Research of CO2-Soluble Surfactants for Enhanced Oil Recovery: Review and Outlook
Abstract
1. Introduction
| Adverse Effect | Mobility Control Method | Shortcoming | Reference | |
|---|---|---|---|---|
| Low viscosity | Viscous fingering | Water alternating gas (WAG) | It may cause tubing corrosion and scaling and not avoid gravity override. | [19,20] |
| Premature breakthrough | Gel profile control | It is not applicable to low-permeability reservoirs. | [21,22,23,49,50] | |
| Unfavorable mobility ratio | Direct thickeners (polymer and surfactant) | High solubility is impossible under reservoir conditions. | [24] | |
| Low density | Gravity override | Carbonated water injection (CWI) or micro and nanobubbles (MNBs) | It leads to low CO2 content and accelerated tubing corrosion. | [25,26] |
| Low swept volume | Surfactant-assisted or nanoparticle-assisted CO2 foam | It induces adsorption during injection and tubing corrosion. | [27,28,29,30] | |
2. CO2-Soluble Surfactants
2.1. Fluorocarbon Surfactants
2.2. Silicone Surfactants
2.3. Hydrocarbon Surfactants
2.4. Oxygenated Hydrocarbon Surfactants
3. Molecular Dynamics Simulation
4. Applications
4.1. CO2 Thickening
4.2. Reducing Miscibility Pressure
4.3. Supercritical CO2 Foam
5. Outlook
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nikolai, P.; Rabiyat, B.; Aslan, A.; Ilmutdin, A. Supercritical CO2: Properties and Technological Applications—A Review. J. Therm. Sci. 2019, 28, 394–430. [Google Scholar] [CrossRef]
- Weibel, G.L.; Ober, C.K. An Overview of Supercritical CO2 Applications in Microelectronics Processing. Microelectron. Eng. 2003, 65, 145–152. [Google Scholar] [CrossRef]
- Sifat, N.S.; Haseli, Y. A Critical Review of CO2 Capture Technologies and Prospects for Clean Power Generation. Energies 2019, 12, 4143. [Google Scholar] [CrossRef]
- Bucio, S.L.; Sanz, M.T.; Beltrán, S.; Melgosa, R.; Solaesa, Á.G.; Ruiz, M.O. Study of the Influence of Process Parameters on Liquid and Supercritical CO2 Extraction of Oil from Rendered Materials: Fish Meal and Oil Characterization. J. Supercrit. Fluids 2016, 107, 270–277. [Google Scholar] [CrossRef]
- Massarweh, O.; Abushaikha, A.S. A Review of Recent Developments in CO2 Mobility Control in Enhanced Oil Recovery. Petroleum 2022, 8, 291–317. [Google Scholar] [CrossRef]
- Hou, Q.; Zhu, Y.; Luo, Y.; Weng, R. Studies on Foam Flooding EOR Technique for Daqing Reservoirs after Polymer Flooding. In All Days, Proceedings of the SPE, Tulsa, OK, USA, 14 April 2012; OnePetro: Richardson, TX, USA, 2012; p. SPE-151955-MS. [Google Scholar] [CrossRef]
- Sun, L.; Wang, B.; Pu, W.; Yang, H.; Shi, M. The Effect of Foam Stability on Foam Flooding Recovery. Pet. Sci. Technol. 2015, 33, 15–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xue, F.; Wang, Y.; Ren, B.; Zhang, L.; Ren, S. CO2 Foam Flooding for Improved Oil Recovery: Reservoir Simulation Models and Influencing Factors. J. Pet. Sci. Eng. 2015, 133, 838–850. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Y.; Dong, Y.; Wen, C.; Yang, Y. High-Pressure Supersonic Carbon Dioxide (CO2) Separation Benefiting Carbon Capture, Utilisation and Storage (CCUS) Technology. Appl. Energy 2023, 339, 120975. [Google Scholar] [CrossRef]
- Zhang, X.; Han, B. Cleaning Using CO2-Based Solvents. CLEAN—Soil Air Water 2007, 35, 223–229. [Google Scholar] [CrossRef]
- Al-Qadri, A.A.; Nasser, G.A.; Adamu, H.; Muraza, O.; Saleh, T.A. CO2 Utilization in Syngas Conversion to Dimethyl Ether and Aromatics: Roles and Challenges of Zeolites-Based Catalysts. J. Energy Chem. 2023, 79, 418–449. [Google Scholar] [CrossRef]
- Daqing, Y.; Qinghua, S.; Shaojing, J.; Chunxia, H.; Ruijia, T. A Study about Influence Law of Permeability on Gas Channeling of CO2 Flooding under Low Permeability Reservoirs. J. Southwest Pet. Univ. Technol. Ed. 2014, 36, 137. [Google Scholar] [CrossRef]
- Kaifeng, J.; Yuxia, W.; Shilu, W.; Dongchao, J.; Bin, L.; Ruiyao, Z.; Jindong, G. Influences of Reservoir Heterogeneity on Gas Channeling during CO2 Flooding in Low Permeability Reservoirs. Xinjiang Pet. Geol. 2019, 40, 208–212. [Google Scholar] [CrossRef]
- Chen, Z.; Su, Y.-L.; Li, L.; Meng, F.-K.; Zhou, X.-M. Characteristics and Mechanisms of Supercritical CO2 Flooding under Different Factors in Low-Permeability Reservoirs. Pet. Sci. 2022, 19, 1174–1184. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, P.; Huang, S.; Hao, H.; Zhang, M.; Lu, G. Performance and Applicable Limits of Multi-Stage Gas Channeling Control System for CO2 Flooding in Ultra-Low Permeability Reservoirs. J. Pet. Sci. Eng. 2020, 192, 107336. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, H.; Nguyen, Q.P. Effect of Surfactant Partitioning on Mobility Control During CO2 Flooding. SPE J. 2013, 18, 752–765. [Google Scholar] [CrossRef]
- Qin, J.; Han, H.; Liu, X. Application and Enlightenment of Carbon Dioxide Flooding in the United States of America. Pet. Explor. Dev. 2015, 42, 232–240. [Google Scholar] [CrossRef]
- Liu, F.; Yue, P.; Wang, Q.; Yu, G.; Zhou, J.; Wang, X.; Fang, Q.; Li, X. Experimental Study of Oil Displacement and Gas Channeling during CO2 Flooding in Ultra—Low Permeability Oil Reservoir. Energies 2022, 15, 5119. [Google Scholar] [CrossRef]
- Khoshsima, A.; Sedighi, M.; Mohammadi, M. Chapter 8—Enhanced Oil Recovery by Water Alternating Gas Injection. In Gas Injection Methods; Li, Z., Husein, M.M., Hemmati-Sarapardeh, A., Eds.; Enhanced Oil Recovery Series; Gulf Professional Publishing: Houston, TX, USA, 2023; pp. 295–316. ISBN 978-0-12-822302-4. [Google Scholar]
- Chai, X.; Tian, L.; Zhang, M.; Shao, H.; Wang, J.; Zhang, K. Production Characteristics, Evaluation, and Prediction of CO2 Water-Alternating-Gas Flooding in Tight Oil Reservoir. J. Energy Resour. Technol. 2022, 144, 033006. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q. Review of Gel Systems for CO2 Geological Storage Leakage and Conformance Control for Enhanced Oil Recovery: Mechanisms, Recent Advances, and Future Perspectives. J. Pet. Sci. Eng. 2022, 219, 111110. [Google Scholar] [CrossRef]
- Song, T.; Zhai, Z.; Liu, J.; Eriyagama, Y.; Ahdaya, M.; Alotibi, A.; Wang, Z.; Schuman, T.; Bai, B. Laboratory Evaluation of a Novel Self-Healable Polymer Gel for CO2 Leakage Remediation during CO2 Storage and CO2 Flooding. Chem. Eng. J. 2022, 444, 136635. [Google Scholar] [CrossRef]
- Zhao, M.; Yan, X.; Wang, X.; Yan, R.; Dai, C. The Development of a Smart Gel for CO2 Mobility Control in Heterogeneity Reservoir. Fuel 2023, 342, 127844. [Google Scholar] [CrossRef]
- Lemaire, P.C.; Alenzi, A.; Lee, J.J.; Beckman, E.J.; Enick, R.M. Thickening CO2 with Direct Thickeners, CO2-in-Oil Emulsions, or Nanoparticle Dispersions: Literature Review and Experimental Validation. Energy Fuels 2021, 35, 8510–8540. [Google Scholar] [CrossRef]
- Tavakolian, M.; Sohrabi, M.; Jami, M.; Ireland, S. Significant Improvement in Oil Recovery and CO2 Storage by Carbonated Water Injection (CWI). In Proceedings of the Third EAGE CO2 Geological Storage Workshop, Edinburgh, UK, 26 March 2012; European Association of Geoscientists & Engineers: Bunnik, The Netherlands, 2012; p. cp-281-00038, ISBN 978-94-6282-054-8. [Google Scholar]
- Li, X.; Peng, B.; Liu, Q.; Liu, J.; Shang, L. Micro and Nanobubbles Technologies as a New Horizon for CO2-EOR and CO2 Geological Storage Techniques: A Review. Fuel 2023, 341, 127661. [Google Scholar] [CrossRef]
- Wellington, S.L.; Vinegar, H.J. CT Studies of Surfactant-Induced CO2 Mobility Control. In Proceedings of the SPE Annual Technical Conference and Exhibition, Las Vegas, NV, USA, 22 September 1985; OnePetro: Richardson, TX, USA, 1985. [Google Scholar] [CrossRef]
- Du, D.; Zitha, P.L.J.; Uijttenhout, M.G.H. Carbon Dioxide Foam Rheology in Porous Media: A CT Scan Study. SPE J. 2007, 12, 245–252. [Google Scholar] [CrossRef]
- Enick, R.M.; Olsen, D.; Ammer, J.; Schuller, W. Mobility and Conformance Control for CO2 EOR via Thickeners, Foams, and Gels—A Literature Review of 40 Years of Research and Pilot Tests. In All Days, Proceedings of the SPE, Tulsa, OK, USA, 14 April 2012; OnePetro: Richardson, TX, USA, 2012; p. SPE-154122-MS. [Google Scholar] [CrossRef]
- Ashoori, E.; Marchesin, D.; Rossen, W.R. Stability Analysis of Uniform Equilibrium Foam States for EOR Processes. Transp. Porous Media 2012, 92, 573–595. [Google Scholar] [CrossRef][Green Version]
- Ren, G. Dynamics of Supercritical CO2 Foam in Porous Media with CO2 Soluble Surfactuants. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2012. [Google Scholar]
- Fernø, M.A.; Gauteplass, J.; Pancharoen, M.; Haugen, A.; Graue, A.; Kovscek, A.R.; Hirasaki, G. Experimental Study of Foam Generation, Sweep Efficiency, and Flow in a Fracture Network. SPE J. 2016, 21, 1140–1150. [Google Scholar] [CrossRef]
- Rossen, W.R. A Critical Review of Roof Snap-off as a Mechanism of Steady-State Foam Generation in Homogeneous Porous Media. Colloids Surf. Physicochem. Eng. Asp. 2003, 225, 1–24. [Google Scholar] [CrossRef]
- Yu, Y.; Saraji, S. Supercritical CO2 Foam Stabilized by a Viscoelastic Surfactant in Fractured Porous Media: The Effect of Fracture Surface Roughness. Energy Fuels 2021, 35, 10051–10061. [Google Scholar] [CrossRef]
- Tsau, J.-S.; Syahputra, A.E.; Grigg, R.B. Economic Evaluation of Surfactant Adsorption in CO2 Foam Application. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 3 April 2000; OnePetro: Richardson, TX, USA, 2000; p. SPE-59365-MS. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, G.; Brewer, M.; Lv, Q.; Sudhölter, E.J.R. Comprehensive Review on Surfactant Adsorption on Mineral Surfaces in Chemical Enhanced Oil Recovery. Adv. Colloid Interface Sci. 2021, 294, 102467. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Hussain, S.M.S.; Norrman, K.; Mahmoud, M.; Patil, S. Adsorption Mechanisms of a Novel Cationic Gemini Surfactant onto Different Rocks. Energy Fuels 2022, 36, 5737–5748. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Muruganathan, R.M.; Rossen, W.R.; Krastev, R. Effect of Gas Type on Foam Film Permeability and Its Implications for Foam Flow in Porous Media. Adv. Colloid Interface Sci. 2011, 168, 71–78. [Google Scholar] [CrossRef]
- Zeng, Y.; Farajzadeh, R.; Eftekhari, A.A.; Vincent-Bonnieu, S.; Muthuswamy, A.; Rossen, W.R.; Hirasaki, G.J.; Biswal, S.L. Role of Gas Type on Foam Transport in Porous Media. Langmuir 2016, 32, 6239–6245. [Google Scholar] [CrossRef] [PubMed]
- Harati, S.; Bayat, A.E.; Sarvestani, M.T. Assessing the Effects of Different Gas Types on Stability of SiO2 Nanoparticle Foam for Enhanced Oil Recovery Purpose. J. Mol. Liq. 2020, 313, 113521. [Google Scholar] [CrossRef]
- Zhou, J.; Srivastava, M.; Hahn, R.; Inouye, A.; Dwarakanath, V. Evaluation of an Amphoteric Surfactant for CO2 Foam Applications: A Comparative Study. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 30 August 2020; OnePetro: Richardson, TX, USA, 2020; p. SPE-200315-MS. [Google Scholar] [CrossRef]
- Xue, Z.; Panthi, K.; Fei, Y.; Johnston, K.P.; Mohanty, K.K. CO2-Soluble Ionic Surfactants and CO2 Foams for High-Temperature and High-Salinity Sandstone Reservoirs. Energy Fuels 2015, 29, 5750–5760. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Ge, J.; Wang, Y.; Ding, L.; Zhang, G. The CO2-in-Water Foam Stabilized with the Mixture of CO2-Soluble Surfactant and Nonionic Surfactant. J. Pet. Sci. Eng. 2021, 198, 108117. [Google Scholar] [CrossRef]
- Føyen, T.; Alcorn, Z.P.; Fernø, M.A.; Barrabino, A.; Holt, T. CO2 Mobility Reduction Using Foam Stabilized by CO2- and Water-Soluble Surfactants. J. Pet. Sci. Eng. 2021, 196, 107651. [Google Scholar] [CrossRef]
- McLendon, W.J.; Koronaios, P.; McNulty, S.; Enick, R.M.; Biesmans, G.; Miller, A.; Salazar, L.; Soong, Y.; Romanov, V.; Crandall, D. Assessment of CO2-Soluble Surfactants for Mobility Reduction using Mobility Measurements and CT Imaging. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14 April 2012; OnePetro: Richardson, TX, USA, 2012; p. SPE-154205-MS. [Google Scholar] [CrossRef]
- Eastoe, J.; Downer, A.M.; Paul, A.; Steytler, D.C.; Rumsey, E. Adsorption of Fluoro Surfactants at Air-Water and Water-Carbon Dioxide Interfaces. In Trends in Colloid and Interface Science XIV; Buckin, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 214–221. [Google Scholar] [CrossRef]
- Eastoe, J.; Dupont, A.; Steytler, D.C. Fluorinated Surfactants in Supercritical CO2. Curr. Opin. Colloid Interface Sci. 2003, 8, 267–273. [Google Scholar] [CrossRef]
- Eastoe, J.; Yan, C.; Mohamed, A. Microemulsions with CO2 as a Solvent. Curr. Opin. Colloid Interface Sci. 2012, 17, 266–273. [Google Scholar] [CrossRef]
- Gu, X.; Cai, G.; Fan, X.; He, Y.; Huang, F.; Gao, Z.; Kang, S. Evaluation of Foam Gel Compound Profile Control and Flooding Technology in Low-Permeability Reservoirs. Processes 2023, 11, 2424. [Google Scholar] [CrossRef]
- Brattekås, B.; Seright, R. A Review of Polymer Gel Utilization in Carbon Dioxide Flow Control at the Core and Field Scale. SPE J. 2023, 1–17. [Google Scholar] [CrossRef]
- Michels, A.; Kleerekoper, L. Measurements on the Dielectric Constant of CO2 at 25°, 50° and 100 °C up to 1700 Atmospheres. Physica 1939, 6, 586–590. [Google Scholar] [CrossRef]
- Consan, K.A.; Smith, R.D. Observations on the Solubility of Surfactants and Related Molecules in Carbon Dioxide at 50 °C. J. Supercrit. Fluids 1990, 3, 51–65. [Google Scholar] [CrossRef]
- Hoefling, T.A.; Beitle, R.R.; Enick, R.M.; Beckman, E.J. Design and Synthesis of Highly CO2-Soluble Surfactants and Chelating Agents. Fluid Phase Equilib. 1993, 83, 203–212. [Google Scholar] [CrossRef]
- Sagisaka, M.; Yoda, S.; Takebayashi, Y.; Otake, K.; Kondo, Y.; Yoshino, N.; Sakai, H.; Abe, M. Effects of CO2-Philic Tail Structure on Phase Behavior of Fluorinated Aerosol-OT Analogue Surfactant/Water/Supercritical CO2 Systems. Langmuir 2003, 19, 8161–8167. [Google Scholar] [CrossRef]
- Sagir, M.; Mushtaq, M.; Tahir, M.B.; Tahir, M.S.; Ullah, S.; Shahzad, K.; Rashid, U. CO2 Foam for Enhanced Oil Recovery (EOR) Applications Using Low Adsorption Surfactant Structure. Arab. J. Geosci. 2018, 11, 789. [Google Scholar] [CrossRef]
- Sanders, A.W.; Jones, R.M.; Linroth, M.A.; Nguyen, Q.P. Implementation of a CO2 Foam Pilot Study in the SACROC Field: Performance Evaluation. In All Days, Proceedings of the SPE, San Antonio, TX, USA, 8 October 2012; OnePetro: Richardson, TX, USA, 2012; p. SPE-160016-MS. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Wang, Y.; Wang, M.; Wang, Y.; Ren, S. Dissolution of Surfactants in Supercritical CO2 with Co-Solvents. Chem. Eng. Res. Des. 2015, 94, 624–631. [Google Scholar] [CrossRef]
- Li, J.; Jin, J.; Zhang, Z.; Wang, Y. Measurement and Correlation of Solubility of Benzamide in Supercritical Carbon Dioxide with and without Cosolvent. Fluid Phase Equilib. 2011, 307, 11–15. [Google Scholar] [CrossRef]
- Liu, J.; Han, B.; Zhang, J.; Mu, T.; Li, G.; Wu, W.; Yang, G. Effect of Cosolvent on the Phase Behavior of Non-Fluorous Ls-54 Surfactant in Supercritical CO2. Fluid Phase Equilib. 2003, 211, 265–271. [Google Scholar] [CrossRef]
- Chennamsetty, N.; Bock, H.; Scanu, L.F.; Siperstein, F.R.; Gubbins, K.E. Cosurfactant and Cosolvent Effects on Surfactant Self-Assembly in Supercritical Carbon Dioxide. J. Chem. Phys. 2005, 122, 094710. [Google Scholar] [CrossRef]
- Cummings, S.; Xing, D.; Enick, R.; Rogers, S.; Heenan, R.; Grillo, I.; Eastoe, J. Design Principles for Supercritical CO2 Viscosifiers. Soft Matter 2012, 8, 7044–7055. [Google Scholar] [CrossRef]
- Temtem, M.; Casimiro, T.; Santos, A.G.; Macedo, A.L.; Cabrita, E.J.; Aguiar-Ricardo, A. Molecular Interactions and CO2-Philicity in Supercritical CO2. A High-Pressure NMR and Molecular Modeling Study of a Perfluorinated Polymer in scCO2. J. Phys. Chem. B 2007, 111, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Sagisaka, M.; Hollamby, M.; Rogers, S.E.; Heenan, R.K.; Dyer, R.; Eastoe, J. Hybrid CO2-Philic Surfactants with Low Fluorine Content. Langmuir 2012, 28, 6299–6306. [Google Scholar] [CrossRef] [PubMed]
- Sagisaka, M.; Ono, S.; James, C.; Yoshizawa, A.; Mohamed, A.; Guittard, F.; Rogers, S.E.; Heenan, R.K.; Yan, C.; Eastoe, J. Effect of Fluorocarbon and Hydrocarbon Chain Lengths in Hybrid Surfactants for Supercritical CO2. Langmuir 2015, 31, 7479–7487. [Google Scholar] [CrossRef] [PubMed]
- Sagisaka, M.; Ogiwara, S.; Ono, S.; James, C.; Yoshizawa, A.; Mohamed, A.; Rogers, S.E.; Heenan, R.K.; Yan, C.; Peach, J.A.; et al. New Class of Amphiphiles Designed for Use in Water-in-Supercritical CO2 Microemulsions. Langmuir 2016, 32, 12413–12422. [Google Scholar] [CrossRef] [PubMed]
- Fink, R.; Beckman, E.J. Phase Behavior of Siloxane-Based Amphiphiles in Supercritical Carbon Dioxide. J. Supercrit. Fluids 2000, 18, 101–110. [Google Scholar] [CrossRef]
- Shi, Q.; Qiao, W. Synthesis of Siloxane Polyether Surfactants and Their Solubility in Supercritical CO2. J. Surfactants Deterg. 2017, 20, 453–458. [Google Scholar] [CrossRef]
- Sagisaka, M.; Kudo, K.; Nagoya, S.; Yoshizawa, A. Highly Methyl-Branched Hydrocarbon Surfactant as a CO2-Philic Solubilizer for Water/Supercritical CO2 Microemulsion. J. Oleo Sci. 2013, 62, 481–488. [Google Scholar] [CrossRef]
- Ihara, T.; Suzuki, N.; Maeda, T.; Sagara, K.; Hobo, T. Extraction of Water-Soluble Vitamins from Pharmaceutical Preparations Using AOT (Sodium Di-2-Ethylhexyl Sulfosuccinate)/Pentane Reversed Micelles. Chem. Pharm. Bull. 1995, 43, 626–630. [Google Scholar] [CrossRef][Green Version]
- Nave, S.; Eastoe, J.; Penfold, J. What Is So Special about Aerosol-OT? 1. Aqueous Systems. Langmuir 2000, 16, 8733–8740. [Google Scholar] [CrossRef]
- Johnston, K.P.; Cho, D.; DaRocha, S.R.P.; Psathas, P.A.; Ryoo, W.; Webber, S.E.; Eastoe, J.; Dupont, A.; Steytler, D.C. Water in Carbon Dioxide Macroemulsions and Miniemulsions with a Hydrocarbon Surfactant. Langmuir 2001, 17, 7191–7193. [Google Scholar] [CrossRef]
- Eastoe, J.; Paul, A.; Nave, S.; Steytler, D.C.; Robinson, B.H.; Rumsey, E.; Thorpe, M.; Heenan, R.K. Micellization of Hydrocarbon Surfactants in Supercritical Carbon Dioxide. J. Am. Chem. Soc. 2001, 123, 988–989. [Google Scholar] [CrossRef] [PubMed]
- Hollamby, M.J.; Trickett, K.; Mohamed, A.; Cummings, S.; Tabor, R.F.; Myakonkaya, O.; Gold, S.; Rogers, S.; Heenan, R.K.; Eastoe, J. Tri-Chain Hydrocarbon Surfactants as Designed Micellar Modifiers for Supercritical CO2. Angew. Chem. Int. Ed. 2009, 48, 4993–4995. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, W.; Webber, S.E.; Johnston, K.P. Water-in-Carbon Dioxide Microemulsions with Methylated Branched Hydrocarbon Surfactants. Ind. Eng. Chem. Res. 2003, 42, 6348–6358. [Google Scholar] [CrossRef]
- Burrows, L.C.; Haeri, F.; Tapriyal, D.; Sanguinito, S.; Shah, P.G.; Lemaire, P.; Crandall, D.; Enick, R.M.; Goodman, A. Dissolving Nonionic Surfactants in CO2 to Improve Oil Recovery in Unconventional Reservoirs via Wettability Alteration. Energy Fuels 2022, 36, 11913–11929. [Google Scholar] [CrossRef]
- Chen, Y.; Elhag, A.S.; Poon, B.M.; Cui, L.; Ma, K.; Liao, S.Y.; Omar, A.; Worthen, A.J.; Hirasaki, G.J.; Nguyen, Q.P.; et al. Ethoxylated Cationic Surfactants for CO2 EOR in High Temperature, High Salinity Reservoirs. In All Days, Proceedings of the SPE, Tulsa, Oklahoma, USA, 14 April 2012; OnePetro: Richardson, TX, USA, 2012; p. SPE-154222-MS. [Google Scholar] [CrossRef]
- Chen, Y.; Elhag, A.S.; Poon, B.M.; Cui, L.; Ma, K.; Liao, S.Y.; Reddy, P.P.; Worthen, A.J.; Hirasaki, G.J.; Nguyen, Q.P.; et al. Switchable Nonionic to Cationic Ethoxylated Amine Surfactants for CO2 Enhanced Oil Recovery in High-Temperature, High-Salinity Carbonate Reservoirs. SPE J. 2014, 19, 249–259. [Google Scholar] [CrossRef]
- Chen, Y.; Elhag, A.S.; Reddy, P.P.; Chen, H.; Cui, L.; Worthen, A.J.; Ma, K.; Quintanilla, H.; Noguera, J.A.; Hirasaki, G.J.; et al. Phase Behavior and Interfacial Properties of a Switchable Ethoxylated Amine Surfactant at High Temperature and Effects on CO2-in-Water Foams. J. Colloid Interface Sci. 2016, 470, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Hoefling, T.A.; Enick, R.M.; Beckman, E.J. Beckman Microemulsions in Near-Critical and Supercritical Carbon Dioxide. J. Phys. Chem. 1991, 95, 7127–7129. [Google Scholar] [CrossRef]
- Hoefling, T.; Stofesky, D.; Reid, M.; Beckman, E.; Enick, R.M. The Incorporation of a Fluorinated Ether Functionality into a Polymer or Surfactant to Enhance C02-Solubility. J. Supercrit. Fluids 1992, 5, 237–241. [Google Scholar] [CrossRef]
- Harrison, K.; Goveas, J.; Johnston, K.P.; O’Rear, E.A.I. Water-in-Carbon Dioxide Microemulsions with a Fluorocarbon-Hydrocarbon Hybrid Surfactant. Langmuir 1994, 10, 3536–3541. [Google Scholar] [CrossRef]
- Dardin, A.; DeSimone, J.M.; Samulski, E.T. Fluorocarbons Dissolved in Supercritical Carbon Dioxide. NMR Evidence for Specific Solute−Solvent Interactions. J. Phys. Chem. B 1998, 102, 1775–1780. [Google Scholar] [CrossRef]
- Beckman, E.J. A Challenge for Green Chemistry: Designing Molecules That Readily Dissolve in Carbon Dioxide. Chem. Commun. 2004, 17, 1885–1888. [Google Scholar] [CrossRef]
- Falandysz, J.; Taniyasu, S.; Gulkowska, A.; Yamashita, N.; Schulte-Oehlmann, U. Is Fish a Major Source of Fluorinated Surfactants and Repellents in Humans Living on the Baltic Coast? Environ. Sci. Technol. 2006, 40, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Butenhoff, J.; Costa, G.; Elcombe, C.; Farrar, D.; Hansen, K.; Iwai, H.; Jung, R.; Kennedy, G.; Lieder, P.; Olsen, G.; et al. Toxicity of Ammonium Perfluorooctanoate in Male Cynomolgus Monkeys after Oral Dosing for 6 Months. Toxicol. Sci. 2002, 69, 244–257. [Google Scholar] [CrossRef]
- Houde, M.; Bujas, T.A.D.; Small, J.; Wells, R.S.; Fair, P.A.; Bossart, G.D.; Solomon, K.R.; Muir, D.C.G. Biomagnification of Perfluoroalkyl Compounds in the Bottlenose Dolphin (Tursiops Truncatus) Food Web. Environ. Sci. Technol. 2006, 40, 4138–4144. [Google Scholar] [CrossRef]
- Johnston, K.P.; Randolph, T.; Bright, F.; Howdle, S. Toxicology of a PFPE Surfactant. Science 1996, 272, 1726. [Google Scholar] [CrossRef] [PubMed]
- Fink, R.; Hancu, D.; Valentine, R.; Beckman, E.J. Toward the Development of “CO2-Philic” Hydrocarbons. 1. Use of Side-Chain Functionalization to Lower the Miscibility Pressure of Polydimethylsiloxanes in CO2. J. Phys. Chem. B 1999, 103, 6441–6444. [Google Scholar] [CrossRef]
- Hoefling, T.A.; Newman, D.A.; Enick, R.M.; Beckman, E.J. Effect of Structure on the Cloud-Point Curves of Silicone-Based Amphiphiles in Supercritical Carbon Dioxide. J. Supercrit. Fluids 1993, 6, 165–171. [Google Scholar] [CrossRef]
- Alzobaidi, S.; Lee, J.; Jiries, S.; Da, C.; Harris, J.; Keene, K.; Rodriguez, G.; Beckman, E.; Perry, R.; Johnston, K.P.; et al. Carbon Dioxide-in-Oil Emulsions Stabilized with Silicone-Alkyl Surfactants for Waterless Hydraulic Fracturing. J. Colloid Interface Sci. 2018, 526, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, S.R.P.; Dickson, J.; Cho, D.; Rossky, P.J.; Johnston, K.P. Stubby Surfactants for Stabilization of Water and CO2 Emulsions: Trisiloxanes. Langmuir 2003, 19, 3114–3120. [Google Scholar] [CrossRef]
- Fan, X.; Potluri, V.K.; McLeod, M.C.; Wang, Y.; Liu, J.; Enick, R.M.; Hamilton, A.D.; Roberts, C.B.; Johnson, J.K.; Beckman, E.J. Oxygenated Hydrocarbon Ionic Surfactants Exhibit CO2 Solubility. J. Am. Chem. Soc. 2005, 127, 11754–11762. [Google Scholar] [CrossRef]
- Liu, J.; Han, B.; Li, G.; Zhang, X.; He, J.; Liu, Z. Investigation of Nonionic Surfactant Dynol-604 Based Reverse Microemulsions Formed in Supercritical Carbon Dioxide. Langmuir 2001, 17, 8040–8043. [Google Scholar] [CrossRef]
- Liu, J.; Han, B.; Wang, Z.; Zhang, J.; Li, G.; Yang, G. Solubility of Ls-36 and Ls-45 Surfactants in Supercritical CO2 and Loading Water in the CO2/Water/Surfactant Systems. Langmuir 2002, 18, 3086–3089. [Google Scholar] [CrossRef]
- Sanders, A.W.; Nguyen, Q.P.; Nguyen, N.M.; Adkins, S.S.; Johnston, K.P. Twin-Tailed Surfactants for Creating CO2-in-Water Macroemulsions for Sweep Enhancement in CO2-EOR. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 1 November 2010; OnePetro: Richardson, TX, USA, 2010; p. SPE-137689-MS. [Google Scholar] [CrossRef]
- Sarbu, T.; Styranec, T.; Beckman, E.J. Non-Fluorous Polymers with Very High Solubility in Supercritical CO2 down to Low Pressures. Nature 2000, 405, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Pitt, A.R.; Morley, S.D.; Burbidge, N.J.; Quickenden, E.L. The Relationship between Surfactant Structure and Limiting Values of Surface Tension, in Aqueous Gelatin Solution, with Particular Regard to Multilayer Coating. Colloids Surf. Physicochem. Eng. Asp. 1996, 114, 321–335. [Google Scholar] [CrossRef]
- Eastoe, J.; Dupont, A.; Steytler, D.C.; Thorpe, M.; Gurgel, A.; Heenan, R.K. Micellization of Economically Viable Surfactants in CO2. J. Colloid Interface Sci. 2003, 258, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Jing, L.; Xiong, C.; Liu, C.; Qiao, W. Solubility of Nonionic Hydrocarbon Surfactants with Different Hydrophobic Tails in Supercritical CO2. J. Chem. Eng. Data 2015, 60, 2469–2476. [Google Scholar] [CrossRef]
- Liebum, M.M.; Hirasaki, G.; Nguyen, Q.P. Solubility of Alkyl Amine Surfactants in Mixed Gas and Pure CO2 Environments. Ind. Eng. Chem. Res. 2017, 56, 10958–10964. [Google Scholar] [CrossRef]
- Rindfleisch, F.; DiNoia, T.P.; McHugh, M.A. Solubility of Polymers and Copolymers in Supercritical CO2. J. Phys. Chem. 1996, 100, 15581–15587. [Google Scholar] [CrossRef]
- Kilic, S.; Michalik, S.; Wang, Y.; Johnson, J.K.; Enick, R.M.; Beckman, E.J. Phase Behavior of Oxygen-Containing Polymers in CO2. Macromolecules 2007, 40, 1332–1341. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Liotta, C.L.; Eckert, C.A. Specific Intermolecular Interaction of Carbon Dioxide with Polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- O’Shea, K.E.; Kirmse, K.M.; Fox, M.A.; Johnston, K.P. Polar and Hydrogen-Bonding Interactions in Supercritical Fluids: Effects on the Tautomeric Equilibrium of 4-(Phenylazo)-1-Naphthol. J. Phys. Chem. 1991, 95, 7863–7867. [Google Scholar] [CrossRef]
- Drohmann, C.; Beckman, E.J. Phase Behavior of Polymers Containing Ether Groups in Carbon Dioxide. J. Supercrit. Fluids 2002, 22, 103–110. [Google Scholar] [CrossRef]
- Raveendran, P.; Wallen, S.L. Cooperative C−H···O Hydrogen Bonding in CO2−Lewis Base Complexes: Implications for Solvation in Supercritical CO2. J. Am. Chem. Soc. 2002, 124, 12590–12599. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, P.; Wallen, S.L. Sugar Acetates as Novel, Renewable CO2-Philes. J. Am. Chem. Soc. 2002, 124, 7274–7275. [Google Scholar] [CrossRef] [PubMed]
- Potluri, V.K.; Hamilton, A.D.; Karanikas, C.F.; Bane, S.E.; Xu, J.; Beckman, E.J.; Enick, R.M. The High CO2-Solubility of per-Acetylated α-, β-, and γ-Cyclodextrin. Fluid Phase Equilib. 2003, 211, 211–217. [Google Scholar] [CrossRef]
- Potluri, V.K.; Xu, J.; Enick, R.; Beckman, E.; Hamilton, A.D. Peracetylated Sugar Derivatives Show High Solubility in Liquid and Supercritical Carbon Dioxide. Org. Lett. 2002, 4, 2333–2335. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Wei, B.; McLendon, W.; Enick, R.; McNulty, S.; Trickett, K.; Mohamed, A.; Cummings, S.; Eastoe, J.; Rogers, S.; et al. CO2-Soluble, Nonionic, Water-Soluble Surfactants That Stabilize CO2-in-Brine Foams. SPE J. 2012, 17, 1172–1185. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Li, S.; Lv, Q.; Wang, P.; Liu, J.; Liu, J. Enhancing Sodium Bis(2-Ethylhexyl) Sulfosuccinate Injectivity for CO2 Foam Formation in Low-Permeability Cores: Dissolving in CO2 with Ethanol. Energy Fuels 2018, 32, 5846–5856. [Google Scholar] [CrossRef]
- Liu, J.; Shervani, Z.; Raveendran, P.; Ikushima, Y. Micellization of Sodium Bis(2-Ethylhexyl)Sulfosuccinate in Supercritical CO2 with Fluorinated Co-Surfactant and Its Solubilization of Hydrophilic Species. J. Supercrit. Fluids 2005, 33, 121–130. [Google Scholar] [CrossRef]
- Gold, S.; Eastoe, J.; Grilli, R.; Steytler, D.C. Branched Trichain Sulfosuccinates as Novel Water in CO2 Dispersants. Colloid Polym. Sci. 2006, 284, 1333–1337. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; Zheng, W.; Zhang, T.; Ge, J.; Jiang, P.; Zhang, G. CO2 in Water Foam Stabilized with CO2-Dissolved Surfactant at High Pressure and High Temperature. J. Pet. Sci. Eng. 2019, 178, 930–936. [Google Scholar] [CrossRef]
- Salaniwal, S.; Cui, S.T.; Cummings, P.T.; Cochran, H.D. Self-Assembly of Reverse Micelles in Water/Surfactant/Carbon Dioxide Systems by Molecular Simulation. Langmuir 1999, 15, 5188–5192. [Google Scholar] [CrossRef]
- Da Rocha, S.R.P.; Johnston, K.P.; Westacott, R.E.; Rossky, P.J. Molecular Structure of the Water−Supercritical CO2 Interface. J. Phys. Chem. B 2001, 105, 12092–12104. [Google Scholar] [CrossRef]
- Lísal, M.; Hall, C.K.; Gubbins, K.E.; Panagiotopoulos, A.Z. Micellar Behavior in Supercritical Solvent–Surfactant Systems from Lattice Monte Carlo Simulations. Fluid Phase Equilib. 2002, 194–197, 233–247. [Google Scholar] [CrossRef]
- Li, Z.; Hall, C.K. Phase Behavior in Model Homopolymer/CO2 and Surfactant/CO2 Systems: Discontinuous Molecular Dynamics Simulations. Langmuir 2004, 20, 8559–8568. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.-R.; Yin, J.-Z. The Self-Assembly and Microscopic Interfacial Properties of a Supercritical CO2 Microemulsion Having Hydrotropes: Atom-Level Observation from Molecular Dynamics Simulation. J. CO2 Util. 2020, 38, 77–87. [Google Scholar] [CrossRef]
- Zhu, H.; Yin, J. Effect of Cosolvent Ethanol on Solubilization of Ionic Liquids in Supercritical CO2 Microemulsions: Experiments and Simulations. J. Chem. Eng. Data 2021, 66, 347–359. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, P.; Li, Z.; Liu, T.; Zhao, L.; Hu, D. Ethanol Enhanced Anionic Surfactant Solubility in CO2 and CO2 Foam Stability: MD Simulation and Experimental Investigations. Fuel 2020, 267, 117162. [Google Scholar] [CrossRef]
- Nan, Y.; Jin, Z. Effect of Alcohol Tail Length on Aggregate Behavior of Alcohol and AOT at the Water-scCO2 Interface: MD Simulation Study. In Nanostructured Materials for Sustainable Energy: Design, Evaluation, and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2022; Volume 1421, pp. 263–288. ISBN 978-0-8412-9753-1. [Google Scholar]
- Nan, Y.; Li, W.; Zhang, M.; Jin, Z. Ethanol Blending to Improve Reverse Micelle Dispersity in Supercritical CO2: A Molecular Dynamics Study. J. Phys. Chem. B 2021, 125, 9621–9628. [Google Scholar] [CrossRef]
- Kobayashi, K.; Firoozabadi, A. Effect of Branching on Mutual Solubility of Alkane–CO2 Systems by Molecular Simulations. J. Phys. Chem. B 2022, 126, 8300–8308. [Google Scholar] [CrossRef]
- Trickett, K.; Xing, D.; Enick, R.; Eastoe, J.; Hollamby, M.J.; Mutch, K.J.; Rogers, S.E.; Heenan, R.K.; Steytler, D.C. Rod-Like Micelles Thicken CO2. Langmuir 2010, 26, 83–88. [Google Scholar] [CrossRef]
- Zhao, M.; Yan, R.; Li, Y.; Wu, Y.; Dai, C.; Yan, H.; Liu, Z.; Cheng, Y.; Guo, X. Study on the Thickening Behavior and Mechanism of Supercritical CO2 by Modified Polysiloxane. Fuel 2022, 323, 124358. [Google Scholar] [CrossRef]
- Dong, Z.; Li, Y.; Lin, M.; Li, M. A Study of the Mechanism of Enhancing Oil Recovery Using Supercritical Carbon Dioxide Microemulsions. Pet. Sci. 2013, 10, 91–96. [Google Scholar] [CrossRef]
- Wang, F. Study on the Decrease of Miscibility Pressure on CO2 Flooding by Fatty Alcohol Polyether Surfactant. Master’s Thesis, China University of Petroleum (East China), Qingdao, China, 2016. [Google Scholar]
- Guo, P.; Hu, Y.; Qin, J.; Li, S.; Jiao, S.; Chen, F.; He, J. Use of Oil-Soluble Surfactant to Reduce Minimum Miscibility Pressure. Pet. Sci. Technol. 2017, 35, 345–350. [Google Scholar] [CrossRef]
- Zhang, C.; Xi, L.; Wu, P.; Li, Z. A Novel System for Reducing CO2-Crude Oil Minimum Miscibility Pressure with CO2-Soluble Surfactants. Fuel 2020, 281, 118690. [Google Scholar] [CrossRef]
- Lv, W.; Gong, H.; Li, Y.; Li, Z.; Dong, M. The Potential and Mechanism of Nonionic Polyether Surfactants Dissolved in CO2 to Improve the Miscibility of CO2–Hydrocarbon Systems. Fuel 2022, 326, 125012. [Google Scholar] [CrossRef]
- Lv, W.; Dong, M.; Sarma, H.; Li, Y.; Li, Z.; Sun, J.; Gong, H. Effects of CO2-Philic Nonionic Polyether Surfactants on Miscibility Behaviors of CO2–Hydrocarbon Systems: Experimental and Simulation Approach. Chem. Eng. J. 2023, 464, 142701. [Google Scholar] [CrossRef]
- Le, V.Q.; Nguyen, Q.P.; Sanders, A.W. A Novel Foam Concept with CO2 Dissolved Surfactants. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20 April 2008; OnePetro: Richardson, TX, USA, 2008; p. SPE-113370-MS. [Google Scholar] [CrossRef]
- Ren, G.; Sanders, A.W.; Nguyen, Q.P. New Method for the Determination of Surfactant Solubility and Partitioning between CO2 and Brine. J. Supercrit. Fluids 2014, 91, 77–83. [Google Scholar] [CrossRef]
- Bi, W.; Zhang, P.; Zhang, Y.; Yang, T.; Liu, X.; Wang, S.; Ren, S. Development and performance evaluation on CO2-soluble surfactant foam system for low permeability reservoir. Pet. Geol. Recovery Effic. 2018, 25, 71–77. [Google Scholar] [CrossRef]
- Ramadhan, G.; Hirasaki, G.; Nguyen, Q.P. Foaming Behavior of CO2-Soluble, Viscoelastic Surfactant in Homogenous Porous Media. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14 April 2018; OnePetro: Richardson, TX, USA, 2018; p. SPE-190302-MS. [Google Scholar] [CrossRef]
- Haeri, F.; Burrows, L.C.; Lemaire, P.; Shah, P.G.; Tapriyal, D.; Enick, R.M.; Crandall, D.M.; Goodman, A. Improving CO2-EOR In Shale Reservoirs Using Dilute Concentrations of Wettability-Altering CO2-Soluble Nonionic Surfactants. In Proceedings of the 8th Unconventional Resources Technology Conference, Virtual, 20 July 2020; OnePetro: Richardson, TX, USA, 2020; p. URTEC-2020-2774-MS. [Google Scholar] [CrossRef]
- Zhou, M.; Ni, R.; Zhao, Y.; Huang, J.; Deng, X. Research Progress on Supercritical CO2 Thickeners. Soft Matter 2021, 17, 5107–5115. [Google Scholar] [CrossRef]
- Pal, N.; Zhang, X.; Ali, M.; Mandal, A.; Hoteit, H. Carbon Dioxide Thickening: A Review of Technological Aspects, Advances and Challenges for Oilfield Application. Fuel 2022, 315, 122947. [Google Scholar] [CrossRef]
- Kuang, N.; Yang, S.; Yuan, Z.; Wang, M.; Zhang, Z.; Zhang, X.; Wang, M.; Zhang, Y.; Li, S.; Wu, J.; et al. Study on Oil and Gas Amphiphilic Surfactants Promoting the Miscibility of CO2 and Crude Oil. ACS Omega 2021, 6, 27170–27182. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, X.; Wang, R.; Zhang, X.; Ma, S.; Su, Y.; Wang, C.; Luo, W.; Sun, H. Microscopic Experiment Study on Mechanisms of Oil-Gas Interaction and CO2 -Surfactant Flooding with Different Temperatures and Pressures. J. CO2 Util. 2023, 69, 102389. [Google Scholar] [CrossRef]
- Tang, J.; Vincent-Bonnieu, S.; Rossen, W.R. Experimental Investigation of the Effect of Oil on Steady-State Foam Flow in Porous Media. SPE J. 2019, 24, 140–157. [Google Scholar] [CrossRef]
- Lehrer, S.E.; Debord, S.B.; Lehmann, M.N.; Means, N.C. Method for Recovering Oil from an Oil Well 2013. U.S. Patent AU-A-2010278850, 30 July 2010. [Google Scholar]
- Ren, G.; Nguyen, Q.P.; Lau, H.C. Laboratory Investigation of Oil Recovery by CO2 Foam in a Fractured Carbonate Reservoir Using CO2-Soluble Surfactants. J. Pet. Sci. Eng. 2018, 169, 277–296. [Google Scholar] [CrossRef]
| Structure | Conditions | Solubility | Co-Solvent | Reference |
|---|---|---|---|---|
 M-F7H4 | 60 MPa 45 °C | 4.4 wt% | - | Cummings [61] |
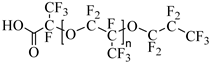 Krytox 157 FSL Mw 2500 | 22 MPa 60.85 °C | 1.72 wt% | - | Temtem [62] |
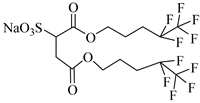 di-CF2 | 22.4 MPa 40 °C | 2.77 wt% | - | Mohamed [63] |
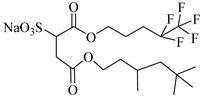 Hybrid CF2/AOT4 | 34 MPa 40 °C | 2.59 wt% | - | Mohamed [63] |
 FC6-FC4 | 35 MPa 45 °C | 1.08 wt% | - | Sagisaka [64,65] |
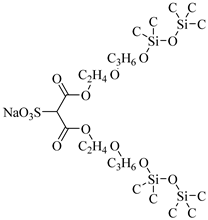 Sulfonated siloxane-functional sulfonate surfactants | 31.7 MPa 65 °C | 1.00 wt% | - | Fink [66] |
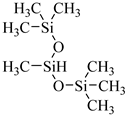 HMTS | 9.25 MPa 50 °C | 0.11 wt% | - | Shi [67] |
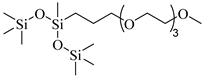 (PEG)3-TS | 10.09 MPa 50 °C | 0.003 wt% | - | Shi [67] |
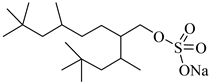 SIS1 | 35–75 °C 10–40 MPa | 0.007 wt% | - | Sagisaka [68] |
 TMN-6 | 35–75 °C 10–40 MPa | 0.004 wt% | - | Sagisaka [68] |
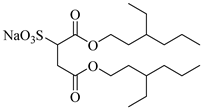 AOT | 30 MPa 60 °C | Dissolved | Ethanol | Ihara [69] |
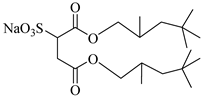 sodium bis(2,4,4-trimethyl-1-pentyl)sulfosuccinate | 25 MPa 33 °C | Dissolved | - | Eastoe [51,52,53] |
 sodium bis(3,5,5-trimethyl-1-hexyl) sulfosuccinate | 25 MPa 33 °C | Dissolved | - | Eastoe [70,71,72] |
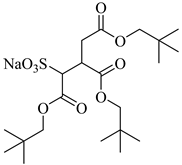 TC14 | 3 MPa 25 °C | Dissolved | - | Hollamby [73] |
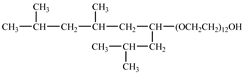 Tergitol TMN ethoxylated nonyl ether | 2.75 MPa 25 °C | 1.0 wt% | - | Ryoo [74] |
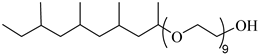 SURFONIC TDA-9 | 29 MPa 77 °C | 1.0 wt% | - | Burrows [75] |
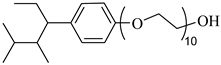 SURFONIC N-100 | 38 MPa 77 °C | 1.0 wt% | - | Burrows [75] |
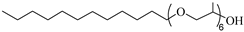 SURFONIC L12-6 | 31 MPa 77 °C | 1.0 wt% | - | Burrows [75] |
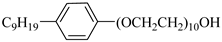 N-NP-10c | 18.4 MPa 40 °C | 1.76 wt% | Ethanol | Zhang [57] |
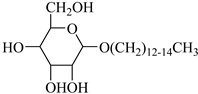 APG-1214 | 17.4 MPa 40 °C | 1.66 wt% | Ethanol and ethylene glycol (3:2) | Zhang [57] |
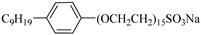 N-NP-15c-H | 17 MPa 40 °C | 0.57 wt% | Ethanol and ethylene glycol (4:1) | Zhang [57] |
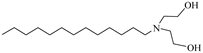 C12–14N(EO)2 | 22.76 MPa 120 °C | 0.2 wt% | - | Chen [76,77,78] |
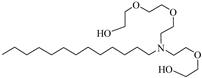 C12–14N(EO)5 | 17.93 MPa 120 °C | 0.2 wt% | - | Chen [76,77,78] |
| Reservoir Type | Conditions | System Composition | Function | Ultimate Oil Recovery | Reference |
|---|---|---|---|---|---|
| \* | 25 °C, 35 MPa | Ni(di-HCF4) (10 wt%) in CO2 | Thicken the system to 0.22 MPa·s | \ | Trickett [125] |
| \ | 40 °C, 39.24 MPa | Vinyl polysiloxane (8 wt%) in CO2 | Thicken the system to 12.57 MPa·s | \ | Zhao [126] |
| Daqing crude oil | 45 °C, 22,7 MPa | AOT (0.005 wt%–0.015 wt%), ethanol (13.76 wt%), water (0.41 wt%–1.61 wt%) in CO2 | Reduce the MMP from 23.8 to 22.7 MPa | About 80% | Dong [127] |
| Shengli crude oil | 60 °C, 13.22 MPa | C12PO6 (0.6 wt%), ethanol (0.7 wt%) in CO2 | Reduce the MMP from 17.79 to 13.22 MPa | \ | Wang [128] |
| \ | 85 °C, 21.2 MPa | CAE (0.2 wt%) in CO2 | Reduce the MMP from 27.3 to 21.2 MPa | 92.06% | Guo [129] |
| Shengli crude oil | 60 °C, 11.41 MPa | TXIB (0.3 wt%%), ethanol (7 wt%) in CO2 | Reduce the MMP from 16.79 to 11.41 MPa | \ | Zhang [130] |
| \ | 50 °C, 13.6 MPa | C4PsO3 (3 wt%) in CO2 | Reduce the MMP from 17.75 to 13.6 MPa | \ | Lv [131,132] |
| QS8 oilfield | 76 °C, 30 MPa | SPO5 (0.5 wt%), n-pentanol (0.25 wt%) in CO2 | Reduce the IFT of the CO2-oil system | 93.47% | Kuang [116] |
| Midland Farm (West Texas) crude oil | 26.67 °C, 10.34 MPa | Surfactant (0.5 wt%) in CO2 | Generate foam, as indicated by the immediate increase of pressure drop at the start of CO2 injection | 60% | Le [133] |
| SACROC Field | 60 °C, 24.13 MPa | ELEVATE™ CO2 EOR Conformance Solutions | Generate foam | Oil production increased by 30% in one month | Sanders [56] |
| Silurian-dolomite cores | 35 °C, 10.34 MPa | CO2-soluble surfactants (S, 4 S, and 15 S) (0.15 wt%) in CO2 | Reduce the delay of foam propagation | \ | Ren [16,134] |
| \ | 120 °C, 20 MPa | N-P-12 (2.99 wt%), ethylene glycol (16.97 wt%) in CO2 | Increase and then decrease the resistance | 92.5% | Bi [135] |
| \ | 40 °C, 11.72 MPa | DTTM (0.5 wt%) in CO2 | Cause delay in foam generation and propagation by viscous fingering | \ | Ramadhan [136] |
| Eagle Ford outcrop rock Eagle Ford crude oil | 80 °C, 27.6 MPa | SURFONIC®N-100 (0.1 wt%) in CO2 | Alter the wettability of unconventional rock | 75% | Haeri [137] |
| Factor | General Effects on CO2 of Increasing the Factor | |
|---|---|---|
| Solubility | Viscosity | |
| Molecular weight | Decrease | Increase |
| Concentration | Increase and then decrease | Increase |
| Shear rate | Increase | Decrease |
| Temperature | Decrease | Decrease |
| Pressure | Increase | Increase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Luo, W.; Luo, Z.; Wang, W.; Xue, X.; Dong, B. Research of CO2-Soluble Surfactants for Enhanced Oil Recovery: Review and Outlook. Molecules 2023, 28, 8042. https://doi.org/10.3390/molecules28248042
Liang S, Luo W, Luo Z, Wang W, Xue X, Dong B. Research of CO2-Soluble Surfactants for Enhanced Oil Recovery: Review and Outlook. Molecules. 2023; 28(24):8042. https://doi.org/10.3390/molecules28248042
Chicago/Turabian StyleLiang, Shisheng, Wenli Luo, Zhixing Luo, Wenjuan Wang, Xiaohu Xue, and Bo Dong. 2023. "Research of CO2-Soluble Surfactants for Enhanced Oil Recovery: Review and Outlook" Molecules 28, no. 24: 8042. https://doi.org/10.3390/molecules28248042
APA StyleLiang, S., Luo, W., Luo, Z., Wang, W., Xue, X., & Dong, B. (2023). Research of CO2-Soluble Surfactants for Enhanced Oil Recovery: Review and Outlook. Molecules, 28(24), 8042. https://doi.org/10.3390/molecules28248042







