–536. [Google Scholar] [CrossRef]
- Stuve, O.; Weideman, R.A.; McMahan, D.M.; Jacob, D.A.; Little, B.B. Diclofenac reduces the risk of Alzheimer’s disease: A pilot analysis of NSAIDs in two US veteran populations. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420935676. [Google Scholar] [CrossRef]
- Khuder, S.A.; Mutgi, A.B. Breast cancer and NSAID use: A meta-analysis. Br. J. Cancer 2001, 84, 1188–1192. [Google Scholar] [CrossRef]
- Choi, S.; Kim, S.; Park, J.; Lee, S.E.; Kim, C.; Kang, D. Diclofenac: A Nonsteroidal Anti-Inflammatory Drug Inducing Cancer Cell Death by Inhibiting Microtubule Polymerization and and Autophagy Flux. Antioxidants 2022, 11, 1009. [Google Scholar] [CrossRef]
- Pantziarka, P.; Sukhatme, V.; Bouche, G.; Meheus, L.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)—Diclofenac as an anti-cancer agent. Ecancermedicalscience 2016, 10, 610. [Google Scholar] [CrossRef]
- Thomas, G.J.; Herranz, P.; Cruz, S.B.; Parodi, A. Treatment of actinic keratosis through inhibition of cyclooxygenase-2: Potential mechanism of actionof diclofenac sodium 3% in hyaluronic acid 2.5%. Dermathologic Ther. 2019, 32, e12800. [Google Scholar] [CrossRef]
- Kroesen, V.M.; Gröschel, M.I.; Martinson, N.; Zumla, A.; Maeurer, M.; van der Werf, T.S.; Vilaplana, C. Non-steroidal anti-inflammatory drugs as host-directed therapy for tuberculosis: A systematic review. Front. Immunol. 2017, 8, 772. [Google Scholar] [CrossRef]
- Haltner-Ukomadu, E.; Sacha, M.; Richter, A.; Hussein, K. Hydrogel increases diclofenac skin permeation and absorption. Biopharm. Drug Dispos. 2019, 40, 217–224. [Google Scholar] [CrossRef]
- Ferreira, D.M.; Silva, C.S.; Souza, M.N. Electrical impedance model for evaluation of skin irritation in rabbits and humans. Skin Res. Technol. 2007, 13, 259–267. [Google Scholar] [CrossRef]
- Hołyńska-Iwan, I.; Smyk, P.; Chrustek, A.; Olszewska-Słonina, D.; Szewczyk-Golec, K. The influence of hydration status on ion transport in the rabbit (Oryctolagus cuniculus) skin—An in vitro study. PLoS ONE 2021, 16, e0255825. [Google Scholar] [CrossRef]
- Hołyńska-Iwan, I.; Szewczyk-Golec, K. Analysis of changes in sodium and chloride ion transport in the skin. Sci. Rep. 2020, 10, 18094. [Google Scholar] [CrossRef]
- Xu, W.; Hong, S.J.; Zeitchek, M.; Cooper, G.; Jia, S.; Xie, P.; Quereshi, H.A.; Zhong, A.; Portetfield, M.D.; Galiano, R.D.; et al. Hydration status regulates sodium flux and inflammatory pathways through epithelial sodium channel (ENaC) in the skin. J. Investig. Dermatol. 2015, 135, 796–806. [Google Scholar] [CrossRef]
- Xu, W.; Hong, S.J.; Zhong, A.; Xie, P.; Jia, S.; Sie, Z.; Zeitchek, M.; Niknam-Bienia, S.; Zhao, J.; Porterfield, D.M.; et al. Sodium channel Nax is a regulator in epithelial sodium homeostasis. Sci. Transl. Med. 2015, 7, 312ra177. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-Y.; Charles, R.-P.; Hummler, E.; Baines, D.L.; Isseroff, R.R. The epithelial sodium channel mediates the directionality of galvanotaxis in human keratinocytes. J. Cell. Sci. 2013, 126, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Hanukoglu, I.; Boggula, V.R.; Vaknine, H.; Sharma, S.; Kleyman, T.; Honukoglu, A. Expression of epithelial sodium channel (ENaC) and CFTR in the human epidermis and epidermal appendages. Histochem. Cell Biol. 2017, 147, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Abdayem, R.; Cellejon, S.; Portes, P.; Kirilov, P.; Demarne, F.; Pirot, F.; Jannin, V.; Haftek, M. Modulation of transepithelial electric resistance (TEER) in reconstructed human epidermis by excipients known to permeate intestinal tight junctions. Exp. Dermatol. 2015, 24, 686–691. [Google Scholar] [CrossRef]
- Frosch, M.; Metze, D.; Foell, D.; Vogl, T.; Sorg, C.; Sunderkötter, C.; Roth, J. Early activation of cutaneous vessels and epithelial cells is characteristic of acute systemic onset juvenile idiopathic arthritis. Exp. Dermatol. 2005, 14, 259–265. [Google Scholar] [CrossRef]
- Mieremet, A.; van Dijk, R.; Boiten, W.; Gooris, G.; Bouwstra, J.A.; Ghalbzouri, A.E. Characterization of human skin equivalents developed at body’s core and surface temperatures. J. Tissue Eng. Regen. Med. 2019, 13, 1122–1133. [Google Scholar] [CrossRef]
- Tucker, M.A.; Six, A.; Moyen, N.E.; Satterfield, A.Z.; Ganio, M.S. Effect of hypohydration on postsynaptic cutaneous vasodilation and sweating in healthy men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R637–R642. [Google Scholar] [CrossRef]
- Aioi, A.; Okuda, M.; Matsui, M.M.; Tonogaito, H.; Hamada, K. Effect of high population density environment on skin barrier function in mice. J. Dermatol. Sci. 2001, 25, 189–197. [Google Scholar] [CrossRef]
- Baumauer, K.M.; DeBarry, J.J.; Adelman, P.C.; Miller, R.H.; Hashisuka, J.; Lee, K.H.; Ross, S.E.; Koerber, H.R.; Davis, B.M.; Albers, K.M. Keratinocytes can modulate and directly initiate nociceptive responses. eLife 2015, 4, e09674. [Google Scholar] [CrossRef]
- Denda, M.; Ashida, Y.; Inoue, K.; Kumazawa, N. Skin surface electric potential induced by ion-flux through epidermal cell layers. Biochem. Biophys. Res. Commun. 2001, 284, 112–117. [Google Scholar] [CrossRef]
- Denda, M.; Tsuchiya, T.; Elias, P.M.; Feingold, K. Stress alters cutaneous permeability barrier homeostasis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R367–R372. [Google Scholar] [CrossRef]
- Nakagawa, N.; Sakai, S.; Matsumoto, M.; Yamada, K.; Nagano, M.; Yuki, T.; Sumida, Y.; Uchiwa, H. Relationship between NMF (lactate and potassium) content and the physical properties of the stratum corneum in healthy subjects. J. Investig. Dermatol. 2004, 122, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Hołyńska-Iwan, I.; Sobiesiak, M. Cisplatin influences the skin ion transport: An in vitro study. Biomed. Pharmacol. 2020, 129, 110502. [Google Scholar] [CrossRef]
- Li, H.; Sheppard, D.N.; Hug, M.J. Transepithelial electrical measurements with the Ussing chamber. J. Cyst. Fibros. 2004, 3, 123–126. [Google Scholar] [CrossRef]
- White, E.A.; Horne, A.; Runciman, J.; Orazem, M.E.; Navidi, W.C.; Roper, C.S.; Bunge, A.L. On the correlation between single-frequency impedance measurements and human skin permeability to water. Toxicol. Vitro 2011, 25, 2095–2104. [Google Scholar] [CrossRef]
- Perez Vallina, J.; Menendez Antolin, L.; Cantabrana, B.; Sanchez, M.; Hidalgo, A. Involvement of Sodium/Calcium Exchange in the Diclofenac-induced Spasmolytic Effect on Rat Uterus. Gen. Phormac. 1995, 26, 1249–1253. [Google Scholar] [CrossRef]
- Tronstad, C.; Kalvøy, H.; Grimnes, S.; Martinsen, Ø.G. Waveform difference between skin conductance and skin potential responses in relation to electrical and evaporative properties of skin. Psychophysiology 2013, 50, 1070–1078. [Google Scholar] [CrossRef]
| 1–10/100 Cases |
|---|
| Rash |
| Erythema |
| Eczema |
| Itching |
| Urticaria |
| Dermatitis |
| 1–10/10,000 Cases |
| Follicular eruptions |
| <1/10,000 Cases |
| Edema |
| Light hypersensitivity |
| R (Ω/cm2) | Control (n = 24) | Diclofenac (n = 31) | Results of the Mann–Whitney Test (p) Control vs. Diclofenac | |
|---|---|---|---|---|
| R initial | median | 488 | 1416 | <0.001 |
| lower quartile | 344 | 835 | ||
| upper quartile | 817 | 1848 | ||
| R final | median | 498 | 1338 | <0.001 |
| lower quartile | 337 | 794 | ||
| upper quartile | 804 | 1801 | ||
| Results of the Wilcoxon test (p) R initial vs. R final | 0.24 | <0.001 | ||
| PD (mV) | Control (n = 24) | Diclofenac (n = 31) | Results of the Mann–Whitney Test (p) Control vs. Diclofenac | |
|---|---|---|---|---|
| PD initial | median | 0 | 0.56 | <0.001 |
| lower quartile | −0.12 | 0 | ||
| upper quartile | 0.15 | 0.51 | ||
| PD final | median | 0 | −0.12 | 0.25 |
| lower quartile | 0 | −0.31 | ||
| upper quartile | 0.23 | 0.19 | ||
| Results of the Wilcoxon test (p) PD initial vs. PD final | 0.44 | <0.001 | ||
| Stimulating Solution | Control (n = 24) | Diclofenac (n = 31) | The Mann-Whitney Test (p) | ||||
|---|---|---|---|---|---|---|---|
| PDmax (mV) | PDmin (mV) | PDmax (mV) | PDmin (mV) | Control vs. Diclofenac | |||
| PDmax | PDmin | ||||||
| RH | median | 0.15 | 0 | 0.37 | 0 | 0.16 | 0.31 |
| lower quartile | 0 | −0.18 | 0.15 | −0.21 | |||
| upper quartile | 0.43 | 0 | 0.67 | 0.43 | |||
| B | median | 0.18 | −0.12 | 0.24 | −0.21 | 0.99 | 0.60 |
| lower quartile | 0.15 | −0.21 | 0 | −0.49 | |||
| upper quartile | 0.4 | 0.18 | 0.67 | 0 | |||
| A | median | 0.18 | 0 | 0.52 | −0.34 | 0.18 | 0.39 |
| lower quartile | 0.15 | −0.24 | 0 | −0.55 | |||
| upper quartile | 0.34 | 0 | 0.98 | 0 | |||
| AB | median | 0.18 | −0.12 | 0.18 | −0.31 | 0.66 | 0.35 |
| lower quartile | 0 | −0.4 | −0.12 | −0.64 | |||
| upper quartile | 0.52 | 0 | 0.88 | 0 | |||
| p-Value | |||
|---|---|---|---|
| Stimulating Solution | Parameters Compared | Control (n = 24) | Diclofenac (n = 31) |
| RH | PD/PDmax | <0.001 | <0.001 |
| PD/PDmin | <0.001 | <0.001 | |
| PDmax/PDmin | <0.001 | <0.001 | |
| B | PD/PDmax | 0.06 | <0.001 |
| PD/PDmin | 0.01 | <0.001 | |
| PDmax/PDmin | <0.001 | <0.001 | |
| A | PD/PDmax | <0.001 | <0.001 |
| PD/PDmin | 0.01 | <0.001 | |
| PDmax/PDmin | <0.001 | <0.001 | |
| AB | PD/PDmax | 0.01 | <0.001 |
| PD/PDmin | 0.03 | <0.001 | |
| PDmax/PDmin | <0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobrzeniecka, W.; Daca, M.; Nowakowska, B.; Sobiesiak, M.; Szewczyk-Golec, K.; Woźniak, A.; Hołyńska-Iwan, I. The Impact of Diclofenac Gel on Ion Transport in the Rabbit (Oryctolagus cuniculus) Skin: An In Vitro Study. Molecules 2023, 28, 1332. https://doi.org/10.3390/molecules28031332
Dobrzeniecka W, Daca M, Nowakowska B, Sobiesiak M, Szewczyk-Golec K, Woźniak A, Hołyńska-Iwan I. The Impact of Diclofenac Gel on Ion Transport in the Rabbit (Oryctolagus cuniculus) Skin: An In Vitro Study. Molecules. 2023; 28(3):1332. https://doi.org/10.3390/molecules28031332
Chicago/Turabian StyleDobrzeniecka, Wioletta, Małgorzata Daca, Barbara Nowakowska, Marta Sobiesiak, Karolina Szewczyk-Golec, Alina Woźniak, and Iga Hołyńska-Iwan. 2023. "The Impact of Diclofenac Gel on Ion Transport in the Rabbit (Oryctolagus cuniculus) Skin: An In Vitro Study" Molecules 28, no. 3: 1332. https://doi.org/10.3390/molecules28031332
APA StyleDobrzeniecka, W., Daca, M., Nowakowska, B., Sobiesiak, M., Szewczyk-Golec, K., Woźniak, A., & Hołyńska-Iwan, I. (2023). The Impact of Diclofenac Gel on Ion Transport in the Rabbit (Oryctolagus cuniculus) Skin: An In Vitro Study. Molecules, 28(3), 1332. https://doi.org/10.3390/molecules28031332






