Infrared Spectral Classification of Natural Bitumens for Their Rheological and Thermophysical Characterization
Abstract
1. Introduction
2. Results and Discussion
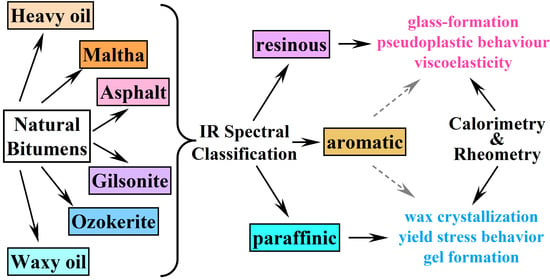
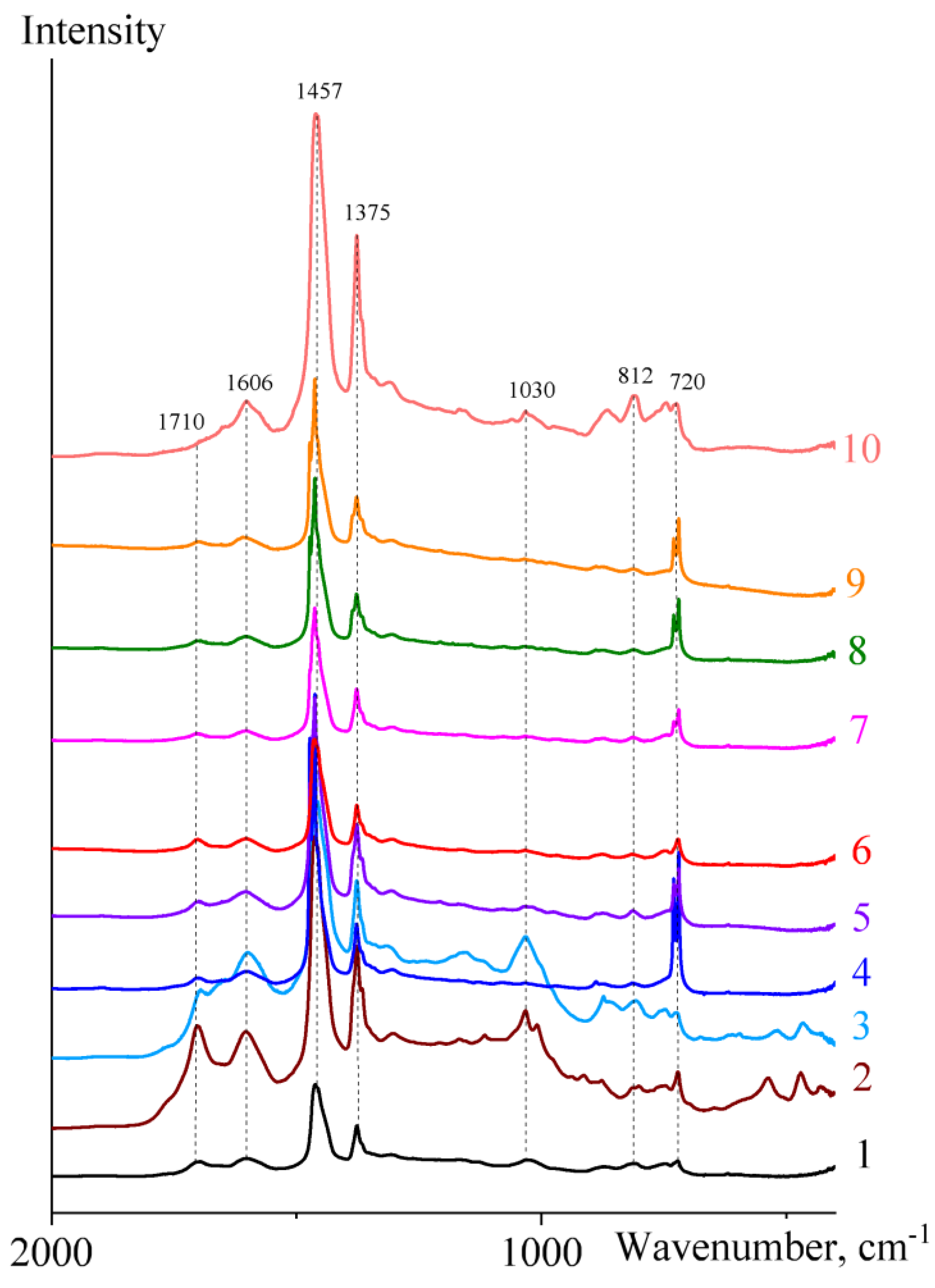
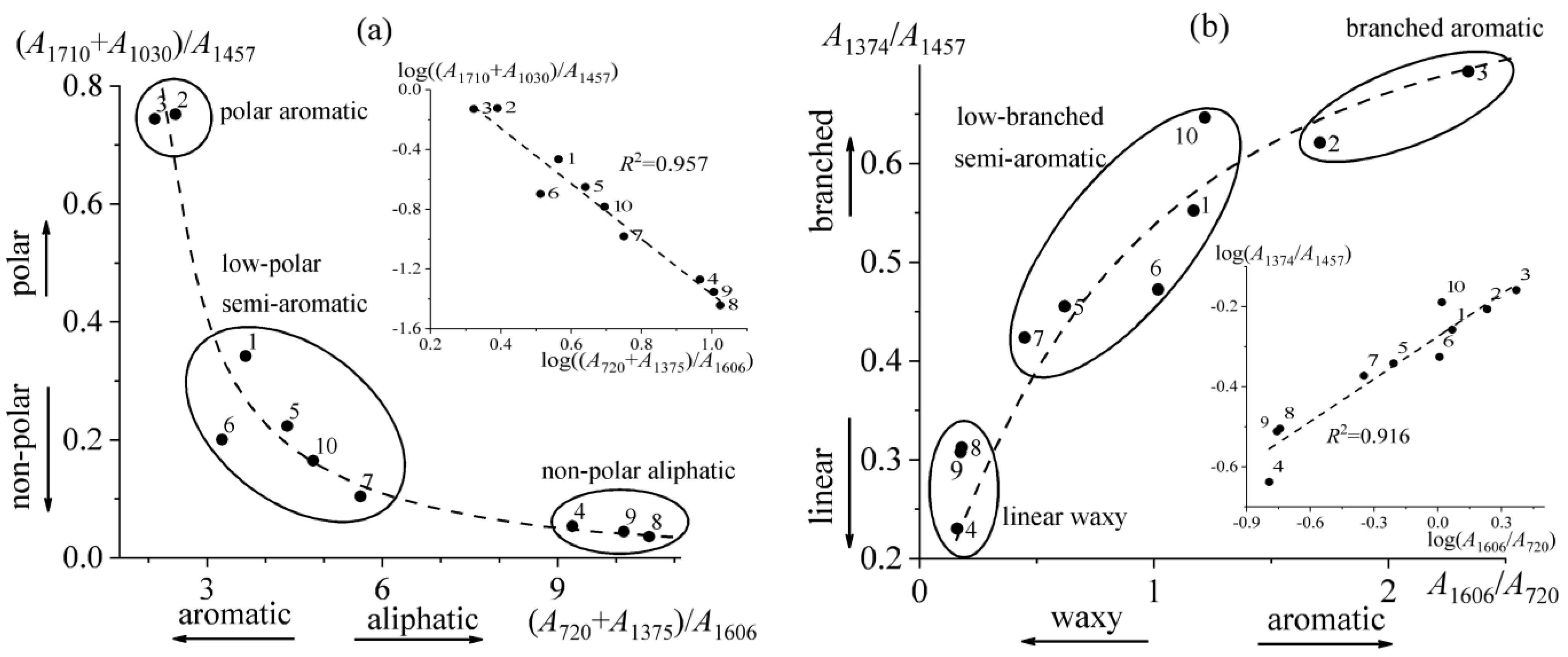
2.1. Infrared Spectral Characterization
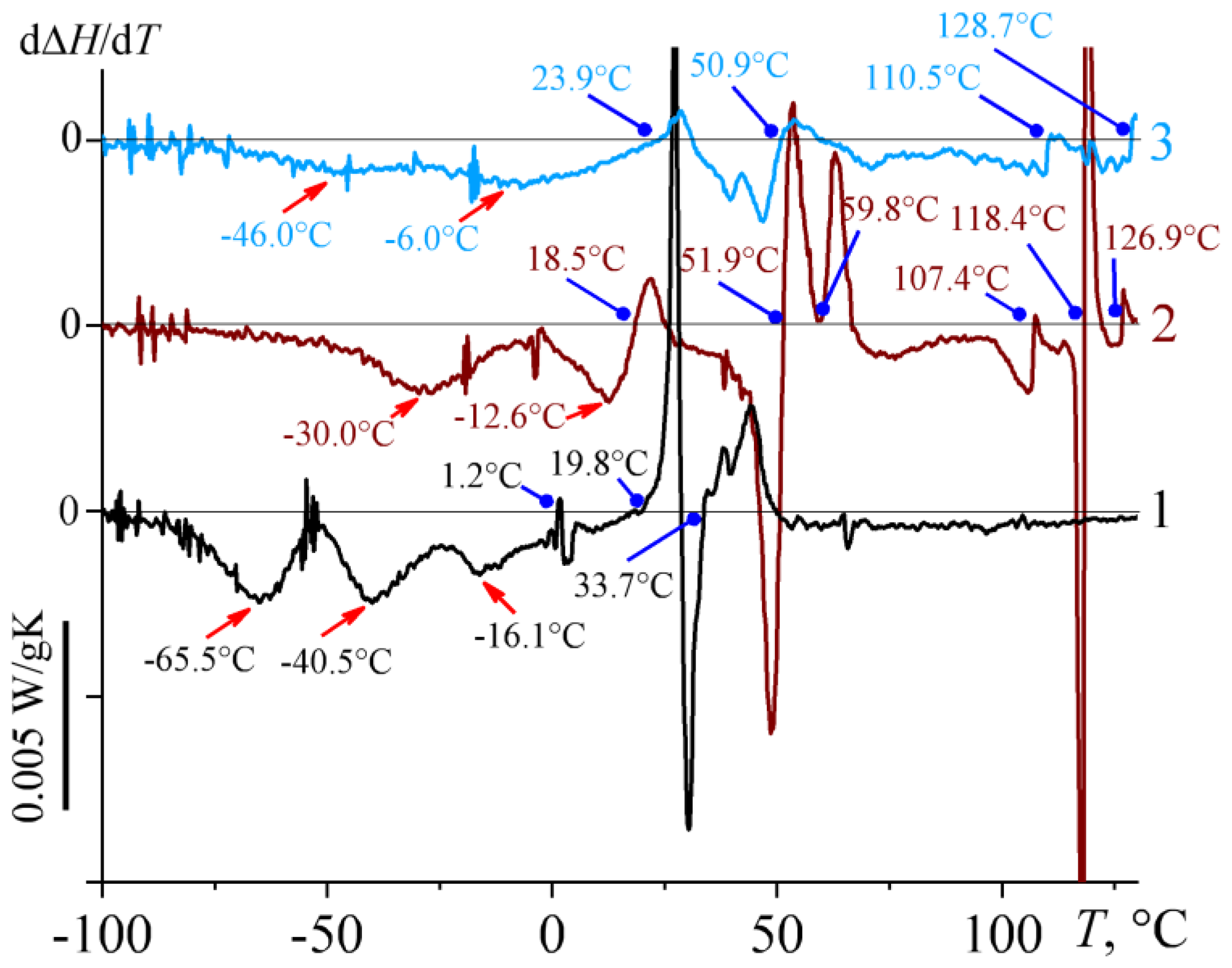
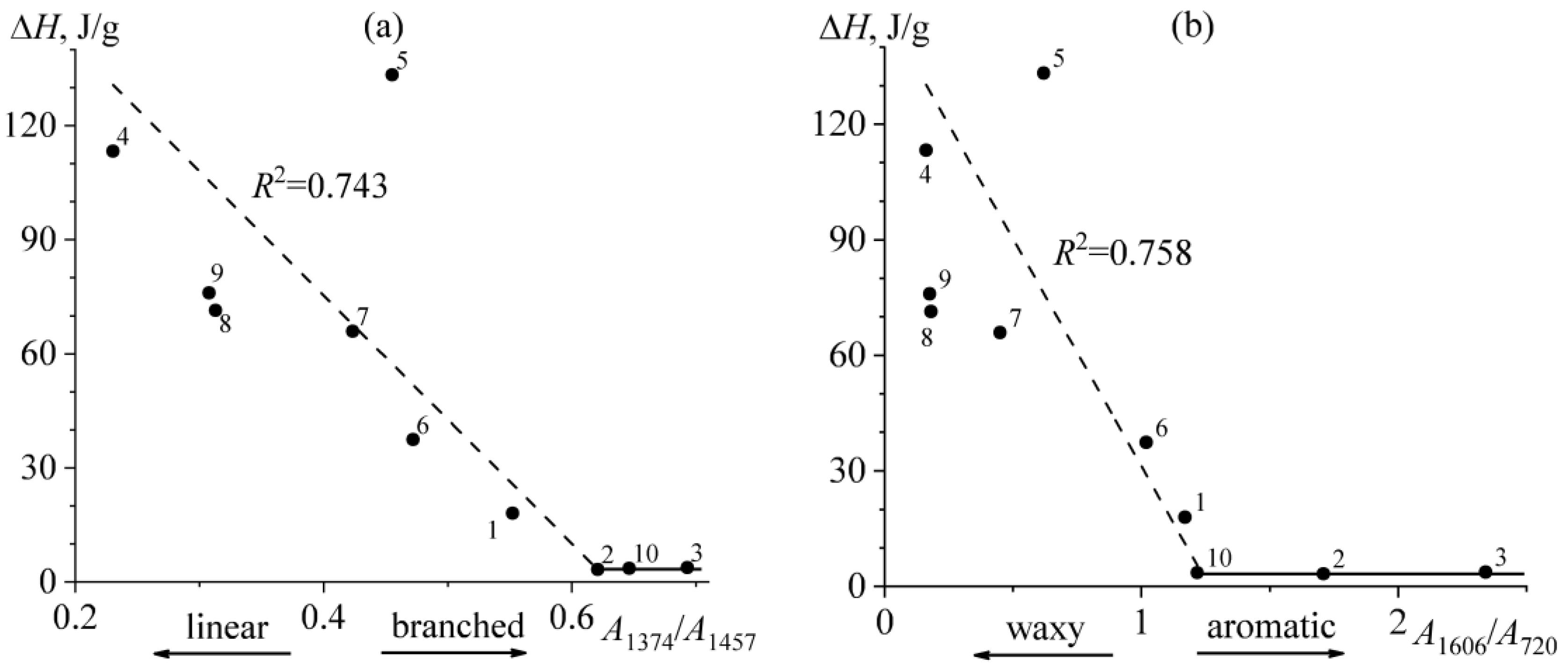
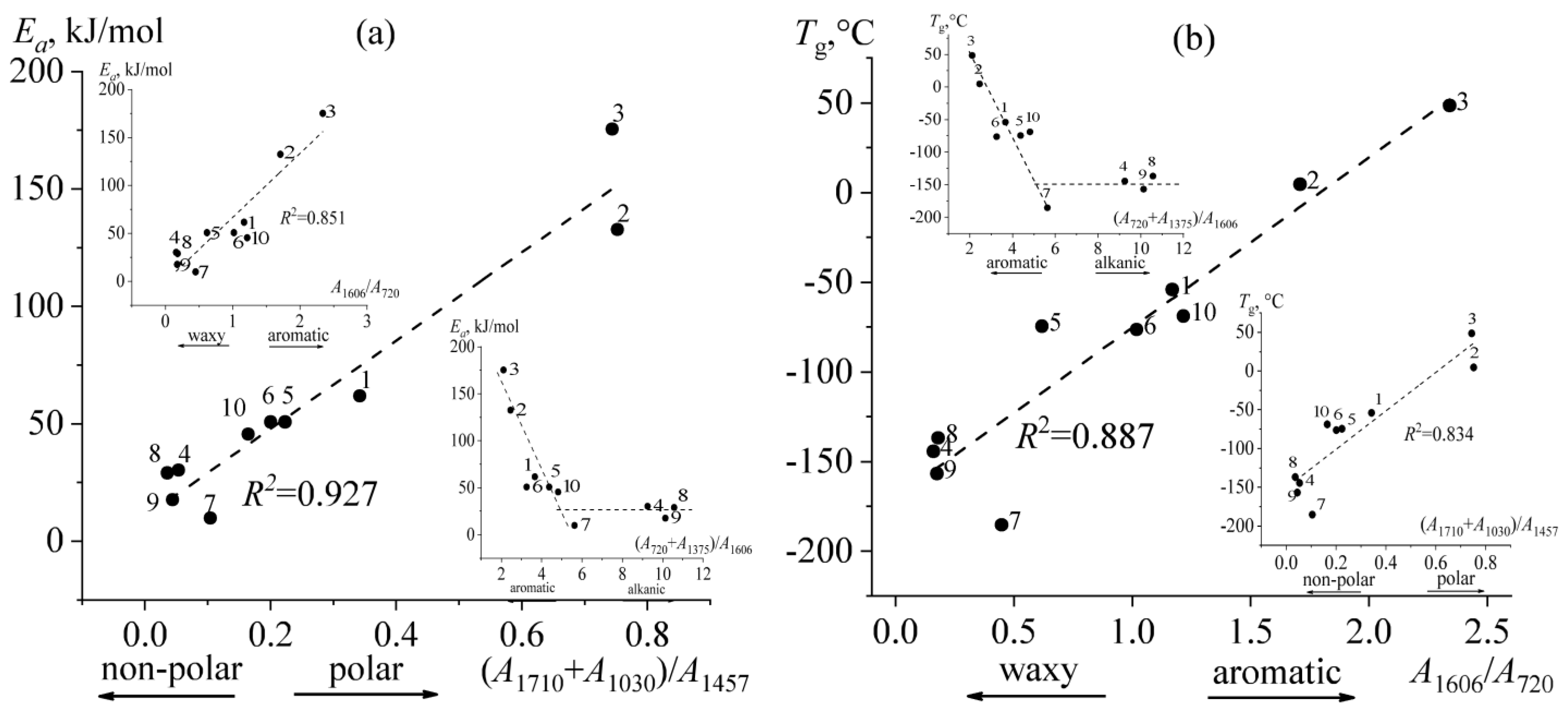
2.2. Thermophysical Properties of Bitumens
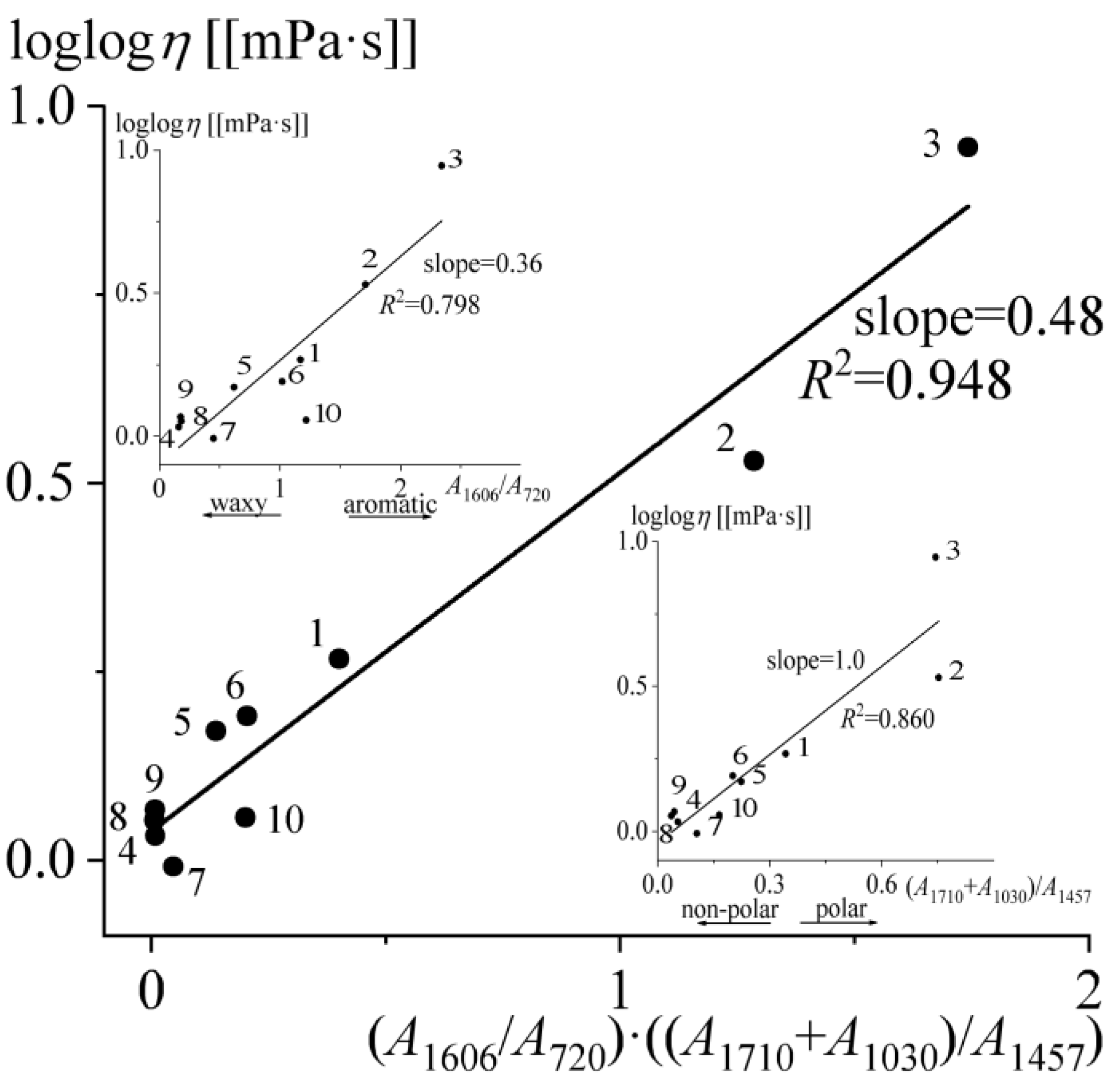
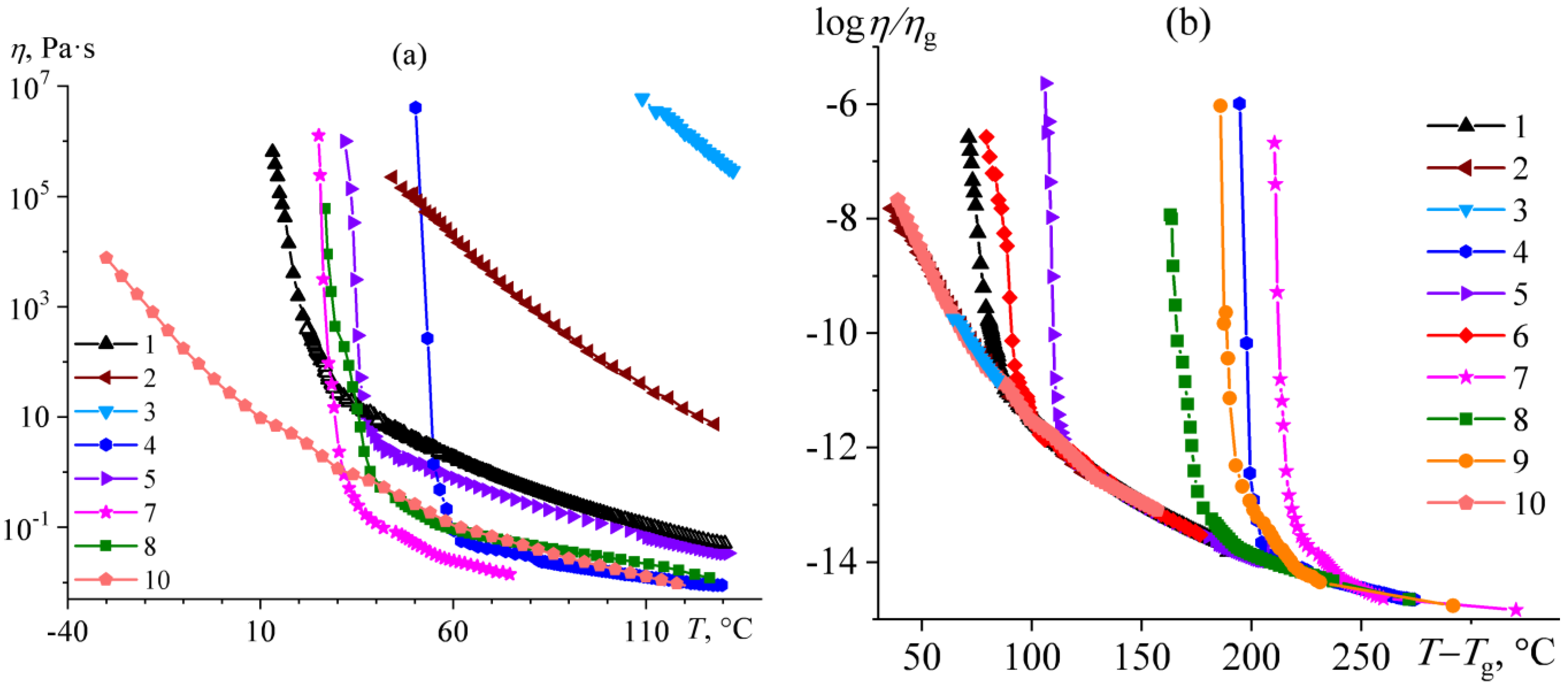
2.3. Flow Behavior of Bitumens
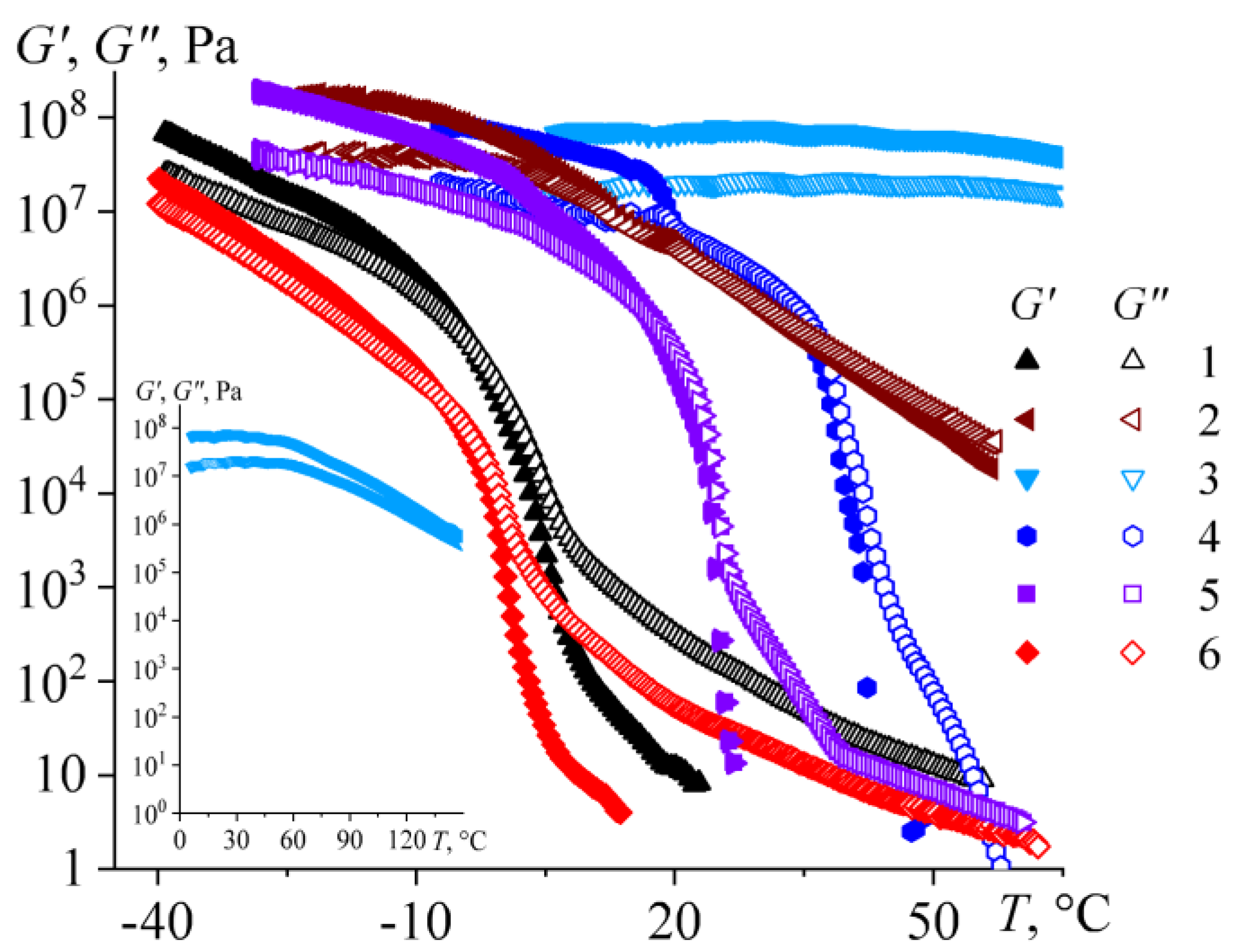
2.4. Viscoelasticity of Bitumens
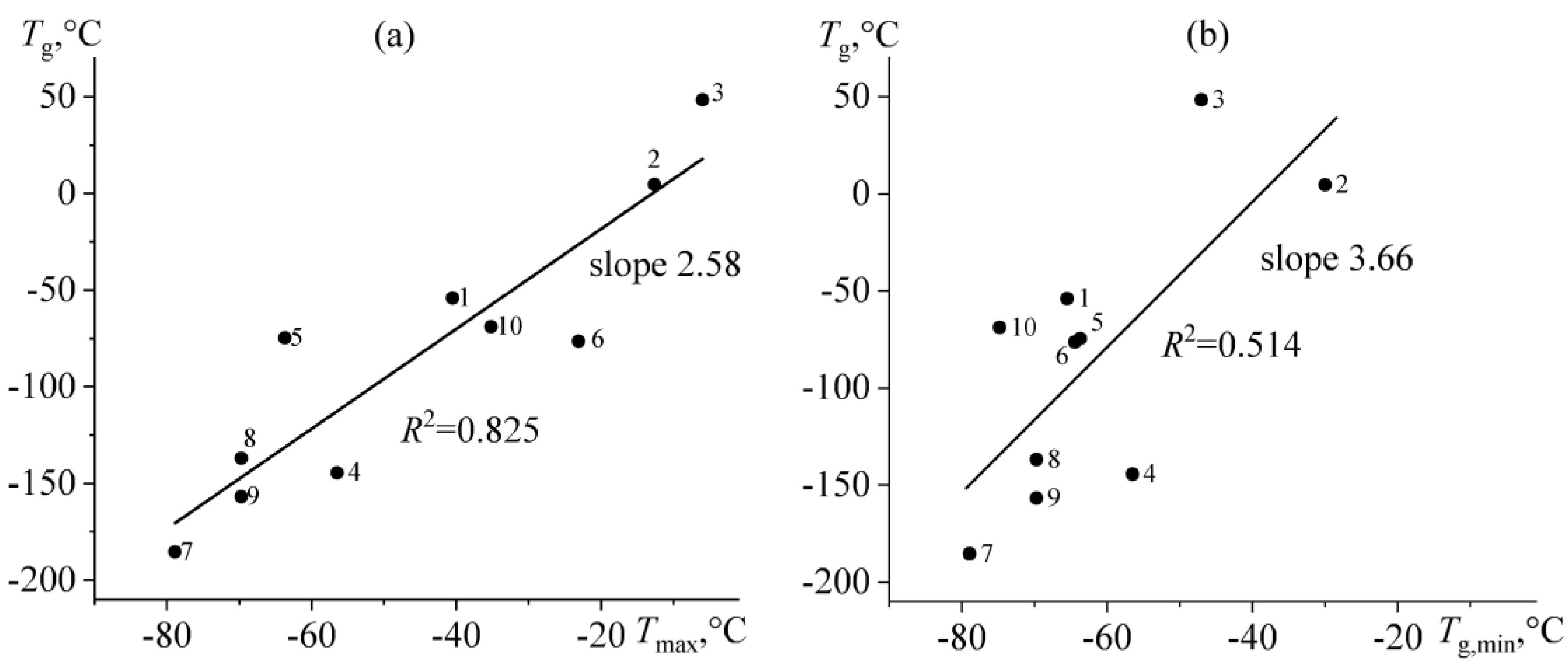
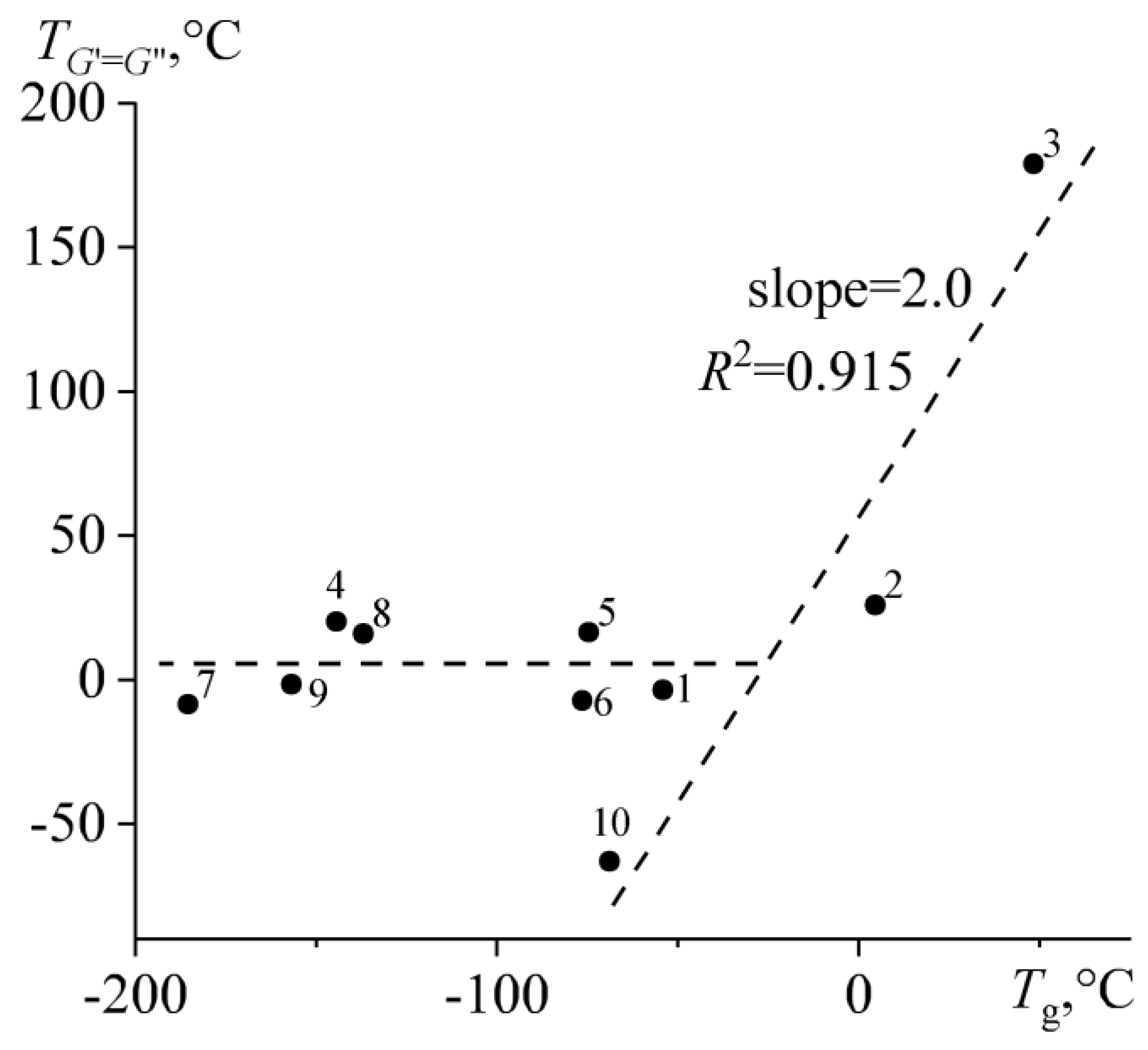
2.5. Rheological Manifestations and the Nature of Temperature Transitions in Bitumens
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
- The dominant constituents of bitumens determine their rheological and thermophysical properties: glass-forming resin-asphaltene compounds, crystallizing paraffin waxes, or aromatics acting as diluents.
- The combination of interrelated infrared spectral characteristics—paraffinicity, aromaticity, polarity, and branchiness—allows for dividing bitumens into three classes: resinous, paraffinic, and aromatic.
- The high glass transition temperature determines the behavior of resinous bitumens (asphalts, gilsonites): they are viscoelastic pseudoplastic glass-forming liquids with a low content of crystallizing paraffin compounds that do not affect bitumens’ rheological properties.
- Crystallizing paraffin compounds give paraffinic bitumens (waxy oils, oxykerites) a gel-like state and a yield stress behavior at low temperatures, disappearing upon heating and melting paraffin crystals.
- Aromatic bitumens (heavy oils, malthas) are Newtonian liquids that can acquire viscoelasticity and non-Newtonian behavior upon cooling due to glass transition or paraffin crystallization.
- The transition of paraffinic and resinous bitumens from a liquid-like to a solid-like state is caused by the paraffin crystallization and the glass-transition of continuous medium, respectively, while the nature of the liquid–solid transition of aromatic bitumens (crystallization or glass-transition) depends on the paraffin content in them.
- The temperature dependence of viscosity is a single universal dependence for bitumens having low paraffinicity or when the test temperature is high, but paraffin crystallization at cooling causes the viscosity deviation from a single dependence.
- An increase in polarity, aromaticity, and branchiness of bitumens (their transition from paraffinic to aromatic and then to resinous) elevates their viscosity, flow activation energy, and glass transition temperature.
- Infrared spectral characteristics allow for predicting such bitumen parameters as melting enthalpy and thus approximate content of crystallizing paraffin compounds, flow activation energy, viscosity, and glass transition temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hein, F.J. Geology of Bitumen and Heavy Oil: An Overview. J. Pet. Sci. Eng. 2017, 154, 551–563. [Google Scholar] [CrossRef]
- Shafiei, A.; Dusseault, M.B. Geomechanics of Thermal Viscous Oil Production in Sandstones. J. Pet. Sci. Eng. 2013, 103, 121–139. [Google Scholar] [CrossRef]
- Huc, A.-Y.; Jones, T. Heavy Crude Oils: From Geology to Upgrading an Overview; IFP, Énergies Nouvelles Publications; Éditions Technip: Paris, France, 2010; ISBN 978-2-7108-0890-9. [Google Scholar]
- Lapidus, A.L.; Kerimov, V.Y.; Mustaev, R.N.; Movsumzade, E.M.; Salikhova, I.M.; Zhagfarov, F.G. Natural Bitumens: Physicochemical Properties and Production Technologies. Solid Fuel Chem. 2018, 52, 344–355. [Google Scholar] [CrossRef]
- Shah, A.; Fishwick, R.; Wood, J.; Leeke, G.; Rigby, S.; Greaves, M. A Review of Novel Techniques for Heavy Oil and Bitumen Extraction and Upgrading. Energy Environ. Sci. 2010, 3, 700. [Google Scholar] [CrossRef]
- Porto, M.; Caputo, P.; Loise, V.; Eskandarsefat, S.; Teltayev, B.; Oliviero Rossi, C. Bitumen and Bitumen Modification: A Review on Latest Advances. Appl. Sci. 2019, 9, 742. [Google Scholar] [CrossRef]
- Lesueur, D. The Colloidal Structure of Bitumen: Consequences on the Rheology and on the Mechanisms of Bitumen Modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef]
- Jacob, H. Classification, Structure, Genesis and Practical Importance of Natural Solid Oil Bitumen (“Migrabitumen”). Int. J. Coal Geol. 1989, 11, 65–79. [Google Scholar] [CrossRef]
- Lesueur, D.; Gerard, J.; Claudy, P.; Letoffe, J.; Planche, J.; Martin, D. A Structure-related Model to Describe Asphalt Linear Viscoelasticity. J. Rheol. 1996, 40, 813–836. [Google Scholar] [CrossRef]
- Porto, M.; Angelico, R.; Caputo, P.; Abe, A.A.; Teltayev, B.; Rossi, C.O. The Structure of Bitumen: Conceptual Models and Experimental Evidences. Materials 2022, 15, 905. [Google Scholar] [CrossRef]
- Speight, J.G. Asphalt Materials Science and Technology; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-800273-5. [Google Scholar] [CrossRef]
- Du, Y.; Chen, J.; Han, Z.; Liu, W. A Review on Solutions for Improving Rutting Resistance of Asphalt Pavement and Test Methods. Constr. Build. Mater. 2018, 168, 893–905. [Google Scholar] [CrossRef]
- Nicholls, C.; Hannah, A. Asphalt Mixture Selection, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2019; ISBN 978-0-429-46183-46185. [Google Scholar] [CrossRef]
- Zaumanis, M.; Mallick, R.B.; Frank, R. 100% Recycled Hot Mix Asphalt: A Review and Analysis. Resour. Conserv. Recycl. 2014, 92, 230–245. [Google Scholar] [CrossRef]
- Tauste, R.; Moreno-Navarro, F.; Sol-Sánchez, M.; Rubio-Gámez, M.C. Understanding the Bitumen Ageing Phenomenon: A Review. Constr. Build. Mater. 2018, 192, 593–609. [Google Scholar] [CrossRef]
- Ganter, D.; Mielke, T.; Maier, M.; Lupascu, D.C. Bitumen Rheology and the Impact of Rejuvenators. Constr. Build. Mater. 2019, 222, 414–423. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Ilyin, S.O. Bitumen Improvement with Bio-Oil and Natural or Organomodified Montmorillonite: Structure, Rheology, and Adhesion of Composite Asphalt Binders. Constr. Build. Mater. 2023, 364, 129919. [Google Scholar] [CrossRef]
- Gasthauer, E.; Mazé, M.; Marchand, J.P.; Amouroux, J. Characterization of Asphalt Fume Composition by GC/MS and Effect of Temperature. Fuel 2008, 87, 1428–1434. [Google Scholar] [CrossRef]
- Ukwuoma, O.; Ademodi, B. The Effects of Temperature and Shear Rate on the Apparent Viscosity of Nigerian Oil Sand Bitumen. Fuel Process. Technol. 1999, 60, 95–101. [Google Scholar] [CrossRef]
- Michalica, P.; Kazatchkov, I.B.; Stastna, J.; Zanzotto, L. Relationship between Chemical and Rheological Properties of Two Asphalts of Different Origins. Fuel 2008, 87, 3247–3253. [Google Scholar] [CrossRef]
- Lemarchand, C.A.; Bailey, N.P.; Todd, B.D.; Daivis, P.J.; Hansen, J.S. Non-Newtonian Behavior and Molecular Structure of Cooee Bitumen under Shear Flow: A Non-Equilibrium Molecular Dynamics Study. J. Chem. Phys. 2015, 142, 244501. [Google Scholar] [CrossRef]
- Zelelew, H.M.; Papagiannakis, A.T. Interpreting Asphalt Concrete Creep Behaviour through Non-Newtonian Mastic Rheology. Road Mater. Pavement Des. 2012, 13, 266–278. [Google Scholar] [CrossRef]
- Vinogradov, G.V.; Isayev, A.I.; Zolotarev, V.A.; Verebskaya, E.A. Rheological Properties of Road Bitumens. Rheol. Acta 1977, 16, 266–281. [Google Scholar] [CrossRef]
- Mouazen, M.; Poulesquen, A.; Vergnes, B. Correlation between Thermal and Rheological Studies to Characterize the Behavior of Bitumen. Rheol. Acta 2011, 50, 169–178. [Google Scholar] [CrossRef]
- Strelets, L.A.; Ilyin, S.O. Effect of Enhanced Oil Recovery on the Composition and Rheological Properties of Heavy Crude Oil. J. Pet. Sci. Eng. 2021, 203, 108641. [Google Scholar] [CrossRef]
- Mouillet, V.; Lamontagne, J.; Durrieu, F.; Planche, J.-P.; Lapalu, L. Infrared Microscopy Investigation of Oxidation and Phase Evolution in Bitumen Modified with Polymers. Fuel 2008, 87, 1270–1280. [Google Scholar] [CrossRef]
- Ma, L.; Varveri, A.; Jing, R.; Erkens, S. Comprehensive Review on the Transport and Reaction of Oxygen and Moisture towards Coupled Oxidative Ageing and Moisture Damage of Bitumen. Constr. Build. Mater. 2021, 283, 122632. [Google Scholar] [CrossRef]
- Soenen, H.; Lu, X.; Laukkanen, O.-V. Oxidation of Bitumen: Molecular Characterization and Influence on Rheological Properties. Rheol. Acta 2016, 55, 315–326. [Google Scholar] [CrossRef]
- Lin, M.-S.; Chaffin, J.M.; Liu, M.; Glover, C.J.; Davison, R.R.; Bullin, J.A. The effect of asphalt composition on the formation of asphaltenes and their contribution to asphalt viscosity. Fuel Sci. Technol. Int. 1996, 14, 139–162. [Google Scholar] [CrossRef]
- Isacsson, U.; Zeng, H. Relationships between Bitumen Chemistry and Low Temperature Behaviour of Asphalt. Constr. Build. Mater. 1997, 11, 83–91. [Google Scholar] [CrossRef]
- Firoozifar, S.H.; Foroutan, S.; Foroutan, S. The Effect of Asphaltene on Thermal Properties of Bitumen. Chem. Eng. Res. Des. 2011, 89, 2044–2048. [Google Scholar] [CrossRef]
- Redelius, P. Asphaltenes in Bitumen, What They Are and What They Are Not. Road Mater. Pavement Des. 2009, 10, 25–43. [Google Scholar] [CrossRef]
- Djimasbe, R.; Galiullin, E.A.; Varfolomeev, M.A.; Fakhrutdinov, R.Z.; Al-Muntaser, A.A.; Farhadian, A. Experimental Study of Non-Oxidized and Oxidized Bitumen Obtained from Heavy Oil. Sci. Rep. 2021, 11, 8107. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Redelius, P. Effect of Bitumen Wax on Asphalt Mixture Performance. Constr. Build. Mater. 2007, 21, 1961–1970. [Google Scholar] [CrossRef]
- Edwards, Y.; Redelius, P. Rheological Effects of Waxes in Bitumen. Energy Fuels 2003, 17, 511–520. [Google Scholar] [CrossRef]
- Bissada, K.K.; Tan, J.; Szymczyk, E.; Darnell, M.; Mei, M. Group-Type Characterization of Crude Oil and Bitumen. Part I: Enhanced Separation and Quantification of Saturates, Aromatics, Resins and Asphaltenes (SARA). Org. Geochem. 2016, 95, 21–28. [Google Scholar] [CrossRef]
- Aske, N.; Kallevik, H.; Sjöblom, J. Determination of Saturate, Aromatic, Resin, and Asphaltenic (SARA) Components in Crude Oils by Means of Infrared and Near-Infrared Spectroscopy. Energy Fuels 2001, 15, 1304–1312. [Google Scholar] [CrossRef]
- Khanmohammadi, M.; Garmarudi, A.B.; Garmarudi, A.B.; de la Guardia, M. Characterization of Petroleum-Based Products by Infrared Spectroscopy and Chemometrics. TrAC Trends Anal. Chem. 2012, 35, 135–149. [Google Scholar] [CrossRef]
- Wieser, M.; Traxl, R.; Unterberger, S.H.; Lackner, R. Assessment of Aging State of Bitumen Based on Peak-Area Evaluation in Infrared Spectroscopy: Influence of Data Processing and Modeling. Constr. Build. Mater. 2022, 326, 126798. [Google Scholar] [CrossRef]
- Curiale, J.A. Origin of Solid Bitumens, with Emphasis on Biological Marker Results. Org. Geochem. 1986, 10, 559–580. [Google Scholar] [CrossRef]
- Muratov, V.N.; Sokoloff, V.P. An experiment in designing genetic classification of organic minerals. Int. Geol. Rev. 1963, 5, 157–167. [Google Scholar] [CrossRef]
- Mossman, D.J.; Nagy, B. Solid Bitumens: An Assessment of Their Characteristics, Genesis, and Role in Geological Processes. Terra Nova 1996, 8, 114–128. [Google Scholar] [CrossRef]
- Klubov, B.A. A new scheme for the formation and classification of bitumens. J. Pet. Geol. 1993, 16, 335–344. [Google Scholar] [CrossRef]
- Khalimov, E.M.; Klimushin, I.M.; Ferdman, L.I.; Gol’dberg, I.S. Geological factors in the formation of deposits of natural bitumens. Int. Geol. Rev. 1985, 27, 187–193. [Google Scholar] [CrossRef]
- Radchenko, O.A.; Uspenskiy, V.A. Genetic types of bitumens and condition of their genesis. Geol. Balc. 1979, 9, 53–70. [Google Scholar]
- Meyerhoff, A.A.; Meyer, R.F. Geology of Heavy Crude Oil and Natural Bitumen in the USSR, Mongolia, and China. In Exploration for Heavy Crude Oil and Natural Bitumen; American Association of Petroleum Geologists: Tulsa, OK, USA, 1987; ISBN 978-0-89181-031-5. [Google Scholar]
- Mastalerz, M.; Drobniak, A.; Stankiewicz, A.B. Origin, Properties, and Implications of Solid Bitumen in Source-Rock Reservoirs: A Review. Int. J. Coal Geol. 2018, 195, 14–36. [Google Scholar] [CrossRef]
- Sanei, H. Genesis of Solid Bitumen. Sci. Rep. 2020, 10, 15595. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Nasyrova, Z.R.; Mikhailova, A.N.; Kosachev, I.P.; Aliev, F.A.; Vakhin, A.V. Composition of Oil after Hydrothermal Treatment of Cabonate-Siliceous and Carbonate Domanic Shale Rocks. Processes 2021, 9, 1798. [Google Scholar] [CrossRef]
- Pikovskiy, Y.I.; Ismailov, N.; Dorokhova, M. Oil and Gas Environmental Ecology, 1st ed.; Academus Publishing: Campbell, CA, USA, 2019; ISBN 978-1-4946-0014-3. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Pakhmanova, O.A.; Kostyuk, A.V.; Antonov, S.V. Effect of the Asphaltene, Resin, and Wax Contents on the Physicochemical Properties and Quality Parameters of Crude Oils. Pet. Chem. 2017, 57, 1141–1143. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Rodionova, G.; Simon, S.; Ilyin, S.O.; Arinina, M.P.; Kulichikhin, V.G.; Sjöblom, J. Some Compositional Viscosity Correlations for Crude Oils from Russia and Norway. Energy Fuels 2016, 30, 9322–9328. [Google Scholar] [CrossRef]
- Bellamy, L.J. The Infrared Spectra of Complex Molecules; Springer: Dordrecht, The Netherlands, 1980; ISBN 978-94-011-6522-8. [Google Scholar] [CrossRef]
- Kostyuk, A.V.; Smirnova, N.M.; Ilyin, S.O. Two-Functional Phase-Change Pressure-Sensitive Adhesives Based on Polyisobutylene Matrix Filled with Paraffin Wax. J. Energy Storage 2022, 52, 104797. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Makarova, V.V.; Ilyin, S.O. Hydrophobic Nanosilica-Stabilized Graphite Particles for Improving Thermal Conductivity of Paraffin Wax-Based Phase-Change Materials. J. Energy Storage 2021, 36, 102417. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Gorbacheva, S.N.; Yadykova, A.Y. Rheology and Tribology of Nanocellulose-Based Biodegradable Greases: Wear and Friction Protection Mechanisms of Cellulose Microfibrils. Tribol. Int. 2023, 178, 108080. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Ilyin, S.O. Compatibility and Rheology of Bio-Oil Blends with Light and Heavy Crude Oils. Fuel 2022, 314, 122761. [Google Scholar] [CrossRef]
- Vinogradov, G.V. Ultimate Regimes of Deformation of Linear Flexible Chain Fluid Polymers. Polymer 1977, 18, 1275–1285. [Google Scholar] [CrossRef]
- Borisenkova, E.K.; Dreval, V.E.; Vinogradov, G.V.; Kurbanaliev, M.K.; Moiseyev, V.V.; Shalganova, V.G. Transition of Polymers from the Fluid to the Forced High-Elastic and Leathery States at Temperatures above the Glass Transition Temperature. Polymer 1982, 23, 91–99. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Ilyin, S.O. Structure, Rheology and Possible Application of Water-in-Oil Emulsions Stabilized by Asphaltenes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126442. [Google Scholar] [CrossRef]
- Lee, S.-J.; Amirkhanian, S.N.; Shatanawi, K.; Kim, K.W. Short-Term Aging Characterization of Asphalt Binders Using Gel Permeation Chromatography and Selected Superpave Binder Tests. Constr. Build. Mater. 2008, 22, 2220–2227. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Arinina, M.P.; Mamulat, Y.S.; Malkin, A.Y.; Kulichikhin, V.G. Rheological Properties of Road Bitumens Modified with Polymer and Solid Nanosized Additives. Colloid J. 2014, 76, 425–434. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Powers, D.P.; Okafor, J.C.; van den Berg, F.G.A. Regular Solution Based Approach to Modeling Asphaltene Precipitation from Native and Reacted Oils: Part 2, Molecular Weight, Density, and Solubility Parameter of Saturates, Aromatics, and Resins. Fuel 2018, 215, 766–777. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Ignatenko, V.Y.; Kostyuk, A.V.; Levin, I.S.; Bondarenko, G.N. Deasphalting of Heavy Crude Oil by Hexamethyldisiloxane: The Effect of a Solvent/Oil Ratio on the Structure, Composition, and Properties of Precipitated Asphaltenes. J. Pet. Sci. Eng. 2022, 208, 109329. [Google Scholar] [CrossRef]
- Ilyin, S.; Kulichikhin, V.; Malkin, A. Characterization of Material Viscoelasticity at Large Deformations. Appl. Rheol. 2014, 24, 13653. [Google Scholar] [CrossRef]
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A Review of Nonlinear Oscillatory Shear Tests: Analysis and Application of Large Amplitude Oscillatory Shear (LAOS). Prog. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; McFarlane, R.; Goual, L.; et al. Advances in Asphaltene Science and the Yen–Mullins Model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Schramm, G. A Practical Approach to Rheology and Rheometry; HAAKE: Karlsruhe, Germany, 2004. [Google Scholar]
- Ilyin, S.O.; Arinina, M.P.; Malkin, A.Y.; Kulichikhin, V.G. Sol–Gel Transition and Rheological Properties of Silica Nanoparticle Dispersions. Colloid J. 2016, 78, 608–615. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Yadykova, A.Y.; Ilyin, S.O. Rheological and Tribological Properties of Low-Temperature Greases Based on Cellulose Acetate Butyrate Gel. Carbohydr. Polym. 2021, 272, 118509. [Google Scholar] [CrossRef] [PubMed]
- Perezlepe, A. Influence of the Processing Conditions on the Rheological Behaviour of Polymer-Modified Bitumen. Fuel 2003, 82, 1339–1348. [Google Scholar] [CrossRef]
- Laukkanen, O.-V.; Winter, H.H.; Soenen, H.; Seppälä, J. An Empirical Constitutive Model for Complex Glass-Forming Liquids Using Bitumen as a Model Material. Rheol. Acta 2018, 57, 57–70. [Google Scholar] [CrossRef]
- Visintin, R.F.G.; Lapasin, R.; Vignati, E.; D’Antona, P.; Lockhart, T.P. Rheological Behavior and Structural Interpretation of Waxy Crude Oil Gels. Langmuir 2005, 21, 6240–6249. [Google Scholar] [CrossRef]
- Mendes, R.; Vinay, G.; Ovarlez, G.; Coussot, P. Modeling the Rheological Behavior of Waxy Crude Oils as a Function of Flow and Temperature History. J. Rheol. 2015, 59, 703–732. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Arinina, M.P.; Polyakova, M.Y.; Kulichikhin, V.G.; Malkin, A.Y. Rheological Comparison of Light and Heavy Crude Oils. Fuel 2016, 186, 157–167. [Google Scholar] [CrossRef]
- Legnani, A.; Santos, T.G.M.; Andrade, D.E.V.; Negrão, C.O.R. Waxy Oils: Deformation-Dependent Materials. J. Non-Newton. Fluid Mech. 2020, 285, 104378. [Google Scholar] [CrossRef]
- Abivin, P.; Taylor, S.D.; Freed, D. Thermal Behavior and Viscoelasticity of Heavy Oils. Energy Fuels 2012, 26, 3448–3461. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Strelets, L.A. Basic Fundamentals of Petroleum Rheology and Their Application for the Investigation of Crude Oils of Different Natures. Energy Fuels 2018, 32, 268–278. [Google Scholar] [CrossRef]
- Souas, F.; Safri, A.; Benmounah, A. A Review on the Rheology of Heavy Crude Oil for Pipeline Transportation. Pet. Res. 2021, 6, 116–136. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Ilyin, S.O. Rheological and Adhesive Properties of Nanocomposite Bitumen Binders Based on Hydrophilic or Hydrophobic Silica and Modified with Bio-Oil. Constr. Build. Mater. 2022, 342, 127946. [Google Scholar] [CrossRef]
- Williams, M.L.; Landel, R.F.; Ferry, J.D. The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-Forming Liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Nifant’Ev, I.; Bagrov, V.; Vinogradov, A.; Vinogradov, A.; Ilyin, S.; Sevostyanova, N.; Batashev, S.; Ivchenko, P. Methylenealkane-Based Low-Viscosity Ester Oils: Synthesis and Outlook. Lubricants 2020, 8, 50. [Google Scholar] [CrossRef]
- Yadykova, A.Y.; Ilyin, S.O. Rheological, Thermophysical, and Morphological Features of Original and Hydrogenated Bio-Oils. Sustain. Energy Fuels 2021, 5, 4425–4433. [Google Scholar] [CrossRef]
- Kerimov, V.Y.; Gordadze, G.N.; Lapidus, A.L.; Giruts, M.V.; Mustaev, R.N.; Movsumzade, E.M.; Zhagfarov, F.G.; Zakharchenko, M.V. Physicochemical Properties and Genesis of the Asphaltites of Orenburg Oblast. Solid Fuel Chem. 2018, 52, 128–137. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Sitnov, S.A.; Mukhamatdinov, I.I.; Aliev, F.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Varfolomeev, M.A.; Nurgaliev, D.K. Aquathermolysis of Heavy Oil in Reservoir Conditions with the Use of Oil-Soluble Catalysts: Part III—Changes in Composition Resins and Asphaltenes. Pet. Sci. Technol. 2018, 36, 1857–1863. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Nasyrova, Z.R.; Gareev, B.I.; Vakhin, A.V. Catalytic Hydrothermal Conversion of Heavy Oil in the Porous Media. Energy Fuels 2021, 35, 1297–1307. [Google Scholar] [CrossRef]
- Basko, A.V.; Pochivalov, K.V.; Chalykh, T.I.; Shandryuk, G.A.; Ezhov, A.A.; Artemov, V.V.; Kudryavtsev, Y.V. Combining Optical Microscopy, Turbidimetry, and DSC to Study Structural Transformations in the Mixtures of Semicrystalline Polymers with Low-Molar-Mass Crystallizable Substances. Thermochim. Acta 2020, 690, 178671. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Malkin, A.Y.; Kulichikhin, V.G. Application of Large Amplitude Oscillatory Shear for the Analysis of Polymer Material Properties in the Nonlinear Mechanical Behavior. Polym. Sci. Ser. A 2014, 56, 98–110. [Google Scholar] [CrossRef]





| # | Oilfield | ρ*, g/mL | C, wt% | H, wt% | S, wt% | N, wt% | H/C, at/at | wr, wt% | wasp, wt% | wwax, wt% | Genetic Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Khaudag, Uzbekistan | 0.98 | 82.09 | 11.24 | 3.8 | 0.28 | 1.64 | 50.4 | 9.4 | 3.9 | Maltha |
| 2 | Bayan-Erkhet, Mongolia | 1.03 | 79.10 | 10.73 | 0.47 | 0.75 | 1.63 | 60.2 | 6.1 | 1.7 | Asphalt |
| 3 | Ivanovka, Russia | 1.12 | 76.15 | 8.60 | 6.07 | 1.05 | 1.36 | 12.7 | 69.2 | 0.4 | Asphaltite (Gilsonite) |
| 4 | Fulaerji, China | 0.92 | 86.38 | 13.32 | <0.1 | 0.17 | 1.85 | 34.1 | 0.2 | 22.4 | Ozokerite |
| 5 | Züünbayan, Mongolia | 0.83 | 80.77 | 12.00 | <0.1 | 0.24 | 1.78 | 8.5 | 1.0 | 26.4 | Waxy oil |
| 6 | Fulaerji, China | 0.93 | 85.57 | 12.39 | <0.1 | 0.20 | 1.74 | 17.9 | 33.0 | 16.3 | Maltha |
| 7 | Toson-Ul, Mongolia | 0.84 | 85.34 | 13.24 | <0.1 | 0.14 | 1.86 | 5.7 | 0.3 | 24.9 | Waxy oil |
| 8 | Züünbayan, Mongolia | 0.90 | 83.77 | 13.09 | <0.1 | 0.23 | 1.88 | 25.5 | 0.4 | 15.7 | Waxy oil |
| 9 | Züünbayan, Mongolia | 0.89 | 85.26 | 13.34 | <0.1 | 0.24 | 1.88 | 14.7 | 0.2 | 24.0 | Waxy oil |
| 10 | Ashalcha, Russia | 0.96 | 82.64 | 11.21 | 3.9 | 0.29 | 1.63 | 23.8 | 7.5 | 0.2 | Heavy oil |
| # | Aa * | Aw | Ab | As | Ap | Character of Material | ||
|---|---|---|---|---|---|---|---|---|
| Ap/Aw | Ab/Aa | Summary | ||||||
| 1 | 1.17 | 3.66 | 0.55 | 0.72 | 0.34 | low-polar semi-aromatic | low-branched semi-aromatic | aromatic |
| 2 | 1.71 | 2.46 | 0.62 | 0.44 | 0.75 | polar aromatic | branched aromatic | resinous |
| 3 | 2.34 | 2.11 | 0.69 | 0.54 | 0.74 | polar aromatic | branched aromatic | resinous |
| 4 | 0.16 | 9.25 | 0.23 | 0.47 | 0.05 | non-polar aliphatic | linear waxy | paraffinic |
| 5 | 0.62 | 4.38 | 0.46 | 0.50 | 0.22 | low-polar semi-aromatic | low-branched semi-aromatic | paraffinic |
| 6 | 1.02 | 3.26 | 0.47 | 0.39 | 0.20 | low-polar semi-aromatic | low-branched semi-aromatic | aromatic |
| 7 | 0.45 | 5.63 | 0.42 | 0.63 | 0.10 | low-polar semi-aromatic | low-branched semi-aromatic | paraffinic |
| 8 | 0.18 | 10.6 | 0.31 | 0.72 | 0.04 | non-polar aliphatic | linear waxy | paraffinic |
| 9 | 0.18 | 10.1 | 0.31 | 0.68 | 0.04 | non-polar aliphatic | linear waxy | paraffinic |
| 10 | 1.22 | 4.82 | 0.65 | 1.10 | 0.16 | low-polar semi-aromatic | low-branched semi-aromatic | aromatic |
| # | TG′ = G″, °C | EA, kJ/mol | logη/ηg | Tg, °C | Tg, min, °C | Tg, max, °C |
|---|---|---|---|---|---|---|
| 1 | −3.4 | 61.7 | 12.39 | −54.1 | −65.5 | −40.5 |
| 2 | 26.0 | 132.6 | 13.3 | 4.6 | −30.0 | −12.6 |
| 3 | 179.0 | 175.4 | 16.29 | 48.4 | −47.0 | −6.0 |
| 4 | 20.2 | 30.2 | 12.56 | −144.4 | −56.5 | −56.5 |
| 5 | 16.5 | 50.8 | 12.50 | −74.6 | −63.7 | −63.7 |
| 6 | −7.2 | 50.8 | 12.19 | −76.4 | −64.4 | −23.1 |
| 7 | −8.5 | 9.87 | 12.78 | −185.4 | −78.9 | −78.9 |
| 8 | 16.1 | 29.0 | 12.23 | −136.9 | −69.7 | −69.7 |
| 9 | −1.5 | 17.6 | 12.93 | −156.8 | −69.7 | −69.7 |
| 10 | −63 | 45.5 | 11.56 | −68.9 | −74.8 | −74.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadykova, A.Y.; Strelets, L.A.; Ilyin, S.O. Infrared Spectral Classification of Natural Bitumens for Their Rheological and Thermophysical Characterization. Molecules 2023, 28, 2065. https://doi.org/10.3390/molecules28052065
Yadykova AY, Strelets LA, Ilyin SO. Infrared Spectral Classification of Natural Bitumens for Their Rheological and Thermophysical Characterization. Molecules. 2023; 28(5):2065. https://doi.org/10.3390/molecules28052065
Chicago/Turabian StyleYadykova, Anastasiya Y., Larisa A. Strelets, and Sergey O. Ilyin. 2023. "Infrared Spectral Classification of Natural Bitumens for Their Rheological and Thermophysical Characterization" Molecules 28, no. 5: 2065. https://doi.org/10.3390/molecules28052065
APA StyleYadykova, A. Y., Strelets, L. A., & Ilyin, S. O. (2023). Infrared Spectral Classification of Natural Bitumens for Their Rheological and Thermophysical Characterization. Molecules, 28(5), 2065. https://doi.org/10.3390/molecules28052065