Evaluation of the Antioxidant Properties of Carvacrol as a Prospective Replacement for Crude Essential Oils and Synthetic Antioxidants in Food Storage
Abstract
1. Introduction
2. Results
2.1. Molecular Interaction between Carvacrol, BHT, and Lipoxygenase (LOX)
2.1.1. Multiple Sequence Alignment (MSA)
2.1.2. Predicted Stable Amino Acids in Non-Conserved Regions
2.1.3. Homology Modelling of LOX
2.1.4. Molecular Docking
2.1.5. Molecular Dynamics Simulation
2.2. In Vitro Antioxidant Capacity of Carvacrol Compared to Seed Essential Oil
2.2.1. Ferrous Metal Chelating Activity of Carvacrol Compared to Seed Essential Oil
2.2.2. Nitric Oxide Scavenging Activity of Carvacrol Compared to Seed Essential Oil
2.2.3. Ferric Reducing Power of Carvacrol Compared to Seed Essential Oil
2.3. Retention of Carvacrol and BHT after Thermal Treatment
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Data Retrieval
4.3. Multiple Sequence Alignment and Prediction of Stable Amino Acids in Non-Conserved Regions
4.4. Homology Modelling
4.5. Docking of Carvacrol and BHT on LOX
4.6. Molecular Dynamics Simulation
4.7. In Vitro Antioxidant Capacity
4.7.1. Ferric Reducing Power Assay
4.7.2. Ferrous Ion Scavenging (Metal Chelating) Activity
4.7.3. Nitric Oxide Scavenging Assay
4.8. Retention of BHT and Carvacrol after Thermal Treatment
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Akula, U.S.; Odhav, B. In vitro 5-Lipoxygenase inhibition of polyphenolic antioxidants from undomesticated plants of South Africa. J. Med. Plants Res. 2008, 2, 207–212. [Google Scholar]
- Atta, E.M.; Mohamed, N.H.; Abdelgawad, A.A.M. Antioxidants: An overview on the natural and synthetic types. Eur. Chem. Bull. 2017, 6, 365–375. [Google Scholar] [CrossRef]
- Edemhanria, L.; Ebhomienlen, J.O.; Ebhohimen, I.E. Antioxidant effect of Aframomum angustifolium seed essential oil in freeze storage of lean meat. SAU Sci.-Tech J. 2020, 5, 97–103. [Google Scholar]
- Lanigan, R.S.; Yamarik, T.A. Final report on the safety assessment of BHT. Int. J. Toxicol. 2002, 21, 19–94. [Google Scholar] [CrossRef]
- Williams, G.M.; Wang, C.X.; Iatropoulos, M.J. Toxicity studies of butylated hydroxyanisole and butylated hydroxytoluene. II. Chronic feeding studies. Food Chem. Toxicol. 1990, 28, 799–806. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 14–16. [Google Scholar] [CrossRef]
- Silveira Alexandre, A.C.; Corrêa Albergaria, F.; dos Santos Ferraz e Silva, L.M.; Carneiro-Fernandes, L.A.; de Sousa Gomes, M.E.; Pimenta, C.J. Effect of natural and synthetic antioxidants on oxidation and storage stability of mechanically separated tilapia meat. LWT-Food Sci. Technol. 2022, 154, 112679. [Google Scholar] [CrossRef]
- Wang, A.; Abrahamsson, D.P.; Jiang, T.; Wang, M.; Morello-Frosch, R.; Park, J.S.; Sirota, M.; Woodruff, T.J. Suspect screening, prioritization, and confirmation of environmental chemicals in maternal-newborn pairs from San Francisco. Environ. Sci. Technol. 2021, 55, 5037–5049. [Google Scholar] [CrossRef]
- Fernández-López, J.; Viuda-martos, M. Application of essential oils in food systems. Foods 2018, 7, 56. [Google Scholar] [CrossRef]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; Andrade, E.H.; Santana de Oliveira, M. Recent trends in the application of essential oils: The next generation of food preservation and food packaging. Trends Food Sci. Technol. 2022, 129, 421–439. [Google Scholar] [CrossRef]
- Asbahani, A.E.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; Mousadik, A.E.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. J. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Ebhohimen, I.E.; Okolie, N.P.; Edemhanria, L.; Onyijen, O.H. In silico prediction of the antioxidant potential and pharmacokinetic parameters of essential oil constituents from Monodora myristica seeds. Nat. Resour. Sustain. Dev. 2021, 11, 23–34. [Google Scholar] [CrossRef]
- Wang, L.; Tan, N.; Hu, J.; Wang, H.; Duan, D.; Ma, L.; Xiao, J.; Wang, X. Analysis of the main active ingredients and bioactivities of essential oil from Osmanthus fragrans Var. thunbergii using a complex network approach. BMC Syst. Biol. 2017, 11, 144. [Google Scholar] [CrossRef]
- Amaral, A.B.; da Sillva, M.V.; Lannes, C.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Phillips, K.M.; Council-Troche, M.A.; McGinty, R.C.; Rasor, A.S.; Tarrago-Trani, M. Stability of vitamin C in fruit and vegetable homogenates stored at different temperatures. J. Food Compos. Anal. 2016, 45, 147–162. [Google Scholar] [CrossRef]
- Hollingsworth, S.A.; Karplus, P.A. A fresh look at the Ramachandran plot and the occurrence of standard structures in proteins. Biomol. Concepts 2010, 1, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef]
- Chalchat, J.; Garry, R.; Lamaty, G.; Malhuret, R. Correlation between chemical composition and antimicrobial activity of some African essential oils. J. Essent. Oil Res. 2011, 9, 67–75. [Google Scholar] [CrossRef]
- Cimanga, K.; Kambu, K.; Tona, L.; Apers, S.; De Bruyne, T.; Hermans, N. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002, 79, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Koudou, J.; Etou-Osibi, A.W.; Aklikokou, K.; Abena, A.A.; Gbeassor, M.; Bessiere, J.M. Chemical composition and hypotensive effects of essential oil of Monodora myristica Gaertn. J. Biol. Sci. 2007, 7, 937–942. [Google Scholar] [CrossRef]
- Cock, I.E. Problems of reproducibility and efficacy of bioassays using crude extracts, with reference to Aloe vera. Pharmacogn. Commun. 2011, 1, 52–62. [Google Scholar] [CrossRef]
- Nelson, M.J.; Seitz, S.P. The structure and function of lipoxygenase. Curr. Opin. Struct. Biol. 1994, 4, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, R.G.; Brüne, B.; Snodgrass, R.G. Regulation and Functions of 15-Lipoxygenases in Human Macrophages. Front. Pharmacol. 2019, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Dominiguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of Oxidative Processes in Meat and Toxicity Induced by Postprandial Degradation Products: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 16, 96–123. [Google Scholar] [CrossRef] [PubMed]
- Daugelaite, J.; Driscoll, A.O.; Sleator, R.D. An Overview of Multiple Sequence Alignments and Cloud Computing in Bioinformatics. ISRN Biomath. 2013, 2013, 615630. [Google Scholar] [CrossRef]
- Bromberg, Y.; Rost, B. Correlating protein function and stability through the analysis of single amino acid substitutions. BMC Bioinform. 2009, 9, S8. [Google Scholar] [CrossRef]
- Ng, P.C.; Henikoff, S. Predicting the Effects of Amino Acid Substitutions on Protein Function. Annu. Rev. Genom. Hum. Genet. 2006, 7, 61–80. [Google Scholar] [CrossRef]
- Parthiban, V.; Gromiha, M.M.; Schomburg, D. CUPSAT: Prediction of protein stability upon point mutations. Nucleic Acids Res. 2006, 34, 239–242. [Google Scholar] [CrossRef]
- Fasnacht, M.; Zhu, J.; Honig, B. Local quality assessment in homology models using statistical potentials and support vector machines. Protein Sci. 2007, 16, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Di Luccio, E.; Koehl, P. A quality metric for homology modelling: The H-factor. BMC Bioinform. 2011, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Al-khayyat, M.Z.S.; Al-dabbagh, A.G.A. In silico Prediction and Docking of Tertiary Structure of LuxI, an Inducer Synthase of Vibrio fischeri. Rep. Biochem. Mol. Biol. 2016, 4, 66–75. [Google Scholar] [PubMed]
- Bell, E.W.; Zhang, Y. DockRMSD: An open-source tool for atom mapping and RMSD calculation of symmetric molecules through graph isomorphism. J. Cheminform. 2019, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, R.; Zengin, G.; Uysal, S.; Ilhan, V.; Kandemir, A.; Anwar, F.; Velen, H. GC-MS analysis and in vitro antioxidant and enzyme inhibitory activities of essential oil from aerial parts of endemic Thymus spathulifolius Hausskn. et Velen. J. Enzym. Inhib. Med. Chem. 2016, 31, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Akise, O.G.; Fasakin, E.A.; Adeparusi, E.O. Chemical composition and in-vitro antimicrobial activity of essential oil of African nutmeg (Monodora myristica (Gaertn) Dunal on microorganisms isolated from smoke-dried catfish (Clarias gariepinus). Afr. J. Microbiol. Res. 2020, 14, 136–147. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Ebhohimen, I.E.; Onyijen, O.H.; Arora, V.; Arora, V.; Adeleke, V.T.; Okpeku, M. Mutation Pattern in the Receptor Binding Motif of SARS-Cov-2 Variants and the Effect on Molecular Interactions in Docked Ligand Complexes. Trop. J. Nat. Prod. Res. 2022, 6, 1262–1267. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP ) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) asinhibitors of Membrane Lipid Peroxidation and Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N] Nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]

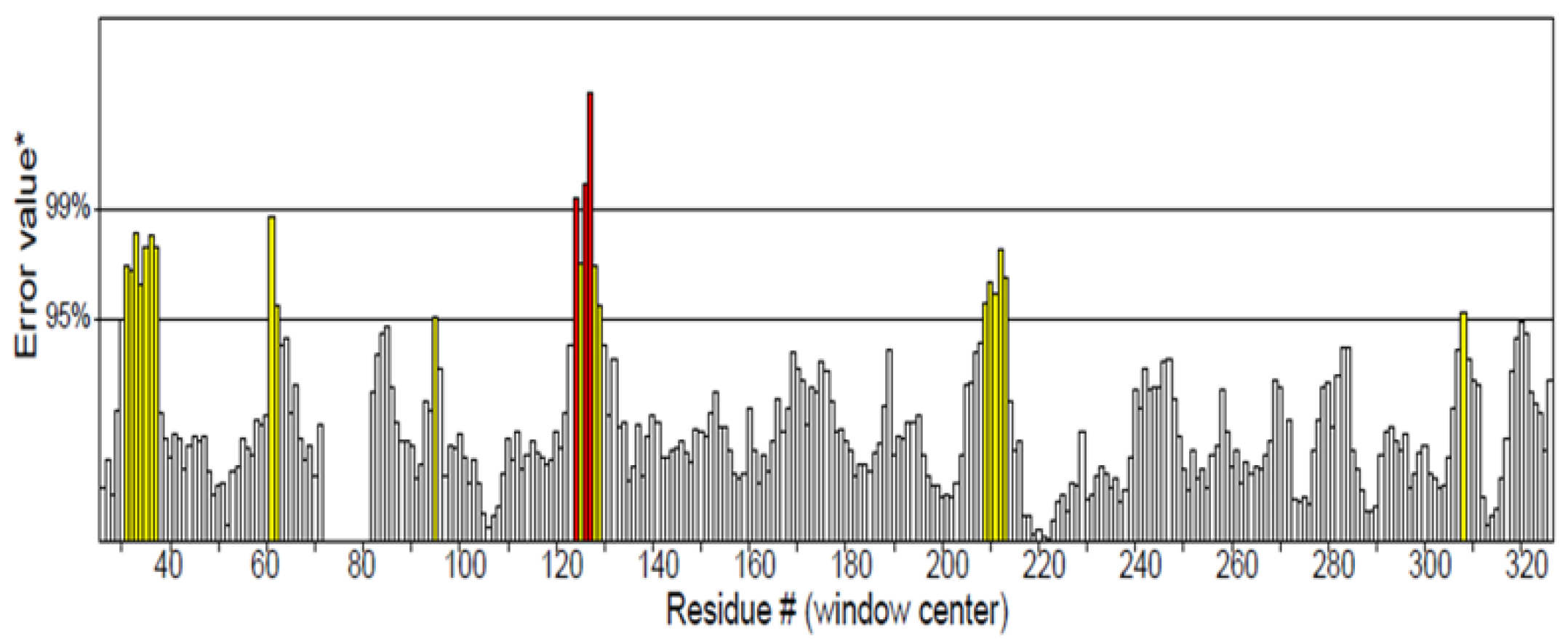
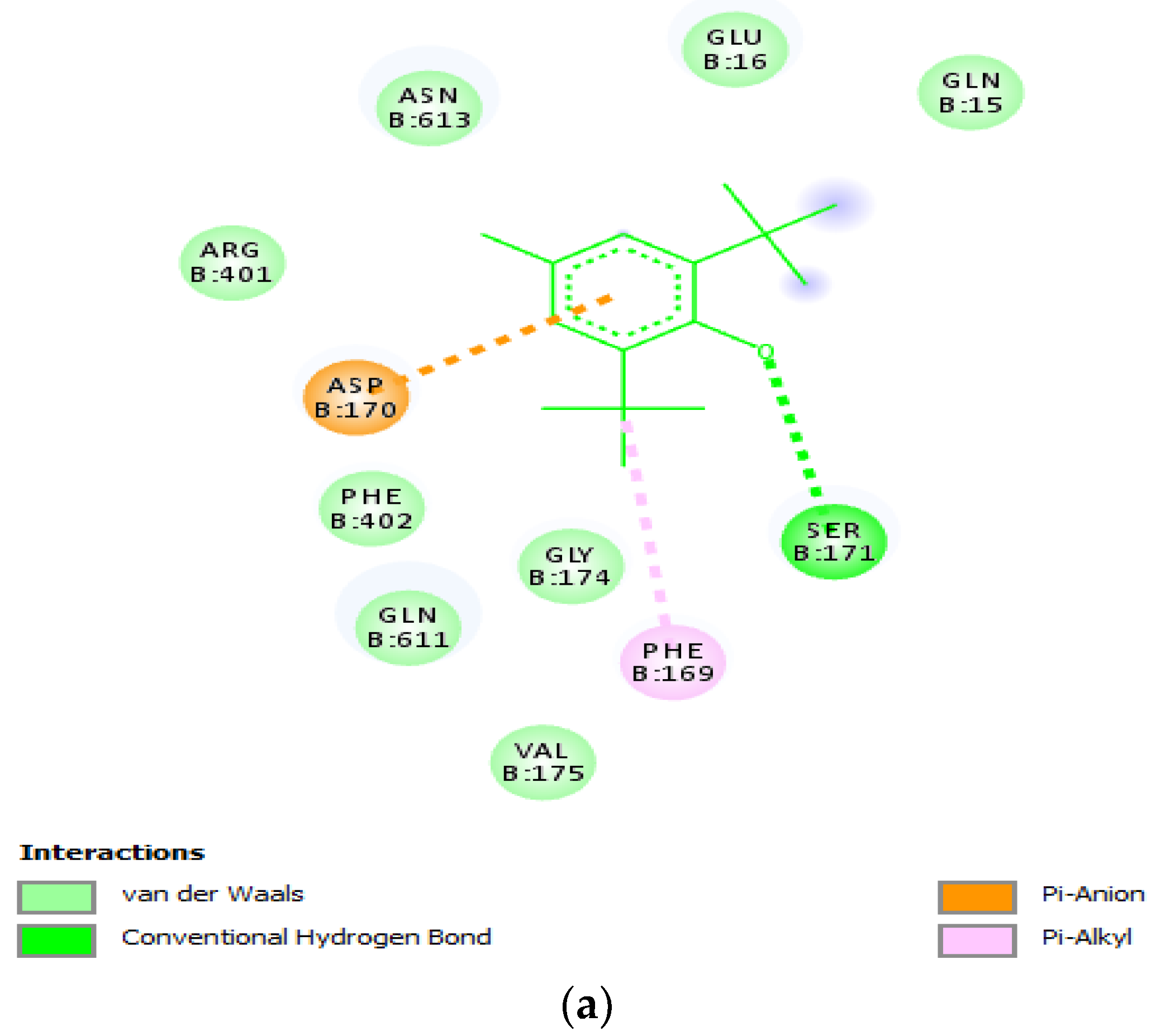


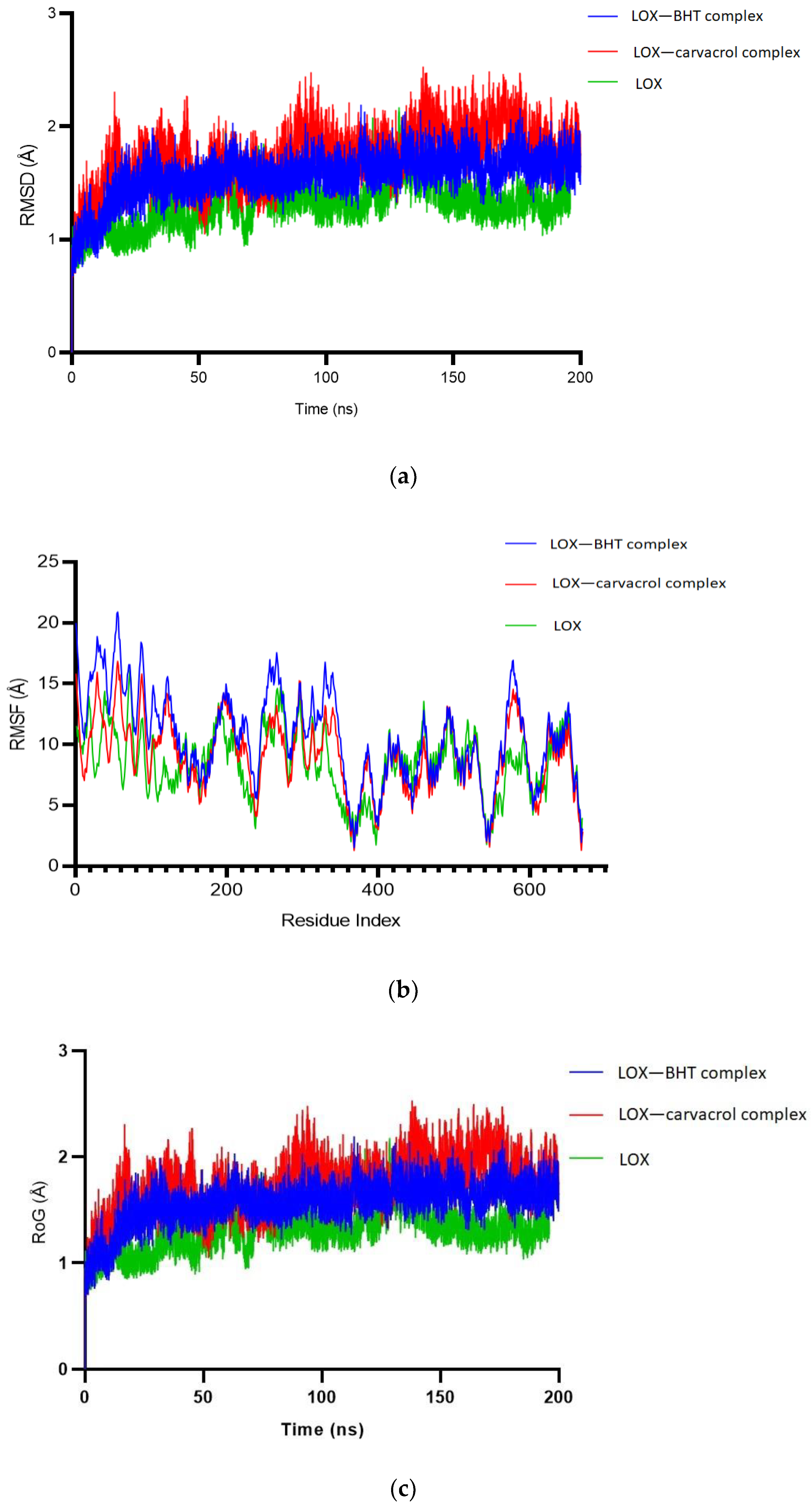
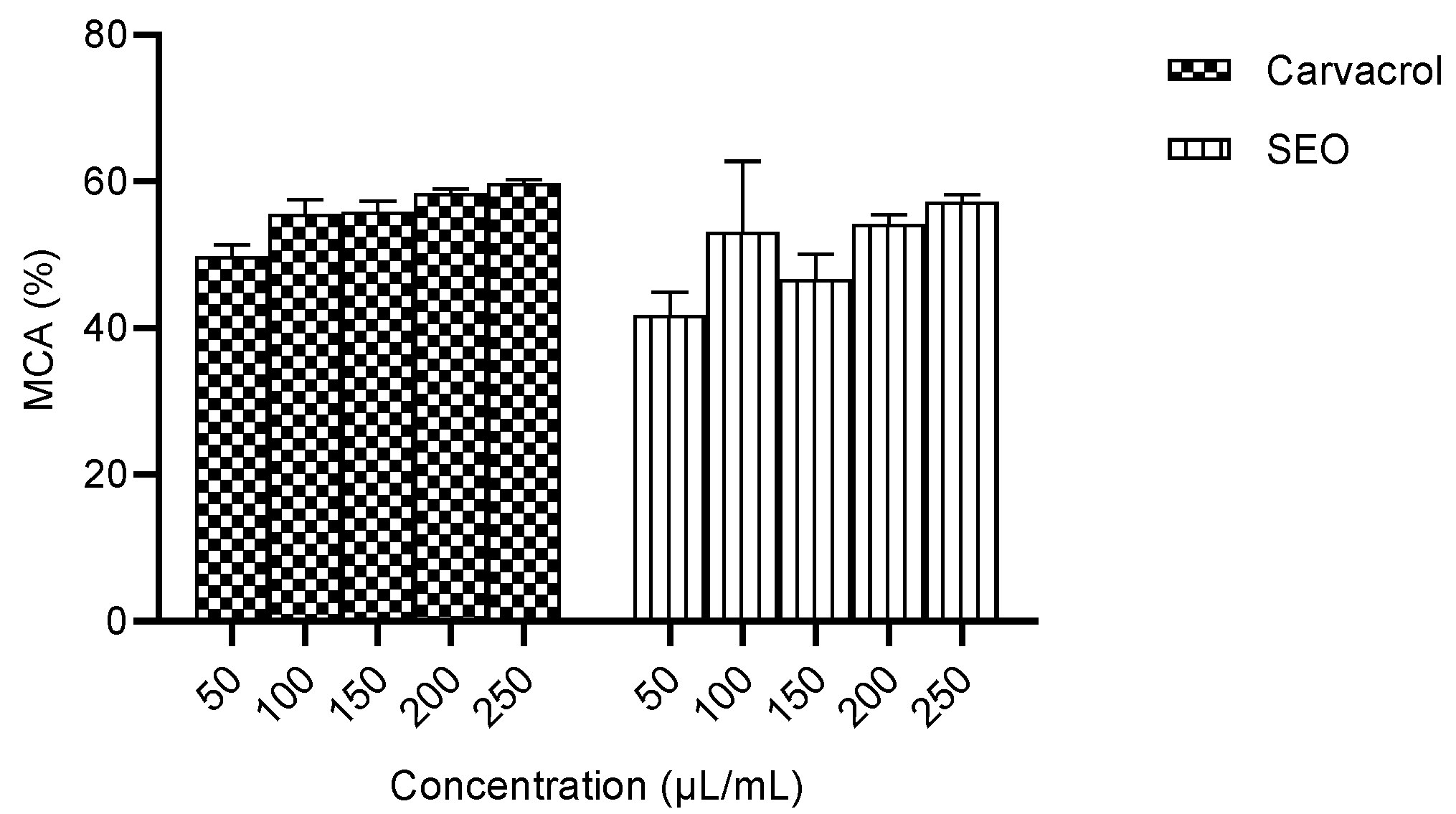


| S/N | Residue Present in 3V92 | Selected Stable and Favourable Mutation | Amino Acid Letter Code |
|---|---|---|---|
| 1 | ILE85 | CYS | C |
| 2 | THR86 | TYR | Y |
| 3 | ILE95 | LYS | K |
| 4 | GLY105 | ASP | D |
| 5 | ASN122 | TRP | W |
| 6 | ILE124 | TRP | W |
| 7 | LEU127 | 1 - | 1 - |
| 8 | LYS133 | TRP | W |
| 9 | TRP144 | PRO | P |
| 10 | MET231 | ILE | I |
| 11 | VAL262 | 1 - | 1 - |
| 12 | CYS264 | 1 - | 1 - |
| 13 | ARG268 | THR | T |
| 14 | SER271 | 1 - | 1 - |
| 15 | LEU272 | 1 - | 1 - |
| 16 | LEU289 | 1 - | 1 - |
| Mode | Affinity (kcal/mol) | |||
|---|---|---|---|---|
| BHT–3V92_B | Carvacrol–3V92_B | BHT–model | Carvacrol–model | |
| 1 | −6.2 | −6.9 | −5.6 | −6.7 |
| 2 | −6.2 | −6.8 | −5.6 | −6.7 |
| 3 | −6.2 | −6.0 | −5.4 | −5.8 |
| 4 | −6.2 | −5.9 | −5.4 | −5.8 |
| 5 | −6.1 | −5.7 | −5.4 | −5.6 |
| 6 | −6.1 | −5.6 | −5.4 | −5.5 |
| 7 | −6.0 | −5.6 | −5.4 | −5.5 |
| 8 | −6.0 | −5.6 | −5.3 | −5.5 |
| 9 | −5.9 | −5.6 | −5.3 | −5.4 |
| RMSD (Å) (Mean ± SEM) | RMSF (Å) (Mean ± SEM) | RoG (Å) (Mean ± SEM) | Binding Affinity (kcal/mol) | |
|---|---|---|---|---|
| LOX-BHT | 1.58 ± 3.12e−4 | 10.62 ± 0.14 | 44.30 ± 4.49e−5 | −19.71 |
| LOX-carvacrol | 1.77 ± 4.57e−4 | 9.08 ± 0.11 | 44.30 ± 4.95e−5 | −22.79 |
| LOX | 1.30 ± 4.33e−4 | 8.50 ± 0.11 | 44.29 ± 5.64e−5 |
| Peak # | Retention Time (Min) | %Composition by Area | Database\NIST11.L: Library/ID | Quality (%) |
|---|---|---|---|---|
| 1 | 2.199 | 5.06 | 1-Propyne | 25 |
| 2 | 2.312 | 4.24 | Cyclopropane | 49 |
| 3 | 2.475 | 0.35 | Pentane | 64 |
| 4 | 2.568 | 4.4 | n-Hexane | 43 |
| 5 | 2.625 | 10.25 | Heptane | 59 |
| 6 | 3.100 | 12.55 | Cyclohexane | 70 |
| 7 | 3.244 | 2.37 | 1-Heptene | 53 |
| 8 | 3.325 | 1.02 | Cyclopentane | 87 |
| 9 | 3.494 | 2.14 | Nonane | 64 |
| 10 | 3.582 | 1.72 | 3-methyl-Heptane | 72 |
| 11 | 3.682 | 4.19 | Cyclohexane | 91 |
| 12 | 3.801 | 2.08 | Cyclopentane | 94 |
| 13 | 3.901 | 1.08 | Cyclohexane | 87 |
| 14 | 3.982 | 1.52 | Cyclohexane | 95 |
| 15 | 4.307 | 0.32 | Cyclohexane | 87 |
| 16 | 10.425 | 0.17 | 2,4-Decadienal | 72 |
| 17 | 10.644 | 0.22 | 2,4-Decadienal | 81 |
| 18 | 10.888 | 37.32 | Carvacrol | 60 |
| 19 | 14.084 | 5.39 | Oleic acid | 84 |
| 20 | 14.579 | 2.85 | 13-octadecadienol | 90 |
| 21 | 14.747 | 0.86 | cis-9-Hexadecanoic acid | 64 |
| Peak # | Retention Time (Min) | %Composition by Area | Database\NIST11.L: Library/ID | Quality (%) |
|---|---|---|---|---|
| 1 | 2.262 | 1.27 | 1-Propyne | 17 |
| 2 | 2.343 | 3.19 | Piperazine | 45 |
| 3 | 2.675 | 11.35 | Hexane | 50 |
| 4 | 3.194 | 12.63 | Cyclohexane | 64 |
| 5 | 3.594 | 1.02 | Heptane | 59 |
| 6 | 3.663 | 1.46 | 4-methyl-Hexane | 58 |
| 7 | 3.801 | 5.95 | Cyclohexane | 87 |
| 8 | 4.082 | 1.62 | Cyclohexane | 94 |
| 9 | 4.439 | 0.21 | 3-Ethylcylopentanone | 58 |
| 10 | 10.419 | −0.28 | Oleic acid | 50 |
| 11 | 10.775 | 0.16 | 2-Hexen-4-yn-1-ol | 52 |
| 12 | 11.013 | 0.16 | 2,4-Decadienal | 74 |
| 13 | 12.446 | 53.09 | Butylated Hydroxytoluene | 95 |
| 14 | 14.047 | 14.53 | Oleic acid | 93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebhohimen, I.E.; Okolie, N.P.; Okpeku, M.; Unweator, M.; Adeleke, V.T.; Edemhanria, L. Evaluation of the Antioxidant Properties of Carvacrol as a Prospective Replacement for Crude Essential Oils and Synthetic Antioxidants in Food Storage. Molecules 2023, 28, 1315. https://doi.org/10.3390/molecules28031315
Ebhohimen IE, Okolie NP, Okpeku M, Unweator M, Adeleke VT, Edemhanria L. Evaluation of the Antioxidant Properties of Carvacrol as a Prospective Replacement for Crude Essential Oils and Synthetic Antioxidants in Food Storage. Molecules. 2023; 28(3):1315. https://doi.org/10.3390/molecules28031315
Chicago/Turabian StyleEbhohimen, Israel Ehizuelen, Ngozi P. Okolie, Moses Okpeku, Mfon Unweator, Victoria T. Adeleke, and Lawrence Edemhanria. 2023. "Evaluation of the Antioxidant Properties of Carvacrol as a Prospective Replacement for Crude Essential Oils and Synthetic Antioxidants in Food Storage" Molecules 28, no. 3: 1315. https://doi.org/10.3390/molecules28031315
APA StyleEbhohimen, I. E., Okolie, N. P., Okpeku, M., Unweator, M., Adeleke, V. T., & Edemhanria, L. (2023). Evaluation of the Antioxidant Properties of Carvacrol as a Prospective Replacement for Crude Essential Oils and Synthetic Antioxidants in Food Storage. Molecules, 28(3), 1315. https://doi.org/10.3390/molecules28031315






