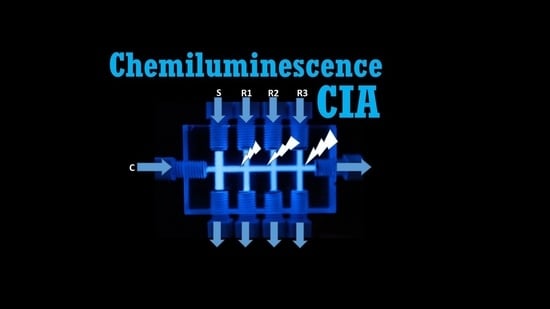
Transparent Cross-Flow Platform as Chemiluminescence Detection Cell in Cross Injection Analysis
Abstract
1. Introduction
2. Results and Discussion
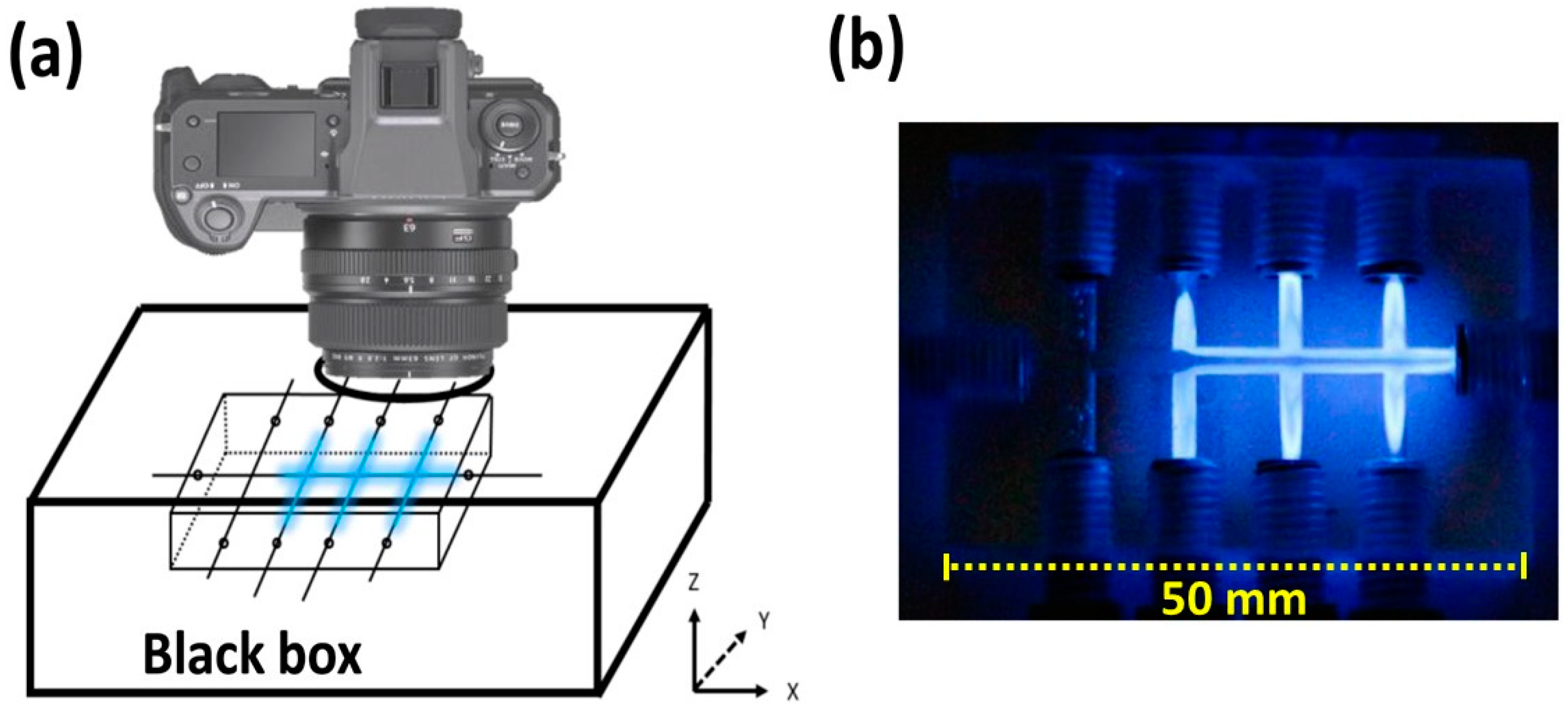
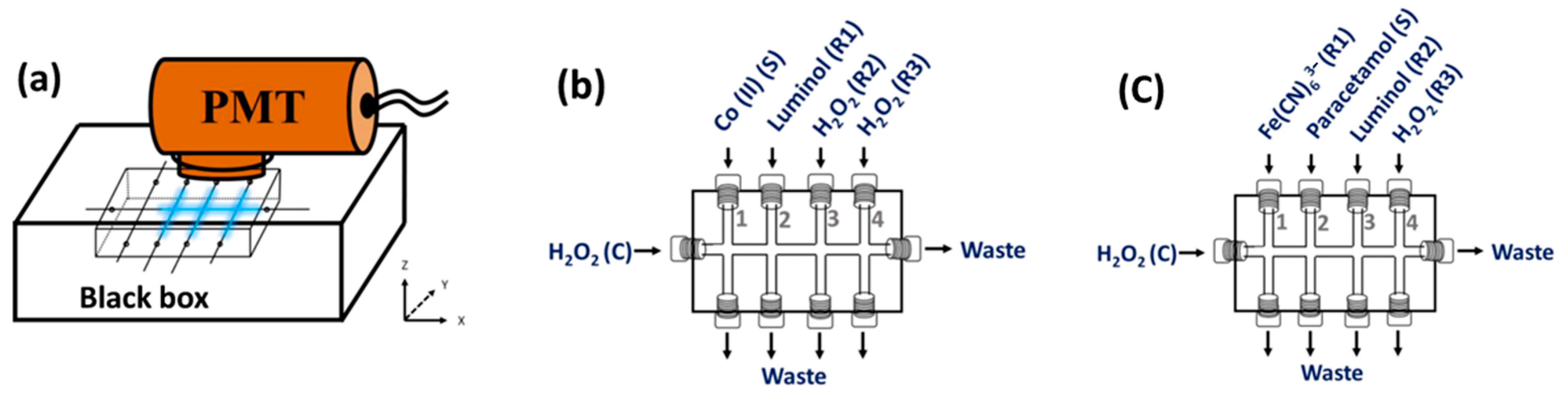
2.1. Preliminary Tests of Light Detection with the CIA Platform
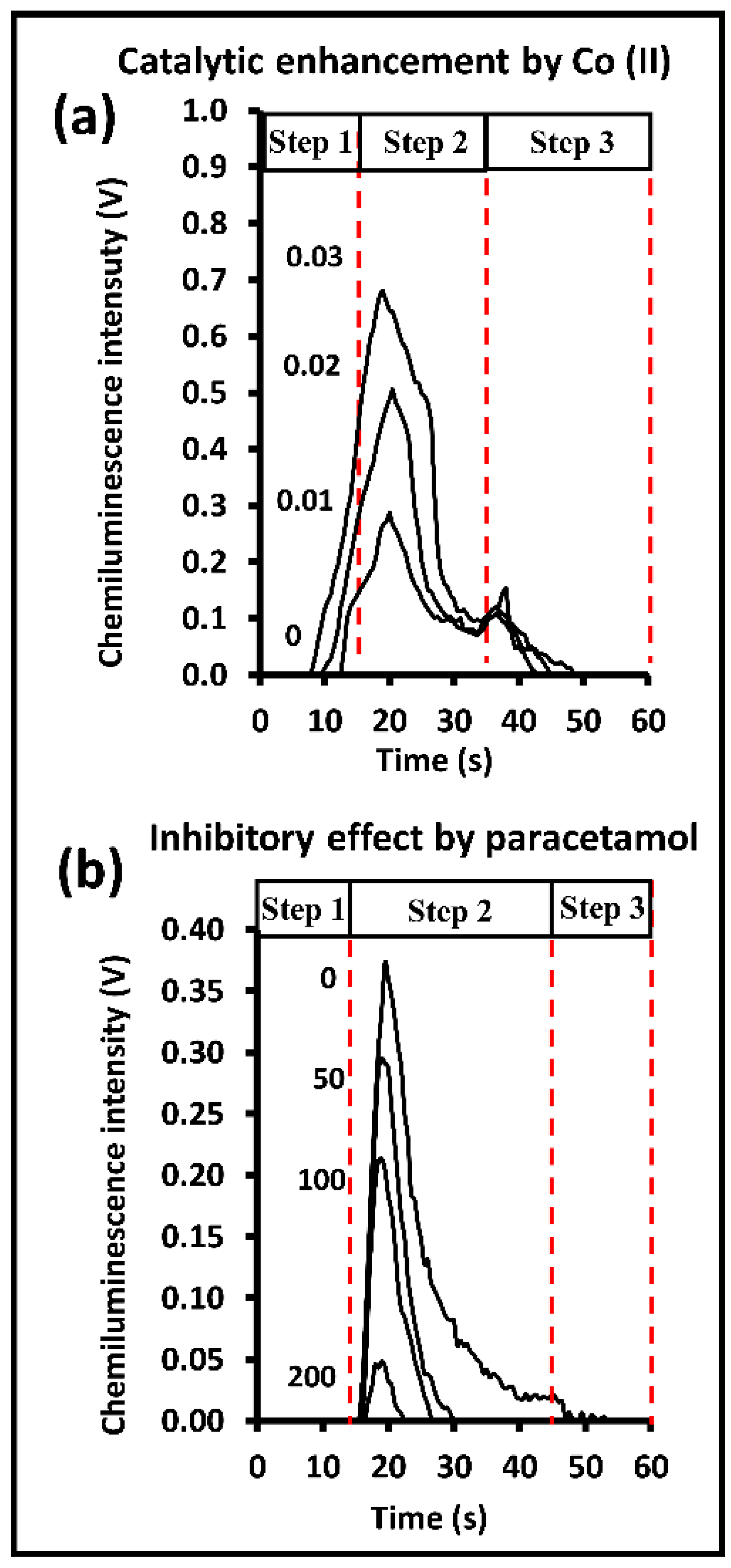
2.2. Optimization of CIA System I for Co(II) Analysis
2.3. CIA System II for Paracetamol Analysis
2.4. Analytical Performance of the CL-CIA Systems
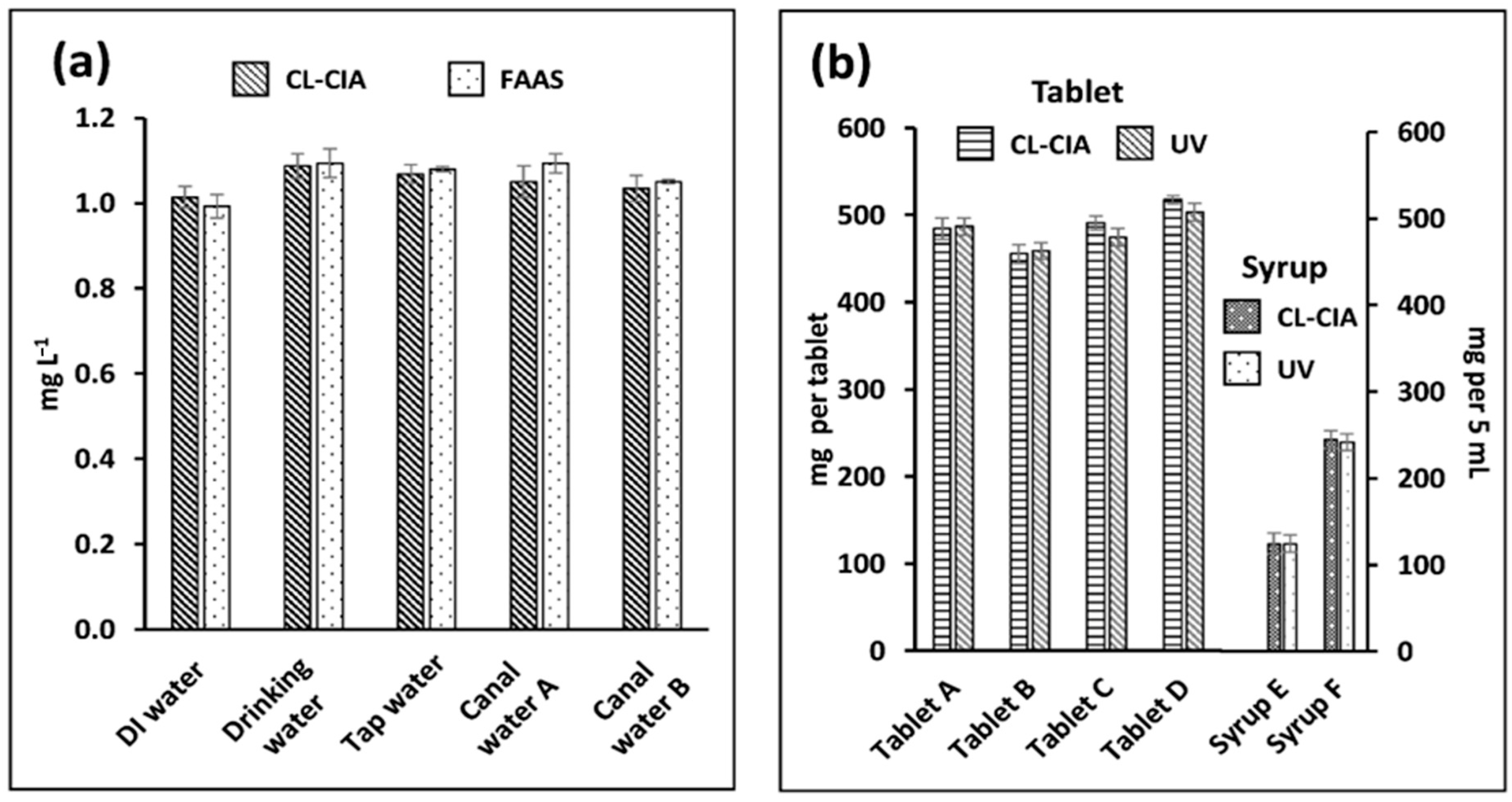
2.5. Applications and Validation
2.6. Comparison with Other Chemiluminescence Flow Configurations
3. Materials and Methods
3.1. The CIA Platform Used for Chemiluminescence Detection
3.2. CIA System I: Manifold and Operating Procedure for Measurement of Co (II)
3.3. CIA System II: Manifold and Operating Procedure for Measurement of Paracetamol
3.4. Standards and Reagents
3.4.1. Analysis of Co (II)
3.4.2. Analysis of Paracetamol
3.5. Sample Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Francis, P.S. Chemiluminescence Detection. In Encyclopedia of Microfluidics and Nanofluidics; Li, D., Ed.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Timofeeva, I.I.; Vakh, C.S.; Bulatov, A.V.; Worsfold, P.J. Flow analysis with chemiluminescence detection: Recent advances and applications. Talanta 2018, 179, 246–270. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Mirasoli, M.; Michelini, E.; Di Fusco, M.; Zangheri, M.; Cevenini, L.; Roda, B.; Simoni, P. Progress in chemical luminescence-based biosensors: A critical review. Biosens. Bioelectron. 2016, 76, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Ocaña-González, J.A.; Ramos-Payán, M.; Fernández-Torres, R.; Villar Navarro, M.; Bello-López, M.Á. Application of chemiluminescence in the analysis of wastewaters—A review. Talanta 2014, 122, 214–222. [Google Scholar] [CrossRef]
- Christodouleas, D.; Fotakis, C.; Economou, A.; Papadopoulos, K.; Timotheou-Potamia, M.; Calokerinos, A. Flow-Based Methods with Chemiluminescence Detection for Food and Environmental Analysis: A Review. Anal. Lett. 2011, 44, 176–215. [Google Scholar] [CrossRef]
- Mervartova, K.; Polasek, M.; Martinez Calatayud, J. Recent applications of flow-injection and sequential-injection analysis techniques to chemiluminescence determination of pharmaceuticals. J. Pharm. Biomed. Anal. 2007, 45, 367–381. [Google Scholar] [CrossRef]
- Marquette, C.A.; Blum, L.J. Applications of the luminol chemiluminescent reaction in analytical chemistry. Anal. Bioanal. Chem. 2006, 385, 546–554. [Google Scholar] [CrossRef]
- Adcock, J.L.; Barnett, N.W.; Barrow, C.J.; Francis, P.S. Advances in the use of acidic potassium permanganate as a chemiluminescence reagent: A review. Anal. Chim. Acta 2004, 807, 9–28. [Google Scholar] [CrossRef]
- Economou, A.; Papargyris, D.; Stratis, J. Automated Flow-Injection Instrument for Chemiluminescence Detection Using a Low-Cost Photodiode Detector: An Interdisciplinary Project in Chemical Instrumentation, Graphical Programming, Computer Interfacing, and Analytical Chemistry. J. Chem. Educ. 2004, 81, 406–410. [Google Scholar] [CrossRef]
- Han, P.; Idrees, D.; Moxley, M.A.; Corbett, J.A.; Ahmad, F.; von Figureura, G.; Sly, W.S.; Waheed, A.; Hassan, M.I. Luminol-Based Chemiluminescent Signals: Clinical and Non-clinical Application and Future Uses. Appl. Biochem. Biotechnol. 2014, 173, 333–355. [Google Scholar]
- Ratanawimarnwong, N.; Amornthammarong, N.; Choengchan, N.; Chaisuwan, P.; Amatatongchai, M.; Wilairat, P.; McKelvie, I.D.; Nacapricha, D. Determination of iodide by detection of iodine using gas-diffusion flow injection and chemiluminescence. Talanta 2005, 65, 756–761. [Google Scholar] [CrossRef]
- Yan, B.; Worsfold, P.J. Determination of cobalt (II), copper (II) and iron (II) by ion chromatography with chemiluminescence detection. Anal. Chim. Acta 1990, 236, 287–292. [Google Scholar] [CrossRef]
- Pizà, N.; Miró, M.; de Armas, G.; Becerra, E.; Estela, J.M.; Cerdà, V. Implementation of chemiluminescence detection in the multisyringe flow injection technique. Anal. Chim. Acta 2002, 467, 155–166. [Google Scholar] [CrossRef]
- Lu, C.; Lin, J.-M.; Huie, C.W.; Yamada, M. Simultaneous Determination of Copper (II) and Cobalt (II) by Ion Chromatography Coupled with Chemiluminescent Detection. Anal. Sci. 2003, 19, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Tortajada-Genaro, L.A.; Campíns-Falcó, P.; Bosch-Reig, F. Analyser of chromium and/or cobalt. Anal. Chim. Acta 2003, 488, 243–254. [Google Scholar] [CrossRef]
- Inpota, P.; Nacapricha, D.; Sunintaboon, P.; Sripumkhai, W.; Jeamsaksiri, W.; Wilairat, P.; Chantiwas, R. Chemiluminescence detection with microfluidics for innovative in situ measurement of unbound cobalt ions in dynamic equilibrium with bound ions in binding study with polyethyleneimine and its functionalized nanoparticles. Talanta 2018, 188, 606–613. [Google Scholar] [CrossRef]
- Gregorio, A.A.; Lahuerta, Z.L.; Martínez, C.J. Indirect determination of paracetamol in pharmaceutical formulations by inhibition of the system luminol–H2O2–Fe(CN)63− chemiluminescence. J. Pharm. Biomed. Anal. 1999, 21, 311–317. [Google Scholar] [CrossRef]
- Easwaramoorthy, D.; Yu, Y.-C.; Huang, H.-J. Chemiluminescence detection of paracetamol by a luminol-permanganate based reaction. Anal. Chim. Acta 2001, 439, 95–100. [Google Scholar] [CrossRef]
- Kubo, H.; Toriba, A. Chemiluminescence flow injection analysis of reducing agents based on the luminol reaction. Anal. Chim. Acta 1997, 353, 345–349. [Google Scholar] [CrossRef]
- Ramos Fernández, J.M.; Bosque-Sendra, J.M.; García-Campaña, A.M.; Alés Barrero, F. Chemiluminescence determination of amikacin based on the inhibition of the luminol reaction catalyzed by copper. J. Pharm. Biomed. Anal. 2005, 36, 969–974. [Google Scholar] [CrossRef]
- Andrade, R.A.N.; Andrade, S.I.E.; Martins, V.L.; Moreira, P.N.T.; Costa, D.J.E.; Lyra, W.S.; Araújo, M.C.U. A flow–batch luminometer. Microchem. J. 2013, 108, 151–155. [Google Scholar] [CrossRef]
- Ibáñez-García, N.; Puyol, M.; Azevedo, C.M.; Martínez-Cisneros, C.S.; Villuendas, F.; Gongora-Rubio, M.R.; Seabra, A.C.; Alonso, J. Vortex Configuration Flow Cell Based on Low-Temperature Cofired Ceramics as a Compact Chemiluminescence Microsystem. Anal. Chem. 2008, 80, 5320–5324. [Google Scholar] [CrossRef] [PubMed]
- Koronkiewicz, S.; Kalinowski, S. Direct-injection chemiluminescence detector. Properties and potential applications in flow analysis. Talanta 2015, 133, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Spilstead, K.B.; Learey, J.J.; Doeven, E.H.; Barbante, G.J.; Mohr, S.; Barnett, N.W.; Terry, J.M.; Hall, R.M.; Francis, P.S. 3D-printed and CNC milled flow-cells for chemiluminescence detection. Talanta 2014, 126, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.; Terry, J.M.; Adcock, J.L.; Fielden, P.R.; Goddard, N.J.; Barnett, N.W.; Wolcott, D.K.; Francis, P.S. Precision milled flow-cells for chemiluminescence detection. Analyst 2009, 134, 2233–2238. [Google Scholar] [CrossRef]
- Terry, J.M.; Adcock, J.L.; Olson, D.C.; Wolcott, D.K.; Schwanger, C.; Hill, L.A.; Barnett, N.W.; Francis, P.S. Chemiluminescence Detector with a Serpentine Flow Cell. Anal. Chem. 2008, 80, 9817–9821. [Google Scholar] [CrossRef] [PubMed]
- Liveira, H.M.; Grand, M.M.; Ruzicka, J.; Measures, C.I. Towards chemiluminescence detection in micro-sequential injection lab-on-valve format: A proof of concept based on the reaction between Fe(II) and luminol in seawater. Talanta 2015, 133, 107–111. [Google Scholar] [CrossRef]
- Rule, G.; Seitz, W.R. Flow-injection analysis with chemiluminescence detection. Clin. Chem. 1979, 25, 1635–1638. [Google Scholar] [CrossRef]
- Burguera, J.L.; Townshend, A.; Greenfield, S. Flow injection analysis for monitoring chemiluminescent reactions. Anal. Chim. Acta 1980, 114, 209–214. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Molina-Díaz, A. Chemiluminescence optosensing implemented with multicommutation: Determination of salicylic acid. Anal. Chim. Acta 2006, 580, 149–154. [Google Scholar] [CrossRef]
- Zeng, H.-J.; Yang, R.; Wang, Q.-W.; Li, J.-J.; Qu, L.-B. Determination of melamine by flow injection analysis based on chemiluminescence system. Food Chem. 2011, 127, 842–846. [Google Scholar] [CrossRef]
- Rocha, F.R.P.; Ródenas-Torralba, E.; Reis, B.F.; Morales-Rubio, Á.; Guardia, M.D.L. A portable and low-cost equipment for flow injection chemiluminescence measurements. Talanta 2005, 67, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Mahbub, P.; Nesterenko, P.N.; Paull, B. A new 3D printed radial flow-cell for chemiluminescence detection: Application in ion chromatographic determination of hydrogen peroxide in urine and coffee extracts. Anal. Chim. Acta 2018, 1005, 81–92. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C.; Estela, J.M.; Cerdà, V. Automatic in Vitro Determination of Hypochlorous Acid Scavenging Capacity Exploiting Multisyringe Flow Injection Analysis and Chemiluminescence. Anal. Chem. 2007, 79, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.; Toivola, B.; Pollema, C.; Růžička, J.; Christian, G.D. The fountain cell: A new tool for chemiluminescence analysis by flow injection. Analyst 1994, 119, 975–979. [Google Scholar] [CrossRef]
- Yang, M.; Xu, Y.; Wang, J.-H. Lab-on-Valve System Integrating a Chemiluminescent Entity and in Situ Generation of Nascent Bromine as Oxidant for Chemiluminescent Determination of Tetracycline. Anal. Chem. 2006, 78, 5900–5905. [Google Scholar] [CrossRef]
- Nacapricha, D.; Sastranurak, P.; Mantim, T.; Amornthammarong, N.; Uraisin, K.; Boonpanaid, C.; Chuyprasartwattana, C.; Wilairat, P. Cross injection analysis: Concept and operation for simultaneous injection of sample and reagents in flow analysis. Talanta 2013, 110, 89–95. [Google Scholar] [CrossRef]
- Choengchan, N.; Mantim, T.; Inpota, P.; Nacapricha, D.; Wilairat, P.; Jittangprasert, P.; Waiyawat, W.; Fucharoen, S.; Sirankpracha, P.; Morales, N.P. Tandem measurements of iron and creatinine by cross injection analysis with application to urine from thalassemic patients. Talanta 2015, 133, 52–58. [Google Scholar] [CrossRef]
- Ruzicka, J. Lab-on-valve: Universal Microflow Analyzer based on Sequential and Bead Injection. Analyst 2000, 125, 1053–1060. [Google Scholar] [CrossRef]
| Feature | Cobalt (II) Analysis | Paracetamol Analysis |
|---|---|---|
| Working range | 0.002–0.025 mg L−1 | 10.0–200 mg L−1 |
| Calibration equation | Signal (volt) = (69.3 ± 2.34) · (Co(II), mg L−1) + (2.50 ± 0.03), r2 = 0.9977 | % decrease = (0.34 ± 0.02) · (paracetamol, mg L−1) + (6 ± 2), r2 = 0.9906 |
| Limit of quantitation | 0.0016 mg L−1 (2 SD of y-intercept/slope) | 3.87 mg L−1 (3SD of blank/slope) |
| Precision (RSD) | 1.9–3.7% (n = 30) | 0.3–4.2% (n = 25) |
| Recovery | 87–103% | 97–110% |
| Sample throughput |  60 samples h−1 60 samples h−1  | |
| Sample and reagent consumption |  167 µL 167 µL  | |
| Design | Fabrication Technique | Body Material/ Cell Volume (µL) | Window Material | Remark | |
|---|---|---|---|---|---|
| 1. Mixing chamber (4 inlets and 1 outlet) [21] | Not mentioned | Teflon block/2000 | Not mentioned |
| |
| 2. Vortex (4 inlets and 1 outlet) [22] | Thermocompression and sintering | Fused multilayer ceramic/38, 152, 271 | Glass sheet |
| |
| 3. Cone-shaped (2 inlets with a cross-flow point and 1 outlet) [23] | Not mentioned | Teflon block/280 | Glass sheet |
| |
| 4. Flat flow cells with channels | |||||
| 4.1. 3D-printed spiral (2 inlets and 1 outlet) [24] | 3D printing | Visijet Ex200 UV curable acrylic plastic as build material/144 | Epoxy acetate film |
| |
| 4.2. Milled spiral (2 inlets and 1 outlet) [25,26] | CNC milling | Poly carbonate/144 [25] White acetal/144 [25] Teflon disk/275 [26] | Epoxy acetate film [25] Sapphire window [26]. |  |
|
| 4.3. Milled sinusoidal (2 inlets and 1 outlet) [25] | CNC milling | White acetal block/133 | Epoxy acetate film | ||
| 4.4. Milled serpentine (2 inlets and 1 outlet) [25,26] | CNC milling | White acetal/144 [25] Teflon disk/275 [26] | Epoxy acetate film [25] Sapphire window [26] | ||
| 5. Lab-on-valve flow unit (4 inlets or more and 3 outlets) [27] | Mesofabrication [39] | Perspex block/ Not mentioned | Not needed |
| |
| 6. Cross-flow platform (5 inlets and 5 outlets) (used in this work) | Milling and drilling | Perspex block/322 | Not needed |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somboonsuk, T.; Saetear, P.; Mantim, T.; Ratanawimarnwong, N.; Wilairat, P.; Choengchan, N.; Nacapricha, D. Transparent Cross-Flow Platform as Chemiluminescence Detection Cell in Cross Injection Analysis. Molecules 2023, 28, 1316. https://doi.org/10.3390/molecules28031316
Somboonsuk T, Saetear P, Mantim T, Ratanawimarnwong N, Wilairat P, Choengchan N, Nacapricha D. Transparent Cross-Flow Platform as Chemiluminescence Detection Cell in Cross Injection Analysis. Molecules. 2023; 28(3):1316. https://doi.org/10.3390/molecules28031316
Chicago/Turabian StyleSomboonsuk, Thachkorn, Phoonthawee Saetear, Thitirat Mantim, Nuanlaor Ratanawimarnwong, Prapin Wilairat, Nathawut Choengchan, and Duangjai Nacapricha. 2023. "Transparent Cross-Flow Platform as Chemiluminescence Detection Cell in Cross Injection Analysis" Molecules 28, no. 3: 1316. https://doi.org/10.3390/molecules28031316
APA StyleSomboonsuk, T., Saetear, P., Mantim, T., Ratanawimarnwong, N., Wilairat, P., Choengchan, N., & Nacapricha, D. (2023). Transparent Cross-Flow Platform as Chemiluminescence Detection Cell in Cross Injection Analysis. Molecules, 28(3), 1316. https://doi.org/10.3390/molecules28031316