Abstract
Two endophytic fungi Trichoderma afroharzianum (HP-3) and Alternaria alstroemeriae (HP-7) were isolated and purified from the fresh root of Dryopteris crassirhizoma. Chemical investigation of the two fungi resulted in the isolation of two new phenols 2,4-dihydroxy-3-farnesyl-5-methoxy benzoic acid (1) and 2-hydroxyphenethyl 2-phenylacetate (2), together with 22 known compounds. Their structures were elucidated by NMR, UV, IR, HRESIMS, and comparison to the literature data. Compounds 15 and 16 showed significant antibacterial activity against Micrococcus lysodeikticus with MIC value of 6.25 μg/mL, while 8 and 14 displayed moderate inhibitory activities against several plant pathogenic fungi and clinically important bacterial strains. This is the first study to report the isolation, identification, and antimicrobial properties of metabolites from endophytic fungi of D. crassirhizoma. Our findings may provide lead compounds for the development of new antibacterial agents.
1. Introduction
Endophytes belong to the mitotic and meiotic ascomyces, which parasitize asymptomatically in healthy tissues below the epidermal cell layer of plants. During the long period of coexistence and evolution, mutualistic relationships have been established between endophytic fungi and host plants. The fungi promote the growth of host plants and enhance their resistance to biotic and abiotic stresses by accumulation of secondary metabolites such as terpenoids, alkaloids, phenols, lignans, and steroids, some of which can be used as drugs or lead compounds [1,2]. Podophyllotoxin, produced by endophytes from Nothapotydes foetida, Podophyllum peltatum, and P. emodi, is a valuable natural product as a precursor to three anticancer drugs, etoposide, teniposide, and etoposide phosphate [3]. In addition, compounds fusarubin, chetomin, and chaetocochin C isolated from plant endophytic fungi were reported to have significant in vitro antimycobacterial activity and could be developed as potential drugs against resistant mycobacterial infections [4].
The rhizome of Dryopteris crassirhizoma is a well-known Chinese herbal medicine used to treat parasitic infestation and viral diseases [5]. So far, there is no report on the chemical constituent or biological activity from endophytic fungus of D. crassirhizoma. Herein, our efforts with the chemical constituents of endophytic fungi Trichoderma afroharzianum and Alternaria alstroemeriae (HP-3 and HP-7) isolated from the rhizome of D. crassirhizoma, led to the isolation of two new (1 and 2) and 22 known (3~24) compounds. In this paper, we mainly describe their structural identification and antimicrobial activities in vitro.
2. Results and Discussion
2.1. Structure Elucidation of the Isolated Compounds
Compound 1 was obtained as a white powder and the molecular formula was determined as C23H32O5 based on the HRESIMS ion at m/z 411.2144 [M + Na]+ (calcd for 411.2142). The 1H and 13C NMR spectra (CDCl3) displayed the characteristic signals of three isopentenyl units at δH 3.41 (2H, d, 7.2)/δC 22.1 (C-1′); 5.26 (1H, t, 7.2)/121.3 (C-2′); 5.07 (2H, m)/124.2, 124.4 (C-6′, C-10′); 1.93, 1.99 (4H, m)/39.7, 39.8 (C-4′, C-8′); 2.00, 2.06 (4H, m)/26.7 (C-5′, C-9′); 1.66 (3H, s)/25.7 (C-12′), 1.58 (3H, s)/17.7 (C-3′); 1.56 (3H, s)/16.0 (C-14′); 1.78 (3H, s)/16.2 (C-15′); 135.9, 2×134.9 (C-3′, C-7′, C-11′) (Table 1). Additional signals observed in the 1D NMR data at δH 7.17 (1H, s)/δC 107.8 (C-6), δC 101.6 (C-1), 157.8 (C-2), 115.6 (C-3), 151.3 (C-4), and 139.8 (C-5) suggested the presence of pentasubstituted benzene. The NMR spectra of 1 and 2,4-dihydroxy-3-farnesyl-5-methoxy benzoic acid [6] were very similar, except for an additional hydroxyl group located at C-2 in 1. This was confirmed by HMBC correlations from H2-1′ (δH 3.41) to C-2 (δC 157.8) and C-4 (δC 151.3), from OCH3 (δH 3.87) to C-5 (δC 139.8), and from H-6 (δH 7.17) to C-1 (δC 101.6), C-2 (δC 157.8), C-3 (δC 115.6), and C-4 (δC 151.3). (Figure 1) Thus, the structure of compound 1 was confirmed as 2,4-dihydroxy-3-farnesyl-5-methoxy benzoic acid. (Figure 2)

Table 1.
1H NMR (600 MHz) and 13C NMR (150 MHz) data for compounds 1 and 2 a.
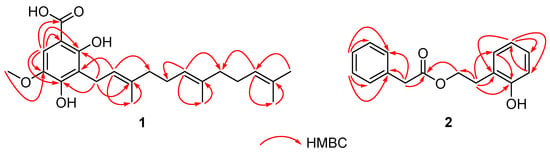
Figure 1.
The key HMBC (red arrows) correlations of 1 and 2.
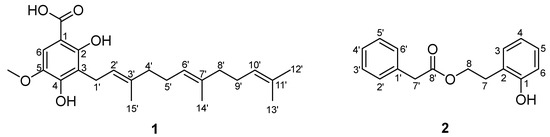
Figure 2.
The structures of compounds 1 and 2.
Compound 2, a white powder, was found to possess the molecular C16H16O3 from the HRESIMS pseudo molecular ion [M + Na]+ at m/z 279.0990 (calcd. 279.0992), with 9 degrees of unsaturation. The 1H NMR and 13C NMR data (CDCl3) of 2 revealed the presence of a phenylacetyl unit [δH 7.25 (2H, m)/δC 129.3 (C-2′, C-6′), 7.31 (2H, m)/128.7 (C-3′, C-5′), 7.27 (1H, m)/127.2 (C-4′), 3.64 (2H, s)/41.4 (C-7′), and 172.0 (C-8′)] supported by HMBC correlations (H-7′/C-1′, C-6′, and C-8′), and a 2-hydroxyphenyl ester unit [δH 7.05 (1H, d, J = 7.6 Hz)/δC 130.9 (C-3), 6.84 (1H, t, J = 7.6 Hz)/δC 120.8 (C-4), 7.13 (1H, t, J = 7.6 Hz)/δC 128.2 (C-5), 6.80 (1H, d, J = 7.6 Hz)/δC 115.9 (C-6), 2.93 (2H, t, J = 6.7 Hz)/δC 29.9 (C-7), 4.29 (2H, t, J = 6.7 Hz)/δC 64.8 (C-8), 154.3 (C-1), and 123.5 (C-2)] proved by HMBC correlations (H-7/C-1, C-2, C-3, and C-8) (Figure 1). Combined with the molecular formula information, HMBC correlations of H-8/C-8′ verified the linkage of the two units via an ester bond, and the structure of 2 was defined as 2-hydroxyphenethyl 2-phenylacetate. (Figure 1 and Figure 2)
The known compounds (Figure 3 and Figure 4) trans-harzialactone A (3) [7], stigmast-4-ene-3,6-dione (4) [8], ergosta-4,6,8(14),22-tetraen-3-one (5) [9], (22E)-5α,8α-epidioxyergosta-6,22-dien-3β-ol (6) [10], penicillazine (7) [11], harzianopyridone (8) [12], 5-hydroxy-2,3-dimethyl-7-methoxychromone (9) [13], 5-hydroxy-3-hydroxymethyl-2-methyl-7-methoxychromone (10) [13], 5-hydroxymethyl-2-furancarboxylic acid (11) [14], 4-hydroxybenzaldehyde (12) [15], 5-methylpyrimidine-2,4-diamin (13) [16], pyrrole-2-carboxylic acid (14) [16], chaetoglobosin F (15) [17], chaetoglobosin B (16) [18], β-sitostenone (17) [19], (22E, 24S)-stigmasta-4,22-dien-3-one (18) [20], (22E, 24S)-stigmasta-4,22,25-trien-3-one (19) [21], 5β-cholestane-3β,5,6β-triol (20) [22], alternariol 9-methyl ether (21) [23], alternariol 4,10-O-dimethyl ether (22) [24], 4-(2-hydroxyethyl) phenol (23) [25], and 3,3′,5′-dihydroxy-2′-methylphenyl-2-butanone (24) [26] were identified by comparison of their spectral data with the literature values.
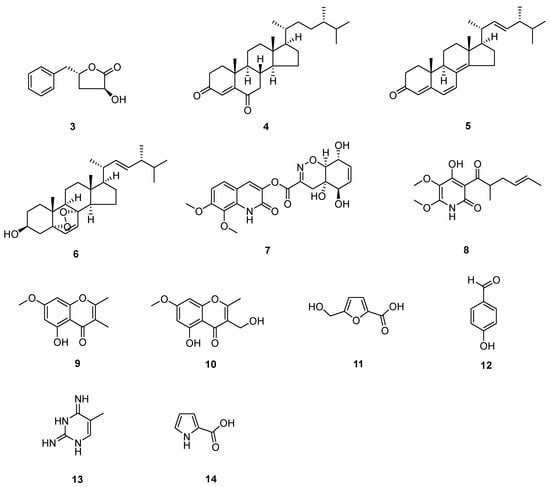
Figure 3.
Compounds from T. afoharzianum.
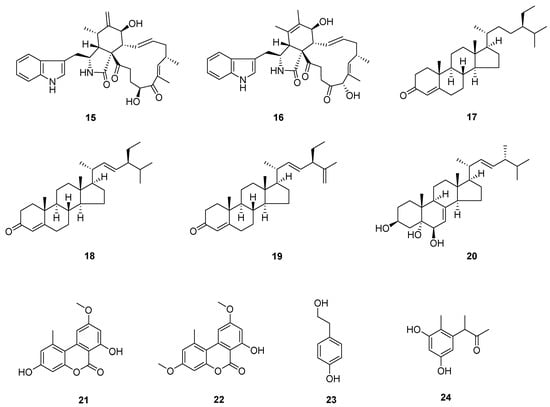
Figure 4.
Compounds from A. alstroemeriae.
2.2. Antifungal Activity of the Isolated Compounds
Antifungal activity of compounds 1~24 was evaluated using four plant pathogenic fungi, including Verticillium dihliae Kleb, Rhizoctonia solani, Sclerotinia sclexotiorum, and Physalospora pixicolg Nose, following the procedures described in the literature [27,28]. Ketoconazole was employed as the reference standard in the experiment. The results (Table S1 in Supplementary Materials) revealed that compound 8 displayed moderate inhibitory activity against three plant pathogenic fungi (V. dahliae Kleb, S. sclexotiorum, and P. pixicolg Nose) with MIC values of 50, 50, and 12.5 μg/mL, respectively.
2.3. Antibacterial Activity of the Isolated Compounds
Compounds 1~24 were tested for their antibacterial activity against clinically important bacterial strains including M. lysodeticus, Bacillus subtilis, M. luteus, Salmonella typhi, Alternaria longipes, and Staphylococcus aureus. The assay was performed using a previously described method, with ciprofloxacin utilized as the reference standard [27,28]. Compounds 15 and 16 exhibited significant inhibitory effects (Table S2 in Supplementary Materials) against M. lysodeikticus with MIC value of 6.25 μg/mL. And 14 showed moderate inhibitory activities against M. lysodeikticus and M. luteus with MIC values of 25 and 50 μg/mL, respectively.
3. Materials and Methods
3.1. Fermentation, Extraction, and Isolation
The study material T. afroharzianum strain HP-3 and A. alstroemeriae strain HP-7 (Figure 5) were isolated from the root of D. crassirhizoma, collected from Fushun, Liaoning province. The endophytic fungi (HP-3 and HP-7) were identified by internal transcribed spacer (ITS) sequencing by Chongqing Biomedicine Biotechnology Co., Ltd, Chongqing, China. The fungi were grown on PDA for 5 days, and DNA templates were prepared by the SDS extraction method [29]. Briefly, mycelium were scraped off from culture plates and transferred into a centrifuge tube. Mycelium were mixed with 500 μL of buffer [50 mM of EDTA (PH 8.0), 100 mM of Tris-HCl (PH 8.0), 500 mM of NaCl, and SDS (20%)] and incubated at 65 °C for 35 min. Then 100 μL of chloroform was mixed into the tube and allowed to stand for 5 min, then centrifuged at 12,000 rpm for 15 min. The aqueous extraction layer (200~400 μL) was transferred into a new tube, followed by the addition of an equal volume of isopropyl alcohol. The tube was gently inverted and left to stand for 10 minutes, then centrifuged at 12,000 rpm for 10 min at RT to precipitate DNA. The DNA pellet was washed with 75% ethanol twice and dried, and their concentration and quality were measured by using UV spectrophotometer at 230, 260, and 280 nm. The ITS regions of the fungus were amplified with the ITS primers ITS-F (TCCGTAGGTGAACCTGCGG) and ITS-R (TCCTCCGCTTATTGATATGC) using the polymerase chain reaction (PCR). The PCR conditions used were as follows: initial denaturation at 95 °C for 3 min, followed by 35 cycles of 95 °C for 10 s, 55 °C for 30 s, 72 °C for 10 s, and a final extension at 72 °C for 5 min. The reaction mixture contained 22 μL of GoldenStar T6 Super PCR Mix, 0.5 μL of each primer (10 μΜ), 2 μL of template DNA, and 25 μL of ddH2O. After electrophoresis at 120 V for 20 min, the amplified products were visualized on 1% (w/v) agarose gel to confirm the presence of a single amplified band (Figure S34 in Supplementary Materials). Then the band due to the PCR products was isolated from the gel slice using the DNA gel purification kit (Tsingke, Beijing, China) according to the manufacturer’s protocol. PCR products were sequenced using electrophoretic sequencing on an ABI 3730XL DNA analyzer (Applied Biosystems, Foster City, CA, USA). The sequences were matched against the nucleotide nucleotide database (BLASTn) of the National Center for Biological Information (NCBI) for final identification of the endophytic isolate. The ITS sequence of strain HP-3 showed high homologies of 99% (in 564 bp) to Trichoderma afroharzianum (CBS 124620), while the sequence of HP-7 showed high homologies of 99% (in 518 bp) to Alternaria alstroemeriae (CBS 118809) (Figures S35 and S36 in Supplementary Materials). The endophytic fungi were stored at the Laboratory of the Department of Materials and Chemistry, Yibin University. The HP-3 and HP-7 strains were mass cultivated in 500 mL sterilized Erlenmeyer flasks, each containing rice (100 g), potato extract broth medium (100 mL), and the seed culture (20.0 mL) at room temperature for 60 days. The two solid fermented materials (200 flasks each strain) were extracted three times with equivoluminal EtOAc to obtain extracts HP-3 (51 g) and HP-7 (113 g), respectively. The HP-3 extracts were then subjected to silica gel CC (200~300 mesh) with petroleum ether PE/EtOAc (from 100:0 to 0:100) to obtain 13 fractions (A~M). Fraction B was rechromatographed on silica gel CC eluted with PE/EtOAc (from 15:0 to 1:1) to yield subfractions (Fr.B1~Fr.B6).

Figure 5.
The plates of T. afroharzianum strain HP-3 and A. alstroemeriae strain HP-7.
Compound 9 (145 mg) was precipitated from subfraction B2. Fr. D (0.50 g) was fractioned over Sephadex LH-20 column (CH2Cl2/MeOH = 1:1) and semi-preparative HPLC (MeOH/H2O = 95:5), to obtain compounds 4 (1.6 mg) and 5 (20 mg). Fr. F (9.0 g) was separated on silica gel column eluted with CH2Cl2/MeOH (20:1) to provide subfractions (F1~F4). Fr. F3 (0.66 g) was fractioned over Sephadex LH-20 column (CH2Cl2/MeOH = 1:1) and semi-preparative HPLC (MeOH/H2O = 62:38), to obtain compounds 2 (1.1 mg) and 8 (13mg, 0.00025%). Fr. G (2.5 g) was separated on silica gel column eluted with CH2Cl2/MeOH = 20:1 to provide subfractions (G1~G6). Compound 10 (20.2 mg) was precipitated from Fr. G2. The mother liquor of Fr. G2 was fractioned over Sephadex LH-20 column (CH2Cl2/MeOH = 1:1) and semi-preparative HPLC (MeOH/H2O = 65:35) to obtain compounds 3 (15 mg), 6 (10 mg), 12 (4 mg), and 14 (2.7 mg). Fr. L was submitted to silica gel column (CH2Cl2/EtOAc = 1:1), Sephadex LH-20 column (CH2Cl2/MeOH = 1:1), and semi-preparative HPLC (MeOH/H2O = 70:30) to afford 7 (23.7 mg), 11 (17.0 mg), and 13 (16.5 mg).
The HP-7 extracts were applied to CC (200~300 mesh), eluting with PE/EtOAc (1:0→0:1 gradient), to yield 11 fractions (A~K). Compound 21 (65 mg) was precipitated from subfractions Fr. B. Fraction D (9.0 g) was subjected to silica gel CC, eluting with PE/EtOAc (20:1) to yield five fractions (D1~D5). Fr. D2 (0.43 g) was fractioned over Sephadex LH-20 column (CH2Cl2/MeOH = 1:1) and semi-preparative HPLC (MeOH/H2O = 88:12), to obtain compound 17 (11 mg). Fraction D3 (2.0 g) was separated on Sephadex LH-20 column (CH2Cl2/MeOH = 1:1) and semi-preparative HPLC with 95% MeOH, to yield 22 (3.1 mg), 18 (4.4 mg), and 19 (3.0 mg). Compound 24 (4.6 mg) was obtained from Fr. G by repeated Sephadex LH-20 column (CH2Cl2/MeOH = 1:1) and semi-preparative HPLC (MeOH/H2O = 55:45). Fr. I (3.0 g) was fractioned over Sephadex LH-20 column (CH2Cl2/MeOH = 1:1) and semi-preparative HPLC (85% MeOH) to obtain compound 1 (6.3 mg). After separation by Sephadex LH-20 column (CH2Cl2/MeOH = 1:1) and then preparative HPLC (45% MeOH), 23 (6.6 mg) was isolated from Fr. J. Fr. K (4.1 g) was separated on MCI gel column (30% to 100% MeOH) and semi-preparative HPLC with 35% MeOH, to yield compounds 15 (20 mg, 0.00018%), 16 (2.0 mg, 0.000018%), and 20 (45.0 mg).
2,4-Dihydroxy-3-farnesyl-5-methoxy benzoic acid (1): white powder; UV (MeOH) λmax (logε): 196 (4.7), 253 (3.9), 310 (3.8) nm; IR (KBr) νmax: 3492, 2925, 1617, 1457, 1236 cm–1; 1H and 13C NMR data see Table 1.
2-Hydroxyphenethyl 2-phenylacetate (2): white powder; UV (MeOH) λmax (logε): 195 (4.7), 274 (3.9) nm; IR (KBr) νmax: 3434, 2922, 2851, 1719, 1456 cm–1; 1H and 13C NMR data see Table 1.
3.2. Assay of Anti-Plant Pathogenic Fungi Activity
Using the microbroth dilution method [27,28], four plant pathogenic fungi (V. dahliae Kleb, R. solani, S. sclexotiorum, and P. pixicolg Nose) were used to evaluate the antifungal activity of all isolated compounds (1~24). These fungi were cultured in potato dextrose agar (PDA) at 28 °C for 72 h to prepare a suspension solution of fungi (104 mycelia fragments/mL). All test compounds and ketoconazole (positive control) were prepared in DMSO at a concentration of 1 mg/mL. The 180 μL of fungi suspension and 20 μL of the above-prepared solutions were added into 96-well flat plates in triplicate. Test compounds and ketoconazole were diluted using 2-fold serial dilution method, resulting in concentrations ranging from 100 to 0.78 μg/mL. Additionally, the medium containing 1% DMSO was used as blank control. The MIC was determined by incubating the plate. The MIC values were defined as the lowest test concentration with no obvious growth after the incubation (28 °C, 72 h).
3.3. Assay of Antibacterial Activity In Vitro
All isolated compounds (1~24) were evaluated for their antibacterial activity against M. lysodeikticus, B. cereus, M. luteus, Salmonella typhimurium, P. aeruginosa, and S. aureus bacteria. Targeted microbes were cultivated in Luria Bertani (LB) medium at 37 °C for 24 h to prepare the suspension solution (1 × 106 CFU/mL) for tests. Compounds and ciprofloxacin (positive control) were prepared in DMSO (1 mg/mL) as stock solution. Bacterial suspension (180 μL) and stock solutions (20 μL) were added to 96-well flat plates in triplicate. The final concentrations of test compounds and ciprofloxacin were 100, 50, 25, 12.5, 6.25, 3.12, 1.56, 0.78 μg/mL in medium. After incubation at 37 °C for 24 h, the MICs were defined as the lowest test concentration that showed no obvious growth.
4. Conclusions
This is the first report about the chemical constituents of endophytic fungi isolated from D. crassirhizoma. A total of 24 compounds including two new ones were isolated from the solid fermentation of the fungi T. afroharzianum and A. alstroemeriae. In the in vitro bioassays, compounds 15 and 16 significantly inhibited M. lysodeikticus with MIC value of 6.25 μg/mL. Compound 8 displayed inhibitory activity against three plant pathogenic fungi (V. dahliae Kleb, S. sclexotiorum, and P. pixicolg Nose) with MIC values of 12.5~50 μg/mL. In addition, 14 showed moderate inhibitory activities against M. lysodeikticus and M. luteus with MIC values of 25 and 50 μg/mL, respectively. The cytochalasin-type alkaloids Chaetoglobosins F (15) and B (16), previously isolated from the genus Chaetomium, Alternaria, Penicillium, etc, were found to have a wide range of biological activities, such as cytotoxic, immunomodulatory, antimicrobial, antioxidative, and neuroprotective properties [30,31,32,33,34,35]. Notably, Andrew and co-workers found that chaetoglobosin B (16) possessed antibacterial activity towards methicillin-resistant Staphylococcus aureus and Mycobacterium tuberculosis H37Ra (IC50: 85 and 22 µM) [36]. In our paper, is the first report that chaetoglobosins F and B (15 and 16) both possessed strong inhibitory effects against M. lysodeikticus. This indicated the possible application of cytochalasan alkaloids in developing new antibacterial agents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28248043/s1, general experimental procedures; Table S1: antifungal activities; Table S2: antibacterial activities; Figures S1~S33: HRESIMS, and 1D, 2D NMR spectra of compounds 1~24; Figure S34: gel electrophoresis of 16S rRNA gene amplicons; Figures S35 and S36: sequence data analysis of HP-3 and HP-7.
Author Contributions
Designing the study: R.L.; drafting the manuscript: P.H.; bioactive assay: M.W. and N.C.; critical revision of the manuscript: Y.G. and J.Y.; assisting the chemical experiment: L.Y., H.J., H.L. and W.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (No. 22067012, 82060637, and 21702181), the Yunnan Innovation Team (2019HC018), the Higher Educational Key Laboratory for New drugs for Viral Respiratory Diseases (Chinese Traditional Medicine) of Yunnan Province, the Scientific and technological innovation project of China Academy of Chinese Medical Sciences (CI2021A04005, CI2021A01809), and the Key Project at Central Government Level: the ability establishment of sustainable use for valuable Chinese medicine resources (2060302).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jia, M.; Chen, L.; Xin, H.L.; Zheng, C.J.; Rahman, K.; Han, T.; Qin, L.P. A friendly relationship between endophytic fungi and medicinal plants: A systematic review. Front. Microbiol. 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Kusari, S.; Hertweck, C.; Spitellert, M. Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef]
- Eyberger, A.L.; Dondapati, R.; Porter, J.R. Endophyte fungal isolates from Podophyllum peltatum produce podophyllotoxin. J. Nat. Prod. 2006, 69, 1121–1124. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Dufosse, L.; Chhipa, H.; Saxena, S.; Mahajan, G.B.; Gupta, M.K. Fungal endophytes: A potential source of antibacterial compounds. Journal of Fungi 2022, 8, 164. [Google Scholar] [CrossRef]
- Yim, N.H.; Lee, J.J.; Lee, B.; Li, W.; Ma, J.Y. Antiplatelet activity of acylphloroglucinol derivatives isolated from Dryopteris crassirhizoma. Molecules 2019, 24, 2212. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.A.; Poon, W.W.; Myles, D.C.; Clarke, C.F. The biosynthesis of ubiquinone: Synthesis and enzymatic modification of biosynthetic precursors. Tetrahedron Lett. 1996, 37, 2395–2398. [Google Scholar] [CrossRef]
- Chen, B.; Yin, H.-F.; Wang, Z.-S.; Xu, J.-H. New synthesis of harzialactone A via kinetic resolution using recombinant Fusarium proliferatum lactonase. Tetrahedron-Asymmetry 2010, 21, 237–240. [Google Scholar] [CrossRef]
- Shen, C.C.; Syu, W.J.; Li, S.Y.; Lin, C.H.; Lee, G.H.; Sun, C.M. Antimicrobial activities of naphthazarins from Arnebia euchroma. J. Nat. Prod. 2002, 65, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, H.; Nakamura, E.; Okuyama, E.; Ishibashi, M. Six immunosuppressive features from an Ascomycete, Zopfiella longicaudata, found in a screening study monitored by immunomodulatory activity. Chem. Pharm. Bull. 2004, 52, 1005–1008. [Google Scholar] [CrossRef]
- Hybelbauerova, S.; Sejbal, J.; Dracinsky, M.; Hahnova, A.; Koutek, B. Chemical constituents of Stereum subtomentosum and two other birch-associated basidiomycetes: An interspecies comparative study. Chem. Biodivers. 2008, 5, 743–750. [Google Scholar] [CrossRef]
- Lin, Y.C.; Shao, Z.Y.; Jiang, G.C.; Zhou, S.N.; Cai, J.W.; Vrijmoed, L.L.P.; Jones, E.B.G. Penicillazine, a unique quinolone derivative with 4H-5,6-dihydro-1,2-oxazine ring system from the marine fungus Penicillium sp (strain #386) from the South China Sea. Tetrahedron 2000, 56, 9607–9609. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Hanson, J.R.; Hitchcock, P.B.; Claydon, N. Structure and biosynthesis of harzianopyridone, an antifungal metabolite of Trichoderma harzianum. J. Chem. Soc. Perkin Trans. 1989, 1, 1885–1887. [Google Scholar] [CrossRef]
- Takenaka, Y.; Tanahashi, T.; Nagakura, N.; Hamada, N. 2,3-Dialkylchromones from mycobiont cultures of the lichen Graphis scripta. Heterocycles 2000, 53, 1589–1593. [Google Scholar] [CrossRef]
- Xie, X.Y.; Wang, R.; Shi, Y.P. Chemical constituents from rizomes of Homalomena occulta. Chin. J. Chin. Materia Med. 2013, 38, 2325–2327. [Google Scholar] [CrossRef]
- Shubina, L.K.; Makar’eva, T.N.; Denisenko, V.A.; Stonik, V.A. 4-hydroxybenzaldehyde from the baikal sponge Lubomirskia baicalensis. Chem. Nat. Compd. 2005, 41, 93–94. [Google Scholar] [CrossRef]
- Dieter, A.; Fiedler, H.P.; Goodfellow, M.; Muller, W.E.G.; Brun, R.; Beil, W.; Bringmann, G. Pyrocoll, an antibiotic, antiparasitic and antitumor compound produced by a novel alkaliphilic Streptomyces strain. J. Antibiot. 2003, 56, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.-M.; Li, X.M.; Li, C.S.; Proksch, P.; Wang, B.G. Cytoglobosins A-G, cytochalasans from a marine-derived endophytic fungus, Chaetomium globosum QEN-14. J. Nat. Prod. 2010, 73, 729–733. [Google Scholar] [CrossRef]
- Guo, Q.F.; Chen, L.; Yin, Z.-H.; Zhang, J.J.; Kang, W.Y.; Wang, X.W.; Ding, G. Secondary metabolites and α-glucosidase inhibitory activities of Chaetomium globosum H6. Mycosystema 2019, 38, 134–144. [Google Scholar] [CrossRef]
- Li, W.H.; Chang, S.T.; Chang, S.C.; Chang, H.T. Isolation of antibacterial diterpenoids from Cryptomeria japonica bark. Nat. Prod. Res. 2008, 22, 1085–1093. [Google Scholar] [CrossRef]
- Georges, P.; Sylvestre, M.; Ruegger, H.; Bourgeois, P. Ketosteroids and hydroxyketosteroids, minor metabolites of sugarcane wax. Steroids 2006, 71, 647–652. [Google Scholar] [CrossRef]
- Layne, T.H.; Reynolds, W.F.; McLean, S.; Tinto, W.F. Secondary metabolites from Clerodendrum chinense. Nat. Prod. Commun. 2008, 3, 1787–1792. [Google Scholar] [CrossRef]
- Piccialli, V.; Sica, D. Four new trihydroxylated sterols from the Sponge Spongionella gracilis. J. Nat. Prod. 1987, 50, 915–920. [Google Scholar] [CrossRef]
- Meng, X.; Mao, Z.; Lou, J.; Xu, L.; Zhong, L.; Peng, Y.; Zhou, L.; Wang, M. Benzopyranones from the endophytic fungus Hyalodendriella sp. ponipodef 12 and their bioactivities. Molecules 2012, 17, 11303–11314. [Google Scholar] [CrossRef]
- Yang, Z.J.; Yang, T.; Luo, M.Y.; Xia, X.; Chen, D.J.; Qian, X.P. A new sesquiterpenoid from fungus Colletotrichum sp. and its cytotoxicity. Acta Pharm. Sin. 2013, 48, 891–895. [Google Scholar]
- Li, X.; Tian, Y.; Dong, Q.; Geng, W.; Song, X.; Luo, D. The secondary metabolites of the endophytic fungi Pestalotiopsis versicolor from mangrove and their biological activities. Chin. Tradit. Pat. Med. 2019, 41, 1054–1058. [Google Scholar]
- Krohn, K.; Florke, U.; Rao, M.S.; Steingrover, K.; Aust, H.J.; Draeger, S.; Schulz, B. Metabolites from fungi 15. New isocoumarins from an endophytic fungus isolated from the Canadian thistle Cirsium arvense. Nat. Prod. Lett. 2001, 15, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Xu, L.L.; Zhang, X.; Gong, X.W.; Zhu, D.L.; Xu, X.H.; Wang, F.; Yang, X.L. Three new phenanthrenes with antimicrobial activities from the aerial parts of Juncus effusus. Fitoterapia 2018, 130, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fang, L.Z.; Liu, F.L.; Pang, X.J.; Qin, H.L.; Zhao, T.; Xu, L.L.; Yang, D.F.; Yang, X.L. New prenylxanthones, polyketide hemiterpenoid pigments from the endophytic fungus Emericella sp XL029 and their anti-agricultural pathogenic fungal and antibacterial activities. Rsc Advances 2017, 7, 31115–31122. [Google Scholar] [CrossRef]
- Li, H.Y.; Fu, T.T.; Zhang, Y.; Lv, T.Y.; Li, Y.; Xu, B.L. Effect comparison of five methods to extract fungal genomic DNA as PCR templates. Chinese Agric. Sci. Bull 2017, 33, 28–35. [Google Scholar]
- Shen, L.; Ju, J.J.; Liu, Q.; Wang, S.S.; Huang, W.Y. Antioxidative and neuroprotective effects of the cytochalasans from endophytes. Nat. Prod. Commun. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Gao, W.X.; He, Y.; Li, F.L.; Chai, C.W.; Zhang, J.W.; Guo, J.R.; Chen, C.M.; Wang, J.P.; Zhu, H.C.; Hua, Z.X.; et al. Antibacterial activity against drug-resistant microbial pathogens of cytochalasan alkaloids from the arthropod-associated fungus Chaetomium globosum TW1-1. Bioorg. Chem. 2019, 98, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Song, Y.C.; Chen, J.R.; Xu, C.; Tan, R.X. Chaetoglobosin U, a cytochalasan alkaloid from endophytic Chaetomium globosum IFB-E019. J. Nat. Prod. 2006, 69, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Yang, Y.; Sun, L.; Dou, H.; Tan, R.; Hou, Y. Chaetoglobosin F, a small molecule compound, possesses immunomodulatory properties on bone marrow-derived dendritic cells via TLR9 signaling pathway. Immunobiology 2013, 218, 292–302. [Google Scholar] [CrossRef]
- Zhu, X.W.; Wu, Z.H.; Liang, F.Y.; Gan, S.X.; Huang, Q.; Ding, W.J.; Li, C.Y. A new L-alanine derivative from the mangrove fungus Penicillium chrysogenum V11. Chem. Nat. Compd. 2018, 54, 520–522. [Google Scholar] [CrossRef]
- Li, H.; Xiao, J.; Gao, Y.Q.; Tang, J.J.; Zhang, A.L.; Gao, J.M. Chaetoglobosins from Chaetomium globosum, an endophytic fungus in Ginkgo biloba, and their phytotoxic and cytotoxic activities. J. Agric. Food Chem. 2014, 62, 3734–3741. [Google Scholar] [CrossRef]
- Andrew, J.F.; Amanda, I.B.; John, A.J.; Christopher, A.G. Polyketides from an endophytic Aspergillus fumigatus isolate inhibit the growth of Mycobacterium tuberculosis and MRSA. Nat. Prod. Commun. 2015, 10, 1661–1662. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).