A Novel Approach to Develop New and Potent Inhibitors for the Simultaneous Inhibition of Protease and Helicase Activities of HCV NS3/4A Protease: A Computational Approach
Abstract
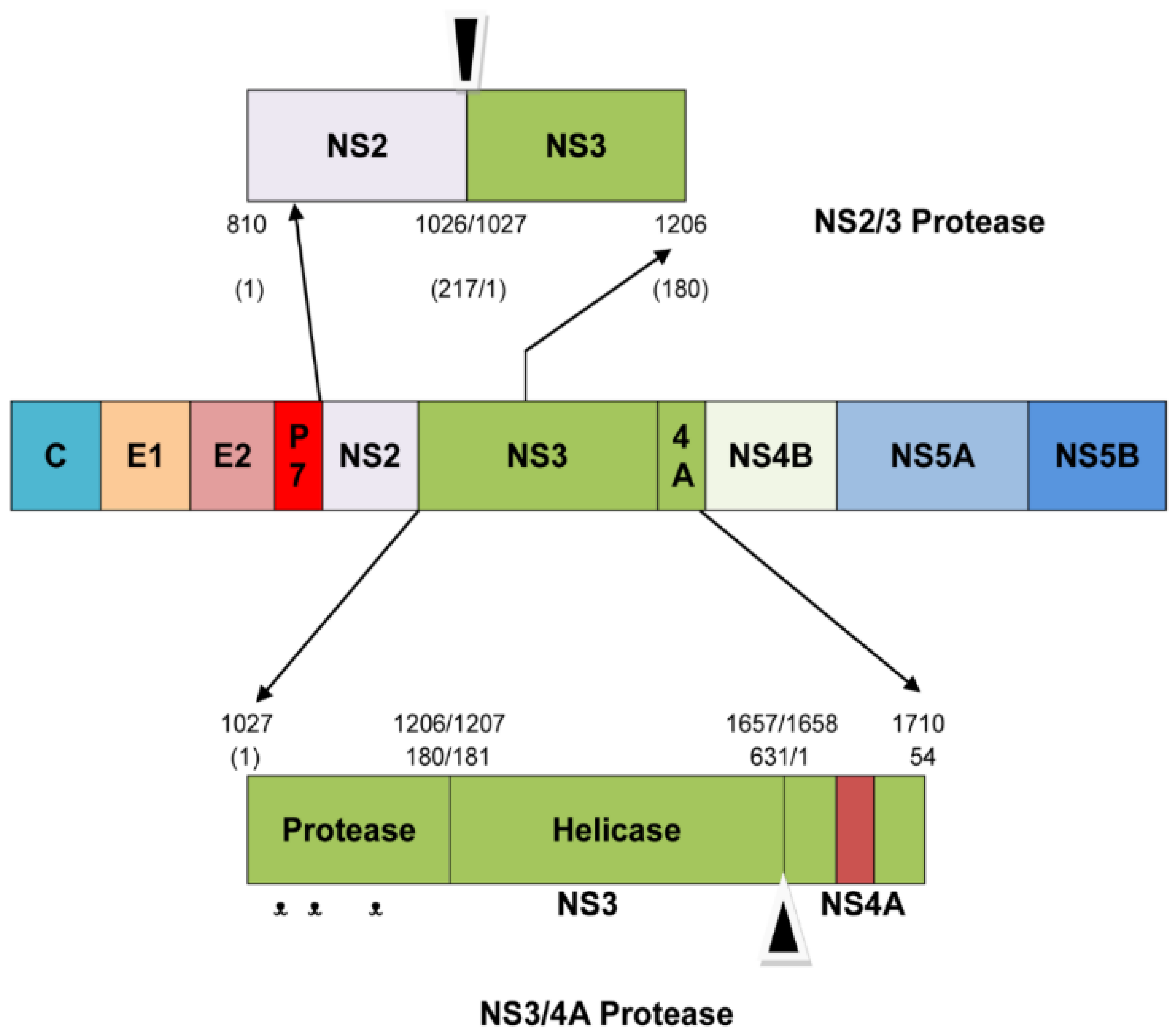
1. Introduction
2. Results and Discussion
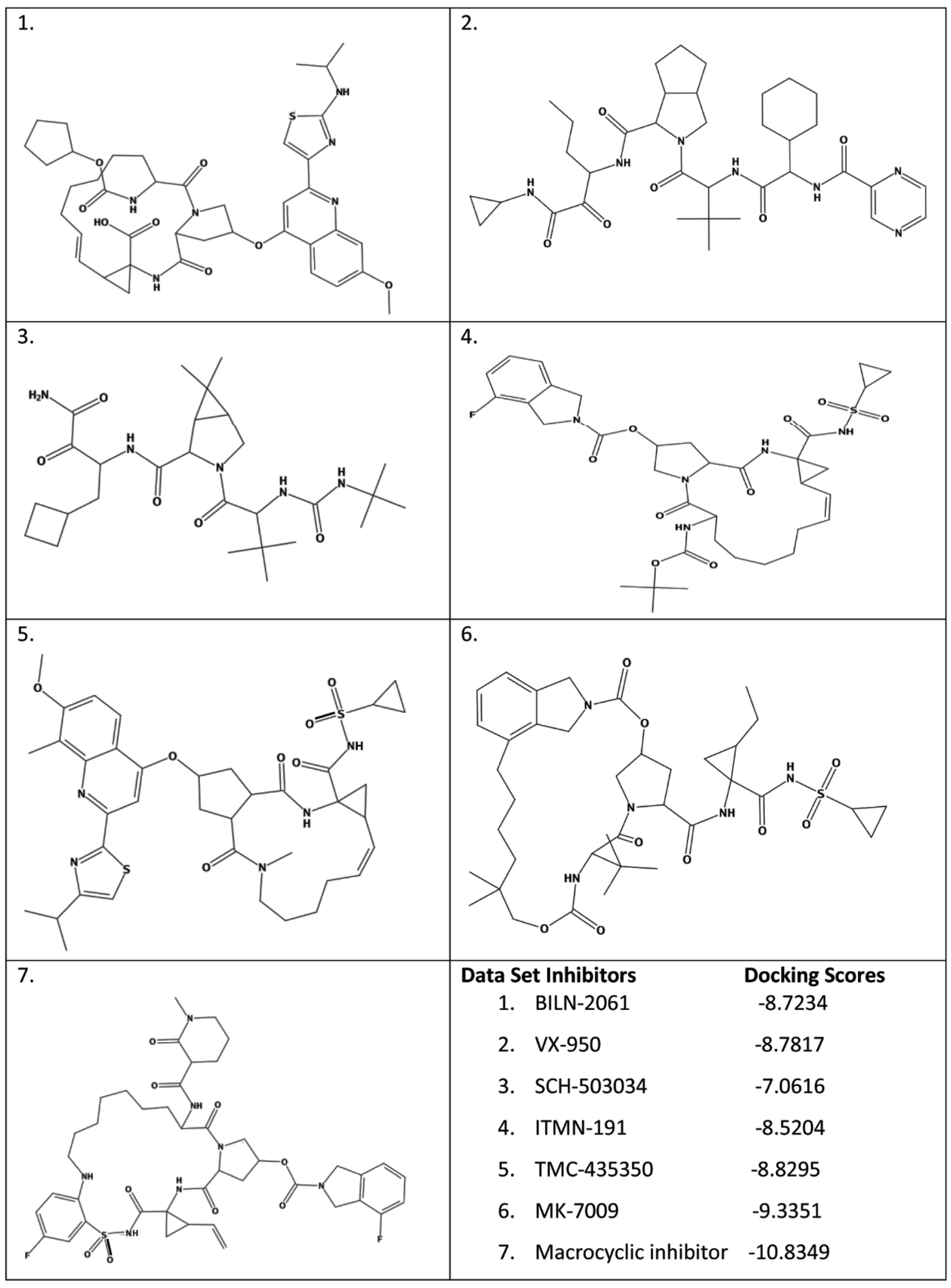
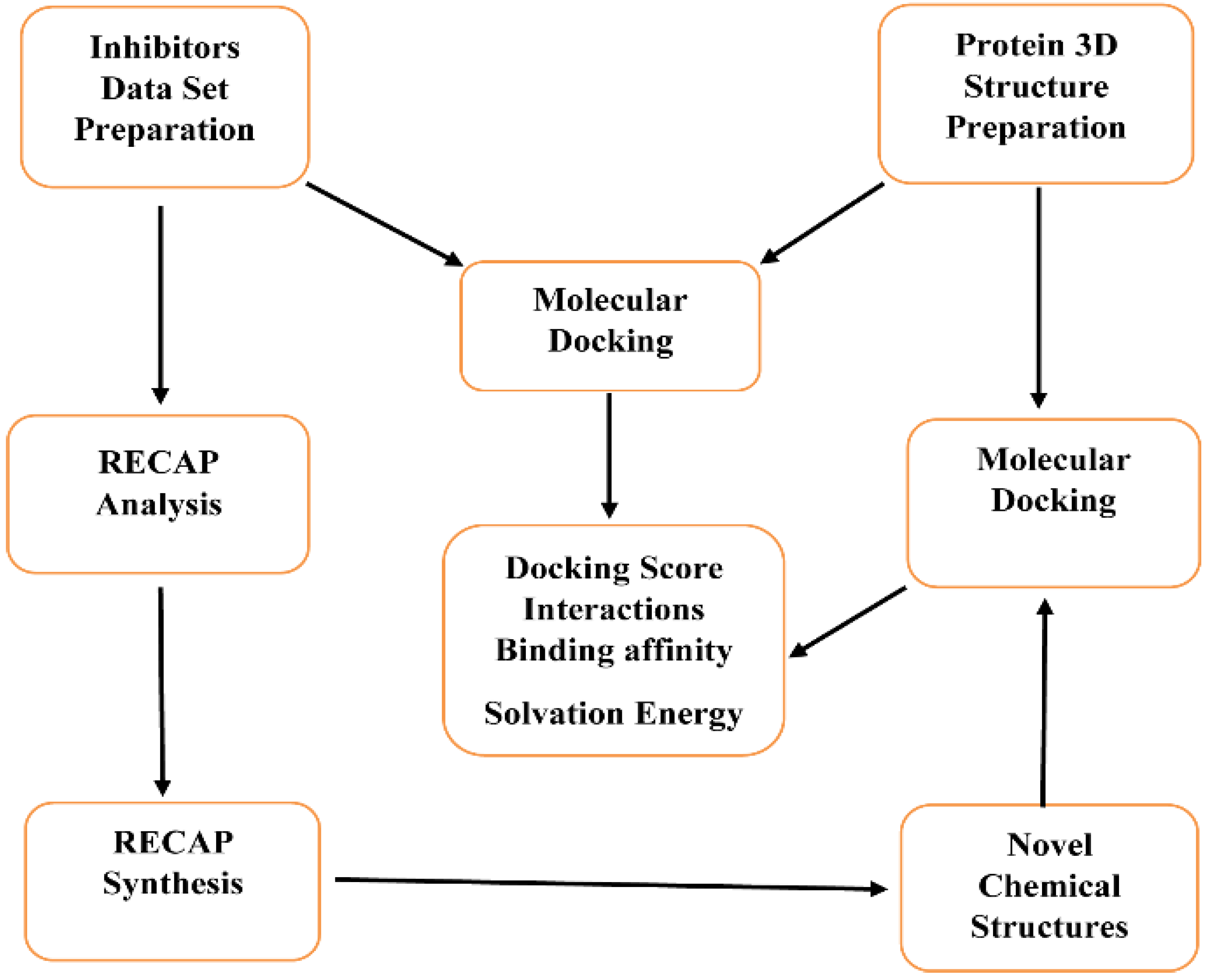
2.1. Molecular Docking on Prepared Data Set Inhibitors
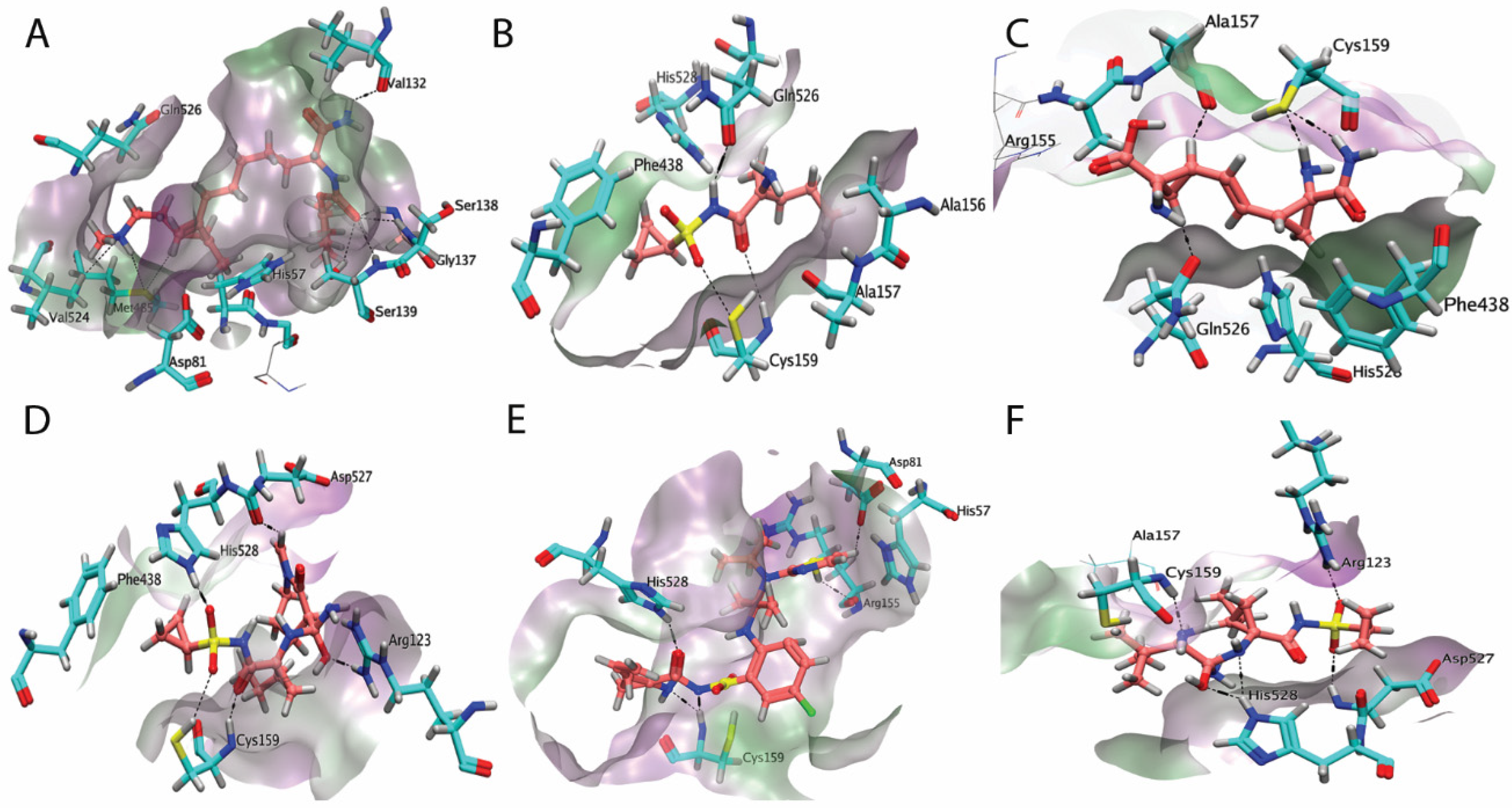
2.2. Analysis of NS3/4A Protease Active Site
2.3. Test of the MOE-Dock Algorithm
2.4. Docking of the Data Set Inhibitors
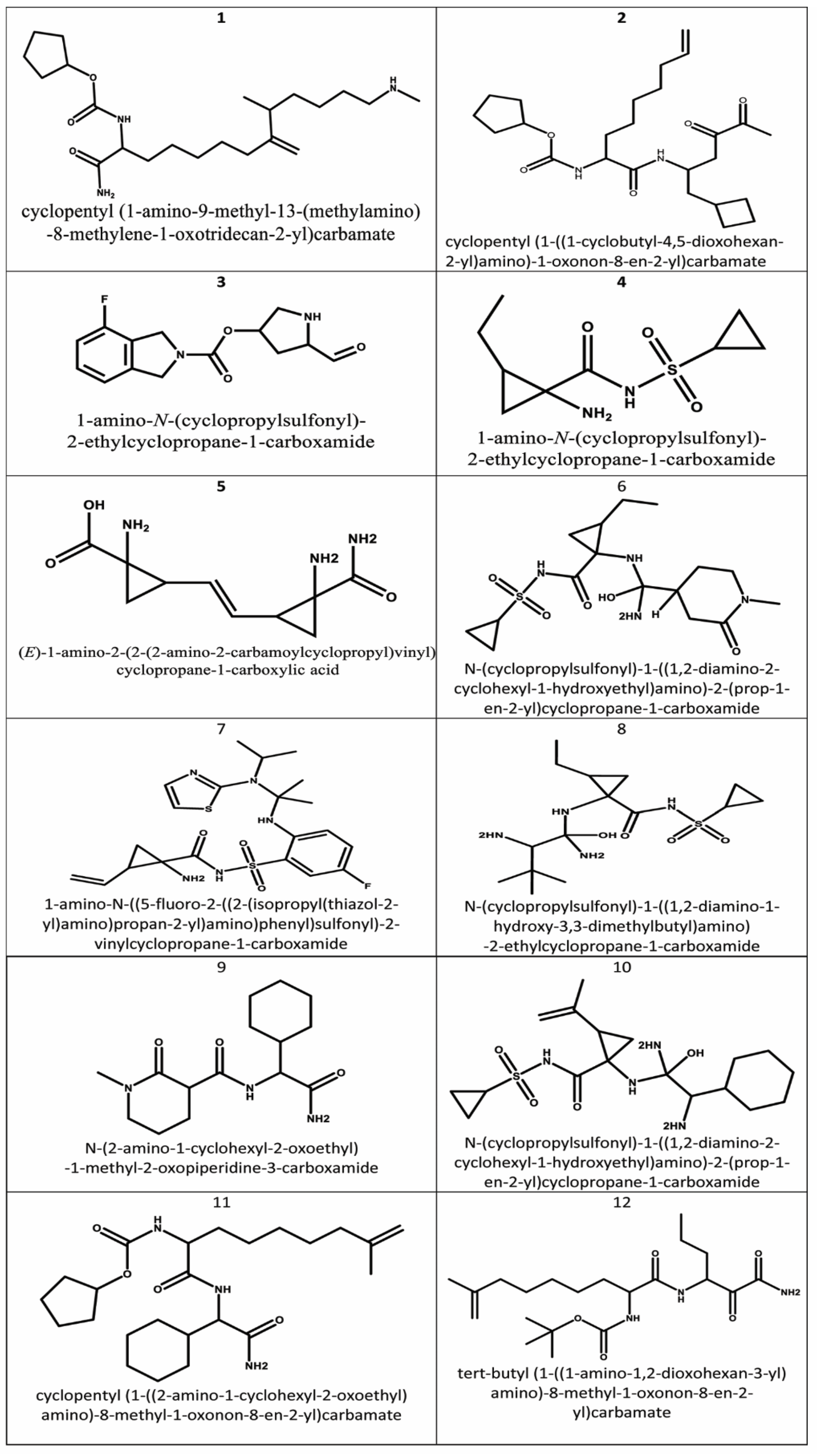
2.5. RECAP Analysis
2.6. RECAP Synthesis and Molecular Docking of the Generated Compounds
2.7. Solvation Energy and Binding Affinity Calculations
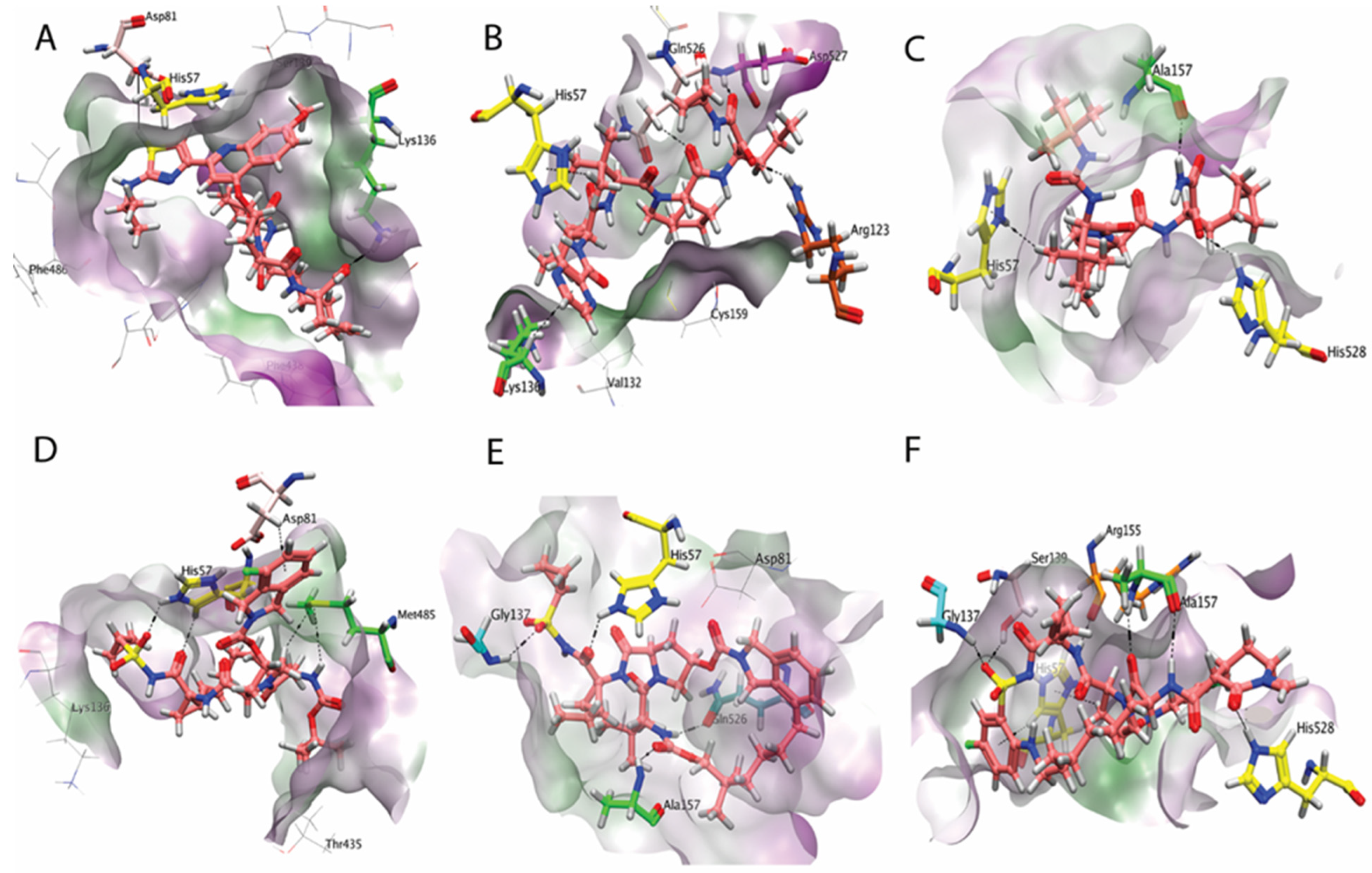
2.8. Binding Interactions of Finally Selected Compounds
3. Materials and Methods
3.1. Inhibitors Data Set Preparation
3.2. Preparation of Protein 3D Structure
3.3. Molecular Docking of Prepared Data Set Inhibitors
3.4. RECAP Analysis
3.5. RECAP Synthesis and Molecular Docking of the Generated Compounds
3.6. Solvation Energy and Binding Affinity Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Schütte, K.; Balbisi, F.; Malfertheiner, P. Prevention of hepatocellular carcinoma. Gastrointest. Tumors 2016, 3, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fattah, M.A.; Al-Marhabi, O.; Matrafi, M.; Babaseel, M.; Asmari, M.; Eletlany, S. Sero-prevalence of Hepatitis B virus infections among blood banking donors in Makkah city, Saudi Arabia: An institutional-based cross-sectional study. J. Umm Al-Qura Univ. Med. Sci. 2020, 6, 2. [Google Scholar]
- Rabban, A.A.; Bakhreba, M.A.; Nasa, M.; Natto, Z.S.; Al-Mutair, A.; Alhumaid, S.; Aljeldah, M.; Garout, M.; Alfouzan, W.A.; Alshahrani, F.S.; et al. Suspected adenovirus causing an emerging hepatitis among children below 10 years: A review. Pathogens 2022, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Houghton, M. The discovery of the hepatitis C virus. In HCV: The Journey from Discovery to a Cure; Springer: Berlin/Heidelberg, Germany, 2019; pp. 19–27. [Google Scholar]
- Ismail, N.S.M.; Hattori, M. Molecular modeling based approach, synthesis and in vitro assay to new indole inhibitors of hepatitis C NS3/4A serine protease. Bioorgan. Med. Chem. 2011, 19, 374–383. [Google Scholar] [CrossRef]
- Hepatitis C General Information—CDC. Available online: http://wwwcdcgov/hepatitis/HCV/PDFs/HepCGeneralFactSheet.pdf (accessed on 16 January 2013).
- Akhtar, S.; Nasir, J.A.; Hinde, A. The prevalence of hepatitis C virus infection in β-thalassemia patients in Pakistan: A systematic review and meta-analysis. BMC Public Health 2020, 20, 587. [Google Scholar] [CrossRef]
- Colvin, H.M.; Mitchell, A.E. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitits B and C; Institute of Medicine Committee on the Prevention and Control of Viral Hepatitis Infections, National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Ghany, M.G.; Strader, D.B.; Thomas, D.L.; Seeff, L.B. Diagnosis management and treatment of hepatitis C: An update. J. Hepatol. 2009, 49, 1335. [Google Scholar] [CrossRef]
- Cacoub, P.; Bourlière, M.; Lübbe, J.; Dupin, N.; Buggisch, P.; Dusheiko, G.; Roujeau, J.C. Dermatological side effects of hepatitis C and its treatment: Patient management in the era of direct-acting antivirals. J. Hepatol. 2012, 56, 455–463. [Google Scholar] [CrossRef]
- Manns, M.P.; McHutchison, J.G.; Gordon, S.C.; Shiffman, M.; Reindollar, R.; Goodman, Z.D.; Koury, K.; Ling, M.; Albrecht, J.K. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet 2001, 358, 958. [Google Scholar] [CrossRef]
- Liu, C.H.; Liu, C.J.; Huang, C.F.; Lin, J.W.; Dai, C.Y.; Liang, C.C.; Kao, J.H. Peginterferon alfa-2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 2 receiving haemodialysis: A randomised trial. Gut 2015, 64, 303. [Google Scholar] [CrossRef]
- Flisiak, R.; Parfieniuk, A. Investigational drugs for hepatitis C. Expert. Opin. Investig. Drugs 2010, 19, 63–75. [Google Scholar] [CrossRef]
- Kwong, A.D.; McNair, L.; Jacobson, I.; George, S. Recent progress in the development of selected hepatitis C virus NS3 4A protease and NS5B polymerase inhibitors. Curr. Opin. Pharmacol. 2008, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Lindenbach, B.D.; Rice, C.M. Flaviviridae: The viruses and their replication. In Field’s Virology; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Lindenbach, B.D.; Rice, C.M. Unraveling hepatitis C virus replication: From genometo function. Nature 2005, 436, 933. [Google Scholar] [CrossRef] [PubMed]
- Assenberg, R.; Mastrangelo, E.; Walter, T.S.; Verma, A.; Milani, M.; Owens, R.J.; Stuart, D.I.; Grimes, J.M.; Mancini, E.J. Crystal structure of a novel conformational state of the flavivirus NS3 protein: Implications for polyprotein processing and viral replication. J. Virol. 2009, 83, 12895–12906. [Google Scholar] [CrossRef]
- Zając, M.; Muszalska, I.; Sobczak, A.; Dadej, A.; Tomczak, S.; Jelińska, A. Hepatitis C—New drugs and treatment prospects. Eur. J. Med. Chem. 2019, 165, 225–249. [Google Scholar] [CrossRef]
- Brass, V.; Berke, J.M.; Montserret, R.; Blum, H.E.; Penin, F.; Moradpour, D. Structural determinants for membrane association and dynamic organization of the Hepatitis C Virus NS3-4A complex. Proc. Natl. Acad. Sci. USA 2008, 105, 14545–14550. [Google Scholar] [CrossRef]
- Schiering, N.; Arcy, A.D.; Villard, F.; Simić, O.; Kamke, M.; Monnet, G.; Hassiepen, U.; Svergun, D.I.; Pulfer, R.; Eder, J.; et al. A macrocyclic HCVNS3/4A protease inhibitor interacts with protease and helicase residues in the complex with its full-length target. Proc. Natl. Acad. Sci. USA 2011, 108, 21052. [Google Scholar] [CrossRef]
- Huang, M.; Phadke, A.; Agarwal, A. Hepatitis C viral proteases and inhibitors. In Proteases in Gastrointestinal Tissue; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Bartenschlager, R.; Ahlborn-Laake, L.; Mous, J.; Jacoben, H. Kinetic and structural analysis of hepatitis C virus polyprotein processing. J. Virol. 1994, 68, 5045. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.X.; Njoroge, F.G. A review of HCV protease inhibitors. Curr. Opin. Investig. Drugs 2009, 10, 821. [Google Scholar]
- Reiser, M.; Timm, J. Serine protease inhibitors as anti-hepatitis C virus agents. Expert Rev. Anti-Infect. Ther. 2009, 7, 537. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, I. Hepatitis C—Pipeline update. Nat. Rev. Drug. Discov. 2011, 10, 93. [Google Scholar] [CrossRef]
- Rong, L.; Dahari, H.; Ribeiro, R.M.; Perelson, A. Rapid emergence of protease inhibitor resistance in hepatitis C virus. Sci. Transl. Med. 2010, 2, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.D.; Lindberg, J.; Lin, T.I.; de Kock, H.; Lenz, O.; Lilja, E.; Felländer, S.; Baraznenok, V.; Nyström, S.; Nilsson, M. Induced-fit binding of the macrocyclic noncovalent inhibitor TMC435 to its HCV NS3/4A protease target. Angew. Chem. Int. Ed. Engl. 2010, 49, 1652. [Google Scholar] [CrossRef]
- Romano, P.; Ali, A.; Royer, W.E.; Schiffer, C.A. Drug resistance against HCV NS3/4A protease inhibitors is defined by the balance of substrate recognition versus inhibitor binding. Proc. Natl. Acad. Sci. USA 2010, 107, 20986. [Google Scholar] [CrossRef] [PubMed]
- Tanji, H.M.; Hirowatari, Y.; Shimotohno, K. Hepatitis C virus polyprotein processin: Kinetic and mutageni analysis of serine proteinase-dependent cleavage. J. Virol. 1994, 68, 8418. [Google Scholar] [CrossRef] [PubMed]
- Beran, R.K.; Serebrov, V.; Pyle, A.M. The serine protease domain of Hepatitis C viral NS3 activates RNA helicase activity by promoting the binding of RNA substrate. J. Biol. Chem. 2007, 282, 34913. [Google Scholar] [CrossRef]
- Beran, R.K.; Pyle, A.M. Hepatitis C viral NS3-4A protease activity is enhanced by the NS3 helicase. J. Biol. Chem. 2008, 283, 29929. [Google Scholar] [CrossRef]
- Love, R.A.; Parge, H.E.; Wickersham, J.A.; Hostomsky, Z.; Habuka, N.; Moomaw, E.W.; Adachi, T.; Hostomska, Z. The crystal structure of hepatitis C virus NS3 proteinase reveals a trypsin-like fold and a structural zinc binding site. Cell 1996, 87, 331–342. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE), version 2016, 08; Chemical Computing Group: Montreal, QC, Canada, 2016; Available online: www.chemcomp.com (accessed on 16 January 2022).
- Satoh, S.; Tanji, Y.; Hiikata, M.; Kimura, K.; Shimotohno, K. The N terminal region of Hepatitis C virus nonstructural protein3 (NS3) is essential for stable complex formation with NS4A. J. Virol. 1995, 69, 4255. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Z.; Wang, M.; Cheng, A. Innate immune evasion mediated by flaviviridae non-structural proteins. Viruses 2017, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, R.A.; Urbani, M.C.; Nardi, M.C.; Tomei, L.; Steinkühler, C.; Tramontano, A. A zinc binding site in viral serine proteinases. Biochemistry 1996, 35, 13282. [Google Scholar] [CrossRef]
- De Francesco, R.A.; Pessi, A.; Steinkühler, C. The Hepatitis C virus NS3 proteinase: Structure and function of a zinc containing serine proteinases. Antivir. Ther. 1988, 3, 99. [Google Scholar] [CrossRef]
- Han, D.S.; Hahm, B.; Rho, H.M.; Jang, S.K. Identification of the protease domain in NS3 of Hepatitis C virus. J. Gen. Virol. 1995, 76, 985. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, N.; Komoda, Y.; Hiikata, M.; Shimotohno, K. Cleavage activity of Hepatitis C virus serine proteinase. J. Biochem. 1997, 122, 749. [Google Scholar] [CrossRef] [PubMed]
- Melagraki, G.; Afantitis, A. Ligand and structure-based virtual screening strategies for hit-finding and optimization of hepatitis C virus (HCV) inhibitors. Curr. Med. Chem. 2011, 18, 2612–2619. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.L.; Iraci, N.; Manfroni, G.; Cecchetti, V. Allosteric inhibition of the hepatitis C virus NS5B polymerase: In silico strategies for drug discovery and development. Future Med. Chem. 2011, 3, 1027–1055. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development setting. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lewell, X.Q.; Judd, D.B.; Watson, S.P.; Hann, M.M. RECAP—Retrosynthetic combinatorial analysis procedure: A powerful new technique for identifying privileged molecular fragments with useful applications in combinatorial chemistry. J. Chem. Inf. Comput. Sci. 1998, 38, 511–522. [Google Scholar] [CrossRef]
- Schneider, G.; Fechner, U. Flux (1): A virtual synthesis scheme for fragment-based de novo design. J. Chem. Inf. Model. 2006, 46, 699–707. [Google Scholar]
- Weininger, D. SMILES 1 introduction and encoding rules. J. Chem. Inf. Comput. 1988, 28, 31–36. [Google Scholar] [CrossRef]
- Labute, P. The generalized born/volume integral (GB/VI) implicit solvent model: Estimation of the free energy of hydration using London dispersion instead of atomic surface area. J. Comput. Chem. 2008, 29, 1693–1698. [Google Scholar] [CrossRef]


| Compounds | Docking Score (KJ/mol) | Binding Affinity (KJ/mol) | Solvation Energy (KJ/mol) | Drug Like Properties |
|---|---|---|---|---|
| 1 | −9.4824 | −8.95 | −58.56 | MW. 393.57 g/mol, TPSA. 93.45 Å2, logP.3.31, logS.−4.10, don.3, acc.3 |
| 2 | −9.4283 | −8.39 | −51–39 | MW. 435.56 g/mol, TPSA. 127.59 Å2, logP. 3.10, logS.−4.52, don.3, acc.4 |
| 3 | −9.3779 | −5.71 | −17.37 | MW. 278.28 g/mol, TPSA. 58.64 Å2, logP.1.74, logS.−2.07, don.1, acc.3 |
| 4 | −8.4715 | −4.21 | −14.95 | MW. 232.30 g/mol, TPSA. 89.26 Å2, logP.−0.28, logS.−1.50, don.2, acc.4 |
| 5 | −9.8573 | −3.92 | −10.33 | MW. 225.25 g/mol, TPSA. 132.43 Å2, logP.−1.45, logS.0.03, don.4, acc.5 |
| 6 | −10.8125 | −4.51 | −13.45 | MW. 387.48 g/mol, TPSA. 141.60 Å2, logP.−1.39, logS.−1.57, don.4, acc.7 |
| 7 | −10.9789 | −6.25 | −19.32 | MW. 481.62 g/mol, TPSA. 105.39 Å2, logP.3.57, logS.−5.57, don.2, acc.6 |
| 8 | −10.7865 | −4.88 | −17.62 | MW. 362.59 g/mol, TPSA. 147.54 Å2, logP.−0.67, logS.−1.80, don.5, acc.7 |
| 9 | −10.5331 | −5.08 | −14.00 | MW. 294.37 g/mol, TPSA. 92.27 Å2, logP.0.19, logS.−2.61, don.2, acc.3 |
| 10 | −10.3632 | −3.92 | −2.56 | MW. 399.54 g/mol, TPSA. 147.54 Å2, logP.−0.30, logS.−2.16, don.5, acc.7 |
| 11 | −10.6646 | −5.39 | −5.37 | MW. 434.60 g/mol, TPSA. 110.52 Å2, logP.3.77, logS.−5.33, don.3, acc.3 |
| 12 | −14.6578 | −7.05 | −50.00 | MW. 410.53 g/mol, TPSA. 127.59 Å2, logP.3.18, logS.−4.38, don.3, acc.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, M.; Rehman, A.U.; Waqas, M.; Khalid, A.; Abdalla, A.N.; Mahmood, A.; Hu, J.; Wadood, A. A Novel Approach to Develop New and Potent Inhibitors for the Simultaneous Inhibition of Protease and Helicase Activities of HCV NS3/4A Protease: A Computational Approach. Molecules 2023, 28, 1300. https://doi.org/10.3390/molecules28031300
Riaz M, Rehman AU, Waqas M, Khalid A, Abdalla AN, Mahmood A, Hu J, Wadood A. A Novel Approach to Develop New and Potent Inhibitors for the Simultaneous Inhibition of Protease and Helicase Activities of HCV NS3/4A Protease: A Computational Approach. Molecules. 2023; 28(3):1300. https://doi.org/10.3390/molecules28031300
Chicago/Turabian StyleRiaz, Muhammad, Ashfaq Ur Rehman, Muhammad Waqas, Asaad Khalid, Ashraf N. Abdalla, Arif Mahmood, Junjian Hu, and Abdul Wadood. 2023. "A Novel Approach to Develop New and Potent Inhibitors for the Simultaneous Inhibition of Protease and Helicase Activities of HCV NS3/4A Protease: A Computational Approach" Molecules 28, no. 3: 1300. https://doi.org/10.3390/molecules28031300
APA StyleRiaz, M., Rehman, A. U., Waqas, M., Khalid, A., Abdalla, A. N., Mahmood, A., Hu, J., & Wadood, A. (2023). A Novel Approach to Develop New and Potent Inhibitors for the Simultaneous Inhibition of Protease and Helicase Activities of HCV NS3/4A Protease: A Computational Approach. Molecules, 28(3), 1300. https://doi.org/10.3390/molecules28031300








