Mechanistic Approaches to the Application of Nano-Zinc in the Poultry and Biomedical Industries: A Comprehensive Review of Future Perspectives and Challenges
Abstract
1. Introduction
2. Different Techniques for Nanoparticles Synthesis
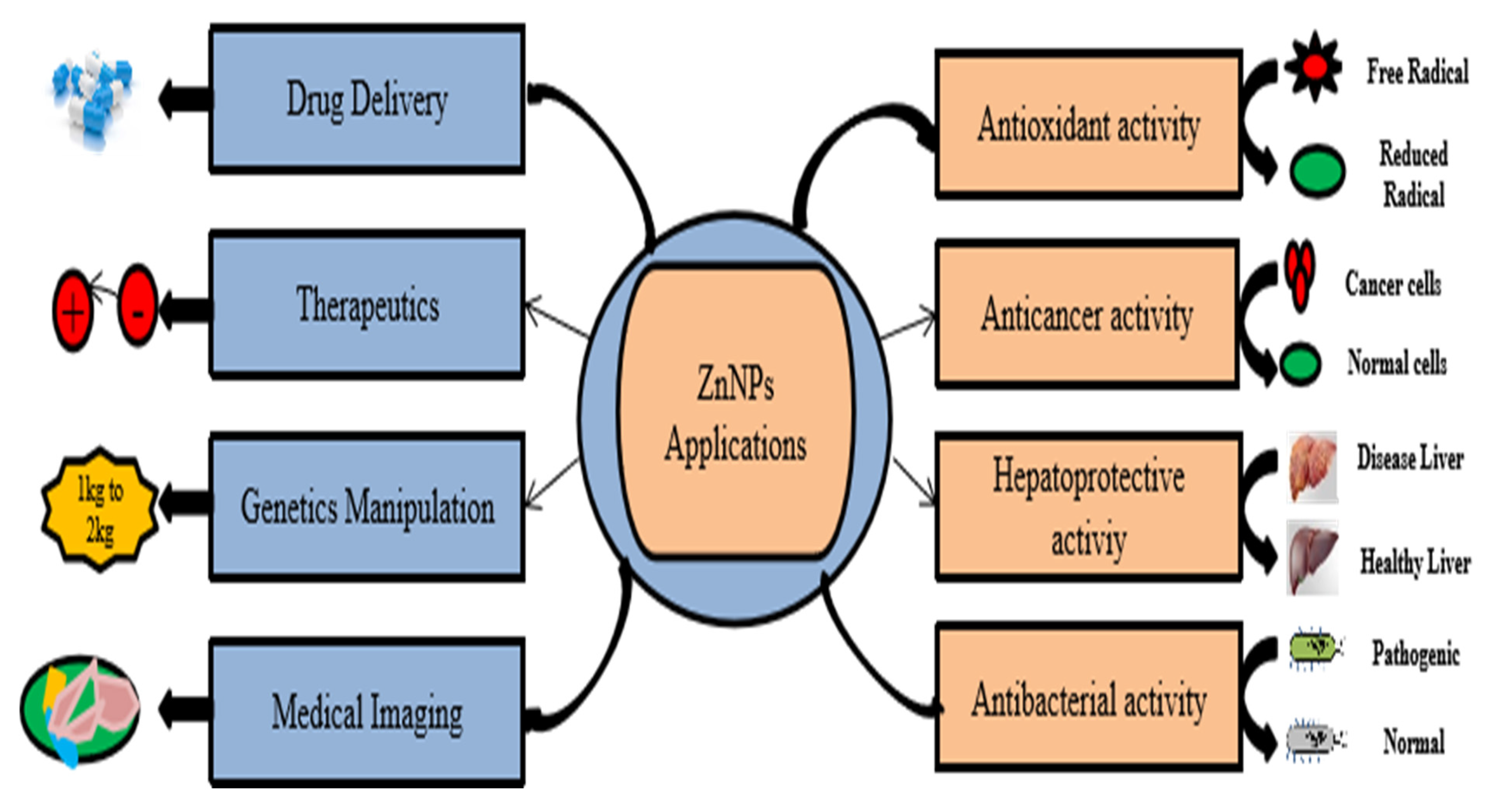
3. Application of ZnO NPs
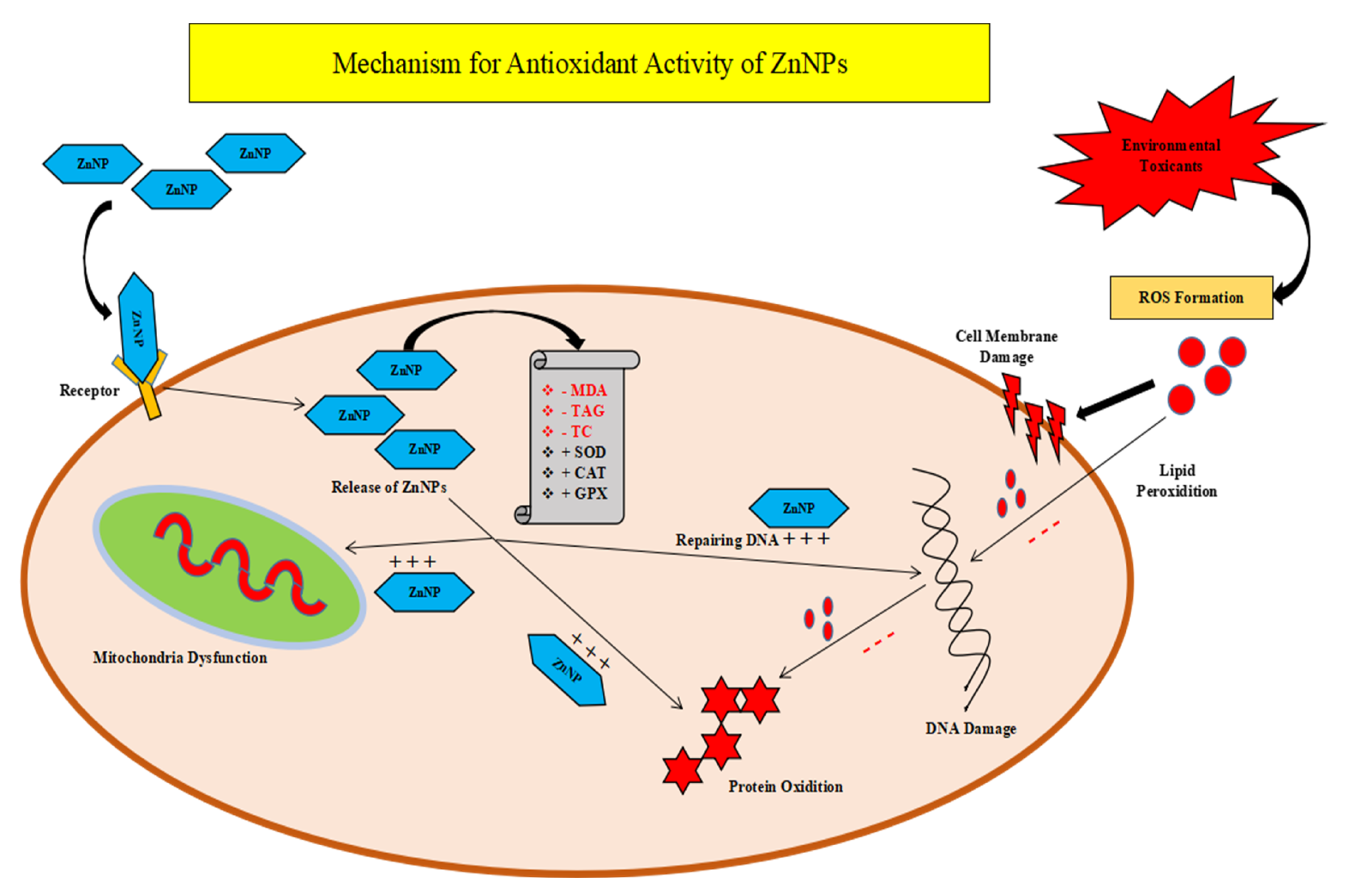
4. Antioxidant Activity of Zn NPs and Their Mechanism
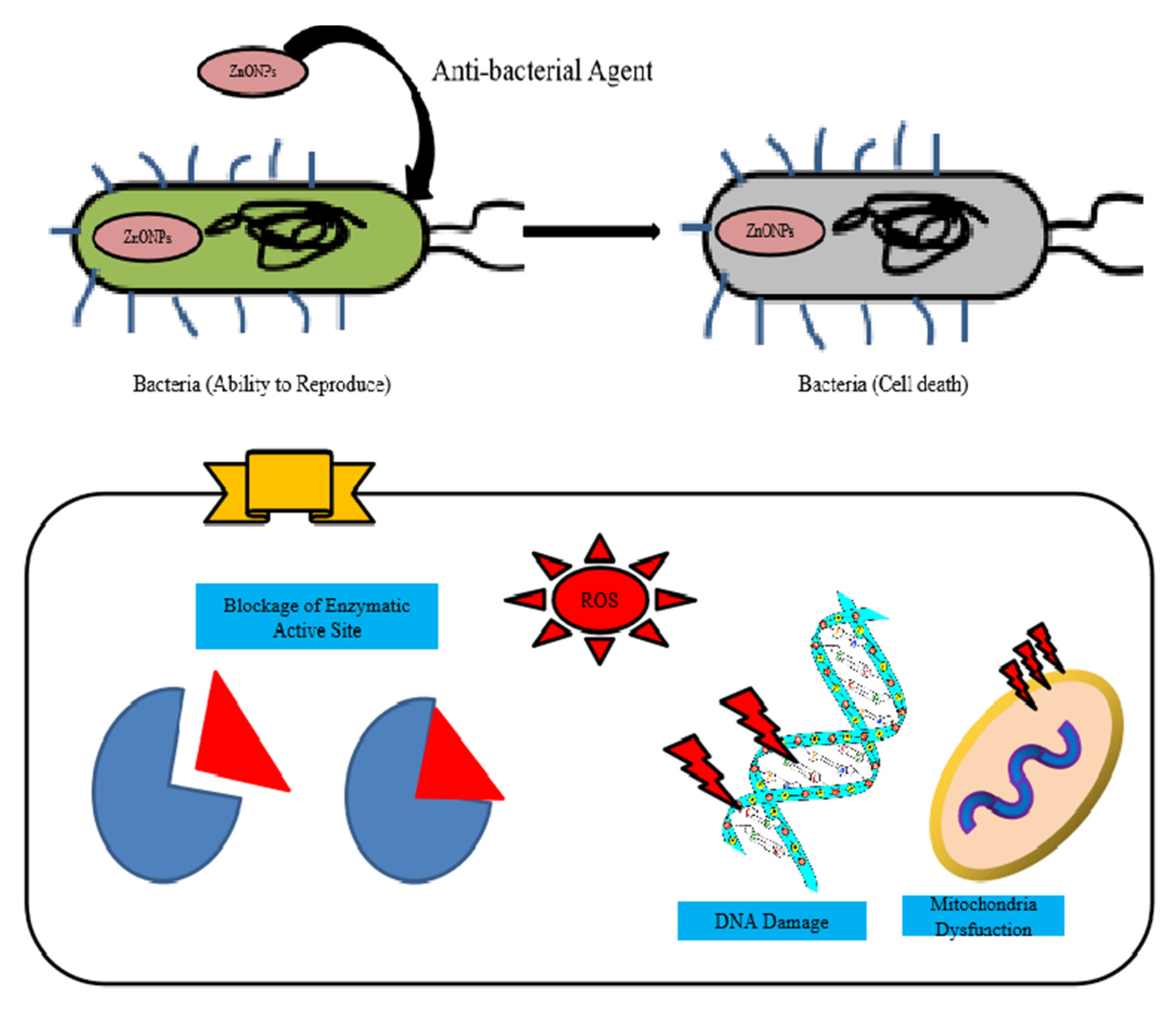
5. Antibacterial Application of Zinc Nanoparticles
6. Hepatoprotective Activity of Zinc Nanoparticles
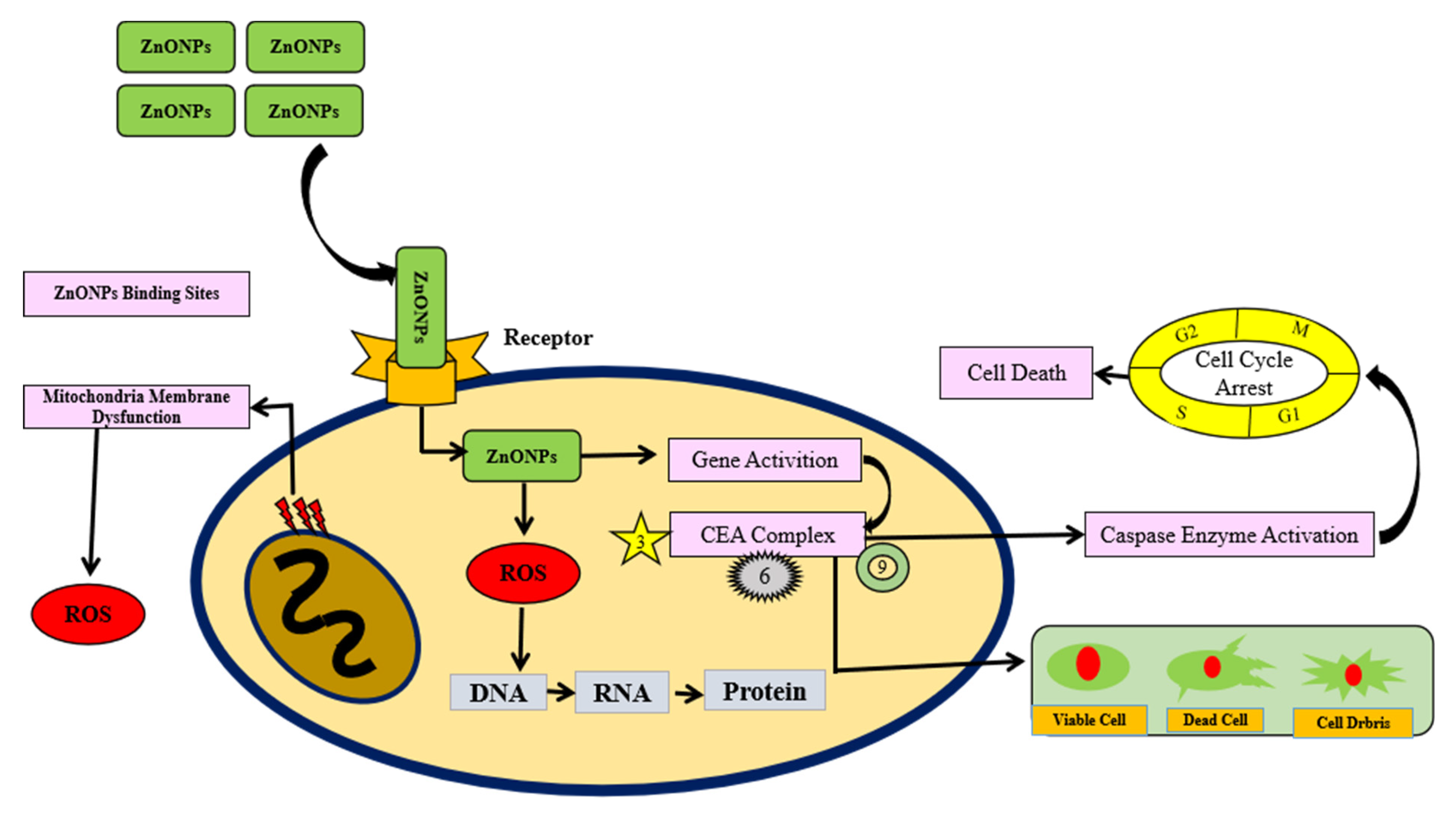
7. Anticancer Activity of Zinc Nanoparticles
| Sr.No | Plant Species | Nanoparticles Synthesized | Plant Part | NPs Size (nm) | Shape/ Morphology | Anticancer Activity | Cell Lines Used | Results | Reference |
|---|---|---|---|---|---|---|---|---|---|
| (1) | Rosa canina | ZnNO3 | Fruit | 30 nm | Spherical | 0.1 mg·mL−1 | Alveolar adenocarcinoma (A549) cells | Toxicity to A549 cells was dose-dependent | [145] |
| (2) | Ziziphus nummularia | ZnNO3 | Leaf | 12–25 nm | Irregular and spherical | 2 and 200 µg·mL−1 | Hela cancer cell lines | Hela cancer cells lines showed dose-dependent toxicity | [142] |
| (3) | Mangifera indica | ZnNO3 | Leaf | 50 nm | hexagonal | 25 µg·mL−1 | Lung cancer A549 cell lines | A549 lung cancer cells possessed significant cytotoxicity | [69] |
| (4) | Costus pictus | ZnNO3 | Leaf | 40 nm | Hexagonal and rectangular | 50 µg·mL−1 | Daltons lymphoma ascites (DLA) cells | DLA-bearing mice cell lines displayed significant anticancer properties | [67] |
| (5) | Anacardium occidentale | Zn(NO3)2•6H2O | leaf | 30 nm | hexagonal | 40 µM (Panc-1) and 30 µM (AsPC-1) | human pancreatic cancer cell lines (Panc-1 and AsPC-1) | Toxicity against human pancreatic cancer cell lines was concentration-dependent | [143] |
| (6) | Gracilaria edulis | Zn(NO3)2•6H2O | aqueous | 20–50 nm | Hexagonal (Wurtzite) rod | 35 µg·mL−1 | Cervical carcinoma cells (SiHa cells) | Cells of SiHa displayed dose-dependent cytotoxicity | [144] |
| (7) | Artocarpus heterophyllus | Zn(NO3)2•6H2O | Leaf | 12–24 nm | Spherical | 20 µg·mL−1 | MDA-MB231 breast cancer cell lines | Dose-dependent nanoparticles suppress breast cancer cell proliferation and induce cytotoxicity | [146] |
| (8) | Cucumis melo inodurus | Zn(CH3CO2)2 | Peel | 25–40 nm | Crystalline but nearly rounded | 40 µg·mL−1 (MCF7) | Human (Michigan Cancer Foundation-7 [MCF7]) | Associated with the induction of apoptosis in human breast cancer cells (MCF7) | [147] |
| (9) | Raphanus sativus | Zn(CH3CO2)2 | leaf | 65 nm | spherical | 40 µg·mL−1 | Lung cancer cell line (A549) | Cell viability has been reduced, indicating improved anticancer efficacy. | [148] |
| (10) | Pongamia pinnata | Zn(CH3COO)2•2H2O | seed | 30–40 nm | Spherical | 50 µg·mL−1 | Human MCF-7 breast cancer cell lines | Inhibits human MCF-7 breast cancer cells more effectively | [149] |
| (11) | Trianthema portulacastrum | ZnSO4 | Root, leaf, stem, flower, fruit | 20–100 nm | Spherical | 100 µg·mL−1 | Mouse pre-osteoblast cell line (MC3T3-E1) | The cells were found to be viable and showed no toxicity | [150] |
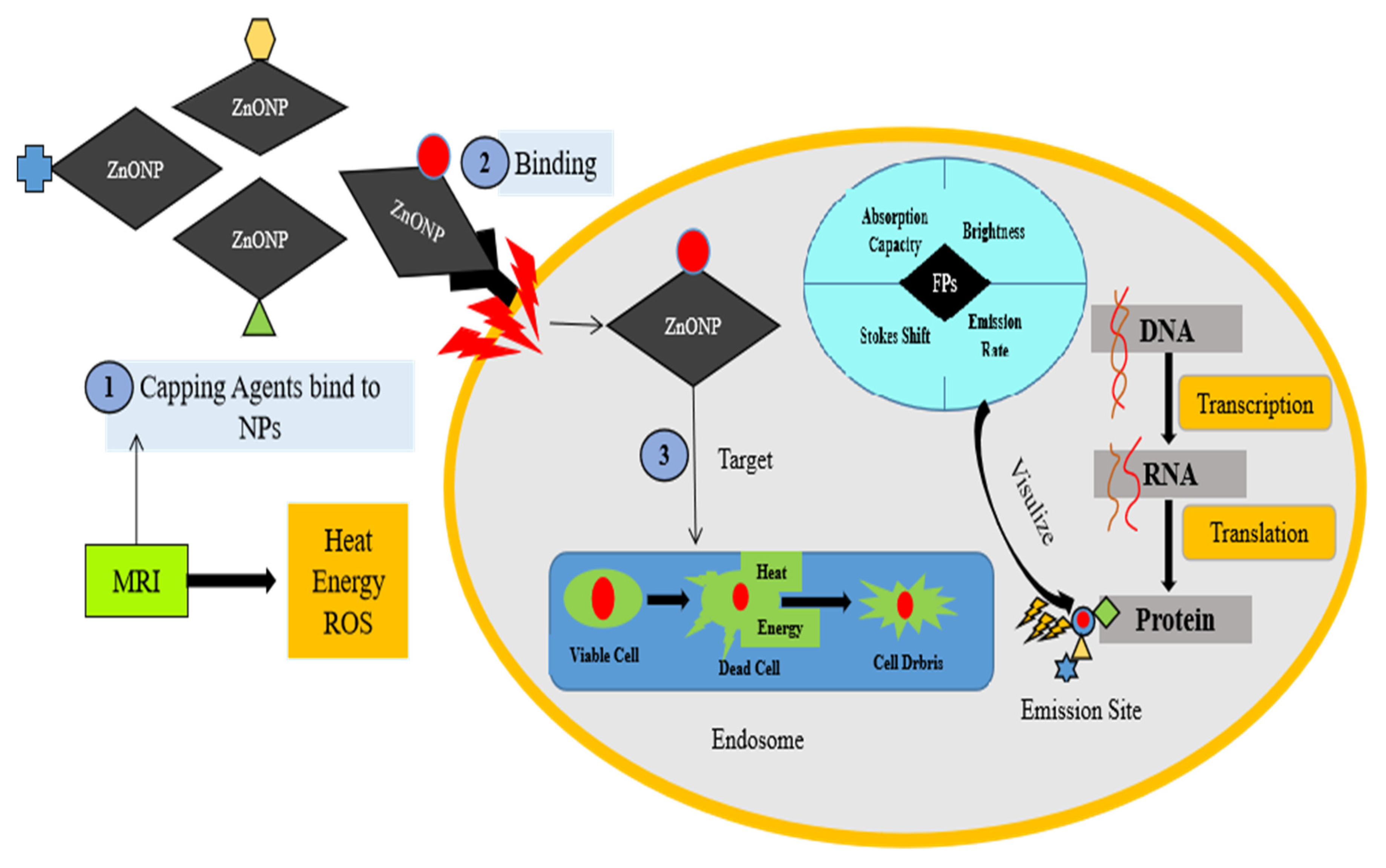
8. Bio-Imaging Application of Zinc Nanoparticles
9. Drug Delivery
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gould, J. Nutrition: A world of insecurity. Nat. Outlook 2017, 544, S7. [Google Scholar] [CrossRef] [PubMed]
- Khush, G.S.; Lee, S.; Cho, J.I.; Jeon, J.S. Biofortification of crops for reducing malnutrition. Plant Biotechnol. Rep. 2012, 6, 195–202. [Google Scholar] [CrossRef]
- Nabi, F.; Arain, M.A.; Hassan, F.; Umar, M.; Rajput, N.; Alagawany, M.; Liu, J. Nutraceutical role of selenium nanoparticles in poultry nutrition: A review. World Poult. Sci. J. 2020, 76, 459–471. [Google Scholar] [CrossRef]
- Reda, F.M.; El-Saadony, M.T.; Elnesr, S.S.; Alagawany, M.; Tufarelli, V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals 2020, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.; Yahia, D.; Abdel-Magiud, D.S.; Darwish, M.H.; Abd-Elkareem, M.; Mahmoud, U.T. Broiler welfare is preserved by long-term low-dose oral exposure to zinc oxide nanoparticles: Preliminary study. Nanotoxicology 2021, 15, 605–620. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Wijnhoven, S.W.; Peijnenburg, W.J.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.; Geertsma, R.E. Nano-silver–a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Taha, T.F.; Najjar, A.A.; Zabermawi, N.M.; Nader, M.M.; Salama, A. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J. Biol. Sci. 2021, 28, 6782–6794. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. A guide to human zinc absorption: General overview and recent advances of in vitro intestinal models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef]
- Chrastinová, Ľ.; Čobanová, K.; Chrenková, M.; Poláčiková, M.; Formelová, Z.; Lauková, A.; Grešáková, Ľ. Effect of dietary zinc supplementation on nutrients digestibility and fermentation characteristics of caecal content in physiological experiment with young rabbits. Slovak J. Anim. Sci. 2016, 49, 23–31. [Google Scholar]
- Venubabu Thati, A.; Roy, S.; Prasad, M.A.; Shivannavar, C.T.; Gaddad, S.M. Nanostructured zinc oxide enhances the activity of antibiotics against Staphylococcus aureus. Biosci. Technol. J. 2010, 1, 64–69. [Google Scholar]
- Bennett, P.M.; Jepson, P.D.; Law, R.J.; Jones, B.R.; Kuiken, T.; Baker, J.R.; Kirkwood, J.K. Exposure to heavy metals and infectious disease mortality in harbour porpoises from England and Wales. Environ. Pollut. 2001, 112, 33–40. [Google Scholar] [CrossRef]
- Mohamed, L.A.; El-Hindawy, M.M.; Alagawany, M.; Salah, A.S.; El-Sayed, S.A. Effect of low-or high-CP diet with cold-pressed oil supplementation on growth, immunity and antioxidant indices of growing quail. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1380–1387. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, J.; Fellner, M.; Zhang, C.; Sui, D.; Hu, J. Crystal structures of a ZIP zinc transporter reveal a binuclear metal center in the transport pathway. Sci. Adv. 2017, 3, e1700344. [Google Scholar] [CrossRef]
- Zaghari, M.; Avazkhanllo, M.; Ganjkhanlou, M. Reevaluation of male broiler zinc requirement by dose-response trial using practical diet with added exogenous phytase. J. Agric. Sci. Technol. 2015, 17, 333–343. [Google Scholar]
- Teow, S.Y.; Wong, M.M.T.; Yap, H.Y.; Peh, S.C.; Shameli, K. Bactericidal properties of plants-derived metal and metal oxide nanoparticles (NPs). Molecules 2018, 23, 1366. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tan, S.X.; Xiao, X.Y.; Qiu, X.S.; Pan, J.Q.; Tang, Z.X. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 2014, 160, 361–367. [Google Scholar] [CrossRef]
- Akhavan-Salamat, H.; Ghasemi, H.A. Effect of different sources and contents of zinc on growth performance, carcass characteristics, humoral immunity and antioxidant status of broiler chickens exposed to high environmental temperatures. Livest. Sci. 2019, 223, 76–83. [Google Scholar] [CrossRef]
- Cesur, S.; Cebeci, S.A.; Kavas, G.O.; Aksaray, S.; Tezeren, D. Serum copper and zinc concentrations in patients with chronic hepatitis. Br. J. Infect. Control. 2005, 51, 38–40. [Google Scholar] [CrossRef]
- Laurenti, M.; Verna, A.; Chiolerio, A. Evidence of negative capacitance in piezoelectric ZnO thin films sputtered on interdigital electrodes. ACS Appl. Mater. Interfaces 2015, 7, 24470–24479. [Google Scholar] [CrossRef]
- FDA, U. Select committee on GRAS substances (SCOGS) opinion: Tannic acid (hydrolyzable gallotannins). GRAS Subst. (SCOGS) Database 2015, 3, 153–166. [Google Scholar]
- Pulit-Prociak, J.; Chwastowski, J.; Kucharski, A.; Banach, M. Functionalization of textiles with silver and zinc oxide nanoparticles. Appl. Surf. Sci. 2016, 385, 543–553. [Google Scholar] [CrossRef]
- Patel, P.; Kansara, K.; Senapati, V.A.; Shanker, R.; Dhawan, A.; Kumar, A. Cell cycle dependent cellular uptake of zinc oxide nanoparticles in human epidermal cells. Mutagenesis 2016, 31, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.R.; Sarvi, M.N. Recent achievements in the microbial synthesis of semiconductor metal sulfide nanoparticles. Mater. Sci. Semicond. Process. 2015, 40, 293–301. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Kundu, D.; Hazra, C.; Chatterjee, A.; Chaudhari, A.; Mishra, S. Extracellular biosynthesis of zinc oxide nanoparticles using Rhodococcus pyridinivorans NT2: Multifunctional textile finishing, biosafety evaluation and in vitro drug delivery in colon carcinoma. J. Photochem. Photobiol. B Biol. 2014, 140, 194–204. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Ariff, A.B.; Saad, W.Z.; Mohamad, R. Biosynthesis of ZnO nanoparticles by a new Pichia kudriavzevii yeast strain and evaluation of their antimicrobial and antioxidant activities. Molecules 2017, 22, 872. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide—From synthesis to application: A review. Materials 2014, 7, 2833. [Google Scholar] [CrossRef]
- Agarwal, H.; Menon, S.; Kumar, S.V.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. -Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Ju, D.; Xu, H.; Qiu, Z.; Guo, J.; Zhang, J.; Cao, B. Highly sensitive and selective triethylamine-sensing properties of nanosheets directly grown on ceramic tube by forming NiO/ZnO PN heterojunction. Sens. Actuators B Chem. 2014, 200, 288–296. [Google Scholar] [CrossRef]
- Mclaren, A.; Valdes-Solis, T.; Li, G.; Tsang, S.C. Shape and size effects of ZnO nanocrystals on photocatalytic activity. J. Am. Chem. Soc. 2009, 131, 12540–12541. [Google Scholar] [CrossRef]
- Aboulaich, A.; Balan, L.; Ghanbaja, J.; Medjahdi, G.; Merlin, C.; Schneider, R. Aqueous route to biocompatible ZnSe: Mn/ZnO core/shell quantum dots using 1-thioglycerol as stabilizer. Chem. Mater. 2011, 23, 3706–3713. [Google Scholar] [CrossRef]
- Nagvenkar, A.P.; Deokar, A.; Perelshtein, I.; Gedanken, A. A one-step sonochemical synthesis of stable ZnO–PVA nanocolloid as a potential biocidal agent. J. Mater. Chem. B 2016, 4, 2124–2132. [Google Scholar] [CrossRef]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016; Volume 1724, p. 020048. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Biosynthesis of gold nanoparticles: A green approach. J. Photochem. Photobiol. B Biol. 2016, 161, 141–153. [Google Scholar] [CrossRef]
- Pantidos, N.; Horsfall, L.E. Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. J. Nanomed. Nanotechnol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Yusof, M.H.; Mohamad, R.; Zaidan, U.H.; Rahman, A. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: A review. J. Anim. Sci. Biotechnol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.M.; Balaji, K.; Kalaichelvan, P.T.; Venkatesan, R. Fungal based synthesis of silver nanoparticles—An effect of temperature on the size of particles. Colloids Surf. B Biointerfaces 2009, 74, 123–126. [Google Scholar] [CrossRef]
- Verma, A.; Mehata, M.S. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Radiat. Res. Appl. Sci. 2016, 9, 109–115. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Ibrahim, H.M. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J. Radiat. Res. Appl. Sci. 2015, 8, 265–275. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Yun, Y.-S. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresour. Technol. 2010, 101, 7958–7965. [Google Scholar] [CrossRef] [PubMed]
- Khanam, S. Toxicological effect of zinc on liver of broiler chicks. Egypt. Liver J. 2020, 10, 1–5. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc oxide nanoparticles for revolutionizing agriculture: Synthesis and applictions. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef] [PubMed]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants-A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Wagner, J.; Kirner, T.; Mayer, G.; Albert, J.; Köhler, J.M. Generation of metal nanoparticles in a microchannel reactor. Chem. Eng. J. 2004, 101, 251–260. [Google Scholar] [CrossRef]
- Fruhwirth, G.O.; Hermetter, A. Mediation of apoptosis by oxidized phospholipids. Lipids Health Dis. 2008, 49, 351–367. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 1–27. [Google Scholar] [CrossRef]
- Mujahid, A.; Akiba, Y.; Toyomizu, M. Acute heat stress induces oxidative stress and decreases adaptation in young white leghorn cockerels by downregulation of avian uncoupling protein. Poult. Sci. 2007, 86, 364–371. [Google Scholar] [CrossRef]
- Mishra, B.; Jha, R. Oxidative stress in the poultry gut: Potential challenges and interventions. Front. Vet. Sci. 2019, 6, 60. [Google Scholar] [CrossRef]
- Ruttkay-Nedecky, B.; Nejdl, L.; Gumulec, J.; Zitka, O.; Masarik, M.; Eckschlager, T.; Kizek, R. The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 2013, 14, 6044. [Google Scholar] [CrossRef]
- Ritchie, S.M.C. Enhanced dechlorination of trichloroethylene by membrane-supported iron and bimetallic nanoparticles. In Nanotechnology Applications for Clean Water; William Andrew: Norwich, NY, USA, 2014; pp. 351–367. [Google Scholar]
- Ramiah, S.K.; Awad, E.A.; Mookiah, S.; Idrus, Z. Effects of zinc oxide nanoparticles on growth performance and concentrations of malondialdehyde, zinc in tissues, and corticosterone in broiler chickens under heat stress conditions. Poult. Sci. 2019, 98, 3828–3838. [Google Scholar] [CrossRef]
- Starkov, A.A. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef]
- Giorgio, M.; Migliaccio, E.; Orsini, F.; Paolucci, D.; Moroni, M.; Contursi, C.; Pelicci, P.G. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 2005, 122, 221–233. [Google Scholar] [CrossRef]
- Hashizawa, Y.; Kubota, M.; Kadowaki, M.; Fujimura, S. Effect of dietary vitamin E on broiler meat qualities, color, water-holding capacity and shear force value, under heat stress conditions. Anim. Sci. J. 2013, 84, 732–736. [Google Scholar] [CrossRef]
- Marchini, C.F.P.; Silva, P.L.; Nascimento, M.R.B.M.; Beletti, M.E.; Silva, N.M.; Guimarães, E.C. Body weight, intestinal morphometry and cell proliferation of broiler chickens submitted to cyclic heat stress. Int. J. Poult. Sci 2011, 10, 455–460. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 1, 1–289. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef]
- Mujahid, A.; Pumford, N.R.; Bottje, W.; Nakagawa, K.; Miyazawa, T.; Akiba, Y.; Toyomizu, M. Mitochondrial oxidative damage in chicken skeletal muscle induced by acute heat stress. J. Poult. Sci. 2007, 44, 439–445. [Google Scholar] [CrossRef]
- Mujahid, A.; Sato, K.; Akiba, Y.; Toyomizu, M. Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult. Sci. 2006, 85, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- El-Orabi, N.F.; Rogers, C.B.; Edwards, H.G.; Schwartz, D.D. Heat-induced inhibition of superoxide dismutase and accumulation of reactive oxygen species leads to HT-22 neuronal cell death. J. Therm. Biol. 2011, 36, 49–56. [Google Scholar] [CrossRef]
- Girish, C.; Smith, T. Impact of feed-borne mycotoxins on avian cell-mediated and humoral immune responses. World Mycotoxin J. 2008, 1, 105–121. [Google Scholar] [CrossRef]
- Das, D.; Nath, B.C.; Phukon, P.; Dolui, S.K. Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surf. B Biointerfaces 2013, 111, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Nethravathi, P.; Lingaraju, K.; Rajanaika, H.; Sharma, S.; Nagabhushana, H. EGCG assisted green synthesis of ZnO nanopowders: Photodegradative, antimicrobial and antioxidant activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 1467–1474. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Cha, S.J.; Yang, I.J.; Sreekanth, T.; Kim, K.J.; Shin, H.M. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J. Photochem. Photobiol. B Biol. 2015, 146, 10–17. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Kumar, S.V.; Ramaiah, A.; Agarwal, H.; Lakshmi, T.; Roopan, S.M. Biosynthesis of zinc oxide nanoparticles usingMangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzym. Microb. Technol. 2018, 117, 91–95. [Google Scholar] [CrossRef]
- Biswas, P.; Adhikari, A.; Mondal, S.; Das, M.; Bhattacharya, S.S.; Pal, D.; Pal, S.K. Synthesis and spectroscopic characterization of a zinc oxide-polyphenol nanohybrid from natural resources for enhanced antioxidant activity with less cytotoxicity. Mater. Today Proc. 2021, 43, 3481–3486. [Google Scholar] [CrossRef]
- Hafez, A.; Nassef, E.; Fahmy, M.; Elsabagh, M.; Bakr, A.; Hegazi, E. Impact of dietary nano-zinc oxide on immune response and antioxidant defense of broiler chickens. Environ. Sci. Pollut. Res. 2020, 27, 19108–19114. [Google Scholar] [CrossRef]
- Rahimi, G.; Mohammad, K.S.; Zarei, M.; Shokoohi, M.; Oskoueian, E.; Poorbagher, M.R.M.; Karimi, E. Zinc oxide nanoparticles synthesized using Hyssopus Officinalis, L. Extract Induced oxidative stress and changes the expression of key genes involved in inflammatory and antioxidant Systems. Biol. Res. 2022, 55, 24. [Google Scholar] [CrossRef]
- Abedini, M.; Shariatmadari, F.; Karimi Torshizi, M.; Ahmadi, H. Effects of zinc oxide nanoparticles on the egg quality, immune response, zinc retention, and blood parameters of laying hens in the late phase of production. J. Anim. Physiol. Anim. Nutr. 2018, 102, 736–745. [Google Scholar] [CrossRef]
- Zhao, H.; He, Y.; Li, S.; Sun, X.; Wang, Y.; Shao, Y.; Xing, M. Subchronic arsenism-induced oxidative stress and inflammation contribute to apoptosis through mitochondrial and death receptor dependent pathways in chicken immune organs. Oncotarget 2017, 8, 40327. [Google Scholar] [CrossRef]
- Wang, H.; Wingett, D.; Engelhard, M.H.; Feris, K.; Reddy, K.M.; Turner, P.; Punnoose, A. Fluorescent dye encapsulated ZnO particles with cell-specific toxicity for potential use in biomedical applications. J. Mater. Sci. Mater. Med. 2009, 20, 11–22. [Google Scholar] [CrossRef]
- Miles, D.M.; Branton, S.L.; Lott, B.D. Atmospheric ammonia is detrimental to the performance of modern commercial broilers. Poult. Sci. 2004, 83, 1650–1654. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Tang, X.; Lu, Q.; Sa, R.; Zhang, H. Proteome changes in the small intestinal mucosa of broilers (Gallus gallus) induced by high concentrations of atmospheric ammonia. Proteome Sci. 2015, 13, 9. [Google Scholar] [CrossRef]
- Wei, F.; Xu, B.; Hu, X.; Li, S.; Liu, F.; Sun, Q.; Wang, L. The effect of ammonia and humidity in poultry houses on intestinal morphology and function of broilers. J. Anim. Vet. Adv. 2012, 11, 3641–3646. [Google Scholar]
- Gabriel, I.; Lessire, M.; Mallet, S.; Guillot, J.F. Microflora of the digestive tract: Critical factors and consequences for poultry. Worlds Poult. Sci. J. 2006, 62, 499–511. [Google Scholar]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2012; Volume 23, pp. 729–737. [Google Scholar] [CrossRef]
- Tang, D.; Wu, J.; Jiao, H.; Wang, X.; Zhao, J.; Lin, H. The development of antioxidant system in the intestinal tract of broiler chickens. Poult. Sci. 2019, 98, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Min, W.H.; Fang, X.B.; Wu, T.; Fang, L.; Liu, C.L.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Borchert, G.L.; Surazynski, A.; Hu, C.A.; Phang, J.M. Proline oxidase activates both intrinsic and extrinsic pathways for apoptosis: The role of ROS/superoxides, NFAT and MEK/ERK signaling. Oncogene 2006, 25, 5640–5647. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Jiang, S.; Jiang, Z.; Zheng, C.; Gou, Z. Effects of equol on H2 O2-induced oxidative stress in primary chicken intestinal epithelial cells. Poult. Sci. 2016, 95, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The role of zinc in antiviral immunity. Adv. Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef]
- Vural, H.; Demirin, H.; Kara, Y.; Eren, I.; Delibas, N. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer’s disease. J. Trace Elem. Med. Biol. 2010, 24, 169–173. [Google Scholar] [CrossRef]
- Pascual, A.S.; Tyszka-Czochara, M.; Gdula-Argasińska, J.; Librowski, T.; Grzywacz, A.; Opoka, W. Zinc, the trace element essential in living organisms. Med. Int. Rev. 2012, 99, 55–59. [Google Scholar]
- Martin, K.M. The Effects of Zinc Supplementation from Two Sources on Egg Quality and Bone Health in Laying Hens. Ph.D. Thesis, University of Nebruska-Lincoln, Lincoln, NE, USA, 2016; pp. 1–74. [Google Scholar]
- Abalaka, M.E.; Daniyan, S.Y.; Mann, A. Evaluation of the antimicrobial activities of two Ziziphus species (Ziziphus mauritiana L. and Ziziphus spinachristi L.) on some microbial pathogens. Afr. J. Pharm. Pharmacol. 2010, 4, 135–139. [Google Scholar]
- Singh, M.; Cowieson, A.J. Range use and pasture consumption in free-range poultry production. Anim. Prod. Sci. 2013, 53, 1202–1208. [Google Scholar] [CrossRef]
- Budkevich, T.V.; El’skaya, A.V.; Nierhaus, K.H. Features of 80S mammalian ribosome and its subunits. Nucleic Acids Res. 2008, 36, 4736–4744. [Google Scholar] [CrossRef]
- Fu, M.C.; Glover, F.W.; April, J. Simulation optimization: A review, new developments, and applications. In Proceedings of the Winter Simulation Conference, Orlando, FL, USA, 4 December 2005. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shehata, A.M.; Arif, M.; Paswan, V.K.; El-Saber Batiha, G.; Khafaga, A.F.; Elbestawy, A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: A review. Environ. Sci. Pollut. Res. 2021, 28, 4989–5004. [Google Scholar] [CrossRef]
- Dorman, C.J. DNA supercoiling and transcription in bacteria: A two-way street. BMC Mol. Cell Biol. 2019, 20, 26. [Google Scholar] [CrossRef]
- Khiralla, G.M.; El-Deeb, B.A. Antimicrobial and antibiofilm effects of selenium nanoparticles on some foodborne pathogens. LWT-Food Sci. Technol. 2015, 63, 1001–1007. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Joshi, A.; Roh, H. The role of context in work team diversity research: A meta-analytic review. Acad. Manag. J. 2009, 52, 599–627. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Yousaf, S.; Akhtar, M.; Sarwar, N.; Ikram, W.; Hussain, S. Sustaining zinc bioavailability in wheat grown on phosphorus amended calcisol. J. Cereal Sci. 2019, 90, 102846. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Kasemets, K.; Ivask, A.; Dubourguier, H.C.; Kahru, A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. In Vitro 2009, 23, 1116–1122. [Google Scholar] [CrossRef]
- Li, L.Z.; Zhou, D.M.; Peijnenburg, W.J.; van Gestel, C.A.; Jin, S.Y.; Wang, Y.J.; Wang, P. Toxicity of zinc oxide nanoparticles in the earthworm, Eisenia fetida and subcellular fractionation of Zn. Environ. Int. 2011, 37, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, Z.; Wai, O.W.; Li, Y.S. Chemical forms of Pb, Zn and Cu in the sediment profiles of the Pearl River Estuary. Mar. Pollut. Bull. 2001, 42, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Deplancke, B.; Gaskins, H.R. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 2001, 73, 1131S–1141S. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, H.N.; Jahanian, R. Immunological responses of broiler chicks can be modulated by dietary supplementation of zinc-methionine in place of inorganic zinc sources. Asian-Australas. J. Anim. Sci. 2009, 22, 396–403. [Google Scholar] [CrossRef]
- Kiarie, E.; Romero, L.F.; Nyachoti, C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 2013, 26, 71–88. [Google Scholar] [CrossRef]
- Haque, M.H.; Sarker, S.; Islam, M.S.; Islam, M.A.; Karim, M.R.; Kayesh, M.E.H.; Anwer, M.S. Sustainable antibiotic-free broiler meat production: Current trends, challenges, and possibilities in a developing country perspective. Biology 2020, 9, 411. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Popova, T. Effect of probiotics in poultry for improving meat quality. Curr. Opin. Food Sci. 2017, 14, 72–77. [Google Scholar] [CrossRef]
- Mehdi, Y.; Létourneau-Montminy, M.P.; Gaucher, M.L.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Godbout, S. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 2018, 4, 170–178. [Google Scholar] [CrossRef]
- Giannenas, I.; Papadopoulos, E.; Tsalie, E.; Triantafillou, E.L.; Henikl, S.; Teichmann, K.; Tontis, D. Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet. Parasitol. 2012, 188, 31–40. [Google Scholar] [CrossRef]
- Zalewski, P.D.; Truong-Tran, A.Q.; Grosser, D.; Jayaram, L.; Murgia, C.; Ruffin, R.E. Zinc metabolism in airway epithelium and airway inflammation: Basic mechanisms and clinical targets. A review. Pharmacol. Ther. 2005, 105, 127–149. [Google Scholar] [CrossRef]
- Salim, H.M.; Jo, C.; Lee, B.D. Zinc in broiler feeding and nutrition. Avian Biol. Res. 2008, 1, 5–18. [Google Scholar] [CrossRef]
- Kamel, D.A.; Abdel-Khalek, A.E.; Gabr, S.A. Effect of Dietary Zinc-Oxide or Nano-Zinc Oxide on Growth Performance, Oxidative Stress, and Immunity of Growing Rabbits under Hot Climate Conditions. J. Anim. Poult. Prod. 2020, 11, 565–571. [Google Scholar] [CrossRef]
- Sahoo, A.; Swain, R.K.; Mishra, S.K.; Jena, B. Serum biochemical indices of broiler birds fed on inorganic, organic and nano zinc supplemented diets. Int. J. Recent Sci. Res. 2014, 5, 2078–2081. [Google Scholar]
- Tsai, Y.H.; Mao, S.Y.; Li, M.Z.; Huang, J.T.; Lien, T.F. Effects of nanosize zinc oxide on zinc retention, eggshell quality, immune response and serum parameters of aged laying hens. Anim. Feed. Sci. Technol. 2016, 213, 99–107. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, L.; Yang, X.; Wen, C.; Zhou, Y. Bioavailability evaluation of zinc-bearing palygorskite as a zinc source for broiler chickens. Appl. Clay Sci. 2016, 119, 155–160. [Google Scholar] [CrossRef]
- Zaefarian, F.; Abdollahi, M.R.; Cowieson, A.; Ravindran, V. Avian liver: The forgotten organ. Animals 2019, 9, 63. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.; Chan, S.L.; Choo, S.P.; Kudo, M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J. Hepatol. 2020, 72, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Nabavi, S.; Habtemariam, S.; Daglia, M.; Braidy, N.; Rosa Loizzo, M.; Tundis, R.; Fazel Nabavi, S. Neuroprotective effects of ginkgolide B against ischemic stroke: A review of current literature. Curr. Top. Med. Chem. 2015, 15, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Javed, B.; Raja, N.I.; Mashwani, Z.U.R. Biomedical potential of plant-based selenium nanoparticles: A comprehensive review on therapeutic and mechanistic aspects. Int. J. Nanomed. 2021, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pi, J.; Cai, J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Zhang, B.; Wang, H.; Xin, Q.; Song, A. Flexible indium–gallium–zinc–oxide Schottky diode operating beyond 2.45 GHz. Nat. Commun. 2015, 6, 1–7. [Google Scholar] [CrossRef]
- Javed, M.; Abbas, K.; Ahmed, T.; Abdullah, S.; Naz, H.; Amjad, H. Metal pollutants induced peroxidase activity in different body tissues of freshwater fish, Labeo rohita. Environ. Chem. Ecotoxicol. 2020, 2, 162–167. [Google Scholar] [CrossRef]
- Sana, S.S.; Kumbhakar, D.V.; Pasha, A.; Pawar, S.C.; Grace, A.N.; Singh, R.P.; Peng, W. Crotalaria verrucosa leaf extract mediated synthesis of zinc oxide nanoparticles: Assessment of antimicrobial and anticancer activity. Molecules 2020, 25, 4896. [Google Scholar] [CrossRef]
- Kim, A. Mitochondria in cancer energy metabolism: Culprits or bystanders? Toxicol. Res. 2015, 31, 323–330. [Google Scholar] [CrossRef]
- Marc, B. An Introduction to Cancer and Basic Cancer Vocabulary. Beth Publ. 2002, 38, 58–72. [Google Scholar]
- Yu, K.N.; Yoon, T.J.; Minai-Tehrani, A.; Kim, J.E.; Park, S.J.; Jeong, M.S.; Cho, M.H. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol. Vitr. 2013, 27, 1187–1195. [Google Scholar] [CrossRef]
- Meghani, N.; Patel, P.; Kansara, K.; Ranjan, S.; Dasgupta, N.; Ramalingam, C.; Kumar, A. Formulation of vitamin D encapsulated cinnamon oil nanoemulsion: Its potential anti-cancerous activity in human alveolar carcinoma cells. Colloids Surf. B Biointerfaces 2018, 166, 349–357. [Google Scholar] [CrossRef]
- Patil, M.P.; Kim, G.D. Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 79–92. [Google Scholar] [CrossRef]
- Kordezangeneh, M.; Irani, S.; Mirfakhraie, R.; Esfandyari-Manesh, M.; Atyabi, F.; Dinarvand, R. Regulation of BAX/BCL2 gene expression in breast cancer cells by docetaxel-loaded human serum albumin nanoparticles. Med. Oncol. 2015, 32, 208. [Google Scholar] [CrossRef]
- Kim, Y.J.; Perumalsamy, H.; Castro-Aceituno, V.; Kim, D.; Markus, J.; Lee, S.; Yang, D.C. Photoluminescent and self-assembled hyaluronic acid-zinc oxide-ginsenoside Rh2 nanoparticles and their potential caspase-9 apoptotic mechanism towards cancer cell lines. Int. J. Nanomed. 2019, 14, 8195. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Z.; Zhang, J.; Wang, W.P.; Zhang, H.; Lu, Q. Zinc oxide nanoparticle synthesized from Euphorbia fischeriana root inhibits the cancer cell growth through modulation of apoptotic signaling pathways in lung cancer cells. Arab. J. Chem. 2020, 13, 6174–6183. [Google Scholar] [CrossRef]
- Jafarirad, S.; Mehrabi, M.; Divband, B.; Kosari-Nasab, M. Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: A mechanistic approach. Mater. Sci. Eng. C 2016, 59, 296–302. [Google Scholar] [CrossRef]
- Padalia, H.; Chanda, S. Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1751–1761. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; Zheng, Y. Biosynthesis of polyphenols functionalized ZnO nanoparticles: Characterization and their effect on human pancreatic cancer cell line. J. Photochem. Photobiol. B Biol. 2018, 183, 142–146. [Google Scholar] [CrossRef]
- Asik, R.M.; Gowdhami, B.; Jaabir, M.S.M.; Archunan, G.; Suganthy, N. Anticancer potential of zinc oxide nanoparticles against cervical carcinoma cells synthesized via biogenic route using aqueous extract of Gracilaria edulis. Mater. Sci. Eng. C 2019, 103, 109840. [Google Scholar] [CrossRef]
- Mirza, A.U.; Kareem, A.; Nami, S.A.; Bhat, S.A.; Mohammad, A.; Nishat, N. Malus pumila and Juglen regia plant species mediated zinc oxide nanoparticles: Synthesis, spectral characterization, antioxidant and antibacterial studies. Microb. Pathog. 2019, 129, 233–241. [Google Scholar] [CrossRef]
- Majeed, S.; Danish, M.; Ismail, M.H.B.; Ansari, M.T.; Ibrahim, M.N.M. Anticancer and apoptotic activity of biologically synthesized zinc oxide nanoparticles against human colon cancer HCT-116 cell line-in vitro study. Sustain. Chem. Pharm. 2019, 14, 100179. [Google Scholar] [CrossRef]
- Mahdizadeh, R.; Homayouni-Tabrizi, M.; Neamati, A.; Seyedi, S.M.R.; Tavakkol Afshari, H.S. Green synthesized-zinc oxide nanoparticles, the strong apoptosis inducer as an exclusive antitumor agent in murine breast tumor model and human breast cancer cell lines (MCF7). J. Cell. Biochem. 2019, 120, 17984–17993. [Google Scholar] [CrossRef] [PubMed]
- Murali, M.; Kalegowda, N.; Gowtham, H.G.; Ansari, M.A.; Alomary, M.N.; Alghamdi, S.; Shilpa, N.; Singh, S.B.; Thriveni, M.C.; Aiyaz, M.; et al. Plant-Mediated Zinc Oxide Nanoparticles: Advances in the New Millennium towards Understanding Their Therapeutic Role in Biomedical Applications. Pharmaceutics 2021, 13, 1662. [Google Scholar] [CrossRef] [PubMed]
- Malaikozhundan, B.; Vaseeharan, B.; Vijayakumar, S.; Pandiselvi, K.; Kalanjiam, M.A.R.; Murugan, K.; Benelli, G. Biological therapeutics of Pongamia pinnata coated zinc oxide nanoparticles against clinically important pathogenic bacteria, fungi and MCF-7 breast cancer cells. Microb. Pathog. 2017, 104, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Javed, M.R.; Adrees, M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. 2019, 26, 19859–19870. [Google Scholar] [CrossRef]
- Xiong, H.M. ZnO nanoparticles applied to bioimaging and drug delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef]
- Zhang, H.J.; Xiong, H.M.; Ren, Q.G.; Xia, Y.Y.; Kong, J.L. ZnO@ silica core–shell nanoparticles with remarkable luminescence and stability in cell imaging. J. Mater. Chem. 2012, 22, 13159–13165. [Google Scholar] [CrossRef]
- Spanhel, L. Colloidal ZnO nanostructures and functional coatings: A survey. J. Sol-Gel Sci. Technol. 2006, 39, 7–24. [Google Scholar] [CrossRef]
- Sanderson, M.J.; Smith, I.; Parker, I.; Bootman, M.D. Fluorescence microscopy. Cold Spring Harb. Protoc. 2014, 2014, pdb-top071795. [Google Scholar] [CrossRef]
- Barui, A.K.; Kotcherlakota, R.; Patra, C.R. Inorganic Frameworks as Smart Nanomedicines. William Publ. 2018, 1, 239–278. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Xiong, H.M.; Wang, Z.D.; Liu, D.P.; Chen, J.S.; Wang, Y.G.; Xia, Y.Y. Bonding polyether onto ZnO nanoparticles: An effective method for preparing polymer nanocomposites with tunable luminescence and stable conductivity. Adv. Funct. Mater. 2005, 15, 1751–1756. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Okur, N.Ü.; Karantas, I.D.; Okur, M.E.; Gündoğdu, E.A. Current update on nanoplatforms as therapeutic and diagnostic tools: A review for the materials used as nanotheranostics and imaging modalities. Asian J. Pharm. Sci. 2021, 16, 24–46. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Rees, P.; Wills, J.W.; Brown, M.R.; Barnes, C.M.; Summers, H.D. The origin of heterogeneous nanoparticle uptake by cells. Nat. Commun. 2019, 10, 2341. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Cremers, G.A.; Rosier, B.J.; Riera Brillas, R.; Albertazzi, L.; de Greef, T.F. Efficient small-scale conjugation of DNA to primary antibodies for multiplexed cellular targeting. Bioconjugate Chem. 2019, 30, 2384–2392. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Jeon, S.; You, D.G.; Park, J.H.; Kwon, I.C.; Koo, H.; Kim, K. Inorganic nanoparticles for image-guided therapy. Bioconjugate Chem. 2017, 28, 124–134. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Romeo, J.; Malavolta, M.; Costarelli, L.; Giacconi, R.; Diaz, L.E.; Marcos, A. Zinc: Dietary intake and impact of supplementation on immune function in elderly. Age 2013, 35, 839–860. [Google Scholar] [CrossRef] [PubMed]
- Swain, P.S.; Rao, S.B.; Rajendran, D.; Dominic, G.; Selvaraju, S. Nano zinc, an alternative to conventional zinc as animal feed supplement: A review. Anim. Nutr. 2016, 2, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Hashim, M.; Malik, S.A.; Khan, M.; Lorenzo, J.M.; Abbasi, B.H.; Hano, C. Recent advances in zinc oxide nanoparticles (ZnO NPs) for cancer diagnosis, target drug delivery, and treatment. Cancers 2021, 13, 4570. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef]
- Muhammad, F.; Guo, M.; Guo, Y.; Qi, W.; Qu, F.; Sun, F.; Zhu, G. Acid degradable ZnO quantum dots as a platform for targeted delivery of an anticancer drug. J. Mater. Chem. 2011, 21, 13406–13412. [Google Scholar] [CrossRef]
- Manaia, E.B. Zinc Oxide (ZnO) Based Quantum Dots for Bioimaging Applications of Lipid Nanocarriers. Ph.D. Thesis, Université Paris Saclay (COmUE), Palaiseau, Paris, Universidade Estadual Paulista, Sao Paulo, Brazil, 2016. [Google Scholar]
- Mitra, S.; Subia, B.; Patra, P.; Chandra, S.; Debnath, N.; Das, S.; Goswami, A. Porous ZnO nanorod for targeted delivery of doxorubicin: In vitro and in vivo response for therapeutic applications. J. Mater. Chem. 2012, 22, 24145–24154. [Google Scholar] [CrossRef]


| Size | Assay/Cell Type/Animal Model | Antioxidant Effects | Reference |
|---|---|---|---|
| 40–50 nm | DPPH |
| [66] |
| 10–20 nm | DPPH assay |
| [67] |
| 33–73 nm | DPPH assay |
| [68] |
| 40–60 nm | DPPH assay |
| [69] |
| 5 nm | DPPH Assay |
| [70] |
| 39.2 nm | Broilers |
| [71] |
| 40 nm | SOD, POD, CAT and GPX |
| [72] |
| 20 nm | Egg laying hens |
| [73] |
| 20 nm | Egg laying hens |
| [73] |
| 35–45 nm | Broilers |
| [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Younas, Z.; Mashwani, Z.U.R.; Ahmad, I.; Khan, M.; Zaman, S.; Sawati, L.; Sohail. Mechanistic Approaches to the Application of Nano-Zinc in the Poultry and Biomedical Industries: A Comprehensive Review of Future Perspectives and Challenges. Molecules 2023, 28, 1064. https://doi.org/10.3390/molecules28031064
Younas Z, Mashwani ZUR, Ahmad I, Khan M, Zaman S, Sawati L, Sohail. Mechanistic Approaches to the Application of Nano-Zinc in the Poultry and Biomedical Industries: A Comprehensive Review of Future Perspectives and Challenges. Molecules. 2023; 28(3):1064. https://doi.org/10.3390/molecules28031064
Chicago/Turabian StyleYounas, Zohaib, Zia Ur Rehman Mashwani, Ilyas Ahmad, Maarij Khan, Shah Zaman, Laraib Sawati, and Sohail. 2023. "Mechanistic Approaches to the Application of Nano-Zinc in the Poultry and Biomedical Industries: A Comprehensive Review of Future Perspectives and Challenges" Molecules 28, no. 3: 1064. https://doi.org/10.3390/molecules28031064
APA StyleYounas, Z., Mashwani, Z. U. R., Ahmad, I., Khan, M., Zaman, S., Sawati, L., & Sohail. (2023). Mechanistic Approaches to the Application of Nano-Zinc in the Poultry and Biomedical Industries: A Comprehensive Review of Future Perspectives and Challenges. Molecules, 28(3), 1064. https://doi.org/10.3390/molecules28031064






