Abstract
Photochemical techniques have recently been revitalized as they can readily be adapted to different polymerization modes to yield a wide range of complex macromolecular structures. However, the implementation of the photoinduced cationic methods in the polymerization of cyclic siloxane monomers has scarcely been investigated. Octamethylcyclotetrasiloxane (D4) is an important monomer for the synthesis of polydimethylsiloxane (PDMS) and its copolymers. In this study, the cationic ring-opening polymerization (ROP) of D4, initiated by diphenyl iodonium hexafluorophosphate (DPI), has been studied. Both direct and indirect initiating systems acting at broad wavelength using benzophenone and pyrene were investigated. In both systems, photochemically generated protonic acids and silylium cations are responsible for the polymerization. The kinetics of the polymerization are followed by viscosimetry and GPC analyses. The reported approach may overcome the problems associated with conventional methods and therefore represents industrial importance for the fabrication of polysiloxanes.
1. Introduction
Polysiloxanes are a class of polymers with a backbone of alternating silicon and oxygen atoms with linear or branched structures. Due to their unique characteristics, such as low glass transition temperature at around −120 °C, high gas permeability, good oxidative and thermal properties, UV stability, high chain flexibility, and low surface energy, polydimethylsiloxanes (PDMS) offer critical solutions for specific needs [1,2,3,4,5]. Cyclosiloxanes, specifically octamethylcyclotetrasiloxane (D4) and hexamethylcyclotrisiloxane (D3), are the two most significant cyclic siloxane monomers for ionic ring-opening polymerization (ROP) to obtain polysiloxanes [6,7,8,9,10,11]. D4 and D3 are widely used in a broad range of applications, including automotive, medicine, personal care, implant, and adhesive industries [12,13,14].
The cationic ROP of cyclosiloxanes, initiated by strong protic acids or bases, including H2SO4, F3CSO3H and (H3C)4NOH, NaOH, and KOH, respectively, has been investigated in detail [15,16,17]. This polymerization propagates through an anionic or cationic route, depending on the type of catalyst. The problems associated with the solubility of catalysts in bulk media have negative effects on the feasibility of the method in industrial applications [18].
Compared to thermal polymerization methods, photopolymerization has a number of benefits, including better adaptability, higher reaction rates and spatiotemporal control, and a lower energy requirement, offering greener, safer, and more environmentally friendly reaction conditions [19,20,21,22,23]. In recent years, photoinitiated cationic ROP has been vastly studied and successfully applied to several industrially important monomers, including lactides, [24] ε-caprolactone, [25] cyclic ethers [26], and epoxides [27]. Obtained polymers in particular have deserved attention because of their influence on commercial applications, including adhesives, coatings, the encapsulation of electronic components, and 3D printing [28,29,30,31,32,33,34]. Even though there are a vast number of studies on the thermally catalyzed synthesis of PDMS, photoinduced polymerization approaches are rarely employed [35,36,37].
In this work, the photoinitiated cationic ROP of D4 by direct and indirect activation mechanisms was investigated. Benzophenone and pyrene acted as photosensitizers (PS) in the presence of diphenyliodonium (DPI) salt in indirect activation. As will be shown below, photochemically generated acids and silylium cations were electrophilic enough to induce the cationic polymerization [38,39,40].
Iodonium salts (DPI) and their photochemistry have been investigated extensively [41,42,43]. As they have significant photolysis quantum yields for the generation of reactive cationic species in the UV region, they have been widely used as a photoinitiator in cationic photopolymerizations, and to extend their spectral sensitivity to longer wavelengths, i.e., visible and NIR regions, several approaches were proposed [44,45,46,47,48,49,50,51,52]. As an oxidizing salt, the most commonly employed DPI was utilized due to its favorable redox potential to oxidize free radicals to reactive cationic species [53,54,55,56]. Such photoinduced electron transfer reactions were already reported by several research groups [57,58,59,60,61,62,63,64,65].
2. Results and Discussion
In the direct system, upon irradiation of DPI, the Ph-I bonds are ruptured to form iodobenzene radical cation and phenyl radical. Following this, the hydrogen abstraction of the radical cation from tetramethyldisoloxane (TMDS) essentially yields Bronsted acid capable of initiating the ROP of D4 through the protonation of oxygen on the monomer [66]. An alternative pathway, the oxidation of silyl radicals formed after hydrogen abstraction, also produces reactive silylium cations. Visible-light-induced cationic polymerization, conducted by silylium cations, was previously reported [62]. These cations are capable of initiating the ROP of cyclic monomers. The overall mechanism is presented in Scheme 1. As can be seen in Table 1, an increase in the concentration of DPI and irradiation time increases conversion and molecular weight. The polymers obtained this way were characterized by FTIR, 1H-NMR, and 29Si-NMR spectral analyses (Figure 1). PDMS and D4 have structural units that only the bending of bands on the IR spectra can differentiate. FTIR spectra presented in Figure 1a indicate a strong shift around 1100 cm−1, which can be attributed to the ring opening of the D4 monomer. Bands belonging to other functional groups exhibit no change that underlines that polymerization occurred and no contamination of impurities present in the formed polymer. 1H-NMR spectra (Figure 1b) further confirm the ring-opening process by the shift of the band from 0.11 ppm to 0.08 ppm. In the 29Si-NMR spectra of the PDMS, one major peak at −22 ppm further confirms the purity of the obtained product.
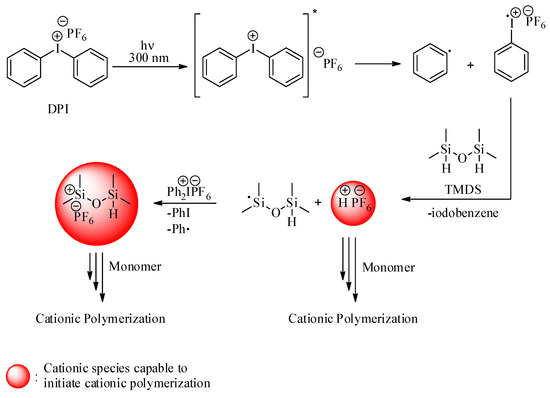
Scheme 1.
Photoinduced generation of cationic species by direct activation.

Table 1.
Photoinduced ROP a of D4 by direct activation using DPI.
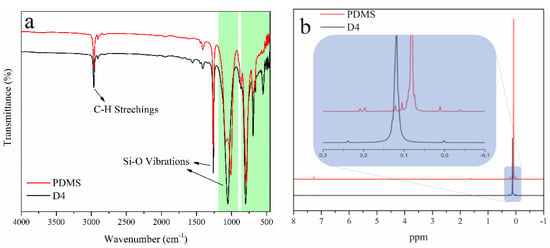
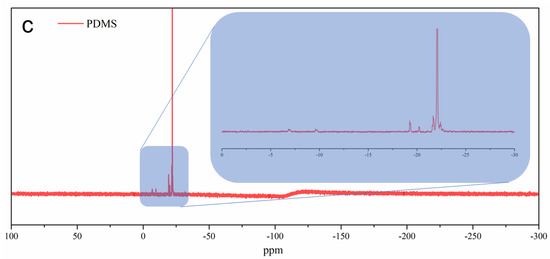
Figure 1.
(a) FTIR and (b) 1H−NMR of PDMS and D4 (c) 29Si−NMR spectra of PDMS.
After the successful implementation of the direct initiation method, sensitized approaches were taken into consideration to extend the activation to longer wavelengths. Pyrene and benzophenone were deliberately selected as PSs so as to benefit from their high wavelength absorption characteristics, high efficiency, and different protonic acid generation mechanisms. Benzophenone is known to be a triplet PS undergoing efficient hydrogen abstraction reactions. In the present case, the silyl radicals formed by the hydrogen abstraction reaction of TMDS can be oxidized to the corresponding cations to initiate the cationic ROP of D4. Concomitantly formed ketyl radicals also undergo redox reactions to essentially generate initiating protonic acids (Scheme 2). Pyrene is a highly conjugated aromatic PS that forms an exciplex with DPI. Electron transfer in the exciplex generates initiating protonic acids as well as silylium cations (Scheme 3).
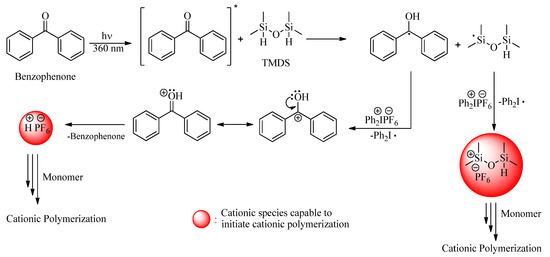
Scheme 2.
Photoinduced generation of cationic species by indirect activation using benzophenone as photosensitizer.
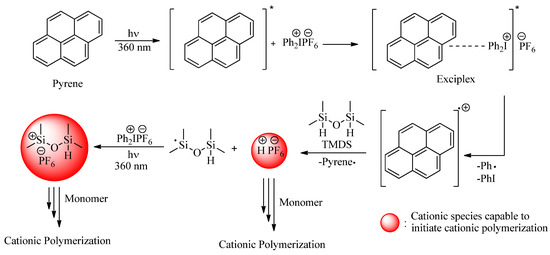
Scheme 3.
Photoinduced generation of cationic species by indirect activation using pyrene as photosensitizer.
Successful polymerizations with high yields were obtained by both benzophenone and pyrene. Control experiments were conducted to confirm that both Ph2I+-PF6 and PSs are indispensable for the polymerization to occur, as no polymers were formed in the absence of either component. The overall results are presented in Table 2. Notably, although a longer wavelength was employed, similar conversion and molecular weight characteristics were attained. Since the monomer and polymer are liquids, the polymerization kinetics for direct and sensitized methods were examined with the viscosity measurements. As can be seen in Figure 2 and Figure S1 (from Supplementary Materials), the polymerization levels off after 6 h of irradiation. The observed levelling off may be due to the attack of iodobenzene produced in the system by the propagating oxonium ions. A similar observation was made in the photoinitiated cationic polymerization of cyclic ethers using iodonium salts [26]. Even though no polymerizations were observed under dark conditions in the control experiments, light on–off experiments revealed that after irradiation, the polymerization also proceeds in the dark, as no termination occurs, due to the non-nucleophilic character of the counter anion (Figure 2d).

Table 2.
Polymerization a of D4 by indirect activation.
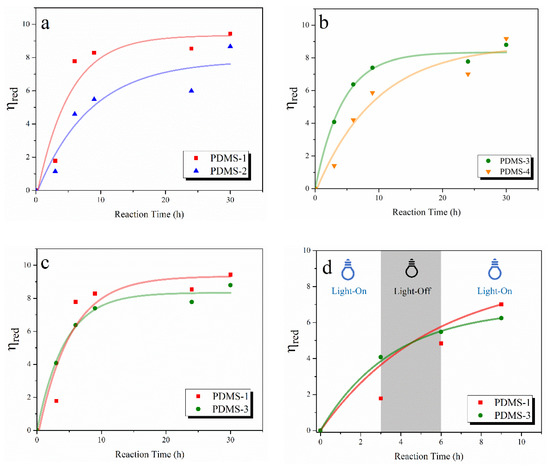
Figure 2.
Reduced viscosity over reaction time graphs of (a) PDMS-1 and PDMS-2 (b) PDMS-3 and PDMS-4 (c) PDMS-1 and PDMS-3 (d) PDMS-1 and PDMS-3.
3. Materials and Methods
3.1. Materials
Octamethylcyclotetrasiloxane (D4) (Denge Kimya, >95%), tetramethyldisiloxane (TMDS) (Denge Kimya, >95%), diphenyl iodonium hexafluorophosphate (DPI) (Aldrich, 98%), benzophenone (Merck, 99%), pyrene (Aldrich, 98%), and methanol (99.8%, Merck) were used as purchased. Dichloromethane (ISOLAB Chemicals, ≥99 %) (DCM) was purified by conventional drying and distillation methods.
3.2. Photoinduced Cationic ROP Procedures
3.2.1. Photoinduced ROP of D4 by Direct Activation
The direct polymerization of D4 was carried out under an inert atmosphere of N2 using standard Schlenk techniques. D4, (0.016 mol), TMDS (1.61 × 10−3 mol), and the photoinitiator DPI (4.46 × 10−3 mol, %0.5 w/w) were placed into a sealed quartz tube. The reaction mixture was irradiated in a Rayonet merry-go-round-type photoreactor equipped with 16 Philips 8 W UV light lamps, emitting light at 300 nm with a light intensity of ~4.0 mW cm−2 at room temperature. The polymer was purified from the resulting reaction mixture by precipitating in methanol. A colorless, viscous liquid was obtained and washed with methanol. It was then dried for 24 h in a vacuum at ambient temperature. Approximately similar conversions and molecular weight distributions were obtained with replicates. The highest numbers were reported. The exact reaction conditions were repeated for a viscosimetric analysis. The measurements were performed by using collected aliquot samples at specified times.
3.2.2. Photoinduced ROP of D4 by Sensitization
The photosensitized polymerization of D4 was carried out under an inert atmosphere of N2 using standard Schlenk techniques. D4, (0.016 mol), TMDS (1.61 × 10−3 mol), DPI (4.46 × 10−3 mol, %0.5 w/w), and the corresponding PS (4.46 × 10−3 mol) were placed into a sealed quartz tube. Polymerizations were performed using a Rayonet merry-go-round-type photoreactor equipped with 16 Philips 8 W/O6 lamps, emitting light nominally at 360 nm. The light intensity was ~3.0 mW cm−2, as measured by the Delta Ohm model HD-9021 radiometer. Approximately similar conversions and molecular weight distributions were obtained with replicates. The highest numbers were reported. The exact reaction conditions were repeated for a viscosimetric analysis. The measurements were performed by using collected aliquot samples at specified times.
3.3. Instrumentation
1H-NMR spectra were recorded at room temperature at 500 MHz (128 scans per analysis) on an Agilent VNMRS 500 spectrometer in CDCl3, using tetramethyl silane as an internal standard. 29Si-NMR spectra were recorded at room temperature using glass tubes at 500 MHz (256 scans; relax time of 30 s) on an Agilent VNMRS 500 spectrometer in CDCl3, without tetramethyl silane as an internal standard. Gel permeation chromatography (GPC) measurements were performed on a TOSOH EcoSEC GPC system equipped with an auto sampler system, a temperature-controlled pump, a column oven, a refractive index (RI) detector, a purge and degasser unit, and a TSKgel superhZ2000, 4.6 mm ID × 15 cm × 2 cm column. Tetrahydrofuran was used as an eluent at a flow rate of 1.0 mL.min−1 at 40 °C. The refractive index detector was calibrated with polystyrene standards, having narrow molecular weight distributions. The data were analyzed using Eco-SEC analysis software. The Fourier transform infrared (FTIR) spectroscopy measurements were recorded on a PerkinElmer FTIR Spectrum One spectrometer. Viscosimetry experiments were conducted at room temperature via Ubbelohde Apparatus SI Analytics Typ 501 01/0a Appr. Nr. 1068985. 5.
4. Conclusions
The photoinitiated polymerization of D4 using DPI salt under ambient temperature has been demonstrated. Both direct and indirect photosensitization approaches were effectively adapted in the polymerization process. Both approaches involved the photogeneration of Bronsted acids and/or silylium cations to initiate the cationic ROP of D4. The polymer structure has been proven by 1H-NMR and FTIR analyses. Kinetic investigations indicated that polymerization reaches a steady level after certain irradiation time. A future practical benefit could be foreseen in connection with the fact that the presented methodology, based on the possibility of photoinduced synthesis of polysiloxanes at desired wavelengths by using common compounds under environmentally friendly and lower energy conditions, has the possibility of conveying its technological aspect on an industrial level. Future studies will be devoted to the photoinduced synthesis of polysiloxanes copolymers in connection with the specialty application areas.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28031299/s1, Figure S1: Details of exponential curve fitting for (a) PDMS-1, (b) PDMS-2, (c) PDMS-3 and (d) PDMS-4.
Author Contributions
Conceptualization, Y.Y. and H.C.K.; methodology, Y.Y. and H.C.K.; software, H.C.K.; validation, Z.G.C. and H.C.K.; formal analysis, Z.G.C. and H.C.K.; investigation, Z.G.C.; resources, Y.Y.; data curation, Z.G.C. and H.C.K.; writing—original draft preparation, H.C.K. and Z.G.C.; writing—review and editing, Y.Y.; visualization, H.C.K.; supervision, Y.Y.; project administration, Y.Y.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Istanbul Technical University Research Fund [43195]; The Scientific and Technological Research Council of Turkey (TUBITAK) [120C121].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data set presented in this study is available in this article.
Acknowledgments
The authors acknowledge Denge Kimya and Deniz Güneş for providing siloxane monomers and the support of TUBITAK (The Scientific and Technical Research Council of Turkey) Project no. 120C121 and Istanbul Technical University Research Fund Project no. 43195. Two of the authors (H.C.K. and Z.G.C.) would like to thank The Scientific and Technological Research Council of Turkey (TUBITAK) 2211-A program for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available on request from the corresponding authors.
Abbreviations
| D3 | hexamethylcyclotrisiloxane |
| D4 | octamethylcyclotetrasiloxane |
| TMDS | tetramethyldisiloxane |
| DPI | diphenyliodonium hexafluorophosphate |
| PDMS | polydimethylsiloxane |
| PS | photosensitizer |
| ROP | ring-opening polymerization |
| NMR | nuclear magnetic resonance |
| FTIR | Fourrier-transform infrared |
| 1H-NMR | proton nuclear magnetic resonance |
| 29Si-NMR | silicon nuclear magnetic resonance |
| GPC | gel-permeation chromatography |
| UV | ultraviolet |
| NIR | near-infrared |
References
- Yilgor, I.; Steckle, W.P.; Yilgor, E.; Freelin, R.G.; Riffle, J.S. Novel triblock siloxane copolymers: Synthesis, characterization, and their use as surface modifying additives. J. Polym. Sci. A Polym. Chem. 1989, 27, 3673–3690. [Google Scholar] [CrossRef]
- Mark, J.E. Some interesting things about polysiloxanes. Acc. Chem. Res. 2004, 37, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Cao, D.; Sun, Y.; Li, F.; Qi, Z. Cationic ring opening polymerization of octamethylcyclotetrasiloxane initiated by solid superacid. Glass Phys. Chem. 2016, 42, 307–311. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Mannsfeld, S.C.B.; Tee, B.C.K.; Stoltenberg, R.M.; Chen, C.V.H.H.; Barman, S.; Muir, B.V.O.; Sokolov, A.N.; Reese, C.; Bao, Z. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat. Mater. 2010, 9, 859–864. [Google Scholar] [CrossRef]
- Grubb, W.T.; Osthoff, R.C. Kinetics of the polymerization of a cyclic dimethylsiloxane. J. Am. Chem. Soc. 1955, 77, 1405–1411. [Google Scholar] [CrossRef]
- Barrère, M.; Ganachaud, F.; Bendejacq, D.; Dourges, M.A.; Maitre, C.; Hémery, P. Anionic polymerization of octamethylcyclotetrasiloxane in miniemulsion II. Molar mass analyses and mechanism scheme. Polymer 2001, 42, 7239–7246. [Google Scholar] [CrossRef]
- Zheng, P.; McCarthy, T.J. A surprise from 1954: Siloxane equilibration is a simple, robust, and obvious polymer self-healing mechanism. J. Am. Chem. Soc. 2012, 134, 2024–2027. [Google Scholar] [CrossRef]
- Rutnakornpituk, M.; Ngamdee, P. Surface and mechanical properties of microporous membranes of poly(ethylene glycol)-polydimethylsiloxane copolymer/chitosan. Polymer 2006, 47, 7909–7917. [Google Scholar] [CrossRef]
- Hao, X.; Jeffery, J.L.; Wilkie, J.S.; Meijs, G.F.; Clayton, A.B.; Watling, J.D.; Ho, A.; Fernandez, V.; Acosta, C.; Yamamoto, H.; et al. Functionalised polysiloxanes as injectable, in situ curable accommodating intraocular lenses. Biomaterials 2010, 31, 8153–8163. [Google Scholar] [CrossRef]
- Rutnakornpituk, M.; Ngamdee, P.; Phinyocheep, P. Synthesis, characterization and properties of chitosan modified with poly(ethylene glycol)-polydimethylsiloxane amphiphilic block copolymers. Polymer 2005, 46, 9742–9752. [Google Scholar] [CrossRef]
- Köhler, T.; Gutacker, A.; Mejiá, E. Industrial synthesis of reactive silicones: Reaction mechanisms and processes. Org. Chem. Front. 2020, 7, 4108–4120. [Google Scholar] [CrossRef]
- Tomanek, A. Silicones & Industry: A Compendium for Practical Use, Instruction and Reference; Wacker-Chemie: Munich, Germany, 1991. [Google Scholar]
- Meals, R.N. Silicones. Ann. N. Y. Acad. Sci. 1965, 125, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Alexandru, M.; Cristea, M.; Cazacu, M.; Ioanid, A.; Simionescu, B.C. Composite materials based on polydimethylsiloxane and in situ generated silica by using the sol–gel technique. Polym. Compos. 2009, 30, 751–759. [Google Scholar] [CrossRef]
- Toskas, G.; Besztercey, G.; Moreau, M.; Masure, M.; Sigwalt, P. Cationic polymerization of hexamethylcyclotrisiloxane by trifluoromethanesulfonic acid and its derivatives, 2. Reaction involving activated trifluoromethylsulfonates. Macromol. Chem. Phys. 1995, 196, 2715–2735. [Google Scholar] [CrossRef]
- Patnode, W.; Wilcock, D.F. Methylpolysiloxanes. J. Am. Chem. Soc. 1946, 68, 358–363. [Google Scholar] [CrossRef]
- Yang, X.; Shao, Q.; Fang, Q.; Yang, L.; Cao, C.; Ren, Z.; Wu, L.; Lai, G.; Han, G. Synthesis of vinyl end-capped polydimethylsiloxane by ring opening polymerization of octamethylcyclotetrasiloxane (D4) catalyzed by rare earth solid super acid SO42−/TiO2/Ln3+. Polym. Int. 2014, 63, 347–351. [Google Scholar] [CrossRef]
- Clark, J.H. Green chemistry: Challenges and opportunities. Green Chem. 1999, 1, 1–8. [Google Scholar] [CrossRef]
- Yilmaz, G.; Yagci, Y. New photochemical processes for macromolecular syntheses. J. Photopoly. Sci. Technol. 2016, 29, 91–98. [Google Scholar] [CrossRef]
- Albini, A.; Fagnoni, M. Green chemistry and photochemistry were born at the same time. Green Chem. 2004, 6, 1–6. [Google Scholar] [CrossRef]
- Zou, X.; Zhu, J.; Zhu, Y.; Yagci, Y.; Liu, R. Photopolymerization of macroscale black 3D objects using near-infrared photochemistry. ACS Appl. Mater. Interfaces 2020, 12, 58287–58294. [Google Scholar] [CrossRef] [PubMed]
- Garra, P.; Fouassier, J.P.; Lakhdar, S.; Yagci, Y.; Lalevée, J. Visible light photoinitiating systems by charge transfer complexes: Photochemistry without dyes. Prog. Polym. Sci. 2020, 107, 101277. [Google Scholar] [CrossRef]
- Kuroishi, P.K.; Dove, A.P. Photoinduced ring-opening polymerisation of L-lactide via a photocaged superbase. Chem. Commun. 2018, 54, 6264–6267. [Google Scholar] [CrossRef] [PubMed]
- Celiker, T.; Ghorbanizamani, F.; Moulahoum, H.; Guler Celik, E.; Tok, K.; Zihnioglu, F.; Cicek, C.; Sertoz, R.; Arda, B.; Goksel, T.; et al. Fluorescent bioassay for SARS-CoV-2 detection using polypyrene-g-poly(ε-caprolactone) prepared by simultaneous photoinduced step-growth and ring-opening polymerizations. Microchim. Acta 2022, 189, 202. [Google Scholar] [CrossRef]
- Yağci, Y.; Ledwith, A. Mechanistic and kinetic studies on the photoinitiated polymerization of tetrahydrofuran. J. Polym. Sci. A Polym. Chem. 1988, 26, 1911–1918. [Google Scholar]
- Kiliclar, H.C.; Altinkok, C.; Yilmaz, G.; Yagci, Y. Visible light induced step-growth polymerization by electrophilic aromatic substitution reactions. Chem. Commun. 2021, 57, 5398–5401. [Google Scholar] [CrossRef]
- Crivello, J.V. The discovery and development of onium salt cationic photoinitiators. J. Polym. Sci. A Polym. Chem. 1999, 37, 4241–4254. [Google Scholar] [CrossRef]
- Klikovits, N.; Sinawehl, L.; Knaack, P.; Koch, T.; Stampfl, J.; Gorsche, C.; Liska, R. UV-induced cationic ring-opening polymerization of 2-oxazolines for hot lithography. ACS Macro Lett. 2020, 9, 546–551. [Google Scholar] [CrossRef]
- Merckle, D.; Constant, E.; Cartwright, Z.; Weems, A.C. Ring opening copolymerization of four-dimensional printed shape memory polyester photopolymers using digital light processing. Macromolecules 2021, 54, 2681–2690. [Google Scholar] [CrossRef]
- Sharma, J.; Ahuja, S.; Arya, R.K. Depth profile study of poly(styrene)-poly(methyl methacrylate)-tetrahydrofuran coatings. Prog. Org. Coat. 2019, 134, 297–302. [Google Scholar] [CrossRef]
- Degirmenci, M.; Izgin, O.; Yagci, Y. Synthesis and characterization of cyclohexene oxide functional poly(ε-caprolactone) macromonomers and their use in photoinitiated cationic homo- and copolymerization. J. Polym. Sci. A Polym. Chem. 2004, 42, 3365–3372. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef]
- Camino, C.; Lomakin, S.M.; Lazzari, M. Polydimethylsiloxane thermal degradation Part 1. Kinetic aspects. Polymer 2001, 42, 2395–2402. [Google Scholar] [CrossRef]
- Schmid, H.; Michel, B. Siloxane polymers for high-resolution, high-accuracy soft lithography. Macromolecules 2000, 33, 3042–3049. [Google Scholar] [CrossRef]
- Lalevée, J.; Mokbel, H.; Fouassier, J.P. Recent developments of versatile photoinitiating systems for cationic ring opening polymerization operating at any wavelengths and under low light intensity sources. Molecules 2015, 20, 7201–7221. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J.; Tehfe, M.; Graff, B.; Lalevée, J.; Dumur, F.; et al. Green-light-induced cationic ring opening polymerization reactions: perylene-3,4:9,10-bis(dicarboximide) as efficient photosensitizers. Macromol. Chem. Phys. 2013, 214, 1052–1060. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Campolo, D.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Copper and iron complexes as visible-light-sensitive photoinitiators of polymerization. J. Polym. Sci. A Polym. Chem 2015, 53, 2673–2684. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Diaryliodonium salts. A new class of photoinitiators for cationic polymerization. Macromolecules 1977, 10, 1307–1315. [Google Scholar] [CrossRef]
- Zhdankin, V.V. Hypervalent iodine chemistry: Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds; Wiley: Hoboken, NJ, USA, 2014; pp. 1–468. [Google Scholar]
- Wirth, T. Hypervalent iodine chemistry in synthesis: Scope and new directions. Angew. Chem. Int. Ed. 2005, 44, 3656–3665. [Google Scholar] [CrossRef] [PubMed]
- Celiker, T.; Kaya, K.; Koyuncu, S.; Yagci, Y. Polypyrenes by photoinduced step-growth polymerization. Macromolecules 2020, 53, 5787–5794. [Google Scholar] [CrossRef]
- Sari, E.; Yilmaz, G.; Koyuncu, S.; Yagci, Y. Photoinduced step-growth polymerization of N-ethylcarbazole. J. Am. Chem. Soc. 2018, 140, 12728–12731. [Google Scholar] [CrossRef]
- Gomurashvili, Z.; Crivello, J.V. Monomeric and polymeric phenothiazine photosensitizers for photoinitiated cationic polymerization. Macromolecules 2002, 35, 2962–2969. [Google Scholar] [CrossRef]
- Sangermano, M.; Sordo, F.; Chiolerio, A.; Yagci, Y. One-pot photoinduced synthesis of conductive polythiophene-epoxy network films. Polymer 2013, 54, 2077–2080. [Google Scholar] [CrossRef]
- Aydogan, B.; Gundogan, A.S.; Ozturk, T.; Yagci, Y. Polythiophene derivatives by step-growth polymerization via photoinduced electron transfer reactions. Chem. Commun. 2009, 41, 6300–6302. [Google Scholar] [CrossRef] [PubMed]
- Topa-Skwarczyńska, M.; Galek, M.; Jankowska, M.; Morlet-Savary, F.; Graff, B.; Lalevée, J.; Popielarz, R.; Ortyl, J. Development of the first panchromatic BODIPY-based one-component iodonium salts for initiating the photopolymerization processes. Polym. Chem. 2021, 12, 6873–6893. [Google Scholar] [CrossRef]
- Yagci, Y.; Yilmaz, F.; Kiralp, S.; Toppare, L. Photoinduced polymerization of thiophene using iodonium salt. Macromol. Chem. Phys. 2005, 206, 1178–1182. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Kumbaraci, V.; Jockusch, S.; Turro, N.J.; Talinli, N.; Yagci, Y. Photoacid generation by stepwise two-photon absorption: Photoinitiated cationic polymerization of cyclohexene oxide by using benzodioxinone in the presence of iodonium salt. Macromolecules 2008, 41, 295–297. [Google Scholar] [CrossRef]
- Topa, M.; Ortyl, J. Moving towards a finer way of light-cured resin-based restorative dental materials: Recent advances in photoinitiating systems based on iodonium salts. Materials 2020, 13, 4093. [Google Scholar] [CrossRef]
- Yagci, Y.; Onen, A.; Schnabel, W. Block copolymers by combination of radical and promoted cationic polymerization routes. Macromolecules 1991, 24, 4620–4623. [Google Scholar] [CrossRef]
- Yağci, Y.; Lukáč, I.; Schnabel, W. Photosensitized cationic polymerization using N-ethoxy-2-methylpyridinium hexafluorophosphate. Polymer 1993, 34, 1130–1133. [Google Scholar] [CrossRef]
- Böttcher, A.; Hasebe, K.; Hizal, G.; Yagci, Y.; Stellberg, P.; Schnabel, W. Initiation of cationic polymerization via oxidation of free radicals using pyridinium salts. Polymer 1991, 32, 2289–2293. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Mechanism of photoinduced step polymerization of thiophene by onium salts: Reactions of phenyliodinium and diphenylsulfinium radical cations with thiophene. Macromolecules 2007, 40, 4481–4485. [Google Scholar] [CrossRef]
- Richardson, R.D.; Wirth, T. Hypervalent iodine goes catalytic. Angew. Chem. Int. Ed. 2006, 45, 4402–4404. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hari, D.P.; Vita, M.V.; Waser, J. Cyclic hypervalent iodine reagents for atom-transfer reactions: Beyond trifluoromethylation. Angew. Chem. Int. Ed. 2016, 55, 4436–4454. [Google Scholar] [CrossRef]
- Merritt, E.A.; Olofsson, B.; Olofsson, B.; Merritt, E.A. Diaryliodonium salts: A journey from obscurity to fame. Angew. Chem. Int. Ed. 2009, 48, 9052–9070. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, Y.; Meng, H.; Shao, Q.; Xu, Z.; Bao, W.; Gu, Y.; Xue, X.S.; Zhao, Y. Metal-free C−H functionalization via diaryliodonium salts with a chemically robust dummy ligand. Angew. Chem. Int. Ed. 2022, 61, e202201240. [Google Scholar]
- Moriarty, R.M.; Prakash, O. Synthesis of heterocyclic compounds using organohypervalent iodine reagents. Adv. Heterocycl. Chem. 1997, 69, 1–87. [Google Scholar]
- Sari, E.; Mitterbauer, M.; Liska, R.; Yagci, Y. Visible light induced free radical promoted cationic polymerization using acylsilanes. Prog. Org. Coat. 2019, 132, 139–143. [Google Scholar] [CrossRef]
- Tasdelen, M.A.; Lalevée, J.; Yagci, Y. Photoinduced free radical promoted cationic polymerization 40 years after its discovery. Polym. Chem. 2020, 11, 1111–1121. [Google Scholar] [CrossRef]
- Wang, D.; Garra, P.; Fouassier, J.P.; Lalevée, J. Silane/iodonium salt as redox/thermal/photoinitiating systems in radical and cationic polymerizations for laser write and composites. Polym. Chem. 2020, 11, 857–866. [Google Scholar] [CrossRef]
- Wang, D.; Szillat, F.; Fouassier, J.P.; Lalevée, J. Remarkable versatility of silane/iodonium salt as redox free radical, cationic, and photopolymerization initiators. Macromolecules 2019, 52, 5638–5645. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J. Photochemical production of interpenetrating polymer networks; simultaneous initiation of radical and cationic polymerization reactions. Polymers 2014, 6, 2588–2610. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).