Laboratory Scale Continuous Flow Systems for the Enantioselective Phase Transfer Catalytic Synthesis of Quaternary Amino Acids
Abstract
1. Introduction
2. Results
2.1. Preliminary Tests in Batch
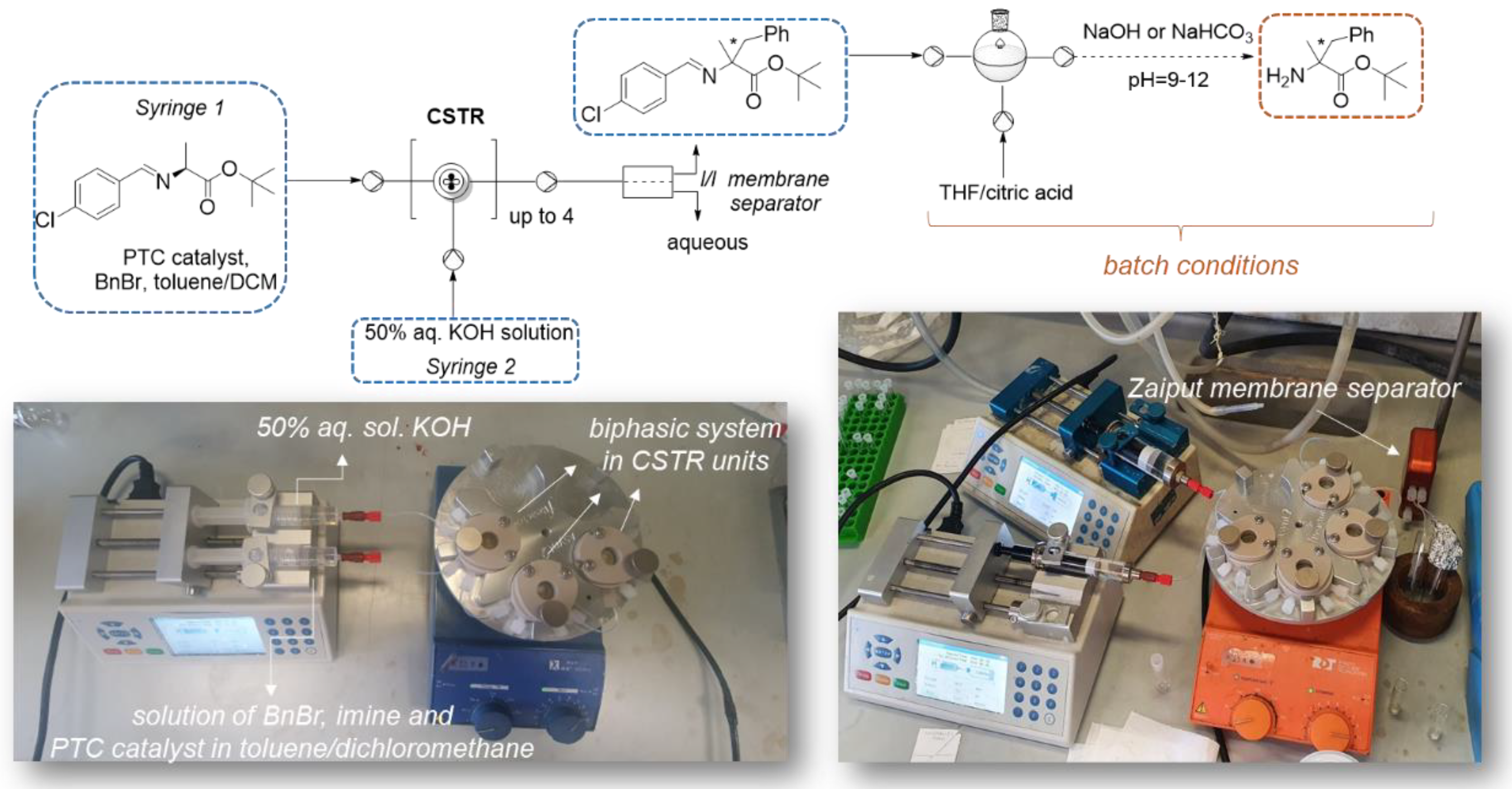
2.2. Liquid-Liquid Enantioselective Phase Transfer Benzylation of L-alanine Imine in Flow
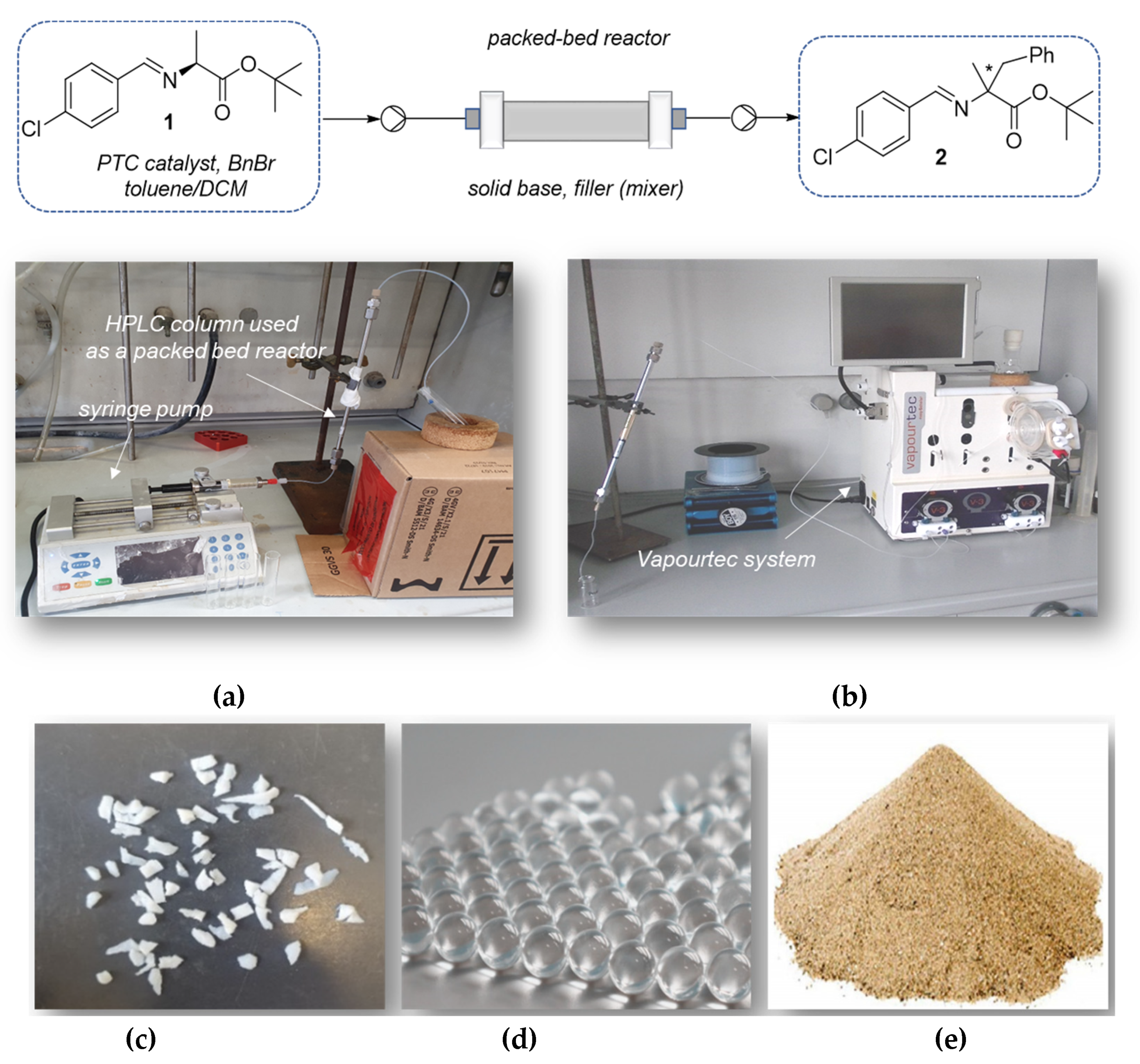
2.3. Solid-Liquid Enantioselective Phase Transfer Benzylation of L-alanine Imine in Flow
2.4. Productivity and Space-Time Yield
3. Materials and Methods
3.1. Materials and Equipment
3.2. Methods
3.2.1. Liquid-Liquid Phase Transfer Benzylation in Flow
3.2.2. Solid-Liquid Phase Transfer Benzylation in Flow
3.2.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Perdih, A.; Dolenc, M.S. Recent Advances in the Synthesis of Unnatural α-Amino Acids. Curr. Org. Chem. 2011, 15, 3750–3799. [Google Scholar] [CrossRef]
- O’Donnell, M.J. Benzophenone Schiff bases of glycine derivatives: Versatile starting materials for the synthesis of amino acids and their derivatives. Tetrahedron 2019, 75, 3667–3696. [Google Scholar] [CrossRef]
- Maruoka, K.; Ooi, T.; Kano, T. Design of chiral organocatalysts for practical asymmetric synthesis of amino acid derivatives. Chem. Commun. 2007, 15, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, K. Highly practical amino acid and alkaloid synthesis using designer chiral phase transfer catalysts as high-performance organocatalysts. Chem. Rec. 2010, 10, 254–259. [Google Scholar] [CrossRef]
- Maruoka, K.; Ooi, T. Enantioselective Amino Acid Synthesis by Chiral Phase transfer Catalysis. Chem. Rev. 2003, 103, 3013–3028. [Google Scholar] [CrossRef]
- Abellán, T.; Chinchilla, R.; Galindo, N.; Guillena, G.; Nájera, C.; Sansano, J.M. Glycine and Alanine Imines as Templates for Asymmetric Synthesis of α-Amino Acids. Eur. J. Org. Chem. 2000, 2000, 2689–2697. [Google Scholar] [CrossRef]
- Ma, J.-A. Recent Developments in the Catalytic Asymmetric Synthesis of α- and β-Amino Acids. Angew. Chem. Int. Ed. 2003, 42, 4290–4299. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, S.; Maruoka, K. Recent Developments in Asymmetric Phase-Transfer Reactions. Angew. Chem. Int. Ed. 2013, 52, 4312–4348. [Google Scholar] [CrossRef] [PubMed]
- Ooi, T.; Maruoka, K. Recent advances in asymmetric phase-transfer catalysis. Angew. Chem. Int. Ed. 2007, 46, 4222–4266. [Google Scholar] [CrossRef]
- Kitamura, M.; Shirakawa, S.; Arimura, Y.; Wang, X.; Maruoka, K. Combinatorial design of simplified high-performance chiral phase-transfer catalysts for practical asymmetric synthesis of α-alkyl- and α,α-dialkyl-α-amino acids. Chem. Asian J. 2008, 3, 1702–1714. [Google Scholar] [CrossRef] [PubMed]
- Jew, S.-s.; Jeong, B.-S.; Lee, J.-H.; Yoo, M.-S.; Lee, Y.-J.; Park, B.-s.; Kim, M.G.; Park, H.-g. Highly Enantioselective Synthesis of α-Alkyl-alanines via the Catalytic Phase-Transfer Alkylation of 2-Naphthyl Aldimine tert-Butyl Ester by Using O (9)-Allyl-N (1)-2′,3′,4′-trifluorobenzylhydro-cinchonidinium Bromide. J. Org. Chem. 2003, 68, 4514–4516. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C.; Ortega, F.J. Convenient conditions for the enantioselective synthesis of α-methyl-α-amino acids with the use of Cinchona ammonium salts as phase-transfer organocatalysts. Eur. J. Org. Chem. 2007, 36, 6034–6038. [Google Scholar] [CrossRef]
- O’Donnell, M.J. The enantioselective synthesis of α-amino acids by phase-transfer catalysis with achiral Schiff base esters. Acc. Chem. Res. 2004, 37, 506–517. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Wu, S.A. Catalytic Enantioselective Synthesis of α-Methyl Amino Acid Derivatives by Phase-Transfer Catalysis. Tetrahedron Asymmetry 1992, 3, 591–594. [Google Scholar] [CrossRef]
- Lygo, B.; Crosby, J.; Peterson, J.A. Enantioselective Alkylation Of Alanine-Derived Imines Using Quaternary Ammonium Catalysts. Tetrahedron Lett. 1999, 40, 8671–8674. [Google Scholar] [CrossRef]
- Ooi, T.; Takeuchi, M.; Kameda, M.; Maruoka, K. Practical Catalytic Enantioselective Synthesis of α,α-Dialkyl- α-amino Acids by Chiral Phase-Transfer Catalysis. J. Am. Chem. Soc. 2000, 122, 5228–5229. [Google Scholar] [CrossRef]
- Kubota, Y.; Shirakawa, S.; Inoue, T.; Maruoka, K. New chiral phase-transfer catalysts possessing a 6,6′-bridged ring on the biphenyl unit: Application to the synthesis of α,α-dialkyl- α-amino acids. Tetrahedron Lett. 2012, 53, 3739–3741. [Google Scholar] [CrossRef]
- Nieves-Remacha, M.J.; Torres, M.; Ruiz-Abad, M.; Rincón, J.A.; Cumming, G.R.; Garcia-Losada, P. Scale-up of N-alkylation reaction using phase-transfer catalysis with integrated separation in flow. React. Chem. Eng. 2019, 4, 334–345. [Google Scholar] [CrossRef]
- Jovanović, J.; Rebrov, E.V.; Nijhuis, T.A.; Hessel, V.; Schouten, J.C. Phase-Transfer Catalysis in Segmented Flow in a Microchannel: Fluidic Control of Selectivity and Productivity. Ind. Eng. Chem. Res. 2010, 49, 2681–2687. [Google Scholar] [CrossRef]
- Ueno, M.; Hisamoto, H.; Kitamori, T.; Kobayashi, S. Phase-transfer alkylation reactions using microreactors. Chem. Commun. 2003, 3, 936–937. [Google Scholar] [CrossRef]
- Okamoto, H. Effect of alternating pumping of two reactants into a microchannel on a phase transfer reaction. Chem. Eng. Technol. 2006, 29, 504–506. [Google Scholar] [CrossRef]
- Naber, J.R.; Buchwald, S.L. Packed-Bed Reactors for Continuous-Flow C-N Cross-Coupling. Angew. Chem. 2010, 122, 9659–9664. [Google Scholar] [CrossRef]
- Šinkovec, E.; Krajnc, M. Phase Transfer Catalyzed Wittig Reaction in the Microtube Reactor under Liquid Liquid Slug-Flow Pattern. Org. Process. Res. Dev. 2011, 15, 817–823. [Google Scholar] [CrossRef]
- De Zani, D.; Colombo, M. Phase-Transfer Catalysis under Continuous Flow Conditions: An Alternative Approach to the Biphasic Liquid/Liquid O-Alkylation of Phenols. J. Flow Chem. 2012, 2, 5–7. [Google Scholar] [CrossRef]
- Reichart, B.; Kappe, C.O.; Glasnov, T.N. Phase-Transfer Catalysis: Mixing Effects in Continuous-Flow Liquid/Liquid O- and S-Alkylation Processes. Synlett. 2013, 24, 2393–2396. [Google Scholar]
- Mo, Y.; Lin, H.; Jensen, K.F. High-performance miniature CSTR for biphasic C–C bond-forming reactions. Chem. Eng. J. 2018, 335, 936–944. [Google Scholar] [CrossRef]
- Hartman, R.L.; McMullen, J.P.; Jensen, K.F. Deciding Whether To Go with the Flow: Evaluating the Merits of Flow Reactors for Synthesis. Angew. Chem. Int. Ed. 2011, 50, 7502–7519. [Google Scholar] [CrossRef]
- Available online: https://www.freactor.com/product_freactorCSTR.html (accessed on 10 January 2023).
- Shirakawa, S.; Yamamoto, K.; Liu, K.; Maruoka, K. Enantioselective Alkylation of 2-[(4- Chlorobenzyliden)Amino]Propanoic Acid tert-Butyl Ester: Synthesis of (R)-2-Amino-2-Methyl-3-Phenylpropanoic Acid tert-Butyl Ester. Org. Synth. 2013, 90, 121–129. [Google Scholar]
- Corey, E.J.; Noe, M.C. Preparation of O-allyl-N-(9-anthracenylmethyl)cinchonidinium bromide as a phase transfer catalyst for the enantioselective alkylation of glycine benzophenone imine tert-butyl ester: (4S)-2-(benzhydrylidenamino)pentanedioic, 1-tert-butyl ester-5-methyl ester. Org. Synth. 2003, 80, 38–45. [Google Scholar]
- Kitamura, M.; Shirakawa, S.; Maruoka, K. Powerful chiral phase-transfer catalysts for the asymmetric synthesis of α-alkyl- and α,α-dialkyl-α-amino acids. Angew. Chem. Int. Ed. 2005, 44, 1549–1551. [Google Scholar]

| Entry | Catalyst | Base | Toluene:DCM Ratio | BnBr (equiv.) | Temperature (°C) | Reaction Time Benzylation (h) 1 | Yield 3 (%) 2 | ee 3 (%) 3 |
|---|---|---|---|---|---|---|---|---|
| 1 | 4 (1 mol%) | 50% KOH | 3:1 | 1.5 | 25 | 28 | 38 | 89 |
| 2 | 5 (5 mol%) | KOH/K2CO3 5 equiv./5 equiv. | toluene | 1.1 | 0 | 3 | 47 | 52 |
| 3 | 5 (5 mol%) | KOH/K2CO3 5 equiv./5 equiv. | 1:1 | 1.1 | 0 | 3 | 62 | 51 |
| 4 | 4 (1 mol%) | CsOH·H2O 5 equiv. | toluene | 1.5 | 0 | 3 | 67 | 87 |
| 5 | 4 (1 mol%) | KOH/K2CO3 5 equiv./5 equiv. | toluene | 1.5 | 0 | 3 | 59 | 90 |
| Entry | Catalyst | BnBr (equiv.) | Toluene:DCM Ratio | C op (mol/dm3) | Flow Rate-op 1 (mL/min) | Flow Rate-wp 2 (mL/min) | CSTR Units (number) | Residence Time (min) | 1H-NMR Conversion to Product 2 (%) 3 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 (1 mol%) | 1.4 | 14:1 | 0.024 | 0.01 | 0.02 | 1 | 60 | 13 |
| 2 | 4 (1 mol%) | 1.5 | 14:1 | 0.024 | 0.0025 | 0.0025 | 1 | 395 | 15 |
| 3 | 4 (1 mol%) | 2.5 | 14:1 | 0.024 | 0.01 | 0.01 | 2 | 220 | 20 |
| 4 | 5 (10 mol%) | 1.4 | 4:1 | 0.025 | 0.1 | 0.2 | 3 | 20 | 10 |
| 5 | 4 (2 mol%) | 1.4 | 4:1 | 0.025 | 0.1 | 0.2 | 3 | 20 | 37 |
| 6 | 4 (1 mol%) | 1.4 | 14:1 | 0.025 | 0.025 | 0.05 | 3 | 80 | 40, (91% ee) 4 |
| 7 | 4 (1 mol%) | 1.4 | 14:1 | 0.025 | 0.025 | 0.05 | 4 | 106 | 38 |
| 8 | 4 (1 mol%) | 3 | 3.6:1 | 0.2 | 0.03 | 0.03 | 2 | 64 | 23 |
| Entry 1 | Catalyst | Toluene:DCM Ratio | Inert Material | Flow Rate-op 2 (mL/min) | Residence Time (min) | Solid Base (equiv.) | 1H-NMR Conversion to the Product 2 (%) 3 |
|---|---|---|---|---|---|---|---|
| 1 | 4 (1 mol%) | 8.5:1 | sand | 0.025 | 40 | KOH/K2CO3 (15/15) | 19 |
| 2 | 4 (1 mol%) | 8.5:1 | PTFE b. s. | 0.025 | 40 | KOH/K2CO3 (10/10) | 35 |
| 3 | 4 (5 mol%) | 5.2:1 | PTFE b. s. | 0.025 | 40 | KOH/K2CO3 (10/10) | 28 |
| 4 | 5 (10 mol%) | 1:2.4 | sand | 0.025 | 40 | KOH/K2CO3 (10/10) | 63 |
| 5 | 5 (10 mol%) | 1:2.4 | sand | 0.015 | 66 | KOH/K2CO3 (10/10) | 65, (52% ee) 4 |
| Entry 1 | Catalyst | BnBr (equiv.) | Toluene:DCM Ratio | Temperature (°C) | C of op (mol/dm3) | Residence Time (min) | Solid Base (equiv.) | 1H-NMR Conversion to the Product 2 (%) 2 | ee% of Product 3 3 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 (1 mol%) | 1.5 | toluene | 0 | 0.2 | 10 | CsOH·H2O (10) | 47 | 93 |
| 2 | 5 (5 mol%) | 1.2 | 1.6:1 | −15 | 0.3 | 15 | KOH/K2CO3 (6/6) | 60 | 72 |
| 3 | 5 (10 mol%) | 1.2 | DCM | 25 | 0.35 | 30 | KOH/K2CO3 (45/45) | 95 | 56 |
| Entry | Method | 1H-NMR Conversion to the Product 2 (%) | Reactor Volume (mL) | Residence Time (min) | Productivity 1 (mmol/h) | Space-Time Yield 2 (mmol/mL*h) |
|---|---|---|---|---|---|---|
| Liquid-liquid phase transfer benzylation in CSTR | ||||||
| Table 1/Entry 1 3 | batch | >98 | 5.43 | 1680 | 0.027 | 0.05 |
| Table 2/Entry 6 | flow | 40 | 25 | 333 | 0.053 | 0.002 |
| Solid-liquid phase transfer benzylation in packed-bed reactor | ||||||
| Table 1/Entry 3 3 | batch | >98 | 4 | 180 | 0.248 | 0.062 |
| Table 3/Entry 5 | flow | 65 | 1.05 | 66 | 0.436 | 0.415 |
| Table 4/Entry 1 | flow | 47 | 0.35 | 5 | 4.2 | 12 |
| Table 4/Entry 2 | flow | 60 | 0.23 | 4.33 | 6.23 | 27 |
| Table 4/Entry 3 | flow | 95 | 1 | 30 | 1.4 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krstić, M.; Rossi, S.; Sanz, M.; Puglisi, A. Laboratory Scale Continuous Flow Systems for the Enantioselective Phase Transfer Catalytic Synthesis of Quaternary Amino Acids. Molecules 2023, 28, 1002. https://doi.org/10.3390/molecules28031002
Krstić M, Rossi S, Sanz M, Puglisi A. Laboratory Scale Continuous Flow Systems for the Enantioselective Phase Transfer Catalytic Synthesis of Quaternary Amino Acids. Molecules. 2023; 28(3):1002. https://doi.org/10.3390/molecules28031002
Chicago/Turabian StyleKrstić, Milena, Sergio Rossi, Miguel Sanz, and Alessandra Puglisi. 2023. "Laboratory Scale Continuous Flow Systems for the Enantioselective Phase Transfer Catalytic Synthesis of Quaternary Amino Acids" Molecules 28, no. 3: 1002. https://doi.org/10.3390/molecules28031002
APA StyleKrstić, M., Rossi, S., Sanz, M., & Puglisi, A. (2023). Laboratory Scale Continuous Flow Systems for the Enantioselective Phase Transfer Catalytic Synthesis of Quaternary Amino Acids. Molecules, 28(3), 1002. https://doi.org/10.3390/molecules28031002









