Quantifying Microalgae Growth by the Optical Detection of Glucose in the NIR Waveband
Abstract
1. Introduction
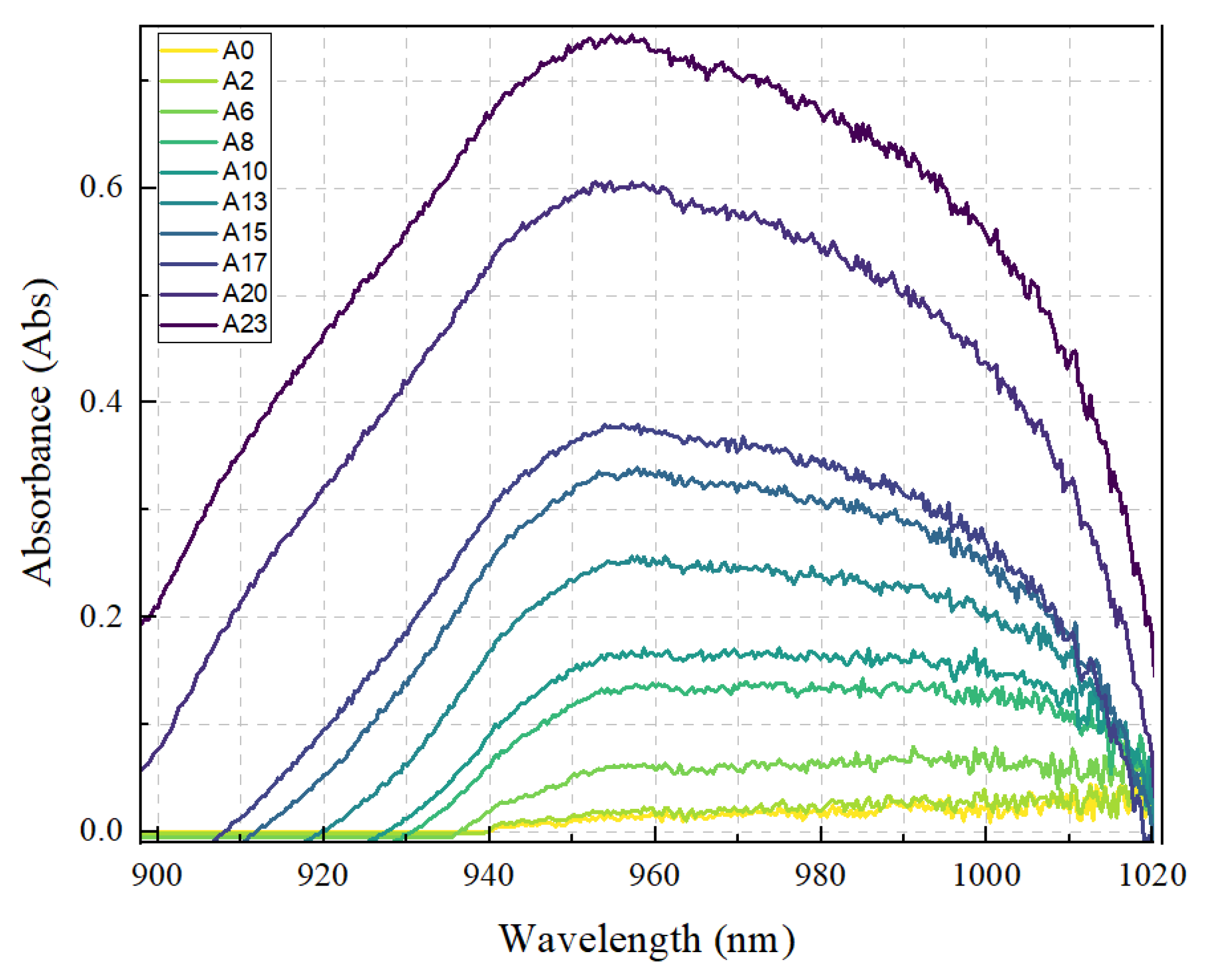
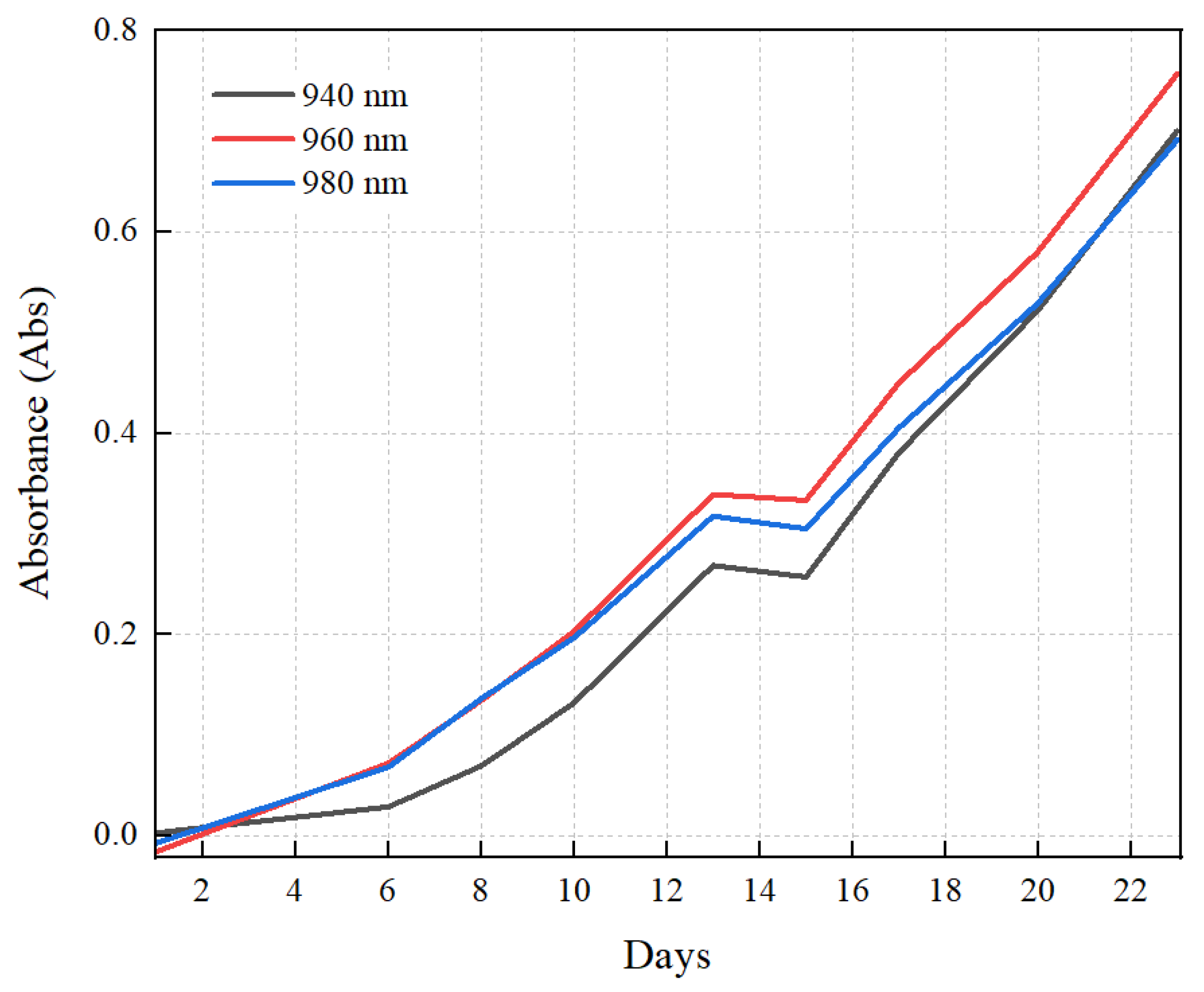
2. Result and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corrêa, P.S.; Morais Júnior, W.G.; Martins, A.A.; Caetano, N.S.; Mata, T.M. Microalgae biomolecules: Extraction, separation and purification methods. Processes 2020, 9, 10. [Google Scholar] [CrossRef]
- Gomes, T.A.; Zanette, C.M.; Spier, M.R. An overview of cell disruption methods for intracellular biomolecules recovery. Prep. Biochem. Biotechnol. 2020, 50, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for high-value products: A way towards green nutraceutical and pharmaceutical compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.d.R.; Leite, M.d.O.; Albino, L.F.T.; Martins, M.A. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- Medipally, S.R.; Yusoff, F.M.; Banerjee, S.; Shariff, M. Microalgae as sustainable renewable energy feedstock for biofuel production. Biomed Res. Int. 2015, 2015, 519513. [Google Scholar] [CrossRef]
- Adnan, A. Apoptotic effects of beta-carotene, alpha-tocopherol and ascorbic acid on PC-3 prostate cancer cells. Hacettepe J. Biol. Chem. 2020, 48, 211–218. [Google Scholar]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-inflammatory and anticancer effects of microalgal carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef] [PubMed]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal carotenoids: A review of production, current markets, regulations, and future direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- Hossain, N.; Mahlia, T.; Saidur, R. Latest development in microalgae-biofuel production with nano-additives. Biotechnol. Biofuels 2019, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Ighalo, J.O.; Dulta, K.; Kurniawan, S.B.; Omoarukhe, F.O.; Ewuzie, U.; Eshiemogie, S.O.; Ojo, A.U.; Abdullah, S.R.S. Progress in microalgae application for CO2 sequestration. Clean. Chem. Eng. 2022. [Google Scholar] [CrossRef]
- Pooja, K.; Priyanka, V.; Rao, B.C.S.; Raghavender, V. Cost-effective treatment of sewage wastewater using microalgae Chlorella vulgaris and its application as bio-fertilizer. Energy Nexus 2022, 7, 100122. [Google Scholar] [CrossRef]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae encapsulation systems for food, pharmaceutical and cosmetics applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Morocho-Jácome, A.L.; Ruscinc, N.; Martinez, R.M.; de Carvalho, J.C.M.; Santos de Almeida, T.; Rosado, C.; Costa, J.G.; Velasco, M.V.R.; Baby, A.R. (Bio) Technological aspects of microalgae pigments for cosmetics. Appl. Biochem. Microbiol. 2020, 104, 9513–9522. [Google Scholar] [CrossRef]
- Schagerl, M.; Siedler, R.; Konopáčová, E.; Ali, S.S. Estimating Biomass and Vitality of Microalgae for Monitoring Cultures: A Roadmap for Reliable Measurements. Cells 2022, 11, 2455. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M. Production of Chlorella vulgaris biomass in tubular photobioreactors during different culture conditions. Appl. Sci. 2021, 11, 3106. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Hu, H.-Y.; Wu, Y.-H.; Wang, T.; Zhang, T.-Y. A novel suspended-solid phase photobioreactor to improve biomass production and separation of microalgae. Bioresour. Technol. 2014, 153, 399–402. [Google Scholar] [CrossRef]
- Sengmee, D.; Cheirsilp, B.; Suksaroge, T.T.; Prasertsan, P. Biophotolysis-based hydrogen and lipid production by oleaginous microalgae using crude glycerol as exogenous carbon source. Int. J. Hydrogen Energy 2017, 42, 1970–1976. [Google Scholar] [CrossRef]
- Hawrot-Paw, M.; Koniuszy, A.; Gałczyńska, M. Sustainable production of Monoraphidium microalgae biomass as a source of bioenergy. Energies 2020, 13, 5975. [Google Scholar] [CrossRef]
- Fu, J.; Huang, Y.; Xia, A.; Zhu, X.; Zhu, X.; Chang, J.-S.; Liao, Q. How the sulfur dioxide in the flue gas influence microalgal carbon dioxide fixation: From gas dissolution to cells growth. Renew. Energy 2022, 198, 114–122. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, W.; Zhou, X.; Jin, W.; Han, W.; Chi, K.; Chen, Y.; Zhao, Z.; He, Z.; Jiang, G. Sub-pilot scale cultivation of Tetradesmus dimorphus in wastewater for biomass production and nutrients removal: Effects of photoperiod, CO2 concentration and aeration intensity. J. Water Process. Eng. 2022, 49, 103003. [Google Scholar] [CrossRef]
- Chioccioli, M.; Hankamer, B.; Ross, I.L. Flow cytometry pulse width data enables rapid and sensitive estimation of biomass dry weight in the microalgae Chlamydomonas reinhardtii and Chlorella vulgaris. PLoS ONE 2014, 9, e97269. [Google Scholar] [CrossRef] [PubMed]
- Sarrafzadeh, M.H.; La, H.-J.; Seo, S.-H.; Asgharnejad, H.; Oh, H.-M. Evaluation of various techniques for microalgal biomass quantification. J. Appl. Phycol. 2015, 216, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Cui, Y.; Yuan, W. Ultrasound for microalgal cell disruption and product extraction: A review. Ultrason. Sonochem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Aquino, A.U.; Fernandez, M.E.M.; Guzman, A.P.; Matias, A.A.; Valenzuela, I.C.; Dadios, E.P. An Artificial Neural Network (ANN) Model for the Cell Density Measurement of Spirulina (A. platensis). In Proceedings of the 2018 IEEE 10th International Conference on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment and Management (HNICEM), Baguio City, Philippines, 29 November–2 December 2018; pp. 1–5. [Google Scholar]
- Giraldo-Zuluaga, J.-H.; Salazar, A.; Diez, G.; Gomez, A.; Martínez, T.; Vargas, J.F.; Peñuela, M. Applications. Automatic identification of Scenedesmus polymorphic microalgae from microscopic images. Pattern Anal. Appl. 2018, 21, 601–612. [Google Scholar] [CrossRef]
- Di Caprio, F. Methods to quantify biological contaminants in microalgae cultures. Algal Res. 2020, 49, 101943. [Google Scholar] [CrossRef]
- Havlik, I.; Beutel, S.; Scheper, T.; Reardon, K.F. On-line monitoring of biological parameters in microalgal bioprocesses using optical methods. Energies 2022, 15, 875. [Google Scholar] [CrossRef]
- Gomes, A.C.; Leandro, T.; Garcia, Y.; Bastos, R.B.; Lopes, R.M. Frame Rhythm: A new cost-effective approach for semi-automatic microalgal imaging and enumeration. Algal Res. 2022, 64, 102659. [Google Scholar] [CrossRef]
- Otálora, P.; Guzmán, J.; Acién, F.; Berenguel, M.; Reul, A. Microalgae classification based on machine learning techniques. Algal Res. 2021, 55, 102256. [Google Scholar] [CrossRef]
- Lojk, J.; Sajn, L.; Ćibej, U.; Pavlin, M. Automatic cell counter for cell viability estimation. In Proceedings of the 2014 37th International Convention on Information and Communication Technology, Electronics and Microelectronics (MIPRO), Opatija, Croatia, 26–30 May 2014; pp. 239–244. [Google Scholar]
- Camacho-Fernández, C.; Hervás, D.; Rivas-Sendra, A.; Marín, M.; Seguí-Simarro, J.M. Comparison of six different methods to calculate cell densities. Plant Methods 2022 14, 1–15. [CrossRef]
- Nielsen, S.L.; Hansen, B.W. Evaluation of the robustness of optical density as a tool for estimation of biomass in microalgal cultivation: The effects of growth conditions and physiological state. Aquacult. Res. 2019, 50, 2698–2706. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, H.; Pan, J.; Jiang, L.; He, Y.; Shag, Y. Chlorophyll content research of Haematococcus pluvialis based on immersed visible/near-infrared spectroscopy. Spectrosc. Spectr. Anal 2017, 37, 3375–3378. [Google Scholar]
- Shao, Y.; Pan, J.; Zhang, C.; Jiang, L.; He, Y. Detection in situ of carotenoid in microalgae by transmission spectroscopy. Comput. Electron. Agric. 2015, 112, 121–127. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, L.; Zhao, Y.; Shao, Y.; Qiu, Z.; He, Y. Study on the Nondestructive Detection Methods for Dynamica Change of Lipid Content in Chlorella sp. Guang Pu Xue Yu Guang Pu Fen Xi Guang Pu 2016, 36, 1352–1357. [Google Scholar] [PubMed]
- Wagner, H.; Liu, Z.; Langner, U.; Stehfest, K.; Wilhelm, C. The use of FTIR spectroscopy to assess quantitative changes in the biochemical composition of microalgae. J. Biophotonics 2010, 3, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, L.; Xie, B.; Wu, M.; Ma, L.; Yang, J.-Y. Optical properties of biochemical compositions of microalgae within the spectral range from 300 to 1700 nm. Appl. Opt. 2021, 60, 10232–10238. [Google Scholar] [CrossRef]
- Katam, K.; Ananthula, R.; Anumala, S.; Sriariyanun, M.; Bhattacharyya, D. The impact of light intensity and wavelength on the performance of algal-bacterial culture treating domestic wastewater. In Proceedings of the E3S Web of Conferences, Bangkok, Thailand, 4–5 August 2022; p. 02003. [Google Scholar]
- Sorgüven, E.; Özilgen, M. Thermodynamic efficiency of synthesis, storage and breakdown of the high-energy metabolites by photosynthetic microalgae. Energy 2013, 58, 679–687. [Google Scholar] [CrossRef]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Fact. 2019, 18, 178. [Google Scholar] [CrossRef]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef]
- Yoo, H.-D.; Kim, D.; Paek, S.-H.; Oh, S.E. Plant cell wall polysaccharides as potential resources for the development of novel prebiotics. Biomol. Ther. 2012, 20, 371. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, N.; Poulhazan, A.; Deligey, F.; Mentink-Vigier, F.; Marcotte, I.; Wang, T. Solid-state NMR investigations of extracellular matrixes and cell walls of algae, bacteria, fungi, and plants. Chem. Rev. 2021, 122, 10036–10086. [Google Scholar] [CrossRef] [PubMed]
- Kienteka, S.S.; Corrêa-Ferreira, M.L.; de Oliveira Petkowicz, C.L. Characterization of cell wall polysaccharides from Sicana odorifera fruit and structural analysis of a galactan-rich fraction pectins as side chains. Carbohydr. Polym. 2018, 197, 395–402. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Z.; Miao, X. Nitrogen and hydrophosphate affects glycolipids composition in microalgae. Sci. Rep. 2016, 6, 30145. [Google Scholar] [CrossRef]
- Golic, M.; Walsh, K.; Lawson, P. Short-wavelength near-infrared spectra of sucrose, glucose, and fructose with respect to sugar concentration and temperature. Appl. Spectrosc. 2003, 57, 139–145. [Google Scholar] [CrossRef]
- Jain, P.; Maddila, R.; Joshi, A.M. A precise non-invasive blood glucose measurement system using NIR spectroscopy and Huber’s regression model. Opt. Quantum Electron 2019, 51, 51. [Google Scholar] [CrossRef]
- Zeb, A.; Qureshi, W.S.; Ghafoor, A.; O’Sullivan, D. Learning fruit class from short wave near infrared spectral features, an AI approach towards determining fruit type. In Proceedings of the 2022 8th International Conference on Mechatronics and Robotics Engineering (ICMRE), Munich, Germany, 10–12 February 2022; pp. 193–196. [Google Scholar]
- Jain, P.; Pancholi, S.; Joshi, A.M. An IoMT based non-invasive precise blood glucose measurement system. In Proceedings of the 2019 IEEE International Symposium on Smart Electronic Systems (iSES)(Formerly iNiS), Rourkela, India, 16–18 December 2019; pp. 111–116. [Google Scholar]
- Yadav, D.; Singh, M.; Sharma, S.; Singh, S.P.; Dubey, P. Dual Wavelength based Approach with Partial Least Square Regression for the Prediction of Glucose Concentration. Indian J. Pure Appl. Phy. 2022, 60, 700–706. [Google Scholar]
- Lai, J.-L.; Huang, S.-Y.; Lin, R.-S.; Tsai, S.-C. Design a non-invasive near-infrared LED blood glucose sensor. In Proceedings of the 2016 International Conference on Applied System Innovation (ICASI), Okinawa, Japan, 26–30 May 2016; pp. 1–4. [Google Scholar]
- Marius, I.; Sever, P. Measuring glucose blood with spectroscopy skin in near infrared. In Proceedings of the 2019 11th International Conference on Electronics, Computers and Artificial Intelligence (ECAI), Pitesti, Romania, 27–29 June 2019; pp. 1–6. [Google Scholar]
- Rady, A.M.; Guyer, D.E.; Watson, N.J. Near-infrared spectroscopy and hyperspectral imaging for sugar content evaluation in potatoes over multiple growing seasons. Food Anal. Methods 2021, 14, 581–595. [Google Scholar] [CrossRef]
- Khamsopha, D.; Woranitta, S.; Teerachaichayut, S.J.F.C. Utilizing near infrared hyperspectral imaging for quantitatively predicting adulteration in tapioca starch. Food Control 2021, 123, 107781. [Google Scholar] [CrossRef]
- Mbanjo, E.; Hershberger, J.; Peteti, P.; Agbona, A.; Ikpan, A.; Ogunpaimo, K.; Kayondo, S.I.; Abioye, R.S.; Nafiu, K.; Alamu, E.O.; et al. Predicting starch content in cassava fresh roots using near-infrared spectroscopy. Front. Plant Sci. 2022, 13, 990250. [Google Scholar] [CrossRef]
- Asrofi, M.; Dwilaksana, D.; Abral, H.; Fajrul, R. Tensile, thermal, and moisture absorption properties of polyvinyl alcohol (PVA)/bengkuang (pachyrhizuserosus) starch blend films. Mat. Sci. Res. India 2019, 16, 70–75. [Google Scholar] [CrossRef]
- Poulhazan, A.; Dickwella Widanage, M.C.; Muszyński, A.; Arnold, A.A.; Warschawski, D.E.; Azadi, P.; Marcotte, I.; Wang, T.J. Identification and quantification of glycans in whole cells: Architecture of microalgal polysaccharides described by solid-state nuclear magnetic resonance. J. Am. Chem. Soc. 2021, 143, 19374–19388. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Morales-Contreras, B.E.; Guerra-Rosas, M.I.; Osorio-Hernández, E.; Culver, C.A.; Morales-Castro, J.; Wicker, L. Huanglongbing disease and quality of pectin and fruit juice extracted from Valencia oranges. LWT 2020, 131, 109692. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Shaheen, H.; Wu, A.-M. Cell wall hemicellulose for sustainable industrial utilization. Renew. Sustain. Energy Rev. 2021, 144, 110996. [Google Scholar] [CrossRef]
- Hollenbach, R.; Völp, A.R.; Höfert, L.; Rudat, J.; Ochsenreither, K.; Willenbacher, N.; Syldatk, C. Interfacial and foaming properties of tailor-made glycolipids—influence of the hydrophilic head group and functional groups in the hydrophobic tail. Molecules 2020, 25, 3797. [Google Scholar] [CrossRef]
- Samaher, C.; Mourad, C.; Alimi, K. Theoretical investigations about the effect of electron-withdrawing groups on proprieties of A-π-D-π-A type small molecules donor for organic solar cells. J. Mol. Model. 2021, 27, 54. [Google Scholar] [CrossRef]
- Omar, A.F.; Yahaya, O.K.M.; Tan, K.C.; Mail, M.H.; Seeni, A. The influence of additional water content towards the spectroscopy and physicochemical properties of genus Apis and stingless bee honey. In Optical Sensing and Detection IV; SPIE: Bellingham, WA, USA, 2016; pp. 223–228. [Google Scholar]
- Raypah, M.E.; Muncan, J.; Sudik, S.; Omar, A.F.; Mail, M.H.; Tsenkova, R.; Seeni, A.J.C.; Systems, I.L. Integration of near-infrared spectroscopy and aquaphotomics for discrimination of cultured cancerous cells using phenol red. Chemom. Intell. Lab. Syst. 2022, 227, 104611. [Google Scholar] [CrossRef]
- Xie, L.; Ye, X.; Liu, D.; Ying, Y. Quantification of glucose, fructose and sucrose in bayberry juice by NIR and PLS. Food Chem. 2009, 114, 1135–1140. [Google Scholar] [CrossRef]
- Masojídek, J.; Ranglová, K.; Lakatos, G.E.; Silva Benavides, A.M.; Torzillo, G. Variables governing photosynthesis and growth in microalgae mass cultures. Processes 2021, 9, 820. [Google Scholar] [CrossRef]
- Rajagopal, R.; Mousavi, S.E.; Goyette, B.; Adhikary, S. Coupling of Microalgae Cultivation with Anaerobic Digestion of Poultry Wastes: Toward Sustainable Value Added Bioproducts. Bioengineering 2021, 8, 57. [Google Scholar] [CrossRef]
- Anyanwu, R.C.; Rodriguez, C.; Durrant, A.; Olabi, A.G.J.S. Evaluation of Growth Rate and Biomass Productivity of Scenedesmus quadricauda and Chlorella vulgaris under Different LED Wavelengths and Photoperiods. Sustainability 2022, 14, 6108. [Google Scholar] [CrossRef]
- Janta, K.; Pekkoh, J.; Tongsiri, S.; Pumas, C.; Peerapornpisal, Y.J. Selection of some native microalgal strains for possibility of bio-oil production in Thailand. Chiang Mai J. Sci. 2013, 40, 593–602. [Google Scholar]
- Yoo, C.; Jun, S.-Y.; Lee, J.-Y.; Ahn, C.-Y.; Oh, H.-M. Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010, 101, S71–S74. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.W.; Quinn, M.; Van Wychen, S.; Hyman, D.; Laurens, L.M. Separation and quantification of microalgal carbohydrates. J. Chromatogr. A 2012, 1270, 225–234. [Google Scholar] [CrossRef]
- Galant, A.; Kaufman, R.; Wilson, J. Glucose: Detection and analysis. Food Chem. 2015, 188, 149–160. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, Q.; Wang, K.; Qian, J.; Dong, X.; Yang, Z.; Du, X.; Qiu, B.J. Enhanced non-enzymatic glucose sensing based on copper nanoparticles decorated nitrogen-doped graphene. Biosens. Bioelectron 2014, 54, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Thomasson, J.A. NIR reflectance and MIR attenuated total reflectance spectroscopy for characterizing algal biomass composition. Trans. ASABE 2016, 59, 435–442. [Google Scholar]
- Liu, B.; Liu, J.; Chen, T.; Yang, B.; Jiang, Y.; Wei, D.; Chen, F. Rapid characterization of fatty acids in oleaginous microalgae by near-infrared spectroscopy. Int. J. Mol. Sci. 2015, 16, 7045–7056. [Google Scholar] [CrossRef]
- Rumin, J.; Bonnefond, H.; Saint-Jean, B.; Rouxel, C.; Sciandra, A.; Bernard, O.; Cadoret, J.-P.; Bougaran, G. The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol. Biofuels 2015, 8, 42. [Google Scholar] [CrossRef]
- Karakach, T.K.; McGinn, P.J.; Choi, J.; MacQuarrie, S.P.; Tartakovsky, B. Real-time monitoring, diagnosis, and time-course analysis of microalgae Scenedesmus AMDD cultivation using dual excitation wavelength fluorometry. J. Appl. Phycol. 2015, 27, 1823–1832. [Google Scholar] [CrossRef]
- Ruiz, C.A.S.; Baca, S.Z.; van den Broek, L.A.; van den Berg, C.; Wijffels, R.H.; Eppink, M.H. Selective fractionation of free glucose and starch from microalgae using aqueous two-phase systems. Algal Res. 2020, 46, 101801. [Google Scholar] [CrossRef]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic engineering of algae for enhanced biofuel production. Algal Res. 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- de Jaeger, L.; Verbeek, R.E.; Draaisma, R.B.; Martens, D.E.; Springer, J.; Eggink, G.; Wijffels, R.H. Superior triacylglycerol (TAG) accumulation in starchless mutants of Scenedesmus obliquus:(I) mutant generation and characterization. Biotechnol. Biofuels 2014, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Scarponi, P.; Ghirardini, A.V.; Bravi, M.; Cavinato, C. Evaluation of Chlorella vulgaris and Scenedesmus obliquus growth on pretreated organic solid waste digestate. Waste Manage. 2021, 119, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Hotos, G.N.; Avramidou, D.; Bekiari, V. Calibration Curves of Culture Density Assessed by Spectrophotometer for Three Microalgae (Nephroselmis sp., Amphidinium carterae and Phormidium sp.). European J. Biol. Biotechnol. 2020, 1. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Garcin, C.; van Hille, R.P.; Harrison, S.T. Interference by pigment in the estimation of microalgal biomass concentration by optical density. J. Microbiol. Methods 2011, 85, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Fazeli Danesh, A.; Mooij, P.; Ebrahimi, S.; Kleerebezem, R.; van Loosdrecht, M. Effective role of medium supplementation in microalgal lipid accumulation. Biotechnol. Bioeng. 2018, 115, 1152–1160. [Google Scholar] [CrossRef]
- Yee, W.; Tang, S.G.H.; Phua, P.S.P.; Megawarnan, H. Long-term maintenance of 23 strains of freshwater microalgae on solid microbiological culture media: A preliminary study. Algal Res. 2019, 41, 101516. [Google Scholar] [CrossRef]

| Stock Solutions and Their Solutes | Per Liter of Distilled Water |
|---|---|
| Major A | |
| Sodium Nitrate (NaNO3) | 25.00 g |
| Calcium Chloride (CaCl2) | 2.50 g |
| Magnesium Sulfate Heptahydrate (MgSO4.7H2O) | 7.50 g |
| Sodium Chloride (NaCl) | 2.50 g |
| Major B | |
| Dipotassium Phosphate (K2HPO4) | 7.50 g |
| Monopotassium Phosphate (KH2PO4) | 17.50 g |
| EDTA-KOH | |
| Ethylenediaminetetraacetic Acid (EDTA) | 50.00 g |
| Potassium Hydroxide (KOH) | 31.00 g |
| Ferric Solution | |
| Iron(II) Sulfate Heptahydrate (FeSO4.7H2O) | 4.98 g |
| Sulfuric Acid (H2SO4) | 1.00 mL |
| Boric Acid | |
| Boric Acid (H3BO3) | 11.42 g |
| Micronutrient Solution | |
| Zinc Sulfate Heptahydrate (ZnSO4.7H2O) | 8.82 g |
| Manganese(II) Chloride Tetrahydrate (MnCl2.4H2O) | 1.44 g |
| Sodium Molybdate Dihydrate (Na2MoO4.2H2O | 0.39 g |
| Copper Sulfate Pentahydrate (CuSO4.5H2O) | 1.57 g |
| Cobalt Nitrate Hexahydrate (Co(NO3)2.6H2O) | 0.49 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiviyanathan, V.A.; Ker, P.J.; Amin, E.P.P.; Tang, S.G.H.; Yee, W.; Jamaludin, M.Z. Quantifying Microalgae Growth by the Optical Detection of Glucose in the NIR Waveband. Molecules 2023, 28, 1318. https://doi.org/10.3390/molecules28031318
Thiviyanathan VA, Ker PJ, Amin EPP, Tang SGH, Yee W, Jamaludin MZ. Quantifying Microalgae Growth by the Optical Detection of Glucose in the NIR Waveband. Molecules. 2023; 28(3):1318. https://doi.org/10.3390/molecules28031318
Chicago/Turabian StyleThiviyanathan, Vimal Angela, Pin Jern Ker, Eric P. P. Amin, Shirley Gee Hoon Tang, Willy Yee, and M. Z. Jamaludin. 2023. "Quantifying Microalgae Growth by the Optical Detection of Glucose in the NIR Waveband" Molecules 28, no. 3: 1318. https://doi.org/10.3390/molecules28031318
APA StyleThiviyanathan, V. A., Ker, P. J., Amin, E. P. P., Tang, S. G. H., Yee, W., & Jamaludin, M. Z. (2023). Quantifying Microalgae Growth by the Optical Detection of Glucose in the NIR Waveband. Molecules, 28(3), 1318. https://doi.org/10.3390/molecules28031318







