Hydrolytic Degradation and Bioactivity of Electrospun PCL-Mg-NPs Fibrous Mats
Abstract
1. Introduction
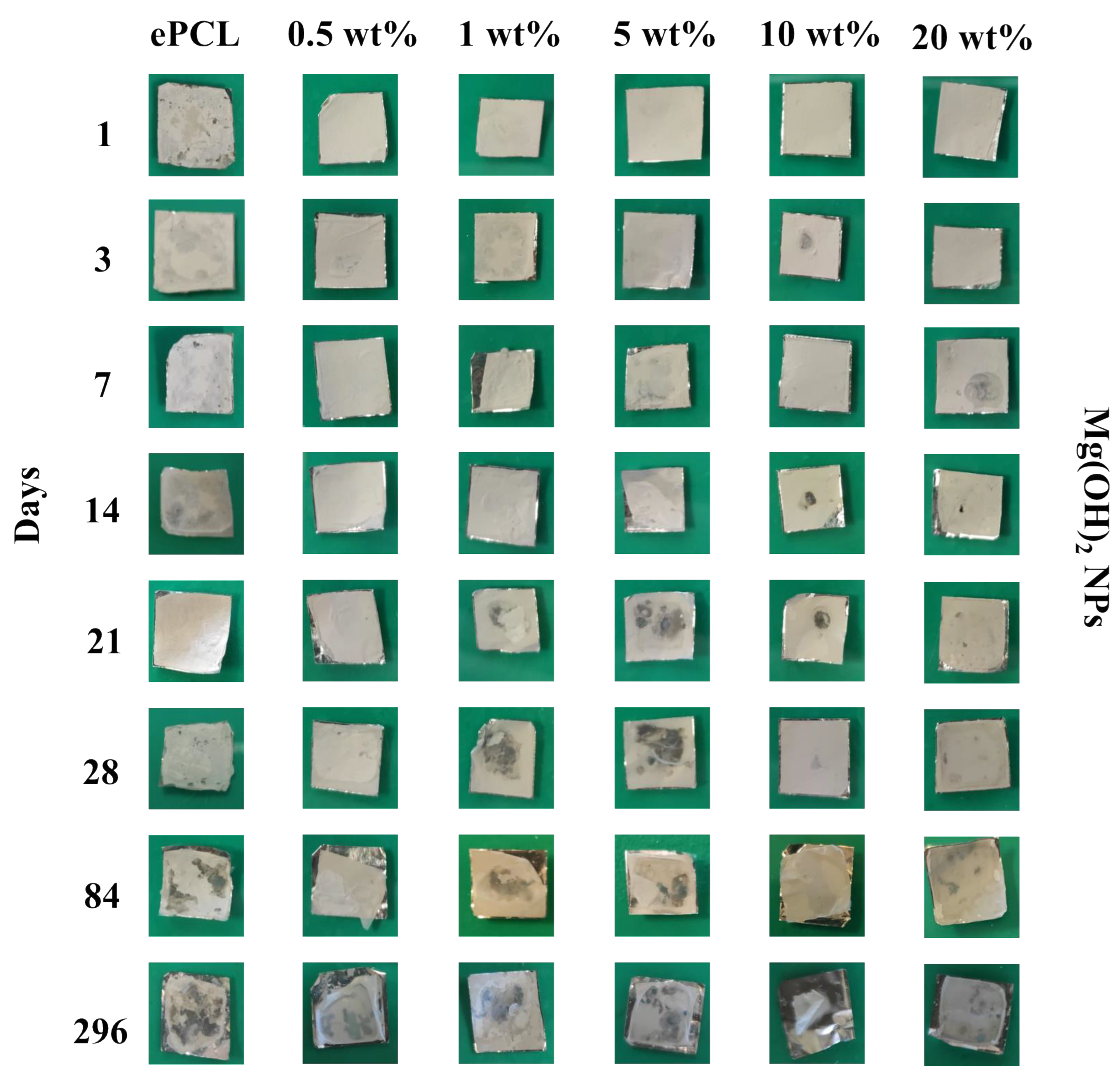
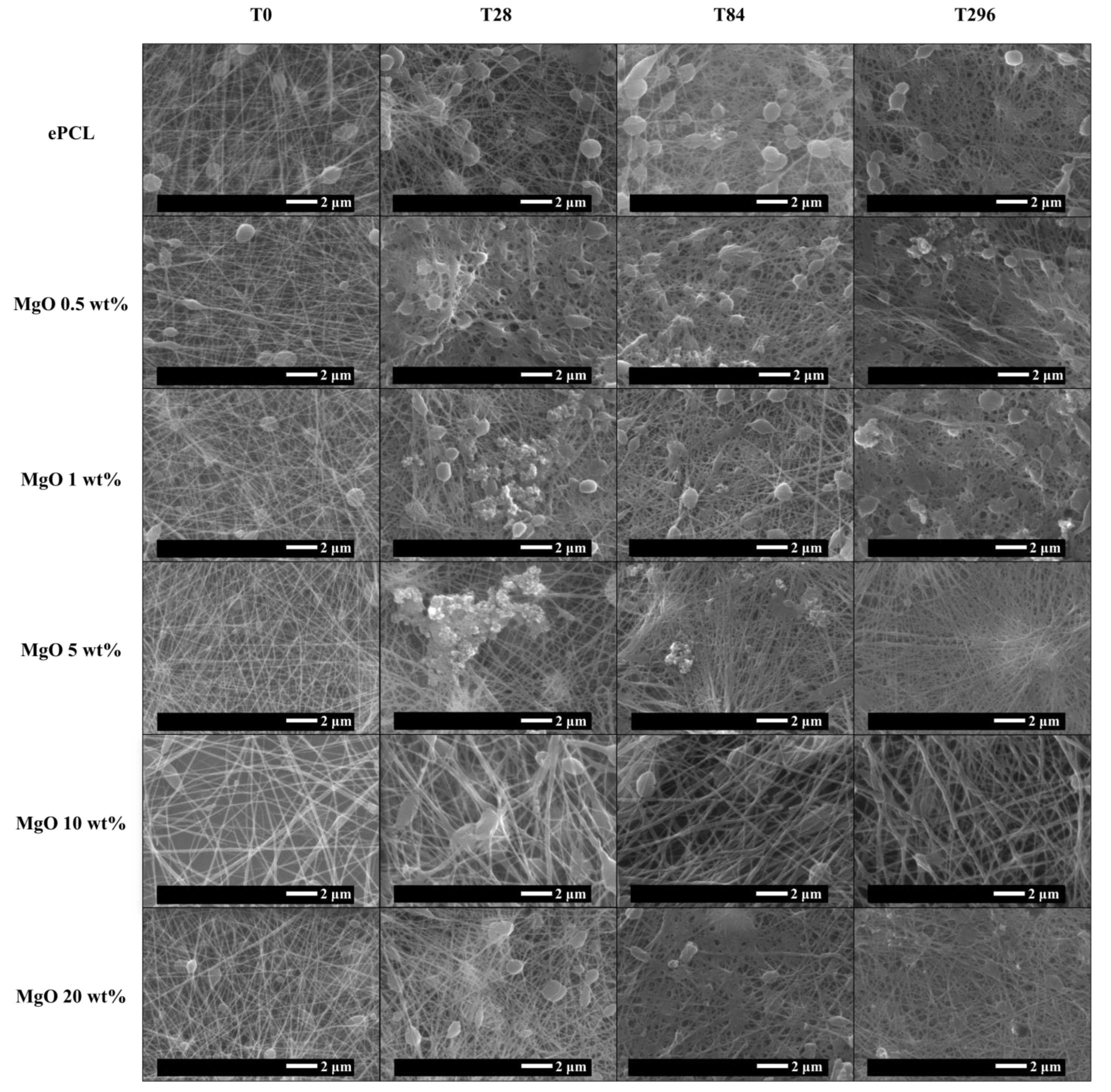
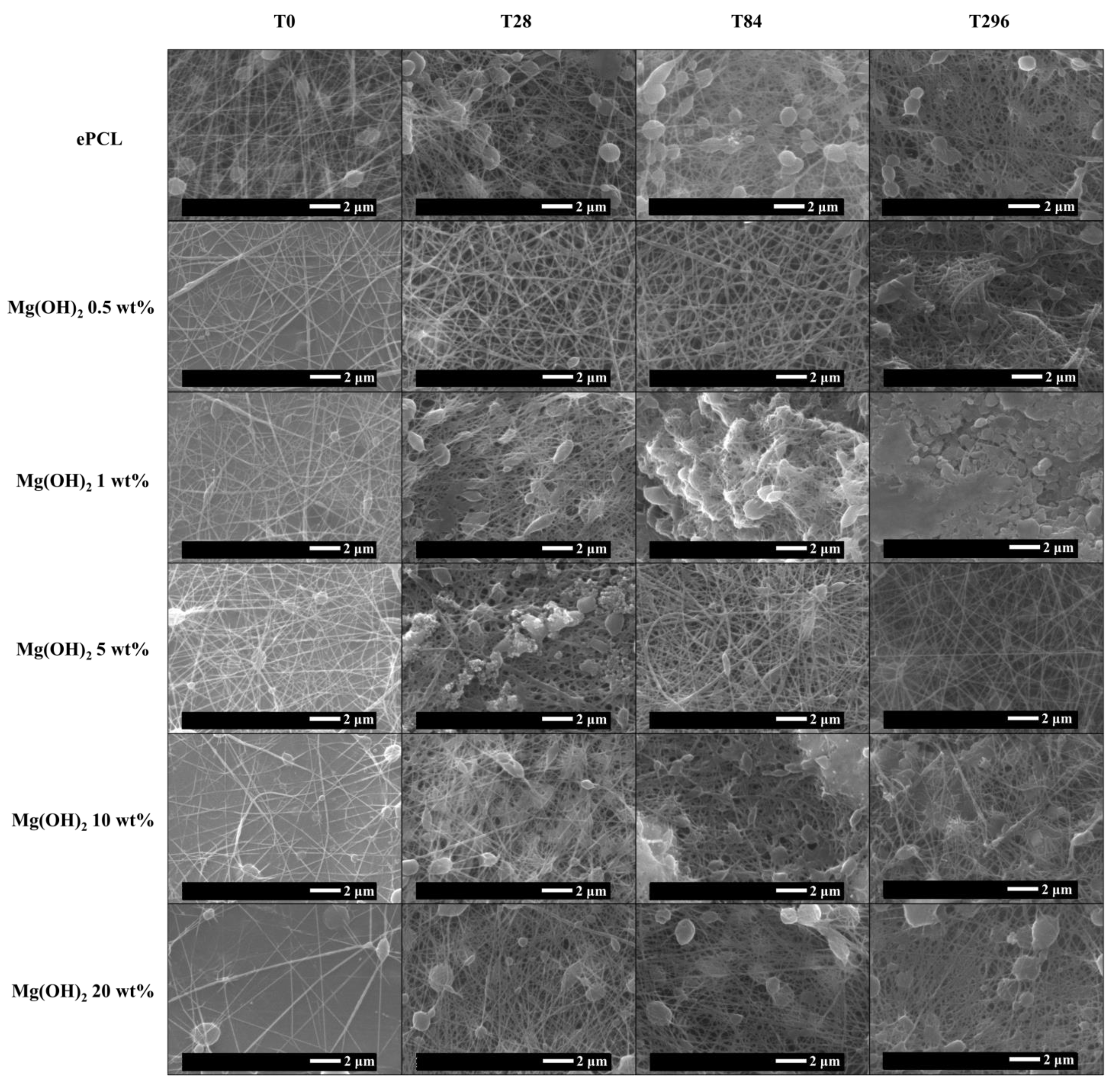
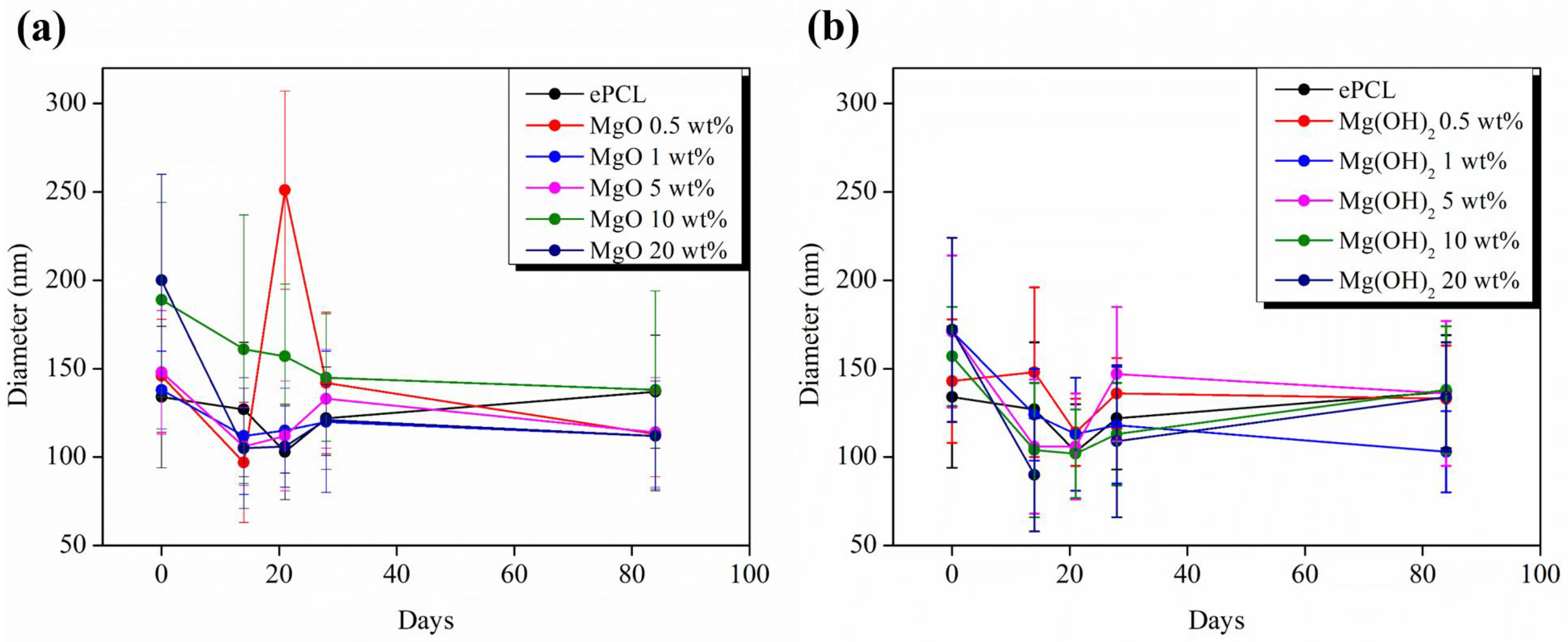
2. Results
3. Materials and Methods
3.1. Degradation Study and Bioactivity
3.2. Characterization Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Echegoyen, Y.; Fabra, M.J.; Castro-Mayorga, J.L.; Cherpinski, A.; Lagaron, J.M. High throughput electro-hydrodynamic processing in food encapsulation and food packaging applications: Viewpoint. Trends Food Sci. Technol. 2017, 60, 71–79. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Anjana, J.; Mony, U.; Jayakumar, R.; Chen, J.P. Process study, development and degradation behavior of different size scale electrospun poly(caprolactone) and poly(lactic acid) fibers. J. Polym. Res. 2018, 25, 259. [Google Scholar] [CrossRef]
- Partheniadis, I.; Nikolakakis, I.; Laidmäe, I.; Heinämäki, J. A mini-review: Needleless electrospinning of nanofibers for pharmaceutical and biomedical applications. Processes 2020, 8, 673. [Google Scholar] [CrossRef]
- Li, Y.; Dong, T.; Li, Z.; Ni, S.; Zhou, F.; Alimi, O.A.; Chen, S.; Duan, B.; Kuss, M.; Wu, S. Review of advances in electrospinning-based strategies for spinal cord regeneration. Mater. Today Chem. 2022, 24, 100944. [Google Scholar] [CrossRef]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.G. Electrospun structural hybrids of acyclovir-polyacrylonitrile at acyclovir for modifying drug release. Polymers 2021, 13, 4283. [Google Scholar] [CrossRef]
- Hameed, M.; Rasul, A.; Waqas, M.K.; Saadullah, M.; Aslam, N.; Abbas, G.; Latif, S.; Afzal, H.; Inam, S.; Shah, P.A. Formulation and evaluation of a clove oil-encapsulated nanofiber formulation for effective wound-healing. Molecules 2021, 26, 2491. [Google Scholar] [CrossRef]
- Xu, C.; Ma, J.; Liu, Z.; Wang, W.; Liu, X.; Qian, S.; Chen, L.; Gu, L.; Sun, C.; Hou, J.; et al. Preparation of shell-core fiber-encapsulated Lactobacillus rhamnosus 1.0320 using coaxial electrospinning. Food Chem. 2023, 402, 134253. [Google Scholar] [CrossRef]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C 2019, 103, 109768. [Google Scholar] [CrossRef]
- Mohandesnezhad, S.; Pilehvar-Soltanahmadi, Y.; Alizadeh, E.; Goodarzi, A.; Davaran, S.; Khatamian, M.; Zarghami, N.; Samiei, M.; Aghazadeh, M.; Akbarzadeh, A. In vitro evaluation of Zeolite-nHA blended PCL/PLA nanofibers for dental tissue engineering. Mater. Chem. Phys. 2020, 252, 123152. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Improvement of mechanical properties and antibacterial activity of electrospun poly(D,L-lactide)-based mats by incorporation of ZnO-graft-poly(D,L-lactide) nanoparticles. Mater. Chem. Phys. 2016, 182, 324–331. [Google Scholar] [CrossRef]
- Thomas, V.; Jagani, S.; Johnson, K.; Jose, M.V.; Dean, D.R.; Vohra, Y.K.; Nyairo, E. Electrospun bioactive nanocomposite scaffolds of polycaprolactone and nanohydroxyapatite for bone tissue engineering. J. Nanosci. Nanotechnol. 2006, 6, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun fibrous scaffolds for tissue engineering: Viewpoints on architecture and fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [PubMed]
- Kai, D.; Liow, S.S.; Loh, X.J. Biodegradable polymers for electrospinning: Towards biomedical applications. Mater. Sci. Eng. C 2015, 45, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, R.; Abbasipour, M.; Bahador, A. Electrospun biodegradable nanofibers scaffolds for bone tissue engineering. J. Appl. Polym. Sci. 2016, 133, 42883. [Google Scholar] [CrossRef]
- Kucinska-Lipka, J.; Gubanska, I.; Janik, H.; Sienkiewicz, M. Fabrication of polyurethane and polyurethane based composite fibres by the electrospinning technique for soft tissue engineering of cardiovascular system. Mater. Sci. Eng. C 2015, 46, 166–176. [Google Scholar] [CrossRef]
- Pitt, C.G.; Zhong-wei, G. Modification of the rates of chain cleavage of poly(ϵ-caprolactone) and related polyesters in the solid state. J. Control. Release 1987, 4, 283–292. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic Degradation and Erosion of Polyester Biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef]
- Domingos, M.; Chiellini, F.; Cometa, S.; De Giglio, E.; Grillo-Fernandes, E.; Bártolo, P.; Chiellini, E. Evaluation of in vitro degradation of pcl scaffolds fabricated via bioextrusion. part 1: Influence of the degradation environment. Virtual Phys. Prototyp. 2010, 5, 65–73. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar]
- Peponi, L.; Puglia, D.; Torre, L.; Valentini, L.; Kenny, J.M. Processing of nanostructured polymers and advanced polymeric based nanocomposites. Mater. Sci. Eng. R Rep. 2014, 85, 1–46. [Google Scholar] [CrossRef]
- Leonés, A.; Mujica-Garcia, A.; Arrieta, M.P.; Salaris, V.; Lopez, D.; Kenny, J.M.; Peponi, L. Organic and inorganic PCL-based electrospun fibers. Polymers 2020, 12, 1325. [Google Scholar] [CrossRef]
- Díaz, E.; Sandonis, I.; Valle, M.B. In Vitro Degradation of Poly ( caprolactone )/nHA Composites. J. Nanomater. 2014, 2014, 185. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, H.; Zhang, S.; Qu, S.; Jiang, Y.; Chen, M. Effects of magnesium oxide (MgO) shapes on in vitro and in vivo degradation behaviors of PLA/MgO composites in long term. Polymers 2020, 12, 1074. [Google Scholar] [CrossRef] [PubMed]
- Rijal, N.P.; Adhikari, U.; Khanal, S.; Pai, D.; Sankar, J.; Bhattarai, N. Magnesium oxide-poly(ε-caprolactone)-chitosan-based composite nanofiber for tissue engineering applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 228, 18–27. [Google Scholar] [CrossRef]
- Jana, A.; Das, M.; Balla, V.K. In vitro and in vivo degradation assessment and preventive measures of biodegradable Mg alloys for biomedical applications. J. Biomed. Mater. Res.-Part A 2022, 110, 462–487. [Google Scholar] [CrossRef] [PubMed]
- Leonés, A.; Lieblich, M.; Benavente, R.; Gonzalez, J.L.; Peponi, L. Potential applications of magnesium-based polymeric nanocomposites obtained by electrospinning technique. Nanomaterials 2020, 10, 1524. [Google Scholar] [CrossRef]
- Leonés, A.; Peponi, L.; Fiori, S.; Lieblich, M. Effect of the Addition of MgO Nanoparticles on the Thermally-Activated Shape Memory Behavior of Plasticized PLA Electrospun Fibers. Polymers 2022, 14, 2657. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.T.; Mantilaka, M.M.M.G.P.G.; Goh, K.L.; Ratnayake, S.P.; Amaratunga, G.A.J.; De Silva, K.M.N. Magnesium Oxide Nanoparticles Reinforced Electrospun Alginate-Based Nanofibrous Scaffolds with Improved Physical Properties. Int. J. Biomater. 2017, 2017, 1391298. [Google Scholar] [CrossRef] [PubMed]
- Salaris, V.; Leonés, A.; López, D.; Kenny, J.M.; Peponi, L. A Comparative Study on the Addition of MgO and Mg(OH)2 Nanoparticles into PCL Electrospun Fibers. Macromol. Chem. Phys. 2022, 224, 2200215. [Google Scholar] [CrossRef]
- Leonés, A.; Peponi, L.; Lieblich, M.; Benavente, R.; Fiori, S. In vitro degradation of plasticized PLA electrospun fiber mats: Morphological, thermal and crystalline evolution. Polymers 2020, 12, 2975. [Google Scholar] [CrossRef]
- Ghazalian, M.; Afshar, S.; Rostami, A.; Rashedi, S.; Bahrami, S.H. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 636, 128163. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Effect of zinc oxide nanoparticles on the in vitro degradation of electrospun polycaprolactone membranes in simulated body fluid. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 28–37. [Google Scholar] [CrossRef]
- Fu, S.Z.; Meng, X.H.; Fan, J.; Yang, L.L.; Lin, S.; Wen, Q.L.; Wang, B.Q.; Chen, L.L.; Wu, J.B.; Chen, Y. In vitro and in vivo degradation behavior of n-HA/PCL-Pluronic-PCL polyurethane composites. J. Biomed. Mater. Res.-Part A 2014, 102, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Miao, X.; Hu, X.; Zhang, Q.; Jie, X.; Zheng, X. Novel synthesis of plasmonic Ag/AgCl@TiO2 continues fibers with enhanced broadband photocatalytic performance. Catalysts 2017, 7, 117. [Google Scholar] [CrossRef]
- Agarwal, M.; Garg, S.K.; Asokan, K.; Kanjilal, D.; Kumar, P. Facile synthesis of KCl:Sm3+ nanophosphor as a new OSL dosimetric material achieved through charge transfer between the defect states. RSC Adv. 2017, 7, 13836–13845. [Google Scholar] [CrossRef]
- Selvam, N.C.S.; Kumar, R.T.; Kennedy, L.J.; Vijaya, J.J. Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO-micro and nano-structures. J. Alloys Compd. 2011, 509, 9809–9815. [Google Scholar] [CrossRef]
- Perinović Jozić, S.; Jozić, D.; Jakić, J.; Andričić, B. Preparation and characterization of PLA composites with modified magnesium hydroxide obtained from seawater. J. Therm. Anal. Calorim. 2020, 142, 1877–1889. [Google Scholar] [CrossRef]
- Kumar, T.S. Effect of Molecular Weight on Electro Spun Pcl Based Composite Fibrous Mats. Biomed. J. Sci. Tech. Res. 2017, 1, 1127. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef]
- Bužarovska, A. Preparation and characterization of poly(ε-caprolactone)/ZnO foams for tissue engineering applications. J. Mater. Sci. 2017, 52, 12067–12078. [Google Scholar] [CrossRef]
- Ali, S.; Ghavimi, A.; Hossein, M. Effect of starch content on the biodegradation of polycaprolactone / starch composite for fabricating in situ pore-forming scaffolds. Polym. Test. 2015, 43, 94–102. [Google Scholar]
- França, D.C.; Bezerra, E.B.; Morais, D.D.S.; Araújo, E.M.; Wellen, R.M.R. Effect of hydrolytic degradation on mechanical properties of PCL. Mater. Sci. Forum 2016, 869, 342–345. [Google Scholar] [CrossRef]
- Höglund, A. Controllable Degradation Product Migration from Cross-Linked Biomedical Polyester-Ethers through Predetermined Alterations in Copolymer Composition. Biomacromolecules 2007, 8, 2025–2032. [Google Scholar]
- Khatiwala, V.K.; Shekhar, N.; Aggarwal, S.; Mandal, U.K. Biodegradation of Poly(ε-caprolactone) (PCL) Film by Alcaligenes faecalis. J. Polym. Environ. 2008, 16, 61–67. [Google Scholar] [CrossRef]
- Suryavanshi, A.; Khanna, K.; Sindhu, K.R.; Bellare, J.; Srivastava, R. Magnesium oxide nanoparticle-loaded polycaprolactone composite electrospun fiber scaffolds for bone-soft tissue engineering applications: In-vitro and in-vivo evaluation. Biomed. Mater. 2017, 12, 055011. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Drouet, C. Apatite formation: Why it may not work as planned, and how to conclusively identify apatite compounds. Biomed Res. Int. 2013, 2013, 490946. [Google Scholar] [CrossRef]
- Chaikina, M.V.; Bulina, N.V.; Vinokurova, O.B.; Gerasimov, K.B.; Prosanov, I.Y.; Kompankov, N.B.; Lapina, O.B.; Papulovskiy, E.S.; Ishchenko, A.V.; Makarova, S.V. Possibilities of Mechanochemical Synthesis of Apatites with Different Ca/P Ratios. Ceramics 2022, 5, 404–422. [Google Scholar] [CrossRef]
- Liu, H.; Yazici, H.; Ergun, C.; Webster, T.J.; Bermek, H. An in vitro evaluation of the Ca/P ratio for the cytocompatibility of nano-to-micron particulate calcium phosphates for bone regeneration. Acta Biomater. 2008, 4, 1472–1479. [Google Scholar] [CrossRef]
- Larrañaga, A.; Aldazabal, P.; Martin, F.J.; Sarasua, J.R. Hydrolytic degradation and bioactivity of lactide and caprolactone based sponge-like scaffolds loaded with bioactive glass particles. Polym. Degrad. Stab. 2014, 110, 121–128. [Google Scholar] [CrossRef]
- Chavan, P.N.; Bahir, M.M.; Mene, R.U.; Mahabole, M.P.; Khairnar, R.S. Study of nanobiomaterial hydroxyapatite in simulated body fluid: Formation and growth of apatite. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 168, 224–230. [Google Scholar] [CrossRef]
- Guang-Mei, C.; Tie-Mei, Z.; Lei, C.; Yi-Ping, H. Crystallization properties of polycaprolactone induced by different hydroxyapatite nano-particles. Asian J. Chem. 2010, 22, 5902–5912. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]

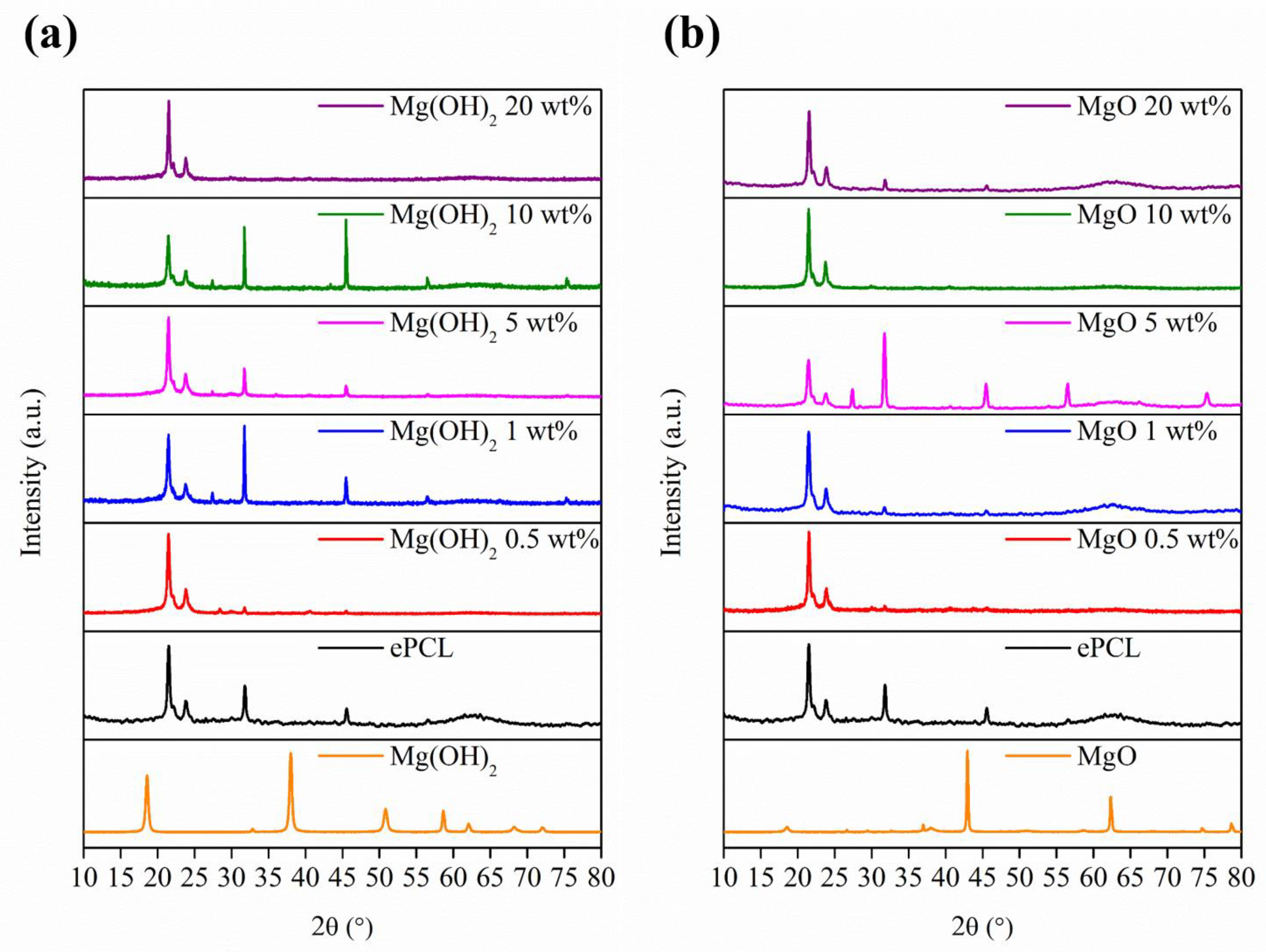
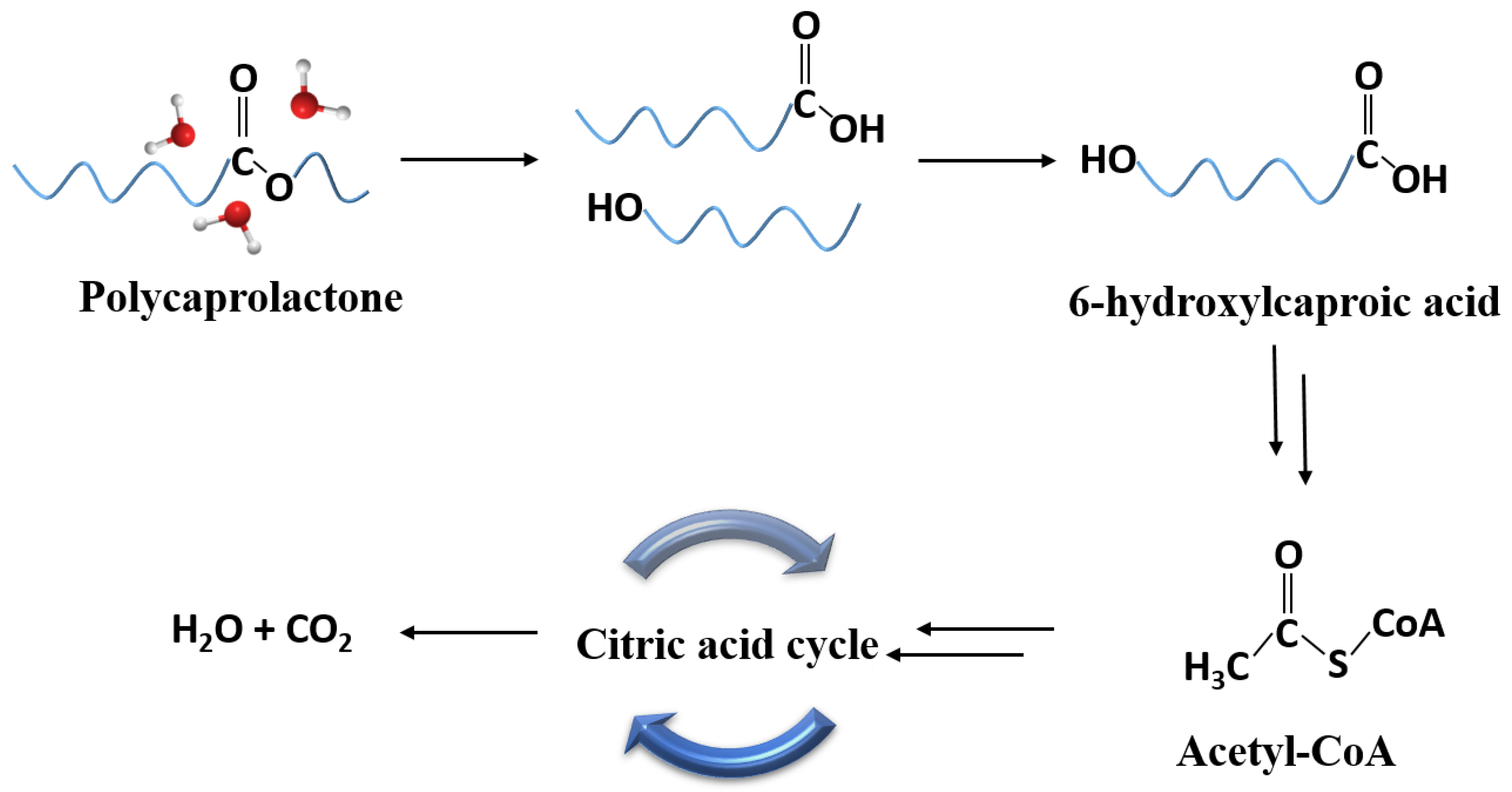
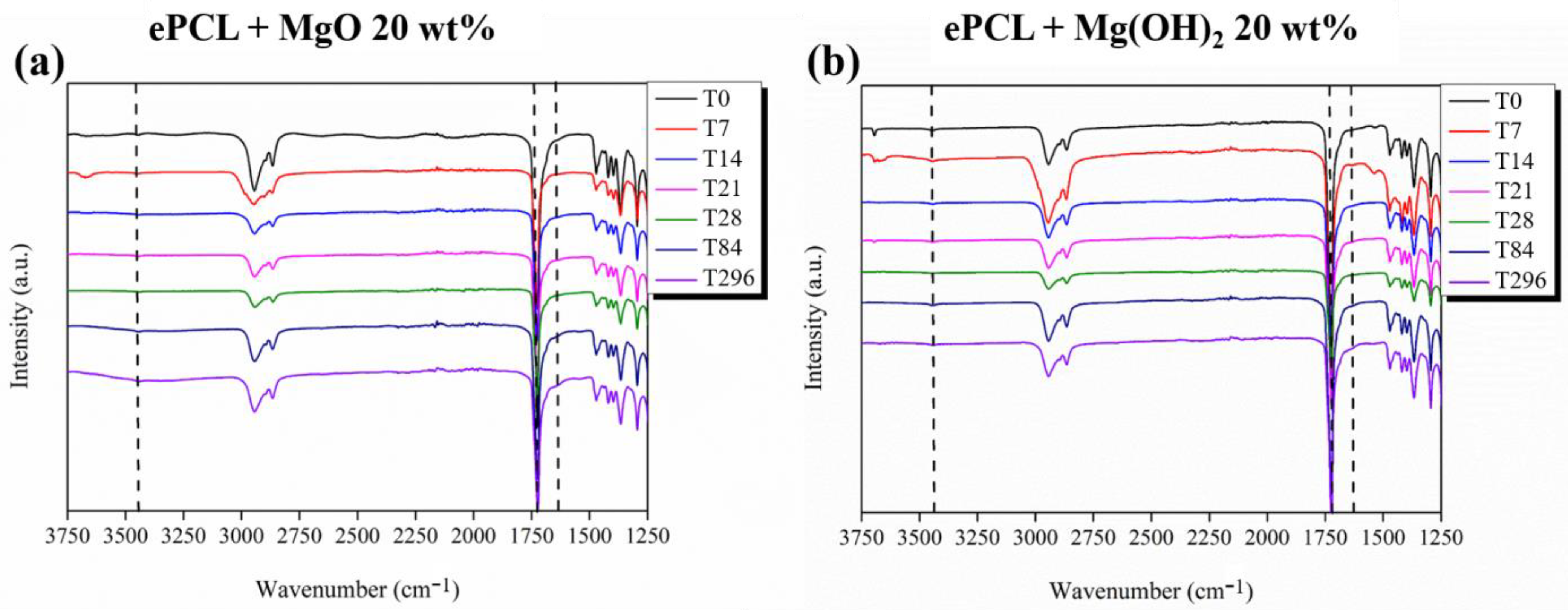
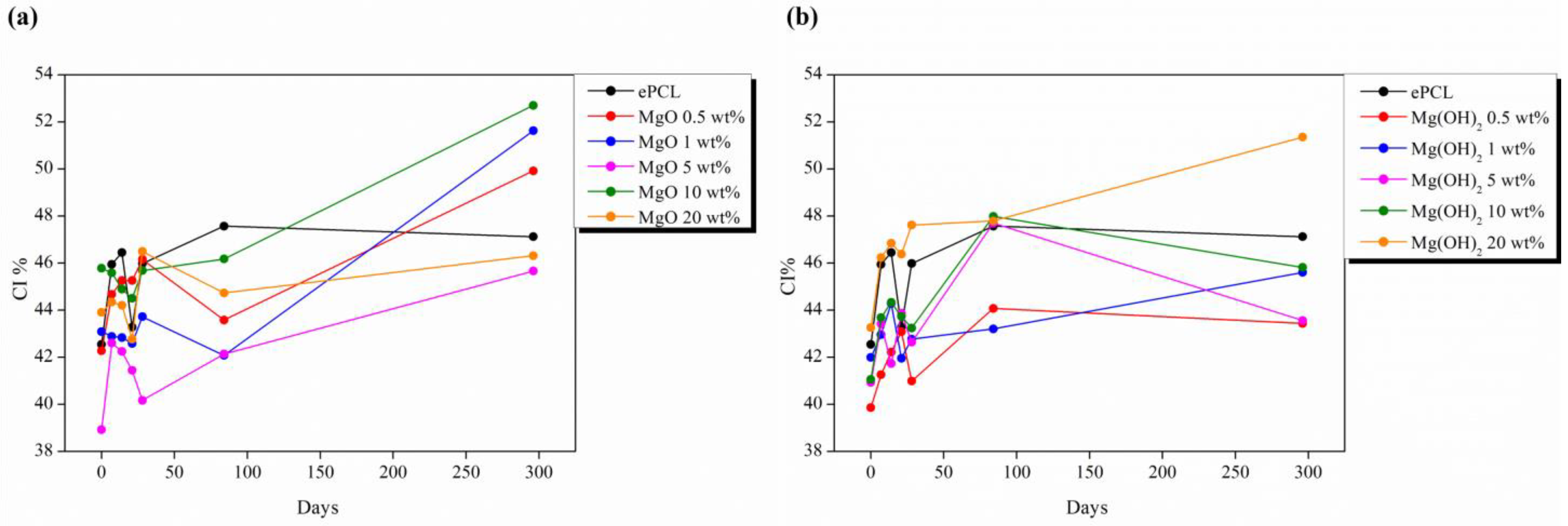
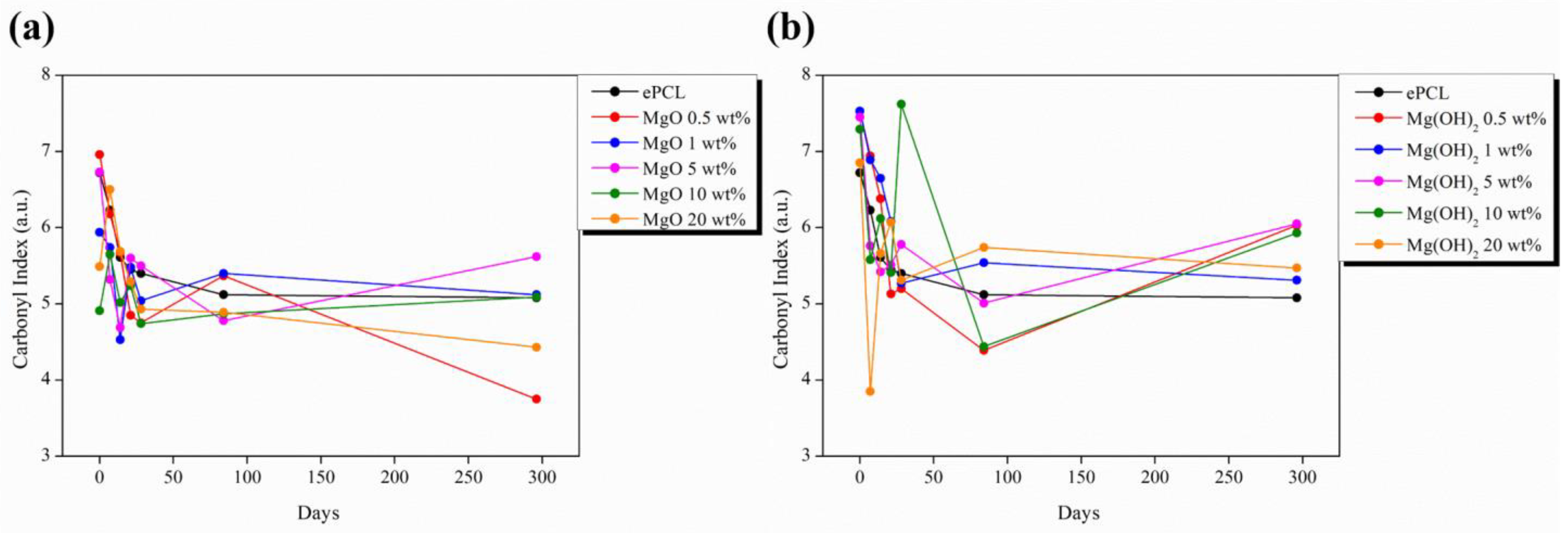




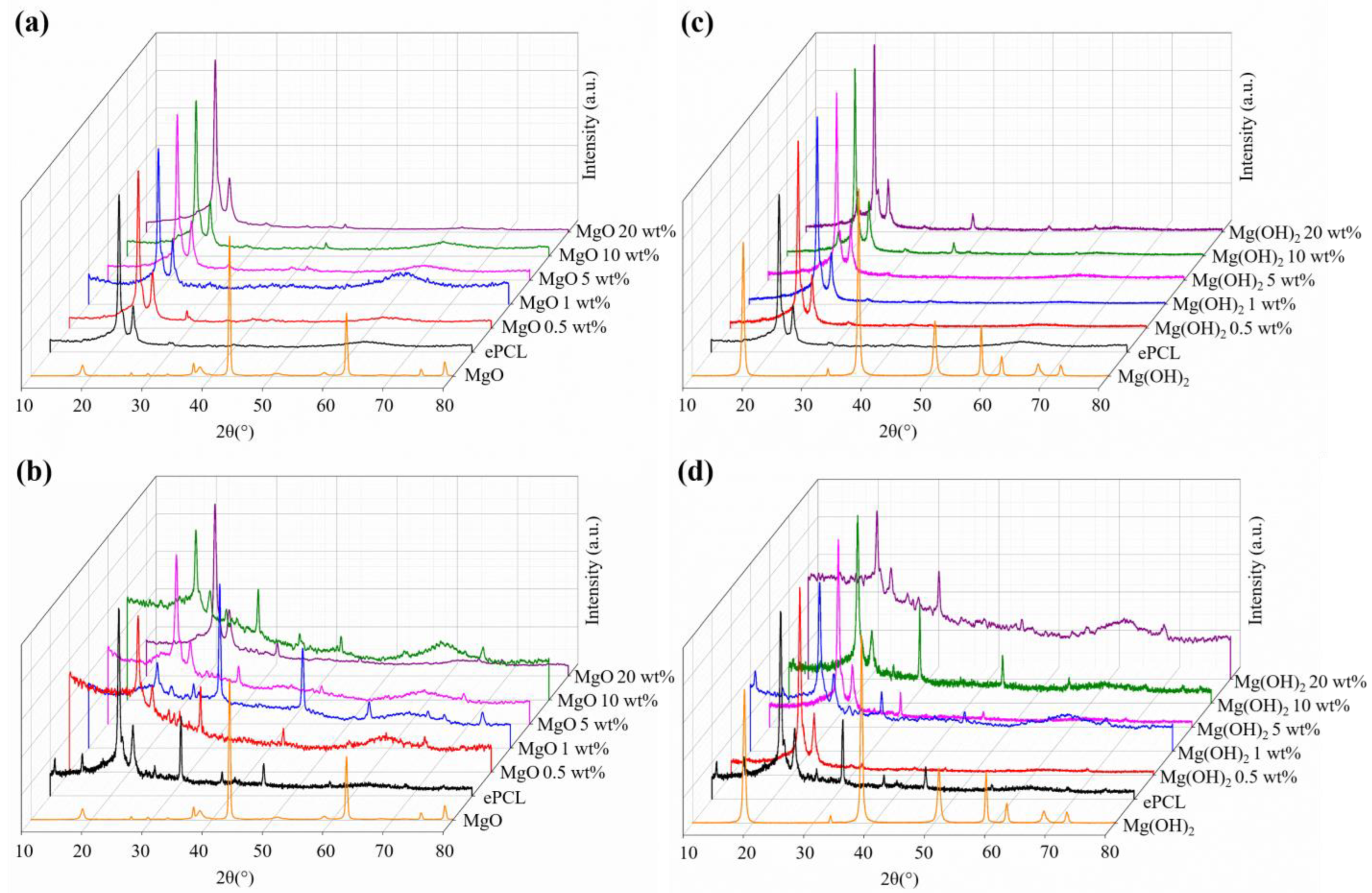
| XRD | FTIR | |||
|---|---|---|---|---|
| Sample | T0 | T296 | T0 | T296 |
| ePCL | 53 | 50 | 43 | 47 |
| ePCL+ MgO 0.5 wt% | 52 | 52 | 42 | 50 |
| ePCL+ MgO 1 wt% | 52 | 51 | 43 | 52 |
| ePCL+ MgO 5 wt% | 52 | 57 | 39 | 46 |
| ePCL+ MgO 10 wt% | 49 | 54 | 46 | 53 |
| ePCL+ MgO 20 wt% | 48 | 57 | 44 | 46 |
| ePCL+ Mg(OH)2 0.5 wt% | 51 | 43 | 40 | 43 |
| ePCL+ Mg(OH)2 1 wt% | 50 | 49 | 42 | 46 |
| ePCL+ Mg(OH)2 5 wt% | 48 | 44 | 41 | 44 |
| ePCL+ Mg(OH)2 10 wt% | 46 | 52 | 41 | 46 |
| ePCL+ Mg(OH)2 20 wt% | 37 | 51 | 43 | 51 |
| T0 | T28 | |
|---|---|---|
| ePCL | - | 0.70 |
| ePCL+ MgO 0.5 wt% | - | 0.18 |
| ePCL+ MgO 1 wt% | - | 0.19 |
| ePCL+ MgO 5 wt% | - | 0.65 |
| ePCL+ MgO 10 wt% | - | 1.17 |
| ePCL+ MgO 20 wt% | - | 1.76 |
| ePCL+ Mg(OH)2 0.5 wt% | - | 0.27 |
| ePCL+ Mg(OH)2 1 wt% | - | 0.56 |
| ePCL+ Mg(OH)2 5 wt% | - | 0.53 |
| ePCL+ Mg(OH)2 10 wt% | - | 0.43 |
| ePCL+ Mg(OH)2 20 wt% | - | 1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salaris, V.; López, D.; Kenny, J.M.; Peponi, L. Hydrolytic Degradation and Bioactivity of Electrospun PCL-Mg-NPs Fibrous Mats. Molecules 2023, 28, 1001. https://doi.org/10.3390/molecules28031001
Salaris V, López D, Kenny JM, Peponi L. Hydrolytic Degradation and Bioactivity of Electrospun PCL-Mg-NPs Fibrous Mats. Molecules. 2023; 28(3):1001. https://doi.org/10.3390/molecules28031001
Chicago/Turabian StyleSalaris, Valentina, Daniel López, José Maria Kenny, and Laura Peponi. 2023. "Hydrolytic Degradation and Bioactivity of Electrospun PCL-Mg-NPs Fibrous Mats" Molecules 28, no. 3: 1001. https://doi.org/10.3390/molecules28031001
APA StyleSalaris, V., López, D., Kenny, J. M., & Peponi, L. (2023). Hydrolytic Degradation and Bioactivity of Electrospun PCL-Mg-NPs Fibrous Mats. Molecules, 28(3), 1001. https://doi.org/10.3390/molecules28031001










