Abstract
Diabetes mellitus is a group of metabolic disorders characterized by hyperglycemia caused by resistance to insulin action, inadequate insulin secretion, or excessive glucagon production. Numerous studies have linked diabetes mellitus and oxidative stress. People with diabetes usually exhibit high oxidative stress due to persistent and chronic hyperglycemia, which impairs the activity of the antioxidant defense system and promotes the formation of free radicals. Recently, several studies have focused on exploring natural antioxidants to improve diabetes mellitus. Fibraurea tinctoria has long been known as the native Borneo used in traditional medicine to treat diabetes. Taxonomically, this plant is part of the Menispermaceae family, widely known for producing various alkaloids. Among them are protoberberine alkaloids such as berberine. Berberine is an isoquinoline alkaloid with many pharmacological activities. Berberine is receiving considerable interest because of its antidiabetic and antioxidant activities, which are based on many biochemical pathways. Therefore, this review explores the pharmacological effects of Fibraurea tinctoria and its active constituent, berberine, against oxidative stress and diabetes, emphasizing its mechanistic aspects. This review also summarizes the pharmacokinetics and toxicity of berberine and in silico studies of berberine in several diseases and its protein targets.
1. Introduction
Diabetes mellitus is a series of physiological dysfunctions characterized by hyperglycemia associated with abnormalities in carbohydrate, fat, and protein metabolism caused by resistance to insulin action, inadequate insulin secretion, or excessive glucagon production [1]. According to the Diabetes Atlas 2021, 537 million people are currently living with diabetes mellitus, which is expected to increase to 643 million by 2030. By 2045, this number will increase by 783 million, or nearly 46%. In addition, 541 million people are estimated to have impaired glucose tolerance by 2021, and more than 6.7 million people between the ages of 20 and 79 will die from diabetes-related causes [2].
Numerous studies have linked diabetes mellitus and oxidative stress [3,4]. Reactive oxygen species (ROS) are produced in cells in excess compared to the capacity of enzymatic and non-enzymatic antioxidants. This imbalance leads to oxidative stress, which damages membranes and essential macromolecules, including DNA, proteins, and lipids [3,5]. In diabetes mellitus, excess free radicals are formed through glucose oxidation and non-enzymatic protein glycation. Because of the cellular damage caused by the breakdown of the antioxidant defense system, diabetes-related problems such as nephropathy, retinopathy, and neuropathy can start to appear [6].
Currently, diabetes mellitus is considered a major global problem, and a truly successful treatment has yet to be found. Despite many current treatment options, hyperglycemia is often poorly controlled [7]. Agarwal et al. reported that the efficacy of antidiabetic drugs in achieving optimal glycemic control was only 41% of total patients [8]. Another study showed that although the use of antihyperglycemic drugs for treating diabetes mellitus has undergone many significant changes and developments, the number of patients who achieved the target HbA1c <7% decreased [9]. Data from the National Health and Nutrition Examination Survey (NHANES) showed that only 57% of people with diabetes achieved the target HbA1c <7% in 2003–2006, which fell to 52.5% in 2007–2010 [10] and fell again to 47.7% in 2011–2014 [11]. In addition, antidiabetic drugs still have limitations, such as disadvantages in the form of side effects and failure to change the course of diabetes complications [12]. For example, metformin, the most commonly prescribed biguanide oral hypoglycemic drug, causes gastrointestinal disturbances such as abdominal discomfort, diarrhea, anorexia, nausea, a metallic taste, and vitamin B12 deficiency [13,14]. Metformin is also linked to lactic acidosis, although the incidence is low [15,16,17].
Natural products are becoming more widely accepted and used as alternative therapies. Several studies have shown the increased use of natural products in patients with diabetes mellitus. This increase is due to the numerous side effects and high costs associated with antidiabetic drugs [18,19]. As targets for combination therapy, natural products have also been discovered to interact synergistically with antidiabetic drugs [20]. In addition, the combination with antidiabetic drugs allows lower drug doses and/or reduces the frequency of administration, thereby reducing side effects and increasing efficacy. Studies on natural products are still being conducted to evaluate their potential as antidiabetic agents and to identify the main structures that can be developed as new antidiabetic drugs [21].
There is a long history of using natural products and their development as antidiabetic drugs. More than 1200 medicinal plants have been tested for their antidiabetic potential, and 200 bioactive substances have been reported to have potent hypoglycemic effects [22]. Many of the currently used drugs are structurally derived from natural compounds. Some compounds with antidiabetic activity are galegine, phlorizine, and ruboxistaurine, obtained from plants; exenatide and trodusquemine, obtained from animals; and acarbose, miglitol, and voglibose, derived from microbes [23,24].
Fibraurea tinctoria is a yellow climbing plant known in Borneo as Akar Kuning (yellow root). This plant has long been known as the native Borneo in traditional medicine. The traditional medicinal uses of this plant include its leaves, roots, stems, and bark [25]. Ethnic groups in Kalimantan have traditionally used yellow root to treat malaria, jaundice, and diabetes [26]. In addition to Fibraurea tinctoria, there are two other plants, Arcangelisia flava (L) Merr and Coscinium fenestratum Colebr, also known as yellow root [27,28]. Taxonomically, these three plants are part of the Menispermaceae family [29]. The Menispermaceae family is widely known for producing a wide variety of alkaloids. Approximately 22 types of alkaloids have been identified in the Menispermacaceae family. Among them are protoberberine alkaloids, such as berberine [30].
Berberine is an isoquinoline alkaloid that has been used for diabetes treatment in traditional Chinese, Indian, and Middle-Eastern folk medicine for more than 400 years. Berberine is widely present in many traditional medicinal plants [31]. Berberine is an important natural alkaloid and is of great interest because of its wide and interesting spectrum of pharmacological activity. Berberine exhibited strong antihyperglycemic activity and positive effects in treating diabetic nephropathy, diabetic neuropathy, and diabetic cardiomyopathy [32]. In addition, several studies showed that berberine has antioxidant activity, which partially contributes to its efficacy against diabetes mellitus [33]. These observations make berberine a potentially promising drug for managing diabetes mellitus.
On this basis, this review focuses on the pharmacological activity of the Fibraurea tinctoria plant and its component, berberine, as an antidiabetic and antioxidant. This review will also discuss the pharmacokinetics and toxicity of berberine and in silico studies of berberine in several diseases and its protein targets.
2. Fibraurea tinctoria
Fibraurea tinctoria is a plant that produces color and is widely distributed in China, Indonesia, Malaysia, Thailand, Laos, Cambodia, and Vietnam [34]. This plant has a long history of use as a medicine in most countries in Asia [35]. In addition to treating dysentery, malaria, and gonorrhea, this plant is also used as an analgesic, antipyretic, antidote, and diuretic [25,34].
Su et al. isolated 45 compounds, including protoberberine alkaloids and furanoditerpenoids, from Fibraurea tinctoria stems. The furanoditerpenoid compounds isolated from this plant included epi-8-hydroxycolumbin (1), fibrauretin B (2), fibrauretin C (3), fibrauretin D (4), fibrauretin E (5), fibrauretin F (6), epi-12-palmatoside G (7), floribundic ester (8), fibraurin (9), fibraurinoside (10), fibleucin (11), fibleucinoside (12), chasmanthin (13), fibrauretin A (14), fibrauretinoside A (15), and epifibrauretinoside A (16). Other compounds that have been identified are palmatine (17), jatrorrhizine (18), columbamine (19), stepharanine (20), 8-trichloromethyl-7,8-dihydropalmatine (21), a mixture of N-(p-trans-coumaroyl)-tyramine (22) and N-(p-cis-coumaroyl)-tyramine (23), N-trans-feruloyl tyramine (24), N-cis-feruloyl tyramine (25), corydaldine (26), thalifoline (27), p-hydroxybenzaldehyde (28), vanillin (29), syrigaldehyde (30), methyl vanillate (31), methyl syringate (32), octadecyl (E)-ferulate (33), vanillic acid (34), syringic acid (35), ferulic acid (36), caffeic acid (37), fibraurecdyside A (38), makisterone A (39), a mixture of β-sitosterol (40) and sigmasterol (41), β-sitosterol 3-O-β-D-glucopyranoside (42), physcion (43), dehydrogormouregine (44), and thomasidioic acid dimethyl ester (45) [34].
Several studies have investigated the pharmacological activities of Fibraurea tinctoria. Nguyen-pouplin et al. conducted antimalarial and cytotoxicity tests on several plants grown in South Vietnam. Fibraurea tinctoria had the highest activity among the 38 plants examined for antimalarial activity, with IC50 values ranging from 0.40 to 1.10 μg/mL [27]. A study by Galappathie et al. on the antimicrobial and antifungal activities of several plants growing on the island of Borneo showed that the leaves and stems of Fibraurea tinctoria extracted using dichloromethane and methanol had activity against Bacillus cereus and Staphylococcus aureus with inhibition zones of 10 mm and 16 mm, respectively, and had a Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) values ranging between 25 and 100 μg/mL [25].
In other studies, floribundic ester and fibraurin from Fibraurea tinctoria showed significant anti-inflammatory activity when administered at 100 mg/kg to reduce carrageenan mouse paw edema. In addition, five components from Fibraurea tinctoria, namely, Epi-12-palmatoside G, fibraurin, fibraurinoside, fibrauretin A, and epifibrauretinoside A, inhibited NO production from macrophages at 1–4 μg/mL [34]. It was also found that the methanol extract of Fibraurea tinctoria inhibited cytochrome P450 3A4 with an IC50 of 5.10 μg/mL [36].
Keawpradub et al. measured the antioxidant activity, acute toxicity, and cytotoxicity of Fibraurea tinctoria grown in Thailand. Antioxidant testing using the DPPH method (1,1-diphenyl-2-picrylhydrazil) showed that the chloroform and methanol extracts of Fibraurea tinctoria exhibited potent antioxidant activity with IC50 values of 78.80 and 83.60 μg/mL. In contrast, petroleum ether and water extracts showed weak antioxidant activity with IC50 > 100 μg/mL. The chloroform extracts of Fibraurea tinctoria showed cytotoxic activity against brine shrimp and the human cancer cell line MCF-7 with LC50 and IC50 values of 252.70 μg/mL and 11.20 μg/mL, respectively. In contrast, petroleum ether, methanol, and water extracts had LC50 values of >1000 μg/mL and IC50 values of >50 μg/mL [37]. Fibraurea tinctoria also showed moderate antiproliferative activity against human cervical cancer (HeLa), oral cancer (KB), and human hepatoma cancer (HepG2), with GI50 values of 39.61 μg/mL, 31.50 μg/mL, and 89.11 μg/mL, respectively [38,39].
3. Berberine
Berberine (18, 5,6-dihydro-9,10-dimethoxybenzo(g)-1,3-benzodioxolo (5,6-a) quino-lizinium) is a benzyl tetra isoquinoline alkaloid that naturally occurs in the plant families Ranunculaceae, Rutaceae, Berberidaceae, Annonaceae, Papaveraceae, and Menispermaceae [30,40,41] and has been widely used in traditional medicine for conditions such as dysentery [42], diarrhea [43,44,45,46,47,48], stomatitis [49], and hepatitis [50,51].
Several studies reported that berberine has many pharmacological activities, including antihyperlipidemic, antidiabetic, antiobesity, anticancer, anti-inflammatory, antioxidant, neuroprotective, cardioprotective, immunomodulatory, and other effects [52,53,54,55]. Recently, many studies have been conducted to examine the role of berberine in COVID-19 [56,57,58,59] (Figure 1).
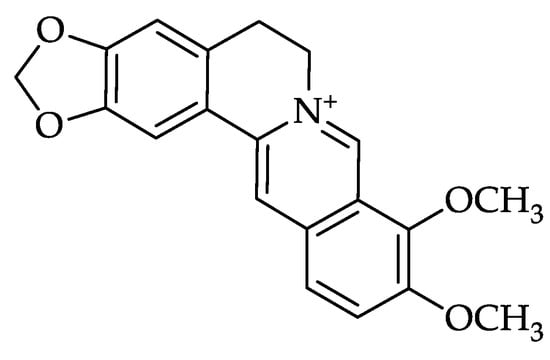
Figure 1.
Molecular structure of berberine.
3.1. Pharmacokinetics of Berberine
Clinically, berberine is administered orally. However, pharmacokinetic studies have shown that berberine has low bioavailability (<5%) [60]. Berberine has an extremely low absolute bioavailability, possibly because of its nature as a quaternary ammonium base. The quaternary ammonium group in its structure exhibits strong hydrophilicity and poor permeability through the cell membrane. Low drug bioavailability results from the restriction of transmembrane transport and intestinal absorption [61].
In addition to being caused by its structure, the low bioavailability of berberine after oral administration is caused by its low permeability, efflux mediated by P-glycoprotein (P-gp-), hepatobiliary excretion, and self-aggregation. The low bioavailability of berberine is also associated with its poor absorption and rapid metabolism in the body (liver and intestine) [62]. As a result of this metabolism, the body produces berberine metabolites known to contribute significantly to the pharmacological effects of berberine [40]. Berberine is also known to have a pKa value = 15, which causes berberine to be in an ionized form in the physiologic condition, so the amount of berberine that can be absorbed after oral administration is low [63].
After oral administration, only 0.50% of the berberine dose can enter the blood circulation, causing low concentrations of berberine in the plasma. Approximately 56% of orally administered berberine is not absorbed owing to P-glycoprotein-mediated efflux and self-aggregation, and 43.50% is metabolized by enterocytes [62]. Liu et al. reported that 99.50% of the oral dose of berberine disappeared during first-pass metabolism in the gastrointestinal tract [64].
The interference of P-glycoprotein with berberine absorption is one of the reasons for its low bioavailability. P-glycoprotein is a vital transporter protein found in epithelial cell membranes that functions as an efflux pump that actively removes berberine from luminal mucosal cells. Therefore, one of the strategies used to increase the bioavailability of berberine is the concurrent administration of berberine with a P-glycoprotein inhibitor. These inhibitors can reduce the efflux of berberine and increase its absorption in the intestine, thereby affecting its pharmacokinetic profile [65]. Some examples of P-glycoprotein inhibitors include tetrandrine [66], D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) [67], glycine (GLY) [68], cyclosporine A (CsA) [69], and oligomeric proanthocyanidins (OPCs) [70].
The gut microbiota plays a vital role in the absorption process. Nitroreductase (NR) from the gut microbiota can convert berberine into a more easily absorbed form, dihydroberberine. Dihydroberberine is absorbed in the intestine 5–10 times more effectively than berberine [71]. However, dihydroberberine is unstable and can be quickly converted back into berberine in the intestinal wall through non-enzymatic oxidation processes and then enters the bloodstream. Therefore, nitroreductase (NR) from the gut microbiota is vital for regulating berberine blood levels [72].
After entering the blood, berberine is distributed mainly to tissues, causing low levels of berberine in the plasma. Berberine is widely distributed in the liver, kidneys, muscles, lungs, brain, heart, pancreas, and fat tissues. Berberine is predominantly found in the liver [73]. The pharmacokinetic profile showed that four hours after administration, the tissue concentration of berberine was 70 times higher than the plasma concentration. In addition, berberine is known to be relatively stable in tissues (e.g., liver, muscle, brain, heart, and pancreas) [64].
Besides absorption and distribution factors, metabolic factors also contribute to the low levels of berberine in the plasma. After oral administration, berberine undergoes several metabolic pathways, resulting in extremely low plasma berberine levels. The major pathways of berberine metabolism are demethylation, demethylenation, reduction, hydroxylation (phase I metabolism), and subsequent glucuronidation, sulfation, and methylation (phase II metabolism) [74,75,76]. Berberine is metabolized mainly by the liver and intestinal flora, and this metabolic process causes the content and structure of berberine to change, which in turn affects its pharmacological activity [77].
Several studies have been conducted on the metabolic processes of berberine and its metabolites. Pan et al. found three metabolites in human urine samples after the administration of berberine chloride, identified as jatrorrhizine 3-sulfate, demethyleneberberine 2-sulfate, and thalifendine 10-sulfate, where the main metabolite is demethyleneberberine 2-sulfate [78]. Tsai et al. reported that berberine is metabolized in the rat liver by phase I (demethylation) and phase II (glucuronidation), which was concluded based on ion peaks at m/z 322 and 498 in bile samples determined using liquid chromatography/mass spectrometry (LC/MS) [78].
In another study in rats, berberine was metabolized in the liver by cytochrome P450 enzymes, including phase I (oxidative demethylation), followed by phase II (glucuronidation). In phase I metabolism, several metabolites, such as berberrubine, thalifendine, demethyleneberberine, and jatrorrhizine, were found, and their respective glucuronide conjugates were found in most tissues, where berberrubine was the main metabolite in plasma [63,74,79].
Research conducted by Xu et al. found 16 berberine metabolites, namely, 10 metabolites resulting from phase I and 6 metabolites from phase II metabolism. These metabolites have been identified in the plasma, feces, bile, and other tissues [80]. Among these metabolites, berberrubine, thalifendine, demethyleneberberine, and jatrorrhizine have relatively high plasma levels [65]. Similar findings were also reported by Wang et al., who identified more than twenty berberine metabolites in humans and mice [40] (Table 1).

Table 1.
Berberine and its metabolites.
Wang et al. identified berberine metabolites in plasma, urine, bile, and feces after the administration of 100 mg/kg/day berberine for three days in rats using ultra-high-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UHPLC/Q-TOF-MS). This study identified 97 metabolites, including 68 in urine, 45 in plasma, 44 in bile, and 41 in feces, where demethylation, demethylenation, reduction, hydroxylation, and subsequent glucuronidation, sulfation, and methylation are the major metabolic pathways of berberine in vivo [76]. In plasma, the phase I metabolites found were berberrubine, demethyleneberberine, and jatrorrhizine, where berberrubine was found at higher levels than demethyleneberberine and jatrorrhizine, whereas jatrorrhizine-3-O-β-D-glucuronide, jatrorrhizine-3-O-sulfate, thalifendine-10-O-β-D-glucuronide, berberrubine-9-O-β-D-glucuronide, demethyleneberberine-2-O-sulfate, and demethyleneberberine-2-O-β-D-glucuronide were phase II metabolites found at high levels in plasma [81].
Berberine is excreted via the hepatobiliary system and kidneys [72]. However, most berberine is excreted through the kidneys (urine) and feces. In some animals, berberine is excreted in the bile, and this excretion pathway occurs very slowly due to hepatoenteric circulation. Studies have shown that in some research subjects, both in test animals and in humans, berberine administered through different routes, such as oral or intravenous administration, will cause different excretion processes [77]. Ma et al. reported that the excretion process of berberine after the oral administration of 200 mg/kg in rats was mostly through feces (22.70%), followed by excretion in lesser amounts through urine (0.09%) and bile (9.20 × 10−6%) in the form of berberine (19.07%) and its metabolites (3.76%) [82]. A similar study by Feng et al. showed that berberine is excreted mainly through feces after oral administration in rats. Berberine is difficult to absorb in the intestine because it is in an ionized form under physiological conditions, so it will be excreted through the feces as its parent component (unabsorbed berberine). Berberine is excreted through feces in the form of berberrubine (18.60%) and bile in the form of berberrubine (2.62%) and berberrubine-9-O-β-D-glucuronide (0.36%). A large amount of excreted berberrubine indicates that berberine undergoes a metabolic process to become berberrubine with the help of metabolizing enzymes in the intestinal wall or intestinal microbes [81]. Studies in humans showed that after berberine administration (0.20 g/day), only approximately 0.01% of berberine is eliminated directly through the urine [83].
The three studies above showed that most berberine is excreted through feces, followed by urine and bile in smaller amounts (feces > urine > bile). However, research conducted by Guo et al. in mice administered berberine at 10 mg/kg intraperitoneally (i.p.) showed that berberine is found at high levels in urine, where the amount of berberine excreted in urine is approximately two times higher than that in feces. In addition, 11 berberine metabolites were found, including 5 in feces and 6 in urine [79] (Figure 2).
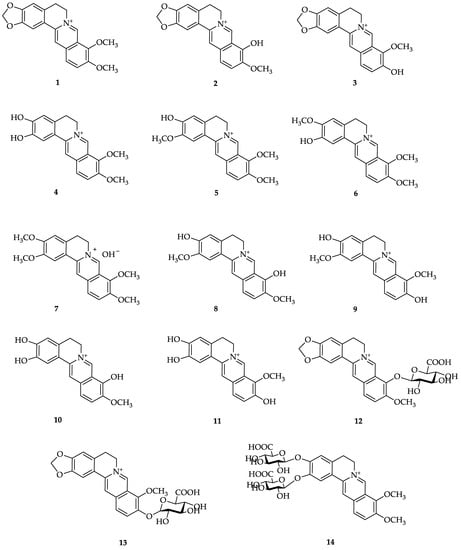
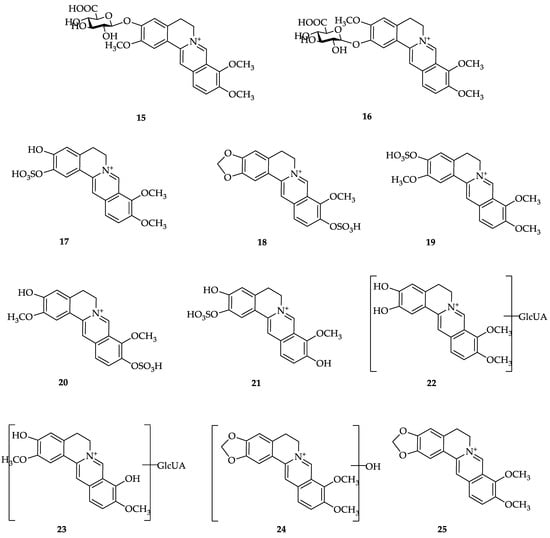
Figure 2.
Structures of berberine and its metabolites. (1) Berberine; (2) berberrubine; (3) thalifendine; (4) demethyleneberberine; (5) jathorrhizine; (6) columbamine; (7) palmatine; (8) 3,9-demethylpalmatine; (9) 3,10-demethylpalmatine; (10) 2,3,9-trihydroxiberberine; (11) 2,3,10-trihydroxiberberine; (12) berberrubine-9-O-β-D-glucuronide; (13) thalifendine-10-O-β-D-glucuronide; (14) demethyleneberberine-2,3-di-O-β-D-glucuronide; (15) jatrorrhizine-3-O-β-D-glucuronide; (16) columbamine-3-O-β-D-glucuronide; (17) demethyleneberberine-2-O-sulfate; (18) thalifendine-10-O-sulfate; (19) jatrorrhizine-3-O-sulfate; (20) 3,10-demethylpalmatine-10-O-sulfate; (21) 2,3,10-trihydroxyberberine-2-O-sulfate; (22) glucuronide of demethyleneberberine; (23) glucuronide of 3,9-demethylpalmatine; (24) hydroxylated berberine; (25) dihydroberberine [40].
3.2. Berberine Toxicity
Kheir et al. conducted a study on the LD50 value of berberine administered in mice using three different routes of administration: intravenous injection (i. v.), intraperitoneal injection (i. p.), and oral administration via intragastric injection (i. g.). Berberine is known to have an LD50 value of 9.04 ± 0.80 mg/kg when administered by intravenous injection and 57.61 ± 23.32 mg/kg by intraperitoneal injection. The safe dose of berberine in mice when administered orally was 20.80 g/kg body weight. It is known that mice have a metabolic rate approximately seven times higher than that of humans, so the safe dose of berberine in humans is 1/7 of 20.81 g/kg, which is 2.97 g/kg body weight. The highest bioavailability of berberine was by intravenous injection, followed by intraperitoneal and intragastric injection (i.v > i.p > i.g). Bioavailability is known to affect LD50 values. Variations in the concentration of berberine in the blood cause different toxicity levels; the greater the bioavailability of berberine, the lower the LD50 value (more toxic) [84].
Another study found that in mice, the LD50 values of berberine upon intraperitoneal and oral administration were 23 and 329 mg/kg, respectively [85]. However, Anis et al. found that the LD50 value of berberine administered via the intraperitoneal injection was 50 mg/kg body weight [86].
Research by Lan et al. on the effects and safety of berberine in patients with type 2 diabetes mellitus, hyperlipidemia, and hypertension showed that the incidence of toxic side effects is strongly correlated with the dose of berberine administered. The higher the dose of berberine, the higher the risk of toxic side effects. A meta-analysis of 27 studies revealed that berberine could cause a few side effects; however, the frequency of these side effects is low, and major side effects during therapy do not harm the body’s vital organs. Berberine can be utilized as an alternative therapy for patients with poor socioeconomic status because it is generally safe for treating diabetes, hyperlipidemia, and hypertension [87].
Kidneys are the primary excretory organs and play an important role in the body. Kidneys mediate the toxicity of various drugs, environmental pollutants, and natural substances that enter the body. Disturbances in kidney function can affect the toxicity of a compound and cause changes in biochemical parameters related to kidney function. Several drugs, environmental pollutants, and natural substances can cause kidney damage, commonly referred to as nephrotoxicity [88,89]. Several studies on the effect of berberine on the kidneys have been conducted. The results showed that berberine had a nephroprotective effect against kidney damage induced by several compounds, including cisplatin, lead, methotrexate, doxorubicin, diclofenac, and gentamicin. The nephroprotective effect of berberine is mediated through many mechanisms, including the inhibition of oxidative/nitrosative stress, inflammation, autophagy, apoptosis, mitochondrial dysfunction, and fibrosis [90,91,92,93,94,95,96,97]. Berberine inhibits oxidative stress, inflammation, apoptosis, and autophagy in the kidney by activating Nrf2/HO-1 and inhibiting the expression of JNK/p38MAPKs/PARP/Beclin-1 [98].
Berberine protects the liver and kidneys from toxicity induced by ferrous sulfate by decreasing lipid peroxidation by scavenging free radicals and by chelating iron, thereby reducing the concentration of iron, which acts as a catalyst for lipid peroxidation [99]. Berberine is also known to protect against mercury-induced liver and kidney damage by increasing the expression of Bcl-2 protein in the liver and kidneys [100].
The most common mRNA modification is N6-methyladenosine (m6A). m6A participates in various physiological and pathological processes, such as metabolism, inflammation, and apoptosis. m6A plays an important role in cisplatin-induced acute renal failure, and berberine can alleviate this process [101]. In addition, berberine relieves cisplatin-induced acute renal failure by regulating mitophagy via the PINK 1/Parkin pathway in renal tubular epithelial cells (RTECs) [102].
Renal tubular epithelial-to-mesenchymal transition (EMT) and renal tubular interstitial fibrosis are the main pathological changes in diabetic nephropathy that cause kidney disease in the final stage. Berberine exerts a therapeutic effect on diabetic nephropathy by inhibiting EMT in the renal tubules and interstitial fibrosis in the kidney. The berberine-mediated inhibition of EMT occurs via the regulation of the Notch/Snail pathway [103]. Berberine can potentially prevent or treat tubulointerstitial fibrosis in diabetic nephropathy by activating the Nrf2 pathway and inhibiting TGF-β/Smad/EMT signaling [104].
Pentoxifylline is a xanthine-derived drug used to treat peripheral arterial disease and nephropathy in patients with diabetes. Al-Kuraishy et al. found that the combined administration of berberine and pentoxifylline provided a significantly more renoprotective effect than either berberine or pentoxifylline alone in diclofenac-induced acute renal failure [105].
Berberine has been reported to reduce the levels of uric acid (UA), blood urea nitrogen (BUN), creatinine (CRE), and pro-inflammatory cytokines (IL-1β and IL-18) in both the serum and kidneys. High uric acid levels are known to cause severe inflammatory effects that can progress to kidney organ dysfunction and even cause kidney damage. Berberine is known to exert nephroprotective effects by inhibiting the expression of URAT1 and suppressing the activation of the NLRP3 inflammasome, which can prevent inflammation in the kidney [106]. Another study showed that berberine has a protective effect against renal fibrosis caused by diabetic nephropathy by decreasing the expression of transforming growth factor (TGF-β) and α-smooth muscle actin (α-SMA) in the kidneys. Therefore, berberine can be used to treat diabetic nephropathy [107].
Several studies on berberine in plants and its effects on the kidneys have also been conducted. Studies have shown that Coptis chinensis has hepatoprotective and nephroprotective effects against cinnabar (HgS)-induced kidney damage [108]. Another study reported that Berberis baluchistanica (Berberidaceae) has nephroprotective effects against gentamicin-induced kidney damage [109], and Berberis vulgaris L (Berberidaceae) has nephroprotective effects against lead acetate-induced kidney damage through its antioxidant and metal-chelating abilities [110].
3.3. In Silico Studies of Berberine
Finding and developing drugs is important because it takes a long time, and it is costly to produce a potential new drug. The new drug discovery process begins with identifying target proteins, validating target proteins, identifying lead compounds, and optimizing lead compounds. In addition, before a new drug is released to the market for sale, it must first go through pre-clinical and clinical trials to obtain a distribution permit. Identifying the target protein takes the longest of these stages [111].
The search for target proteins and lead compounds is an important stage in the early research involving drug search processes. The search for target proteins aims to identify and validate suitable target proteins for disease therapies. Target proteins include receptors, enzymes, transporters, ion channels, transcription factors, and other therapeutic targets [112]. Conversely, the search for lead compounds aims to identify new molecules and compounds that act on target proteins. The target protein validation stage usually requires in vitro and in vivo tests using animal models, which can proceed to human testing after passing these two tests [113].
In silico is a term that means performed on a computer or through computer simulation. In silico drug design is a form of computer-based modeling that applies technology to the drug discovery process [111]. In silico methods have been widely developed and used extensively in pharmacology to search for new drugs because they significantly contribute to initial drug research, especially in identifying target proteins and lead compounds [112]. This method can predict the affinity between drug molecules and target proteins, explaining a drug molecule’s absorption, distribution, metabolism, excretion, and toxicity (ADMET) and its physicochemical characteristics [114].
The in silico method can reduce the number of test animals used in pharmacological tests. In addition, this method assists in the rational and safe design of new drug candidates and supports chemists and pharmacologists during the drug search process. It would be impossible to test millions of molecules in a lab against a given target, but the computerized screening process helps researchers choose a small set of molecules that can then be tested in a wet lab. Therefore, the in silico approach provides a great opportunity to identify target proteins quickly and accurately, thereby saving time and money in discovering new drugs [115] (Table 2).

Table 2.
In silico studies of berberine in several diseases and the protein targets.
4. Antioxidant Activity of Berberine
Oxidative stress in experimental diabetic animal models was first observed in 1982. Studies have shown that oxidative stress plays an important role in all cases of diabetes mellitus, especially type 2 diabetes mellitus and the pathogenesis of diabetes complications [169]. Oxidative stress can occur because of glucose and lipid metabolism abnormalities, leading to hyperglycemia and dyslipidemia. Hyperglycemic conditions are known to worsen and exacerbate the formation of ROS and constitute a significant factor in oxidative stress and glucotoxicity in pancreatic beta cells [170].
Diabetes causes severe metabolic imbalances and non-physiological changes in many tissues, especially in the pancreas. Markers of oxidative stress are elevated in the pancreatic islets of Langerhans in diabetic rats, and oxidative stress is related to impaired insulin secretion and insulin action [3,171]. Another study showed that ROS are strongly associated with the incidence of insulin resistance, resulting in pancreatic beta-cell damage and apoptosis in type 2 diabetes mellitus [31]. ROS are also known to increase the expression of tumor necrosis factor-α (TNF-α), which causes oxidative stress. Increased TNF secretion is associated with insulin resistance caused by obesity, a risk factor for type 2 diabetes mellitus [4].
The increased number of ROS can also act as second messengers and regulate the function of several proteins, including IκB kinase (IKKβ), protein kinase C (PKC), and Kelch-like ECH-associated protein 1 (Keap1), through interactions with cysteine residues (known as a “redox sensor”) of this protein. Redox modification is the term for modulating intracellular redox sensors by ROS. These protein redox changes activate different downstream signaling pathways that play important roles in impaired insulin secretion and insulin resistance, critical for the onset of diabetes mellitus and its consequences [172,173].
Since oxidative stress and diabetes are closely related, various initiatives to promote the health of diabetic patients have been carried out, one of which is to provide dietary supplements containing antioxidants [174]. Berberine is an isoquinoline alkaloid with antioxidant activity and can inhibit the formation of free radicals (ROS) and increase the activity of antioxidant enzymes. In addition, berberine improves mitochondrial function by decreasing oxygen consumption and increasing the mitochondrial membrane potential to prevent oxidative damage [175].
Several mechanisms by which berberine reduces oxidative stress in diabetes mellitus have been reported.
4.1. Berberine Scavenges ROS and Blocks ROS Generation
Shirwaikar et al. investigated the antioxidant activity of berberine in vitro. Berberine has potential as an antioxidant because it can capture superoxide free radicals directly in systems containing alkaline dimethyl sulfoxide (DMSO). Other antioxidant mechanisms of berberine include removing oxygen, capturing ROS and RNS or their precursors, inhibiting the formation of ROS and RNS, binding metal ions needed as catalysts for ROS formation, and regulating endogenous antioxidants [170]. Similar results were shown in a study by Jung et al., where berberine has ONOO– scavenging activity [176]. However, a study by Choi et al. showed that berberine could not stabilize hydroxyl radicals but showed a strong ability to stabilize superoxide anions [177].
4.2. Berberine Chelates Metal Ions
Several metals, such as iron (Fe), copper (Cu), and calcium (Ca), are involved in the oxidation process. Fe plays a role in lipid peroxidation reactions and produces free radicals [178]. The ability of berberine to chelate Fe2+ is still debated. Studies conducted by Shiwarkal et al. demonstrated that berberine shows metal-chelating activity [170], whereas other studies showed otherwise [179,180]. An in vivo study on iron-overloaded mice by Aalikhani et al. showed that berberine decreased total iron levels in the liver, kidney, and lung tissues. Berberine prevents lipid peroxidation by reducing excess iron and chelating it in tissues, which is equivalent to DFO, an iron-chelating drug [181].
4.3. Berberine Regulates Enzyme Activity
Free radicals are physiologically active biomolecules produced by metabolic pathways and immune cells. Free radicals can be derived from molecular oxygen derivatives such as reactive oxygen species (ROS) or molecular nitrogen derivatives such as reactive nitrogen species (RNS). The enzymes responsible for forming free radicals are the mitochondrial enzymes NADPH oxidase (NOX), xanthine oxidase (XO), cyclooxygenase (COX), lipoxygenase (LOX), nitric oxide synthases (NOS), monoamine oxidase (MAO), myeloperoxidase (MPO), and cytochrome P450 enzymes. The body requires specific quantities of free radicals to regulate several cell physiological activities. However, when the number of free radicals increases above the physiological range, it will cause a condition called oxidative stress [182]. Most cells have an intrinsic defense mechanism involving various enzymes, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), GSH reductase (GR), and glutathione transferase (GST), which can protect cells from free radical attack [183].
4.3.1. Berberine Enhances Antioxidant Enzyme Activity and Inhibits Lipid/Protein Peroxidation
In the body, free radicals are converted to hydrogen peroxide by superoxide dismutase (SOD). Hydrogen peroxide is converted into hydroxyl radicals (*OH) during the propagation stage. These hydroxyl radicals cause lipid peroxidation in cell membranes, resulting in cell damage. If allowed to continue, this situation causes an imbalance between free radicals and endogenous antioxidants, known as oxidative stress. Therefore, one of the oxidative stress markers is increased malondialdehyde (MDA), a lipid peroxidation product formed under oxidative stress conditions [184]. Hydrogen peroxide can also be converted into oxygen and water by catalase (CAT) or glutathione peroxidase (GPx). Glutathione (GSH) is a substrate required for the antioxidant activity of GPx. When catalyzing this process, the disulfide bonds of GSH bind to form oxidized glutathione (GSSG), and glutathione reductase (GR) can recycle GSSG into GSH again by oxidizing NADPH. When cells are subjected to oxidative stress, GSSG accumulation occurs, and the GSH/GSSG ratio decreases [185].
GST is generally considered a phase 2 enzyme, mainly in electrophilic compounds for detoxification. Additionally, numerous investigations have demonstrated that GST can catalyze the breakdown of lipid hydrogen peroxide created by oxidative damage to lipid molecules [186].
Protein carbonyl is a biomarker of protein oxidation. Protein carbonylation is an irreversible oxidative protein modification that is an early marker of oxidative-stress-related disorders. Protein carbonyl levels are elevated in many tissues because of acute or prolonged oxidative stress. Protein carbonyl levels are significantly increased in type 2 diabetes [187].
It is known that in diabetes mellitus, there is an increase in GPx mRNA expression and, conversely, a decrease in SOD levels caused by a reduction in SOD mRNA expression. Berberine was reported to restore the GPx levels and SOD mRNA expression in type 2 diabetic animal models back to normal conditions, whereas this ability was not shown by metformin or glibenclamide [188,189].
Several studies related to the antioxidant activity of berberine, both in vitro using cell cultures grown on media containing high glucose and in vivo in experimental animals with streptozotocin- or alloxan-induced diabetes mellitus, have been carried out. Berberine reduces lipid/protein peroxidation biomarkers and increases antioxidant enzyme activity, which counteracts free radicals and overcomes oxidative stress (Table 3).

Table 3.
Effect of berberine on antioxidant parameters in diabetic animal models.
4.3.2. Berberine Inhibits the Production of Oxidase
The NADPH oxidase (NOX) family is a major source of ROS in eukaryotic cells. NOX consists of several isoforms associated with electron transfer and is involved in many essential human physiological functions [209]. NOX activation is related to the onset of diabetes [210]. NOX is a potential target for treating diabetes mellitus and its complications [211].
Berberine can reduce oxidative stress by decreasing the expression of NOX. Berberine suppresses NOX4 overexpression and decreases ROS production in macrophages, endothelial cells, and the vascular endothelium [212,213,214]. Another study showed that NOX2 and NOX4 were detected in the aorta of streptozotocin-induced apolipoprotein E-deficient (apoE−/−) in type 1 diabetic mice [215]. NOX1 and NOX4 were overexpressed in diabetic rats (db/db) [216]. NOX4 is the main NOX isoform in human endothelial cells. The overexpression of NOX4 activates ROS in human endothelial cells [217]. NOX4 is also the primary source of ROS formation in blood vessel walls [218,219].
NOX is negatively regulated by AMPK activation [220,221]. AMPK plays an important role in the antioxidant [188,189] and antidiabetic activity of berberine [222]. In diabetes mellitus, AMPK becomes inactive [223]. Studies have shown that oxidative stress can regulate AMPK activity [224]. AMPKα1 is more sensitive to oxidative stress [225], while AMPKα2 is more sensitive to increased AMPK concentrations [225,226]. Berberine is an AMP-activated protein kinase (AMPK) activator [222]. AMPK activation causes the downregulation of NOX, inhibits ROS formation, and increases SOD expression [227].
It has been demonstrated that XO is a significant source of H2O2 and free radical production in cells and tissues. XO produces peroxide free radicals by catalyzing the oxidation of hypoxanthine and xanthine. Several studies have shown that XO activity is associated with diabetes mellitus [228,229]. Berberine showed the inhibition of xanthine oxidase with an IC50 of 23.64 μM [230].
Nitric oxide (NO) is a free radical produced by nitric oxide synthase (NOS) and is a biomarker of oxidative stress. NOS consists of three isoforms: neuronal (nNOS), inducible (iNOS), and endothelial (eNOS). Studies have shown an increase in NO levels in patients with diabetes mellitus [231]. Berberine has been reported to reduce NO levels [196,205]. Under hyperglycemic conditions, NO levels increase through the upregulation of iNOS. In vivo studies have shown that berberine reduced iNOS levels in diabetic rats [203,204]. In another study, berberine reduced monoamine oxidase (MAO) activity in STZ-induced diabetic rats [208]. Recently, MAO has been identified as a source of reactive oxygen species (ROS) that cause oxidative stress in diabetes mellitus [232].
4.4. Berberine Effects on Signal Transduction Pathway and Cell Antioxidant Response
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that activates antioxidant enzymes to protect cells from damage induced by oxidative stress. Nrf2 deficiency increased ROS production [233]. Berberine is an Nrf2 activator. Berberine protects against oxidative stress by increasing Nrf2 and Hmox-1 expression [234]. Hsu et al. showed that berberine acts against oxidative stress by activating the PI3K/Akt/Nrf2-dependent pathway [235]. In addition, a further study indicated that berberine enhanced nuclear Nrf2 translocation and HO-1 expression [236]. Another study reported that berberine increased the transcription of Nrf2-targeted antioxidative genes (NADPH quinone oxidoreductase-1 (NQO-1) and heme oxygenase-1 (HO-1)), as well as the nuclear localization and phosphorylation of the Nrf2 protein. The Nrf2-mediated activity of berberine depends on AMPK activation [237]. Deng et al. investigated the effects of berberine in rats with NAFLD. Berberine alleviates hepatic oxidative stress, which may be partly attributed to the activation of the Nrf2/ARE signaling pathway [238].
A study by Zhu et al. showed that berberine protects the liver from oxidative stress by increasing sirtuin 1 (SIRT1) levels [239]. SIRT1 is a class III histone deacetylase belonging to the sirtuin family. It regulates several important processes, such as metabolism, oxidative stress, aging, and apoptosis, through the deacetylation of various substrates. SIRT1 inhibition increases ROS levels, and conversely, oxidative stress conditions such as excessive ROS can reduce SIRT1 expression/activity [240]. SIRT1 stimulates antioxidant expression, repairs ROS-induced cell damage, and prevents cell dysfunction [241]. SIRT1 is currently receiving much attention because it fights oxidative stress through several mechanisms, such as the Sirt1/FOXOs, Sirt1/NF-κB, Sirt1/NOX, Sirt1/SOD, and Sirt1/eNOs pathways [242,243]. SIRT1 also protects cells from oxidative stress by increasing catalase activity and blocking p53-induced apoptosis through p53 deacetylation and manganese SOD (MnSOD) induction [244]. Recent studies have shown that SIRT1 can fight oxidative stress in diabetes mellitus by mediating important signals, such as AMPK, NADPH oxidase, endothelial NO synthase, mTOR, and miRNAs [245].
Berberine increases the expression of UCP2 (uncoupling protein 2) [246]. UCP2 is an important member of the uncoupling protein (UCP) family, mitochondrial transport proteins located in the inner mitochondrial membrane, constituting a vital link between ATP and ROS production [247]. UCP2 protects against mitochondrial oxidative damage by reducing ROS [248]. UCP2 overexpression studies have confirmed its essential role in reducing oxidative stress, as ROS production was successfully decreased [249,250]. UCP2 is associated with DM [251]. UCP2 may be a promising therapeutic target for treating type 2 diabetes mellitus because it regulates insulin secretion and beta-cell dysfunction [252]. Since UCP2 plays a role in reducing ROS production, the selective activation of UCP2 may have therapeutic potential in patients with diabetes [253].
NF-κB, also known as nuclear factor-κB, is a nuclear transcription factor found in all cell types. ROS can activate NF-κB, which can modulate oxidative stress [254]. Studies performed in various cells and experimental animals have shown the role of NF-κB in the pathogenesis of diabetes mellitus. Increased NF-κB activation in the basal state and in response to TNF-α was observed at elevated glucose concentrations, indicating its potential relevance to diabetic complications [255]. Berberine can reduce NF-κB levels in the pancreas and liver of diabetic rats [203,204]. In other studies, the administration of berberine to diabetic animal models was reported to inhibit the activation of the NF-κB signaling pathway [256,257].
5. Antidiabetic Activity of Berberine
The effect of berberine on type 2 diabetes mellitus was first reported by Chen and Xie in 1986. Berberine, the main component of Coptis chinensis, showed a hypoglycemic effect in diabetic test animals [258]. In 1988, berberine was reported to have a hypoglycemic effect when used to treat diarrhea in patients with diabetes in China [259]. Since then, berberine has often been used as an antihyperglycemic agent in China. Several clinical trials related to the hypoglycemic effect of berberine have been reported in the literature in China.
Yin et al. compared the effects of berberine and metformin. In a three-month trial, 36 patients with type 2 diabetes mellitus were randomly assigned to berberine or metformin intervention groups. Berberine exhibited a hypoglycemic effect comparable to metformin, as indicated by decreased HbA1c, fasting blood glucose, and postprandial blood glucose. The total cholesterol and LDL cholesterol also significantly decreased. According to these findings, berberine may have potential as an oral hypoglycemic agent and is known to exert beneficial effects on lipid metabolism [260]. A similar clinical experiment by Zhang et al. showed that berberine dramatically lowered the fasting blood glucose, HbA1c, triglyceride, and insulin levels in patients with type 2 diabetes. Berberine can lower fasting blood sugar and HbA1c, similar to metformin and rosiglitazone [261].
A meta-analysis of 27 clinical trials by Lan et al. revealed that berberine has a therapeutic effect on type 2 diabetes mellitus, hyperlipidemia, and hypertension compared with other therapeutic regimens [87]. In another meta-analysis by Wei et al., seventeen studies involving 1198 patients were analyzed. Berberine significantly improved fasting blood glucose, postprandial blood glucose, HbA1c, and insulin resistance. Berberine showed a beneficial effect on the control of blood glucose levels. Although not better than metformin, berberine showed a better effect on fasting blood glucose levels than rosiglitazone. The combination of berberine with other oral hypoglycemic drugs is a new alternative treatment for diabetes mellitus [262]. Liang et al. performed a meta-analysis of 28 studies related to berberine clinical trials involving 2313 patients with type 2 diabetes mellitus. Berberine administered alone or in combination with oral hypoglycemic drugs improved fasting blood glucose, postprandial blood glucose, and HbA1c in patients with type 2 diabetes mellitus. However, berberine combined with oral hypoglycemic drugs showed a better reduction in fasting blood glucose and postprandial blood glucose levels than berberine or oral hypoglycemic drugs alone [12].
In addition to type 2 diabetes mellitus, several studies on berberine activity in gestational diabetes mellitus have also been conducted. It is known that all levels of glucose intolerance during pregnancy or first detected during pregnancy are referred to as gestational diabetes mellitus; however, this type of diabetes is usually diagnosed in the second or third trimester of pregnancy. This definition does not rule out the possibility that glucose intolerance exists before pregnancy or starts with pregnancy [1]. Gestational diabetes mellitus usually appears as a temporary disorder during pregnancy and resolves after pregnancy. Women with gestational diabetes mellitus are at greater risk of adverse pregnancy outcomes. These include high blood pressure (including pre-eclampsia) and a large baby for gestational age (macrosomia), which can make a normal birth difficult and hazardous, with the baby more prone to fractures and nerve damage. Babies born to mothers with gestational diabetes mellitus also have a higher lifetime risk of obesity and type 2 diabetes mellitus. Gestational diabetes mellitus is caused by relative insulin resistance and deficiency that accompanies pregnancy and occurs in nearly 3–5% of pregnant women. In 2021, 21.1 million (16.7%) live births to women involved some form of hyperglycemia during pregnancy. Of these, 80.3% were caused by gestational diabetes mellitus, 10.6% were caused by diabetes detected before pregnancy, and 9.1% were caused by diabetes (including type 1 and type 2) that was first detected in pregnancy [2].
Berberine is reported to improve insulin resistance conditions and maternal–fetal outcomes of gestational diabetes mellitus rats, such as the number of dead and absorbed fetuses, maternal body weight gain, and fetal and placental weight [263]. Maternal obesity and diabetes during pregnancy predispose to the development of cardiometabolic diseases. Berberine treatments protected against obesity, increased glucose-stimulated insulin secretion from pancreatic islets, and improved cardiac dysfunction [264]. Berberine protects against cardiac dysfunction in gestational diabetes mellitus, involving improved mitochondrial function mediated through increased cardiolipin synthesis [265]. Cardiolipin is a key mitochondrial membrane phospholipid that regulates cardiac mitochondrial bioenergetics [266].
Many studies on berberine’s mechanism as an antidiabetic have been conducted. Berberine is reported to act by several mechanisms, including increasing insulin secretion, improving insulin resistance, inhibiting gluconeogenesis, increasing glucose uptake, inducing glycolysis, inhibiting the action of several important enzymes, and regulating the gut microbiota [77,267].
5.1. Berberine Increases Insulin Secretion
Several in vitro and in vivo studies have shown that berberine increases insulin secretion. Lang et al. conducted a study on the effect of berberine in experimental animals with impaired glucose tolerance and its effect on insulin secretion in HIT-T15 cells and pancreatic islets. HIT-T15 is a cell culture sensitive to glucose and plays a role in secreting insulin. Berberine concentrations of 1–10 mol/L increased insulin secretion in HIT-T15 and pancreatic islet cell cultures [268]. Another study by Zhou et al. showed that berberine could increase insulin secretion and expression, and regenerate pancreatic beta cells in experimental rats with diabetes mellitus [171].
HNF-4α, a steroid hormone receptor superfamily member, is expressed in the liver, kidneys, small intestine, and pancreas (including pancreatic beta cells). HNF-4α directly activates the insulin gene. In addition, HNF-4α plays an important role in regulating and maintaining normal insulin secretion from pancreatic beta cells. The decrease in HNF-4α levels causes pancreatic beta cell damage and GSIS disorders and is associated with the emergence of type 2 diabetes mellitus [269]. Berberine increased glucose-stimulated insulin secretion (GSIS) in isolated experimental animal islets. The insulinotropic effects of berberine are mediated by increased nuclear factor 4-alpha (HNF-4α) expression and glucokinase (GK) activity. These two factors are not known to be involved in sulfonylurea-induced insulin secretion. Therefore, berberine is a promising insulin secretagogue that acts via a mechanism different from that of sulfonylureas [270].
Berberine also increased insulin secretion and glucokinase (GK) activity in NIT-1 cells. Berberine acts as a GK activator that increases glucose utilization, increases GK protein activity and expression, and decreases glucokinase regulatory protein (GKRP), an endogenous GK inhibitor [271]. GK activation is an alternative approach to improve glycemic control in patients with type 2 diabetes mellitus. Studies have shown low GK activity in patients with type 2 diabetes mellitus. GK acts as a “glucose sensor” or “glucose receptor” in pancreatic beta cells. The activation of GK triggers insulin secretion, increases glucose uptake in the liver, and plays a role in the synthesis and storage of glycogen in the liver, thereby reducing hepatic glucose expenditure. When GK is activated, glucose (a substrate of GK) is converted into glucose-6-phosphate (G6P). G6P can activate glycogen synthase and act as a substrate for glycogen synthesis [272].
Lu et al. studied the mechanism of action of berberine as an antidiabetic agent. Berberine increases insulin secretion by increasing GLP-1 secretion [273]. Similar findings have been reported by Zhang et al. Berberine was shown to reduce blood glucose levels through the mitogen-activated protein kinase (MAPK) pathway and increase GLP-1 secretion in the intestine [274]. GLP-1 is an incretin hormone in addition to the glucose-dependent insulinotropic peptide (GIP) [275]. The incretin hormone is a polypeptide of the glucagon superfamily synthesized in the small intestine and regulates glucose levels by stimulating insulin secretion in response to the presence of food [276,277].
However, a clinical trial by Pérez-Rubio showed different results from previous studies. Berberine treatment caused a decrease in insulin secretion, as indicated by the insulinogenic index value decreasing from 0.78 ± 0.69 (baseline) to 0.62 ± 0.46 (three months). On the other hand, berberine was also reported to be able to increase insulin sensitivity, as indicated by the Matsuda index value, which increased from 2.10 ± 1.00 (baseline) to 3.10 ± 1.60 (three months). This result suggests that the ability of berberine to improve metabolic control is not through an increase in insulin secretion but through an increase in insulin sensitivity [278]. These findings are similar to those from Bai et al. Berberine inhibited glucose oxidation and ATP synthesis in the pancreatic islets of experimental animals by inhibiting mitochondrial respiration. The inhibition of ATP synthesis alters the ATP/ADP ratio, causes AMPK activation, and decreases insulin secretion from pancreatic beta cells via a KATP-dependent pathway [279].
The role of AMPK in insulin secretion is still under debate [280]. Several studies have shown that AMPK activation inhibits glucose-induced insulin secretion [281,282,283,284]. However, other studies have shown the opposite, where AMPK activation increased insulin secretion [285,286,287].
According to Zhao et al., the ability of berberine to increase insulin secretion is related to the KCNH6 channel. Berberine is reported to be able to bind to the KCNH6 channel and close the KCNH6 channel. KCNH6 channel closure prolongs the depolarization of high-glucose-dependent cell membranes, thereby increasing insulin secretion [288]. As mentioned earlier, insulin secretion from pancreatic beta cells is mediated by KATP channels, which cause membrane depolarization. However, in addition to the KATP channel, another potassium channel also plays a role in insulin secretion in humans and animals, namely, KCNH6 (non-KATP K+ channel). KCNH6 is a voltage-dependent K+ (Kv) channel [289]. In vitro studies have demonstrated the effect of non-KATP K+ channels on insulin secretion, particularly the voltage-dependent K+ (Kv) channel, which modulates insulin secretion [290,291]. Glucose-induced insulin secretion due to Kv channel closure has also been reported in experimental animal islets and insulinoma cells [292,293]. Other studies have also shown that glucose-induced insulin secretion from pancreatic beta cells is affected by high-glucose-dependent repolarization caused by Kv channels such as KCNH6 [294].
5.2. Berberine Improves Insulin Resistance
Several studies have been conducted on AMPK activation by berberine and its relationship with insulin resistance. AMPK plays an important role in regulating the energy homeostasis of cells and is activated by binding an ADP or AMP molecule to the regulatory site of the γ subunit [295]. AMPK plays a role in many metabolic processes, such as reducing energy stores and increasing energy production [296]. AMPK is known to be one of the potential therapeutic targets to improve insulin resistance [297].
Chang et al. conducted a study on the effects and mechanisms of berberine on glucose consumption and glucose uptake in insulin-resistant H9c2 cells. Berberine improves insulin resistance in H9c2 cells by increasing AMPK activity. Under conditions of insulin resistance, AMPK activation by berberine can increase GLUT4 translocation and glucose transport, thereby increasing glucose consumption and glucose uptake in insulin-resistant H9c2 cells [298]. In another study by Lee et al., berberine increased AMP-activated protein kinase (AMPK) activity in 3T3-L1 adipocytes and L6 myotubes, increased GLUT4 translocation in L6 cells, and decreased lipid accumulation in 3T3-L1 adipocytes. This result suggests that berberine has a beneficial effect on treating diabetes and obesity through the stimulation of AMPK activity [299]. Berberine can activate AMPK by inhibiting mitochondrial respiratory complex I and increasing insulin sensitivity in insulin-resistant experimental animals [300]. Mitochondrial complex I is one of the targets in treating diabetes mellitus. Drugs such as metformin and thiazolidinedione have also been reported to inhibit mitochondrial respiratory complex I [301].
Li et al. reported that berberine could increase insulin sensitivity and improve metabolic abnormalities in fructose-induced insulin resistance in experimental animals through activation of the LKB1/AMPK/PGC1α pathway. LKB1 is one of the kinases that play a role in AMPK activation, while PGC1α is involved in insulin-stimulated AKT phosphorylation. AKT is a key molecule in the insulin-signaling pathway that stimulates glycogen synthesis. PGC1α is known to increase AKT and p-AKT expression in muscles. PGC1α is an important transcriptional coactivator that plays a role in stimulating mitochondrial biogenesis and regulating energy metabolism. In this study, there was a decrease in the expression of PGC1α in insulin-resistant experimental animals, which could be improved by administering berberine. This result suggests that the protective effect of berberine against insulin resistance may be related to the upregulation of PGC1α [302].
HIF-2α plays a crucial role in regulating ceramide synthesis. Several recent clinical trials have shown that ceramide is strongly associated with insulin resistance. The ratio of ceramide C18/16 has been identified as a biomarker to predict diabetes and is a risk factor for diabetes mellitus. Ceramide affects glucose metabolism in the liver, and increased ceramide levels can induce insulin resistance. The inhibition of ceramide synthase can reduce ceramide production, thereby improving insulin resistance and obesity [303,304,305]. In vivo studies have shown that berberine can inhibit the accumulation of HIF-2α through the downregulation of the HIF-2α target gene, thereby reducing ceramide production and improving insulin resistance induced by high-fat diets in experimental animals [306].
Under hyperglycemic conditions, toxic compounds are produced from glucose, including methylglyoxal (MGO). MGO influences the pathogenesis of microvascular and macrovascular complications in diabetes mellitus. MGO causes vascular damage to endothelial cells and plays a role in developing insulin resistance, hypertension, diabetic neuropathy, and nephropathy. An increase in MGO levels has been reported in patients with type 2 diabetes mellitus [307]. Memon et al. conducted a clinical trial on the effect of berberine on methylglyoxal (MGO) levels and insulin resistance in patients with newly diagnosed type 2 diabetes mellitus compared with metformin. Metformin and berberine reduced the MGO levels by 43% and 56%, respectively. Likewise, the HOMA-IR value for berberine decreased by 29.50% compared to metformin, which was only 8.12%. This result suggests that berberine is more effective in lowering MGO levels and improving insulin resistance by improving glycemic control in newly diagnosed type 2 diabetes mellitus [308]. Similarly, berberine increased insulin sensitivity in obese experimental animals, reduced fasting insulin levels by 46%, and reduced the homeostatic model assessment of insulin resistance index (HOMA-IR) by 48% [309].
Kong et al. studied the molecular mechanism by which berberine improves insulin resistance. In this study, the insulin receptor was used as a target of berberine to increase insulin sensitivity. The results showed that berberine improved insulin resistance by increasing insulin receptor expression by activating protein kinase C (PKC). In vivo tests on experimental animals with type 2 diabetes mellitus showed that berberine reduced fasting blood glucose levels and fasting serum insulin levels, increased insulin sensitivity, increased the amount of insulin receptor mRNA, and increased PKC activity in the liver [310]. Similar findings were also reported by Gu et al., who reported that berberine prevented insulin resistance by regulating the expression of insulin receptors, insulin receptor substrate-1 (IRS-1), and glucagon in insulin-resistant experimental animals induced by a high-fat diet [311].
An increase in circulating branched-chain amino acids is implicated in the pathogenesis of obesity and insulin resistance. Berberine can reduce insulin resistance by increasing branched-chain amino acid catabolism in peripheral tissues and reducing branched-chain amino acid-producing bacteria, including the order Clostridiales, families Streptococcaceae, Clostridiaceae, and Prevotellaceae, and genera Streptococcus and Prevotella [312]. In another study, the effects of berberine on insulin-induced signal transduction and glucose uptake in insulin-sensitive and insulin-resistant rat skeletal muscle cells were observed. Berberine increases insulin-induced tyrosine phosphorylation of insulin receptors. Under insulin resistance conditions, berberine exhibited a synergistic effect on insulin-induced glucose uptake and GLUT4 translocation. This result suggests that berberine can overcome insulin resistance through the modulation of key molecules in insulin signaling pathways, resulting in increased glucose uptake in insulin-resistant cells [313].
Protein phosphatase Mg2+/Mn2+-dependent 1B (PPM1B) is a single-subunit enzyme that requires magnesium/manganese to function. PPM1B, also known as PP2Cβ, belongs to the Ser/Thr protein phosphatase (PP2C) family. Endogenous PPM1B is present in the nucleus of mature 3T3-L1 adipocytes and can bind to the PPARγ receptor [314]. Berberine improves insulin resistance through mechanisms related to regulating the PPM1B signaling pathway, including the cAMP/PKA/PPM1B, PPM1B/GLUT4, PPM1B/IKKβ/NF-κB, and PPM1B/PI3K/AKT pathways. Therefore, the ability of berberine to protect experimental animals with type 2 diabetes mellitus from insulin resistance is due to the regulation of the expression of cAMP, PKA, PPM1B, PPARγ, LRP1, GLUT4, NF-B p65, JNK, IKKβ, IRS-1, IRS -2, PI3K, and AKT in the heart [315].
In women with gestational diabetes, berberine is known to reduce insulin resistance by inhibiting hypoxia-inducible factor-3α (HIF3A) methylation [316]. It has been reported that HIF3A methylation in women with gestational diabetes is higher than in normal pregnant women. Studies have shown a significant correlation between HIF3A methylation and insulin resistance in gestational diabetes mellitus [317].
5.3. Berberine Inhibits Gluconeogenesis
Hepatic glucose production is the sum of gluconeogenesis. Increased hepatic glucose production, specifically gluconeogenesis, leads to increased glucose release into the blood, which can cause hyperglycemia and subsequent diabetic organ damage [318]. In patients with type 2 diabetes, gluconeogenesis has been identified as the primary source of glucose production, while glycogenolysis was found not to contribute [319,320].
In a study by Xie et al., berberine decreased gluconeogenic genes in the liver [321]. The activity of transcription factors such as forkhead transcription factor O1 (FoxO1) was also decreased. FoxO1 is an important transcription factor in the control of gluconeogenesis in the liver. FoxO1 induces PEPCK and G6Pase gene transcription to initiate gluconeogenesis [322].
In cultured hepatocytes, berberine inhibited oxygen consumption and decreased intracellular ATP levels, thereby inhibiting gluconeogenesis and lipogenesis in the liver. Berberine can reduce fasting blood glucose levels by inhibiting gluconeogenesis in the liver independent of insulin action [321]. Berberine inhibits gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway [323,324].
AMPK is a heteromeric protein consisting of the α catalytic subunit and β and γ regulatory subunits that regulate glucose and lipid metabolism, including gluconeogenesis and lipogenesis processes in the liver. AMPK is strongly associated with gluconeogenesis and is activated by liver kinase B1 (LKB1), transforming growth factor-activated kinase 1 (TAK1), and Ca2+/calmodulin-dependent protein kinase (CaMKKβ). AMPK also plays a role in regulating glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK), which affect gluconeogenesis to improve diabetes mellitus [325]. LKB1 is a serine/threonine kinase with a molecular weight of 50 kDa that plays a role in the phosphorylation and activation of the AMPK catalytic subunit at the T-loop residue of Thr172. In adult rat liver tissue, LKB1 regulates AMPK phosphorylation and controls the rate of gluconeogenesis [326]. TORC2 also plays an important role in gluconeogenesis. TORC2 is required for fasting hepatic gluconeogenesis. Under fasting conditions, there is an increase in glucagon released by the pancreas, which stimulates gluconeogenesis through the increased expression of several gluconeogenic genes through the cAMP-responsive CREB factor. Glucagon stimulates CREB activity by inducing the coactivator TORC2 [327,328].
Other studies have demonstrated that berberine suppresses gluconeogenesis in the liver by blocking the glucagon pathway. Berberine inhibits CREB phosphorylation in hepatocytes and the liver and causes the downregulation of gluconeogenic genes (PEPCK and G6Pase), leading to decreased hepatic glucose production [329]. Zhang et al. also reported that berberine inhibits gluconeogenesis via PEPCK1 [330]. PEPCK1 is an important enzyme in gluconeogenesis and plays a role in regulating glucose homeostasis [331].
5.4. Berberine Increases Glucose Uptake
Berberine can increase glucose uptake by inhibiting SIRT3 deacetylation [330]. Berberine also significantly increased the mitochondrial depolarization ratio and intracellular AMP levels via SIRT3 ubiquitination. The oxidation potential of berberine, which stimulates glucose uptake by activating the AMPK pathway, is promoted by SIRT3 protein breakdown via ubiquitination [332].
SIRT3 is a protein in the mitochondrial matrix that interacts with subunits in complex I mitochondrial respiration [332]. SIRT3 plays a role in regulating the function of complex I in the electron transport chain and maintaining basal ATP [333]. SIRT3 inhibition results in mitochondrial dysfunction and an increased AMP/ATP ratio, which activates AMPK [334]. The activation of the AMPK signaling pathway further enhances glucose uptake [332].
A different mechanism of berberine was demonstrated by Cok et al. In this study, the ability of berberine to induce glucose uptake in L929 fibroblast cells, a cell line that only expresses the GLUT1 transporter protein, was observed. Berberine increased glucose uptake by increasing the activity of GLUT1. However, the ability of berberine to increase glucose uptake in fibroblasts is not associated with AMPK activity [335].
Cheng et al. studied the effect of berberine on L6 myotubes in the skeletal muscles of experimental animals with diabetes mellitus. The L6 cell culture was used to investigate the insulin-stimulated glucose transport mechanism. Berberine has been reported to stimulate glucose uptake in L6 myotubes, and this effect is not via the insulin signaling pathway but via the AMP-AMPK-p38 MAPK pathway. In muscle cells, p38 mitogen-activated protein kinase (p38 MAPK) is strongly associated with GLUT4 [336].
Zhou et al. demonstrated that berberine increased glucose uptake in 3T3-L1 adipocytes in the absence of insulin. Berberine enhanced GLUT1-mediated glucose uptake in 3T3-L1 adipocytes by activating GLUT1 via the AMPK pathway. However, berberine did not significantly stimulate GLUT4 protein translocation. Berberine does not affect GLUT1 or GLUT4 protein gene expression [337]. In contrast, another study showed that berberine increased glucose transport activity in 3T3-L1 adipocytes by increasing the expression of GLUT1 without decreasing GLUT4 expression [338]. Berberine stimulated glucose uptake in 3T3-L1 adipocytes to the same extent as rosiglitazone [339].
5.5. Berberine Induces Glycolysis
Glycolysis has been associated with type 2 diabetes mellitus [340]. Glycolysis is one of the glucose metabolism pathways that regulate insulin secretion and the physiological functions of some tissues and organs. Therefore, it is essential to maintain glucose metabolism homeostasis in a cell-type-dependent manner. Under insulin deficiency or resistance conditions, glycolysis can become dysregulated, caused by improper quantities and activity of metabolic and regulatory enzymes. One of the goals of diabetes treatment is to increase the rate of glycolysis in important tissues and cells involved in regulating systemic glucose homeostasis by targeting key metabolic and regulatory enzymes [341].
Glycolysis is a process modulated by a set of enzymes and factors, including AMPK [342]. AMPK stimulates glycolysis by activating phosphofructokinase (PFK), an important enzyme that plays an important role in glycolysis [343,344].
Berberine can increase glucose metabolism by increasing glycolysis. The increase in glycolysis results from the inhibition of mitochondrial respiratory chain complex I, which causes the suppression of ATP synthesis, thereby inhibiting glucose oxidation in the mitochondria, where this effect is independent of AMPK activation. Increased glycolysis is characterized by increased glucose consumption in HepG2 hepatocytes and C2C12 myotube cells [345]. In vitro studies have shown that berberine can increase glucose metabolism, as indicated by increased glucose consumption in HepG2 hepatocytes that are not insulin-dependent. This action is similar to metformin. HepG2 cells were used to observe the glucose-lowering effect on hepatocytes through glucose consumption [346]. Ren et al. reported a similar finding. Berberine was able to increase glucose metabolism through the activation of AMPKα1 in HepG2 cells [347].
5.6. Berberine Inhibits Enzyme Activity
Two important enzymes are involved in the carbohydrate digestion process: α-amylase and α-glucosidase [348]. The inhibition of these two enzymes causes a delay in the digestion of carbohydrates in the digestive tract, slows glucose absorption into the blood, and causes a decrease in blood glucose levels. Therefore, inhibiting α-amylase and α-glucosidase is a strategy or therapeutic approach for patients with type 2 diabetes mellitus [349,350].
Berberine may exert an antihyperglycemic action in the digestive tract prior to absorption because of its poor gut wall absorption and low bioavailability. It is similar to acarbose, an antihyperglycemic drug with low bioavailability due to poor absorption, and its therapeutic activity is within the gastrointestinal tract [351].
Several studies have shown that berberine inhibits α-amylase and α-glucosidase activities. A study conducted by Zhao et al. showed that berberine has strong inhibitory activity against both α-amylase and α-glucosidase in vitro, with IC50 values of 50.83 µg/mL and 198.40 µg/mL, respectively. The inhibition type in α-amylase is non-competitive, whereas, in α-glucosidase, competitive inhibition is observed [352]. In Caco-2 cells, berberine reduces glucose absorption in the gastrointestinal tract by inhibiting α-glucosidase activity [353]. The inhibition of α-glucosidase by berberine was still lower than that by acarbose [354].
An in vivo study by Liu et al. reported that berberine administration significantly reduced the activity of disaccharide enzymes (lactase, maltase, and sucrase) and β-glucuronidase in the small intestine of streptozotocin-induced diabetic rats. In addition, the activity of this disaccharide enzyme still decreased 24 h after the last administration of berberine [355]. The half-life of berberine after oral administration is approximately six hours [356]. This result suggests that the long-term administration of berberine may reduce the expression of disaccharide enzyme genes [355].
In patients with type 2 diabetes mellitus, decreased levels of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) have been reported to cause hyperglycemia [357]. GLP-1 and GIP are incretin hormones synthesized in the small intestine in response to food. These two hormones regulate blood glucose levels by stimulating insulin secretion from pancreatic beta cells (GLP-1 and GIP) and suppressing glucagon secretion from pancreatic alpha cells (GLP-1). However, both hormones have short half-lives (less than two minutes) due to rapid inactivation by the DPP-4 (dipeptidyl peptidase-4) enzyme. DPP-4 inhibitors play a role in inhibiting the degradation of GLP-1 and GIP [358]. DPP-4 inhibitors can control blood glucose levels by increasing insulin secretion, inhibiting glucagon secretion, and slowing gastric emptying [359]. DPP-4 inhibitors can also induce pancreatic beta-cell regeneration [360] and inhibit pancreatic beta-cell apoptosis [361,362]. Al Masri et al. reported that the human recombinant DPP-4 enzyme was inhibited by berberine with an IC50 of 13.30 μM, where this effect is close to hypoglycemic activity [363].
5.7. Berberine Regulates Gut Microbiota
The gut microbiota plays a vital role in metabolic diseases, including diabetes. Currently, the gut microbiota is a new research area in diabetes management. Studies in animals and humans have shown differences in the gut microbiota composition in patients with diabetes. Disturbances in the gut microbiota can directly or indirectly cause insulin resistance and type 2 diabetes mellitus [364]. In a clinical trial by Zhang et al., the administration of berberine (either alone or in combination with probiotics) significantly changed the gut microbiota composition. Approximately 78 species were induced by berberine treatment. Two of these were Bacteroides spp. and the taxa Proteobacteria induced by metformin treatment. Further metagenomic and metabolomic studies revealed that the hypoglycemic effect of berberine was mediated by the inhibition of deoxycholic acid biotransformation by Ruminococcus bromii [365]. Several taxa have been identified as antidiabetic bacteria. Ruminococcus strains have been reported to regulate bile acid metabolism [366,367]. The bile acid transformation pathway by microbes has become one of the targets of action of several antidiabetic agents, either to decrease 7α/β dehydroxylation or to alter bile salt deconjugation, leading to the modulation of host bile acids and ultimately mediating the hypoglycemic effect of drugs [368].
The normal human gut microbiota consists of two main phyla, Bacteroidetes and Firmicutes, which can degrade many complex glycans and increase energy metabolism. Proteobacteria have been reported to be a common factor associated with human diseases, whereas Lactobacillaceae are effective in treating metabolic disorders. An in vivo study by Yao et al. reported that the gut microbiota community richness and diversity were significantly increased by berberine administration. The berberine intervention group showed an increase in Bacteroidetes and Lactobacillaceae and a decrease in Proteobacteria and Verrucomicrobia. In addition, berberine administration reduces the concentration of aromatic amino acids in the large intestine and blood. High concentrations of aromatic amino acids in the blood are strongly associated with the risk of developing type 2 diabetes mellitus [369]. Similar findings by Zhao et al. showed that Firmicutes, Bacteroidetes, and the Bacteroidetes/Firmicutes ratio were modified after the oral administration of berberine [370].
In another study by Tian et al., the administration of berberine affected the gut microbiota composition by decreasing Clostridium clusters XIVa and IV and bile salt hydrolase (BSH) activity, which caused the accumulation of taurocholic acid (TCA). TCA accumulation is associated with the activation of Farnesoid X receptors in the gut, which can mediate bile salt, lipid, and glucose metabolism [371]. Berberine fumarate (BF) exerts a hypoglycemic effect by regulating the gut microbiota. BF increased the populations of Bacteroidetes, Clostridium, Lactobacillales, Prevotellaceae, and Alloprevotella and decreased Bacteroidales, Lachnospiraceae, Rikenellaceae, and Desulfovibrio [372]. On the other hand, berberine chloride can affect the composition of the gut microbiota, increasing the number of bacteria that produce short-chain fatty acids (e.g., Butyricimonas, Coprococcus, and Ruminococcus), reducing the number of pathogenic bacteria (e.g., Prevotella and Proteus), and increasing some probiotics (e.g., Lactobacillus and Akkermansia) [373].
6. Conclusions
The use of natural products as complementary or alternative medicines is gaining worldwide popularity. Berberine is an isoquinoline alkaloid with several pharmacological activities. In silico studies have shown that berberine has the potential to treat many diseases. As a natural compound, berberine is present in several medicinal plants. One of these is Fibraurea tinctoria, which has a long history of use in traditional medicine in Borneo to treat diabetes. Due to its antioxidant properties, berberine has attracted considerable attention. These qualities may help explain its effectiveness against diabetes mellitus through several biochemical pathways. Therefore, extensive studies on the potency of berberine-containing plants with demonstrated pharmacological activity should involve additional in vitro and in vivo studies.
Author Contributions
Conceptualization, I.P.M., D.S. and I.P.; methodology, I.P.M. and S.S.; validation, I.P.M., D.S. and S.S.; formal analysis, I.P.; resources, I.P.M. and I.P.; data curation, I.P.; writing—original draft preparation, I.P.; writing—review and editing, I.P. and I.P.M.; visualization, I.P.; supervision, I.P.M., D.S. and S.S.; project administration, I.P.M.; funding acquisition, I.P.M. and D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universitas Padjadjaran and also thanks to The Education Endowment Fund investment managed by Lembaga Pengelola Dana Pendidikan (LPDP), Indonesia (20200811303200).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any data.
Acknowledgments
The authors are grateful to the Central Library of Universitas Padjadjaran for depositing supplementary material, The Education Endowment Fund investment managed by Lembaga Pengelola Dana Pendidikan (LPDP), Indonesia, and are also grateful to Universitas Padjadjaran for supporting all research facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- ADA. Standards of Medical Care in Diabetes—2020. Diabetes Care J. Clin. Appl. Res. Educ. 2020, 43, S1–S212. [Google Scholar]
- IDF. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; ISBN 9782930229980. [Google Scholar]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Tabatabaei-Malazy, O.; Fakhrzadeh, H.; Sharifi, F.; Mirarefin, M.; Arzaghi, S.M.; Badamchizadeh, Z.; Khoee, M.A.; Larijani, B. Effect of metabolic control on oxidative stress, subclinical atherosclerosis and peripheral artery disease in diabetic patients. J. Diabetes Metab. Disord. 2015, 14, 84. [Google Scholar] [CrossRef]
- Khan, A.N.; Khan, R.A.; Ahmad, M.; Mushtaq, N. Role of antioxidant in oxidative stress and diabetes mellitus. J. Pharmacogn. Phytochem. 2015, 3, 217–220. [Google Scholar] [CrossRef]
- Zonszein, J.; Groop, P.H. Strategies for diabetes management: Using newer oral combination therapies early in the disease. Diabetes Ther. 2016, 7, 621–639. [Google Scholar] [CrossRef]
- Agarwal, A.; Jadhav, P.; Deshmukh, Y. Prescribing pattern and efficacy of anti-diabetic drugs in maintaining optimal glycemic levels in diabetic patients. J. Basic Clin. Pharm. 2014, 5, 79. [Google Scholar] [CrossRef]
- Lipska, K.J.; Yao, X.; Herrin, J.; McCoy, R.G.; Ross, J.S.; Steinman, M.A.; Inzucchi, S.E.; Gill, T.M.; Krumholz, H.M.; Shah, N.D. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care 2017, 40, 468–475. [Google Scholar] [CrossRef]
- Casagrande, S.S.; Fradkin, J.E.; Saydah, S.H.; Rust, K.F.; Cowie, C.C. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care 2013, 36, 2271–2279. [Google Scholar] [CrossRef]
- Thorpe, L.E.; Kanchi, R.; Chamany, S.; Rodriguez-Lopez, J.S.; Chernov, C.; Freeman, A.; Perlman, S.E. Change in Diabetes Prevalence and Control among New York City Adults: NYC Health and Nutrition Examination Surveys 2004–2014. J. Urban Health 2018, 95, 826–831. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Yin, M.; Zhang, Y.; Huang, L.; Chen, R.; Ni, J. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: A systematic literature review and a meta-analysis. Endocr. J. 2019, 66, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Triplitt, C.; Repas, T.; Alvarez, C. Chapter 74: Diabetes Mellitus. In Pharmacotherapy a Pathophysiologic Approach; Mc Graw Hill Education: New York, NY, USA, 2016; pp. 3211–3292. [Google Scholar]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Sease, J.; Shealy, K. Chapter 43: Diabetes Mellitus. In Pharmacotherapy Principles & Practice; Chicholm-Burns, M., Schwinghammer, T., Wells, B., Malone, P., Kolesar, J., Dipiro, J., Eds.; Mc Graw Hill Education: New York, NY, USA, 2016; pp. 651–678. ISBN 9781626239777. [Google Scholar]
- Defronzo, R.; Fleming, G.A.; Chen, K.; Bicsak, T.A. Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism 2016, 65, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Ezuruike, U.F.; Prieto, J.M. The use of plants in the traditional management of diabetes in Nigeria: Pharmacological and toxicological considerations. J. Ethnopharmacol. 2014, 155, 857–924. [Google Scholar] [CrossRef]
- Lee, A.L.; Chen, B.C.; Mou, C.H.; Sun, M.F.; Yen, H.R. Association of traditional Chinese medicine therapy and the risk of vascular complications in patients with type II diabetes mellitus: A nationwide, retrospective, Taiwanese-registry, cohort study. Medicine 2016, 95, e2536. [Google Scholar] [CrossRef]
- Alam, F.; Islam, M.A.; Kamal, M.A.; Gan, S.H. Updates on managing type 2 diabetes mellitus with natural products: Towards antidiabetic drug development. Curr. Med. Chem. 2016, 23, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhao, L.; Zhou, Q.; Zhao, T.; Wang, H.; Gu, C.; Tong, X. Application of Berberine on Treating Type 2 Diabetes Mellitus. Int. J. Endocrinol. 2015, 2015, 905749. [Google Scholar] [CrossRef]
- Teoh, S.L.; Das, S. Phytochemicals and their effective role in the treatment of diabetes mellitus: A short review. Phytochem. Rev. 2018, 17, 1111–1128. [Google Scholar] [CrossRef]
- Rios, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef]
- Ota, A.; Ulrih, N.P. An overview of herbal products and secondary metabolites used for management of type two diabetes. Front. Pharmacol. 2017, 8, 436. [Google Scholar] [CrossRef]
- Galappathie, S.; Palombo, E.A.; Chia, T.; Lim, D.; Ley, S.; Lee, C.; Mahon, P.J. Comparative antimicrobial activity of South East Asian plants used in Bornean folkloric medicine. J. Herb. Med. 2014, 4, 96–105. [Google Scholar] [CrossRef]
- Noorcahyati, N.; Sulandjari, S.; Dewi, W.S. Asosiasi Akar Kuning (Fibraurea tinctoria Lour.) dengan tumbuhan berpotensi obat di Samboja, Kalimantan Timur. J. Hutan Trop. 2016, 4, 232–239. [Google Scholar]
- Nguyen-pouplin, J.; Tran, H.; Tran, H.; Anh, T.; Dolecek, C. Antimalarial and cytotoxic activities of ethnopharmacologically selected medicinal plants from South Vietnam. J. Ethnopharmacol. 2007, 109, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kiyotani, T.; Maeda, M.; Katayama, T.; Tomita-yokotani, K.; Syafii, W.; Muladi, S. Furanoditerpenes from Arcangelisia flava (L.) Merr. and their antifungal activity. Phytochem. Lett. 2011, 4, 333–336. [Google Scholar] [CrossRef]
- TPL. The Plant List. Available online: http://www.theplantlist.org/ (accessed on 16 August 2022).
- Barbosa-Filho, J.M.; Da-Cunha, E.V.L.; Gray, A.I. Alkaloids of the Menispermaceae. Alkaloids Chem. Biol. 2000, 54, 1–190. [Google Scholar] [CrossRef]
- Chang, W.; Chen, L.; Hatch, G.M. Berberine as a therapy for type 2 diabetes and its complications: From mechanism of action to clinical studies. Biochem. Cell Biol. 2015, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, N.; Zhao, L.; Lu, F. Berberine in the treatment of type 2 diabetes mellitus: A systemic review and meta-analysis. Evid.-Based Complement. Altern. Med. 2012, 2012, 591654. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Geng, Y.N.; Jiang, J.D.; Kong, W.J. Antioxidant and anti-inflammatory activities of Berberine in the treatment of diabetes mellitus. Evid.-Based Complement. Altern. Med. 2014, 2014, 289264. [Google Scholar] [CrossRef]
- Su, C.; Chen, Y.; Liou, M.; Tsai, H.; Chang, W.; Wu, T. Anti-inflammatory activities of furanoditerpenoids and other constituents from Fibraurea tinctoria. Bioorg. Med. Chem. 2008, 16, 9603–9609. [Google Scholar] [CrossRef] [PubMed]
- Watthanachaiyingcharoen, R.; Komatsu, K.; Zhu, S.; Vajragupta, O.; Leelamanit, W. Authentication of Coscinium fenestratum among the other Menispermaceae plants prescribed in Thai folk medicines. Biol. Pharm. Bull. 2010, 33, 91–94. [Google Scholar] [CrossRef]
- Su, C.; Ueng, Y.; Nguyen, X.; Reddy, M.; Wu, T. Cytochrome P3A4 inhibitors and other constituents of Fibraurea tinctoria. J. Nat. Prod. 2007, 70, 1930–1933. [Google Scholar] [CrossRef]
- Keawpradub, N.; Dej-Adisai, S.; Yuenyongsawad, S. Antioxidant and cytotoxic activities of Thai medicinal plants named Khaminkhruea: Arcangelisia flava, Coscinium blumeanum and Fibraurea tinctoria. Songklanakarin J. Sci. Technol. 2005, 27, 455–467. [Google Scholar]
- Manosroi, A.; Akazawa, H.; Pattamapun, K.; Akihisa, T.; Manosroi, W.; Manosroi, J. Potent anti-proliferative effects against oral and cervical cancers of Thai medicinal plants selected from the Thai/Lanna medicinal plant recipe database “MANOSROI III” Potent anti-proliferative effects against oral and cervical cancers of Thai medici. Pharm. Biol. 2015, 53, 1075–1081. [Google Scholar] [CrossRef]
- Manosroi, A.; Akazawa, H.; Kitdamrongtham, W.; Akihisa, T.; Manosroi, W.; Manosroi, J. Potent antiproliferative effect on liver cancer of medicinal plants selected from the Thai/Lanna medicinal plant recipe database “MANOSROI III”. Evid.-Based Complement. Altern. Med. 2015, 2015, 397181. [Google Scholar] [CrossRef]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef]
- Ehteshamfar, S.M.; Akhbari, M.; Afshari, J.T.; Seyedi, M.; Nikfar, B.; Shapouri-Moghaddam, A.; Ghanbarzadeh, E.; Momtazi-Borojeni, A.A. Anti-inflammatory and immune-modulatory impacts of berberine on activation of autoreactive T cells in autoimmune inflammation. J. Cell. Mol. Med. 2020, 24, 13573–13588. [Google Scholar] [CrossRef]
- Chang, Y. Effectiveness of berberine in bacillary dysentery. Zhonghua Nei Ke Za Zhi 1959, 7, 741–743. [Google Scholar]
- Xia, X.; Wang, H.; Niu, X.; Wang, H.; Liu, Z.; Liu, Y.; Qi, Z.; Wang, S.; Liu, S.; Liu, S. Assessment of the anti-diarrhea function of compound Chinese herbal medicine Cangpo Oral Liquid. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 140–147. [Google Scholar] [CrossRef]
- Yu, M.; Jin, X.; Liang, C.; Bu, F.; Pan, D.; He, Q.; Ming, Y.; Little, P.; Du, H.; Liang, S.; et al. Berberine for diarrhea in children and adults: A systematic review and meta-analysis. Therap. Adv. Gastroenterol. 2020, 13, 1756284820961299. [Google Scholar] [CrossRef]
- Yue, S.J.; Liu, J.; Wang, W.X.; Wang, A.T.; Yang, X.Y.; Guan, H.S.; Wang, C.Y.; Yan, D. Berberine treatment-emergent mild diarrhea associated with gut microbiota dysbiosis. Biomed. Pharmacother. 2019, 116, 109002. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, Z.; Li, Y.; Fichna, J.; Storr, M. Effects of berberine in the gastrointestinal tract-A review of actions and therapeutic implications. Am. J. Chin. Med. 2014, 42, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Bertuccioli, A.; Giuberti, R.; Saponara, M.; Ivaldi, L. Role of a berberine-based nutritional supplement in reducing diarrhea in subjects with functional gastrointestinal disorders. Minerva Gastroenterol. Dietol. 2020, 66, 29–34. [Google Scholar] [CrossRef]
- Li, L.; Cui, H.; Li, T.; Qi, J.; Chen, H.; Gao, F.; Tian, X.; Mu, Y.; He, R.; Lv, S.; et al. Synergistic effect of berberine-based Chinese medicine assembled nanostructures on diarrhea-predominant irritable bowel syndrome in vivo. Front. Pharmacol. 2020, 11, 1210. [Google Scholar] [CrossRef]
- Jiang, X.W.; Zhang, Y.; Zhu, Y.L.; Zhang, H.; Lu, K.; Li, F.F.; Peng, H.Y. Effects of berberine gelatin on recurrent aphthous stomatitis: A randomized, placebo-controlled, double-blind trial in a Chinese cohort. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 212–217. [Google Scholar] [CrossRef]
- Li, H.L.; Han, T.; Liu, R.H.; Zhang, C.; Chen, H.S.; Zhang, W.D. Alkaloids from Corydalis saxicola and their anti-hepatitis B virus activity. Chem. Biodivers. 2008, 5, 777–783. [Google Scholar] [CrossRef]
- Hung, T.C.; Jassey, A.; Liu, C.H.; Lin, C.J.; Lin, C.C.; Wong, S.H.; Wang, J.Y.; Yen, M.H.; Lin, L.T. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine 2019, 53, 62–69. [Google Scholar] [CrossRef]
- Och, A.; Podgórski, R.; Nowak, R. Biological activity of Berberine-A summary update. Toxins 2020, 12, 713. [Google Scholar] [CrossRef]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.S.; Smejkal, K.; Malaník, M.; et al. Berberine in cardiovascular and metabolic diseases: From mechanisms to therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef]
- Jin, Y.; Khadka, D.B.; Cho, W.J. Pharmacological effects of berberine and its derivatives: A patent update. Expert Opin. Ther. Pat. 2016, 26, 229–243. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crisan, G.; Buzoianu, A.D. Berberine: Botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Gu, I.; Zhu, W. Dose-dependent effect of berberine on SARS-CoV-2 spike protein induced inflammatory host cell response. Int. J. Med. Sci. Health Res. 2021, 5, 169–181. [Google Scholar] [CrossRef]
- Varghese, F.S.; van Woudenbergh, E.; Overheul, G.J.; Eleveld, M.J.; Kurver, L.; van Heerbeek, N.; van Laarhoven, A.; Miesen, P.; Den Hartog, G.; De Jonge, M.I.; et al. Berberine and Obatoclax inhibit SARS-CoV-2 replication in primary human nasal epithelial cells in vitro. Viruses 2021, 13, 282. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, B.A.; Noval, M.G.; Kaczmarek, M.E.; Jang, K.K.; Thannickal, S.A.; Kottkamp, A.C.; Brown, R.S.; Kielian, M.; Cadwell, K.; Stapleford, K.A. Atovaquone and Berberine Chloride reduce SARS-CoV-2 replication in vitro. Viruses 2021, 13, 2437. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Chen, M.; Chen, X.C.; Cao, K.; You, Y.; Qian, Y.J.; Yu, W.K. Berberine reduces circulating inflammatory mediators in patients with severe COVID-19. Br. J. Surg. 2021, 108, e9–e11. [Google Scholar] [CrossRef]
- Xue, M.; Yang, M.; Zhang, W.; Li, X.; Gao, D.; Ou, Z.; Li, Z.; Liu, S.; Li, X.; Yang, S. Characterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticles. Int. J. Nanomed. 2013, 8, 4677–4687. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.X.; Hu, Y.N.; Li, J.W.; Yuan, K.; Guo, Y. Preparation and evaluation of antidiabetic agents of berberine organic acid salts for enhancing the bioavailability. Molecules 2019, 24, 103. [Google Scholar] [CrossRef]
- Liu, C.S.; Zheng, Y.R.; Zhang, Y.F.; Long, X.Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, S.; Colliva, C.; Camborata, C.; Roberti, M.; Ianni, C.; Neri, F.; Calvarese, C.; Lisotti, A.; Mazzella, G.; Roda, A. Berberine and its metabolites: Relationship between physicochemical properties and plasma levels after administration to human subjects. J. Nat. Prod. 2014, 77, 766–772. [Google Scholar] [CrossRef]
- Liu, Y.T.; Hao, H.P.; Xie, H.G.; Lai, L.; Wang, Q.; Liu, C.X.; Wang, G.J. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef]
- Kwon, M.; Lim, D.Y.; Lee, C.H.; Jeon, J.H.; Choi, M.K.; Song, I.S. Enhanced intestinal absorption and pharmacokinetic modulation of berberine and its metabolites through the inhibition of p-glycoprotein and intestinal metabolism in rats using a berberine mixed micelle formulation. Pharmaceutics 2020, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.-Q.; Zhu, Y.-P.; Pang, J.; Wang, Y.-X.; Song, D.-Q.; Kong, W.-J.; Jiang, J.-D. Tetrandrine potentiates the hypoglycemic efficacy of berberine by inhibiting P-glycoprotein function. Biol. Pharm. Bull. 2013, 36, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Miao, Y.Q.; Fan, D.J.; Yang, S.S.; Lin, X.; Meng, L.K.; Tang, X. Bioavailability study of berberine and the enhancing effects of TPGS on intestinal absorption in rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Wang, Q.; Wang, S.; Kuang, Y.; Li, K.; Song, W.; Ye, M. A 42-markers pharmacokinetic study reveals interactions of berberine and glycyrrhizic acid in the anti-diabetic chinese medicine formula Gegen-Qinlian decoction. Front. Pharmacol. 2018, 9, 622. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, F.; Jiang, J.; Gao, C.; Tan, Y. Intestinal absorption mechanisms of berberine, palmatine, jateorhizine, and coptisine: Involvement of P-glycoprotein. Xenobiotica 2011, 41, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Wang, T.; Chen, K.; Wang, H.; Jia, Q.; Li, Y. Enhancement of berberine hypoglycemic activity by oligomeric proanthocyanidins. Molecules 2018, 23, 3318. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Q.; Shou, J.W.; Zhao, Z.X.; Li, X.Y.; Zhang, X.F.; Ma, S.R.; He, C.Y.; Lin, Y.; Wen, B.Y.; et al. Gut microbiota-mediated personalized treatment of hyperlipidemia using berberine. Theranostics 2017, 7, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Shou, J.W.; Zhao, Z.X.; He, C.Y.; Ma, C.; Huang, M.; Fu, J.; Tan, X.S.; Li, X.Y.; Wen, B.Y.; et al. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci. Rep. 2015, 5, 12155. [Google Scholar] [CrossRef]
- Tan, X.S.; Ma, J.Y.; Feng, R.; Ma, C.; Chen, W.J.; Sun, Y.P.; Fu, J.; Huang, M.; He, C.Y.; Shou, J.W.; et al. Tissue distribution of berberine and its metabolites after oral administration in rats. PLoS ONE 2013, 8, e77969. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, H.; Xie, H.; LV, H.; Liu, C.; Wang, G. Oxidative demethylenation and subsequent glucuronidation are the major metabolic pathways of berberine in rats. J. Pharm. Sci. 2009, 99, 4391–4401. [Google Scholar] [CrossRef]
- Alolga, R.N.; Fan, Y.; Chen, Z.; Liu, L.W.; Zhao, Y.J.; Li, J.; Chen, Y.; Lai, M.D.; Li, P.; Qi, L.W. Significant pharmacokinetic differences of berberine are attributable to variations in gut microbiota between Africans and Chinese. Sci. Rep. 2016, 6, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chai, L.; Feng, X.; Liu, Z.; Liu, H.; Ding, L.; Qiu, F. Metabolites identification of berberine in rats using ultra-high performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2017, 139, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xiang, Y.; Shi, Y.; Tang, X.; Pan, L.; Gao, J.; Bi, R.; Lai, X. Pharmacokinetics and pharmacological activities of berberine in diabetes mellitus treatment. Evid.-Based Complement. Altern. Med. 2021, 2021, 9987097. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-L.; Tsai, T.-H. Hepatobiliary excretion of berberine. Drug Metab. Dispos. 2004, 32, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, F.; Ma, X.; Cheng, X.; Zhou, H.; Klaassen, C.D. CYP2D plays a major role in berberine metabolism in liver of mice and humans. Xenobiotica 2011, 41, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xu, C.; Li, X.; Li, D.; Li, Y.; Jiang, J.; Yang, P.; Duan, G. Rapid identification of berberine metabolites in rat plasma by UHPLC-Q-TOF-MS. Molecules 2019, 24, 1994. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, K.; Cao, S.; Ding, L.; Qiu, F. Pharmacokinetics and excretion of berberine and its nine metabolites in rats. Front. Pharmacol. 2021, 11, 594852. [Google Scholar] [CrossRef]
- Ma, J.Y.; Feng, R.; Tan, X.S.; Ma, C.; Shou, J.W.; Fu, J.; Huang, M.; He, C.Y.; Chen, S.N.; Zhao, Z.X.; et al. Excretion of berberine and its metabolites in oral administration in rats. J. Pharm. Sci. 2013, 102, 4181–4192. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, H.; Ren, J.-Y.; Pan, J.-F.; Hong, Y.-C.; Zhu, D.-Y.; Xu, X.-R. Determination and preliminary studies of metabolism of berberine in human urine after oral administration. Chin. J. Clin. Pharmacol. 2000, 16, 36–39. [Google Scholar]
- Kheir, M.M.; Wang, Y.; Hua, L.; Hu, J.; Li, L.; Lei, F.; Du, L. Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem. Toxicol. 2010, 48, 1105–1110. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, B. Toxicological effects of berberine and sanguinarine. Front. Mol. Biosci. 2018, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Anis, K.V.; Kuttan, G.; Kuttan, R. Role of berberine as an adjuvant response modifier during tumour therapy in mice. Pharm. Pharmacol. Commun. 1999, 5, 697–700. [Google Scholar] [CrossRef]
- Lan, J.; Zhao, Y.; Dong, F.; Yan, Z.; Zheng, W.; Fan, J.; Sun, G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015, 161, 69–81. [Google Scholar] [CrossRef]
- Barnett, L.M.A.; Cummings, B.S. Nephrotoxicity and renal pathophysiology: A contemporary perspective. Toxicol. Sci. 2018, 164, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Lee, T.W.; Park, D.J. Drug-induced nephrotoxicity. J. Korean Med. Assoc. 2020, 63, 30–35. [Google Scholar] [CrossRef]
- Domitrović, R.; Cvijanović, O.; Pernjak-Pugel, E.; Škoda, M.; Mikelić, L.; Crnčević-Orlić, Ž. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol. 2013, 62, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Riahi, H. Preventive use of berberine in inhibition of lead-induced renal injury in rats. Environ. Sci. Pollut. Res. 2018, 25, 4896–4903. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.M.; Shalkami, A.G.S.; Khalaf, M.M.; Mohamed, W.R.; Hemeida, R.A.M. The impact of Keap1/Nrf2, P 38 MAPK/NF-κB and Bax/Bcl2/caspase-3 signaling pathways in the protective effects of berberine against methotrexate-induced nephrotoxicity. Biomed. Pharmacother. 2019, 109, 47–56. [Google Scholar] [CrossRef]
- Allameh, H.; Fatemi, I.; Malayeri, A.R.; Nesari, A.; Mehrzadi, S.; Goudarzi, M. Pretreatment with berberine protects against cisplatin-induced renal injury in male Wistar rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1825–1833. [Google Scholar] [CrossRef]
- Zhao, X.; Tong, N. Protective effects of berberine on doxorubicin-induced nephrotoxicity in mice. J. Transl. Med. 2012, 10, 400716. [Google Scholar] [CrossRef]
- Hussien, N.R.; Al-Kuraishy, H.M.; Al-Gareeb, A.I. Reno-protective effect of berberine. J. Pak. Med. Assoc. 2019, 69, S83–S87. [Google Scholar]
- Adil, M.; Kandhare, A.D.; Dalvi, G.; Ghosh, P.; Venkata, S.; Raygude, K.S.; Bodhankar, S.L. Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren. Fail. 2016, 38, 996–1006. [Google Scholar] [CrossRef]
- Ibrahim Fouad, G.; Ahmed, K.A. The protective impact of berberine against doxorubicin-induced nephrotoxicity in rats. Tissue Cell 2021, 73, 101612. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.K.; Malik, S.; Mutneja, E.; Sahu, A.K.; Rupashi, K.; Dinda, A.K.; Arya, D.S.; Bhatia, J. Mechanism involved in fortification by berberine in CDDP-induced nephrotoxicity. Curr. Mol. Pharmacol. 2020, 13, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Gholampour, F.; Keikha, S. Berberine protects the liver and kidney against functional disorders and histological damages induced by ferrous sulfate. Iran. J. Basic Med. Sci. 2018, 21, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.S.; Safwat, G.; Aboulkhair, M.; Abdel Moneim, A.E. The potential effect of berberine in mercury-induced hepatorenal toxicity in albino rats. Food Chem. Toxicol. 2014, 69, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, W.; Shao, X.; Wu, J.; Li, S.; Che, X.; Ni, Z. Integrated analysis of m6A methylome in cisplatin-induced acute kidney injury and berberine alleviation in mouse. Front. Genet. 2020, 11, 584460. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Xue, Q.; Kuang, L.; Xie, L.; Luo, R.; Nie, X. Berberine alleviates cisplatin-induced acute kidney injury by regulating mitophagy via PINK 1/Parkin pathway. Transl. Androl. Urol. 2020, 9, 1712–1724. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, Z.; Zhang, X.; Wu, A.; Huang, Y.; Miao, Y.; Yang, M. Effect of berberine on the renal tubular epithelial-to-mesenchymal transition by inhibition of the notch/snail pathway in diabetic nephropathy model KKAy mice. Drug Des. Dev. Ther. 2017, 11, 1065–1079. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Liang, D.; Jiang, Y.; Liang, W.; Chi, Z.H.; Ma, J. Protective effects of berberine on renal injury in streptozotocin (STZ)-Induced diabetic mice. Int. J. Mol. Sci. 2016, 17, 1327. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Hussien, N.R. Synergistic effect of berberine and pentoxifylline in attenuation of acute kidney injury. Int. J. Crit. Illn. Inj. Sci. 2019, 9, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huang, Z.; Liu, D.; Zheng, J.; Xie, J.; Chen, J.; Zeng, H.; Su, Z.; Li, Y. Effect of berberine on hyperuricemia and kidney injury: A network pharmacology analysis and experimental validation in a mouse model. Drug Des. Dev. Ther. 2021, 15, 3241–3254. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, W. Protective effect of berberine on renal fibrosis caused by diabetic nephropathy. Mol. Med. Rep. 2017, 16, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Wang, H.; Gao, Y.; Chen, G.; Pei, Y.; Bai, J. 1H-NMR-Based metabonomics of the protective effect of coptis chinensis and berberine on cinnabar-induced hepatotoxicity and nephrotoxicity in rats. Molecules 2017, 22, 1855. [Google Scholar] [CrossRef]
- Pervez, S.; Saeed, M.; Khan, H.; Shahid, M.; Ullah, I. Nephroprotective effect of berberis baluchistanica against gentamicin-induced nephrotoxicity in rabbit. Bangladesh J. Pharmacol. 2018, 13, 222–230. [Google Scholar] [CrossRef]
- Laamech, J.; El-hilaly, J.; Fetoui, H.; Chtourou, Y.; Tahraoui, A.; Lyoussi, B. Nephroprotective effects of Berberis Vulgaris L. total extract on lead acetate-induced toxicity in mice. Indian J. Pharm. Sci. 2016, 78, 326–333. [Google Scholar] [CrossRef]
- Rao, V.S.; Srinivas, K. Modern drug discovery process: An in silico approach. J. Bioinform. Seq. Anal. 2011, 2, 89–94. [Google Scholar]
- Ekins, S.; Mestres, J.; Testa, B. In silico pharmacology for drug discovery: Applications to targets and beyond. Br. J. Pharmacol. 2007, 152, 21–37. [Google Scholar] [CrossRef]
- Terstappen, G.C.; Reggiani, A. In silico research in drug discovery. Trends Pharmacol. Sci. 2001, 22, 23–26. [Google Scholar] [CrossRef]
- Kazmi, S.R.; Jun, R.; Yu, M.S.; Jung, C.; Na, D. In silico approaches and tools for the prediction of drug metabolism and fate: A review. Comput. Biol. Med. 2019, 106, 54–64. [Google Scholar] [CrossRef]
- Brogi, S.; Ramalho, T.C.; Kuca, K.; Medina-Franco, J.L.; Valko, M. Editorial: In silico methods for drug design and discovery. Front. Chem. 2020, 8, 612. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Discussing the mechanism of Dahuang Huanglian Xiexin decoction in the treatment of type 2 diabetes mellitus via network pharmacology and molecular docking. Res. Sq. 2021. preprint. [Google Scholar] [CrossRef]
- Zabidi, N.A.; Ishak, N.A.; Hamid, M.; Ashari, S.E.; Mohammad Latif, M.A. Inhibitory evaluation of Curculigo latifolia on α-glucosidase, DPP (IV) and in vitro studies in antidiabetic with molecular docking relevance to type 2 diabetes mellitus. J. Enzyme Inhib. Med. Chem. 2021, 36, 109–121. [Google Scholar] [CrossRef]
- Jhong, C.H.; Riyaphan, J.; Lin, S.H.; Chia, Y.C.; Weng, C.F. Screening alpha-glucosidase and alpha-amylase inhibitors from natural compounds by molecular docking in silico. BioFactors 2015, 41, 242–251. [Google Scholar] [CrossRef]
- Mandar, B.K.; Khanal, P.; Patil, B.M.; Dey, Y.N.; Pasha, I. In silico analysis of phytoconstituents from Tinospora cordifolia with targets related to diabetes and obesity. In Silico Pharmacol. 2021, 9, 3. [Google Scholar] [CrossRef]
- Rahman, N.; Muhammad, I.; Nayab, G.E.; Khan, H.; Aschner, M.; Filosa, R.; Daglia, M. Molecular docking of isolated alkaloids for possible α-Glucosidase inhibition. Biomolecules 2019, 9, 544. [Google Scholar] [CrossRef]
- Mohanty, I.; Kumar, S.; Rajesh, S. Dipeptidyl Peptidase IV inhibitory activity of Berberine and Mangiferin: An in silico approach. Int. J. Clin. Endocrinol. Metab. 2017, 3, 18–22. [Google Scholar] [CrossRef]
- Herowati, R.; Widodo, G.P. Molecular docking studies of chemical constituents of Tinospora cordifolia on glycogen phosphorylase. Procedia Chem. 2014, 13, 63–68. [Google Scholar] [CrossRef]
- Dou, Y.; Huang, R.; Li, Q.; Liu, Y.; Li, Y.; Chen, H.; Ai, G.; Xie, J.; Zeng, H.; Chen, J.; et al. Oxyberberine, an absorbed metabolite of berberine, possess superior hypoglycemic effect via regulating the PI3K/Akt and Nrf2 signaling pathways. Biomed. Pharmacother. 2021, 137, 111312. [Google Scholar] [CrossRef]
- Kaboli, P.J.; Ismail, P.; Ling, K.H. Molecular modeling, dynamics simulations, and binding efficiency of berberine derivatives: A new group of RAF inhibitors for cancer treatment. PLoS ONE 2018, 13, e0193941. [Google Scholar] [CrossRef]
- Song, L.; Luo, Y.; Wang, X.; Almutairi, M.M.; Pan, H.; Li, W.; Liu, Y.; Wang, Q.; Hong, M. Exploring the active mechanism of berberine against HCC by systematic pharmacology and experimental validation. Mol. Med. Rep. 2019, 20, 4654–4664. [Google Scholar] [CrossRef] [PubMed]
- Jabbarzadeh Kaboli, P.; Leong, M.P.Y.; Ismail, P.; Ling, K.H. Antitumor effects of berberine against EGFR, ERK1/2, P38 and AKT in MDA-MB231 and MCF-7 breast cancer cells using molecular modelling and in vitro study. Pharmacol. Rep. 2019, 71, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Khan, I.A.; Rehman, A.; Bibi, Z. Determination of effectiveness of berberine, a characteristic phytochemical of Berberis species, against human proteome using in-silico analysis. J. Biodivers. Environ. Sci. 2014, 4, 53–63. [Google Scholar]
- Foroughi, K.; Jahanbani, S.; Nazarnezhad, S.; Khastar, H.; Jafarisani, M. Survivin as a target for anti-cancer phytochemicals according to the molecular docking analysis. Int. J. Pept. Res. Ther. 2019, 26, 1115–1126. [Google Scholar] [CrossRef]
- Subair, T.I.; Soremekun, O.S.; Olotu, F.A.; Soliman, M.E.S. Prospecting the therapeutic edge of a novel compound (B12) over berberine in the selective targeting of Retinoid X Receptor in colon cancer. J. Mol. Model. 2021, 27, 231. [Google Scholar] [CrossRef] [PubMed]
- Rajasekhar, K.; Samanta, S.; Bagoband, V.; Murugan, N.A.; Govindaraju, T. Antioxidant berberine-derivative inhibits multifaceted amyloid toxicity. iScience 2020, 23, 101005. [Google Scholar] [CrossRef]
- Chu, M.; Chen, X.; Wang, J.; Guo, L.; Wang, Q.; Gao, Z.; Kang, J.; Zhang, M.; Feng, J.; Guo, Q.; et al. Polypharmacology of berberine based on multi-target binding motifs. Front. Pharmacol. 2018, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Marasco, D.; Vicidomini, C.; Krupa, P.; Cioffi, F.; Dinh, P.; Huy, Q.; Suan, M.; Florio, D.; Broersen, K.; Francesca, M.; et al. Plant isoquinoline alkaloids as potential neurodrugs: A comparative study of the effects of benzo[c]phenanthridine and berberine-based compounds on β-amyloid aggregation. Chem. Biol. Interact. 2021, 334, 109300. [Google Scholar] [CrossRef]
- Hussien, H.M.; Abd-Elmegied, A.; Ghareeb, D.A.; Hafez, H.S.; Ahmed, H.E.A.; El-Moneam, N.A. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem. Toxicol. 2018, 111, 432–444. [Google Scholar] [CrossRef]
- Sarkar, B.; Ullah, M.A.; Prottoy, M.N.I. A computational approach for exploring herbal inhibitors of acetylcholinesterase in Alzheimer’s disease. medRxiv 2020. [Google Scholar] [CrossRef]
- Amat-ur-rasool, H.; Ahmed, M.; Hasnain, S.; Ahmed, A.; Carter, W.G. In silico design of dual-binding site anti-cholinesterase phytochemical heterodimers as treatment options for Alzheimer’s disease. Curr. Issues Mol. Biol. 2022, 44, 152–175. [Google Scholar] [CrossRef]
- Ribaudo, G.; Zanforlin, E.; Canton, M.; Bova, S.; Zagotto, G. Preliminary studies of berberine and its semi-synthetic derivatives as a promising class of multi-target anti-parkinson agents. Nat. Prod. Res. 2018, 32, 1395–1401. [Google Scholar] [CrossRef]
- Jaitrong, M.; Boonsri, P.; Samosorn, S. Molecular docking studies of berberine derivative as novel multitarget Pcsk9 and Hmgcr inhibitors. Srinakharinwirot Sci. J. 2021, 37, 124–142. [Google Scholar]
- Olǧaç, A.; Orhan, I.E.; Banoglu, E. The potential role of in silico approaches to identify novel bioactive molecules from natural resources. Future Med. Chem. 2017, 9, 1663–1684. [Google Scholar] [CrossRef] [PubMed]
- Jenny, J.; Kumar, P.B. Dihydroxy berberine from tinospora cordifolia: In silico evidences for the mechanism of anti-inflammatory action through dual inhibition of lipoxygenase and cyclooxygenase. Indian J. Biochem. Biophys. 2021, 58, 244–252. [Google Scholar]
- Liu, X.; Fan, Y.; Du, L.; Mei, Z.; Fu, Y. In silico and in vivo studies on the mechanisms of chinese medicine formula (Gegen Qinlian decoction) in the treatment of ulcerative colitis. Front. Pharmacol. 2021, 12, 665102. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, T.; Zhao, J.; Li, C.; Zou, H.; Li, F.; Zhang, J.; Ren, L. Glucocorticoid receptor-mediated alleviation of inflammation by berberine: In vitro, in silico and in vivo investigations. Food Funct. 2021, 12, 11974–11986. [Google Scholar] [CrossRef] [PubMed]
- Shalkami, A.-G.S.; Hassanein, E.H.M.; Sayed, A.M.; Mohamed, W.R.; Khalaf, M.M.; Hemeida, R.A.M. Hepatoprotective effects of phytochemicals berberine and umbelliferone against methotrexate-induced hepatic intoxication: Experimental studies and in silico evidence. Environ. Sci. Pollut. Res. 2021, 28, 67593–67607. [Google Scholar] [CrossRef]
- Shinde, S.D.; Cheke, R.S.; Tathe, P.R.; Jain, P.G.; Narkhede, R.R. The Berberis aristata ameliorates oxazolone induced contact dermatitis: In-vivo and in silico evidences. Adv. Tradit. Med. 2021, 21, 685–692. [Google Scholar] [CrossRef]
- Alamzeb, M.; Ali, S.; Mamoon-Ur-Rashid; Khan, B.; Ihsanullah; Adnan; Omer, M.; Ullah, A.; Ali, J.; Setzer, W.N.; et al. Antileishmanial potential of berberine alkaloids from Berberis glaucocarpa roots: Molecular docking suggests relevant leishmania protein targets. Nat. Prod. Commun. 2021, 16, 1934578X211031148. [Google Scholar] [CrossRef]
- Nguyen, C.Q.; Nguyen, T.H.M.; Nguyen, T.T.T.; Bui, T.B.H.; Nguyen, T.T.; Huynh, N.T.; Le, T.D.; Nguyen, T.M.P.; Nguyen, D.T.; Nguyen, M.T.; et al. Designs, synthesis, docking studies, and biological evaluation of novel berberine derivatives targeting zika virus. J. Chem. 2021, 2021, 5567111. [Google Scholar] [CrossRef]
- Jhanji, R.; Bhati, V.; Singh, A.; Kumar, A. Phytomolecules against bacterial biofilm and efflux pump: An in silico and in vitro study. J. Biomol. Struct. Dyn. 2020, 38, 5500–5512. [Google Scholar] [CrossRef] [PubMed]
- Laudadio, E.; Cedraro, N.; Mangiaterra, G.; Citterio, B.; Mobbili, G.; Minnelli, C.; Bizzaro, D.; Biavasco, F.; Galeazzi, R. Natural alkaloid berberine activity against Pseudomonas aeruginosa MexXY-mediated aminoglycoside resistance: In silico and in vitro studies. J. Nat. Prod. 2019, 82, 1935–1944. [Google Scholar] [CrossRef]
- Giorgini, G.; Mangiaterra, G.; Cedraro, N.; Laudadio, E.; Sabbatini, G.; Cantarini, M.; Minnelli, C.; Mobbili, G.; Frangipani, E.; Biavasco, F.; et al. Berberine derivatives as pseudomonas aeruginosa mexxy-oprm inhibitors: Activity and in silico insights. Molecules 2021, 26, 6644. [Google Scholar] [CrossRef] [PubMed]
- Milani, G.; Cavalluzzi, M.M.; Solidoro, R.; Salvagno, L.; Quintieri, L.; Di Somma, A.; Rosato, A.; Corbo, F.; Franchini, C.; Duilio, A.; et al. Molecular simplification of natural products: Synthesis, antibacterial activity, and molecular docking studies of berberine open models. Biomedicines 2021, 9, 452. [Google Scholar] [CrossRef]
- Sun, N.; Chan, F.Y.; Lu, Y.J.; Neves, M.A.C.; Lui, H.K.; Wang, Y.; Chow, K.Y.; Chan, K.F.; Yan, S.C.; Leung, Y.C.; et al. Rational design of berberine-based FtsZ inhibitors with broad-spectrum antibacterial activity. PLoS ONE 2014, 9, e97514. [Google Scholar] [CrossRef]
- Domadia, P.N.; Bhunia, A.; Sivaraman, J.; Swarup, S.; Dasgupta, D. Berberine targets assembly of Escherichia coli cell division protein FtsZ. Biochemistry 2008, 47, 3225–3234. [Google Scholar] [CrossRef]
- Kwatra, B.; Bhattacharya, B.; Khokhawat, T.; Raphael Jes, A.; Bhati, M.; Ahuja, S. Drug repurposing: In silico modeling of Mucormycosis. J. Sci. Res. Rep. 2021, 27, 53–61. [Google Scholar] [CrossRef]
- Da Silva, G.D.; de Lima, H.G.; de Freitas, H.F.; da Rocha Pita, S.S.; dos Santos Luz, Y.; de Figueiredo, M.P.; Uzêda, R.S.; Branco, A.; Costa, S.L.; Batatinha, M.J.M.; et al. In vitro and in silico studies of the larvicidal and anticholinesterase activities of berberine and piperine alkaloids on Rhipicephalus microplus. Ticks Tick-Borne Dis. 2021, 12, 101643. [Google Scholar] [CrossRef]
- Kumar, M.; Chung, S.M.; Enkhtaivan, G.; Patel, R.V.; Shin, H.S.; Mistry, B.M. Molecular docking studies and biological evaluation of berberine–benzothiazole derivatives as an anti-influenza agent via blocking of neuraminidase. Int. J. Mol. Sci. 2021, 22, 2368. [Google Scholar] [CrossRef]
- Enkhtaivan, G.; Muthuraman, P.; Kim, D.H.; Mistry, B. Discovery of berberine based derivatives as anti-influenza agent through blocking of neuraminidase. Bioorg. Med. Chem. 2017, 25, 5185–5193. [Google Scholar] [CrossRef] [PubMed]
- Le, K.; Tran, D.; Nguyen, A.; Le, L. A screening of neuraminidase inhibition activities of isoquinolone alkaloids in Coptis chinensis using molecular docking and pharmacophore analysis. ACS Omega 2020, 5, 30315–30322. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Chaturvedi, A.; Shrivastava, A.; Dubey, N.; Jain, S.; Saxena, N.; Gupta, P.; Mujariya, R. In silico interaction of Berberine with some immunomodulatory targets: A docking analysis. Indian J. Biochem. Biophys. 2022, 59, 848–853. [Google Scholar] [CrossRef]
- Yu, R.; Li, P. Screening of potential spike glycoprotein/ACE2 dual antagonists against COVID-19 in silico molecular docking. J. Virol. Methods 2020, 301, 114424. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Kumar, S.; Prasad, A.K.; Bhatt, M.L.B.; Saxena, S.K. Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor. VirusDisease 2020, 31, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Li, K.; Maskey, A.R.; Huang, W.; Toutov, A.A.; Yang, N.; Srivastava, K.; Geliebter, J.; Tiwari, R.; Miao, M.; et al. A small molecule compound berberine as an orally active therapeutic candidate against COVID-19 and SARS: A computational and mechanistic study. FASEB J. 2021, 35, e21360. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P. In silico investigation of phytoconstituents from Indian medicinal herb ‘Tinospora cordifolia (giloy)’ against SARS-CoV-2 (COVID-19) by molecular dynamics approach. J. Biomol. Struct. Dyn. 2021, 39, 6792–6809. [Google Scholar] [CrossRef]
- Agrawal, A.; Jain, N.K.; Kumar, N.; Kulkarni, G.T. Molecular Docking Study to Identify Potential Inhibitor of COVID-19 Main Protease Enzyme: An In-Silico Approach; Cambridge University Press: Washington, DC, USA, 2020. [Google Scholar]
- Narkhede, R.R.; Pise, A.V.; Cheke, R.S.; Shinde, S.D. Recognition of natural products as potential inhibitors of COVID-19 Main Protease (Mpro): In silico evidences. Nat. Prod. Bioprospect. 2020, 10, 297–306. [Google Scholar] [CrossRef]
- Krupanidhi, S.; Abraham Peele, K.; Venkateswarulu, T.C.; Ayyagari, V.S.; Nazneen Bobby, M.; John Babu, D.; Venkata Narayana, A.; Aishwarya, G. Screening of phytochemical compounds of Tinospora cordifolia for their inhibitory activity on SARS-CoV-2: An in silico study. J. Biomol. Struct. Dyn. 2020, 39, 5799–5803. [Google Scholar] [CrossRef]
- Garg, S.; Roy, A. In silico analysis of selected alkaloids against main protease (Mpro) of SARS-CoV-2. Chem. Biol. Interact. 2020, 332, 109309. [Google Scholar] [CrossRef]
- Gopalasatheeskumar, K.; Lakshmanan, K.; Moulishankar, A.; Suresh, J.; Kalaichelvan, V.K.; Kumudhaveni, B. Screening of Kabasura Kudineer Chooranam against COVID-19 through targeting of main protease and RNA-dependent RNA polymerase of SARS-CoV-2 by molecular docking studies. SSRN Electron. J. 2020, 2020, 3625653. [Google Scholar] [CrossRef]
- Cheke, R.S.; Narkhede, R.R.; Shinde, S.D.; Ambhore, J.P.; Jain, P.G. Natural product emerging as potential sars spike glycoproteins-ace2 inhibitors to combat COVID-19 attributed by in-silico investigations. Biointerface Res. Appl. Chem. 2021, 11, 10628–10639. [Google Scholar] [CrossRef]
- Kumar, S.; Kashyap, P.; Chowdhury, S.; Kumar, S.; Panwar, A.; Kumar, A. Identification of phytochemicals as potential therapeutic agents that binds to Nsp15 protein target of coronavirus (SARS-CoV-2) that are capable of inhibiting virus replication. Phytomedicine 2020, 85, 153317. [Google Scholar] [CrossRef]
- Matkovics, B.; Varga, S.I.; Szabo, L.; Witas, H. The effect of diabetes on the activities of the peroxide metabolism enzymes. Horm. Metab. Res. 1982, 14, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.H.; Pang, Q.Q.; Jung, P.; Cho, E.J. Antioxidant activity and acteoside analysis of Abeliophyllum distichum. Antioxidants 2020, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhou, S.; Tang, J.; Zhang, K.; Guang, L.; Huang, Y.; Xu, Y.; Ying, Y. Protective effect of berberine on beta cells in streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Eur. J. Pharmacol. 2009, 606, 262–268. [Google Scholar] [CrossRef]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J. Diabetes Res. 2017, 2017, 4826724. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine and barberry (Berberis vulgaris): A clinical review. Phytother. Res. 2019, 33, 504–523. [Google Scholar] [CrossRef]
- Jung, H.A.; Min, B.S.; Yokozawa, T.; Lee, J.H.; Kim, Y.S.; Choi, J.S. Anti-Alzheimer and antioxidant activities of coptidis rhizoma alkaloids. Biol. Pharm. Bull. 2009, 32, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-S.; Kim, S.-J.; Jung, M.Y. Inhibitory activity of berberine on DNA strand cleavage induced by hydrogen peroxide and cytochrome c. Biosci. Biotechnol. Biochem. 2001, 65, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Stoyanovsky, D.; Tyurina, Y.; Shrivastava, I.; Bahar, I.; Tyurin, V.; Protchenko, O.; Jadhav, S.; Bolevich, S.; Kozlov, A.; Vladimirov, Y.; et al. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Radic. Biol. Med. 2019, 133, 153–161. [Google Scholar] [CrossRef]
- Jang, M.H.; Kim, H.Y.; Kang, K.S.; Yokozawa, T.; Park, J.H. Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Arch. Pharm. Res. 2009, 32, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Parvin, M.S.; Chlebek, J.; Hošťálková, A.; Catapano, M.C.; Lomozová, Z.; Macáková, K.; Mladěnka, P. Interactions of isoquinoline alkaloids with transition metals iron and copper. Molecules 2022, 27, 6429. [Google Scholar] [CrossRef]
- Aalikhani, M.; Alikhani, M.; Jahanshahi, M.; Elyasi, L.; Khalili, M. Berberine is a promising alkaloid to attenuate iron toxicity efficiently in iron-overloaded mice. Nat. Prod. Commun. 2022, 17, 1934578X211029522. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxidative Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, A.; Kumar, A. Targeting oxidative stress through antioxidants in diabetes mellitus. J. Drug Target. 2018, 26, 766–776. [Google Scholar] [CrossRef]
- Ji, M.; Gong, X.; Li, X.; Wang, C.; Li, M. Mechanism of polyphenols from Hippophae species—A review. Molecules 2020, 25, 917. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Wagner, A.H. Role of protein carbonylation in diabetes. J. Inherit. Metab. Dis. 2018, 41, 29–38. [Google Scholar] [CrossRef]
- Lao-Ong, T.; Chatuphonprasert, W.; Nemoto, N.; Jarukamjorn, K. Alteration of hepatic glutathione peroxidase and superoxide dismutase expression in streptozotocin-induced diabetic mice by berberine. Pharm. Biol. 2012, 50, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Chatuphonprasert, W.; Lao-Ong, T.; Jarukamjorn, K. Improvement of superoxide dismutase and catalase in streptozotocin-nicotinamide-induced type 2-diabetes in mice by berberine and glibenclamide. Pharm. Biol. 2014, 52, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, P.; Tao, S.; Deng, Y.; Li, X.; Lan, T.; Zhang, X.; Guo, F.; Huang, W.; Chen, F.; et al. Berberine inhibits aldose reductase and oxidative stress in rat mesangial cells cultured under high glucose. Arch. Biochem. Biophys. 2008, 475, 128–134. [Google Scholar] [CrossRef]
- Xie, X.; Chang, X.; Chen, L.; Huang, K.; Huang, J.; Wang, S.; Shen, X.; Liu, P.; Huang, H. Berberine ameliorates experimental diabetes-induced renal inflammation and fibronectin by inhibiting the activation of RhoA/ROCK signaling. Mol. Cell. Endocrinol. 2013, 381, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Zhou, S.W. Protective effect of berberine on antioxidant enzymes and positive transcription elongation factor b expression in diabetic rat liver. Fitoterapia 2011, 82, 184–189. [Google Scholar] [CrossRef]
- Wu, D.; Wen, W.; Qi, C.L.; Zhao, R.X.; Lü, J.H.; Zhong, C.Y.; Chen, Y.Y. Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine 2012, 19, 712–718. [Google Scholar] [CrossRef]
- Tang, L.Q.; Wei, W.; Chen, L.M.; Liu, S. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J. Ethnopharmacol. 2006, 108, 109–115. [Google Scholar] [CrossRef]
- Bhutada, P.; Mundhada, Y.; Bansod, K.; Tawari, S.; Patil, S.; Dixit, P.; Umathe, S.; Mundhada, D. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav. Brain Res. 2011, 220, 30–41. [Google Scholar] [CrossRef]
- Moghaddam, H.K.; Baluchnejadmojarad, T.; Roghani, M.; Khaksari, M.; Norouzi, P.; Ahooie, M.; Mahboobi, F. Berberine ameliorate oxidative stress and astrogliosis in the hippocampus of STZ-induced diabetic rats. Mol. Neurobiol. 2014, 49, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Z.; Song, Y.; Wu, D.; Zheng, X.; Li, P.; Jin, J.; Xu, N.; Li, L. Effects of berberine on amelioration of hyperglycemia and oxidative stress in high glucose and high fat diet-induced diabetic hamsters in vivo. Biomed. Res. Int. 2015, 2015, 313808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Campbell, T.; Perry, B.; Beaurepaire, C.; Qin, L. Hypoglycemic and insulin-sensitizing effects of berberine in high-fat diet- and streptozotocin-induced diabetic rats. Metabolism. 2011, 60, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kakkar, P. Antihyperglycemic and antioxidant effect of Berberis aristata root extract and its role in regulating carbohydrate metabolism in diabetic rats. J. Ethnopharmacol. 2009, 123, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Hei, Z.Q.; Nie, H.; Tang, F.T.; Huang, H.Q.; Li, X.J.; Deng, Y.H.; Chen, S.R.; Guo, F.F.; Huang, W.G.; et al. Berberine ameliorates renal injury in streptopzotocin-induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin. Med. J. 2008, 121, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.O.; Kim, H.J. Berberine ameliorates cold and mechanical allodynia in a rat model of diabetic neuropathy. J. Med. Food 2013, 16, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, K. Berberine alleviates oxidative stress in islets of diabetic mice by inhibiting miR-106b expression and up-regulating SIRT1. J. Cell. Biochem. 2017, 118, 4349–4357. [Google Scholar] [CrossRef]
- Chandirasegaran, G.; Elanchezhiyan, C.; Ghosh, K. Berberine chloride ameliorates oxidative stress, inflammation and apoptosis in the pancreas of streptozotocin induced diabetic rats. Biomed. Pharmacother. 2017, 95, 175–185. [Google Scholar] [CrossRef]
- Chandirasegaran, G.; Elanchezhiyana, C.; Ghosh, K. Effects of berberine chloride on the liver of streptozotocin-induced diabetes in albino wistar rats. Biomed. Pharmacother. 2018, 99, 227–236. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Abdel-rahman, M.M.; Bastawy, N.A.; Eissa, H.M. Modulatory effect of berberine on adipose tissue PPAR γ, adipocytokines and oxidative stress in high fat diet/streptozotocin-induced diabetic rats. J. Appl. Pharm. Sci. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Q.; Zhang, X.; Wang, T.; Hu, W.; Manicum, T.; Chen, H.; Sun, L. Possible therapeutic potential of berberine in the treatment of STZ plus HFD-induced diabetic osteoporosis. Biomed. Pharmacother. 2018, 108, 280–287. [Google Scholar] [CrossRef]
- Zych, M.; Wojnar, W.; Kielanowska, M.; Folwarczna, J.; Kaczmarczyk-sedlak, I. Effect of berberine on glycation, aldose reductase activity, and oxidative stress in the lenses of streptozotocin-induced diabetic rats in vivo—A preliminary study. Int. J. Mol. Sci. 2020, 21, 4278. [Google Scholar] [CrossRef] [PubMed]
- Adefegha, S.A.; Dada, F.A.; Oyeleye, S.I.; Oboh, G. Effects of berberine on cholinesterases and monoamine oxidase activities, and antioxidant status in the brain of streptozotocin (STZ)-induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2022, 33, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Elumalai, S.; Karunakaran, U.; Moon, J.S.; Won, K.C. NADPH Oxidase (NOX) targeting in diabetes: A special emphasis on pancreatic β-cell dysfunction. Cells 2021, 10, 1573. [Google Scholar] [CrossRef]
- Sedeek, M.; Montezano, A.C.; Hebert, R.L.; Gray, S.P.; Di Marco, E.; Jha, J.C.; Cooper, M.E.; Jandeleit-Dahm, K.; Schiffrin, E.L.; Wilkinson-Berka, J.L.; et al. Oxidative stress, Nox isoforms and complications of diabetes-potential targets for novel therapies. J. Cardiovasc. Transl. Res. 2012, 5, 509–518. [Google Scholar] [CrossRef]
- Sarna, L.K.; Wu, N.; Hwang, S.Y.; Siow, Y.L.; Karmin, O. Berberine inhibits NADPH oxidase mediated superoxide anion production in macrophages. Can. J. Physiol. Pharmacol. 2010, 88, 369–378. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, Y.; Li, J.; Su, C.; Wu, F.; Xia, W.H.; Yang, Z.; Yu, B.B.; Qiu, Y.X.; Tao, J. Berberine improves endothelial function by reducing endothelial microparticles-mediated oxidative stress in humans. Int. J. Cardiol. 2013, 167, 936–942. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Lv, X.; Zhang, M.; Song, Y.; Chen, L.; Liu, Y. Ameliorative effect of berberine on endothelial dysfunction in diabetic rats induced by high-fat diet and streptozotocin. Eur. J. Pharmacol. 2009, 620, 131–137. [Google Scholar] [CrossRef]
- Ding, H.; Hashem, M.; Triggle, C. Increased oxidative stress in the streptozotocin-induced diabetic apoE-deficient mouse: Changes in expression of NADPH oxidase subunits and eNOS. Eur. J. Pharmacol. 2007, 561, 121–128. [Google Scholar] [CrossRef]
- Liang, C.F.; Liu, J.T.; Wang, Y.; Xu, A.; Vanhoutte, P.M. Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 777–784. [Google Scholar] [CrossRef]
- Goettsch, C.; Goettsch, W.; Muller, G.; Seebach, J.; Schnittler, H.J.; Morawietz, H. Nox4 overexpression activates reactive oxygen species and p38 MAPK in human endothelial cells. Biochem. Biophys. Res. Commun. 2009, 380, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Rueckschloss, U.; Duerrschmidt, N.; Morawietz, H. NADPH oxidase in endothelial cells: Impact on atherosclerosis. Antioxidants Redox Signal. 2003, 5, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Inoue, N.; Kawashima, S. Role of the vascular NADH/NADPH oxidase system in atherosclerosis. Ann. N. Y. Acad. Sci. 2000, 902, 241–248. [Google Scholar] [CrossRef]
- Eid, A.A.; Ford, B.M.; Block, K.; Kasinath, B.S.; Gorin, Y.; Ghosh-Choudhury, G.; Barnes, J.L.; Abboud, H.E. AMP-activated Protein Kinase (AMPK) negatively regulates Nox4-dependent activation of p53 and epithelial cell apoptosis in diabetes. J. Biol. Chem. 2010, 285, 37503–37512. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Liang, B.; Xu, J.; Xie, Z.; Liu, C.; Viollet, B.; Yan, D.; Zou, M.H. AMPKα2 deletion causes aberrant expression and activation of NAD(P)H Oxidase and consequent endothelial dysfunction in vivo: Role of 26S proteasomes. Circ. Res. 2010, 106, 1117–1128. [Google Scholar] [CrossRef]
- Yin, J.; Ye, J.; Jia, W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharm. Sin. B 2012, 2, 327–334. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Song, P.; Viollet, B.; Zou, M.H. In vivo activation of AMP-activated protein kinase attenuates diabetes-enhanced degradation of GTP cyclohydrolase I. Diabetes 2009, 58, 1893–1901. [Google Scholar] [CrossRef]
- Song, P.; Zou, M.-H. Regulation of NAD(P)H Oxidase by AMPK in Cardiovascular Systems. Free Radic. Biol. Med. 2012, 52, 1607–1619. [Google Scholar] [CrossRef]
- Toyoda, T.; Hayashi, T.; Miyamoto, L.; Yonemitsu, S.; Nakano, M.; Tanaka, S.; Ebihara, K.; Masuzaki, H.; Hosoda, K.; Inoue, G.; et al. Possible involvement of the α1 isoform of 5′AMP-activated protein kinase in oxidative stress-stimulated glucose transport in skeletal muscle. Am. J. Physiol.—Endocrinol. Metab. 2004, 287, 166–173. [Google Scholar] [CrossRef]
- Salt, I.; Celler, J.W.; Hawley, S.A.; Prescott, A.; Woods, A.; Carling, D.; Hardie, D.G. AMP-activated protein kinase: Greater AMP dependence, and preferential nuclear localization, of complexes containing the α2 isoform. Biochem. J. 1998, 334, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Kukidome, D.; Nishikawa, T.; Sonoda, K.; Imoto, K.; Fujisawa, K.; Yano, M.; Motoshima, H.; Taguchi, T.; Matsumura, T.; Araki, E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 2006, 55, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Meng, X.; Gao, X.; Pang, X.; Wang, Y.; Wu, X.; Deng, X.; Zhang, Q.; Sun, C.; Li, Y. Elevated serum xanthine oxidase activity is associated with the development of type 2 diabetes: A prospective cohort study. Diabetes Care 2018, 41, 884–890. [Google Scholar] [CrossRef]
- Sunagawa, S.; Shirakura, T.; Hokama, N.; Kozuka, C.; Yonamine, M.; Namba, T.; Morishima, S.; Nakachi, S.; Nishi, Y.; Ikema, T.; et al. Activity of xanthine oxidase in plasma correlates with indices of insulin resistance and liver dysfunction in patients with type 2 diabetes mellitus and metabolic syndrome: A pilot exploratory study. J. Diabetes Investig. 2019, 10, 94–103. [Google Scholar] [CrossRef]
- Liu, S.; Xing, J.; Zheng, Z.; Song, F.; Liu, Z.; Liu, S. Ultrahigh performance liquid chromatography-triple quadrupole mass spectrometry inhibitors fishing assay: A novel method for simultaneously screening of xanthine oxidase inhibitor and superoxide anion scavenger in a single analysis. Anal. Chim. Acta 2012, 715, 64–70. [Google Scholar] [CrossRef]
- Assmann, T.S.; Brondani, L.A.; Bouças, A.P.; Rheinheimer, J.; de Souza, B.M.; Canani, L.H.; Bauer, A.C.; Crispim, D. Nitric oxide levels in patients with diabetes mellitus: A systematic review and meta-analysis. Nitric Oxide—Biol. Chem. 2016, 61, 1–9. [Google Scholar] [CrossRef]
- Duicu, O.M.; Lighezan, R.; Sturza, A.; Balica, R.; Vaduva, A.; Feier, H.; Gaspar, M.; Ionac, A.; Noveanu, L.; Borza, C.; et al. Assessment of mitochondrial dysfunction and monoamine oxidase contribution to oxidative stress in human diabetic hearts. Oxidative Med. Cell. Longev. 2016, 2016, 8470394. [Google Scholar] [CrossRef]
- Vomhof-DeKrey, E.E.; Picklo, M.J. The Nrf2-antioxidant response element pathway: A target for regulating energy metabolism. J. Nutr. Biochem. 2012, 23, 1201–1206. [Google Scholar] [CrossRef]
- Jia, L.; Xue, K.; Liu, J.; Habotta, O.A.; Hu, L.; Abdel Moneim, A.E. Anticolitic effect of berberine in rat experimental model: Impact of PGE2/p38 MAPK pathways. Mediat. Inflamm. 2020, 2020, 9419085. [Google Scholar] [CrossRef]
- Hsu, Y.Y.; Chen, C.S.; Wu, S.N.; Jong, Y.J.; Lo, Y.C. Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur. J. Pharm. Sci. 2012, 46, 415–425. [Google Scholar] [CrossRef]
- Hsu, Y.Y.; Tseng, Y.T.; Lo, Y.C. Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth. Toxicol. Appl. Pharmacol. 2013, 272, 787–796. [Google Scholar] [CrossRef]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of Berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Tang, K.; Chen, R.; Nie, H.; Liang, S.; Zhang, J.; Zhang, Y.; Yang, Q. Berberine attenuates hepatic oxidative stress in rats with non-alcoholic fatty liver disease via the Nrf2/ARE signalling pathway. Exp. Ther. Med. 2019, 17, 2091–2098. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, X.; Mao, G.; Gao, Z.; Wang, H.; He, Q.; Li, D. Hepatoprotection of berberine against hydrogen peroxide-induced apoptosis by upregulation of sirtuin 1. Phyther. Res. 2013, 27, 417–421. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between oxidative stress and SIRT1: Impact on the aging process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef]
- Alam, F.; Syed, H.; Amjad, S.; Baig, M.; Khan, T.A.; Rehman, R. Interplay between oxidative stress, SIRT1, reproductive and metabolic functions. Curr. Res. Physiol. 2021, 4, 119–124. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Q.; Zeng, Z.; Wu, J.; Zhang, Y.; Chen, Z. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxidative Med. Cell. Longev. 2017, 2017, 7543973. [Google Scholar] [CrossRef]
- Chen, Z.; Shentu, T.P.; Wen, L.; Johnson, D.A.; Shyy, J.Y.J. Regulation of SIRT1 by oxidative stress-responsive miRNAs and a systematic approach to identify its role in the endothelium. Antioxid. Redox Signal. 2013, 19, 1522–1538. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Shang, Y.C.; Wang, S.; Maiese, K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert Opin. Ther. Targets 2012, 16, 167–178. [Google Scholar] [CrossRef]
- Meng, T.; Qin, W.; Liu, B. SIRT1 antagonizes oxidative stress in diabetic vascular complication. Front. Endocrinol. 2020, 11, 568861. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, X.; Feng, K.; Wang, T.; Li, W.; Yuan, T.; Sun, X.; Sun, Q.; Xiang, H.; Wang, H. Berberine moderates glucose and lipid metabolism through multipathway mechanism. Evid.-Based Complement. Altern. Med. 2011, 2011, 924851. [Google Scholar] [CrossRef]
- Donadelli, M.; Dando, I.; Fiorini, C.; Palmieri, M. UCP2, a mitochondrial protein regulated at multiple levels. Cell. Mol. Life Sci. 2014, 71, 1171–1190. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zheng, Y.; Huang, J.; Peng, W.; Chen, X.; Kang, X.; Zeng, Q. UCP2 ameliorates mitochondrial dysfunction, inflammation, and oxidative stress in lipopolysaccharide-induced acute kidney injury. Int. Immunopharmacol. 2019, 71, 336–349. [Google Scholar] [CrossRef]
- Lee, K.U.; Lee, I.K.; Han, J.; Song, D.K.; Kim, Y.M.; Song, H.S.; Kim, H.S.; Lee, W.J.; Koh, E.H.; Song, K.H.; et al. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ. Res. 2005, 96, 1200–1207. [Google Scholar] [CrossRef]
- Teshima, Y.; Akao, M.; Jones, S.P.; Marbán, E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ. Res. 2003, 93, 192–200. [Google Scholar] [CrossRef]
- Hou, G.; Jin, Y.; Liu, M.; Wang, C.; Song, G. UCP2–866G/A polymorphism is associated with prediabetes and type 2 diabetes. Arch. Med. Res. 2020, 51, 556–563. [Google Scholar] [CrossRef]
- Čater, M.; Bombek, L.K. Protective role of mitochondrial uncoupling proteins against age-related oxidative stress in type 2 diabetes mellitus. Antioxidants 2022, 11, 1473. [Google Scholar] [CrossRef]
- Hass, D.T.; Barnstable, C.J. Uncoupling proteins in the mitochondrial defense against oxidative stress. Prog. Retin. Eye Res. 2021, 83, 100941. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Patel, S.; Santani, D. Role of NF-κB in the pathogenesis of diabetes and its associated complications. Pharmacol. Rep. 2009, 61, 595–603. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Liu, P.; Shen, X.; Lan, T.; Li, W.; Jiang, Q.; Xie, X.; Huang, H. Effects of berberine on matrix accumulation and NF-kappa B signal pathway in alloxan-induced diabetic mice with renal injury. Eur. J. Pharmacol. 2010, 638, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Li, Z.; Zhang, H.; Ma, L.; Ma, Z.; Zhang, Y.; Zou, J.; Li, M.; Ma, L.; Wang, X.; et al. Berberine protects against diabetic retinopathy by inhibiting cell apoptosis via deactivation of the NF-κB signaling pathway. Mol. Med. Rep. 2020, 22, 4227–4235. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xie, M. Studies on the hypoglycemic effect of Coptis chinensis and berberine. Acta Pharm. Sin. 1986, 21, 401–406. [Google Scholar] [CrossRef]
- Ni, Y.X. Therapeutic effect of berberine on 60 patients with type II diabetes mellitus and experimental research. Chin. J. Mod. Dev. Tradit. 1988, 8, 707, 711–713. [Google Scholar]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, J.; Xue, R.; Wu, J.; Zhao, W.; Wang, Z.; Kong, W.; Jiang, J. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metab. Clin. Exp. 2010, 59, 285–292. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, L.; Wang, C. Efficacy and safety of berberine in patients with type 2 diabetes mellitus: A meta-analysis. Chin. Herb. Med. 2015, 7, 344–353. [Google Scholar] [CrossRef]
- Li, A.; Lin, C.; Xie, F.; Jin, M.; Lin, F. Berberine ameliorates insulin resistance by inhibiting IKK/NF-κB, JNK, and IRS-1/AKT signaling pathway in liver of gestational diabetes mellitus rats. Metab. Syndr. Relat. Disord. 2022, 20, 480–488. [Google Scholar] [CrossRef]
- Cole, L.K.; Zhang, M.; Chen, L.; Sparagna, G.C.; Vandel, M.; Xiang, B.; Dolinsky, V.W.; Hatch, G.M. Supplemental berberine in a high-fat diet reduces adiposity and cardiac dysfunction in offspring of mouse dams with gestational diabetes mellitus. J. Nutr. 2021, 151, 892–901. [Google Scholar] [CrossRef]
- Cole, L.K.; Sparagna, G.C.; Vandel, M.; Xiang, B.; Dolinsky, V.W.; Hatch, G.M. Berberine elevates cardiolipin in heart of offspring from mouse dams with high fat diet-induced gestational diabetes mellitus. Sci. Rep. 2021, 11, 15770. [Google Scholar] [CrossRef]
- Mejia, E.M.; Hatch, G.M. Mitochondrial phospholipids: Role in mitochondrial function. J. Bioenerg. Biomembr. 2016, 48, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yi, H.; Wu, J.; Kuang, T.; Zhang, J.; Li, Q.; Du, H.; Xu, T.; Jiang, G.; Fan, G. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed. Pharmacother. 2021, 133, 110984. [Google Scholar] [CrossRef]
- Leng, S.H.; Lu, F.E.; Xu, L.J. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol. Sin. 2004, 25, 496–502. [Google Scholar] [PubMed]
- Yamagata, K. Roles of HNF1α and HNF4α in pancreatic β-cells: Lessons from a monogenic form of diabetes (MODY). Vitam. Horm. 2014, 95, 407–423. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Lu, F.E.; Leng, S.H.; Fang, X.S.; Chen, G.; Wang, Z.S.; Dong, L.P.; Yan, Z.Q. Facilitating effects of berberine on rat pancreatic islets through modulating hepatic nuclear factor 4 alpha expression and glucokinase activity. World J. Gastroenterol. 2008, 14, 6004–6011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Lu, F.E.; Chen, G.; Xu, L.J.; Wang, K.F.; Zou, X. Effect of berberine on insulin secretion and glucokinase activity of NIT-1 cells. Acta Pharm. Sin. 2007, 42, 1045–01049. [Google Scholar]
- Toulis, K.A.; Nirantharakumar, K.; Pourzitaki, C.; Barnett, A.H.; Tahrani, A.A. Glucokinase activators for type 2 diabetes: Challenges and future developments. Drugs 2020, 80, 467–475. [Google Scholar] [CrossRef]
- Lu, S.S.; Yu, Y.L.; Zhu, H.J.; Liu, X.D.; Liu, L.; Liu, Y.W.; Wang, P.; Xie, L.; Wang, G.J. Berberine promotes glucagon-like peptide-1 (7-36) amide secretion in streptozotocin-induced diabetic rats. J. Endocrinol. 2009, 200, 159–165. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, X.; Li, M.; Li, W.; Yu, M.; Zhang, H.; Ping, F.; Wang, Z.; Zheng, J. Berberine moderates glucose metabolism through the GnRH-GLP-1 and MAPK pathways in the intestine. BMC Complement. Altern. Med. 2014, 14, 188. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Boer, G.A.; Holst, J.J. Increatin hormones and type 2 diabetes mellitus—Mechanistic insights and therapeutic approches. Biology 2020, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rubio, K.G.; González-Ortiz, M.; Martínez-Abundis, E.; Robles-Cervantes, J.A.; Espinel-Bermúdez, M.C. Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab. Syndr. Relat. Disord. 2013, 11, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Liu, Y.; Zhou, F.; Zhang, Y.; Zhu, Q.; Zhang, L.; Zhang, Q.; Wang, S.; Zhu, K.; Wang, X.; et al. Berberine inhibits glucose oxidation and insulin secretion in rat islets. Endocr. J. 2018, 65, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. The relevance of AMP-activated protein kinase in insulin-secreting β cells: A potential target for improving β cell function? J. Physiol. Biochem. 2019, 75, 423–432. [Google Scholar] [CrossRef]
- Da Silva Xavier, G.; Leclerc, I.; Varadi, A.; Tsuboi, T.; Moule, S.K.; Rutter, G.A. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem. J. 2003, 371, 761–774. [Google Scholar] [CrossRef]
- Fu, A.; Eberhard, C.E.; Screaton, R.A. Role of AMPK in pancreatic beta cell function. Mol. Cell. Endocrinol. 2013, 366, 127–134. [Google Scholar] [CrossRef]
- Salt, I.P.; Johnson, G.; Ashcroft, S.J.H.; Hardie, D.G. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic β cells, and may regulate insulin release. Biochem. J. 1998, 335, 533–539. [Google Scholar] [CrossRef]
- Targonsky, E.D.; Dai, F.; Koshkin, V.; Karaman, G.T.; Gyulkhandanyan, A.V.; Zhang, Y.; Chan, C.B.; Wheeler, M.B. α-Lipoic acid regulates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Diabetologia 2006, 49, 1587–1598. [Google Scholar] [CrossRef]
- Langelueddecke, C.; Jakab, M.; Ketterl, N.; Lehner, L.; Hufnagl, C.; Schmidt, S.; Geibel, J.P.; Fuerst, J.; Ritter, M. Effect of the AMP-kinase modulators AICAR, metformin and compound C on insulin secretion of INS-1E rat insulinoma cells under standard cell culture conditions. Cell. Physiol. Biochem. 2012, 29, 75–86. [Google Scholar] [CrossRef]
- Zhang, F.; Dey, D.; Bränstrom, R.; Forsberg, L.; Lu, M.; Zhang, Q.; Sjöholm, Å. BLX-1002, a novel thiazolidinedione with no PPAR affinity, stimulates AMP- Activated protein kinase activity, raises cytosolic Ca2+, and enhances glucose- Stimulated insulin secretion in a PI3K-dependent manner. Am. J. Physiol.—Cell Physiol. 2009, 296, 346–354. [Google Scholar] [CrossRef]
- Elazzouny, M.A.; Evans, C.R.; Burant, C.F.; Kennedy, R.T. Metabolomics analysis reveals that AICAR affects glycerolipid, ceramide and nucleotide synthesis pathways in INS-1 cells. PLoS ONE 2015, 10, e0129029. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Lu, J.; Li, S.; Wang, H.; Cao, X.; Li, Q.; Shi, T.T.; Matsunaga, K.; Chen, C.; Huang, H.; et al. Berberine is an insulin secretagogue targeting the KCNH6 potassium channel. Nat. Commun. 2021, 12, 5616. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Lu, J.; Yuan, S.S.; Asan; Cao, X.; Qiu, H.Y.; Shi, T.T.; Yang, F.Y.; Li, Q.; Liu, C.P.; et al. From hyper- to hypoinsulinemia and diabetes: Effect of KCNH6 on insulin secretion. Cell Rep. 2018, 25, 3800–3810.e6. [Google Scholar] [CrossRef] [PubMed]
- Félix-Martínez, G.J.; Godínez-Fernández, J.R. Mathematical models of electrical activity of the pancreatic β-cell: A physiological review. Islets 2014, 6, e949195. [Google Scholar] [CrossRef]
- Yang, S.N.; Shi, Y.; Yang, G.; Li, Y.; Yu, J.; Berggren, P.O. Ionic mechanisms in pancreatic β cell signaling. Cell. Mol. Life Sci. 2014, 71, 4149–4177. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.E.; Sewing, S.; Wang, J.; Joseph, J.W.; Smukler, S.R.; Sakellaropoulos, G.; Wang, J.; Saleh, M.C.; Chan, C.B.; Tsushima, R.G.; et al. Inhibition of Kv2.1 voltage-dependent K+ channels in pancreatic β-cells enhances glucose-dependent insulin secretion. J. Biol. Chem. 2002, 277, 44938–44945. [Google Scholar] [CrossRef]
- Macdonald, P.E.; Ha, X.F.; Wang, J.; Smukler, S.R.; Sun, A.M.; Gaisano, H.Y.; Salapatek, A.M.F.; Backx, P.H.; Wheeler, M.B. Members of the Kv1 and Kv2 voltage-dependent K+ channel families regulate insulin secretion. Mol. Endocrinol. 2001, 15, 1423–1435. [Google Scholar] [CrossRef]
- Herrington, J.; Zhou, Y.P.; Bugianesi, R.M.; Dulski, P.M.; Feng, Y.; Warren, V.A.; Smith, M.M.; Kohler, M.G.; Garsky, V.M.; Sanchez, M.; et al. Blockers of the delayed-rectifier potassium current in pancreatic β-cells enhance glucose-dependent insulin secretion. Diabetes 2006, 55, 1034–1042. [Google Scholar] [CrossRef]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing glucose as well as cellular energy status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef]
- Nawrocki, A.R.; Rajala, M.W.; Tomas, E.; Pajvani, U.B.; Saha, A.K.; Trumbauer, M.E.; Pang, Z.; Chen, A.S.; Ruderman, N.B.; Chen, H.; et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J. Biol. Chem. 2006, 281, 2654–2660. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Zhang, M.; Li, J.; Meng, Z.; Wei, S.; Du, H.; Chen, L.; Hatch, G.M. Berberine improves insulin resistance in cardiomyocytes via activation of 5′-adenosine monophosphate-activated protein kinase. Metabolism 2013, 62, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.M.; Lee, C.H.; Oh, W.K.; Kim, C.T.; et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef]
- Turner, N.; Li, J.; Gosby, A.; To, S.W.C.; Cheng, Z.; Miyoshi, H.; Taketo, M.M.; Cooney, G.J.; Kraegen, E.W.; James, D.E.; et al. Berberine and Its More Biologically Available Derivative, Dihydroberberine, Inhibit Mitochondrial Respiratory Complex I. Diabetes 2008, 57, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.L.; Yin, J.; Alimujiang, M.; Yu, X.Y.; Ai, L.G.; Bao, Y.Q.; Liu, F.; Jia, W.P. Inhibition of mitochondrial complex I improves glucose metabolism independently of AMPK activation. J. Cell. Mol. Med. 2018, 22, 1316–1328. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Shen, J.; Bai, M.; Xu, E. Berberine attenuates fructose-induced insulin resistance by stimulating the hepatic LKB1/AMPK/PGC1α pathway in mice. Pharm. Biol. 2020, 58, 385–392. [Google Scholar] [CrossRef]
- Neeland, I.J.; Singh, S.; McGuire, D.K.; Vega, G.L.; Roddy, T.; Reilly, D.F.; Castro-Perez, J.; Kozlitina, J.; Scherer, P.E. Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: The Dallas Heart Study. Diabetologia 2018, 61, 2570–2579. [Google Scholar] [CrossRef]
- Hilvo, M.; Salonurmi, T.; Havulinna, A.S.; Kauhanen, D.; Pedersen, E.R.; Tell, G.S.; Meyer, K.; Teeriniemi, A.M.; Laatikainen, T.; Jousilahti, P.; et al. Ceramide stearic to palmitic acid ratio predicts incident diabetes. Diabetologia 2018, 61, 1424–1434. [Google Scholar] [CrossRef]
- Adams, J.M.; Pratipanawatr, T.; Berria, R.; Wang, E.; DeFronzo, R.A.; Sullards, M.C.; Mandarino, L.J. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 2004, 53, 25–31. [Google Scholar] [CrossRef]
- Xia, Q.; Wu, F.; Wu, W.; Dong, H.; Huang, Z.; Xu, L.; Lu, F.; Gong, J. Berberine reduces hepatic ceramide levels to improve insulin resistance in HFD-fed mice by inhibiting HIF-2α. Biomed. Pharmacother. 2022, 150, 112955. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Nakayama, K.; Nakayama, M.; Mori, T.; Matsushima, M.; Okamura, M.; Senda, M.; Nako, K.; Miyata, T.; Ito, S. Methylglyoxal is a predictor in type 2 diabetic patients of intima-media thickening and elevation of blood pressure. Hypertension 2010, 56, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-B.; Tian, M.; Jin, J.; Zou, G.-L.; Sui, Y.-B.; Peng, P.; Liu, L. Berberine improves metabolic syndrome insulin resistance by inducing macrophage M2 polarization. Int. J. Clin. Exp. Med. 2018, 11, 11191–11197. [Google Scholar]
- Yin, J.; Gao, Z.; Liu, D.; Liu, Z.; Ye, J. Berberine improves glucose metabolism through induction of glycolysis. Am. J. Physiol.—Endocrinol. Metab. 2008, 294, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Zhang, H.; Song, D.Q.; Xue, R.; Zhao, W.; Wei, J.; Wang, Y.M.; Shan, N.; Zhou, Z.X.; Yang, P.; et al. Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metabolism 2009, 58, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Fy, G.; Ty, Z. A preliminary investigation of the mechanisms underlying the effect of berberine in preventing high-fat diet-induced insulin resistance in rats. J. Physiol. Pharmacol. 2012, 63, 505–513. [Google Scholar] [PubMed]
- Yue, S.J.; Liu, J.; Wang, A.T.; Meng, X.T.; Yang, Z.R.; Peng, C.; Guan, H.S.; Wang, C.Y.; Yan, D. Berberine alleviates insulin resistance by reducing peripheral branched-chain amino acids. Am. J. Physiol.—Endocrinol. Metab. 2019, 316, E73–E85. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Cheung, S.C.K.; Lan, L.L.; Ho, S.K.S.; Xu, H.X.; Chan, J.C.N.; Tong, P.C.Y. Berberine modulates insulin signaling transduction in insulin-resistant cells. Mol. Cell. Endocrinol. 2010, 317, 148–153. [Google Scholar] [CrossRef]
- Tasdelen, I.; Van Beekum, O.; Gorbenko, O.; Fleskens, V.; Van Den Broek, N.J.F.; Koppen, A.; Hamers, N.; Berger, R.; Coffer, P.J.; Brenkman, A.B.; et al. The serine/threonine phosphatase PPM1B (PP2Cβ) selectively modulates PPARγ activity. Biochem. J. 2013, 451, 45–53. [Google Scholar] [CrossRef]
- Wu, Y.S.; Li, Z.M.; Chen, Y.T.; Dai, S.J.; Zhou, X.J.; Yang, Y.X.; Lou, J.S.; Ji, L.T.; Bao, Y.T.; Xuan, L.; et al. Berberine improves inflammatory responses of diabetes mellitus in zucker diabetic fatty rats and insulin-resistant HepG2 cells through the PPM1B pathway. J. Immunol. Res. 2020, 2020, 2141508. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, W.; Lv, S.; Qu, H.; He, Y. Berberine improves insulin resistance in adipocyte models by regulating the methylation of hypoxia-inducible factor-3a. Biosci. Rep. 2019, 39, BSR20192059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Qu, H.; Wang, Y. Methylation of HIF3A promoter CpG islands contributes to insulin resistance in gestational diabetes mellitus. Mol. Genet. Genom. Med. 2019, 7, e00583. [Google Scholar] [CrossRef] [PubMed]
- Hatting, M.; Tavares, C.D.J.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Gluconeogenesis and glycogenolysis in health and diabetes. J. Investig. Med. 2004, 52, 375–378. [Google Scholar] [CrossRef]
- Chung, S.T.; Hsia, D.S.; Chacko, S.K.; Rodriguez, L.M.; Haymond, M.W. Increased gluconeogenesis in youth with newly diagnosed type 2 diabetes. Diabetologia 2015, 58, 596–603. [Google Scholar] [CrossRef]
- Xia, X.; Yan, J.; Shen, Y.; Tang, K.; Yin, J.; Zhang, Y.; Yang, D.; Liang, H.; Ye, J.; Weng, J. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS ONE 2011, 6, e16556. [Google Scholar] [CrossRef]
- Benchoula, K.; Arya, A.; Parhar, I.S.; Hwa, W.E. FoxO1 signaling as a therapeutic target for type 2 diabetes and obesity. Eur. J. Pharmacol. 2021, 891, 173758. [Google Scholar] [CrossRef]
- Jiang, S.J.; Dong, H.; Li, J.B.; Xu, L.J.; Zou, X.; Wang, K.F.; Lu, F.E.; Yi, P. Berberine inhibits hepatic gluconeogenesis via the LKB1-AMPK-TORC2 signaling pathway in streptozotocin-induced diabetic rats. World J. Gastroenterol. 2015, 21, 7777–7785. [Google Scholar] [CrossRef]
- Xu, X.H.; Hu, Q.; Zhou, L.S.; Xu, L.J.; Zou, X.; Lu, F.E.; Yi, P. Berberine inhibits gluconeogenesis in skeletal muscles and adipose tissues in streptozotocin-induced diabetic rats via LKB1-AMPK-TORC2 signaling pathway. Curr. Med. Sci. 2020, 40, 530–538. [Google Scholar] [CrossRef]
- Chen, M.; Huang, N.; Liu, J.; Huang, J.; Shi, J.; Jin, F. AMPK: A bridge between diabetes mellitus and Alzheimer’s disease. Behav. Brain Res. 2021, 400, 113043. [Google Scholar] [CrossRef]
- Miller, R.; Chu, Q.; Le Lay, J.; Scherer, P.; Ahima, R.; Kaestner, K.; Foretz, M.; Viollet, B.; Birnbaum, M. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J. Clin. Investig. 2011, 121, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Canettieri, G.; Koo, S.H.; Berdeaux, R.; Heredia, J.; Hedrick, S.; Zhang, X.; Montminy, M. Dual role of the coactivator TORC2 in modulating hepatic glucose output and insulin signaling. Cell Metab. 2005, 2, 331–338. [Google Scholar] [CrossRef]
- Wang, Y.; Vera, L.; Fischer, W.H.; Montminy, M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 2009, 460, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Jin, J.; Liu, P.; Song, Y.; Zhang, H.; Sheng, L.; Zhou, H.; Jiang, B. Berberine attenuates hyperglycemia by inhibiting the hepatic glucagon pathway in diabetic mice. Oxidative Med. Cell. Longev. 2020, 2020, 6210526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Pan, Y.; Xu, L.; Tang, D.; Dorfman, R.G.; Zhou, Q.; Yin, Y.; Li, Y.; Zhou, L.; Zhao, S.; et al. Berberine promotes glucose uptake and inhibits gluconeogenesis by inhibiting deacetylase SIRT3. Endocrine 2018, 62, 576–587. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Shima, A.; Kuramoto, D.; Kikumoto, D.; Matsui, T.; Michihara, A. Phosphoenolpyruvate carboxykinase, a key enzyme that controls blood glucose, is a target of retinoic acid receptor-related orphan receptor α. PLoS ONE 2015, 10, e0137955. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Kuang, H.; Feng, X.; Ai, W.; Wang, Y.; Shi, S.; Chen, J.; Fan, R. Berberine increases glucose uptake and intracellular ROS levels by promoting Sirtuin 3 ubiquitination. Biomed. Pharmacother. 2020, 121, 109563. [Google Scholar] [CrossRef]
- Xu, S.; Liu, C.X.; Xu, W.; Huang, L.; Zhao, J.Y.; Zhao, S.M. Butyrate induces apoptosis by activating PDC and inhibiting complex i through SIRT3 inactivation. Signal Transduct. Target. Ther. 2017, 2, 16035. [Google Scholar] [CrossRef]
- Ahn, B.H.; Kim, H.S.; Song, S.; In, H.L.; Liu, J.; Vassilopoulos, A.; Deng, C.X.; Finkel, T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef]
- Cok, A.; Plaisier, C.; Salie, M.J.; Oram, D.S.; Chenge, J.; Louters, L.L. Berberine acutely activates the glucose transport activity of GLUT1. Biochimie 2011, 93, 1187–1192. [Google Scholar] [CrossRef]
- Cheng, Z.; Pang, T.; Gu, M.; Gao, A.-H.; Xie, C.-M.; Li, J.-Y.; Nan, F.-J.; Li, J. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim. Biophys. Acta 2006, 1760, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, Y.; Wang, X.; Liu, S.; Shang, W.; Yuan, G.; Li, F.; Tang, J.; Chen, M.; Chen, J. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism 2007, 56, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, E.J.; Kim, E.D.; Bayaraa, T.; Frost, S.C.; Hyun, C.K. Berberine activates GLUT1-mediated glucose uptake in 3T3-L1 adipocytes. Biol. Pharm. Bull. 2007, 30, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, W.; Wang, X.; Chen, W. Effect of Astragalus polysaccharides and berberine on carbohydrate metabolism and cell differentiation in 3T3-L1 adipocytes. Zhongguo Zhong Xi Yi Jie He Za Zhi 2004, 24, 926–928. [Google Scholar] [PubMed]
- Guasch-ferré, M.; Santos, J.L.; Martínez-gonzález, M.A.; Clish, C.B.; Razquin, C.; Wang, D. Glycolysis/gluconeogenesis- and tricarboxylic acid cycle—Related metabolites, Mediterranean diet, and type 2 diabetes. Am. J. Clin. Nutr. 2020, 111, 835–844. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Xu, H.; Woo, S.; Dong, H.; Lu, F.; Lange, A.J.; Wu, C. Glycolysis in the control of blood glucose homeostasis. Acta Pharm. Sin. B 2012, 2, 358–367. [Google Scholar] [CrossRef]
- Li, X.B.; Gu, J.D.; Zhou, Q.H. Review of aerobic glycolysis and its key enzymes—New targets for lung cancer therapy. Thorac. Cancer 2015, 6, 17–24. [Google Scholar] [CrossRef]
- Yan, S.; Wei, X.; Xu, S.; Sun, H.; Wang, W.; Liu, L.; Jiang, X.; Zhang, Y.; Che, Y. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoform 3 spatially mediates autophagy through the AMPK signaling pathway. Oncotarget 2017, 8, 80909–80922. [Google Scholar] [CrossRef]
- Marsin, A.S.; Bertrand, L.; Rider, M.H.; Deprez, J.; Beauloye, C.; Vincent, M.F.; Van den Berghe, G.; Carling, D.; Hue, L. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr. Biol. 2000, 10, 1247–1255. [Google Scholar] [CrossRef]
- Xu, M.; Xiao, Y.; Yin, J.; Hou, W.; Yu, X.; Shen, L.; Liu, F.; Wei, L.; Jia, W. Berberine promotes glucose consumption independently of AMP-activated protein kinase activation. PLoS ONE 2014, 9, e103702. [Google Scholar] [CrossRef]
- Yin, J.; Hu, R.; Chen, M.; Li, F.; Yang, Y.; Chen, J. Effects of berberine on glucose metabolism in vitro. Metab. Clin. Exp. 2002, 51, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Guo, J.H.; Qian, Y.Z.; Kong, W.J.; Jiang, J.D. Berberine improves glucose and lipid metabolism in HepG2 cells through AMPKα1 activation. Front. Pharmacol. 2020, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Magaji, U.F.; Sacan, O.; Yanardag, R. Alpha amylase, alpha glucosidase and glycation inhibitory activity of Moringa oleifera extracts. S. Afr. J. Bot. 2020, 128, 225–230. [Google Scholar] [CrossRef]
- Riyaphan, J.; Jhong, C.-H.; Tsai, M.-J.; Lee, D.-N.; Leong, M.K.; Weng, C.-F. Potent natural inhibitors of alpha-glucosidase and alpha-amylase against hyperglycemia in vitro and in vivo. Preprints 2017, 2017, 2017030116. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-Safi, I.; Louba, A.; Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alharbi, M.S.; Alqahtani, A.; Bari, A.; et al. In vitro alpha-amylase and alpha-glucosidase inhibitory activity and in vivo antidiabetic activity of withania frutescens l. Foliar extract. Molecules 2021, 26, 293. [Google Scholar] [CrossRef]
- Que, L.; Huang, K.; Ding, Y.; Chu, N.; Yang, J.; Qian, Z.; He, Q. Acarbose bioequivalence: Exploration of eligible protocol design. J. Clin. Pharm. Ther. 2021, 46, 492–503. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Karrar, E.; Xu, D.; Sun, X. Inhibition mechanism of berberine on α-Amylase and α-glucosidase in vitro. Starch 2022, 74, 2100231. [Google Scholar] [CrossRef]
- Pan, G.-Y.; Huang, Z.-J.; Wang, G.-J.; Fawcett, J.; Liu, X.-D.; Zhao, X.-C.; Sun, J.-G.; Xie, Y.-Y. The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med. 2003, 69, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Zuo, D.Y.; Qie, X.D.; Qi, H.; Zhao, M.Q.; Wu, Y.L. Berberine acutely inhibits the digestion of maltose in the intestine. J. Ethnopharmacol. 2012, 142, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Deng, Y.; Yu, S.; Lu, S.; Xie, L.; Liu, X. Berberine attenuates intestinal disaccharidases in streptozotocin-induced diabetic rats. Pharmazie 2008, 63, 384–388. [Google Scholar] [CrossRef]
- Lu, T.; Liang, Y.; Song, J.; Xie, L.; Wang, G.J.; Liu, X.D. Simultaneous determination of berberine and palmatine in rat plasma by HPLC-ESI-MS after oral administration of traditional Chinese medicinal preparation Huang-Lian-Jie-Du decoction and the pharmacokinetic application of the method. J. Pharm. Biomed. Anal. 2006, 40, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: Physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.P.; Pratley, R.E. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: Review of head-to-head clinical trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef]
- Makrilakis, K. The role of dpp-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: When to select, what to expect. Int. J. Environ. Res. Public Health 2019, 16, 2720. [Google Scholar] [CrossRef] [PubMed]
- Argun-Kurum, G.; Kaya-Dagistanli, F.; Ozturk, M. DPP4 inhibitor induces beta cell regeneration and DDR-1 protein expression as an endocrine progenitor cell marker in neonatal STZ-diabetic rats. Pharmacol. Rep. 2019, 71, 721–731. [Google Scholar] [CrossRef]
- Wu, Y.J.; Guo, X.; Li, C.J.; Li, D.Q.; Zhang, J.; Yang, Y.; Kong, Y.; Guo, H.; Liu, D.M.; Chen, L.M. Dipeptidyl peptidase-4 inhibitor, vildagliptin, inhibits pancreatic beta cell apoptosis in association with its effects suppressing endoplasmic reticulum stress in db/db mice. Metabolism 2015, 64, 226–235. [Google Scholar] [CrossRef]
- Bugliani, M.; Syed, F.; Paula, F.M.M.; Omar, B.A.; Suleiman, M.; Mossuto, S.; Grano, F.; Cardarelli, F.; Boggi, U.; Vistoli, F.; et al. DPP-4 is expressed in human pancreatic beta cells and its direct inhibition improves beta cell function and survival in type 2 diabetes. Mol. Cell. Endocrinol. 2018, 473, 186–193. [Google Scholar] [CrossRef]
- Al-Masri, I.M.; Mohammad, M.K.; Tahaa, M.O. Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. J. Enzym. Inhib. Med. Chem. 2009, 24, 1061–1066. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P.-F. Diabetes and gut microbiota. World J. Diabetes 2021, 12, 1693–1703. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Ren, H.; Wang, S.; Zhong, H.; Zhao, X.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat. Commun. 2020, 11, 5015. [Google Scholar] [CrossRef]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.M.; Cree, I.A.; Covington, J.A.; Arasaradnam, R.P. The interplay of the gut microbiome, bile acids, and volatile organic compounds. Gastroenterol. Res. Pract. 2015, 2015, 398585. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, X.; Li, J.; Zhang, Y.; Zhong, H.; Liu, R.; Zhang, D.; Feng, Q.; Xie, X.; Hong, J.; et al. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun. 2017, 8, 1785. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, H.; Yan, L.; Wang, W.; Wang, D. Berberine alleviates type 2 diabetic symptoms by altering gut microbiota and reducing aromatic amino acids. Biomed. Pharmacother. 2020, 131, 110669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.D.; Yu, C.J.; Li, J.Y.; Wang, S.H.; Yang, D.; Guo, C.L.; Du, X.; Zhang, W.J.; Cheng, R.D.; Diao, X.C.; et al. Effect of berberine on hyperglycaemia and gut microbiota composition in type 2 diabetic Goto-Kakizaki rats. World J. Gastroenterol. 2021, 27, 708–724. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Cai, J.; Gui, W.; Nichols, R.G.; Koo, I.; Zhang, J.; Anitha, M.; Patterson, A.D. Berberine directly affects the gut microbiota to promote intestinal farnesoid X receptor activation. Drug Metab. Dispos. 2019, 47, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.X.; Hu, Y.N.; Li, J.W.; Yuan, K. Hypoglycemic mechanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxidative Med. Cell. Longev. 2018, 2018, 8930374. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J.H.; Yu, T.; Chen, Q.K. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharmacother. 2019, 118, 109131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).