Solid-Phase Parallel Synthesis of Dual Histone Deacetylase-Cyclooxygenase Inhibitors
Abstract
1. Introduction
2. Results and Discussion
2.1. Design and Synthesis of Dual HDAC-COX Inhibitors
2.2. Determination of COX Inhibition
2.3. Inhibition of HDAC1 and HDAC6
2.4. Determination of Lipophilicity
2.5. Antiproliferative Properties of Dual HDAC-COX Inhibitors
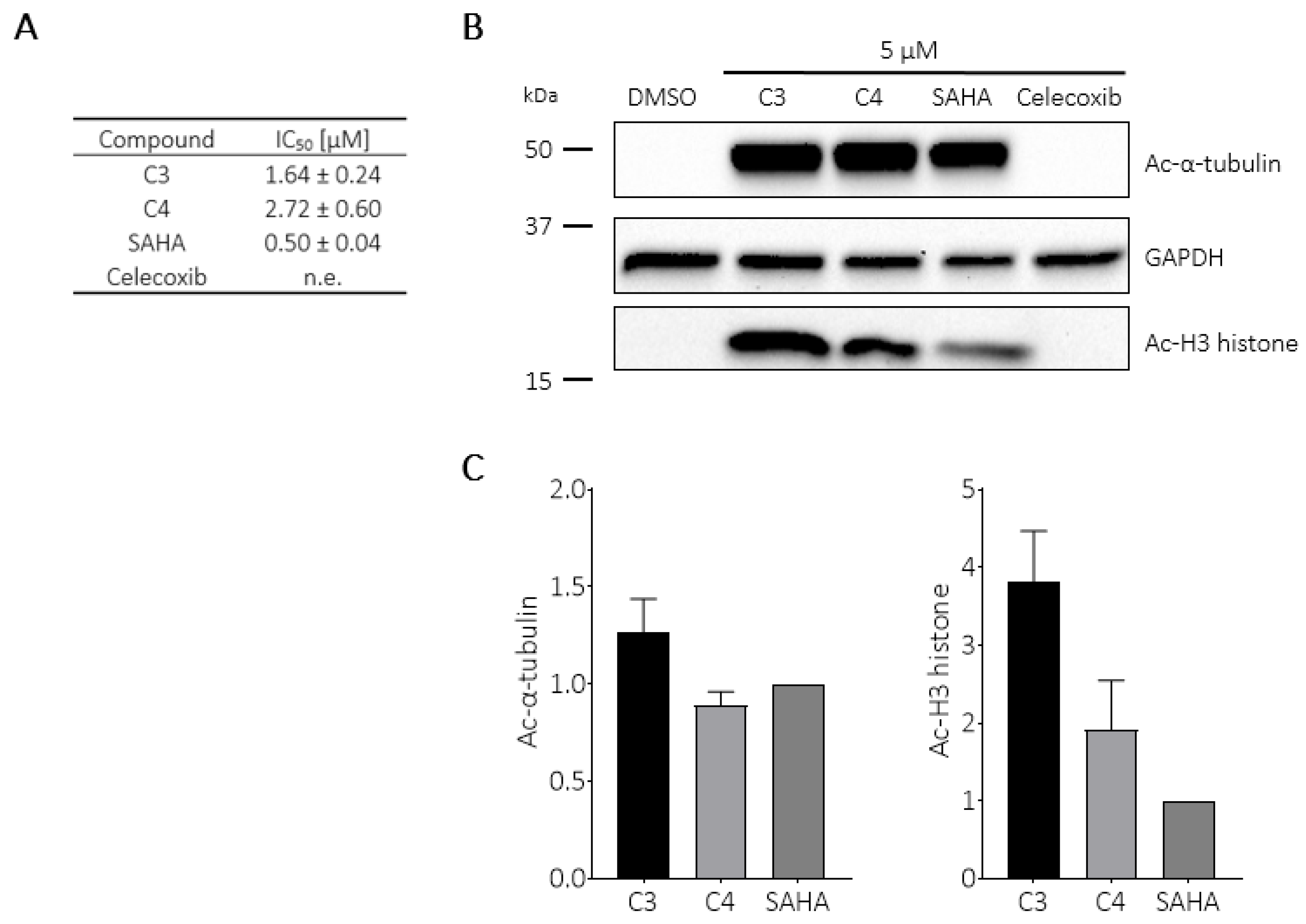
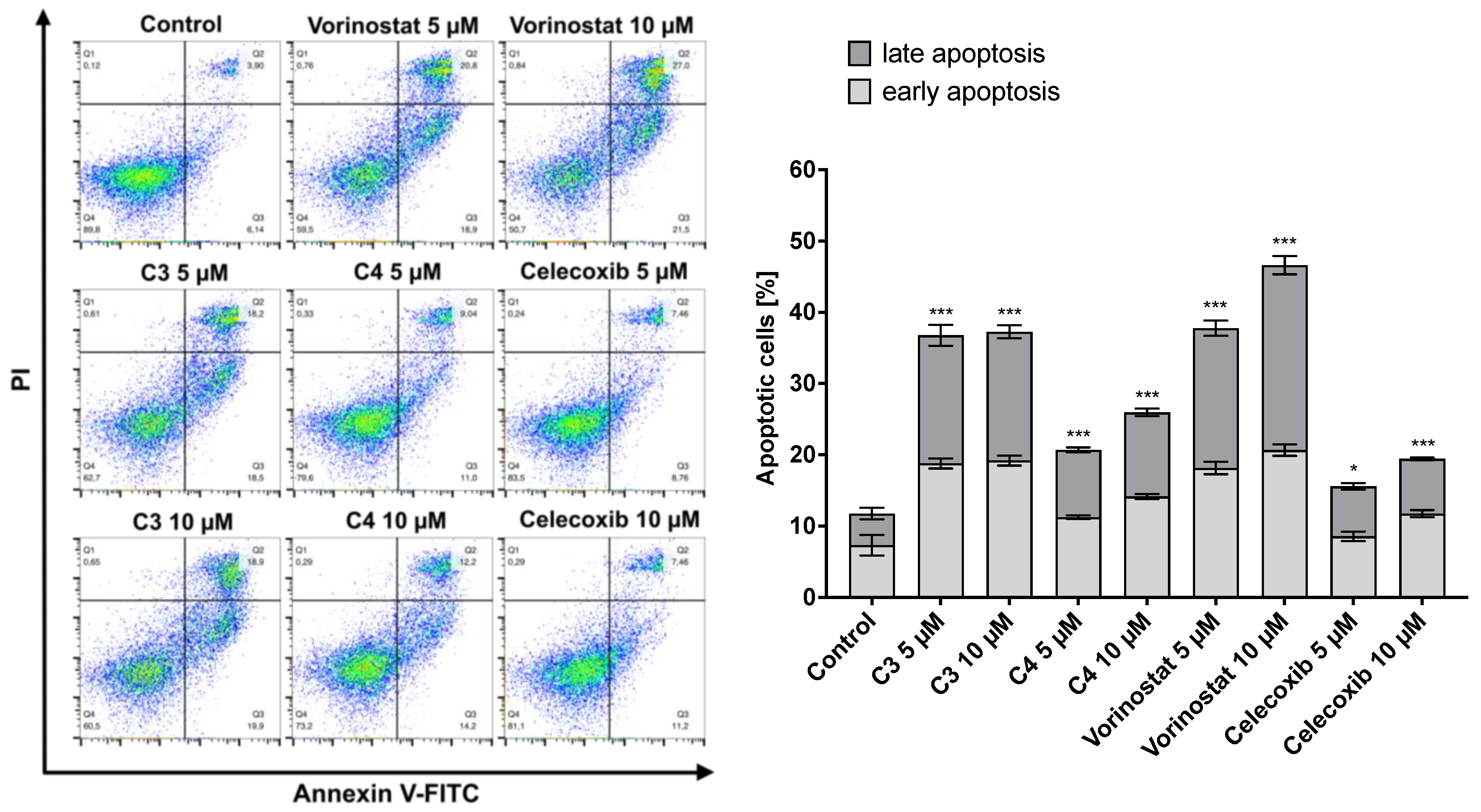
2.6. Intracellular Target Engagement and Apoptosis Induction
3. Materials and Methods
3.1. General Remarks
3.1.1. Materials and Experimental Procedures
3.1.2. High Performance Liquid Chromatography (HPLC)
3.1.3. Nuclear Magnetic Resonance Spectroscopy (NMR)
3.1.4. Mass Spectrometry (MS)
3.1.5. TNBS-Test
3.1.6. Determination of the Loading
3.1.7. Determination of the LogD7.4HPLC
3.2. General Procedures
General Procedure A: Coupling of the Cap Group
3.3. Syntheses
3.3.1. Building Block Synthesis
3.3.2. Library Synthesis
3.4. Biological Evaluation
3.4.1. Cell Lines and Cell Culture
3.4.2. MTT Cell Viability Assay
3.4.3. Apoptosis Assay
3.4.4. HDAC Whole Cell Assay
3.4.5. Immunoblot
3.4.6. In Vitro HDAC1 and HDAC6 Cell Assay
3.4.7. Determination of COX Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- All cancers excluding non-melanoma skin cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/40-All-cancers-excluding-non-melanoma-skin-cancer-fact-sheet.pdf (accessed on 4 November 2022).
- de Lera, A.R.; Ganesan, A. Epigenetic polypharmacology: From combination therapy to multitargeted drugs. Clin. Epigenetics 2016, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Anighoro, A.; Bajorath, J.; Rastelli, G. Polypharmacology: Challenges and opportunities in drug discovery. J. Med. Chem. 2014, 57, 7874–7887. [Google Scholar] [CrossRef]
- Proschak, E.; Stark, H.; Merk, D. Polypharmacology by design: A medicinal chemist’s perspective on multitargeting compounds. J. Med. Chem. 2019, 62, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Rosini, M. Polypharmacology: The rise of multitarget drugs over combination therapies. Future Med. Chem. 2014, 6, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef]
- Schobert, R.; Biersack, B. Multimodal HDAC inhibitors with improved anticancer activity. Curr. Cancer Drug Targets 2018, 18, 39–56. [Google Scholar] [CrossRef]
- Ganesan, A. Multitarget drugs: An epigenetic epiphany. ChemMedChem 2016, 11, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, N.M.; Pingili, D.; Kadasi, S.; Mettu, A.; Prasad, S.V.U.M. Dual or multi-targeting inhibitors: The next generation anticancer agents. Eur. J. Med. Chem. 2018, 143, 1277–1300. [Google Scholar] [CrossRef]
- Fu, R.-G.; Sun, Y.; Sheng, W.-B.; Liao, D.-F. Designing multi-targeted agents: An emerging anticancer drug discovery paradigm. Eur. J. Med. Chem. 2017, 136, 195–211. [Google Scholar] [CrossRef]
- Bhatia, S.; Krieger, V.; Groll, M.; Osko, J.D.; Reßing, N.; Ahlert, H.; Borkhardt, A.; Kurz, T.; Christianson, D.W.; Hauer, J.; et al. Discovery of the first-in-class dual histone deacetylase-proteasome inhibitor. J. Med. Chem. 2018, 61, 10299–10309. [Google Scholar] [CrossRef]
- Pang, L.Y.; Hurst, E.A.; Argyle, D.J. Cyclooxygenase-2: A role in cancer stem cell survival and repopulation of cancer cells during therapy. Stem Cells Int. 2016, 2016, 2048731. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yue, W.; Wang, H.; Lai, B.; Yang, X.; Zhang, C.; Wang, Y.; Gu, M. Cyclooxygenase-2 is associated with malignant phenotypes in human lung cancer. Oncol. Lett. 2016, 12, 3836–3844. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Khan, O.; La Thangue, N.B. HDAC inhibitors in cancer biology: Emerging mechanisms and clinical applications. Immunol. Cell Biol. 2012, 90, 85–94. [Google Scholar] [CrossRef]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone deacetylase inhibitors as anticancer drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef]
- Kaur, J.; Daoud, A.; Eblen, S.T. Targeting chromatin remodeling for cancer therapy. Curr. Mol. Pharmacol. 2019, 12, 215–229. [Google Scholar] [CrossRef]
- Fardi, M.; Solali, S.; Farshdousti Hagh, M. Epigenetic mechanisms as a new approach in cancer treatment: An updated review. Genes Dis. 2018, 5, 304–311. [Google Scholar] [CrossRef]
- Martínez-Iglesias, O.; Ruiz-Llorente, L.; Sánchez-Martínez, R.; García, L.; Zambrano, A.; Aranda, A. Histone deacetylase inhibitors: Mechanism of action and therapeutic use in cancer. Clin. Transl. Oncol. 2008, 10, 395–398. [Google Scholar] [CrossRef]
- Roche, J.; Bertrand, P. Inside HDACs with more selective HDAC inhibitors. Eur. J. Med. Chem. 2016, 121, 451–483. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Busquets-Cortés, C.; Capó, X.; Tejada, S.; Tur, J.A.; Pons, A.; Sureda, A. Cyclooxygenase-2 inhibitors as a therapeutic target in inflammatory diseases. Curr. Med. Chem. 2019, 26, 3225–3241. [Google Scholar] [CrossRef]
- Kirkby, N.S.; Chan, M.V.; Zaiss, A.K.; Garcia-Vaz, E.; Jiao, J.; Berglund, L.M.; Verdu, E.F.; Ahmetaj-Shala, B.; Wallace, J.L.; Herschman, H.R.; et al. Systematic study of constitutive cyclooxygenase-2 expression: Role of NF-κB and NFAT transcriptional pathways. Proc. Natl. Acad. Sci. USA 2016, 113, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, M.; Richter, S.; Seifert, V.; Hauser, S.; Calsina, B.; Martínez-Montes, Á.M.; Ter Laak, M.; Ziegler, C.G.; Timmers, H.; Eisenhofer, G.; et al. Targeting cyclooxygenase-2 in pheochromocytoma and paraganglioma: Focus on genetic background. Cancers 2019, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Laube, M.; Kniess, T.; Pietzsch, J. Radiolabeled COX-2 inhibitors for non-invasive visualization of COX-2 expression and activity—a critical update. Molecules 2013, 18, 6311–6355. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yousef, A.; Grandis, J.R.; Johnson, D.E. NSAID therapy for PIK3CA-altered colorectal, breast, and head and neck cancer. Adv. Biol. Regul. 2020, 75, 100653. [Google Scholar] [CrossRef]
- Ralph, S.J.; Nozuhur, S.; Moreno-Sánchez, R.; Rodríguez-Enríquez, S.; Pritchard, R. NSAID celecoxib: A potent mitochondrial pro-oxidant cytotoxic agent sensitizing metastatic cancers and cancer stem cells to chemotherapy. J. Cancer Metastasis Treat. 2018, 4, 49. [Google Scholar] [CrossRef]
- Harris, R.E.; Beebe-Donk, J.; Alshafie, G.A. Cancer chemoprevention by cyclooxygenase 2 (COX-2) blockade: Results of case control studies. Subcell. Biochem. 2007, 42, 193–212. [Google Scholar] [CrossRef]
- Fischer, S.M.; Hawk, E.T.; Lubet, R.A. Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer Prev. Res. 2011, 4, 1728–1735. [Google Scholar] [CrossRef]
- Haase-Kohn, C.; Laube, M.; Donat, C.K.; Belter, B.; Pietzsch, J. CRISPR/Cas9 mediated knockout of cyclooxygenase-2 gene inhibits invasiveness in A2058 melanoma cells. Cells 2022, 11, 749. [Google Scholar] [CrossRef]
- Zhang, G.H.; Gan, Y.H. Combination of pan-HDAC inhibitor and COX-2 inhibitor produces synergistic anticancer effects in human salivary adenoid cystic cancer cells. Chin. J. Dent. Res. 2019, 22, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gan, Y.-H. Synergistic antitumor effects of the combined treatment with an HDAC6 inhibitor and a COX-2 inhibitor through activation of PTEN. Oncol. Rep. 2017, 38, 2657–2666. [Google Scholar] [CrossRef] [PubMed]
- Peulen, O.; Gonzalez, A.; Peixoto, P.; Turtoi, A.; Mottet, D.; Delvenne, P.; Castronovo, V. The anti-tumor effect of HDAC inhibition in a human pancreas cancer model is significantly improved by the simultaneous inhibition of cyclooxygenase 2. PLoS One 2013, 8, e75102. [Google Scholar] [CrossRef] [PubMed]
- Raji, I.; Yadudu, F.; Janeira, E.; Fathi, S.; Szymczak, L.; Kornacki, J.R.; Komatsu, K.; Li, J.-D.; Mrksich, M.; Oyelere, A.K. Bifunctional conjugates with potent inhibitory activity towards cyclooxygenase and histone deacetylase. Bioorg. Med. Chem. 2017, 25, 1202–1218. [Google Scholar] [CrossRef] [PubMed]
- Reßing, N.; Sönnichsen, M.; Osko, J.D.; Schöler, A.; Schliehe-Diecks, J.; Skerhut, A.; Borkhardt, A.; Hauer, J.; Kassack, M.U.; Christianson, D.W.; et al. Multicomponent synthesis, binding mode, and structure-activity relationship of selective histone deacetylase 6 (HDAC6) inhibitors with bifurcated capping groups. J. Med. Chem. 2020, 63, 10339–10351. [Google Scholar] [CrossRef]
- Mackwitz, M.K.W.; Hesping, E.; Antonova-Koch, Y.; Diedrich, D.; Woldearegai, T.G.; Skinner-Adams, T.; Clarke, M.; Schöler, A.; Limbach, L.; Kurz, T.; et al. Structure-activity and structure-toxicity relationships of peptoid-based histone deacetylase inhibitors with dual-stage antiplasmodial activity. ChemMedChem 2019, 14, 912–926. [Google Scholar] [CrossRef]
- Shen, S.; Benoy, V.; Bergman, J.A.; Kalin, J.H.; Frojuello, M.; Vistoli, G.; Haeck, W.; Van Den Bosch, L.; Kozikowski, A.P. Bicyclic-capped histone deacetylase 6 inhibitors with improved activity in a model of axonal Charcot−Marie−Tooth disease. ACS Chem. Neurosci. 2016, 7, 240–258. [Google Scholar] [CrossRef]
- Sinatra, L.; Bandolik, J.J.; Roatsch, M.; Sönnichsen, M.; Schoeder Clara, T.; Hamacher, A.; Schöler, A.; Borkhardt, A.; Meiler, J.; Bhatia, S.; et al. Hydroxamic acids immobilized on resins (HAIRs): Synthesis of dual-targeting HDAC inhibitors and HDAC degraders (PROTACs). Angew. Chem. Int. Ed. 2020, 59, 22494–22499. [Google Scholar] [CrossRef]
- Useini, L.; Mojić, M.; Laube, M.; Lönnecke, P.; Dahme, J.; Sárosi, M.B.; Mijatović, S.; Maksimović-Ivanić, D.; Pietzsch, J.; Hey-Hawkins, E. Carboranyl analogues of mefenamic acid and their biological evaluation. ACS Omega 2022, 7, 24282–24291. [Google Scholar] [CrossRef]
- Brandt, F.; Ullrich, M.; Seifert, V.; Haase-Kohn, C.; Richter, S.; Kniess, T.; Pietzsch, J.; Laube, M. Exploring nitric oxide (NO)-releasing celecoxib derivatives as modulators of radioresponse in pheochromocytoma cells. Molecules 2022, 27, 6587. [Google Scholar] [CrossRef]
- Selg, C.; Schöler, A.; Schliehe-Diecks, J.; Hanl, M.; Sinatra, L.; Borkhardt, A.; Sárosi, M.B.; Bhatia, S.; Hey-Hawkins, E.; Hansen, F.K. Borinostats: Solid-phase synthesis of carborane-capped histone deacetylase inhibitors with a tailor-made selectivity profile. Chem. Sci. 2021, 12, 11873–11881. [Google Scholar] [CrossRef] [PubMed]
- Reßing, N.; Schliehe-Diecks, J.; Watson, P.R.; Sönnichsen, M.; Cragin, A.D.; Schöler, A.; Yang, J.; Schäker-Hübner, L.; Borkhardt, A.; Christianson, D.W.; et al. Development of fluorinated peptoid-based histone deacetylase (HDAC) inhibitors for therapy-resistant acute leukemia. J. Med. Chem. 2022, 65, 15457–15472. [Google Scholar] [CrossRef] [PubMed]
- Sandrone, G.; Cukier, C.D.; Zrubek, K.; Marchini, M.; Vergani, B.; Caprini, G.; Fossati, G.; Steinkühler, C.; Stevenazzi, A. Role of fluorination in the histone deacetylase 6 (HDAC6) selectivity of benzohydroxamate-based inhibitors. ACS Med. Chem. Lett. 2021, 12, 1810–1817. [Google Scholar] [CrossRef]
- Sinatra, L.; Yang, J.; Schliehe-Diecks, J.; Dienstbier, N.; Vogt, M.; Gebing, P.; Bachmann, L.M.; Sönnichsen, M.; Lenz, T.; Stühler, K.; et al. Solid-phase synthesis of cereblon-recruiting selective histone deacetylase 6 degraders (HDAC6 PROTACs) with antileukemic activity. J. Med. Chem. 2022, 65, 16860–16878. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S.F.; Pescatore, M.C. Method for measuring the logarithm of the octanol–water partition coefficient by using short octadecyl–poly(vinyl alcohol) high-performance liquid chromatography columns. J. Chromatogr. A 2002, 952, 47–61. [Google Scholar] [CrossRef]
- Stocks, M. The small molecule drug discovery process—from target selection to candidate selection. In Introduction to Biological and Small Molecule Drug Research and Development: Theory and Case Studies; Ganellin, C.R., Ed.; Elsevier Academic Press: Waltham, MA, USA, 2013; pp. 81–126. ISBN 9780123971760. [Google Scholar]
- Shen, F.-Q.; Wang, Z.-C.; Wu, S.-Y.; Ren, S.-Z.; Man, R.-J.; Wang, B.-Z.; Zhu, H.-L. Synthesis of novel hybrids of pyrazole and coumarin as dual inhibitors of COX-2 and 5-LOX. Bioorg. Med. Chem. Lett. 2017, 27, 3653–3660. [Google Scholar] [CrossRef]
- Krieger, V.; Hamacher, A.; Cao, F.; Stenzel, K.; Gertzen, C.G.W.; Schäker-Hübner, L.; Kurz, T.; Gohlke, H.; Dekker, F.J.; Kassack, M.U.; et al. Synthesis of peptoid-based class I-selective histone deacetylase inhibitors with chemosensitizing properties. J. Med. Chem. 2019, 62, 11260–11279. [Google Scholar] [CrossRef]
- Erdeljac, N.; Bussmann, K.; Schöler, A.; Hansen, F.K.; Gilmour, R. Fluorinated analogues of the histone deacetylase inhibitor vorinostat (Zolinza): Validation of a chiral hybrid bioisostere, BITE. ACS Med. Chem. Lett. 2019, 10, 1336–1340. [Google Scholar] [CrossRef]
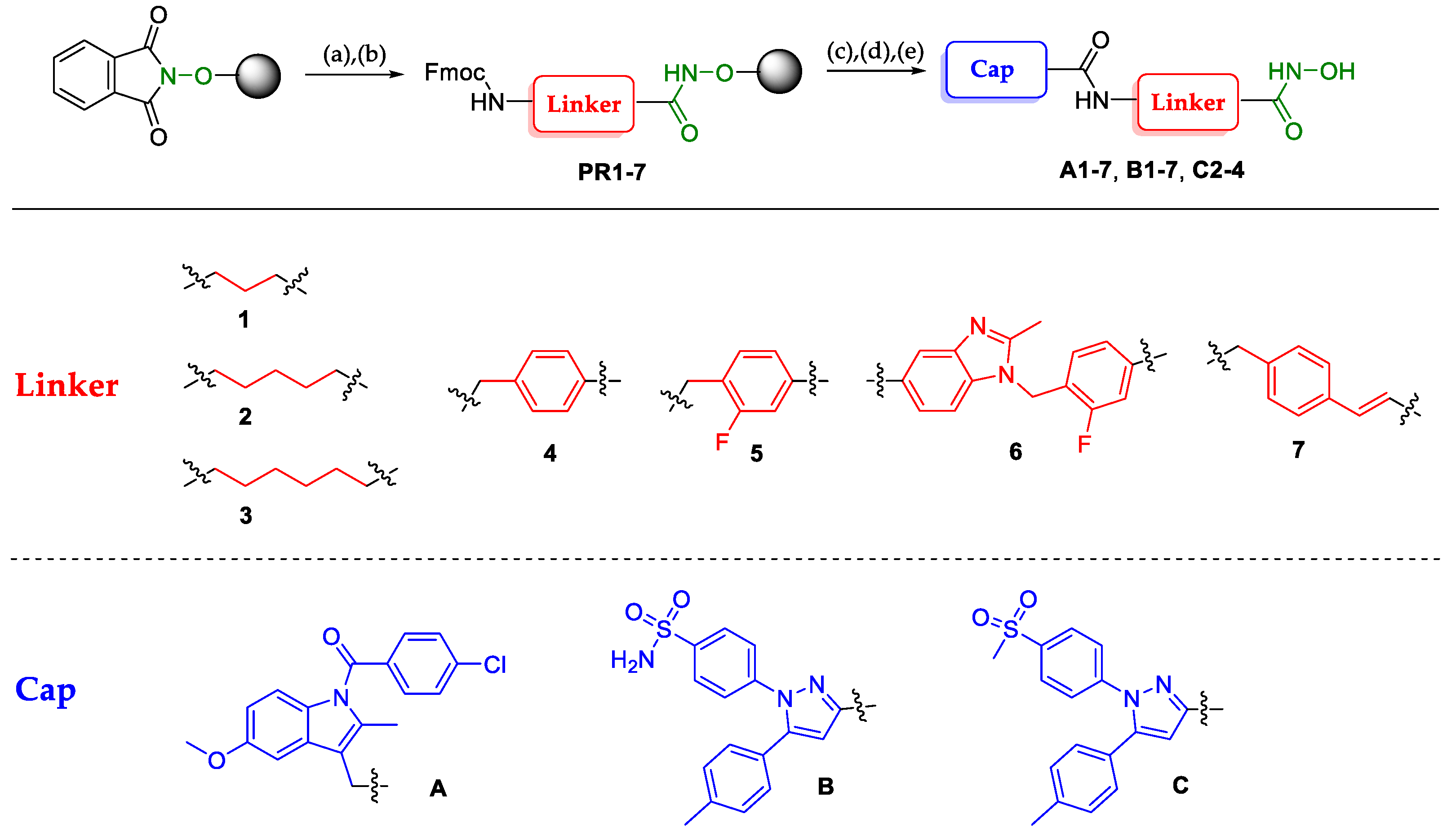
 A1 | 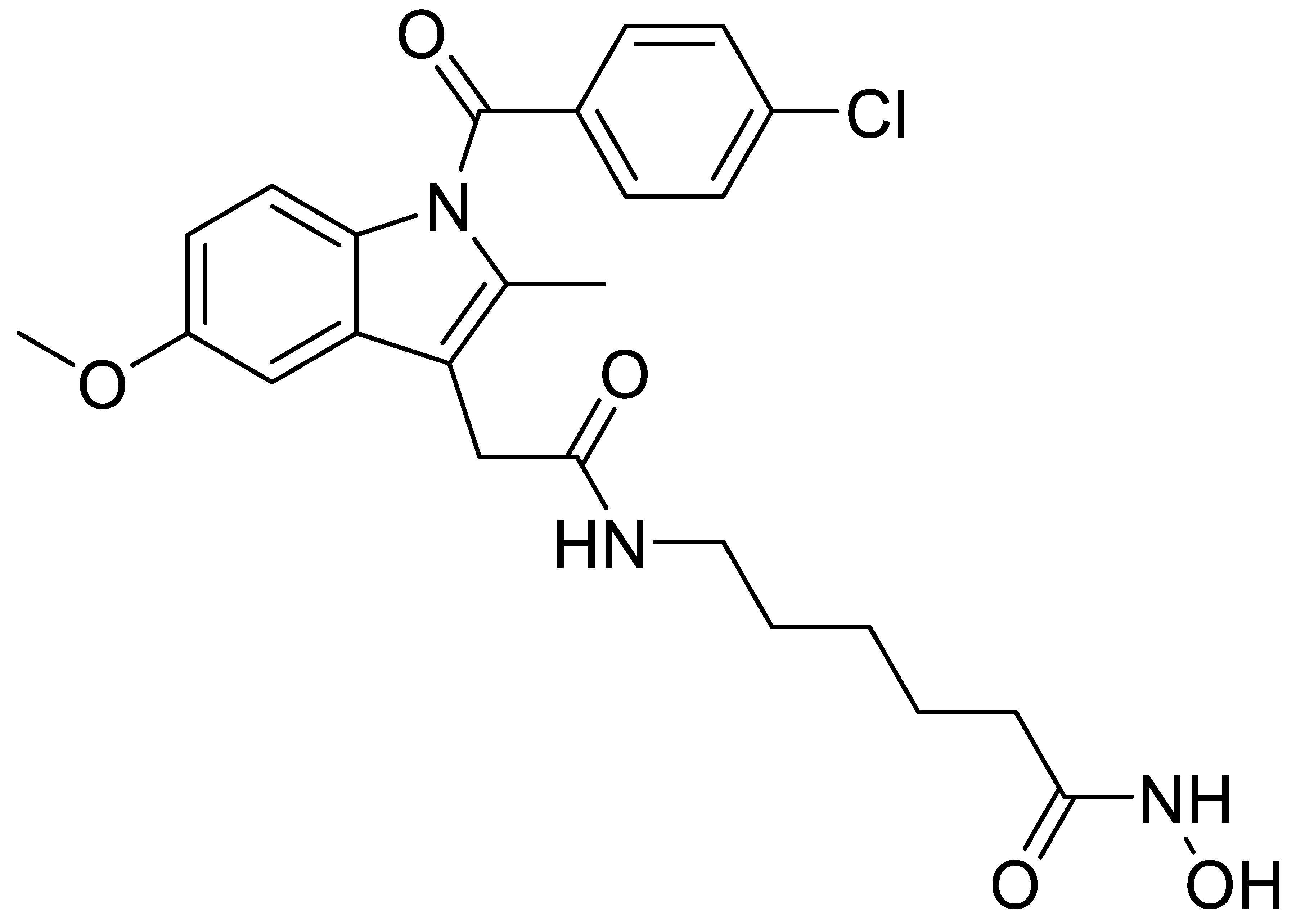 A2 |  A3 |
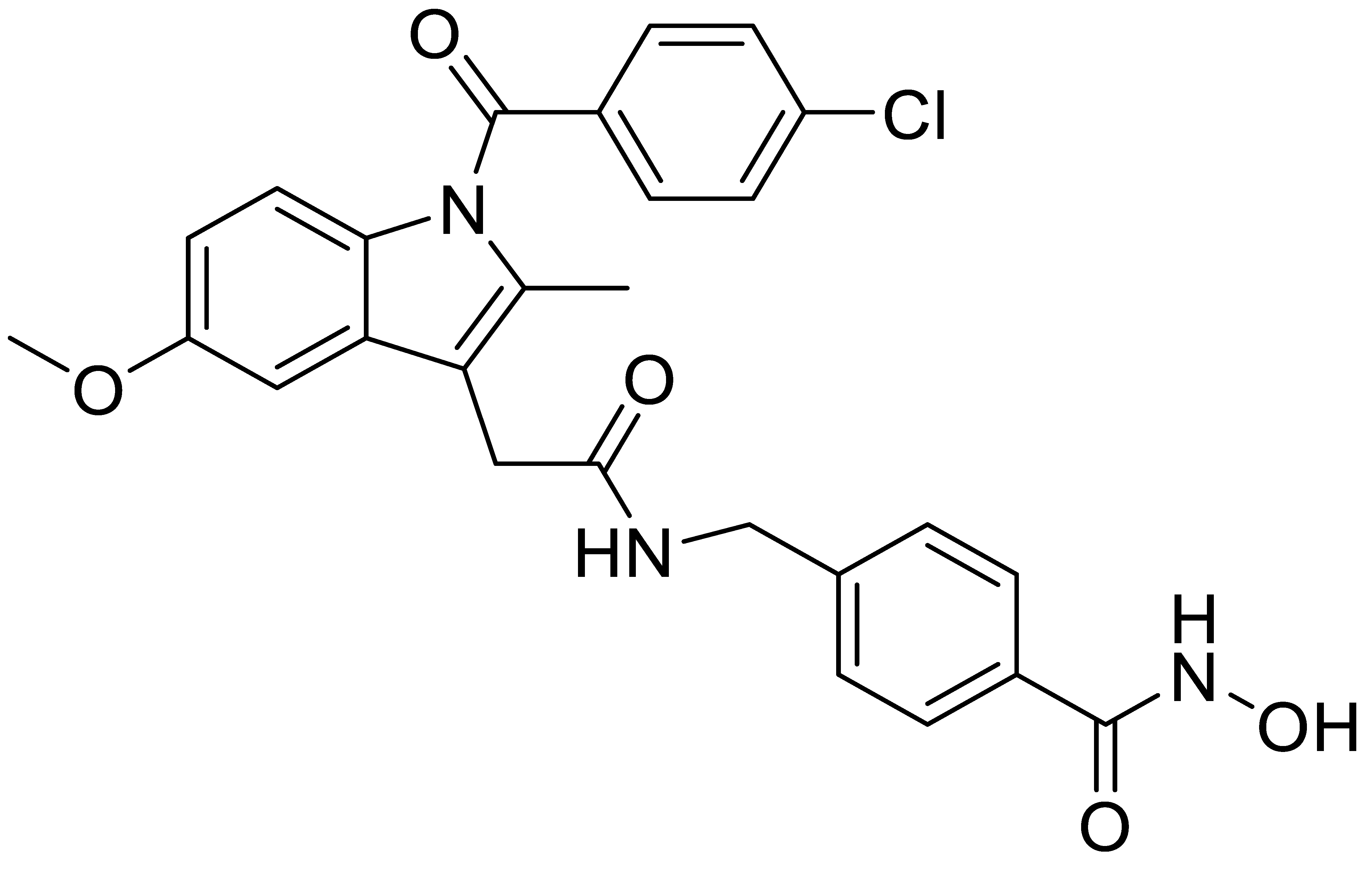 A4 | 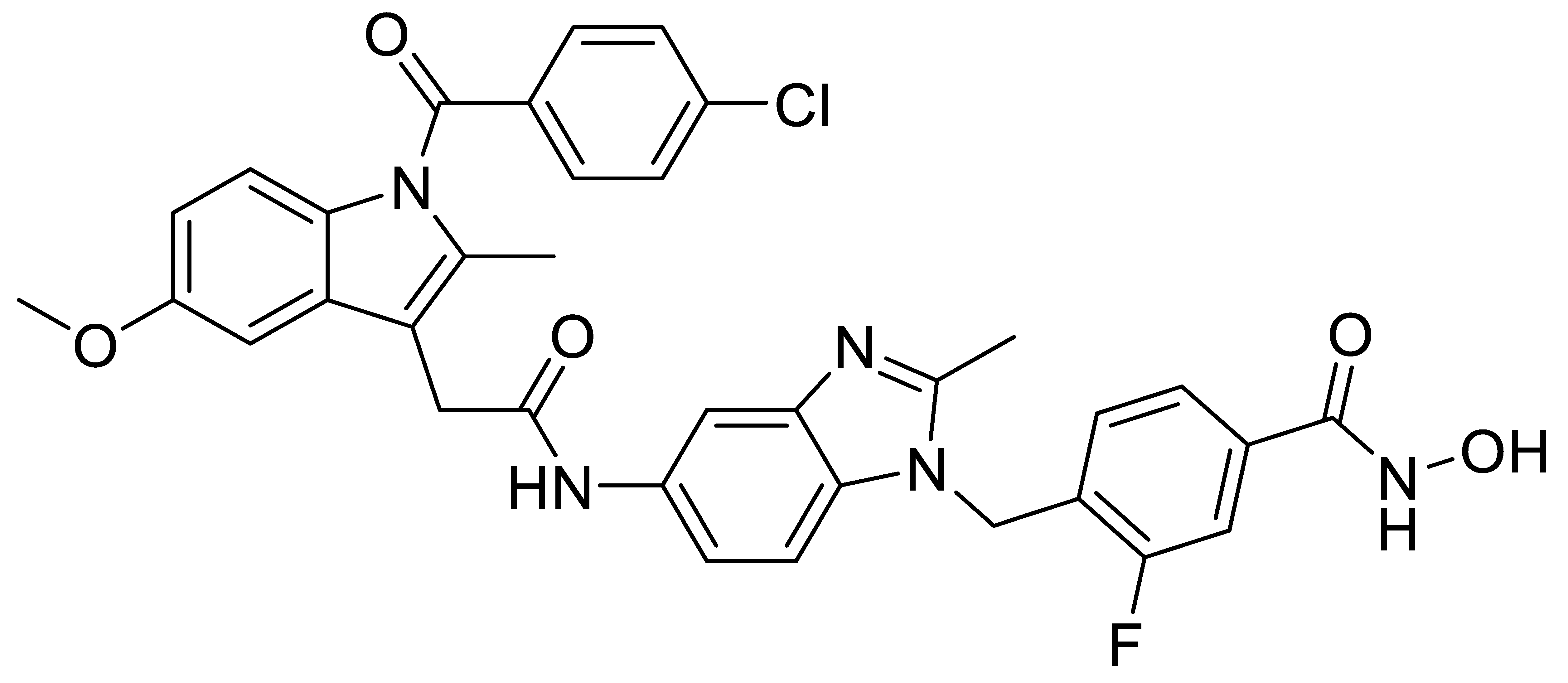 A5 | 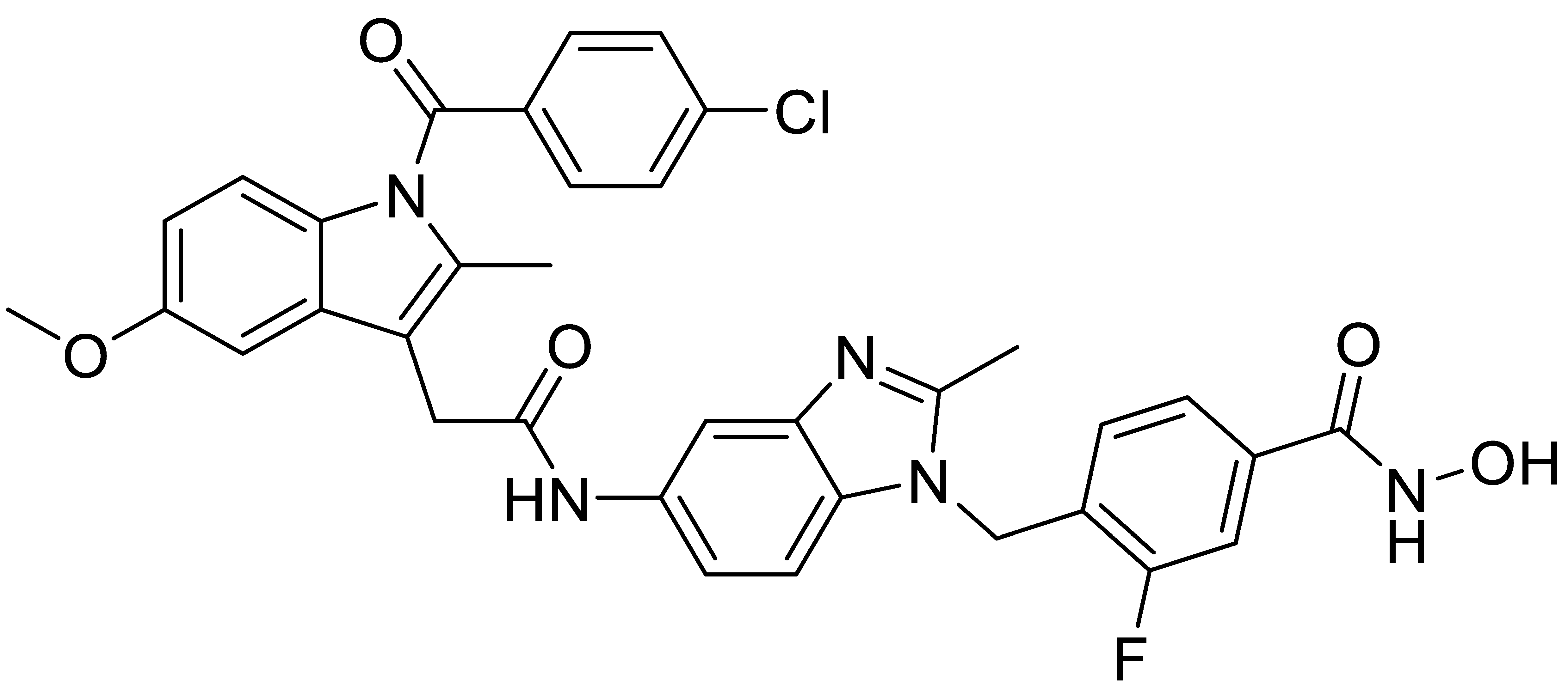 A6 |
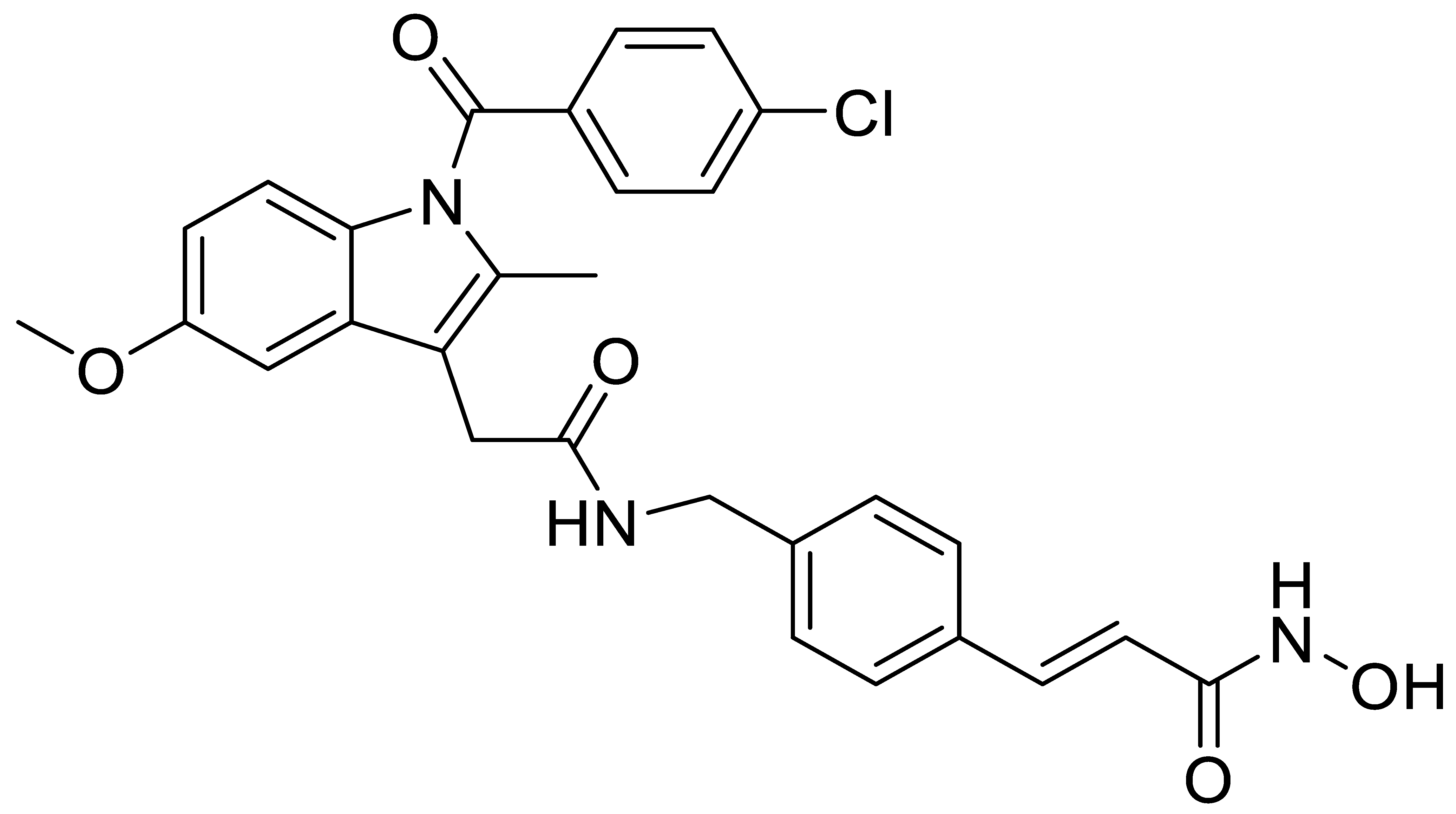 A7 | 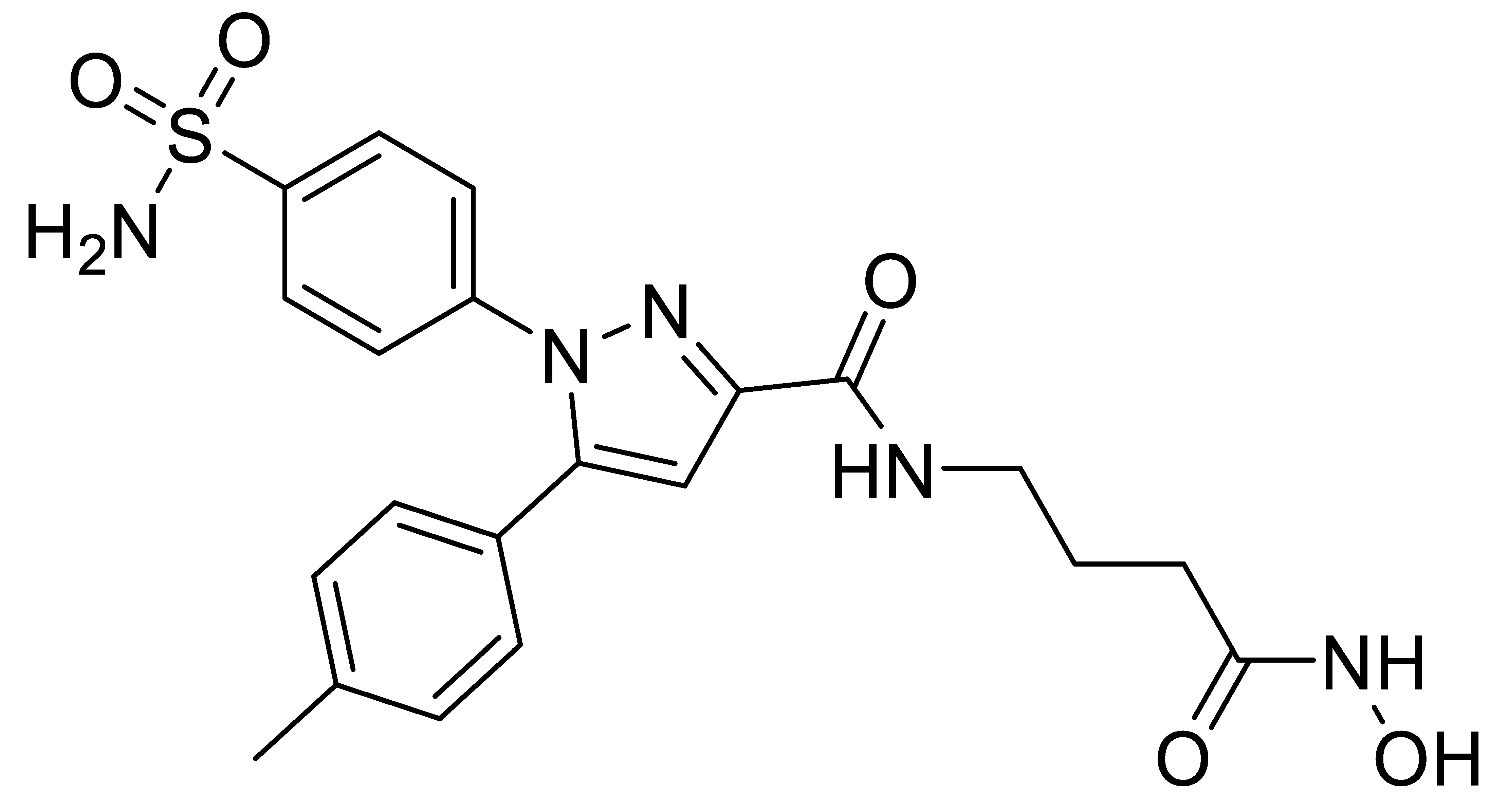 B1 |  B2 |
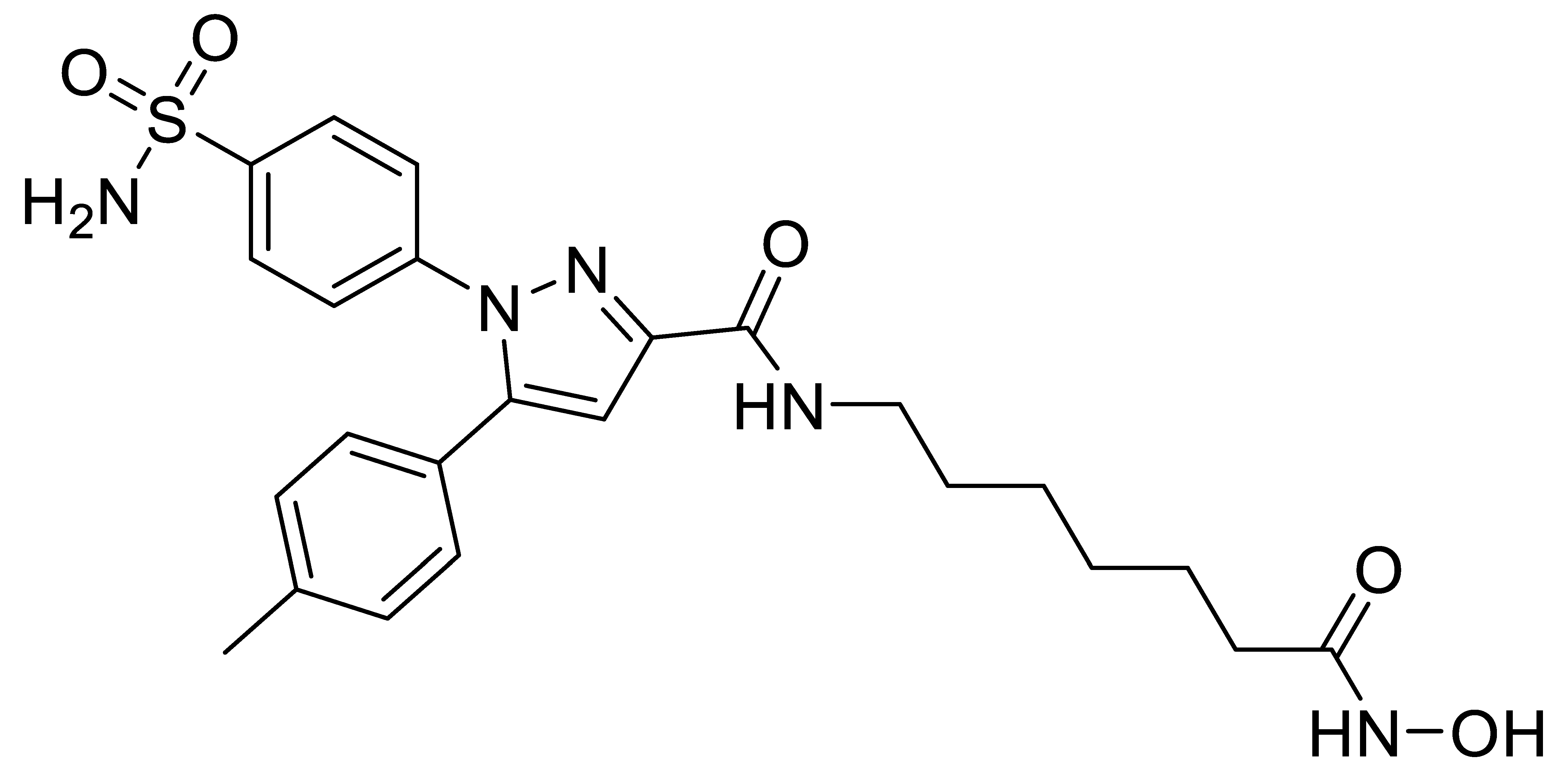 B3 |  B4 |  B5 |
 B6 | 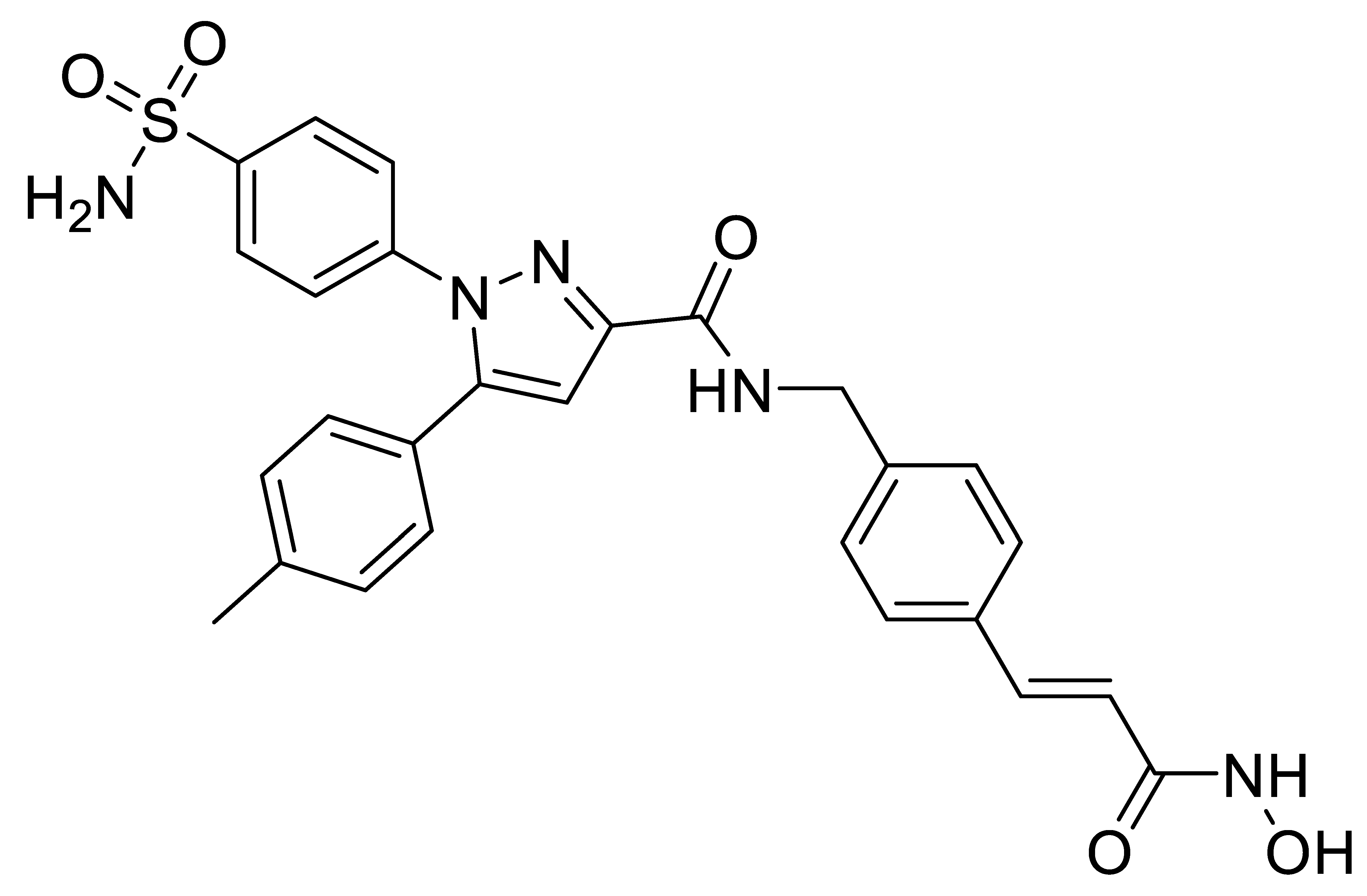 B7 | 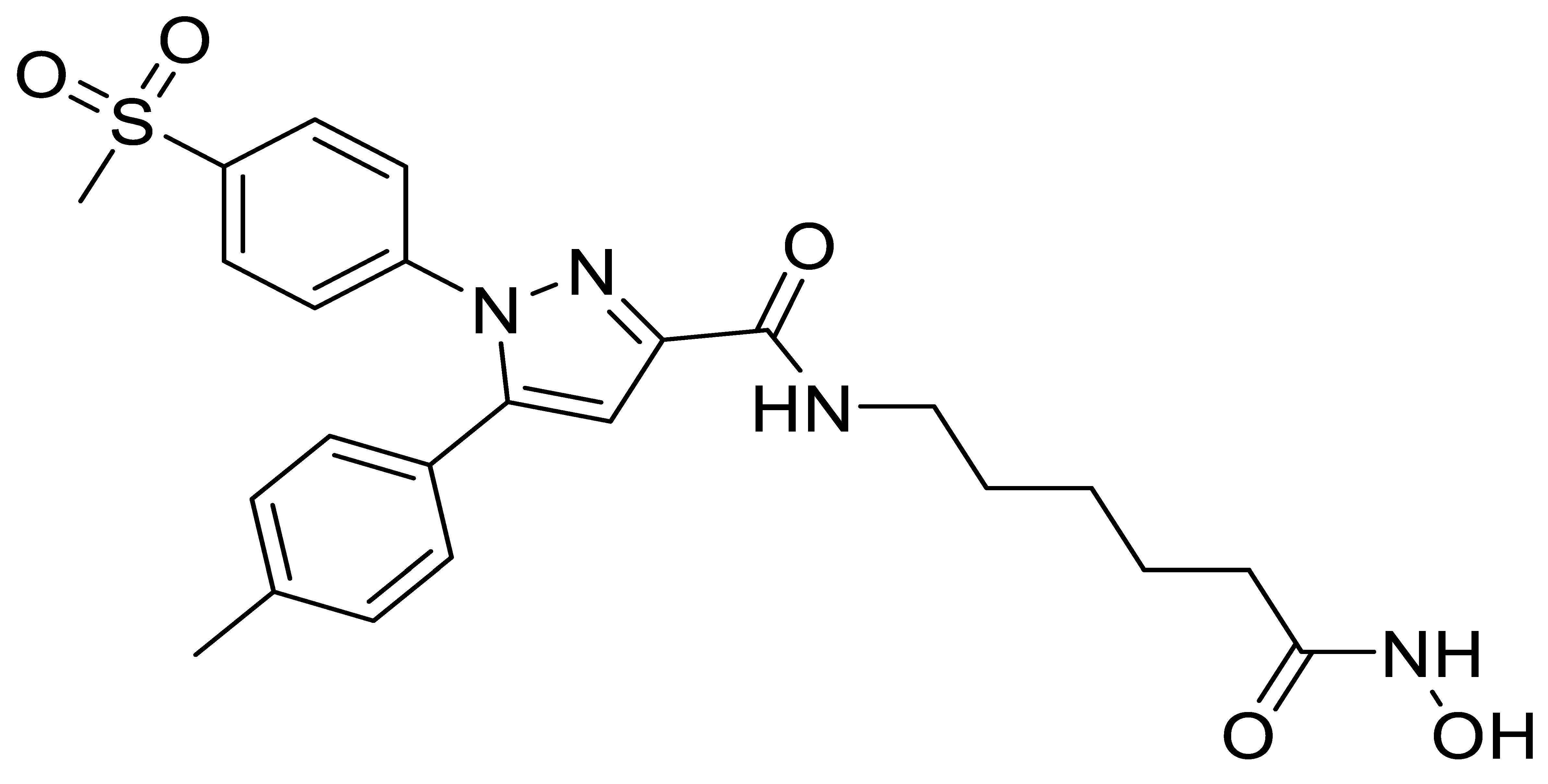 C2 |
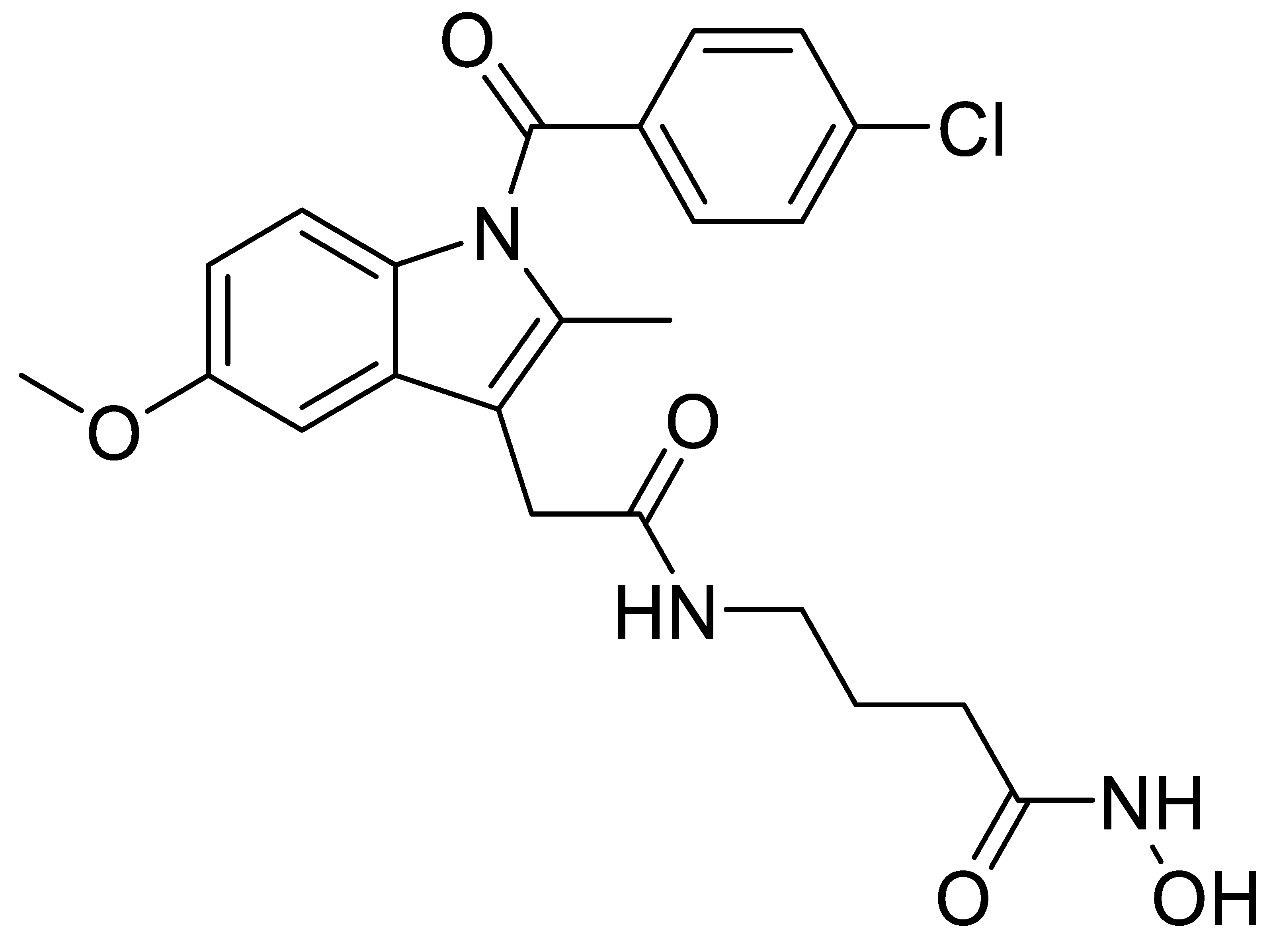
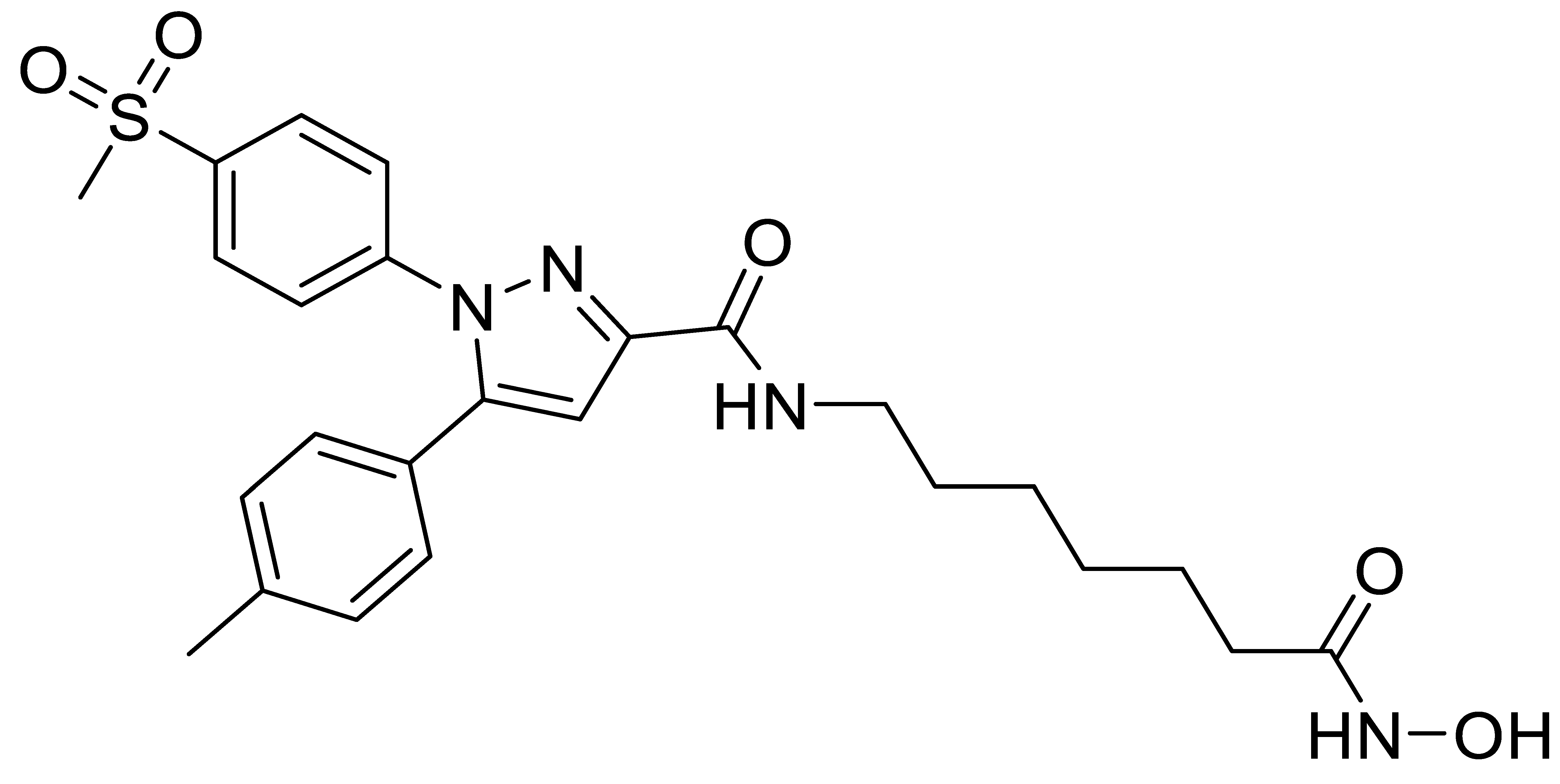 C3 | 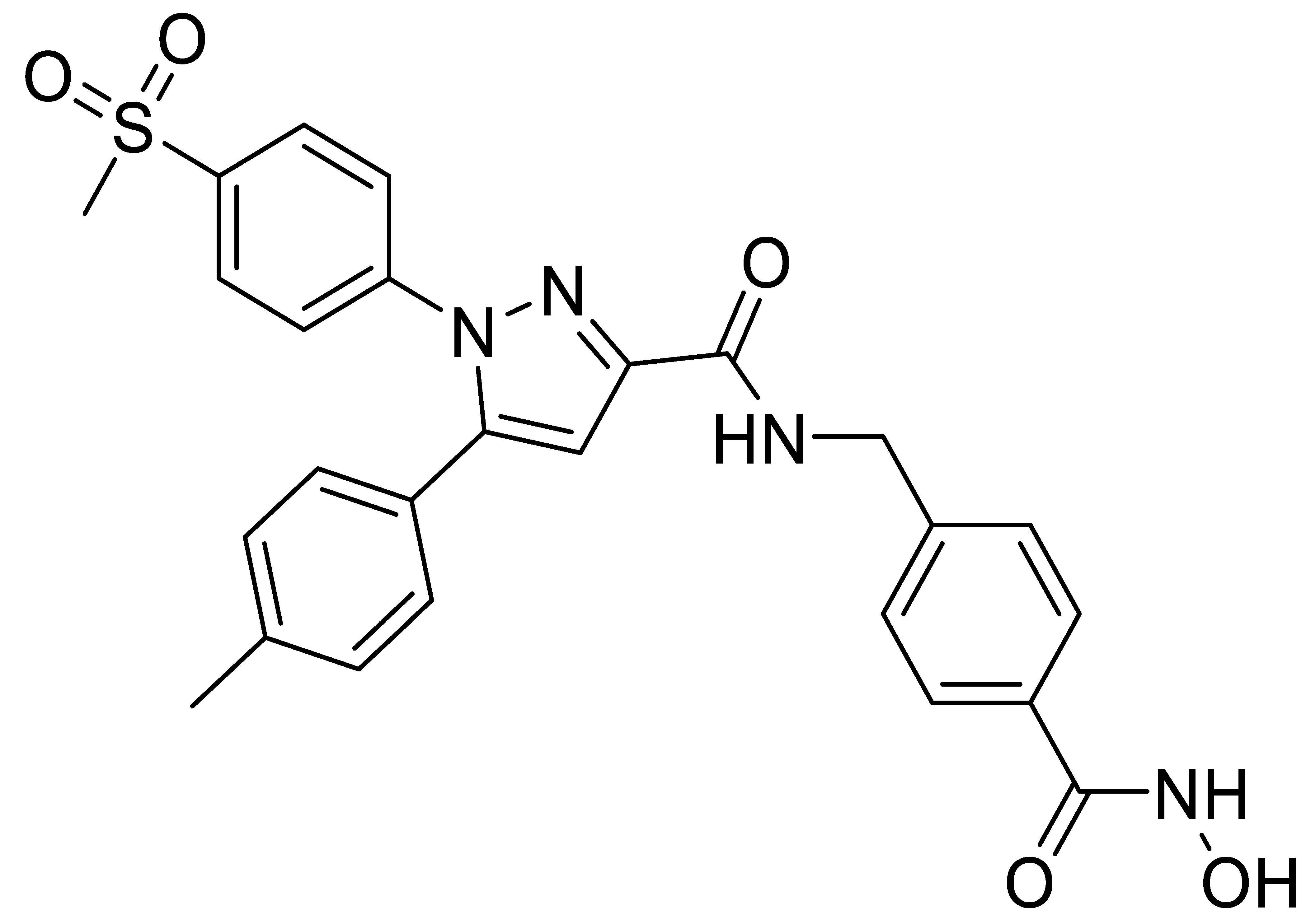 C4 |
| Cmpd 1 | COX-1 2 IC50 [µM] | COX-2 2 IC50 [µM] | SI COX-1/2 | HDAC1 IC50 [µM] | HDAC6 IC50 [µM] | SI HDAC1/6 | logD7.4HPLC 3 | AsPC1 IC50 [µM] 4 |
|---|---|---|---|---|---|---|---|---|
| A1 | >100 | 5.7 | >17 | 3.990 ± 0.143 | 3.039 ± 0.147 | 1.3 | 2.48 | 36.46 ± 6.36 |
| A2 | 38.1 | 4.1 | 9.3 | 0.554 ± 0.059 | 0.055 ± 0.004 | 10 | 2.63 | 10.28 ± 1.95 |
| A3 | 27.2 | 2.0 | 13.6 | 0.377 ± 0.004 | 0.084 ± 0.012 | 4 | 2.76 | 6.25 ± 1.07 |
| A4 | 18.1 | 1.2 | 15.1 | >3.33 | 0.174 ± 0.012 | >19 | 2.89 | n.e. |
| A5 | 6.5 | 1.6 | 4.0 | >3.33 | 0.052 ± 0.007 | >64 | 3.00 | 35.02 ± 5.08 |
| A6 | 2.4 | 1.9 | 1.2 | >2.80 | 0.011 ± 0.001 | >254 | 3.14 | n.e. |
| A7 | 3.5 | 1.8 | 2.2 | 0.542 ± 0.052 | 0.112 ± 0.008 | 5 | 3.31 | 19.20 ± 2.87 |
| B1 | >100 | 43.0 | >2.3 | 2.502 ± 0.260 | 0.695 ± 0.017 | 4 | 1.23 | n.e. |
| B2 | >100 | 3.3 | >30.3 | 0.099 ± 0.004 | 0.023 ± 0.0001 | 4 | 1.53 | 45.51 ± 15.83 |
| B3 | 11.6 | 7.5 | 1.5 | 0.034 ± 0.001 | 0.006 ± 0.00001 | 6 | 1.79 | 3.49 ± 1.29 |
| B4 | 10.8 | 1.4 | 7.7 | 0.039 ± 0.004 | 0.005 ± 0.0001 | 8 | 1.89 | 15.84 ± 4.26 |
| B5 | 8.8 | 2.7 | 3.3 | 0.155 ± 0.032 | 0.003 ± 0.0004 | 52 | 2.05 | n.e. |
| B6 | 1.8 | 1.4 | 1.3 | 0.829 ± 0.038 | 0.005 ± 0.0001 | 166 | 2.39 | n.e. |
| B7 | 7.5 | 1.9 | 3.9 | 0.014 ± 0.001 | 0.060 ± 0.011 | 0.2 | 2.28 | 2.54 ± 0.68 |
| C2 | 70.9 | 3.3 | 21.5 | 0.123 ± 0.004 | 0.038 ± 0.002 | 3 | 1.75 | 59.32 ± 8.47 |
| C3 | 93.8 | 0.98 | 95.7 | 0.043 ± 0.002 | 0.009 ± 0.001 | 5 | 1.99 | 2.39 ± 0.92 |
| C4 | 11.5 | 4.1 | 2.8 | 0.058 ± 0.001 | 0.010 ± 0.001 | 6 | 2.11 | 4.28 ± 0.93 |
| Vorinostat | n.d. | n.d. | - | 0.094 ± 0.013 | 0.027 ± 0.004 | 3 | n.d. | 1.04 ± 0.37 |
| Cele-coxib | >100 | 0.04 ± 0.02 (n = 6) | >2500 | n.d. | n.d. | n.d. | n.d. | n.e. |
| Compound | AsPC1 IC50 [µM] | MV3 IC50 [µM] | PC-3 IC50 [µM] | MDA-MB-231 IC50 [µM] | FaDu IC50 [µM] | HT-29 IC50 [µM] | U-87 IC50 [µM] |
|---|---|---|---|---|---|---|---|
| A7 | 19.20 ± 2.87 | 20.94 ± 5.75 | 10.59 ± 1.75 | 24.45 ± 2.03 | 6.61 ± 0.74 | 6.18 ± 1.26 | 17.46 ± 4.96 |
| B7 | 2.54 ± 0.68 | 11.59 ± 5.33 | 7.75 ± 2.30 | 12.47 ± 4.33 | 4.79 ± 0.45 | 6.40 ± 0.67 | 17.76 ± 2.25 |
| C3 | 2.39 ± 0.92 | 3.37 ± 0.98 | 2.67 ± 0.10 | 3.55 ± 0.62 | 2.21 ± 0.73 | 3.56 ± 0.74 | 6.91 ± 0.31 |
| C4 | 4.28 ± 0.93 | 9.82 ± 3.02 | 7.59 ± 1.98 | 9.57 ± 2.06 | 3.67 ± 0.50 | 5.46 ± 0.80 | 10.63 ± 0.97 |
| Vorinostat | 1.04 ± 0.37 | 1.72 ± 0.35 | 1.40 ± 0.57 | 1.60 ± 0.46 | 1.50 ± 0.28 | 1.58 ± 0.71 | 4.25 ± 0.84 |
| Celecoxib | n.e. | n.e. | n.e. | n.e. | n.e. | n.e. | n.e. |
| V + C (1:1) 2 | 1.21 ± 0.12 | 1.62 ± 0.34 | 1.30 ± 0.50 | 1.46 ± 0.29 | 1.50 ± 0.41 | 1.52 ± 0.54 | 4.94 ± 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachmann, L.M.; Hanl, M.; Feller, F.; Sinatra, L.; Schöler, A.; Pietzsch, J.; Laube, M.; Hansen, F.K. Solid-Phase Parallel Synthesis of Dual Histone Deacetylase-Cyclooxygenase Inhibitors. Molecules 2023, 28, 1061. https://doi.org/10.3390/molecules28031061
Bachmann LM, Hanl M, Feller F, Sinatra L, Schöler A, Pietzsch J, Laube M, Hansen FK. Solid-Phase Parallel Synthesis of Dual Histone Deacetylase-Cyclooxygenase Inhibitors. Molecules. 2023; 28(3):1061. https://doi.org/10.3390/molecules28031061
Chicago/Turabian StyleBachmann, Luisa M., Maria Hanl, Felix Feller, Laura Sinatra, Andrea Schöler, Jens Pietzsch, Markus Laube, and Finn K. Hansen. 2023. "Solid-Phase Parallel Synthesis of Dual Histone Deacetylase-Cyclooxygenase Inhibitors" Molecules 28, no. 3: 1061. https://doi.org/10.3390/molecules28031061
APA StyleBachmann, L. M., Hanl, M., Feller, F., Sinatra, L., Schöler, A., Pietzsch, J., Laube, M., & Hansen, F. K. (2023). Solid-Phase Parallel Synthesis of Dual Histone Deacetylase-Cyclooxygenase Inhibitors. Molecules, 28(3), 1061. https://doi.org/10.3390/molecules28031061







