Sustainable Coating Based on Zwitterionic Functionalized Polyurushiol with Antifouling and Antibacterial Properties
Abstract
:1. Introduction
2. Results and Discussions
2.1. Synthesis and Structural Characterization of the HUDM-SB and IPUDM-SB Monomers
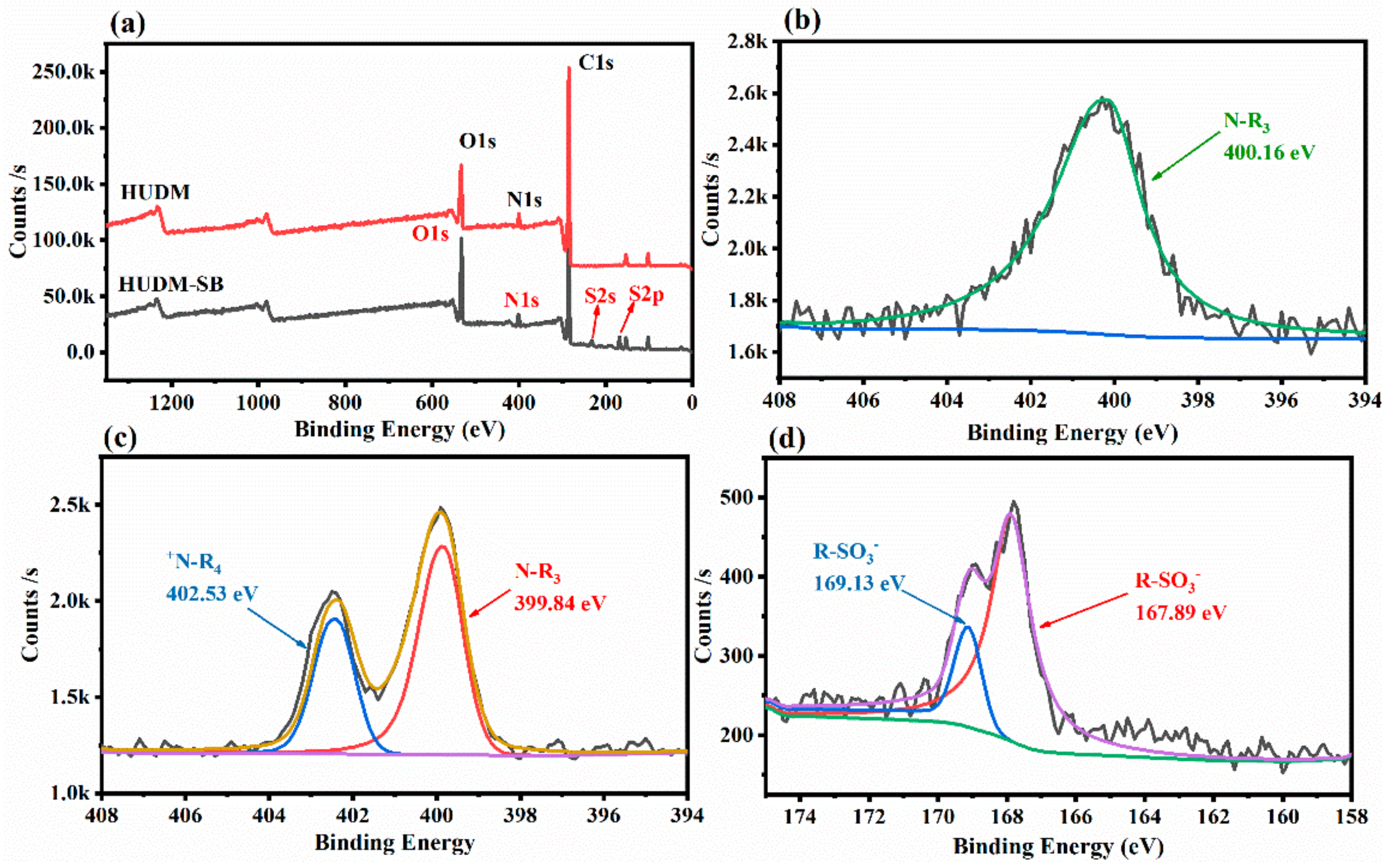
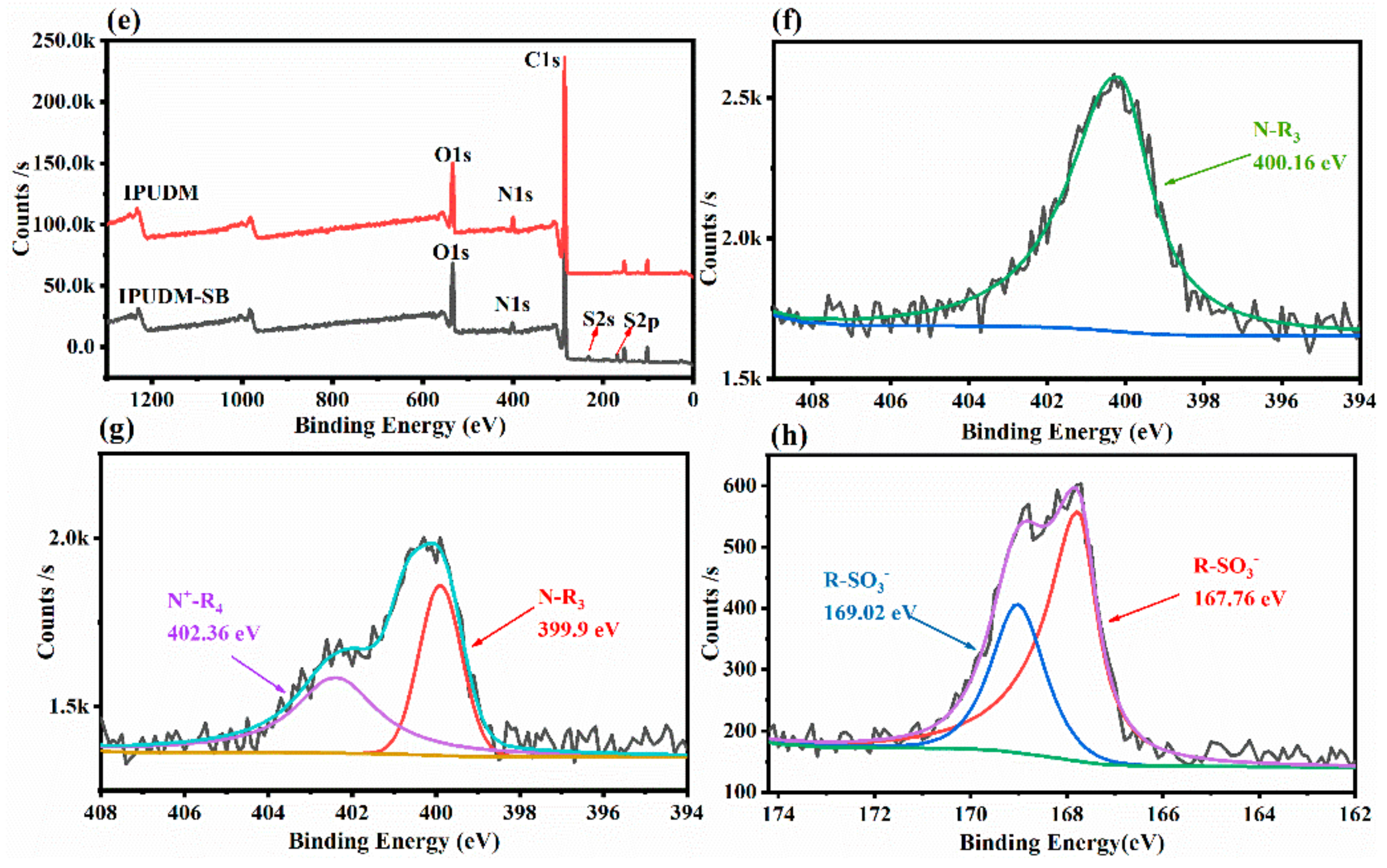
2.2. Surface Elemental Composition of the HUDM-SB and IPUDM-SB Coatings
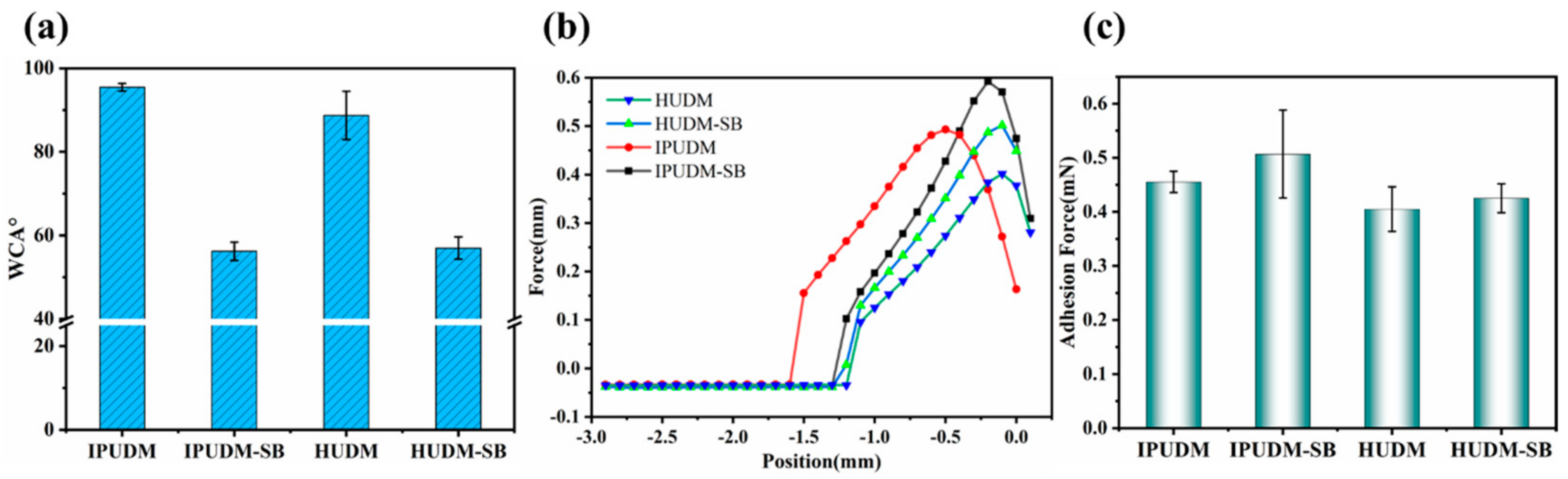
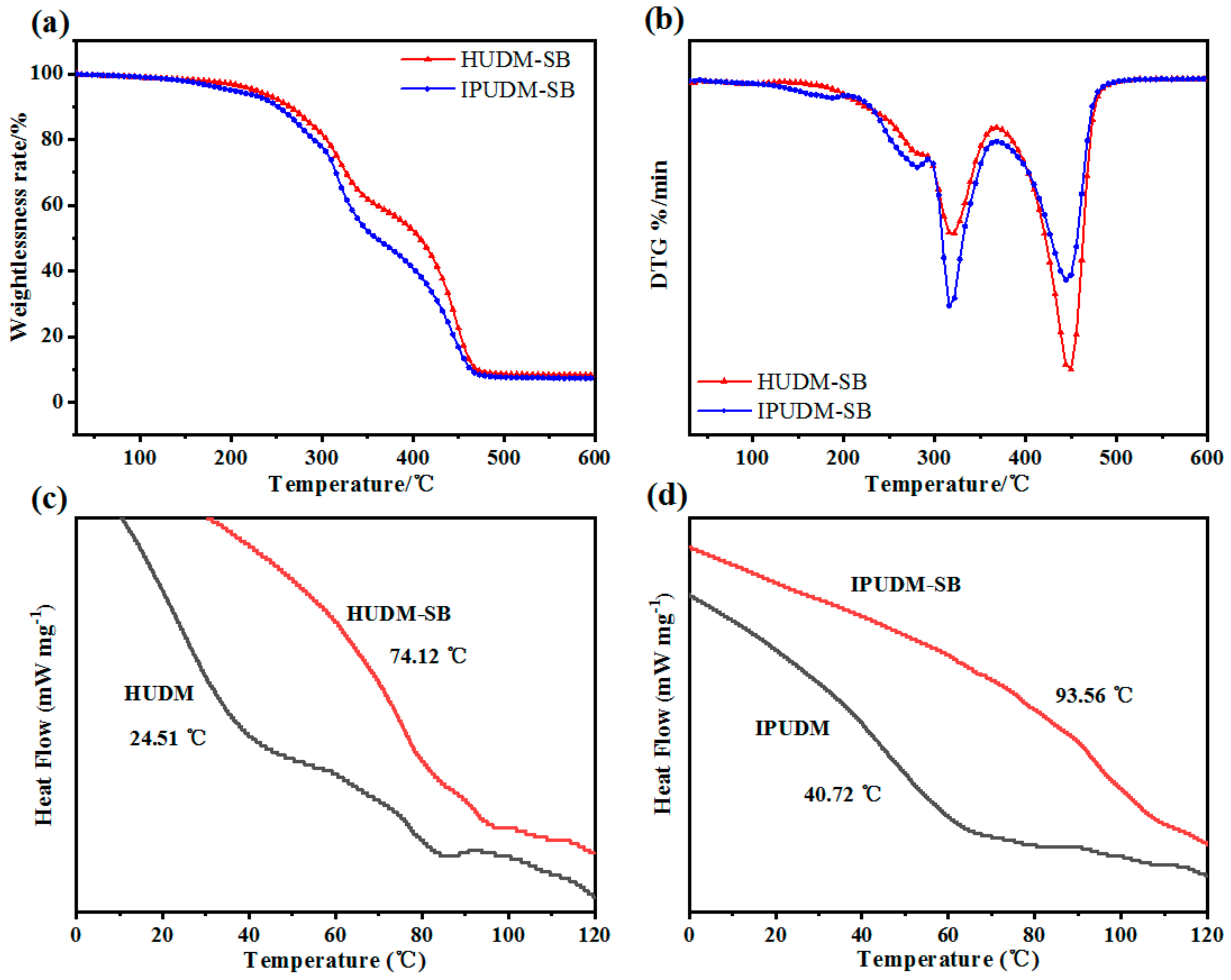
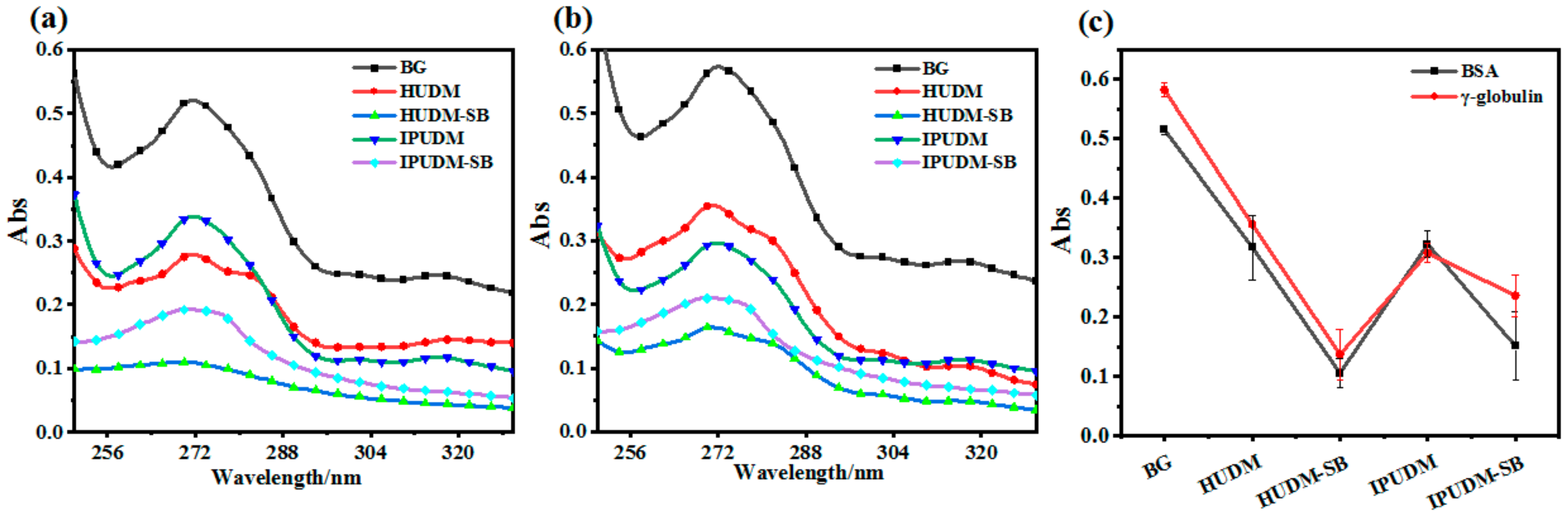
2.3. Surface and Physicochemical Properties of the Coatings
2.4. Surface and Physicochemical Properties of the Coatings
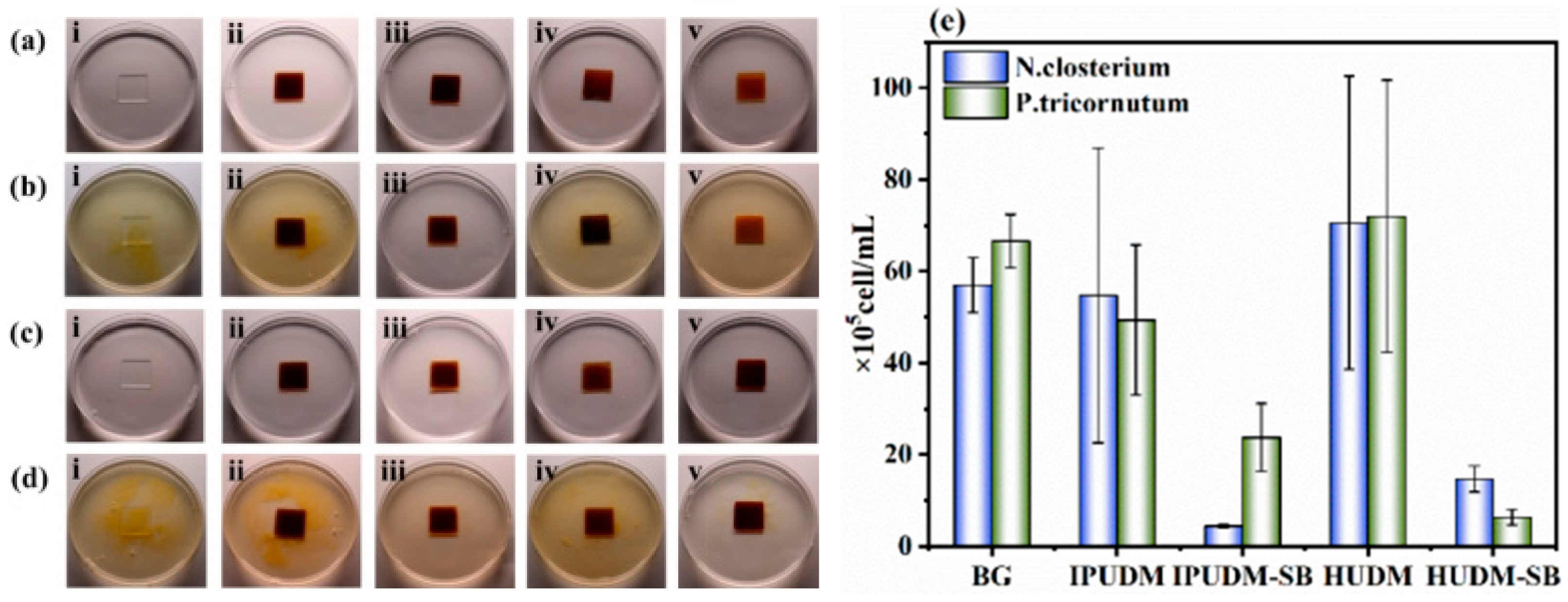
2.5. Antibacterial Performance of the Coatings
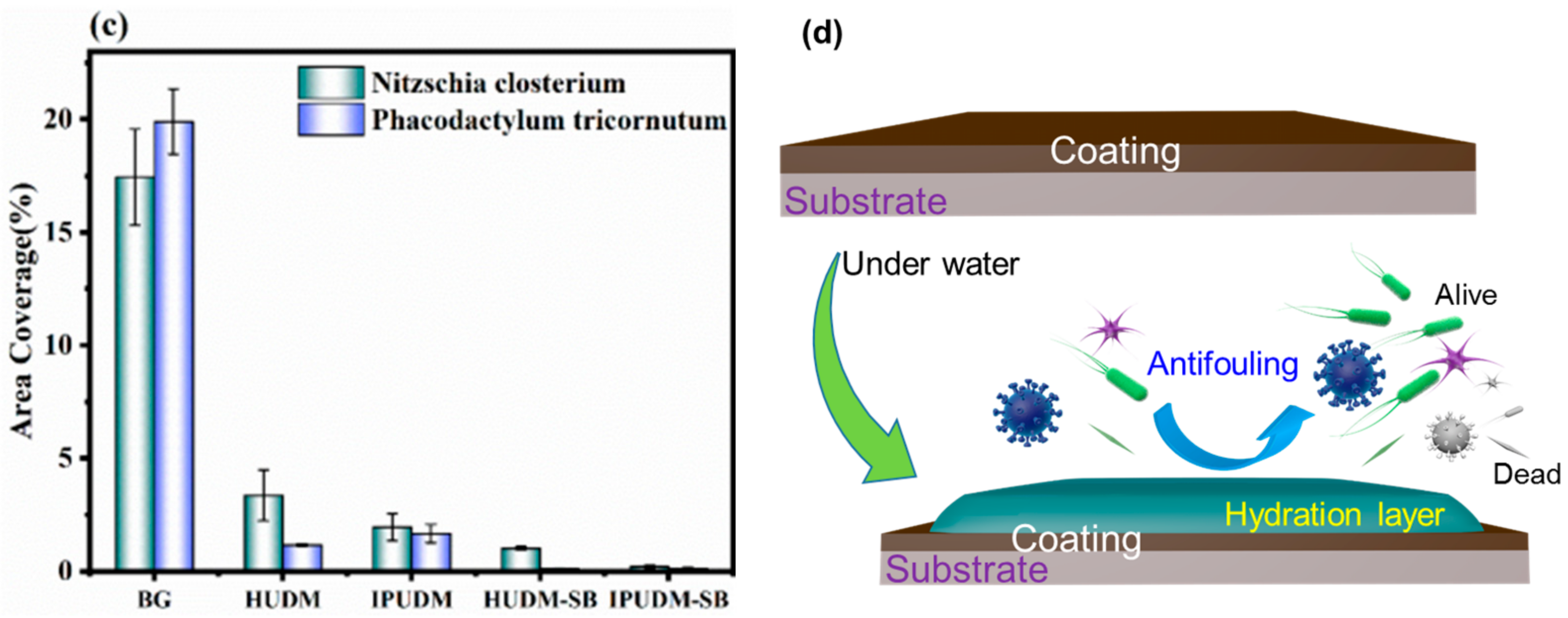
2.6. Algal-Fouling Resistance of the Coatings
3. Materials and Methods
3.1. Materials
3.2. Synthesis of HUDM-SB Monomers
3.3. Synthesis of IPUDM-SB Monomers
3.4. Synthesis of HUDM-SB and IPUDM-SB Coatings
3.5. Characterizations
3.6. Testing of Surface-Protein Adsorption
3.7. Assessment of Antibacterial Activity
3.8. Algal Biofouling Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, H.; Tian, L.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Pourhashem, S.; Seif, A.; Saba, F.; Nezhad, E.G.; Ji, X.; Zhou, Z.; Zhai, X.; Mirzaee, M.; Duan, J.; Rashidi, A. Antifouling nanocomposite polymer coatings for marine applications: A review on experiments, mechanisms, and theoretical studies. J. Mater. Sci. Technol. 2022, 118, 73–113. [Google Scholar] [CrossRef]
- Romeu, M.J.; Mergulhão, F. Development of Antifouling Strategies for Marine Applications. Microorganisms 2023, 11, 1568. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, W.; Li, W.; Sun, T.; Feng, H.; Lv, G.; Chen, S. Full solar spectrum-driven Cu2O/PDINH heterostructure with enhanced photocatalytic antibacterial activity and mechanism insight. J. Hazard. Mater. 2023, 448, 130851. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Xu, L. A Cu2O-based marine antifouling coating with controlled release of copper ion mediated by amphiphilic PLMA-b-PDMAEMA copolymers. Prog. Org. Coat. 2022, 170, 107003. [Google Scholar] [CrossRef]
- Soon, Z.Y.; Jung, J.; Jang, M.; Kang, J.; Jang, M.; Lee, J.; Kim, M. Zinc pyrithione (ZnPT) as an antifouling biocide in the marine environment—A literature review of its toxicity, environmental fates, and analytical methods. Water Air Soil Pollut. 2019, 230, 310. [Google Scholar] [CrossRef]
- Lee, S.; Haque, M.N.; Lee, D.; Rhee, J. Comparison of the effects of sublethal concentrations of biofoulants, copper pyrithione and zinc pyrithione on a marine mysid-A multigenerational study. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 271, 109694. [Google Scholar] [CrossRef]
- Almond, K.M.; Trombetta, L.D. The effects of copper pyrithione, an antifouling agent, on developing zebrafish embryos. Ecotoxicology 2016, 25, 389–398. [Google Scholar] [CrossRef]
- Li, Q.; Huang, M.; Li, F.; Ling, Z.; Meng, Y.; Chen, F.; Ji, Z.; Wang, S. Biomimetic stable cellulose based superhydrophobic Janus paper sheets engineered with industrial lignin residues/nano-silica for efficient oil-water separation. Ind. Crops Prod. 2024, 207, 117774. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Q.; Li, X.; Meng, Y.; Ling, Z.; Ji, Z.; Chen, F. Preparation and Characterization of Degradable Cellulose−Based Paper with Superhydrophobic, Antibacterial, and Barrier Properties for Food Packaging. Int. J. Mol. Sci. 2022, 23, 11158. [Google Scholar] [CrossRef]
- Chen, J.; Jian, R.; Yang, K.; Bai, W.; Huang, C.; Lin, Y.; Zheng, B.; Wei, F.; Lin, Q.; Xu, Y. Urushiol-based benzoxazine copper polymer with low surface energy, strong substrate adhesion and antibacterial for marine antifouling application. J. Clean. Prod. 2021, 318, 128527. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Gopinath, K.; Zhang, K.; Kang, E.T.; Xu, L.; Yu, Y. Recent progress in tannic acid-driven antibacterial/antifouling surface coating strategies. J. Mater. Chem. B 2022, 10, 2296–2315. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, J.; Lin, F.; Zheng, X.; Jian, R.; Lin, Y.; Wei, F.; Lin, Q.; Bai, W.; Xu, Y. Polymerized tung oil toughened urushiol-based benzoxazine copper polymer coatings with excellent antifouling performances. Prog. Org. Coat. 2023, 177, 107411. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, Y.; Zhang, S.; Xiong, G.; Wang, K.; Zhang, Z. Anti-marine biofouling adhesion performance and mechanism of PDMS fouling-release coating containing PS-PEG hydrogel. Mar. Pollut. Bull. 2023, 194, 115345. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Review of the research on anti-protein fouling coatings materials. Prog. Org. Coat. 2020, 147, 105860. [Google Scholar] [CrossRef]
- Li, K.; Qi, Y.; Zhou, Y.; Sun, X.; Zhang, Z. Microstructure and properties of poly (ethylene glycol)-segmented polyurethane antifouling coatings after immersion in seawater. Polymers 2021, 13, 573. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, P.; Li, X.; Wang, X. Protein-resistant surface based on zwitterion-functionalized nanoparticles for marine antifouling applications. New J. Chem. 2020, 44, 2059–2069. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, P.; Zhang, J.; Deng, L.; Zhang, J.; Lin, C.; Guo, R.; Dong, A. Novel dual-functional coating with underwater self-healing and anti-protein-fouling properties by combining two kinds of microcapsules and a zwitterionic copolymer. Prog. Org. Coat. 2019, 127, 211–221. [Google Scholar] [CrossRef]
- Zheng, L.; Sundaram, H.S.; Wei, Z.; Li, C.; Yuan, Z. Applications of zwitterionic polymers. React. Funct. Polym. 2017, 118, 51–61. [Google Scholar] [CrossRef]
- Chen, P.; Lang, J.; Zhou, Y.; Khlyustova, A.; Zhang, Z.; Ma, X.; Liu, S.; Cheng, Y.; Yang, R. An imidazolium-based zwitterionic polymer for antiviral and antibacterial dual functional coatings. Sci. Adv. 2022, 8, abl8812. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, M.; Peng, P.; Liu, J.; Lu, J.; Qian, S.; Feng, J. “Self-Defensive” antifouling zwitterionic hydrogel coatings on polymeric substrates. ACS Appl. Mater. Interfaces 2022, 14, 56097–56109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Tian, S.; Qi, X.; Yang, J.; Li, Q.; Lin, C.; Zhang, J.; Zhang, L. A switchable zwitterionic ester and capsaicin copolymer for multifunctional marine antibiofouling coating. Chem. Eng. J. 2022, 436, 135072. [Google Scholar] [CrossRef]
- Guan, Y.; Li, S.-L.; Fu, Z.; Qin, Y.; Wang, J.; Gong, G.; Hu, Y. Preparation of antifouling TFC RO membranes by facile grafting zwitterionic polymer PEI-CA. Desalination 2022, 539, 115972. [Google Scholar] [CrossRef]
- Li, D.; Li, K.; Fang, J. Research Progress on Modification and Application of Raw Lacquer. ChemistrySelect 2022, 7, e202200943. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, G.; Zhang, G.; Ma, C. Rapid curing and self-stratifying lacquer coating with antifouling and anticorrosive properties. Chem. Eng. J. 2021, 421, 129755. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Lin, X.; Ni, P.; Liu, H.; Li, S. Catechol derivative urushiol’s reactivity and applications beyond traditional coating. Ind. Crops Prod. 2023, 197, 116598. [Google Scholar] [CrossRef]
- Ma, J.; Lee, G.H.; Kim, J.H.; Kim, S.W.; Jo, S.; Kim, C. A transparent self-healing polyurethane–isophorone-diisocyanate elastomer based on hydrogen-bonding interactions. ACS Appl. Polym. Mater. 2022, 4, 2497–2505. [Google Scholar] [CrossRef]
- Oh, J.; Kim, Y.K.; Hwang, S.; Kim, H.; Jung, J.; Jeon, C.; Kim, J.; Lim, S.K. Synthesis of thermoplastic polyurethanes containing bio-based polyester polyol and their fiber property. Polymers 2022, 14, 2033. [Google Scholar] [CrossRef]
- Gaddam, S.K.; Arukula, R. Renewable soft segment-induced anionic waterborne polyurethane dispersions with enriched bio-content. J. Polym. Res. 2022, 29, 59. [Google Scholar] [CrossRef]
- Cheng, Q.; Jia, X.; Cheng, P.; Zhou, P.; Hu, W.; Cheng, C.; Hu, H.; Xia, M.; Liu, K.; Wang, D. Improvement of the filtration and antifouling performance of a nanofibrous sterile membrane by a one-step grafting zwitterionic compound. New J. Chem. 2022, 46, 15423–15433. [Google Scholar] [CrossRef]
- Yuan, Y.; Tan, W.; Zhang, J.; Li, Q.; Guo, Z. Water-soluble amino functionalized chitosan: Preparation, characterization, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2022, 217, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, J.; Zheng, X.; Lin, Q.; Zheng, G.; Xu, Y.; Lin, F. Urushiol-Based Benzoxazine Containing Sulfobetaine Groups for Sustainable Marine Antifouling Applications. Polymers 2023, 15, 2383. [Google Scholar] [CrossRef]
- Morang, S.; Karak, N. Nanocomposites of waterborne polyurethanes. In Eco-Friendly Waterborne Polyurethanes; CRC Press: Boca Raton, FL, USA, 2022; pp. 83–100. [Google Scholar]
- Bi, J.; Yan, Z.; Hao, L.; Elnaggar, A.Y.; El-Bahy, S.M.; Zhang, F.; Azab, I.H.E.; Shao, Q.; Mersal, G.A.; Wang, J. Improving water resistance and mechanical properties of waterborne acrylic resin modified by octafluoropentyl methacrylate. J. Mater. Sci. 2023, 58, 1452–1464. [Google Scholar] [CrossRef]
- Chou, Y.-N.; Ou, M. Zwitterionic Surface Modification of Aldehydated Sulfobetaine Copolymers for the Formation of Bioinert Interfaces. ACS Appl. Polym. Mater. 2023, 5, 5411–5428. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, Y.; Ma, X.; Geng, X.; Ye, L.; Zhang, A.; Feng, Z. Preparation and characterisation of zwitterionic sulfobetaine containing siloxane-based biostable polyurethanes. Mater. Adv. 2022, 3, 4608–4621. [Google Scholar] [CrossRef]
- Chen, Z. Surface hydration and antifouling activity of zwitterionic polymers. Langmuir 2022, 38, 4483–4489. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Cai, Y.; Xu, L.; Yi, L. Protein-resistant amphiphilic copolymers containing fluorosiloxane side chains with controllable length. ACS Appl. Polym. Mater. 2022, 4, 7903–7910. [Google Scholar] [CrossRef]
- Sarker, P.; Lu, T.; Liu, D.; Wu, G.; Chen, H.; Sajib, M.S.J.; Jiang, S.; Chen, Z.; Wei, T. Hydration Behaviors of Nonfouling Zwitterionic Materials. Chem. Sci. 2023, 14, 7500–7511. [Google Scholar] [CrossRef]
- Chen, C.; Li, Z.; Li, X.; Kuang, C.; Liu, X.; Song, Z.; Liu, H.; Shan, Y. Dual-functional antimicrobial coating based on the combination of zwitterionic and quaternary ammonium cation from rosin acid. Compos. Part B 2022, 232, 109623. [Google Scholar] [CrossRef]
- Peng, J.; Li, K.; Du, Y.; Yi, F.; Wu, L.; Liu, G. A robust mixed-charge zwitterionic polyurethane coating integrated with antibacterial and anticoagulant functions for interventional blood-contacting devices. J. Mater. Chem. B 2023, 11, 8020–8032. [Google Scholar] [CrossRef]
- Malouch, D.; Berchel, M.; Dreanno, C.; Stachowski-Haberkorn, S.; Chalopin, M.; Godfrin, Y.; Jaffrès, P. Evaluation of lipophosphoramidates-based amphiphilic compounds on the formation of biofilms of marine bacteria. Biofouling 2023, 39, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Gu, J.; Li, J.; Wang, Y.; Chen, S. Tailorable antibacterial and cytotoxic chitosan derivatives by introducing quaternary ammonium salt and sulfobetaine. Int. J. Biol. Macromol. 2022, 218, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Yuan, Z.; Wang, Y.; Song, Z.; Gong, X.; Zhao, Y.; Mu, Q.; Zhan, Q.; Xu, W.; Wang, L. Structures, properties, and applications of zwitterionic polymers. ChemPhysMater 2022, 1, 294–309. [Google Scholar] [CrossRef]
- Xu, H.; Lu, Z.; Zhang, G. Synthesis and properties of thermosetting resin based on urushiol. RSC Adv. 2012, 2, 2768–2772. [Google Scholar] [CrossRef]
- GB/T 6739-2006/ISO 15184–2012; Paints and Varnishes-Determination of Film Hardness by Pencil Test. ISO: Geneva, Switzerland, 2012.
- GB/T 1720–1979; Method of Test for Adhesion of Paint Films. China Petroleum and Chemical Industry Federation: Beijing, China, 1979.



| Samples | Adhesion (Grade) | Pencil Hardness | Glossiness (%) |
|---|---|---|---|
| IPUDM | 7 | 1H | 93.7 |
| IPUDM-SB | 3 | 2H | 88.7 |
| HUDM | 6 | 4B | 65.8 |
| HUDM-SB | 4 | HB | 44.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Xie, H.; Sun, C.; Lin, W.; You, Z.; Zheng, G.; Zheng, X.; Xu, Y.; Chen, J.; Lin, F. Sustainable Coating Based on Zwitterionic Functionalized Polyurushiol with Antifouling and Antibacterial Properties. Molecules 2023, 28, 8040. https://doi.org/10.3390/molecules28248040
Xu K, Xie H, Sun C, Lin W, You Z, Zheng G, Zheng X, Xu Y, Chen J, Lin F. Sustainable Coating Based on Zwitterionic Functionalized Polyurushiol with Antifouling and Antibacterial Properties. Molecules. 2023; 28(24):8040. https://doi.org/10.3390/molecules28248040
Chicago/Turabian StyleXu, Kaiyue, Huimin Xie, Chenyi Sun, Wenyan Lin, Zixuan You, Guocai Zheng, Xiaoxiao Zheng, Yanlian Xu, Jipeng Chen, and Fengcai Lin. 2023. "Sustainable Coating Based on Zwitterionic Functionalized Polyurushiol with Antifouling and Antibacterial Properties" Molecules 28, no. 24: 8040. https://doi.org/10.3390/molecules28248040
APA StyleXu, K., Xie, H., Sun, C., Lin, W., You, Z., Zheng, G., Zheng, X., Xu, Y., Chen, J., & Lin, F. (2023). Sustainable Coating Based on Zwitterionic Functionalized Polyurushiol with Antifouling and Antibacterial Properties. Molecules, 28(24), 8040. https://doi.org/10.3390/molecules28248040







